Abstract

Lovastatin is a standard therapy for dyslipidemia. Alternatively, some ethnomedicines, such as Coptidis preparation, have been used for the treatment of dyslipidemia. Statins and complementary and alternative medicines may possess individual mechanisms of action against dyslipidemia. We hypothesize that the combination of Coptidis preparation and lovastatin may have synergistic effects for the treatment of dyslipidemia. To investigate this hypothesis, we developed a validated ultra-high-performance liquid chromatography-tandem mass spectrometry method to monitor lovastatin and its metabolites for pharmacokinetic studies in rats. This study was divided into four groups: lovastatin (10 mg/kg, p.o.) alone and lovastatin (10 mg/kg, p.o.) + Coptidis preparation (0.3, 1, or 3 g/kg, p.o.) for five consecutive days. In pharmacodynamic studies, a high-fat diet (HFD) was used to induce dyslipidemia in experimental rat models. The HFD rats were divided into four groups: treatment with HFD, HFD + lovastatin (100 mg/kg, p.o.), HFD + Coptidis preparation (1 g/kg, p.o.), and HFD + lovastatin (50 mg/kg, p.o.) + Coptidis preparation (1 g/kg, p.o.) for 28 consecutive days. The pharmacokinetic results demonstrated that Coptidis preparation significantly augmented the conversion of lovastatin into its main metabolite lovastatin acid in vivo. The pharmacodynamic results revealed that the Coptidis preparation and half-dose lovastatin group reduced the body weight, liver weight, and visceral fat in HFD rats. These findings provide constructive preclinical pharmacokinetic and pharmacodynamic applications of Coptidis preparation on the benefit of hyperlipidemia.
1. Introduction
Primary dyslipidemia is usually caused by genetic factors, while secondary dyslipidemia may have other underlying causes, such as diabetes.1 Dyslipidemia is the leading cause of atherosclerosis, which can cause cardiovascular disease,2 the most common cause of death in developed countries. To date, the best treatments for dyslipidemia are lipid-lowering drugs and lifestyle changes.3 The U.S. Food and Drug Administration has approved statins and a variety of other nonstatin drugs for the treatment of dyslipidemia, including bile acid sequestrants, cholesterol absorption inhibitors, fibrates, niacin, and omega-3;3 however, statins are among the most widely used prescription drugs for dyslipidemia. For most patients who do not meet the criteria for treatment with statins, supplementary or alternative therapies that complement the intake of a plant-based diet and limit the intake of sweets have been proven to be effective.3
Lovastatin is a cholesterol-lowering drug that can competitively inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase.4 This drug can reduce cholesterol synthesis, reduce the concentration of apolipoprotein B, and increase the activity of low-density lipoprotein (LDL) receptors without adverse effects on other products in the cholesterol synthesis pathway.4 Lovastatin can also reduce the concentrations of triglycerides and increase the concentration of high-density lipoprotein (HDL)4 in plasma. However, a small number of patients experience symptoms such as headache, rash, insomnia, and myalgia, as well as gastrointestinal discomfort, such as flatulence, irregular bowel movements, and nausea.4 In addition, lovastatin is a water-insoluble drug with low oral bioavailability because it cannot be completely absorbed from the gastrointestinal tract, which affects its therapeutic effects.5 Thus, the development of a herbal drug combination dosing regimen with lovastatin should be a good strategy to provide synergistic effects to improve the efficacy of drug treatment.
Many patients with dyslipidemia seek complementary or alternative medicines.6 Traditional Chinese medicine can provide preventative effects for complementary and alternative methods of cardiovascular disease treatment, and it has been widely used in clinical practice.7 Coptidis preparation consists of Coptidis rhizome, Scutellariae radix, and Rhei rhizome with a weight ratio of 2:1:1 (similar to the traditional Chinese medicine, San-Huang-Xie-Xin-Tang). The berberine contained within the Coptidis rhizome can inhibit the production of fat and LDL and has antiobesity and anti-dyslipidemic effects.8 Herbs contain many ingredients and can cause many effects. For example, if certain drugs can increase the absorption rate of rhein, berberine, and baicalein, then these drugs can simultaneously help promote the oral bioavailability of Coptidis preparation.9 The ingredients of Coptidis preparation mutually reinforce drug interactions at the level of pharmacodynamics and pharmacokinetics.9
Coptidis preparation, the traditional Chinese medicine, was first recorded in the ancient Chinese medical literature “The Synopsis of the Golden Chamber” (in Chinese, Jingui Yaolue); its clinical uses include the prevention and treatment of atherosclerosis and constipation,10 and it protects gastric mucosa.11 Numerous studies have also found that Coptidis preparation has other biological activities and therapeutic effects, such as antihypertensive effects,12 antiatherosclerosis effects, and cardioprotective effects.9
Based on the literature survey above, outpatients with dyslipidemia can consider combining lovastatin with Coptidis preparation. Our hypothesis is that Coptidis preparation may have a lipid-lowering effect on the lovastatin treatment group. The aim of this study was to investigate the herb–drug interactions between lovastatin and Coptidis preparation and the lipid-lowering effects in obese rats. For the pharmacokinetic studies, Coptidis preparation (0.3, 1, or 3 g/kg, p.o.) was administered for five consecutive days before lovastatin administration (10 mg/kg, p.o.). In the pharmacodynamic study, four groups of 5-week-old rats on high-fat diet (HFD) received either no additional medication, lovastatin (100 mg/kg, p.o.), Coptidis preparation (1 g/kg, p.o.), or Coptidis preparation (1 g/kg, p.o.) in combination with lovastatin (50 mg/kg, p.o.), respectively, for 28 days to study the effects of different treatment schemes on weight gain. Total cholesterol (TC), triglycerides (TG), high-density-lipoprotein cholesterol (HDL-C), low-density-lipoprotein cholesterol (LDL-C), and body weights of rats were measured. In addition, a histopathological examination of hepatic lipids, perirenal fat tissue, and epididymal fat tissue was performed to determine the lipid-lowering functions of the Coptidis preparation on HFD-induced dyslipidemia.
2. Results and Discussion
2.1. Chromatographic Analysis
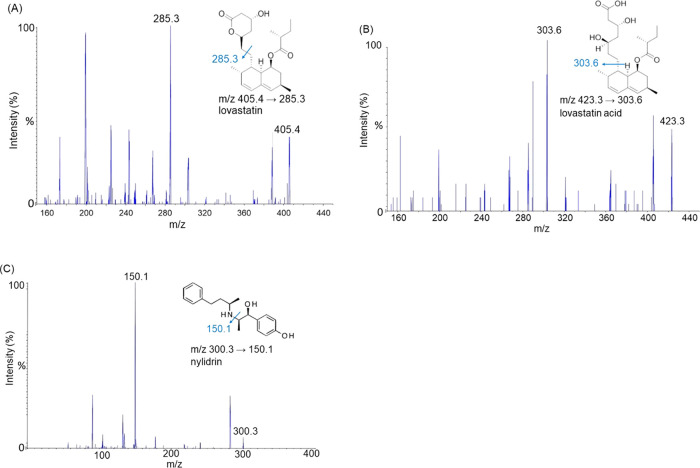
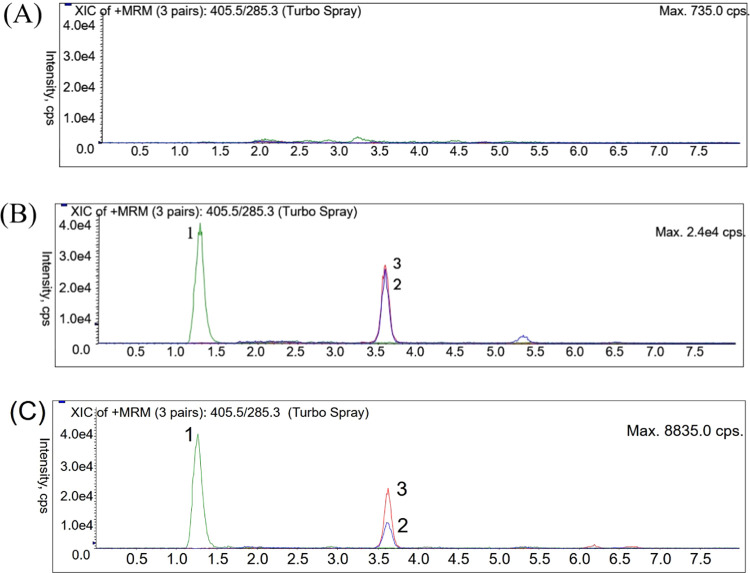
In terms of chromatographic analysis, we optimized the composition of the analytical column and the mobile phase by adjusting the concentration of formic acid and the percentage of organic solvents to improve the resolution of the analytes, including lovastatin, lovastatin acid, and nylidrin. After elution, the symmetry of the chromatographic peak was generated by the detector system. It was found that using 0.1% formic acid/methanol (10:90) gave the best conditions for isolating lovastatin and lovastatin acid from rat plasma. According to the mass and abundance of peaks in the ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) spectrum, we could obtain information on the exact mass and the molecular structure of each analyzed molecule, including m/z 405.4 → 285.3 for lovastatin, m/z 423.3 → 303.6 for lovastatin acid, and m/z 300.3 → 150.1 for nylidrin, as shown in Figure 1. The highest peak symmetry indicates that the analytes lovastatin and lovastatin acid are sufficiently separated. As shown in Figure 2, the retention times of lovastatin, lovastatin acid, and nylidrin (internal standard) were approximately 3.6, 3.6, and 1.3 min, respectively, and the chromatograms of the following three groups showed no obvious interference with the signal peaks including group A, blank plasma; group B, lovastatin standard (10 ng/mL) and lovastatin acid (100 ng/mL) spiked in plasma; and group C, lovastatin (4.33 ng/mL) and lovastatin acid (87.07 ng/mL) collected 60 min after lovastatin administration (10 mg/kg, orally) in a rat plasma sample.
Figure 1.
Chemical structures and mass spectra of (A) lovastatin, (B) lovastatin acid, and (C) nylidrin (internal standard; IS); molecular weights: 405, 423, and 300, respectively. The mass transitions of lovastatin, lovastatin acid, and nylidrin were m/z 405.4 → 285.3, 423.3 → 303.6, and 300.3 → 150.1, respectively.
Figure 2.
Typical multiple reaction monitoring (MRM) chromatograms of (A) rat blank plasma and (B) lovastatin (10 ng/mL), lovastatin acid (100 ng/mL), and IS: nylidrin (1 ng/mL) spiked in rat plasma. (C) Rat plasma sample containing lovastatin (4.33 ng/mL) and lovastatin acid (87.07 ng/mL) collected 60 min after lovastatin oral administration (10 mg/kg). (1) IS: nylidrin (RT: 1.3 min), (2) lovastatin (RT: 3.6 min), and (3) lovastatin acid (RT: 3.6 min).
2.2. Validation of Selectivity, Specificity, Linearity, Precision, Accuracy, Recovery, Matrix Effect, and Stability
To validate the selectivity of analytes, we compared the signals of blank plasma with no analytes to six different concentrations of analytes to assess the selectivity of the bioanalytical method and confirm that the measured substance had no interference with blank plasma.
The UHPLC-MS/MS ion fragmentation acquisition was used to determine the specificity of analytes. The fragmentation of lovastatin was m/z 405.4 → 285.3, and the fragmentation of lovastatin acid was m/z 423.3 → 303.6. The multiple reaction monitoring mode was used to determine the specificity of the parent compound and its metabolite. The signals of rat plasma samples to six different concentrations of quality control (QC) analytes were compared, and it was confirmed that the measured substance had no interference for carryover, as shown in Figure 2.
After establishing a calibration curve with a standard sample of known concentration, a regression equation was used to quantify the properties of the test substance. The regression equations of lovastatin and lovastatin acid in rat plasma were y = 0.0453x + 0.0202 (r2 = 0.9998) and y = 0.0047x + 0.0201 (r2 = 1), respectively. The results demonstrated that the regression equation had good linearity within the concentration range of 0.5–100 ng/mL for lovastatin and 10–1000 ng/mL for lovastatin acid. The data are presented in Table S1.
The extraction recoveries were assessed by comparing the peak areas of the extracted samples vs samples with standards added after extraction. The analyte is added to three standard solutions of different concentrations, and the recovery rate is calculated. Of the three concentrations of stock solutions (1, 10, 100 ng/mL) of lovastatin and lovastatin acid, the recovery rates were 68.4 ± 7.4 to 70.9 ± 3.8% and 68.1 ± 2.5 to 76.3 ± 6.4%, respectively. The data are presented in Table S2.
The intraday precision and accuracy of lovastatin ranged from 1.16 to 9.11% and −1.55 to 4.21%, and that of lovastatin acid ranged from 3.16 to 18.58% and −6.03 to 3.98%. The interday precision and accuracy of lovastatin ranged from 1.74 to 3.64% and −8.40 to 7.70%, and that of lovastatin acid ranged from 2.66 to 9.54% and −0.52 to 7.96%. The data are presented in Tables S3 and S4, and the relative standard deviation (RSD) and bias values were all within acceptable limits of ±15% (±20% for lower limit of quantification [LLOQ]). The intra- and interday precision and accuracy values of the analytes at various concentrations were within the scope of the bioanalytical method validation by USFDA guidelines,13 revealing that the analytical method was considered reliable.
The matrix effects were assessed by comparing the peak areas of the samples extracted with blank plasma vs without blank plasma. Of the three concentrations of stock solutions (1, 10, 100 ng/mL) of lovastatin and lovastatin acid, the matrix effects were 95.2 ± 6.0 to 100.1 ± 2.9% and 121.6 ± 6.3 to 129.8 ± 5.0%, respectively. The data are presented in Table S2.
To evaluate the stability of the samples during storage, preparation, and analysis, we evaluated the stability under different storage conditions and usage conditions, including the short-term, autosampler, freeze–thaw, and long-term stability. Of the three concentrations of stock solutions (1, 10, 100 ng/mL) of lovastatin and lovastatin acid, the stabilities were 69.7 ± 2.3 to 92.8 ± 0.5% and 87.1 ± 3.0 to 111.1 ± 6.9%, respectively. The data are presented in Table S5.
2.3. Pharmacokinetic Interactions of the Herbal Drug with Lovastatin
To investigate the pharmacokinetic herb–drug interaction, single-dose lovastatin (10 mg/kg, p.o.) was administered to compare the dose-dependently pretreated with Coptidis preparation (0.3, 1, or 3 g/kg, p.o.) for five consecutive days before lovastatin administration (10 mg/kg, p.o.). In clinical practice, patients with hyperlipidemia take 10–80 mg of lovastatin daily. The standard of dose conversion from human to animal research is based on the human body surface area (BSA).14 Through the time vs concentration curve, the pharmacokinetic characteristics of oral lovastatin and Coptidis preparation in rat plasma were evaluated in terms of absorption, distribution, metabolism, and excretion.
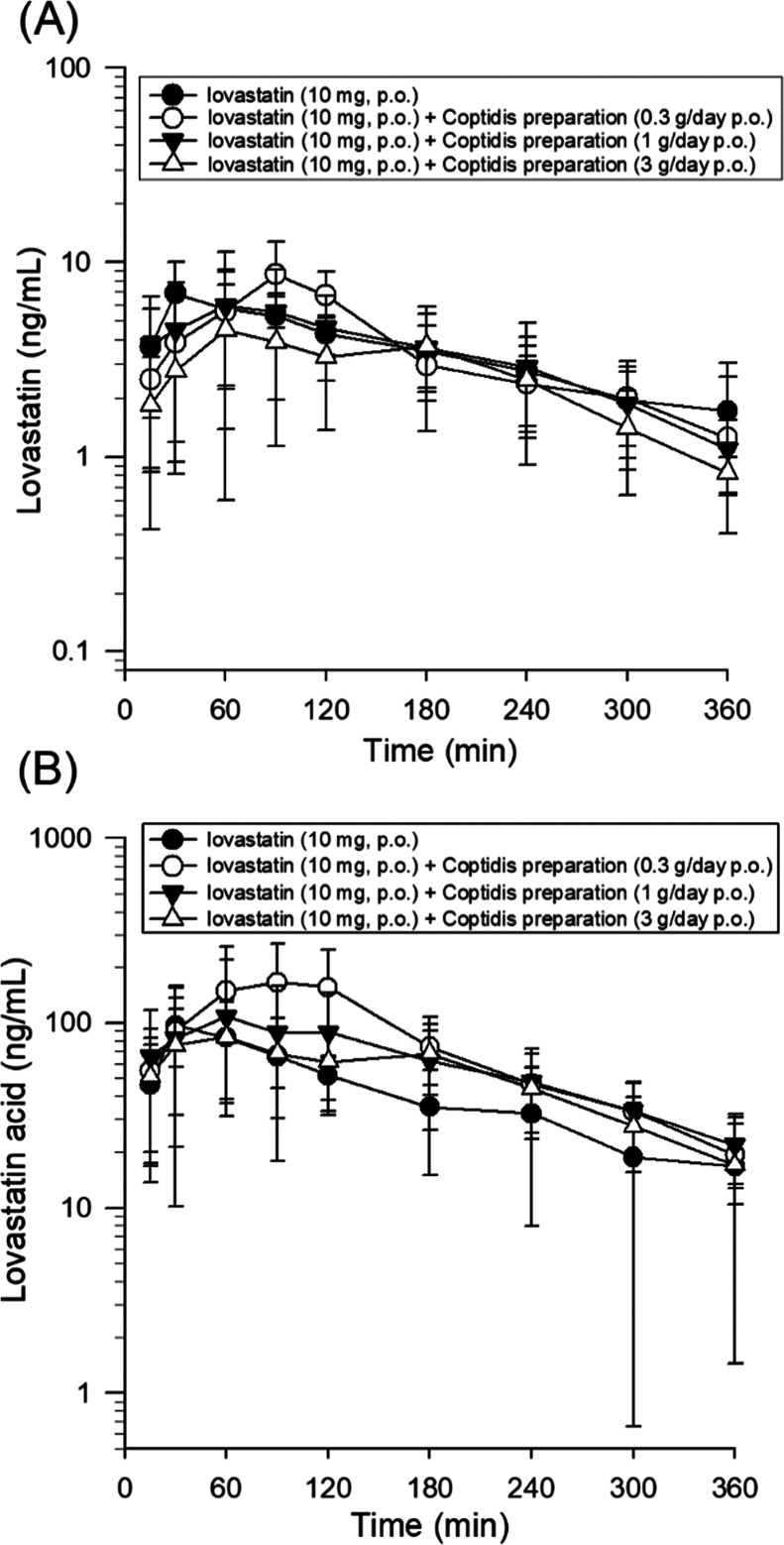
Figure 3A shows the representative change in the curve of the lovastatin concentration in rat plasma. The plasma reached Cmax of 10 mg/kg for lovastatin within 30 min after taking the drug, and after adding Coptidis preparation, there was a delay of 60–90 min before reaching the Cmax of lovastatin. Table 1 summarizes the pharmacokinetic parameters calculated from lovastatin concentration observed in lovastatin combined with different doses of Coptidis preparation. Many pharmacokinetic parameters of lovastatin and lovastatin acid, including the area under the concentration curve measured to infinity (AUCinf), maximum concentrations (Cmax), elimination half-life (t1/2), clearance (CL), volume of distribution (Vd), and mean residence time (MRT), were measured.
Figure 3.
Concentration–time profiles of (A) lovastatin and (B) lovastatin acid in rat plasma following oral administration of 10 mg/kg lovastatin for the control group and the groups treated with dried decoctions of Coptidis preparation at 0.3, 1, and 3 g/kg. Data are expressed as the mean ± SD (n = 6).
Table 1. Pharmacokinetic Parameters of (A) Lovastatin and (B) Lovastatin Acid in Rat Plasma after Administration of Lovastatin and Coptidis Preparationa.
| parameter | lovastatin (10 mg/kg) | lovastatin (10 mg/kg) + Coptidis preparation (0.3 g/kg) | lovastatin (10 mg/kg) + Coptidis preparation (1 g/kg) | lovastatin (10 mg/kg) + Coptidis preparation (3 g/kg) |
|---|---|---|---|---|
| Lovastatin | ||||
| AUC0-t (min ng/mL) | 1279 ± 252 | 1330 ± 438 | 1245 ± 739 | 1025 ± 431 |
| AUC0-inf (min ng/mL) | 1700 ± 636 | 1504 ± 330 | 1438 ± 797 | 1099 ± 394 |
| Cmax (ng/mL) | 7.85 ± 3.22 | 9.38 ± 3.58 | 7.36 ± 4.49 | 5.07 ± 2.61 |
| t1/2 (min) | 132 ± 71 | 102 ± 46 | 118 ± 32 | 93 ± 35 |
| tmax (min) | 36 ± 13 | 90 ± 18 | 95 ± 48 | 115 ± 72 |
| CL (L/h/kg) | 0.39 ± 0.13 | 0.44 ± 0.14 | 0.50 ± 0.18 | 0.62 ± 0.19* |
| Vd (L/kg) | 1.15 ± 0.53 | 1.06 ± 0.59 | 1.42 ± 0.71 | 1.44 ± 0.77 |
| MRT (min) | 238 ± 124 | 192 ± 58 | 208 ± 53 | 218 ± 62 |
| Lovastatin Acid | ||||
| AUC0-t (min ng/mL) | 15 380 ± 2572 | 29 110 ± 13 860 | 22 370 ± 11 649 | 20 390 ± 6927 |
| AUC0-inf (min ng/mL) | 18 850 ± 6206 | 30 460 ± 12 490 | 25 660 ± 12 390 | 22 760 ± 6112 |
| Cmax (ng/mL) | 116 ± 42.1 | 172 ± 101 | 131 ± 103 | 93.81 ± 36.57 |
| t1/2 (min) | 102 ± 56 | 108 ± 47 | 113 ± 40 | 137 ± 54 |
| tmax (min) | 42 ± 15 | 95 ± 23 | 95 ± 48 | 95 ± 67 |
| MRT (min) | 191 ± 105 | 212 ± 92.8 | 210 ± 44 | 239 ± 77 |
| AUClovastatin acid/AUClovastatin | 12.06 ± 5.91 | 20.78 ± 5.96* | 19.91 ± 8.02 | 21.76 ± 6.22* |
* indicates a significant difference when compared with the lovastatin group (*p < 0.05). All data are expressed as the mean ± SD. Area under the concentration curve (AUC) was measured to infinity; maximum concentration (Cmax); elimination half-life (t1/2); clearance (CL); volume of distribution (Vd); and mean residence time (MRT).
The AUCs of lovastatin in lovastatin (10 mg/kg, p.o.) alone group and lovastatin (10 mg/kg, p.o.) + Coptidis preparation (0.3, 1, or 3 g/kg, p.o.) group were 1700 ± 636 (min ng/mL), 1504 ± 330 (min ng/mL), 1438 ± 797 (min ng/mL), and 1099 ± 394 (min ng/mL), respectively (Table 1). The AUCs of lovastatin acid in lovastatin (10 mg/kg, p.o.) alone group and lovastatin (10 mg/kg, p.o.) + Coptidis preparation (0.3, 1, or 3 g/kg, p.o.) group were 18 850 ± 6206 (min ng/mL), 30 460 ± 12 490 (min ng/mL), 25 660 ± 12 390 (min ng/mL), and 22 760 ± 6112 (min ng/mL), respectively (Table 1).
The results showed that the three doses of Coptidis preparation (0.3, 1, or 3 g/kg) combined with lovastatin resulted in a decrease in the t1/2, MRT, and Cmax of lovastatin in the plasma (in the medium- and high-dose Coptidis preparation groups), leading to a decrease in the AUCinf in the plasma (in medium- and high-dose Coptidis preparation groups). This phenomenon was predominant in the high-dose Coptidis preparation group (3 g/kg, p.o.). The clearance of lovastatin showed a significant increase after combining with high-dose Coptidis preparation (3 g/kg, p.o.), and the volume of distribution also showed an increase after combining with the three doses of Coptidis preparation (0.3, 1, or 3 g/kg). The level of lovastatin acid, the active metabolite of lovastatin, showed the opposite results. The lovastatin acid level with the three doses of Coptidis preparation (0.3, 1, or 3 g/kg) resulted in an increase in the t1/2, MRT, and Cmax in the plasma (in low and medium Coptidis preparation dose groups), leading to an increase in the AUC in the plasma. This phenomenon was predominant in the low-dose Coptidis preparation group (0.3 g/kg, p.o.).
To investigate the effects of herbal drug biotransformation, the metabolic ratio was defined as the AUC ratio of lovastatin/lovastatin acid (AUClovastatin acid/AUClovastatin). The results demonstrated that the metabolic ratios of lovastatin acid/lovastatin for the experimental groups of lovastatin (10 mg/kg), lovastatin (10 mg/kg) + Coptidis preparation (0.3 g/kg), lovastatin (10 mg/kg) + Coptidis preparation (1 g/kg), and lovastatin (10 mg/kg) + Coptidis preparation (3 g/kg) groups were 12.06 ± 5.91, 20.78 ± 5.96, 19.91 ± 8.02, and 21.76 ± 6.22, respectively (Table 1). The metabolic ratios reached a plateau dose-dependently, which may due to the saturation.
Lovastatin is a prodrug lactone, and its open chain 3,5-dihydroxy acid is an effective competitive inhibitor, HMG-CoA (the cholesterol biochemical synthesis rate-limiting enzyme) reductase. This biotransformation of lovastatin was observed in rats; that is, the pharmacologically active dihydroxy acid, lovastatin acid, was produced by hydrolysis of the lactone ring.15 After combining with the three doses of Coptidis preparation (0.3, 1, or 3 g/kg), an increase in the Cmax, t1/2, MRT, and AUC of lovastatin acid was observed, which indicated the rapid absorption of lovastatin along with its rapid metabolism into lovastatin acid. Compared with the lovastatin (10 mg/kg) group, the decrease in AUC0-inf and t1/2 and the increase in CL in the lovastatin (10 mg/kg) + Coptidis preparation (3 g/kg) group indicated that lovastatin quickly hydrolyzed to lovastatin acid. These results are consistent with a previous report that gemfibrozil markedly increases plasma concentrations of lovastatin acid.16
Herein, we have revealed for the first time the biotransformation of lovastatin to lovastatin acid, and its metabolic rate was defined as AUClovastatin acid/AUClovastatin, the metabolic ratio. The metabolic ratio of lovastatin (10 mg, p.o.) combined with high-dose Coptidis preparation (3 g/kg, p.o., 21.76 ± 6.22) showed a significant increase compared to that of the lovastatin group (10 mg, p.o., 12.06 ± 5.91). Here, we comprehensively reviewed the progress of the metabolic pathways of lovastatin and the regulation of hydrolysis for reference for the regulation of lovastatin metabolism by Coptidis preparations.
2.4. Effects of Body Weight, Food Efficiency, and Tissue Weight
To evaluate the effects of the herbal drug combination on body weight, the rats were fed a HFD for 4 weeks to cause diet-induced obesity. The results demonstrated that during the experimental period, the body weight gains in experimental groups fed a normal diet (ND), HFD, HFD + lovastatin (100 mg/kg), HFD + Coptidis preparation (1 g/kg), and HFD + lovastatin (50 mg/kg) + Coptidis preparation (1 g/kg) for 4 weeks were 201 ± 17.8, 252.4 ± 29.0, 221.8 ± 20.3, 230.6 ± 16.8, and 205.4 ± 19.4 g, respectively. The weights in HFD groups of rats were significantly higher than those of ND rats. In addition, compared to the HFD group, the half dose of lovastatin (50 mg/kg) + Coptidis preparation (1 g/kg) showed a significant reduction in body weight gain.
To investigate how food consumption shifts body weight gain, feed efficiency, which refers to the ratio between the weight gained by the animal and the weight of the feed consumed by the animal over a period of time [feed efficiency = (weight gain/food intake) × 100%], was applied.17 The results demonstrated that during the experimental period, the feed efficiencies of ND, HFD, HFD + lovastatin (100 mg/kg), HFD + Coptidis preparation (1 g/kg), and HFD + lovastatin (50 mg/kg) + Coptidis preparation (1 g/kg) experimental groups were 32.66 ± 1.34, 58.91 ± 5.21, 51.33 ± 4.04, 55.03 ± 4.16, and 53.33 ± 4.95%, respectively. The feed efficiencies of the HFD group and drug treatment groups were significantly higher than that of the ND group.
To explore the weights of the liver and visceral, epididymal and perirenal fat, tissue samples were collected and measured. The results showed that the epididymal and perirenal fats in ND, HFD, HFD + lovastatin (100 mg/kg), HFD + Coptidis preparation (1 g/kg), and HFD + lovastatin (50 mg/kg) + Coptidis preparation (1 g/kg) groups were 4.41 ± 0.54 and 3.96 ± 0.37 g; 8.37 ± 2.07 and 10.48 ± 2.76 g; 7.15 ± 1.24 and 9.57 ± 1.81 g; 7.10 ± 0.86 and 9.54 ± 2.24 g; and 5.70 ± 0.53 and 7.18 ± 1.35 g, respectively. The results demonstrated that the weights of the epididymal and perirenal fat tissues in the HFD group were significantly higher than those in ND rats. The half-dose lovastatin (50 mg/kg) + Coptidis preparation (1 g/kg) group showed a significant suppression of the weights of the epididymal and perirenal fats compared to the HFD group.
Previous studies have found that excessive energy intake can cause excess fat to accumulate in surrounding tissues,18 and the obesity induction model of experimental animals fed a HFD can lead to more visceral fat accumulation.19 After the rats in each test group were sacrificed, their epididymal adipose tissue and perirenal adipose tissue were taken as representatives of visceral fat. Currently, Coptidis preparation has been reported to be beneficial for preventing and treating hyperlipidemia20 and an effective and harmless treatment option in certain clinical trials.8 Our study considers previous reports to attenuate the degree of fatty changes. In addition, a combination therapeutic recipe of half-dose lovastatin combined with Coptidis preparation showed a decrease in body weight, epididymal adipose weight, and perirenal adipose weight compared with the HFD group.
2.5. Effects of the Serum Lipid Profile
To explore lipid synthesis, blood lipid levels, TC, TG, HDL-C, and LDL-C were monitored in liver cells.21 TC and HDL levels increased significantly in the HFD group compared to the ND group (63.35 ± 12.16 and 22.58 ± 1.66 mg/dL to 34.74 ± 4.19 and 13.66 ± 2.54 mg/dL). TG and LDL levels increased in the HFD group compared to the ND group (25.68 ± 7.12 and 9.5 ± 2.54 mg/dL to 23.58 ± 7.47 and 6.90 ± 1.24 mg/dL) (Table 2). The rat species does not have cholesteryl ester transfer protein, which can transfer cholesteryl esters from HDL to cholesterol-rich LDL and convert TG to HDL. Therefore, a rat model of hyperlipidemia induced by a high-fat diet may show excessive total cholesterol and HDL cholesterol concentrations. These data are consistent with previous reports on dyslipidemia-induced animal models.22,23 Notably, a half dose of lovastatin combined with Coptidis preparation decreased plasma TC (44.78 ± 4.77 mg/dL), triglycerides (15.22 ± 12.65 mg/dL), and LDL-C (8.46 ± 1.07 mg/dL) compared with the HFD group (63.35 ± 12.16, 25.68 ± 7.12, and 9.50 ± 2.54 mg/dL, respectively) (Table 2). The blood lipid levels demonstrated a decreasing trend for the group treated with a half dose of lovastatin combined with Coptidis preparation.
Table 2. Effects of Lovastatin and Coptidis Preparation on Serum Biochemical Parameters in HFD-Induced Obese Rats (n = 5)a.
| ND | HFD | lovastatin (100 mg/kg) | Coptidis preparation (1 g/kg) | lovastatin (50 mg/kg) + Coptidis preparation (1 g/kg) | |
|---|---|---|---|---|---|
| TC (mg/dL) | 34.74 ± 4.19 | 63.35 ± 12.16# | 48.13 ± 13.73 | 49.08 ± 8.55 | 44.78 ± 4.77 |
| TG (mg/dL) | 23.58 ± 7.47 | 25.68 ± 7.12 | 18.92 ± 14.07 | 21.58 ± 5.24 | 15.22 ± 12.65 |
| HDL-C (mg/dL) | 13.66 ± 2.54 | 22. 58 ± 1.66## | 16.76 ± 3.40 | 19.34 ± 3.24 | 15.52 ± 1.50** |
| LDL-C (mg/dL) | 6.90 ± 1.24 | 9.50 ± 2.54 | 7.60 ± 3.97 | 6.96 ± 2.06 | 8.46 ± 1.07 |
indicates a significant difference when compared with the high-fat diet (HFD) group (*p < 0.05, **p < 0.01). # indicates a significant difference when compared with the normal diet (ND) group (#p < 0.05, ##p < 0.01). All data are expressed as the mean ± SD. TC, total cholesterol; TG, triglyceride; HDL-C, high-density-lipoprotein cholesterol; and LDL-C, low-density lipoprotein cholesterol.
Previous reports have proven that the expression of liver LDL receptors regulates the homeostasis of human plasma LDL-C.24 In herbal medicine, Coptidis preparation has been reported to have hypolipidemic effects.25 These studies have shown that different alkaloids exhibit different anti-hypercholesterolemic activities through different molecular mechanisms, like activating CYP7A1 catalytic activity by strongly interacting with receptors and ligands, thus, promoting cholesterol catabolism and accelerating the excretion of bile acids.26 Berberine is an active herbal ingredient of Coptidis preparation that has been reported to be a promising lipid-lowering drug and has been shown in human experiments to reduce triglycerides and cholesterol levels and has also demonstrated a decrease in triglycerides and cholesterol levels in rat experiments.27 This study suggests that berberine should be the active component of Coptidis preparation. Our previous report demonstrated the hepatobiliary excretion of berberine.28 Berberine, an alkaloid originally extracted from Coptidis, showed the highest activity in increasing the expression of LDL receptors.29 Therefore, we expect that other therapeutic interventions can be used to increase the expression of liver LDL receptors through a mechanism that is different from that of the current statin therapy to increase the success rate of dyslipidemia treatment. Our studies provide an alternative remedy recipe of a half dose of lovastatin combined with Coptidis preparation for the treatment of lipid synthesis and blood lipid levels.
2.6. Histopathological Analyses of Lovastatin and Coptidis Preparation
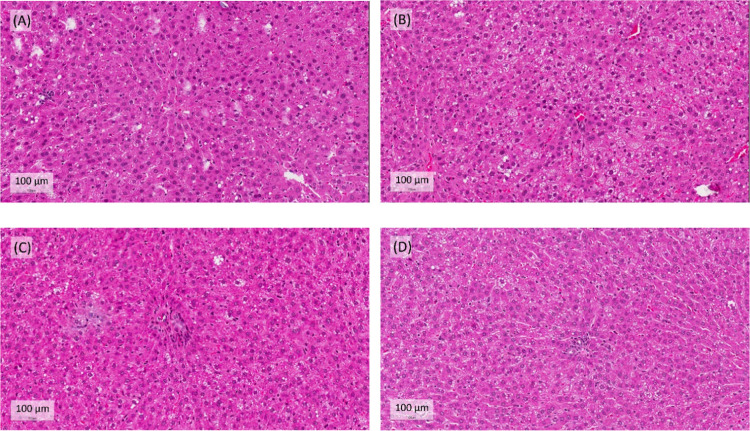
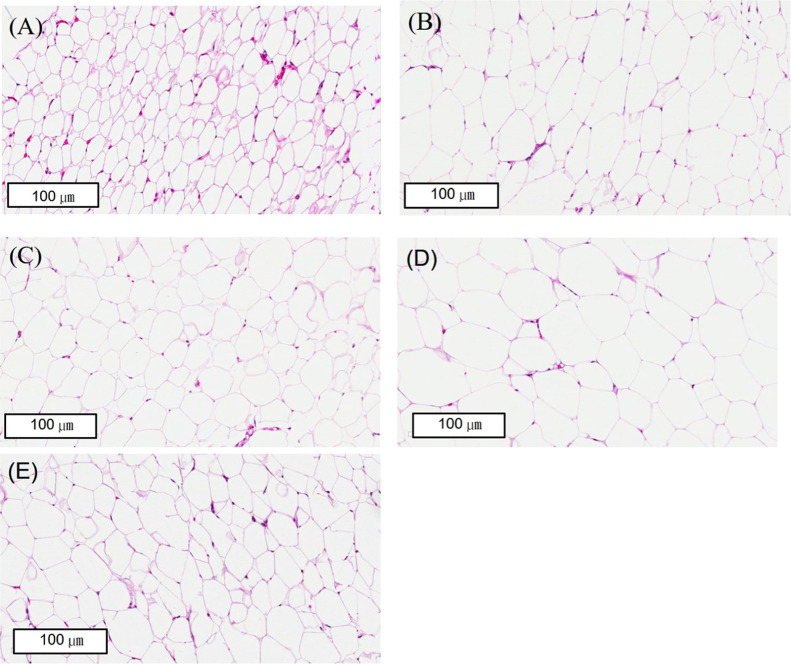
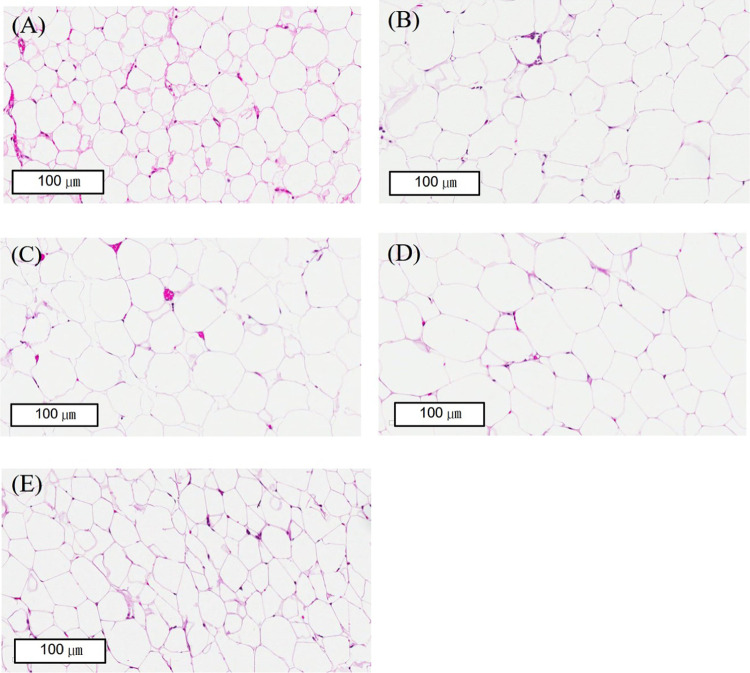
Long-term intake of a HFD can lead to abnormal endogenous lipid metabolism, which in turn causes lipid accumulation in the liver and even the formation of pathological fats.30 The rat livers were stained with H&E to observe whether the liver tissue had lesions.31 The liver slices from the HFD group showed that the liver cells had fat granules, fatty hypertrophy, steatosis, and inflammation. The degree of fat changes was attenuated in the half dose of lovastatin combined with the Coptidis preparation group (Figure 4). We stained the white adipose tissue of the epididymal and the perirenal tissue with H&E and found that the fat cells in the HFD group were significantly larger than those in the ND group (Figures 5 and 6). The half dose of lovastatin combined with the Coptidis preparation group showed a significant decrease in the fat content in the epididymal adipose tissue and perirenal adipose tissue compared with the HFD group (5.70 ± 0.53 vs 8.37 ± 2.07 g and 7.18 ± 1.35 vs 10.48 ± 2.76 g, respectively) (Figures 5 and 6).
Figure 4.
Effects of lovastatin and Coptidis preparation on hepatic lipids in HFD-induced obese rats. Livers were stained with hematoxylin and eosin (H&E). Original magnification: 400× (scale bars, 100 μm). (A) HFD, (B) lovastatin (100 mg/kg), (C) Coptidis preparation (1 g/kg), and (D) lovastatin (50 mg/kg) and Coptidis preparation (1 g/kg).
Figure 5.
Effects of lovastatin and Coptidis preparation on the epididymal fat size in HFD-induced obese rats. Epididymal fat tissues were stained with hematoxylin and eosin (H&E). Original magnification: 400× (scale bars, 100 μm). (A) ND group, (B) HFD group, (C) lovastatin 100 mg/kg group, (D) Coptidis preparation 1 g/kg group, and (E) epididymal fat in lovastatin 50 mg/kg and Coptidis preparation 1 g/kg group.
Figure 6.
Effects of lovastatin and Coptidis preparation on the perirenal fat size in HFD-induced obese rats. Perirenal fat tissues were stained with hematoxylin and eosin (H&E). Original magnification: 400× (scale bars, 100 μm). (A) ND group, (B) HFD group, (C) lovastatin 100 mg/kg group, (D) Coptidis preparation 1 g/kg group, and (E) epididymal fat in lovastatin 50 mg/kg and Coptidis preparation 1 g/kg group.
After we stained the adipose tissue with H&E, we found that in the half dose of lovastatin combined with the Coptidis preparation group, the cell sizes and shapes were smaller than those in the HFD group (Figure 6). We calculated the cell area and perimeter by Wimasis image analysis and noticed that the half dose of lovastatin combined with Coptidis preparation significantly decreased the cell area and cell perimeter in perirenal adipose cells compared with the HFD group (2122.72 ± 1787.96 μm2 and 175.09 ± 83.35 μm vs 4156.39 ± 1077.98 μm2 and 244.21 ± 38.59 μm, respectively) (Table 3).
Table 3. Fat Cell Area and Perimeter of Epidydimal and Perirenal Tissues in HFD-Induced Obese Rats (n = 5)a.
| ND | HFD | lovastatin (100 mg/kg) | Coptidis preparation (1 g/kg) | lovastatin (50 mg/kg) + Coptidis preparation (1 g/kg) | |
|---|---|---|---|---|---|
| Epididymal | |||||
| cell area (μm2) | 1712 ± 299.6 | 2492 ± 547.4# | 2293 ± 460.1# | 2009 ± 197 | 2944 ± 769.7# |
| cell perimeter (μm) | 160 ± 13.23 | 192.0 ± 23.04# | 180.1 ± 18.18 | 168.5 ± 8.24 | 205.5 ± 29.01# |
| Perirenal | |||||
| cell area (μm2) | 2521 ± 476 | 4156 ± 1078# | 4343 ± 911.9## | 4458 ± 1251# | 2123 ± 1788** |
| cell perimeter (μm) | 192.0 ± 18.55 | 244.2 ± 38.59# | 249.9 ± 34.29# | 249.3 ± 42.67# | 175.1 ± 83.35** |
indicates a significant difference when compared with the high-fat diet (HFD) group (*p < 0.05, **p < 0.01). # indicates a significant difference when compared with the normal diet (ND) group (#p < 0.05). All data are expressed as the mean ± SD.
3. Conclusions
Due to herb–drug interactions between herbal medicines and Western medicines, the pharmacological or toxicological effects of each preparation may be increased or decreased, and pharmacokinetic studies are needed to clarify the clinical efficacy of this combination.32 A validated UHPLC-MS/MS method was developed to monitor lovastatin and lovastatin acid levels in rat plasma. The pharmacokinetic results demonstrated that the biotransformation ratio of lovastatin/lovastatin acid (AUClovastatin acid/AUClovastatin) was significantly enhanced by treatment with Coptidis preparation, suggesting an enzymatic herbal drug interaction. The pharmacodynamic results showed that feed efficiency, lipid synthesis, and blood lipid levels were significantly ameliorated by the combination of half dose of lovastatin (50 mg/kg) + Coptidis preparation (1 g/kg) compared to the HFD group. Integrated treatment with ethnomedicine and low-dose Western medicine should be a new trend for therapeutic recipes. The role of ethnomedicine not only benefits lowering body weights but also decreases the fat content. In conclusion, this study provides a potential therapeutic recipe to reduce the dose of lovastatin and combine it with Coptidis preparation for the treatment of hyperlipidemia.
4. Materials and Methods
4.1. Chemicals and Reagents
Lovastatin, lovastatin acid, and nylidrin were purchased from Sigma Aldrich Chemicals (St. Louis, MO). Liquid chromatographic grade solvents (methanol, sodium dihydrogen phosphate (NaH2PO4), and orthophosphoric acid (H3PO4, 85%)) were purchased from E. Merck (Darmstadt, Germany). Triple-deionized water (Millipore, Bedford, MA) was used in this study. The roots and stems of Rhei rhizome, Scutellariae radix, and Coptidis rhizome were purchased from Lu-An traditional Chinese medicine Pharmacy (Taipei, Taiwan) (product lot no. HL12108 for Coptidis rhizome, product lot no. HQ12108 for Scutellariae radix, and product lot no. DH12108 for Rhei rhizome). These products were imported from herbal companies complying with good manufacturing practice regulations. After comparison of the purchased specimens from the National Institute of Traditional Chinese Medicine in Taiwan, the two sets of samples were determined to be the same. The content of berberine from the original Coptidis preparation was 1.03 ± 0.01 mg/g, and its extraction ratio was 15%.33
Lovastatin, lovastatin acid, and internal standard stock solutions were prepared in methanol and diluted to the required concentrations with 50% methanol. Both the analyte standard solutions and internal standard solutions were stored at −20 °C before the experiments were performed. Coptidis preparation was dissolved in warm triple-deionized water and was ready to be used in animal experiments.
4.2. Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry (UHPLC-MS/MS) Analysis
We used an MDS Sciex API 3000 tandem quadrupole mass spectrometer (Toronto, Canada) for UHPLC-MS/MS analysis. This mass spectrometer was equipped with an electrospray ionization interface and was integrated into an UHPLC system (Agilent Technologies 1100 series, Waldbronn, Germany). We took 10 μL of the sample, performed sample preprocessing, and then proceeded to the testing process.
After injecting our sample with an autosampler, a C18 reversed-phase column (150 mm × 2.0 mm i.d.; particle size 5 μm, Phenomenex, Torrance, CA) was used and the mobile phase was allowed to carry the analyte into the column to mix with the stationary phase and produce different polar forces to achieve separation. Our mobile phase consisted of 0.1% formic acid in water and methanol, and the flow rate was set to 0.2 mL/min.
An electrospray ionization source was used, the ion spraying voltage was set to 5500 V, and the other common parameters were 10, 6, and 10 for the nebulizer gas, curtain gas, and collision gas, respectively, and the compound parameters, viz., the values of the declustering potential (DP), focusing potential (FP), entrance potential (EP), collision energy (CE), and collision exit potential (CXP) were 35, 120, 11, 14, and 20; 55, 180, 13, 13, and 22; and 30, 130, 10, 28, and 13 for lovastatin, lovastatin acid, and nylidrin, respectively.
Two-stage quadrupole mass spectrometers (Quadrupole Analyzer) Q1 & Q3 in tandem were the mass spectrometers that were used in multiple reaction monitoring (MRM) mode to detect ions. The following ion pair transitions were monitored: m/z 405.40 for the lovastatin precursor ion to the m/z 285.3 product ion; m/z 423.3 for the lovastatin acid precursor ion to the m/z 303.5 product ion; and m/z 300.3 for the nylidrin precursor ion to the m/z 150.1 product ion. The data obtained by the detector were analyzed using Analyst software (version 1.4.1).
4.3. Method Validation
First, stock solution and quality control (QC) samples were prepared. Stock solutions of lovastatin and lovastatin acid were prepared in methanol. The concentration of both stock solutions was 1 mg/mL, and then the stock solutions were diluted with 50% methanol to obtain the working standard solutions. The concentration ranges of working solutions were 1, 5, 10, 50, 100, 500, and 1000 ng/mL. Nylidrin was chosen as the internal standard. The analytes and internal standard solutions were then stored at −20 ± 2 °C. The blank rat plasma was spiked with different concentrations of working solutions to construct the calibration curve. The calibration curves in rat plasma ranged from 0.5 to 100 ng/mL for lovastatin and 10 to 1000 ng/mL for lovastatin acid. The coefficient correlation (r2) of each calibration curve was used to evaluate the linearity of the measurements. The extraction recovery of lovastatin was calculated at low, medium, and high QC levels (1, 10, and 100 ng/mL), and the extraction recovery of lovastatin acid was also calculated at low, medium, and high QC levels (10, 100, and 1000 ng/mL). Lovastatin was diluted to 10 and 100 ng/mL and lovastatin acid was diluted to 10, 100, and 1000 ng/mL in working solution. The linearity, precision, accuracy, and stability assessments were based on the bioanalytical method validation by USFDA guidelines.13 Accuracy indicates how close the determined value (observed value) is to the known true value (nominal value) according to the following equation: (bias %) = [(Cobserved – Cnominal)/(Cnominal)] × 100%. Precision represents the closeness between a series of measurements obtained from multiple samplings with the same nominal concentration, according to the following equation: (relative standard deviation, RSD %) = [standard deviation (SD)/mean of Cobserved] × 100%. Accuracy and precision were established at four QC levels per run (LLOQ, L, M, and H QC) and five replicates per QC levels. The LLOQ was defined as 1 ng/mL for lovastatin and 10 ng/mL for lovastatin acid; Low QC was defined as five times the LLOQ, including 5 ng/mL for lovastatin and 50 ng/mL for lovastatin acid; Mid QC was defined as mid-range, including 50 ng/mL for lovastatin and 500 ng/mL for lovastatin acid; and high QC was defined as high range, including 100 ng/mL for lovastatin and 1000 ng/mL for lovastatin acid. Under the following four conditions, short-term stability, autosampler stability, freeze–thaw stability, and long-term stability, the stability of lovastatin was tested at low (1 ng/mL), medium (10 ng/mL), and high (100 ng/mL) concentrations in rat plasma, and the stability of lovastatin acid at low (10 ng/mL), medium (100 ng/mL), and high (1000 ng/mL) concentrations in rat plasma was also tested.
4.4. Animal Experiment
After being reviewed and approved by the Institutional Animal Care and Use Committee of National Yang Ming Chiao Tung University (IACUC number: 1080327), all animal experiments were performed. Six-week-old male Sprague–Dawley rats with an average weight of 220 ± 20 g obtained from the Animal Center of National Yang Ming Chiao Tung University were housed in different cages. The surrounding environment was maintained at a 12-h light/dark cycle while continuously providing food (Laboratory Autoclavable Rodent Diet 5010, PMI Feeds, Richmond, Indiana) and water. After the experimental rats were anesthetized with pentobarbital34 (50 mg/kg, i.p.), they were cannulated. First, we proceeded to implant a venous tube to facilitate the collection of plasma samples for subsequent animal experiments. We implanted a polyethylene tube (PE50) into the left jugular vein, fixed the cannula on the dorsal area of the neck, and flushed it with heparinized saline (20 IU/mL) to keep the tube open. After the operation, the rat was left alone in the cage for a day for recovery.
First, different doses of Coptidis preparation were prepared with water, including 0.3, 1, or 3 g/kg. The rats were fed directly by gavage tube, and blood was collected from the jugular vein cannula for analysis at specific time intervals of 0, 15, 30, 60, 90, 120, 180, 240, 300, and 360 min. The total amount of blood taken was approximately 200 μL, and the samples were placed in heparin-containing bottles. Next, the same procedure as that described previously (sample liquid–liquid extraction method) was used to extract the analytes from rat plasma.35
4.5. Experimental Design for the Pharmacokinetic Study
Rats were given the Coptidis preparation suspension solution and the experiment was divided into the following two parts: pharmacokinetic and pharmacodynamic studies. Pharmacokinetic calculations were performed on each individual set of data using the pharmacokinetic software WinNonlin Standard Edition, version 5.3 (Pharsight Corp., Mountain View, CA) in noncompartmental mode.
Part A: Herbal drug pharmacokinetics interaction study.
Group A1, lovastatin (10 mg/kg, p.o.) alone.
Group A2, lovastatin (10 mg/kg, p.o.) + pretreatment with Coptidis preparation (0.3 g/kg p.o. for five consecutive days; this dose is equivalent to a berberine dose of 0.31 mg/kg).
Group A3, lovastatin (10 mg/kg, p.o.) + pretreatment with Coptidis preparation (1 g/kg p.o. for five consecutive days; this dose is equivalent to a berberine dose of 1.03 mg/kg).
Group A4, lovastatin (10 mg/kg, p.o.) + pretreatment with Coptidis preparation (3 g/kg p.o. for five consecutive days; this dose is equivalent to a berberine dose of 3.09 mg/kg).
Rats undergoing neck catheterization were randomly divided into four groups, namely, groups A1–A4. Each group of rats was given an aqueous solution of lovastatin (10 mg/kg) via oral gavage, and three groups were fed different concentrations of the aqueous solution of Coptidis preparation (0.3, 1, or 3 g/kg). After drug administration, blood was collected from the jugular vein of each rat at specific time intervals (0, 15, 30, 60, 90, 120, 180, 240, 300, and 360 min after administration). After the collected blood was centrifuged (13 000g for 10 min), the plasma was stored at −20 °C until UHPLC analysis. The data obtained from these samples were plotted as a graph of the drug concentration vs time to construct a pharmacokinetic curve. The AUCinf represents the time from zero to infinity of drug exposure across time.
4.6. Experimental Design for the Pharmacodynamic Study in Rats
The Animal Care and Use Committee of National Yang Ming Chiao Tung University (IACUC number: 1070113) reviewed all animal experiment protocols, and the experiments were performed after approval. Five-week-old male Sprague–Dawley rats weighing 150 ± 20 g were housed in different cages at the National Yang Ming Chiao Tung University Animal Center, Taipei, Taiwan. The surrounding environment was maintained at a 12-h light/dark cycle providing HFD food (E.A. Ulman, Ph.D., Research Diets, Rodent Diet, Inc., D12492 with 60 kcal% fat) and water to cause obesity. The rats were fed once a day by gavage tube for 4 weeks. The contents of the gavage included lovastatin (dose of 50 or 100 mg/kg) or Coptidis preparation (dose of 1 g/kg body weight).
Part B: Herbal drug pharmacodynamics interaction study.
Group B1, HFD for 28 consecutive days.
Group B2, HFD and lovastatin (100 mg/kg, p.o. for 28 consecutive days).
Group B3, HFD and Coptidis preparation (1 g/kg, p.o. for 28 consecutive days).
Group B4, HFD, lovastatin (50 mg/kg, p.o.) and Coptidis preparation (1 g/kg, p.o. for 28 consecutive days).
The 24 rats were divided into four groups (six rats in each group) and were fed a HFD starting at the age of 5 weeks (n = 24) for 4 weeks. Previous studies have found that eating a HFD (>30% fat energy) is associated with a high incidence rate of being overweight, central obesity, and dyslipidemia in rat.36 To establish animal models that mimic the structural and functional characteristics of dyslipidemia, experimental animals were usually fed HFD. Other studies have shown that feeding a HFD for 4 weeks can cause diet-induced obesity and aggravate hyperlipidemia.37 During the study, food intake and body weights were measured once a day. After the study, blood was drawn from the rats, and the blood lipid data were measured in the Redox Medical Laboratory, including total cholesterol, triglycerides, high-density-lipoprotein cholesterol, and low-density-lipoprotein cholesterol.
After the experimental rats were sacrificed by exsanguination under anesthesia, the livers, epididymal fat tissues, and perirenal fat tissues were collected and weighed. A histopathological examination of the hepatic lipid, perirenal fat tissues, and epididymal fat tissues was performed. Briefly, the liver, perirenal tissue, and epididymal tissue of the rats were fixed overnight in 10% neutral buffered formalin (pH 7.4) and embedded in paraffin. The paraffin-embedded tissue was cut into thin slices, fixed on a processed microscope slide, and then stained with hematoxylin and eosin (H&E) at Taipei City Hospital (Taipei, Taiwan).
The cell area and cell perimeter of the epididymal adipose and perirenal adipose tissues were determined by Wimasis image analysis (Edificio Centauro, 14014 Córdoba, Spain). The Wimasis Adipose tool was used to calculate the cross-sectional area distribution of the fat cells. By observing the tissue sections stained with H&E, the cell area and diameter data were calculated, which is an objective and repeatable quantitative method. The calculation method used a phase contrast microscope (Olympus 1X51) to take photomicrographs (20×) of the hole at the FD time point and analyze the relevant parameters, including circularity, convexity, and elongation, to distinguish the fat droplets. The criteria for judging are as follows: area ≥ 10 pixels (Px), circularity > elongation, and convexity > 0.95. Fat drops that do not meet the conditions were deleted and not included in the calculation.
4.7. Statistical Analysis
For drug analysis, we used the noncompartmental model in the software program WinNonlin version 5.0 (Pharsight Corporation, Mountain View, CA) to calculate the relevant pharmacokinetic parameters, including the AUC and Cmax of lovastatin and t1/2 and MRT of lovastatin acid. In terms of statistical analysis of data, we used the variance function of SPSS 18.0 (SPSS Inc., Chicago, Illinois) and one-way analysis of variance (one-way ANOVA) to compare between groups. All data are expressed as the mean ± SD. Significant differences between the data are expressed as *p < 0.05 or **p < 0.01.
Acknowledgments
The authors thank the Department of Teaching and Research in the Taipei City Hospital for supporting them with UHPLC-MS/MS for inspection.
Glossary
Abbreviations
- AUC
area under the concentration curve
- Cmax
maximum concentrations
- t1/2
elimination half-life
- CL
clearance
- Vd
volume of distribution
- MRT
mean residence time
- ND
normal diet
- HFD
high-fat diet
- TC
total cholesterol
- TG
triglyceride
- HDL-C
high-density-lipoprotein cholesterol
- LDL-C
low-density-lipoprotein cholesterol
- UHPLC-MS/MS
ultra-high-performance liquid chromatography-tandem mass spectrometry
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme A
- QC
quality control
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01201.
Method validation of linear range, calibration curve, and r2 of lovastatin and lovastatin acid (Table S1); extraction recovery and matrix effect of lovastatin and lovastatin acid in rat plasma (Table S2); intraday precision and accuracy for the determination of lovastatin and lovastatin acid in plasma samples (Table S3); interday precision and accuracy for the determination of lovastatin and lovastatin acid in plasma samples (Table S4); and stability of lovastatin and lovastatin acid in rat plasma (Table S5) (PDF)
Author Contributions
W.-Y.P. performed the experiments, analyzed the data, and prepared the manuscript; W.-Y.P., A.C.H., and C.-T.T. performed the chemical analysis; and T.-H.T. designed the experiments, secured the funding, and edited the paper.
Funding for this study was provided in part by research grants from the Ministry of Science and Technology of Taiwan (MOST 109-2113-M-010-007).
The authors declare no competing financial interest.
Supplementary Material
References
- Sukhorukov V. N.; Karagodin V. P.; Orekhov A. N. Modern methods of diagnosis dyslipidemia. Patol. Fiziol. Eksp. Ter. 2016, 60, 65–72. [PubMed] [Google Scholar]
- Lauer M. S.; Fontanarosa P. B. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA, J. Am. Med. Assoc. 2001, 285, 2486–2497. 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Kopin L.; Lowenstein C. J. Dyslipidemia. Ann. Intern. Med. 2017, 167, ITC81 10.7326/AITC201712050. [DOI] [PubMed] [Google Scholar]
- Henwood J. M.; Heel R. C. Lovastatin. A preliminary review of its pharmacodynamic properties and therapeutic use in hyperlipidaemia. Drugs 1988, 36, 429–454. 10.2165/00003495-198836040-00003. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Zhang H.; Chec E.; Zhang L.; Han J.; Yang Y.; Wang S.; Zhang M.; Gao C. Development of novel mesoporous nanomatrix-supported lipid bilayers for oral sustained delivery of the water-insoluble drug, lovastatin. Colloids Surf., B 2015, 128, 77–85. 10.1016/j.colsurfb.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Nies L. K.; Cymbala A. A.; Kasten S. L.; Lamprecht D. G.; Olson K. L. Complementary and alternative therapies for the management of dyslipidemia. Ann. Pharmacother. 2006, 40, 1984–1992. 10.1345/aph.1H040. [DOI] [PubMed] [Google Scholar]
- Hao P.; Jiang F.; Cheng J.; Ma L.; Zhang Y.; Zhao Y. Traditional Chinese medicine for cardiovascular disease: evidence and potential mechanisms. J. Am. Coll. Cardiol. 2017, 69, 2952–2966. 10.1016/j.jacc.2017.04.041. [DOI] [PubMed] [Google Scholar]
- Cicero A. F. G.; Rovati L. C.; Setnikar I. Eulipidemic effects of berberine administered alone or in combination with other natural cholesterol-lowering agents. A single-blind clinical investigation. Arzneim. Forsch. 2007, 57, 26–30. 10.1055/s-0031-1296582. [DOI] [PubMed] [Google Scholar]
- Liou S. F.; Hsu J. H.; Liang J. C.; Ke H. J.; Chen lJ.; Wu J. R.; Yeh J. L. San-Huang-Xie-Xin-Tang protects cardiomyocytes against hypoxia/reoxygenation injury via inhibition of oxidative stress-induced apoptosis. J. Nat. Med. 2012, 66, 311–320. 10.1007/s11418-011-0592-0. [DOI] [PubMed] [Google Scholar]
- Wu T. Y.; Chang F. R.; Liou J. R.; Lo I. W.; Chung T. C.; Lee L. Y.; Chi C. C.; Du Y. C.; Wong M. H.; Juo S. H.; Lee C. C.; Wu Y. C. Rapid HPLC quantification approach for detection of active constituents in modern combinatorial formula, San-Huang-Xie-Xin-Tang (SHXXT). Front. Pharmacol. 2016, 7, 374 10.3389/fphar.2016.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. C.; Tan T. W. The role of gastric muscle relaxation in cytoprotection induced by San-Huang-Xie-Xin-Tang in rats. J. Ethnopharmacol. 1994, 44, 171–179. 10.1016/0378-8741(94)01184-2. [DOI] [PubMed] [Google Scholar]
- Tsai H. H.; Chen I. J.; Lo Y. C. Effects of San-Huang-Xie-Xin-Tang on U46619-induced increase in pulmonary arterial blood pressure. J. Ethnopharmacol. 2008, 117, 457–462. 10.1016/j.jep.2008.02.024. [DOI] [PubMed] [Google Scholar]
- USFDA. Bioanalytical Method Validation Guidance for Industry, 2018. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry.
- Reagan-Shaw S.; Nihal M.; Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Halpin R.; Ulm E.; Till A.; Kari P.; Vyas K.; Hunninghake D.; Duggan D. E. Biotransformation of lovastatin. V. Species differences in in vivo metabolite profiles of mouse, rat, dog, and human. Drug Metab. Dispos. 1993, 21, 1003–1011. [PubMed] [Google Scholar]
- Kyrklund C.; Backman J. T.; Kivistö K. T.; Neuvonen M.; Laitila J.; Neuvonen P. J. Plasma concentrations of active lovastatin acid are markedly increased by gemfibrozil but not by bezafibrate. Clin. Pharmacol. Ther. 2001, 69, 340–345. 10.1067/mcp.2001.115542. [DOI] [PubMed] [Google Scholar]
- Lushbough C. H.; Schweigert B. S. The effect of diet on growth rate and feed efficiency in the normal rat. J. Nutr. 1960, 70, 252–256. 10.1093/jn/70.2.252. [DOI] [PubMed] [Google Scholar]
- Large V.; Peroni O.; Letexier D.; Ray H.; Beylot M. Metabolism of lipids in human white adipocyte. Diabetes Metab. 2004, 30, 294–309. 10.1016/S1262-3636(07)70121-0. [DOI] [PubMed] [Google Scholar]
- Yao Y.; Li X. B.; Zhao W.; Zeng Y. Y.; Shen H.; Xiang H.; Xiao H. Anti-obesity effect of an isoflavone fatty acid ester on obese mice induced by high fat diet and its potential mechanism. Lipids Health Dis. 2010, 9, 49 10.1186/1476-511X-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X. X.; Yan H. M.; Xu Q.; Xia M. F.; Bian H.; Zhu T. F.; Gao X. The effects of berberine on hyperhomocysteinemia and hyperlipidemia in rats fed with a long-term high-fat diet. Lipids Health Dis. 2012, 11, 86 10.1186/1476-511X-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves-Bezerra M.; Cohen D. E. Triglyceride metabolism in the liver. Compr. Physiol. 2019, 8, 1–22. 10.1002/cphy.c170012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.; Fu M. Alterations of HDL subclasses in hyperlipidemia. Clin. Chim. Acta 2003, 332, 95–102. 10.1016/S0009-8981(03)00138-4. [DOI] [PubMed] [Google Scholar]
- Poledne R.; Jurcikova-Novotna L. Experimental models of hyperlipoproteinemia and atherosclerosis. Physiol. Res. 2017, 66, S69–S75. 10.33549/physiolres.933585. [DOI] [PubMed] [Google Scholar]
- Brown M. S.; Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science 1986, 232, 34–47. 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Xu Z.; Feng W.; Shen Q.; Yu N.; Yu K.; Wang S.; Chen Z.; Shioda S.; Guo Y. Rhizoma coptidis and berberine as a natural drug to combat aging and aging-related diseases via anti-oxidation and AMPK activation. Aging Dis. 2017, 8, 760–777. 10.14336/AD.2016.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Han Y.; Chai F.; Xiang H.; Huang T.; Kou S.; Han B.; Gong X.; Ye X. The antihypercholesterolemic effect of columbamine from Rhizoma Coptidis in HFHC-diet induced hamsters through HNF-4α/FTF-mediated CYP7A1 activation. Fitoterapia 2016, 115, 111–121. 10.1016/j.fitote.2016.09.019. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Ehli E. A.; Kittelsrud J.; Ronan P. J.; Munger K.; Downey T.; Bohlen K.; Callahan L.; Munson V.; Jahnke M.; Marshall L. L.; Nelson K.; Huizenga P.; Hansen R.; Soundy T. J.; Davies G. E. Lipid-lowering effect of berberine in human subjects and rats. Phytomedicine 2012, 19, 861–867. 10.1016/j.phymed.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Tsai P. L.; Tsai T. H. Hepatobiliary excretion of berberine. Drug Metab. Dispos. 2004, 32, 405–412. 10.1124/dmd.32.4.405. [DOI] [PubMed] [Google Scholar]
- Kong W.; Wei J.; Abidi P.; Lin M.; Inaba S.; Li C.; Wang Y.; Wang Z.; Si S.; Pan H.; Wang S.; Wu J.; Wang Y.; Li Z.; Liu J.; Jiang J. D. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from stains. Nat. Med. 2004, 10, 1344–1351. 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- Kempaiah R. K.; Srinivasan K. Beneficial influence of dietary curcumin, capsaicin and garlic on erythrocyte integrity in high-fat fed rats. J. Nutr. Biochem. 2006, 17, 471–478. 10.1016/j.jnutbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Bataller R.; Brenner D. A. Liver fibrosis. J. Clin. Invest. 2005, 115, 209–218. 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelingwani R.; Masimirembwa C. Evaluation of herbal medicines: value addition to traditional medicines through metabolism, pharmacokinetic and safety studies. Curr. Drug Metab. 2015, 15, 942–952. 10.2174/1389200216666150206125727. [DOI] [PubMed] [Google Scholar]
- Peng W. Y.; Tsai T. H. Scanning electron microscopy and liquid chromatography for physical and chemical inspection of industrial harmaceutical traditional Chinese herbal medicine. ACS Omega 2020, 5, 11563–11569. 10.1021/acsomega.0c00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen J. J.; Remie R.; Rensema J. W.; Van Wunnik G. H. J.. Manual of microsurgery on the Laboratory Rat. Elsevier Science 1990, Part 1: General Information and Experimental Techniques. USA. ISBN 0-444-81139-7.
- Hou M. L.; Chang L. W.; Lin C. H.; Lin L. C.; Tsai T. H. Determination of bioactive components in Chinese herbal formulae and pharmacokinetics of rhein in rats by UPLC-MS/MS. Molecules 2014, 19, 4058–4075. 10.3390/molecules19044058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. H.; Chen C. L.; Chang S. H.; Tsai G. J. Evaluation of antiobesity activity of soybean meal products fermented by lactobacillus plantarum FPS 2520 and Bacillus subtilis N1 in rats fed with high-fat diet. J. Med. Food 2020, 23, 667–675. 10.1089/jmf.2019.4643. [DOI] [PubMed] [Google Scholar]
- Namekawa J.; Takagi Y.; Wakabayashi K.; Nakamura Y.; Watanabe A.; Nagakubo D.; Shirai M.; Asai F. Effects of high-fat diet and fructose-rich diet on obesity, dyslipidemia and hyperglycemia in the WBN/Kob-Leprfa rat, a new model of type 2 diabetes mellitus. J. Vet. Med. Sci. 2017, 79, 988–991. 10.1292/jvms.17-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.