Abstract
In the absence of ligand, Cs2CO3-promoted cross-coupling reaction of arenes with cyano-/nitro-substituted aryl halides in DMSO affording biaryls is reported. The cyano/nitro group in biaryls is useful and convenient for further transformation. The formation of dibenzofurans resulting from the reactions between arenes and 1-bromo-2-iodobenzene is also reported. On the basis of control experiments and theoretical studies, a radical mechanism is proposed for the formation of biaryls.
Introduction
Arene carbon–hydrogen bond activation and its cross-coupling reactions have opened new avenues in synthetic chemistry with atom- and step-economy.1 Among many applications, the carbon–carbon bond formation via cross-coupling reactions between arene and aryl halides is particularly interesting as it provides an efficient access to functionalized biaryls, which have been well developed with the use of transition-metal complexes as catalysts.2 In the past 2 decades, under transition-metal-free conditions, efficient procedures for biaryl syntheses from cross-coupling reactions of arene and aryl halides promoted by bases have also been developed.3 As shown in Scheme 1, with the use of different ligands, the alkali salts of KOtBu, NaOtBu, and NaH have been found to be efficient promoters or catalysts for this transformation.4
Scheme 1. Base-Promoted Biaryl Syntheses via Coupling Reactions of Arenes with Aryl Halides.
Recently, without a ligand, Cs2CO3 in dimethyl sulfoxide (DMSO) has been found to be an efficient and versatile promoter in different transformations reported by our group5e,5f and others.5a−5d In continuation of our earlier studies on arene C–H arylation,6 and C–H activation/annulations,7 we would like to describe herein the biaryl synthesis via the cross-coupling of arenes with cyano-/nitro-substituted aryl halides in DMSO promoted by Cs2CO3 under ligand-free conditions (Scheme 1). The synthesis of biaryls bearing cyano and nitro groups is rarely reported with low to fair yields by transition-metal-free cross-coupling reactions of arenes with aryl halides.4a,4f,4g,4i
Results and Discussion
We selected the reaction between naphthalene (1a, 3.0 equiv) and 2-iodobenzonitrile (2a, 1.0 equiv) in DMSO at 100 °C in a sealed tube to optimize the reaction conditions. As shown in Table 1, in the presence of 2.5 equiv of KOtBu, KOH, and K2CO3, the reactions under nitrogen for 24 h gave low yields or trace amounts of the desired coupling product 2-(naphthalen-1-yl)benzonitrile (3aa) (entries 1–3). When Cs2CO3 was chosen as a base, the yield of 3aa could be increased to 65% (entry 4). However, if tetrahydrofuran (THF), 1,4-dioxane, dimethylformamide (DMF), or toluene was used as the solvent to replace DMSO, 3aa formed in trace amounts only (entries 5–8). The decrease of either 1a (3.0 equiv to 2.0 equiv) or Cs2CO3 (2.5 equiv to 0.5 equiv) resulted in a considerable decrease in yields (entries 9–12). Therefore, the use of 3.0 equiv of Cs2CO3 and 1a is the best reaction condition to obtain 3aa in 72% yield (entry 13). In addition, when organic bases such as DABCO (1,4-diazabicyclo[2.2.2]octane) and DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) were used, or without any base, no reaction occurred at all (entries 14–16).
Table 1. Optimizing Reaction Conditions for Biaryl Formationa.
| entry | 1a (equiv) | base (equiv) | solvent | yield (%)b |
|---|---|---|---|---|
| 1 | 3.0 | KOtBu (2.5) | DMSO | 16 |
| 2 | 3.0 | KOH (2.5) | DMSO | 10 |
| 3 | 3.0 | K2CO3 (2.5) | DMSO | trace |
| 4 | 3.0 | Cs2CO3 (2.5) | DMSO | 65 |
| 5 | 3.0 | Cs2CO3 (2.5) | THF | trace |
| 6 | 3.0 | Cs2CO3 (2.5) | 1,4-dioxane | trace |
| 7 | 3.0 | Cs2CO3 (2.5) | DMF | trace |
| 8 | 3.0 | Cs2CO3 (2.5) | toluene | trace |
| 9 | 2.0 | Cs2CO3 (2.5) | DMSO | 53 |
| 10 | 2.5 | Cs2CO3 (2.5) | DMSO | 60 |
| 11 | 3.0 | Cs2CO3 (0.5) | DMSO | 33 |
| 12 | 3.0 | Cs2CO3 (1.0) | DMSO | 51 |
| 13 | 3.0 | Cs2CO3(3.0) | DMSO | 72 |
| 14 | 3.0 | DABCO (3.0) | DMSO | 0 |
| 15 | 3.0 | DBU (3.0) | DMSO | 0 |
| 16 | 3.0 | DMSO | 0 |
Naphthalene (1a, 3.0 mmol) and 2-iodobenzonitrile (2a, 1.0 mmol) in 4.0 mL of solvent in a seated tube under N2 at 100 °C for 24.
Yields are isolated yields.
Table 2 summarizes the substrate scope for biaryl syntheses with the reaction conditions indicated in entry 13 of Table 1. The cross-coupling reactions of 1a with 2-bromobenzonitrile, 1-iodo-, and 1-bromo-2-nitrobenzene gave the corresponding biaryls (3aa and 3ab) in 58–73% yields. The bromobenzenes show a somewhat lower reactivity compared with iodobenzenes. The use of 1-iodo-4-methyl-2-nitrobenzene resulted in 51% yield of 3ac, indicating that aryl halides bearing an electron-donating group is unfavorable for biaryl formation. 1a reacted with 1-bromo-4-nitrobenzene affording 3ad in 68% yields. In addition, the reactions 1a with meta-iodobenzonitrile and meta-iodonitrobenzene afforded the corresponding biaryls 3ae and 3af in 51 and 55% yields, respectively. When benzene (1b) was used, the corresponding biaryls (3ba–3be) were obtained in 41–75% yields, resulting from the cross-coupling reactions with cyano- or nitro-substituted aryl bromides and iodides. When p-xylene and mesitylene were employed, the cross-coupling reactions with aryl halides produces the expected biaryls (3cc–3dc) in moderate to good yields. In addition, the reactions of anthracene and phenanthrene were also examined, and the cross-coupling reactions occurred with high regioselectivity to give 9-substituted anthracenes and phenanthrenes.
Table 2. Substrate Scope of Biaryl Formation.
Noted that under the standard conditions, aryl chlorides such as 2-chlorobenzonitrile and 1-chloro-4-nitrobenzene cannot undergo the cross-coupling reactions with 1a or 1b.
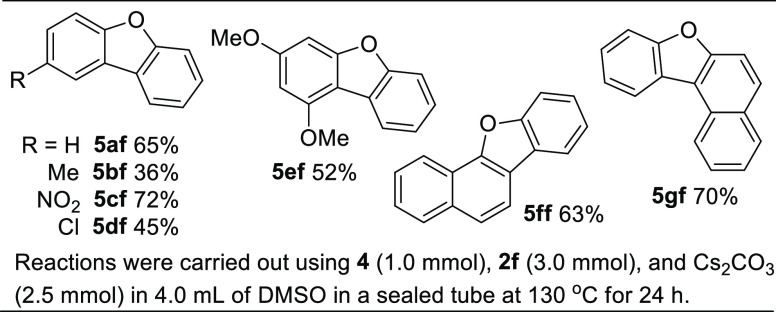
Although the cross-coupling reactions between arenes and bromo-/iodo-benzene or electron-rich aryl halides bearing methoxy groups do not occur smoothly under the standard conditions, the intramolecular coupling reaction of 1-iodo-2-phenoxybenzene can produce dibenzofuran (5af) (eq 1). Therefore, as shown in Table 3, under the modified reaction conditions, dibenzofuran derivatives can be prepared in fair to good yields from a two-component reaction between 1-bromo-2-iodobenzene and phenols.
 |
1 |
Table 3. Dibenzofuran Formationa.
Reactions were carried out using 4 (1.0 mmol), 2f (3.0 mmol), and Cs2CO3 (2.5 mmol) in 4.0 mL of DMSO in a sealed tube at 130 °C for 24 h.
The radical mechanism for biaryl formation via cross-coupling of arenes with aryl halides under different conditions has been proposed in several literature studies.4,8 In the present work, a radical mechanism is also proposed on the basis of the control experiments and the computational studies.
At first, a free radical trapping experiment was conducted in the reaction of 1b with 2b in the presence of the free radical scavengers 2,2,6,6-tetramethyl-1-piperidin-1-oxyl (TEMPO) under the standard conditions. Although the corresponding O-aryl-TEMPO could not be detected by gas chromatography (GC)–mass spectrometry (MS) analysis of the reaction mixture, 3bb was formed in trace amounts only. The results indicate that Cs2CO3/DMSO-promoted biaryl formation resulted from a free radical way via the coupling reactions of arenes with aryl halides.
In addition, we also performed the reaction of 1b with 2b using 1:1 of 1b and 1b-d6 and found that the kinetic isotopic effect value is 1.06 for the formation of 3bb, indicating that the rate-determining step for biaryl formation is the formation of 2b′s radical anion, not the activation or cleavage of C–H/C–D (eq 2).
 |
2 |
The purposes of computational studies are to investigate the role of Cs2CO3 in the cross-coupling reactions between arenes and haloarenes without the assistance of the ligand. The computational studies on the formation of 3bb from the reaction of benzene (1b) with 1-iodo-2-nitrobenzene (2b) as the model reaction have been done, the energy profile is shown in Figure 1.
Figure 1.
Energy Profile for 2b′ Radical Formation.
In the radical mechanism, M06/def2-TZVP was used with the SMD solvation model in DMSO, and thermal correction.9 The computational studies show that the vertical single electron transfer (SET) from cesium carbonate to the electron acceptor of 2b, without structure relaxation, requires 45.9 kcal/mol of electronic energy or 39.4 kcal/mol of free energy change. After the relaxation of the structures of the radical cation of cesium carbonate and the radical anion of 2-iodonitrobenzene, the electronic energy change is 39.4 kcal/mol or the free energy change is 20.9 kcal/mol. Namely, the way from [Cs2CO3 + 2b] to [S* + ν] to S* to form an active radical 2b (S*) should overcome a relatively high energy ([S* + ν]). However, the other way via the vibrational excited state ([S + ν′]) requires 16.6 kcal/mol electronic energy or 17.8 kcal/mol free energy only and to give S* with 20.9 kcal/mol free energy change, which can be achieved easily in the reaction temperature. Therefore, the SET radical pair can be readily realized via the vibrational excitation process to promote the cross-coupling reaction.
Therefore, a proposed mechanism for the formation of 3bb is depicted in Scheme 2. Cs2CO3 provides signal election to 2b, generating anion radical and then 2b radical. 2b radical interacts with benzene (1b) to form C–C bond and transfer radical to benzene ring. The interaction between the iodine radical and the benzene radical affords the final product 3bb, accompanied by the formation of HI.
Scheme 2. Radical Mechanism for 3bb Formation.
Conclusions
In conclusion, in the absence of a ligand, Cs2CO3-promoted cross-coupling reactions between arenes and aryl halides in DMSO affording biaryls in good yields is developed. Although aryl halides are limited to electron-deficient substrates, the electron-withdrawing groups (−CN and −NO2) in biaryls are important and useful for further synthetic applications. Dibenzofuran derivatives can also be synthesized in fair to good yields from the reactions between 1-bromo-2-iodobenzene and phenols. In addition, from the results of the controlling experiments and the computational studies on the role of Cs2CO3, a radical mechanism is proposed for the formation of biaryls.
Experimental Section
General Methods
All commercial reagents are analytically pure and used without further purification. The purity of Cs2CO3 is 99.99%. Nuclear magnetic resonance (NMR) spectra were recorded using CDCl3 at 298 K. 1H NMR (400 and 600 MHz) chemical shifts (δ) were referenced to internal standard TMS (for1 H, δ = 0.00 ppm). 13C NMR (100 and 125 MHz) chemical shifts were referenced to internal solvent CDCl3 (for 13C, δ = 77.16 ppm). Mass spectra were obtained on a low-resolution GC–MS spectrometer, and high-resolution mass spectra were recorded on a high-resolution magnetic sector mass spectrometer with an electrospray ionization (ESI) source.
Typical Experimental Procedure for the Synthesis of 2-(Naphthalen-1-yl)benzonitrile (3aa)
To a 25 mL tube equipped with a magnetic stirrer were added naphthalene (1a) (384.1 mg, 3.0 mmol), 2-iodobenzonitrile (2a) (229.4 mg, 1.0 mmol), Cs2CO3 (978.3 mg, 3.0 mmol), and DMSO (4.0 mL) under nitrogen. The tube was sealed and stirred at 100 °C for 24 h in an oil bath. After the reaction mixture was cooled to room temperature, it was poured into a solvent mixture of water (50.0 mL) and ethyl acetate (20.0 mL), and the two phases were then separated. The aqueous layer was extracted with ethyl acetate (3 × 20.0 mL). The combined organic extracts were dried over anhydrous Na2SO4. After removal of the solvent under reduced pressure, the residue was purified by column chromatography on silica gel with petroleum ether/ethyl acetate (gradient mixture ratio from 100:0 to 95:05) as an eluent to afford 3aa as a white solid 72% (165.3 mg, 0.72 mmol).
Typical Experimental Procedure for the Synthesis of Dibenzofuran (5af)
To a 25 mL tube equipped with a magnetic stirrer were added phenol (4a) (94.1 mg, 1.0 mmol), 1-bromo-2-iodobenzene (2f) (849.1 mg, 3.0 mmol), Cs2CO3 (978.3 mg, 3.0 mmol), and DMSO (4.0 mL) under nitrogen. The tube was sealed and stirred at 130 °C for 24 h in an oil bath. After the reaction mixture was cooled to room temperature, it was poured into a solvent mixture of water (50.0 mL) and ethyl acetate (20.0 mL), and the two phases were then separated. The aqueous layer was extracted with ethyl acetate (3 × 20.0 mL). The combined organic extracts were dried over anhydrous Na2SO4. After removal of the solvent under reduced pressure, the residue was purified by column chromatography on silica gel with petroleum ether/ethyl acetate (gradient mixture ratio from 100:0 to 90:10) as an eluent to afford 5af in 65% (109.3 mg, 0.65 mmol).
2-(Naphthalen-1-yl)benzonitrile (3aa)10
White solid (X = I, 165.3 mg, 72%; X = Br, 132.9 mg, 58%); 1H NMR (400 MHz, CDCl3): δ 7.96 (t, J = 6.9 Hz, 2H), 7.85 (d, J = 7.7 Hz, 1H), 7.70 (t, J = 7.5 Hz,1H), 7.63–7.44 (m, 7H); 13C NMR (100 MHz, CDCl3): δ 144.4, 135.9, 133.7, 133.2, 132.3, 131.6, 131.5, 129.2, 128.6, 128.0, 127.6, 126.7, 126.2, 125.2, 118.1, 113.6; GC–MS m/z: 229 (M+).
1-(2-Nitrophenyl)naphthalene (3ab)11
Orange solid (X = I, 181.7 mg, 73%; X = Br, 152.0 mg, 61%); 1H NMR (400 MHz, CDCl3): δ 8.09 (d, J = 8.1 Hz, 1H), 7.94 (d, J = 7.9 Hz, 2H), 7.69 (t, J = 7.0 Hz, 1H), 7.62–7.41 (m, 6H), 7.38 (d, J = 6.9 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 149.8, 135.5, 135.2, 133.5, 133.1, 132.6, 131.5, 128.7, 128.6, 128.5, 126.6, 126.1, 125.3, 124.9, 124.3; GC–MS m/z: 249 (M+).
1-(4-Methyl-2-nitrophenyl)naphthalene (3ac)
Orange waxy oil (134.2 mg, 51%); 1H NMR (400 MHz, CDCl3): δ 7.93–7.87 (m, 3H), 7.55–7.45 (m, 4H), 7.44–7.32 (m, 3H), 2.54 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 149.6, 139.2, 135.7, 133.5, 133.4, 132.9, 132.4, 131.7, 128.5, 126.6, 126.2, 126.1, 125.3, 125.0, 124.6, 21.1; HRMS (ESI) m/z: [M + H]+ calcd for C17H14NO2, 264.0946; found, 264.0949.
1-(4-Nitrophenyl)naphthalene (3ad)12
White waxy oil (169.4 mg, 68%); 1H NMR (400 MHz, CDCl3): δ 8.37 (d, J = 8.2 Hz, 2H), 7.97–7.90 (m, 2H), 7.79 (d, J = 8.4 Hz, 1H), 7.68 (d, J = 8.8 Hz, 2H), 7.60–7.40 (m, 4H); 13C NMR (100 MHz, CDCl3): δ 147.8, 147.3, 137.9, 133.9, 131.1, 129.1, 128.7, 127.2, 126.9, 126.4, 125.4, 125.2, 123.7; GC–MS m/z: 249 (M+).
3-(Naphthalen-1-yl)benzonitrile (3ae)13
White solid (116.3 mg, 51%); 1H NMR (400 MHz, CDCl3): δ 8.05–7.86 (m, 6H), 7.71–7.64 (m, 2H), 7.62–7.50 (m, 3H); 13C NMR (100 MHz, CDCl3): δ 142.5, 136.2, 133.6, 133.1, 131.8, 131.0, 130.8, 129.8, 129.1, 128.4, 127.8, 126.8, 126.7, 126.3, 124.9, 119.0, 113.2; GC–MS m/z: 229 (M+).
1-(3-Nitrophenyl)naphthalene (3af)13
Yellow solid (136.7 mg, 55%);1H NMR (400 MHz, CDCl3): δ 8.58 (s, 1H), 8.23 (d, J = 8.1 Hz, 1H), 8.09 (s, 1H), 8.04 (d, J = 7.7 Hz, 1H), 8.00–7.87 (m, 3H), 7.75 (d, J = 8.8 Hz, 1H), 7.64 (t, J = 8.0 Hz, 1H), 7.59–7.52 (m, 2H), 13C NMR (100 MHz, CDCl3): δ 148.9, 142.9, 136.0, 133.6, 133.3, 133.2, 129.9, 129.1, 128.4, 127.8, 126.9, 126.8, 126.5, 124.9, 122.2, 122.1; GC–MS m/z: 249 (M+).
(1,1′-Biphenyl)-2-carbonitrile (3ba)3c
Yellow waxy oil (X = I, 98.3 mg, 55%; X = Br, 78.5 mg, 44%); 1H NMR (400 MHz, CDCl3): δ 7.77 (d, J = 7.7 Hz, 1H), 7.65 (t, J = 7.6 Hz, 1H), 7.62–7.41 (m, 7H); 13C NMR (100 MHz, CDCl3): δ 145.6, 138.2, 133.8, 132.9, 130.2, 128.8, 127.6, 118.8, 111.4; GC–MS m/z: 179 (M+).
2-Nitro-1,1′-biphenyl (3bb)3c
Yellow solid (X = I, 125.2 mg, 63%; X = Br, 105.5 mg, 58%); 1H NMR (400 MHz, CDCl3): δ 7.86 (d, J = 8.1 Hz, 1H), 7.62 (t, J = 7.5 Hz, 1H), 7.51–7.39 (m, 5H), 7.36–7.30 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 149.4, 137.5, 136.4, 132.4, 132.0, 128.8, 128.3, 128.2, 128.0, 124.2; GC–MS m/z: 199 (M+).
4-Methyl-2-nitro-1,1′-biphenyl (3bc)14
Pale orange oil (87.2 mg, 41%); 1H NMR (400 MHz, CDCl3): δ 7.67 (s, 1H), 7.44–7.36 (m, 4H), 7.34–7.28 (m, 3H), 2.47 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 149.2, 138.8, 137.5, 133.6, 133.1, 131.8, 128.7, 128.1, 128.0, 124.5, 21.0; GC–MS m/z: 213 (M+).
4-Nitro-1,1′-biphenyl (3bd)3c
Pale yellow solid (149.3 mg, 75%); 1H NMR (400 MHz, CDCl3): δ 8.30 (d, J = 8.7 Hz, 2H), 7.74 (d, J = 8.7 Hz, 2H), 7.63 (d, J = 7.2 Hz, 2H), 7.54–7.41 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 147.7, 147.1, 138.8, 129.2, 129.0, 127.9, 127.5, 124.2; GC–MS m/z: 199 (M+).
3-Nitro-1,1′-biphenyl (3be)13
Yellow solid (121.2 mg, 61%): 1H NMR (400 MHz, CDCl3): δ 8.43 (s, 1H), 8.18 (d, J = 8.1 Hz, 1H), 7.90 (d, J = 7.7 Hz, 1H), 7.65–7.39 (m, 6H); 13C NMR (100 MHz, CDCl3): δ 148.7, 142.2, 138.6, 133.0, 129.7, 129.1, 128.5, 127.1, 122.0, 121.8; GC–MS m/z: 199 (M+).
2′,4,5′-Trimethyl-2-nitro-1,1′-biphenyl (3cc)
Pale yellow oil (101.3 mg, 42%); 1H NMR (400 MHz, CDCl3): δ 7.79 (s, 1H), 7.42 (d, J = 7.7 Hz, 1H), 7.20 (d, J = 7.7 Hz, 1H), 7.16–7.07 (m, 2H), 6.90 (s, 1H), 2.49 (s, 3H), 2.32 (s, 3H), 2.05 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 149.0, 138.6, 137.4, 135.2, 133.9, 133.4, 132.7, 132.1, 129.9, 129.1, 128.8, 124.4, 21.0, 19.5; HRMS (ESI) m/z: [M + H]+ calcd for C15H16NO2, 242.1103; found, 242.1106.
2′,4′,6′-Trimethyl-[1,1′-biphenyl]-2-carbonitrile (3da)10
White solid (103.7 mg, 47%); 1H NMR (400 MHz, CDCl3): δ 7.77 (d, J = 7.7 Hz, 1H), 7.65 (t, J = 7.4 Hz, 1H), 7.45 (t, J = 7.6 Hz, 1H), 7.29 (d, J = 7.7 Hz, 1H), 6.98 (s, 2H), 2.34 (s, 3H), 1.99 (s, 6H); 13C NMR (100 MHz, CDCl3): δ 146.6, 138.1, 135.7, 135.0,133.0, 132.9, 130.6, 128.5, 127.5, 118.0, 113.4, 21.2, 20.3; GC–MS m/z: 221 (M+).
2,4,6-Trimethyl-2′-nitro-1,1′-biphenyl (3db)15
Yellow oil (X = I, 168.6 mg, 70%; X = Br, 159.0 mg, 66%); 1H NMR (400 MHz, CDCl3): δ 8.01 (d, J = 8.1 Hz, 1H), 7.66 (t, J = 7.4 Hz, 1H), 7.52 (t, J = 7.4 Hz, 1H), 7.27–7.23 (m, 2H), 6.93 (s, 2H), 2.33 (s, 3H), 1.95 (s, 6H); 13C NMR (100 MHz, CDCl3): δ 149.4, 137.5, 135.9, 135.5, 134.2, 133.1, 132.1, 128.2, 124.2, 21.2, 20.5; GC–MS m/z: 241 (M+).
2,4,4′,6-Tetramethyl-2′-nitro-1,1′-biphenyl (3dc)
Waxy yellow oil (96.8 mg, 38%); 1H NMR (400 MHz, CDCl3): δ 7.81 (s, 1H), 7.45 (d, J = 7.7 Hz, 1H), 7.12 (d, J = 7.8 Hz, 1H), 6.92 (s, 2H), 2.49 (s, 3H), 2.32 (s, 3H), 1.94 (s, 6H); 13C NMR (100 MHz, CDCl3): δ 149.2, 138.6, 137.4, 135.7, 134.3, 133.9, 132.9, 128.2, 124.6, 21.2, 21.1, 20.5; HRMS (ESI) m/z: [M + H]+ calcd for C16H18NO2, 256.1259; found, 256.1263.
2-(Anthracen-9-yl)benzonitrile (3ea)16
White solid (206.3 mg, 74%); 1H NMR (400 MHz, CDCl3): δ 8.59 (s, 1H), 8.09 (d, J = 8.5 Hz, 2H), 7.94 (d, J = 7.7 Hz, 1H), 7.82 (t, J = 7.7 Hz, 1H), 7.66 (t, J = 7.5 Hz, 1H), 7.58–7.37 (m, 7H); 13C NMR (100 MHz, CDCl3): δ 143.2, 133.3, 132.8, 132.6, 132.0, 131.3, 130.2, 128.6, 128.48, 128.44, 126.4, 125.6, 125.4, 117.7, 115.2; GC–MS m/z: 279 (M+).
9-(2-Nitrophenyl)anthracene (3eb)11
Pale yellow solid (236.3 mg, 79%); 1H NMR (400 MHz, CDCl3): δ 8.54 (s, 1H), 8.27 (d, J = 7.8 Hz, 1H), 8.07 (d, J = 8.5 Hz, 2H), 7.80 (t, J = 7.4 Hz, 1H), 7.73 (t, J = 7.7 Hz, 1H), 7.53–7.33 (m, 7H); 13C NMR (100 MHz, CDCl3): δ 150.4, 134.1, 134.0, 133.1, 131.8, 131.3, 129.9, 129.2, 128.8, 127.6, 126.2, 125.5, 125.3, 124.7; GC–MS m/z: 299 (M+)
2-(Phenanthren-9-yl)benzonitrile (3fa)
White solid (203.5 mg, 73%); 1H NMR (400 MHz, CDCl3): δ 8.80 (d, J = 8.3 Hz, 1H), 8.75 (d, J = 8.1 Hz, 1H), 7.93 (d, J = 7.8 Hz, 1H), 7.87 (d, J = 7.8 Hz, 1H), 7.77–7.52 (m, 9H); 13C NMR (100 MHz, CDCl3): δ 144.6, 134.7, 133.3, 132.5, 131.7, 131.1, 130.7, 130.69, 130.66, 129.1, 128.7, 128.1, 127.5, 127.1, 127.0, 126.2, 123.2, 122.7, 118.1, 113.8; HRMS (ESI) m/z: [M + H]+ calcd for C21H14N, 280.1048; found, 280.1052.
9-(4-Nitrophenyl)phenanthrene (3fd)17
Pale yellow solid (212.3 mg, 71%); 1H NMR (400 MHz, CDCl3): δ 8.81 (d, J = 8.3 Hz, 1H), 8.75 (d, J = 8.3 Hz, 1H), 8.39 (d, J = 8.5 Hz, 2H), 7.93 (d, J = 7.7 Hz, 1H), 7.79 (d, J = 8.2 Hz, 1H), 7.77–7.63 (m, 6H), 7.58 (t, J = 7.6 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 147.9, 136.5, 131.1, 130.8, 130.4, 130.2, 129.0, 128.2, 127.5, 127.3, 127.1, 127.0, 126.3, 125.1, 123.8, 123.3, 122.7; GC–MS m/z: 299 (M+)
Dibenzofuran (5af)17
White solid (109.1 mg, 65%); 1H NMR (400 MHz, CDCl3): δ 7.97 (d, J = 7.6 Hz, 2H), 7.59 (d, J = 8.2 Hz, 2H), 7.47 (t, J = 7.7 Hz, 2H), 7.36 (t, J = 7.4 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 156.2, 127.2, 124.3, 122.8, 120.7, 111.7; GC–MS m/z: 168 (M+).
2-Methyldibenzofuran (5bf)18
Waxy oil (65.5 mg, 36%); 1H NMR (400 MHz, CDCl3): δ 7.94 (d, J = 7.0 Hz, 1H), 7.76 (s, 1H), 7.57 (d, J = 8.2 Hz, 1H), 7.50–7.42 (m, 2H), 7.34 (t, J = 7.4 Hz, 1H), 7.30–7.23 (m, 1H), 2.53 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 156.5, 154.4, 132.4, 132.2, 128.3, 127.7, 127.0, 122.6, 120.7, 120.6, 111.7, 111.2, 21.4; GC–MS m/z: 182 (M+).
2-Nitrodibenzofuran (5cf)19
Pale yellow solid (153.2 mg, 72%); 1H NMR (400 MHz, CDCl3): δ 8.88 (d, J = 2.3 Hz, 1H), 8.44–8.38 (m, 1H), 8.04 (d, J = 7.7 Hz, 1H), 7.68–7.62 (m, 2H), 7.58 (t, J = 7.7 Hz, 1H), 7.46 (t, J = 7.5 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 159.3, 157.6, 144.0, 129.1, 125.1, 124.0, 123.2, 123.1, 121.4, 117.3, 112.4, 112.1; GC–MS m/z: 213 (M+).
2-Chlorodibenzofuran (5df)18
White solid (90.8 mg, 45%); 1H NMR (400 MHz, CDCl3): δ 7.92–7.87 (m, 2H), 7.57 (d, J = 8.2 Hz, 1H), 7.52–7.46 (m, 2H), 7.43–7.33 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 156.8, 154.5, 128.2, 127.9, 127.2, 125.7, 123.4, 123.1, 120.9, 120.5, 112.7, 111.9; GC–MS m/z: 202 (M+).
1,3-Dimethoxydibenzofuran (5ef)
White solid (118.4 mg, 52%); 1H NMR (400 MHz, CDCl3): δ 8.01 (d, J = 8.3 Hz, 1H), 7.49 (d, J = 7.4 Hz, 1H), 7.37–7.26 (m, 2H), 6.71 (s, 1H), 6.41 (s, 1H), 4.01 (s, 3H), 3.90 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 161.1, 158.3, 156.1, 155.6, 124.8, 123.9, 122.9, 121.8, 110.8, 107.3, 94.0, 88.5, 55.9, 55.8; HRMS (ESI) m/z: [M + H]+ calcd for C14H13O3, 229.0786; found, 229.0789.
Naphtho[1,2-b]benzofuran (5ff)18
White solid (137.4 mg, 63%); 1H NMR (400 MHz, CDCl3): δ 8.47 (d, J = 8.2 Hz, 1H), 8.06–7.95 (m, 3H), 7.80 (d, J = 8.5 Hz, 1H), 7.74 (d, J = 8.2 Hz, 1H), 7.67 (t, J = 7.5 Hz, 1H), 7.59 (t, J = 7.5 Hz, 1H), 7.50 (t, J = 7.7 Hz, 1H), 7.42 (t, J = 7.4 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 156.0, 152.1, 133.1, 128.5, 126.5, 126.3, 126.1, 125.1, 123.4, 123.0, 121.4, 121.0, 120.4, 119.2, 118.6, 111.9; GC–MS m/z: 218 (M+).
Naphtho[2,1-b]benzofuran (5gf)18
White solid (152.7 mg, 70%); 1H NMR (400 MHz, CDCl3): δ 8.63 (d, J = 8.3 Hz, 1H), 8.40 (d, J = 8.4 Hz, 1H), 8.04 (d, J = 8.2 Hz, 1H), 7.93 (d, J = 8.9 Hz, 1H), 7.81–7.69 (m, 3H), 7.60–7.45 (m, 3H); 13C NMR (100 MHz, CDCl3): δ 155.9, 154.4, 130.5, 129.3, 129.1, 128.6, 127.2, 125.9, 125.0, 124.5, 123.5, 122.0, 117.4, 112.8, 112.0; GC–MS m/z: 218 (M+)..
Acknowledgments
This project was supported by the National Natural Science Foundation of China (21673124). M.A.I. and H.M. thank the China Scholarship Council (CSC) for offering generous support for their study at Tsinghua University as PhD candidates.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01736.
Copies of 1H and 13C NMR spectra of all products (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Selected reviews, see:; a Colby D. A.; Bergman R. G.; Ellman J. A. Rhodium-catalyzed C-C bond formation via heteroatom-directed C-H bond activation. Chem. Rev. 2010, 110, 624–655. 10.1021/cr900005n. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wencel-Delord J.; Dröge T.; Liu F.; Glorius F. Towards mild metal-catalyzed C–H bond activation. Chem. Soc. Rev. 2011, 40, 4740–4761. 10.1039/c1cs15083a. [DOI] [PubMed] [Google Scholar]; c Arockiam P. B.; Bruneau C.; Dixneuf P. H. Ruthenium(II)-catalyzed C–H bond activation and functionalization. Chem. Rev. 2012, 112, 5879–5918. 10.1021/cr300153j. [DOI] [PubMed] [Google Scholar]; d Liu C.; Yuan J.; Gao M.; Tang S.; Li W.; Shi R.; Lei A. Oxidative coupling between two hydrocarbons: An update of recent C–H functionalizations. Chem. Rev. 2015, 115, 12138–12204. 10.1021/cr500431s. [DOI] [PubMed] [Google Scholar]; e Gensch T.; Hopkinson M. N.; Glorius F.; Wencel-Delord J. Mild metal-catalyzed C–H activation: examples and concepts. Chem. Soc. Rev. 2016, 45, 2900–2936. 10.1039/c6cs00075d. [DOI] [PubMed] [Google Scholar]; f Shang R.; Ilies L.; Nakamura E. Iron-catalyzed C–H bond activation. Chem. Rev. 2017, 117, 9086–9139. 10.1021/acs.chemrev.6b00772. [DOI] [PubMed] [Google Scholar]; g Niu B.; Yang K.; Lawrence B.; Ge H. Transient ligand-enabled transition metal-catalyzed C-H functionalization. ChemSusChem 2019, 12, 2955–2969. 10.1002/cssc.201900151. [DOI] [PubMed] [Google Scholar]; h Ghosh K.; Rit R. K.; Shankar M.; Mukherjee K.; Sahoo A. K. Directing group assisted unsymmetrical multiple functionalization of arene C-H bonds. Chem. Rec. 2020, 20, 1017–1042. 10.1002/tcr.202000063. [DOI] [PubMed] [Google Scholar]; i Rej S.; Das A.; Chatani N. Strategic evolution in transition metal-catalyzed directed C–H bond activation and future directions. Coord. Chem. Rev. 2021, 431, 213683. 10.1016/j.ccr.2020.213683. [DOI] [Google Scholar]
- Selected reviews on biaryl syntheses, see:; a Hassan J.; Sévignon M.; Gozzi C.; Schulz E.; Lemaire M. Aryl-aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002, 102, 1359–1470. 10.1021/cr000664r. [DOI] [PubMed] [Google Scholar]; b Campeau L.-C.; Fagnou K. Palladium-catalyzed direct arylation of simple arenes in synthesis of biaryl molecules. Chem. Commun. 2006, 1253–1264. 10.1039/b515481m. [DOI] [PubMed] [Google Scholar]; c Alberico D.; Scott M. E.; Lautens M. Aryl-aryl bond formation by transition-metal-catalyzed direct arylation. Chem. Rev. 2007, 107, 174–238. 10.1021/cr0509760. [DOI] [PubMed] [Google Scholar]; d Zhang Y.-F.; Shi Z.-J. Upgrading cross-coupling reactions for biaryl syntheses. Acc. Chem. Res. 2019, 52, 161–169. 10.1021/acs.accounts.8b00408. [DOI] [PubMed] [Google Scholar]; e Yuan S.; Chang J.; Yu B. Construction of biologically important biaryl scaffolds through direct C–H bond activation: Advances and prospects. Top. Curr. Chem. 2020, 378, 23. 10.1007/s41061-020-0285-9. [DOI] [PubMed] [Google Scholar]
- Selected reports, see:; a Sun C.-L.; Shi Z.-J. Transition-metal-free coupling reactions. Chem. Rev. 2014, 114, 9219–9280. 10.1021/cr400274j. [DOI] [PubMed] [Google Scholar]; b Shigeno M.; Kai Y.; Yamada T.; Hayashi K.; Nozawa-Kumada K.; Denneval C.; Kondo Y. Construction of biaryl scaffolds from iodoarenes and C-H heteroarenes using an amide base generated in situ from aminosilane and fluoride anion. Asian J. Org. Chem. 2018, 7, 2082–2086. 10.1002/ajoc.201800438. [DOI] [Google Scholar]; c Nozawa-Kumada K.; Nakamura K.; Kurosu S.; Iwakawa Y.; Denneval C.; Shigeno M.; Kondo Y. Tetramethylammonium fluoride tetrahydrate-mediated transition metal-free coupling of aryl iodides with unactivated arenes in air. Chem. Pharm. Bull. 2019, 67, 1042–1045. 10.1248/cpb.c19-00452. [DOI] [PubMed] [Google Scholar]; d Music A.; Baumann A. N.; Spieß P.; Plantefol A.; Jagau T. C.; Didier D. Electrochemical synthesis of biaryls via oxidative intramolecular coupling of tetra(hetero)arylborates. J. Am. Chem. Soc. 2020, 142, 4341–4348. 10.1021/jacs.9b12300. [DOI] [PubMed] [Google Scholar]; e Gerleve C.; Studer A. Transition-metal-free oxidative cross-coupling of tetraarylborates to biaryls using organic oxidants. Angew. Chem., Int. Ed. 2020, 59, 15468–15473. 10.1002/anie.202002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Sun C.-L.; Li H.; Yu D.-G.; Yu M.; Zhou X.; Lu X.-Y.; Huang K.; Zheng S.-F.; Li B.-J.; Shi Z.-J. An efficient organocatalytic method for constructing biaryls through aromatic C–H activation. Nat. Chem. 2010, 2, 1044–1049. 10.1038/nchem.862. [DOI] [PubMed] [Google Scholar]; b Shirakawa E.; Itoh K.-i.; Higashino T.; Hayashi T. tert-Butoxide-mediated arylation of benzene with aryl halides in the presence of a catalytic 1,10-phenanthroline derivative. J. Am. Chem. Soc. 2010, 132, 15537–15539. 10.1021/ja1080822. [DOI] [PubMed] [Google Scholar]; c Liu W.; Cao H.; Zhang H.; Zhang H.; Chung K. H.; He C.; Wang H.; Kwong F. Y.; Lei A. Organocatalysis in cross-coupling: DMEDA-catalyzed direct C-H arylation of unactivated benzene. J. Am. Chem. Soc. 2010, 132, 16737–16740. 10.1021/ja103050x. [DOI] [PubMed] [Google Scholar]; d Liu H.; Yin B.; Gao Z.; Li Y.; Jiang H. Transition-metal-free highly chemo- and regioselective arylation of unactivated arenes with aryl halides over recyclable heterogeneous catalysts. Chem. Commun. 2012, 48, 2033–2035. 10.1039/c2cc16790e. [DOI] [PubMed] [Google Scholar]; e Zhao H.; Shen J.; Guo J.; Ye R.; Zeng H. A macrocyclic aromatic pyridone pentamer as a highly efficient organocatalyst for the direct arylations of unactivated arenes. Chem. Commun. 2013, 49, 2323–2325. 10.1039/c3cc00019b. [DOI] [PubMed] [Google Scholar]; f Ghonchepour E.; Islami M. R.; Mostafavi H.; Tikdari A. M.; Sheikhshoaie I. Transition metal-free and base-mediated transformation arylation of unactivated benzene with aryl halides in presence of N,N′-bis(salicylidene)ethylenediamine as organocatalyst. Catal. Commun. 2018, 107, 87–91. 10.1016/j.catcom.2018.01.007. [DOI] [Google Scholar]; g Zhao H.; Xu X.; Wu W.; Zhang W.; Zhang Y. Urea-based organocatalyst catalyzed direct C-H bond arylations of unactivated arenes. Catal. Commun. 2018, 111, 95–99. 10.1016/j.catcom.2018.04.008. [DOI] [Google Scholar]; h Ghonchepour E.; Islami M. R.; Tikdari A. M. Methyl red as organocatalyst for arylation of unactivated benzene derivatives with aryl halides. ChemistrySelect 2018, 3, 11517–11521. 10.1002/slct.201802643. [DOI] [Google Scholar]; i Nozawa-Kumada K.; Iwakawa Y.; Onuma S.; Shigeno M.; Kondo Y. NaH-mediated direct C–H arylation in the presence of 1,10-phenanthroline. Chem. Commun. 2020, 56, 7773–7776. 10.1039/d0cc00730g. [DOI] [PubMed] [Google Scholar]; j Liu Z.; Wang P.; Chen Y.; Yan Z.; Chen S.; Chen W.; Mu T. Small organic molecules with tailored structures: initiators in the transition-metal-free C–H arylation of unactivated arenes. RSC Adv. 2020, 10, 14500–14509. 10.1039/d0ra01845g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Joshi M.; Patel M.; Tiwari R.; Verma A. K. Base-mediated selective synthesis of diversely substituted N-heterocyclic enamines and enaminones by the hydroamination of alkynes. J. Org. Chem. 2012, 77, 5633–5645. 10.1021/jo300782n. [DOI] [PubMed] [Google Scholar]; b Majumdar K. C.; Ganai S.; Nandi R. K.; Ray K. Catalyst-free synthesis of coumarin-, quinolone- and pyridine-annulated oxazole derivatives. Tetrahedron Lett. 2012, 53, 1553–1557. 10.1016/j.tetlet.2012.01.015. [DOI] [Google Scholar]; c Jia F.-C.; Xu C.; Zhou Z.-W.; Cai Q.; Wu Y.-D.; Wu A.-X. Substrates as electron-donor precursors: synthesis of naphtho-fused oxindoles via benzannulation of 2-halobenzaldehydes and indolin-2-ones. Org. Lett. 2016, 18, 5232–5235. 10.1021/acs.orglett.6b02515. [DOI] [PubMed] [Google Scholar]; d Irudayanathan F. M.; Kim J.; Song K. H.; Lee S. Transition-metal-free decarboxylative coupling reactions for the synthesis of propargyl alcohols. Asian J. Org. Chem. 2016, 5, 1148–1154. 10.1002/ajoc.201600265. [DOI] [Google Scholar]; e Iqbal M. A.; Lu L.; Mehmood H.; Khan D. M.; Hua R. Quinazolinone synthesis through base-promoted SNAr reaction of ortho-fluorobenzamides with amides followed by cyclization. ACS Omega 2019, 4, 8207–8213. 10.1021/acsomega.9b00699. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Mehmood H.; Iqbal M. A.; Lu L.; Hua R. Base-promoted annulation of amidoximes with alkynes: Simple access to 2,4-disubstituted imidazoles. Molecules 2020, 25, 3621. 10.3390/molecules25163621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Li M.; Hua R. Gold(I)-catalyzed direct C-H arylation of pyrazine and pyridine with aryl bromides. Tetrahedron Lett. 2009, 50, 1478–1481. 10.1016/j.tetlet.2009.01.059. [DOI] [Google Scholar]; b Yin T.; Hua R. Straightforward approach to synthesize 3,3’-bipyrroles by oxidative homocoupling of 1,2,5-trisubstituted pyrroles. Chem. Lett. 2013, 42, 836–837. 10.1246/cl.130253. [DOI] [Google Scholar]
- a Zheng L.; Hua R.R. C–H activation and alkyne annulation via automatic or intrinsic directing groups: towards high step economy. Chem. Rec. 2018, 18, 556–569. 10.1002/tcr.201700024. [DOI] [PubMed] [Google Scholar]; b Zheng L.; Hua R. Recent advances in construction of polycyclic natural product scaffolds via one-pot reactions involving alkyne annulation. Front. Chem. 2020, 8, 580355. 10.3389/fchem.2020.580355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Tang S.; Lei A. Tuning radical reactivity for selective radical/radical cross-coupling. Sci. Bull. 2018, 63, 1006–1009. 10.1016/j.scib.2018.06.004. [DOI] [PubMed] [Google Scholar]
- a Zhao Y.; Truhlar D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. 10.1007/s00214-007-0310-x. [DOI] [Google Scholar]; b Pritchard B. P.; Altarawy D.; Didier B.; Gibson T. D.; Windus T. L. New basis set exchange: An open, up-to-date resource for the molecular sciences community. J. Chem. Inf. Model. 2019, 59, 4814–4820. 10.1021/acs.jcim.9b00725. [DOI] [PubMed] [Google Scholar]; c Marenich A. V.; Cramer C. J.; Truhlar D. G. Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. 10.1021/jp810292n. [DOI] [PubMed] [Google Scholar]; d Grimme S.; Antony J.; Ehrlich S.; Krieg H. A consistent and accurate ab initio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
- Anbarasan P.; Neumann H.; Beller M. A convenient synthesis of benzonitriles via electrophilic cyanation with N-cyanobenzimidazole. Chem.—Eur. J. 2010, 16, 4725–4728. 10.1002/chem.201000086. [DOI] [PubMed] [Google Scholar]
- Yan M.-Q.; Yuan J.; Lan F.; Zeng S.-H.; Gao M.-Y.; Liu S.-H.; Chen J.; Yu G.-A. An active catalytic system for Suzuki-Miyaura cross-coupling reactions using low levels of palladium loading. Org. Biomol. Chem. 2017, 15, 3924–3929. 10.1039/c7ob00178a. [DOI] [PubMed] [Google Scholar]
- Novikov R. A.; Tarasova A. V.; Denisov D. A.; Borisov D. D.; Korolev V. A.; Timofeev V. P.; Tomilov Y. V. [4 + 2] Annulation of donor-acceptor cyclopropanes with acetylenes using 1,2-zwitterionic reactivity. J. Org. Chem. 2017, 82, 2724–2738. 10.1021/acs.joc.7b00209. [DOI] [PubMed] [Google Scholar]
- Hassan J.; Hathroubi C.; Gozzi C.; Lemaire M. Preparation of unsymmetrical biaryls via palladium-catalyzed coupling reaction of aryl halides. Tetrahedron 2001, 57, 7845–7855. 10.1016/s0040-4020(01)00752-9. [DOI] [Google Scholar]
- Hong W.; Qiu Y.; Yao Z.; Wang Z.; Jiang S. Palladium-catalyzed direct C–H arylation of unactivated arenes with aryl halidesPalladium-catalyzed direct C-H arylation of unactivated arenes with aryl Halides. Tetrahedron Lett. 2011, 52, 4916–4919. 10.1016/j.tetlet.2011.07.046. [DOI] [Google Scholar]
- Zhang S.; Tang Z.; Bao W.; Li J.; Guo B.; Huang S.; Zhang Y.; Rao Y. Perylenequinonoid-catalyzed photoredox activation for the direct arylation of (het)arenes with sunlight. Org. Biomol. Chem. 2019, 17, 4364–4369. 10.1039/c9ob00659a. [DOI] [PubMed] [Google Scholar]
- Zehm D.; Fudickar W.; Hans M.; Schilde U.; Kelling A.; Linker T. 9,10-Diarylanthracenes as molecular switches: Syntheses, properties, isomerisations and their reactions with singlet oxygen. Chem.—Eur. J. 2008, 14, 11429–11441. 10.1002/chem.200801355. [DOI] [PubMed] [Google Scholar]
- Xiao T.; Dong X.; Tang Y.; Zhou L. Phenanthrene synthesis by eosin Y-catalyzed, visible light-induced [4+2] benzannulation of biaryldiazonium salts with alkynes. Adv. Synth. Catal. 2012, 354, 3195–3199. 10.1002/adsc.201200569. [DOI] [Google Scholar]
- Maetani S.; Fukuyama T.; Ryu I. Rhodium-catalyzed decarbonylative C-H arylation of 2-aryloxybenzoic acids leading to dibenzofuran derivatives. Org. Lett. 2013, 15, 2754–2757. 10.1021/ol4010905. [DOI] [PubMed] [Google Scholar]
- Panda N.; Mattan I.; Nayak D. K. Synthesis of dibenzofurans via C-H activation of o-iodo diaryl ethers. J. Org. Chem. 2015, 80, 6590–6597. 10.1021/acs.joc.5b00634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.