Abstract

Reverse osmosis (RO)
concentrate produced in the municipal solid
waste (MSW) leachate treatment process is extremely hard to be treated
because of its high color, high salt content, and high concentration
of recalcitrant organic compounds. A new multichannel flow reactor
with electrode gaps of 5 mm was designed to desalinate and remove
organics simultaneously from the RO leachate concentrate (ROLC) by
electrochemical oxidation process using the RuO2/IrO2-coated titanium plate (RuO2/IrO2-Ti)
as the anodes. The effects of the process parameters of current density
(IA), superficial circulating velocity
(UL), etc. on the removal efficiency (RE)
of the chemical oxygen demand (COD) and average energy consumption
(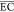 ) were investigated.
The results illustrated
that after 3 h of treatment, the RE of COD, Cl–, and color could reach as high
as 96.5, 96.7, and 99.6%, respectively. Besides, the
) were investigated.
The results illustrated
that after 3 h of treatment, the RE of COD, Cl–, and color could reach as high
as 96.5, 96.7, and 99.6%, respectively. Besides, the 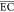 of the electrochemical
oxidation treatment process is as low as 40.98 kWh/(kg COD), and a
new mechanism of the simultaneous removal of COD and desalination
has been proposed. This work provides an alternative technology for
the treatment of MSW leachate RO concentrate.
of the electrochemical
oxidation treatment process is as low as 40.98 kWh/(kg COD), and a
new mechanism of the simultaneous removal of COD and desalination
has been proposed. This work provides an alternative technology for
the treatment of MSW leachate RO concentrate.
1. Introduction
Incineration and landfill are the mainstream treatments of municipal solid waste (MSW) in China.1 However, a large amount of leachate that accounts for 10–25% mass weight of MSW would be produced during its storage stage because of the inadequate MSW classification in China.2 The MSW leachate is a kind of dark gray, foul-smelling organic wastewater, which contains high concentrations of organic matter, ammonia, dissolved solids, and heavy metals.3−6 It is a considerable threat to the ecological environment and human beings.7,8 Reverse osmosis (RO) technology is often used to treat the biologically treated MSW leachate due to its advantages of excellent effluent quality, smaller footprint, automatic control, and low cost.9 However, 20% and even up to 50% volume of the treated wastewater is concentrated as the RO concentrate with extremely high concentrations of color, alkalinity, salinity, and recalcitrant organic compounds.10 As a result, the RO leachate concentrate (ROLC) is extremely hard to be treated. In practical wastewater treatment, recirculating spraying and combustion treatment are often used in landfills and MSW incineration plants in China, respectively. However, the above-mentioned treatments of ROLC are costly, have low efficiency, and exhibit adverse side effects. Especially for the landfills, recirculating spraying treatment is the temporary disposal of ROLC, causing the salt and recalcitrant organic compounds to accumulate continually. As a result, the whole landfill system faces a threat of collapse, and treatment technology for ROLC is urgently needed.
To develop a new efficient treatment technology of ROLC, researchers around the world have turned to advanced oxidation processes (AOPs) for a solution. The AOPs are characterized as producing strong oxidation species such as •OH/•O2–/•HO2–/ during the treatment process, exhibiting superiority in recalcitrant organic removal and biodegradability improvement.11 The AOPs commonly used in ROLC include electrochemical oxidation,12,13 Fenton-active persulfate-coupling oxidation,14,15 coagulation–flocculation Fenton method,16,17 coagulation–ozonation method,18,19 electro-Fenton oxidation,20−22 and photoelectro-Fenton oxidation.23 However, many kinds of research studies on AOPs are still at the laboratory stage because of the high treatment cost, complicated operation, large generation of sludge, and secondary pollution.
Compared with the other AOPs, electrochemical oxidation is considered to be an eye-catching technology for the treatment of ROLC owing to no sludge generation, no addition of chemical oxidation reagent, convenient operation, versatility, and low cost.13,24−27 Furthermore, the high concentration of inorganic ions, particularly the chloride ions (Cl–) in ROLC, can improve the conductivity of wastewater and benefit the formation of active chlorine species (Cl2/OCl–).26,28 As active chlorine has been proved to be a good intensifier to remove organic substances,28 leading to an anticipation of a highly efficient but low-energy-consuming treatment of ROLC by electrochemical oxidation. Last but not the least, desalination, which is vital for the treatment of ROLC, is also expected during the electrochemical treatment process because of the consumption of Cl–.
In this work, ROLC was electrochemically oxidized in a homemade
multichannel flow reactor (MCFR) with an electrode gap of 5 mm using
a RuO2/IrO2-coated titanium plate (RuO2/IrO2-Ti) as the anode and a 304 stainless steel plate
as the cathode. The effects of current density (IA), wastewater circulating velocity (UL), and treatment capacity per electrode area (CE) on the chemical oxygen demand (COD) removal
efficiency (RE) were studied. Then, the average energy consumption
(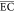 ) of this electrochemical
oxidation process
was comprehensively evaluated and compared with other works. Besides,
gas chromatography–mass spectrometry (GC–MS) was employed
to determine the original and final treated products of ROLC. Finally,
the precipitate generated in the treatment process was collected and
analyzed, and a new mechanism of the simultaneous removal of COD and
desalination by electrochemical oxidation was proposed correspondingly.
) of this electrochemical
oxidation process
was comprehensively evaluated and compared with other works. Besides,
gas chromatography–mass spectrometry (GC–MS) was employed
to determine the original and final treated products of ROLC. Finally,
the precipitate generated in the treatment process was collected and
analyzed, and a new mechanism of the simultaneous removal of COD and
desalination by electrochemical oxidation was proposed correspondingly.
2. Results and Discussion
2.1. Effects of Process Parameters on Organics Removal
2.1.1. Effect of IA
The effect of IA on the COD removal of ROLC by electrochemical oxidation was investigated and the results are shown in Figure 1. As can be seen in the figure, the increase of IA from 4.39 to 10.96 mA/cm2 accelerated the COD elimination rate but no significant influence of IA on COD removal was observed when IA exceeded 13.16 mA/cm2. The anode of RuO2/IrO2-Ti plates in this work is the so-called chlorine evolution electrode. The mechanism of the electrochemical oxidation of the recalcitrant organic compounds in the ROLC can be described by the following eqs 1–329,30
| 1 |
| 2 |
| 3 |
Higher current densities (4.39–10.96 mA/cm2) promoted the generation of oxidants of active chlorine at the surface of the electrode, resulting in the rapid removal of COD,31 which is also confirmed by the detection of residual active chlorine as shown in Figure 1c. As illustrated in Figure 1c, a higher concentration of residual active chlorine was detected with a higher current density. However, further increasing IA to 13.16 mA/cm2 did not significantly affect the removal rate of COD may due because the control steps of the electrochemical oxidation reaction depended on the mass transfer between the oxidants and organic pollutants.32,33 This phenomenon is also consistent with the concentration changes of residual active chlorine produced in the electrochemical oxidation process shown in Figure 1c. In the first 80 min of the electrochemical treatment, oxidant species of Cl2/OCl– reacted rapidly with the organics due to the high content of organic compounds. As a result, the content of the residual active chlorine in the reaction system is particularly low, and the limiting factor of the treatment is the production of oxidants. However, when the treatment time exceeds 80 min, the residual active chlorine content in the system increased greatly with the enhancement of current density. Thus, at this stage of the treatment, the limiting factor of the electrochemical reaction changed into the mass-transfer process.
Figure 1.
Effect of IA on COD concentration (a), RECOD (b), and active chlorine (c) in the electrochemical treatment of ROLC. Reaction conditions: wastewater volume = 2.5 L, UL = 0.44 cm/s, CE = 1.10 mL/cm2, initial Cl– concentration = 15 396 mg/L, pH = 8.1–9.6, and wastewater temperature 25.3–28.6 °C.
The decolorization of ROLC by the electrochemical oxidation process was also investigated, and the result is shown in Figure 2. It can be noted that the color of ROLC was nearly completely removed after 180 min treatment by the electrochemical process. This is mainly due to the production of active chlorine (reactions 1–2) that oxidizes the colored substances.
Figure 2.

Decolorization of the ROLC by electrochemical oxidation treatment. Reaction conditions: wastewater volume = 2.5 L, IA = 10.96 mA/cm2, UL = 0.44 cm/s, CE = 1.10 mL/cm2, initial Cl– concentration = 15 396 mg/L, pH = 8.1–9.6, wastewater temperature 25.3–28.6 °C.
2.1.2. Effect of UL
The UL affects the residence time, solubility of chlorine, mix and mass transfer of organic compounds, inorganic ions, and oxidant (Cl2/OCl–) in the electrochemical treatment process.33,34 The effect of UL on the electrochemical oxidation efficiency of the recalcitrant organic pollutants was studied and presented in Figure 3. As illustrated in Figure 3, the efficiency of COD removal decreased continuously, and the RECOD at 180 min decreased from 94.9 to 77.4% with the increase of UL from 0.29 to 0.73 cm/s. This result is a little different from that reported by the previous literature,29,35,36 probably because a higher UL led to a shortening of the residence time of the wastewater in the multichannel electrolytic unit. Besides, increasing UL also improves the convection effect of the solution between the electrode plates, which promotes the mass transfer of OCl– to the cathode plates and strengthens the cathode reaction of the consumption of oxidant (OCl–),37 which results in a decrease of the COD removal rate. Finally, excessive UL can harm the solubility of electrogenerated chlorine,29 which may not benefit the electrochemical degradation of organics in the ROLC.
Figure 3.
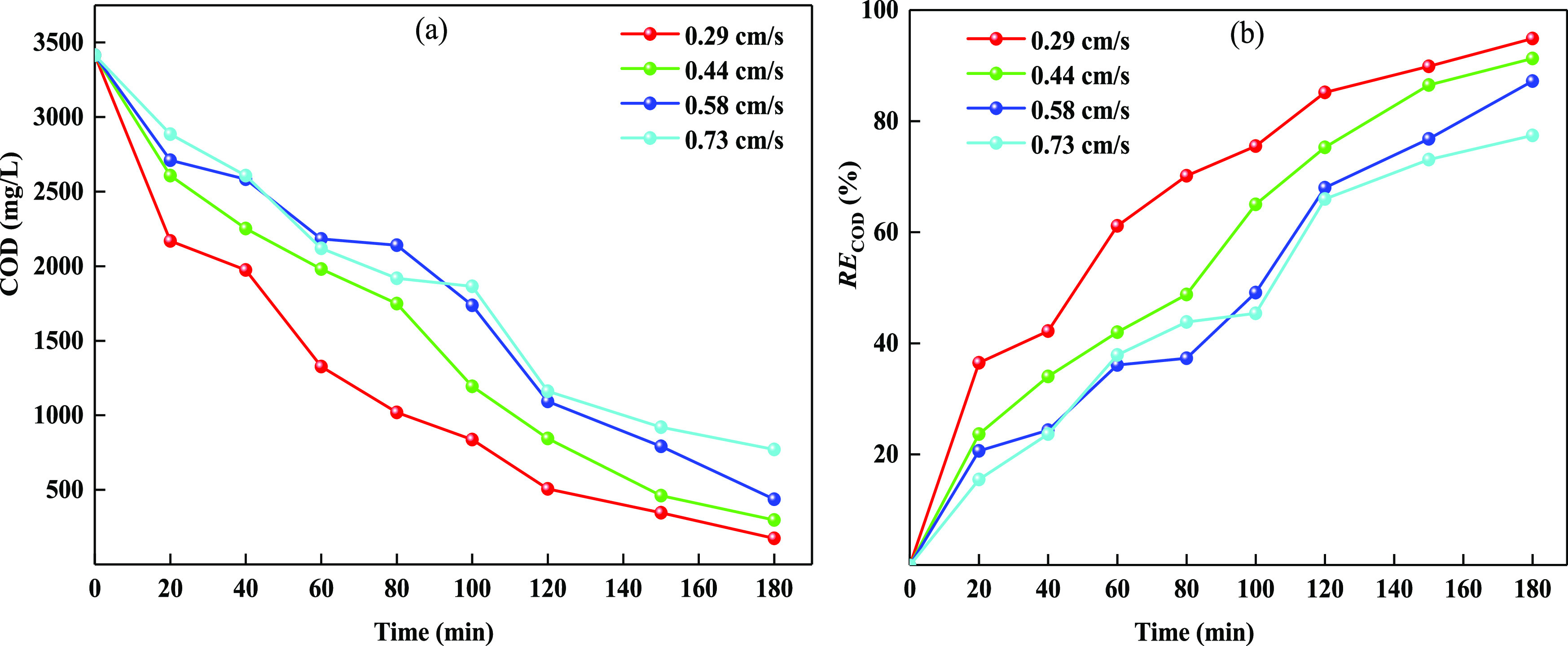
Effect of UL on the COD concentration (a) and RECOD (b) in the electrochemical treatment of ROLC. Reaction conditions: wastewater volume = 2.5 L, IA = 10.96 mA/cm2, CE = 1.10 mL/cm2, initial Cl– concentration = 15 396 mg/L, pH = 8.1– 9.6, and wastewater temperature 25.3–28.6 °C.
2.1.3. Effect of CE
The CE (mL/cm2) is defined as the corresponding wastewater treatment capacity per electrode area of the electrochemical oxidation process for the first time, which is a good index that can be used for the evaluation to scale up an electrochemical reactor. The effect of CE on the electrochemical degradation efficiency of recalcitrant organic pollutants is indicated in Figure 4. The figure shows that the COD elimination rate increased first with an increase in CE from 0.88 to 1.10 mL/cm2 but decreased rapidly and sharply with a further increase in CE from 1.10 to 1.54 mL/cm2. Thus, the optimal CE was 1.10 mL/cm2. The results possibly imply that for a certain electrode area, there may exist a relatively good wastewater treatment capacity to match. For a smaller CE, maybe the electrode plates were not in their best conditions to produce chlorine, and a little larger CE may improve the generating efficiency of chlorine. However, extremely larger CE means that an excessive organic matter needs to be treated with limited active chlorine because of the fixed anode plate area, which also agrees with our previous research studies.38,39 Moreover, a larger CE also causes a reduction in the treatment cycles of wastewater in the electrolytic cell, which can significantly affect the electrochemical treatment efficiency.
Figure 4.

Effect of CE on the COD concentration (a) and RECOD (b) in the electrochemical treatment of ROLC. Reaction conditions: IA = 10.96 mA/cm2, UL = 0.44 cm/s, initial Cl– concentration = 15 396 mg/L, pH = 8.1–9.6, and wastewater temperature 25.3–28.6 °C.
2.2. Energy Consumption Evaluation
The energy consumption of the electrochemical treatment of ROLC was evaluated under a better condition of IA of 10.96 mA/cm2, UL of 0.44 cm/s, and CE of 1.10 mL/cm2, and water quality of the treated ROLC by electrochemical oxidation process for 3 h is listed in Table 1. As shown in Table 1, the MCFR exhibited excellent performance of simultaneous desalination and removal of recalcitrant organic compounds from ROLC, and the REs of the COD, total organic carbon (TOC), Cl–, and color could reach as high as 96.5, 73.9, 96.7, and 99.6%, respectively.
Table 1. Properties of the Treated ROLC by Electrochemical Oxidation in MCFRa.
| parameters | treated ROLC | RE (%) |
|---|---|---|
| pH | 9.60 | |
| COD | 120.7 mg/L | 96.5 |
| TOC | 519.1 mg/L | 73.9 |
| Cl– | 502.0 mg/L | 96.7 |
| color | 10 times | 99.6 |
Reaction conditions: wastewater volume = 2.5 L, IA = 10.96 mA/cm2, UL = 0.44 cm/s, CE = 1.10 mL/cm2, initial Cl– concentration = 15 396 mg/L, pH = 8.1–9.6, and wastewater temperature 25.3–28.6 °C.
The 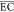 (kWh/(kg
COD)) of the treatment process
was calculated by eq 16, and the result is presented in Figure 5. As noted in Figure 5,
(kWh/(kg
COD)) of the treatment process
was calculated by eq 16, and the result is presented in Figure 5. As noted in Figure 5, 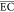 of the electrochemical
oxidation of ROLC
increased from the initial 9.15 kW/(kg COD) to 40.98 kW/(kg COD) gradually
when the electrochemical treatment process continued from 0.5 to 3
h. At the first stage of electrochemical oxidation of 30 min, the
of the electrochemical
oxidation of ROLC
increased from the initial 9.15 kW/(kg COD) to 40.98 kW/(kg COD) gradually
when the electrochemical treatment process continued from 0.5 to 3
h. At the first stage of electrochemical oxidation of 30 min, the 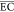 was as low as only 9.15
kW/(kg COD). The
reason for this is not difficult to understand that the initial COD,
TOC, and Cl– were as high as 3414.7, 1987.5, and
15396.4 mg/L, respectively, which are easy to electrolyze and oxidize,
and the COD sharply decreased to 1791.4 mg/L of RECOD of
47.5% as a result of a lowest
was as low as only 9.15
kW/(kg COD). The
reason for this is not difficult to understand that the initial COD,
TOC, and Cl– were as high as 3414.7, 1987.5, and
15396.4 mg/L, respectively, which are easy to electrolyze and oxidize,
and the COD sharply decreased to 1791.4 mg/L of RECOD of
47.5% as a result of a lowest  of the treatment process.
However, with
the prolongation of the treatment time, the degradation rate of recalcitrant
organics in the ROLC was slowed down, resulting in a correspondingly
sharp increase in the energy consumption of the electrochemical oxidation
process.29 This phenomenon was also observed
by many other researchers.35,40 However, it is worth
mentioning that the COD degradation rate was much quicker than that
of the TOC, which indicated that a larger proportion of organics in
the ROLC may be volatile compounds and were difficult to degrade by
the electrochemical treatment process. This hypothesis is also confirmed
by the GC–MS results of a large number of esters, alkanes,
etc. To exhibit the energy-saving and advanced organics removal performance
of the MCFR, a comparison of energy consumption of different electrochemical
oxidation processes was made and is presented in Table 2. As can be seen in the table,
the MCFR reveals higher COD removal efficiency, good energy-saving
performance, and larger wastewater treatment capacity for the electrochemical
oxidation process of recalcitrant organic wastewater of landfill leachate
and ROLC.
of the treatment process.
However, with
the prolongation of the treatment time, the degradation rate of recalcitrant
organics in the ROLC was slowed down, resulting in a correspondingly
sharp increase in the energy consumption of the electrochemical oxidation
process.29 This phenomenon was also observed
by many other researchers.35,40 However, it is worth
mentioning that the COD degradation rate was much quicker than that
of the TOC, which indicated that a larger proportion of organics in
the ROLC may be volatile compounds and were difficult to degrade by
the electrochemical treatment process. This hypothesis is also confirmed
by the GC–MS results of a large number of esters, alkanes,
etc. To exhibit the energy-saving and advanced organics removal performance
of the MCFR, a comparison of energy consumption of different electrochemical
oxidation processes was made and is presented in Table 2. As can be seen in the table,
the MCFR reveals higher COD removal efficiency, good energy-saving
performance, and larger wastewater treatment capacity for the electrochemical
oxidation process of recalcitrant organic wastewater of landfill leachate
and ROLC.
Figure 5.
Average energy consumption (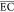 ) of the electrochemical
oxidation of ROLC.
Reaction conditions: wastewater volume = 2.5 L, IA = 10.96 mA/cm2, UL = 0.44 cm/s, CE = 1.10 mL/cm2, initial Cl– concentration = 15 396 mg/L,
pH = 8.1–9.6, and wastewater temperature 25.3–28.6 °C.
) of the electrochemical
oxidation of ROLC.
Reaction conditions: wastewater volume = 2.5 L, IA = 10.96 mA/cm2, UL = 0.44 cm/s, CE = 1.10 mL/cm2, initial Cl– concentration = 15 396 mg/L,
pH = 8.1–9.6, and wastewater temperature 25.3–28.6 °C.
Table 2. Comparison of Average Energy Consumption
(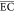 ) of This Study with
the Other Electrochemical
Oxidation Processes.
) of This Study with
the Other Electrochemical
Oxidation Processes.
| reactors | electrodes/oxidant species | electrolysis channels | wastewater/treatment capacity (L) | other experimental condition | RE and 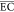
|
reference |
|---|---|---|---|---|---|---|
| differential column batch reactor | anode: boron-doped diamond (BDD), cathode: stainless steel electrode; Cl2/OCl– | 1 | ultrafiltration effluent of landfill leachate/0.4 | initial COD = 600 mg/L, flow velocity = 5.6 cm/s, electrode area = 50.0 cm2, reaction time = 12 h, current density = 1–80 mA/cm2 | RECOD = 88.3%, 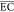 = 135.1 kWh/(kg COD) = 135.1 kWh/(kg COD) |
(27) |
| joint electrochemical system | anode: iron or Ti/RuO2, cathode: Cu/Zn; Cl2/OCl– | 1 | biologically treated landfill leachate/0.3 | initial COD = 2237.5 mg/L, flow rate = 40 mL/min, reaction time = 4 h, electrode area = 51.6 cm2, current density = 30–70 mA/cm2 | RECOD = 90.2%, 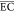 = 1091.3 kWh/(kg COD) = 1091.3 kWh/(kg COD) |
(41) |
| flow sequencing batch reactor | anode: Ti/BDD, cathode: Pt; Cl2/OCl–/•OH | 1 | biologically landfill leachate/0.05 | initial COD = 3308–3540 mg/L, wastewater volume = 50 mL, flow rate = 50 L/h, reaction time = 4–8 h, electrode area = 6 cm2, current density = 83 mA/cm2 | RECOD = 95%, 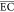 = 110 kWh/(kg COD) = 110 kWh/(kg COD) |
(35) |
| semipilot plant operating in batch mode with recirculation | anode: BDD, cathode: carbon-felt; Cl2/OCl–/•OH | 1 | RO leachate concentrate/10 | initial COD = 9900 mg/L, flow rate = 500 L/h, reaction time = 8 h, electrode area = 70 cm2, current density = 4.3 mA/cm2 | RECOD = 34%, 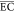 = 58 kWh/(kg COD) = 58 kWh/(kg COD) |
(26) |
| multichannel flow reactor | anode: RuO2/IrO2 coated Ti plates, cathode: 304 stainless steel plates; Cl2/OCl– | 6 | RO leachate concentrate/2.5 | initial COD = 3414.7 mg/L, circulating velocity = 0.44 cm/s, reaction time = 3 h, electrode area = 380 cm2 × 6, current density = 10.96 mA/cm2 | RECOD = 96%,  = 41 kWh/(kg COD) = 41 kWh/(kg COD) |
this study |
2.3. GC–MS Analysis
The organic compounds of the original ROLC and the treated ROLC for 3 h were detected by GC–MS under the conditions of IA of 10.96 mA/cm2, UL of 0.44 cm/s, and CE of 1.10 mL/cm2. The GC–MS results show that the organic species of the original and the treated ROLC were 104 and 176 kinds, respectively. Furthermore, the organics with the credibility of ≥60% and the relative percentage content of ≥0.1% are separately listed in Figure 6. Compared with the GC–MS results of the original ROLC sample, we found that after 3 h of electrochemical oxidation treatment, the organics in the treated ROLC were greatly reduced with their content from 46.8 to 17.1%. Besides, the species of organics (credibility of ≥60% and percentage content of ≥0.1%) were also significantly eliminated from 33 kinds (10 alkanes, 10 esters, 3 ketones, 2 alcohols, and 8 others) of the original wastewater compared to 13 kinds (7 chlorinated organics, 2 alkanes, 1 ester, 1 alcohol, 1 organic acid, and 1 alkene) of the treated ROLC. These results can be explained because of the electrochemical oxidation degradation of macromolecular organics into small molecular substances, chlorinated organic intermediates, and even completely oxidated into CO2 and H2O.41 The results also agree with RECOD and RETOC of the electrochemical oxidation process of 96.5 and 73.9%, respectively, indicating the good removal performance of recalcitrant organic compounds from ROLC with MCFR.
Figure 6.
Variations of organics in ROLC before and after electrochemical oxidation in the MCFR. Reaction conditions: wastewater volume = 2.5 L, IA = 10.96 mA/cm2, UL = 0.44 cm/s, CE = 1.10 mL/cm2, initial Cl– concentration = 15 396 mg/L, pH = 8.1–9.6, and wastewater temperature 25.3– 28.6 °C.
2.4. Mechanism Analysis
In addition to the excellent elimination performance of recalcitrant organics in the ROLC, the electrochemical treatment with MCFR also showed a commendable ability of desalination. During the electrochemical oxidation process, white precipitates were observed to be generated in the treated ROLC solution and on the surface of cathode plates, which were separated for further X-ray diffraction (XRD) analysis. The phase compositions of the white precipitates were analyzed by XRD, and the results are shown in Figure 7. Compared with the standard XRD diffraction patterns, it can be preliminarily determined that the white precipitates (shown in the inset of Figure 7) are NaCl and CaCO3. Furthermore, the precipitates were confirmed to be NaCl and CaCO3 by qualitative analysis experiments of the insoluble part of the precipitates through precipitation by silver nitrate and dissolving the other part with a hydrochloric acid solution that could release CO2 gas.
Figure 7.
XRD patterns of precipitates generated in the electrochemical treatment of ROLC.
For the electrochemical wastewater treatment system, the removal effect of recalcitrant organic compounds mainly happened on anodes because Cl2 and OCl– with strong oxidizability were generated on the anode plates.42 As a result, the dissolved organics in the ROLC were oxidized effectively and achieved good RECOD and RETOC of 96.5 and 73.9%, respectively. However, a large number of chlorinated organics were also generated in the anodic oxidation reactions, which led to the detection of as many as 176 kinds of organics in the treated ROLC by GC–MS analysis. The desalination of the electrochemical process was achieved partly because of the reactions on the cathode of 304 stainless steel plates, which generated Cl– and OH–. Due to the high salinity of ROLC, which contains a lot of Na+ and Ca2+, resulting in the precipitation of Na+ and Cl– on the cathode plates. Second, a large amount of CO2 was produced in the wastewater solution because of the complete oxidation of organic compounds by the electrogenerated Cl2 and OCl–. The CO2 could be dissolved in ROLC and reacted with the OH– generated in cathodes to form CO32–, which was precipitated with Ca2+ to form CaCO3.
Combined with other related studies,27,31,42−45 the mechanisms of electrochemical oxidation of ROLC in the MCFR are summarized and presented in Figure 8 and eqs 4–14
Figure 8.

Mechanism diagram of the electrochemical oxidation treatment of ROLC in MCFR.
Anode reaction
| 4 |
| 5 |
| 6 |
Cathode reaction
| 7 |
| 8 |
| 9 |
Solution reaction
| 10 |
| 11 |
| 12 |
| 13 |
| 14 |
3. Conclusions
An easy scale-up multichannel flow reactor (MCFR) with electrode gaps of 5 mm was developed to simultaneously desalinate and eliminate recalcitrant organic compounds from RO leachate concentrate (ROLC) by the electrochemical oxidation process. High current density (IA) favors high organic substance removal efficiencies, but higher superficial circulating velocity (UL) and treatment capacity per electrode area (CE) restrict pollutant removal efficiency. The better conditions for the electrochemical oxidation of ROLC with MCFR are IA of 10.96 mA/cm2, UL of 0.44 cm/s, CE of 1.10 mL/cm2, and the removal efficiency of COD, TOC, Cl–, and color as high as 96.5, 73.9, 96.7, and 99.6%, respectively, for 3 h of electrochemical oxidation treatment. The MCFR also shows an energy-saving property with an average energy consumption of only 40.98 kW/(kg COD). After the electrochemical oxidation treatment, the organic species in ROLC reduce from 33 kinds to 13 kinds with relatively higher credibility (≥60%) and content (≥0.1%). The desalination mechanism of the electrochemical treatment process is mainly the precipitation of NaCl and CaCO3 and the consumption of Cl–. Therefore, MCFR provides a promising solution for the treatment of ROLC and exhibits prospects of industrial application.
4. Materials and Methods
4.1. Water Quality of ROLC
ROLC is a dark-colored and poisonous organic wastewater, which is classified as a hazardous waste in China. The ROLC used in this paper was collected from a local MSW incineration plant in Banan district, Chongqing City. The main physicochemical characteristics of the ROLC are summarized in Table 3. As illustrated in the table, the ROLC has a high color of 2500 times, high salt content with Cl– of 15 396.4 mg/L, total alkalinity (CO32–/HCO3–) of 11 500.0 mg/L, high concentration of organic compounds with COD and TOC of 3414.7 and 1987.5 mg/L, respectively, and extreme refractory biodegradation with BOD5/COD of 0.06.
Table 3. Properties of ROLC.
| parameters | RO concentrate |
|---|---|
| pH | 8.10 ± 0.02 |
| COD | 3414.7 ± 30 mg/L |
| TOC | 1987.5 ± 20 mg/L |
| BOD5 /COD | 0.06 |
| Cl– | 15 396.4 ± 100 mg/L |
| color | 2500.0 ± 50 times |
| total alkalinity (CaCO3) | 11 500.0 ± 300 mg/L |
4.2. Experimental Setup and Operation
The electrochemical oxidation of ROLC was conducted in MCFR, and the experimental setup is shown in Figure 9. The MCFR consisted of the main body of an acrylic multichannel electrolytic cell (190 mm × 50 mm × 320 mm, L × W × H), a direct current (DC) power supply (KXN-6050D, Zhaoxin Co.), a circulation pump (HQB-2200, Sensen Co.), a water tank (10 L), and a rotameter. The electrodes were placed in the main body with three RuO2/IrO2-Ti plates as the anode and four 304 stainless steel plates as the cathode (20 cm × 19 cm, L × W; thickness of 1 mm), and the distance between the electrode plates was fixed at 5 mm.
Figure 9.

Diagram of the electrochemical oxidation of ROLC in MCFR.
During experiments, a certain volume (2.0, 2.5, 3.0, 3.5 L) of ROLC was added into the water tank. Then, it was circulated in the system by a circulation pump for 10 min, and 10 mL of sample was collected and labeled as the zero-time sample to detect the original water quality. After that, the DC power supplier was turned on to start the electrochemical treatment process. The electrooxidation process was carried out at a constant ambient temperature (25.0 ± 1.0 °C), different IA (4.39, 6.58, 8.77, 10.96, and 13.16 mA/cm2), UL (0.29, 0.44, 0.58, and 0.73 cm/s), and CE (0.88, 1.10, 1.32, and 1.54 mL/cm2). The samples were taken out from the reactor after a fixed time interval to detect the water quality. Each experiment was performed in duplicate, and the deviation of the results between the same experiments was controlled at ±3%.
4.3. Analytical Method
The COD of the ROLC was analyzed according to the Chinese water quality determination of the COD of the fast digestion spectrophotometric method. The absorbance was measured using a UV–vis spectrophotometer (TU-1901, Beijing Puxi Analyzing Devices Ltd. Co., China). The TOC of the wastewater was detected using a TOC analyzer (TOC-LCPH, Shimadzu, Japan). The concentrations of active chlorine (Cl2/ClO–) and chloride ions (Cl–) were measured by iodimetry29 and silver nitrate titration methods, respectively. The color and alkalinity were determined by multiple dilution and acid–base titration methods, respectively.
An X-ray diffractometer (XRD-7000, Shimadzu, Japan) with Cu Kα radiation (λ = 1.5418 Å, 40 kV, 30 mA) at a scan step of 0.02° and a scan speed of 2°/min was used to determine the phase compositions of the precipitate generated and collected during the electrochemical oxidation process.
Gas chromatography–mass spectrometry (GC–MS, Agilent 7890A-5975C) was used to determine the organic compounds of the ROLC before and after electrochemical treatment with an OV1701 capillary column (30 m × 0.25 mm × 0.25 μm). Before the GC–MS test, 500 mL of ROLC or treated ROLC sample was extracted with trichloromethane (CHCl3, high-performance liquid chromatography (HPLC) grade) under the original pH of 8.1, then under acidic condition (pH 2.0), and at last under alkaline condition (pH 12.0) using a separating funnel. Each extraction of the same pH was performed three times with 25 mL of CHCl3, respectively. The combined extract organic phases were concentrated to 2–3 mL at 40 °C with a rotary evaporator and then dehydrated with anhydrous Na2SO4 for GC–MS analysis. During the test, the sample injection volume was 0.5 μL, and high-purity helium (He, 99.999%) was used as the carrier gas with a flow rate of 1.0 mL/min. The heating program of the GC–MS test is kept at 40 °C for 5 min, then heated at a rate of 4 °C/min to 250 °C, and then kept for 5 min. The voltage of the electron multiplier is 1341 eV, and the emission electron energy is 69.9 eV.46,47
4.4. Calculation of RE and 
The REs of COD, TOC, and Cl– by the electrochemical oxidation process were calculated as eq 15
| 15 |
where C0 and Ct (mg/L) are the concentrations of COD, TOC, and Cl– at the initial and t time of the electrochemical treatment, respectively.
The 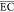 of the electrochemical
oxidation process
can be calculated with eq 16(42)
of the electrochemical
oxidation process
can be calculated with eq 16(42)
| 16 |
where 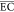 (kWh/(kg COD)) is the
average energy consumption
of electrochemical treatment process; U (V) is the
DC voltage; I (A) the is the DC current; t (h) is the electrochemical oxidation time; V (L) is the volume of the treated wastewater; and C0 and Ct (mg/L) are the COD
of ROLC at the initial and t time of the electrochemical
treatment, respectively.
(kWh/(kg COD)) is the
average energy consumption
of electrochemical treatment process; U (V) is the
DC voltage; I (A) the is the DC current; t (h) is the electrochemical oxidation time; V (L) is the volume of the treated wastewater; and C0 and Ct (mg/L) are the COD
of ROLC at the initial and t time of the electrochemical
treatment, respectively.
Acknowledgments
This research was funded by the Natural Science Foundation of Chongqing, China (cstc2020jcyj-msxmX0308), the Youth Project of Science and Technology Research Program of Chongqing Education Commission of China (KJQN202001148), the Special Project of Scientific and Technological of Banan District, Chongqing, China (2020TJZ003 and 2020QC385), and the Postgraduate Innovation Project of Chongqing University of Technology (clgycx 20203067).
Glossary
Abbreviations Used
- AOPs
advanced oxidation processes
- BOD
biochemical oxygen demand
- COD
chemical oxygen demand
- CE
treatment capacity per electrode area
- DC
direct current
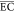
average energy consumption
- IA
current density
- MSW
municipal solid waste
- MCFR
multichannel flow reactor
- RE
removal efficiency
- RO
reverse osmosis
- ROLC
reverse osmosis leachate concentrate
- RuO2/IrO2-Ti
RuO2/IrO2-coated titanium plate
- TOC
total organic carbon
- UL
superficial circulating velocity
Author Contributions
§ C.Y. and Y.T. contributed equally to the work.
The authors declare no competing financial interest.
References
- Cheng W.; Quan X.; Huang X.; Cheng C.; Yang L.; Cheng Z. Enhancement of micro-filtration performance for biologically-treated leachate from municipal solid waste by ozonation in a micro bubble reactor. Sep. Purif. Technol. 2018, 207, 535–542. 10.1016/j.seppur.2018.07.005. [DOI] [Google Scholar]
- Umamaheswari J.; Bharathkumar T.; Shanthakumar S.; Gothandam K. M. A feasibility study on optimization of combined advanced oxidation processes for municipal solid waste leachate treatment. Process Saf. Environ. Prot. 2020, 143, 212–221. 10.1016/j.psep.2020.06.040. [DOI] [Google Scholar]
- Miao L.; Yang G.; Tao T.; Peng Y. Recent advances in nitrogen removal from landfill leachate using biological treatments - A review. J. Environ. Manage. 2019, 235, 178–185. 10.1016/j.jenvman.2019.01.057. [DOI] [PubMed] [Google Scholar]
- Gomes A. I.; Santos S. G. S.; Silva T. F. C. V.; Boaventura R. A. R.; Vilar V. J. P. Treatment train for mature landfill leachates: Optimization studies. Sci. Total Environ. 2019, 673, 470–479. 10.1016/j.scitotenv.2019.04.027. [DOI] [PubMed] [Google Scholar]
- Ferraz F. M.; Bruni A. T.; Povinelli J.; Vieira E. M. Leachate/domestic wastewater aerobic co-treatment: A pilot-scale study using multivariate analysis. J. Environ. Manage. 2016, 166, 414–419. 10.1016/j.jenvman.2015.10.034. [DOI] [PubMed] [Google Scholar]
- Ren Y.; Ferraz F. M.; Ferraz Q. Landfill Leachate Treatment Using Aerobic Granular Sludge. J. Environ. Eng. 2017, 143, 04017060 10.1061/(ASCE)EE.1943-7870.0001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulikowska D.; Klimiuk E. The effect of landfill age on municipal leachate composition. Bioresour. Technol. 2008, 99, 5981–5985. 10.1016/j.biortech.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Wang J. Treatment of fresh leachate from a municipal solid waste incineration plant by combined radiation with coagulation process. Radiat. Phys. Chem. 2020, 166, 108501–108510. 10.1016/j.radphyschem.2019.108501. [DOI] [Google Scholar]
- Calabrò P. S.; Gentili E.; Meoni C.; Orsi S.; Komilis D. Effect of the recirculation of a reverse osmosis concentrate on leachate generation: A case study in an Italian landfill. Waste Manage. 2018, 76, 643–651. 10.1016/j.wasman.2018.03.007. [DOI] [PubMed] [Google Scholar]
- Labiadh L.; Fernandes A.; Ciríaco L.; Pacheco M. J.; Gadri A.; Ammar S.; Lopes A. Electrochemical treatment of concentrate from reverse osmosis of sanitary landfill leachate. J. Environ. Manage. 2016, 181, 515–521. 10.1016/j.jenvman.2016.06.069. [DOI] [PubMed] [Google Scholar]
- Wang J. L.; Xu L. J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. 10.1080/10643389.2010.507698. [DOI] [Google Scholar]
- Moreira F. C.; Soler J.; Fonseca A.; Saraiva I.; Boaventura R. A. R.; Brillas E.; Vilar V. J. P. Incorporation of electrochemical advanced oxidation processes in a multistage treatment system for sanitary landfill leachate. Water Res. 2015, 81, 375–387. 10.1016/j.watres.2015.05.036. [DOI] [PubMed] [Google Scholar]
- Deng Y.; Zhu X.; Chen N.; Feng C.; Wang H.; Kuang P.; Hu W. Review on electrochemical system for landfill leachate treatment: Performance, mechanism, application, shortcoming, and improvement scheme. Sci. Total Environ. 2020, 745, 140768–140825. 10.1016/j.scitotenv.2020.140768. [DOI] [PubMed] [Google Scholar]
- Silveira J. E.; Zazo J. A.; Pliego G.; Casas J. A. Landfill leachate treatment by sequential combination of activated persulfate and Fenton oxidation. Waste Manage. 2018, 81, 220–225. 10.1016/j.wasman.2018.10.007. [DOI] [PubMed] [Google Scholar]
- Wang J.; Zhuan R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023–135070. 10.1016/j.scitotenv.2019.135023. [DOI] [PubMed] [Google Scholar]
- Ishak A. R.; Hamid F. S.; Mohamad S.; Tay K. S. Removal of organic matter from stabilized landfill leachate using Coagulation-Flocculation-Fenton coupled with activated charcoal adsorption. Waste Manage. Res. 2017, 35, 739–746. 10.1177/0734242X17707572. [DOI] [PubMed] [Google Scholar]
- Amor C.; Torres-Socías E. D.; Peres J. A.; Maldonado M. I.; Oller I.; Malato S.; Lucas M. S. Mature landfill leachate treatment by coagulation/flocculation combined with Fenton and solar photo-Fenton processes. J. Hazard. Mater. 2015, 286, 261–268. 10.1016/j.jhazmat.2014.12.036. [DOI] [PubMed] [Google Scholar]
- Chen W.; Gu Z.; Wen P.; Li Q. Degradation of refractory organic contaminants in membrane concentrates from landfill leachate by a combined coagulation-ozonation process. Chemosphere 2019, 217, 411–422. 10.1016/j.chemosphere.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Wang J.; Chen H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249–135324. 10.1016/j.scitotenv.2019.135249. [DOI] [PubMed] [Google Scholar]
- Dolatabadi M.; Świergosz T.; Ahmadzadeh S. Electro-Fenton approach in oxidative degradation of dimethyl phthalate - The treatment of aqueous leachate from landfills. Sci. Total Environ. 2021, 772, 145323 10.1016/j.scitotenv.2021.145323. [DOI] [PubMed] [Google Scholar]
- Liu X.; Novak J. T.; He Z. Synergistically coupling membrane electrochemical reactor with Fenton process to enhance landfill leachate treatment. Chemosphere 2020, 247, 125954 10.1016/j.chemosphere.2020.125954. [DOI] [PubMed] [Google Scholar]
- Mohajeri; Hamidi A. A.; M A. M. H. Landfill Leachate Treatment through Electro-Fenton Oxidation. Pollution. 2019, 5, 199–209. [Google Scholar]
- Pellenz L.; Borba F. H.; Daroit D. J.; Lassen M. F. M.; Baroni S.; Zorzo C. F.; Guimarães R. E.; Espinoza-Quiñones F. R.; Seibert D. Landfill leachate treatment by a boron-doped diamond-based photo-electro-Fenton system integrated with biological oxidation: A toxicity, genotoxicity and by products assessment. J. Environ. Manage. 2020, 264, 110473 10.1016/j.jenvman.2020.110473. [DOI] [PubMed] [Google Scholar]
- Ganiyu S. O.; Martinez H. C. A.; Oturan M. A. Electrochemical advanced oxidation processes for wastewater treatment: Advances in formation and detection of reactive species and mechanisms. Curr. Opin. Electrochem. 2020, 27, 100678 10.1016/j.coelec.2020.100678. [DOI] [Google Scholar]
- Mandal P.; Dubey B. K.; Gupta A. K. Review on landfill leachate treatment by electrochemical oxidation: Drawbacks, challenges and future scope. Waste Manage. 2017, 69, 250–273. 10.1016/j.wasman.2017.08.034. [DOI] [PubMed] [Google Scholar]
- Labiadh L.; Fernandes A.; Ciríaco L.; Pacheco M. J.; Gadri A.; Ammar S.; Lopes A. Electrochemical treatment of concentrate from reverse osmosis of sanitary landfill leachate. J. Environ. Manage. 2016, 181, 515–521. 10.1016/j.jenvman.2016.06.069. [DOI] [PubMed] [Google Scholar]
- Feng H.; Chen Z.; Wang X.; Chen S.; Crittenden J. Electrochemical advanced oxidation for treating ultrafiltration effluent of a landfill leachate system: Impacts of organics and inorganics and economic evaluation. Chem. Eng. J. 2021, 413, 127492–127499. 10.1016/j.cej.2020.127492. [DOI] [Google Scholar]
- Liu K.; Yi Y.; Zhang N. Anodic oxidation produces active chlorine to treat oilfield wastewater and prepare ferrate (VI). J. Water Process Eng. 2021, 41, 101998 10.1016/j.jwpe.2021.101998. [DOI] [Google Scholar]
- Quan X.; Cheng Z.; Chen B.; Zhu X. Electrochemical oxidation of recalcitrant organic compounds in biologically treated municipal solid waste leachate in a flow reactor. J. Environ. Sci. 2013, 25, 2023–2030. 10.1016/S1001-0742(12)60253-8. [DOI] [PubMed] [Google Scholar]
- Fernandes A.; Santos D.; Pacheco M. J.; Ciríaco L.; Lopes A. Electrochemical oxidation of humic acid and sanitary landfill leachate: Influence of anode material, chloride concentration and current density. Sci. Total Environ. 2016, 541, 282–291. 10.1016/j.scitotenv.2015.09.052. [DOI] [PubMed] [Google Scholar]
- Cui Y.-H.; Xue W.-J..; Yang S. Q.; Tu J.-L.; Guo G.-L.; Liu Z.-Q. Electrochemical/peroxydisulfate/Fe 3+ treatment of landfill leachate nanofiltration concentrate after ultrafiltration. Chem. Eng. J. 2018, 353, 208–217. 10.1016/j.cej.2018.07.101. [DOI] [Google Scholar]
- Zhang H.; Ran X.; Wu X.; Zhang D. Evaluation of electro-oxidation of biologically treated landfill leachate using response surface methodology. J. Hazard. Mater. 2011, 188, 261–268. 10.1016/j.jhazmat.2011.01.097. [DOI] [PubMed] [Google Scholar]
- Zhou B.; Yu Z.; Wei Q.; Long H.; Xie Y.; Wang Y. Electrochemical oxidation of biological pretreated and membrane separated landfill leachate concentrates on boron doped diamond anode. Appl. Surf. Sci. 2016, 377, 406–415. 10.1016/j.apsusc.2016.03.045. [DOI] [Google Scholar]
- Turro E.; Giannis A.; Cossu R.; Gidarakos E.; Mantzavinos D.; Katsaounis A. Electrochemical oxidation of stabilized landfill leachate on DSA electrodes. J. Hazard. Mater. 2011, 190, 460–465. 10.1016/j.jhazmat.2011.03.085. [DOI] [PubMed] [Google Scholar]
- Luu T. L. Post treatment of ICEAS-biologically landfill leachate using electrochemical oxidation with Ti/BDD and Ti/RuO2 anodes. Environ. Technol. Innovation 2020, 20, 101099–101131. 10.1016/j.eti.2020.101099. [DOI] [Google Scholar]
- Panizza M.Importance of Electrode Material in the Electrochemical Treatment of Wastewater Containing Organic Pollutants. In Electrochemistry for the Environment; Springer, 2009; Vol. 2, pp 25–54. [Google Scholar]
- Cotillas S.; Llanos J.; Cañizares P.; Mateo S.; Rodrigo M. A. Optimization of an integrated electrodisinfection/electrocoagulation process with Al bipolar electrodes for urban wastewater reclamation. Water Res. 2013, 47, 1741–1750. 10.1016/j.watres.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Huaiqin T.; Xuejun Q.; Bo C.; Zhiliang C. Electrochemical treatment of biologically treated leachate from municipal solid waste incinerator. Chin. J. Environ. Eng 2013, 7, 4823–4828. 10.3969/j.issn.0438-1157.2013.04.037. [DOI] [Google Scholar]
- Chaoqun Y.; Zhiliang C.; Xuejun Q.; Chengfei F.; Geng C. Electrochemical pretreatment of landfill leachate RO concentrate with multi-channel mesh elecrode. Environ. Chem. 2021, 40, 603–613. [Google Scholar]
- Le Luu T.; Stephane D. D. F.; Minh N. H.; Canh N. D.; Thanh B. X. Electrochemical oxidation as a post treatment for biologically tannery wastewater in batch reactor. Water Sci. Technol. 2019, 80, 1326–1337. 10.2166/wst.2019.380. [DOI] [PubMed] [Google Scholar]
- Deng Y.; Feng C.; Chen N.; Hu W.; Kuang P.; Liu H.; Hu Z.; Li R. Research on the treatment of biologically treated landfill leachate by joint electrochemical system. Waste Manage. 2018, 82, 177–187. 10.1016/j.wasman.2018.10.028. [DOI] [PubMed] [Google Scholar]
- Mandal P.; Gupta A. K.; Dubey B. K. Role of inorganic anions on the performance of landfill leachate treatment by electrochemical oxidation using graphite/PbO2 electrode. J. Water Process Eng. 2020, 33, 101119–101129. 10.1016/j.jwpe.2019.101119. [DOI] [Google Scholar]
- Xue W. J.; Cui Y.-H.; Liu Z.-Q.; Yang S.-Q.; Li J.-Y.; Guo X.-L. Treatment of landfill leachate nanofiltration concentrate after ultrafiltration by electrochemically assisted heat activation of peroxydisulfate. Sep. Purif. Technol. 2020, 231, 115928 10.1016/j.seppur.2019.115928. [DOI] [Google Scholar]
- Wang J.; Wang S. Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants. Chem. Eng. J. 2021, 411, 128392 10.1016/j.cej.2020.128392. [DOI] [Google Scholar]
- Wang J.; Wang S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158 10.1016/j.cej.2020.126158. [DOI] [Google Scholar]
- Cheng W.; Quan X.; Huang X.; Cheng C.; Yang L.; Cheng Z. Enhancement of micro-filtration performance for biologically-treated leachate from municipal solid waste by ozonation in a micro bubble reactor. Sep. Purif. Technol. 2018, 207, 535–542. 10.1016/j.seppur.2018.07.005. [DOI] [Google Scholar]
- Cheng Z.; Dai M.; Quan X.; Li S.; Zheng D.; Liu Y.; Yao R. Synthesis and Catalytic Activity of Activated Carbon Supported Sulfonated Cobalt Phthalocyanine in the Preparation of Dimethyl Disulfide. Appl. Sci. 2019, 9, 124. 10.3390/app9010124. [DOI] [Google Scholar]






