Abstract
Button batteries (BB) are found in common household items and can lead to significant morbidity and mortality in the pediatric population when ingested. BBs are made of various chemistries and have a unique size and shape that yield significant injury when lodged in the pediatric esophagus. BBs create a local tissue pH environment of 10 to 13 and can induce liquefactive necrosis at the negative pole. This initial injury can progress with further tissue breakdown even after removal. Unfortunately, patients may present with vague symptoms similar to viral illnesses and there is not always a known history of ingestion. Plain film X‐ray can be diagnostic. Exposure can lead to caustic injury within 2 hours. Thus, timely endoscopic removal is the mainstay of treatment. Novel mitigation and neutralization strategies have been implemented into treatment guidelines. These include the preremoval ingestion of honey or sucralfate and intraoperative irrigation with acetic acid. Depending on the severity of injury following removal, careful consideration should be given for potential delayed complications including fistulization into major vessels which often leads to death. The National Button Battery Taskforce and several industry members have implemented prevention strategies such as educational safety outreach campaigns, child‐resistant packaging changes, and warning labels. Governmental regulation and industry changes are key to limit not only the amount of BB ingestions, but also the devastating consequences that can result. Anonymous reporting of BB injuries through the Global Injury Research Collaborative has been made convenient and centralized through the advent of a user‐friendly smartphone iOS/App Store and Android/GooglePlay application called the “GIRC App”; all specialists who manage foreign body cases should contribute their cases to help prevent future injuries. BB ingestion must be recognized and treated promptly using a multidisciplinary approach to optimize outcomes for these patients. Ultimately, a safer BB technology is critically needed to reduce or eliminate the severe and life‐threatening injuries in children.
Level of Evidence
5
Keywords: battery injury, button battery, disc battery, esophageal foreign body, pediatric injury
1. INTRODUCTION
Button battery (BB) ingestion is an important topic of concern for many medical professionals who may commonly encounter these patients in practice. Although a majority of ingestions occur in the pediatric population, an extensive analysis of over 8000 patients revealed approximately 1600 adult ingestions from 1990 to 2008. 1 Therefore, current review of this topic is relevant to both pediatric and adult health care professionals. The provider must quickly recognize and appropriately manage BB aspiration, ingestion, or insertion to prevent severe complications that may arise as a result of prolonged exposure. In addition, providers should be aware of the latest additions to the treatment guidelines including mitigation and neutralization strategies based on the latest available data. Smaller BBs can also be inserted into the nasal cavity, ear canal, or aspirated into the tracheobronchial tree which can lead to morbidities such as nasal septal or tympanic membrane perforations and airway obstruction issues. In addition, severe esophageal injuries and death have been reported with both lithium and non‐lithium BB. This topic has also been presented in detail for emergency medicine providers. 2 For the purposes of this review, we will focus on the most relevant current management information for those medical professionals who manage these cases. This article provides an overview on BB ingestion, aspiration, and insertion, summarizes the latest management guidelines, and discusses the latest innovations in BB injury prevention.
1.1. Historical context and timeline
BBs trace their history to as early as 1945, when Ruben and Mallory who founded the original company that eventually went on to become Duracell invented the zinc‐mercuric oxide alkaline button‐type cell. 3 As BBs were introduced into mainstream culture over the next several decades, more and more BB injuries were noted prompting a need for systematic tracking and guidance. In 1982, the National Battery Ingestion Hotline (NBIH) was formed through the National Capital Poison Center (NCPC) as a 24/7 hotline. Health care providers or the public with suspected BB injuries are able to call 1‐800‐498‐8666 for assistance. The data that has been collected and published since its inception has informed clinical triage, treatment algorithms, and prevention strategies. In 2018, the NBIH was eventually transitioned to the Rocky Mountain Poison Center. BB injury statistics and treatment algorithms can be found at www.poison.org/battery. 4
In 1983, a year after formation of the NBIH, the U.S. Consumer Product Safety Commission (CPSC) issued a warning on BBs. 5 After several decades of extensive BB injury, outcomes data collection and analysis by the NBIH and NCPC, 4 , 6 and publication of a comprehensive review of over 8000 cases in the journal Pediatrics, 1 a group of physicians presented the concerning information on BB injuries to the CPSC in March 2011. 7 Just a few months later in June, S. 1165, the Button Cell Battery Safety Act of 2011 was introduced to the U.S. Senate. This legislation aimed to enforce BB industry safety standards by mandating child‐resistant packaging and consumer warnings with CPSC regulation. 8 The legislation was not enacted by Congress despite in‐person lobbying efforts by multiple pediatric otolaryngologists. In 2012, the Centers for Disease Control published an extensive report highlighting a 2.5‐fold increase in battery‐related injuries in children <13 years old from 1995 to 2010, citing over 20 000 children treated in Emergency Departments (EDs) for BB injuries. 9 Also in 2012, the National Button Battery Task Force (BBTF) was formed through the American Academy of Pediatrics (AAP) and the American Broncho‐Esophagological Association with the following mission statement 10 :
A collaborative effort of representatives from relevant organizations in industry, medicine, public health, and government to develop, coordinate, and implement strategies to reduce the incidence of button battery injuries in children.
Importantly, the BBTF has for several years employed a multidisciplinary approach to education, research collaboration, data collection and analysis, management algorithms and practice guideline development and dissemination, governmental advocacy, industry standard development, and hazard elimination which has resulted in numerous meaningful changes to minimize the risk of BB ingestion injuries. The work completed by the BBTF has also resulted in several national and international awards. 11 , 12 A central tenet of the BBTF is that consumers including parents and childcare providers must be aware of the hazard to help with primary prevention of the injuries.
1.2. BBs: What are they?
BBs are disc‐shaped metallic objects with similar appearance to coins and can become lodged in the pediatric esophagus leading to significant injury given their unique size, shape, and chemical properties (Figure 1). 11 The most commonly ingested lithium BB imprint code is the CR 2032. 11 “CR” refers to the chemical identification for lithium/manganese dioxide and “2032” refers to a diameter of 20 mm and a thickness of 3.2 mm. Lithium is popular due to its ability to store 3 V, long shelf life, and largest capacitance relative to size. 13 , 14 As a result, the 20‐mm diameter lithium BB has become increasingly prevalent in the marketplace. When this battery is ingested, the size of the BB can predispose to esophageal impaction and the effects of lithium can lead to significant caustic injury to surrounding tissues and serious complications. Thus, there has been an increase in severity of BB ingestion injuries thought to be related to this spike in lithium, larger diameter BBs. 1 , 11 , 15 Other common BB chemistries include alkaline, zinc air, and silver oxide. Smaller diameter, lower voltage, and nonlithium BBs can also contribute to similar types of severe injury and death, 16 , 17 but at a slightly slower rate. 15 , 18 Diameter is not absolute, as even new smaller lithium 12.5‐mm BBs have been lodged in the esophagus of an otherwise healthy 2.5‐year‐old girl with no underlying esophageal pathology. 19
FIGURE 1.

As shown, button batteries can vary significantly in shape, size, chemistry, and voltage (1.5‐3 V). Reproduced with permission from K. R. Jatana
1.3. BBs: Where are they found?
BBs are found in a wide range of common household electronic devices which poses a risk of BB injury to many children with access to these items. In a large retrospective analysis of over 8000 battery ingestion cases from the NBIH data, the most common intended use of ingested batteries was hearing aid or cochlear implant at 36.3% followed by game/toy, watch, calculator, flashlight or other similar small light source, remote control, and key chain. Although rare, even unexpected items such as toothbrush, lighted shoe, bookmark, and thermometer have been implicated in BB ingestion. 1 Table 1 highlights the most common items associated with BB ingestion, overall for both nonlithium and lithium types. More recent data on intended use of ingested 20‐mm lithium BBs analyzed from 2014 to 2016 revealed the most common product sources were remote controls (25.0%), lights (14.7%), and candles (14.0%), Table 2. 12 In children <6 years of age, 61.8% of batteries were obtained directly from a product, 29.8% were loose, and 8.2% were obtained directly from battery packaging. Of all ingestions, 15.5% occurred in the elderly with batteries mostly intended for hearing aids that were mistaken for pills. 1
TABLE 1.
Most common intended use of both nonlithium and lithium of ingested button batteries in a National Battery Ingestion Hotline review of 8648 cases 1
| Intended use | % |
|---|---|
| Hearing aid or cochlear implant | 36.27 |
| Game or toy | 22.07 |
| Watch | 11.12 |
| Calculator | 5.74 |
| Small light source | 4.59 |
| Remote control | 2.87 |
| Key chain | 2.40 |
| Clock/watch/timer | 1.79 |
| Jewelry | 1.74 |
| Unknown | 19.4 |
Note: Other identified objects include: candle, camera, pen, toothbrush, dog collar, music/video player, thermometer, exercise equipment, greeting card, medical equipment, ornament, clothing.
TABLE 2.
Intended use of ingested “20‐mm diameter lithium button batteries,” National Battery Ingestion Hotline, July 2016 to June 2018
| Intended use | Frequency | Percent (%) | Valid percent (%) |
|---|---|---|---|
| Remote control (garage door openers, TV, media) | 39 | 17.6 | 30.5 |
| Game/toy | 22 | 10.0 | 17.2 |
| Watch | 14 | 6.3 | 10.9 |
|
Light Booklight (1), hat light (2), flashlight (2), headlamp (3), other (3) |
11 | 5.0 | 8.6 |
|
Miscellaneous Glasses (3), locator (2), camera (1), computer (1), metronome (1), other (2) |
10 | 4.5 | 7.8 |
| Scale | 7 | 3.2 | 5.5 |
| Candle (flameless, tea) | 7 | 3.2 | 5.5 |
| Car remote, key fob | 5 | 2.3 | 3.9 |
| Meters/gauges/tools/medical devices | 4 | 1.8 | 3.1 |
| Thermometer | 3 | 1.4 | 2.3 |
| Accessories or clothing (flashing/musical) | 2 | 0.9 | 1.6 |
| Alarm | 1 | 0.5 | 0.8 |
| Calculator | 1 | 0.5 | 0.8 |
| Clock/timer | 1 | 0.5 | 0.8 |
| Music/media player | 1 | 0.5 | 0.8 |
| Unknown | 93 | 42.1 | |
| Total | 221 | 100.0 | 100.0 |
Source: Adapted with permission from Dr T. Litovitz, National Capital Poison Center, https://www.poison.org/battery/stats.
1.4. Epidemiology
Annually, it is estimated children ingest 70 000 foreign bodies (FB). 20 In the United States, approximately 3500 known BB ingestions occur each year. 6 , 10 In one large retrospective NCPC study, children under the age of 6 years comprised 62.5% of BB injuries and the greatest frequency occurred in toddlers age 1 to 3 years. 1 A more recent analysis showed over 75% of BB ingestions occurred in children under 6 years and confirmed toddlers as the most vulnerable population. 21 Although the number of FB ingestions in children under 6 years increased approximately 92% from 1995 to 2015, the incidence of BB ingestion as a percentage of all FB ingestions disproportionately increased from 0.14% to 8.4%, with nearly 10% requiring hospitalization. 22 Importantly, this population appears to be especially vulnerable to more severe complications. 15 Figure 2 summarizes national data on moderate, major, or fatal outcomes associated with BB ingestion.
FIGURE 2.
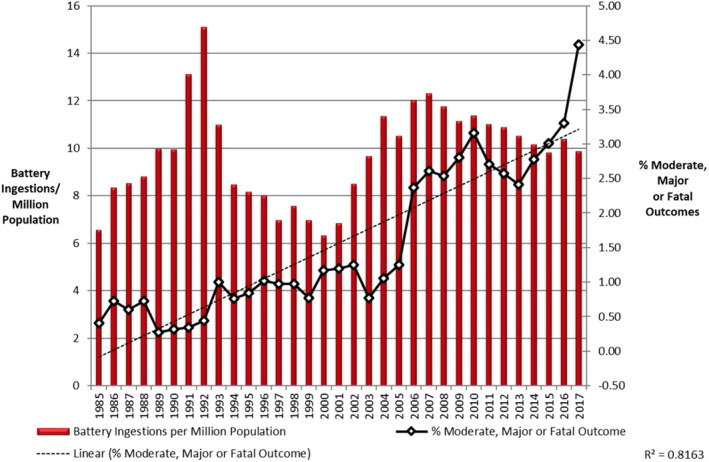
National data from the National Capital Poison Center on reported button battery ingestions with moderate, major, or fatal outcomes. Reproduced with permission from T. Litovitz
Data from the U.S. National Electronic Injury Surveillance System analyzed from 1990 to 2009 have shown an increasing incidence of ED visits related to BB ingestions, reporting a child may present to the ED with a BB every 3 hours. 23 This trend has also been shown in analysis of French ED visits from 1999 to 2015. 24 With increasing incidence of BB ingestion, especially in the young population, an increase in morbidity and severe mortality is expected. Numerous reports have confirmed increasing incidence of the severity of BB ingestions. 9 , 15 , 24 This is likely attributed to an increase in more technologically advanced electronics in the household over the past two decades combined with the increase in production of the larger‐diameter, 3‐V lithium batteries in the marketplace. 11 , 15 , 25
According to the NCPC large retrospective study, an alarming 12.6% of children younger than 6 years who ingested a 20‐mm diameter lithium BB developed a major complication such as esophageal perforation or stricture, tracheoesophageal fistula, fistulization into major vessels, vocal cord paresis and paralysis, or spondylodiscitis. 1 Since 1977, there have been at least 65 fatalities associated with BB ingestion. All of the reported fatalities occurred in patients ≤4 years of age. 26 Younger children are at greatest risk given smaller size of the pediatric esophagus trapping FBs as they explore new objects with their mouths which can lead to devastating consequences. Especially concerning is that 56.2% of the major outcome cases associated with BB injuries were unwitnessed. 15 In addition, the prevalence of these injuries is very likely to be underreported. 27 , 28 Therefore, review of the epidemiologic data highlights the importance of BB ingestion as an increasingly important and severe topic in health care, especially in the pediatric population.
1.5. Impact of COVID‐19 Pandemic
During the COVID‐19 pandemic with school closures mandating virtual home education, children have spent more time in the home setting. The Regina Margherita Children's Hospital in Italy did a retrospective study of foreign body ingestions during February 24 to April 24, 2020, comparing this to the same time period the prior 4 years. This went from 0‐3 battery ingestion cases in this time period annually in 2016‐2019, to 9 cases in the same period in 2020; this was a statistically significant increase (p < 0.001). 29 A recent report from the U.S. CPSC demonstrated a 93% increase in battery related emergency room visits during March to September 2020 compared to same time period, in 2019 in children ages 5‐9. 30 This was mostly related to battery ingestion but also cases of insertion into the ear or nose. This is quite concerning and demonstrates that this age group is also at risk.
1.6. Mechanism of injury
The esophagus contains anatomically narrowed regions more likely to harbor BB impaction, most commonly at the level of the thoracic inlet. 31 Although new BBs are 3.2 times more likely to produce a clinically significant outcome of tissue damage than used BBs, any BB with a residual charge of at least 1.2 V has the ability to cause tissue damage. 11 Thus, even “dead” or “spent” BB unable to power electronics have enough retained capacitance to cause significant injury. A multitude of factors contribute to BB damage including the duration and location of the impaction, the size, orientation, and voltage of the BB, the size of the esophagus, and any potential underlying esophageal pathology of the patient. 32
Previous theories have posited that BB tissue damage occurs secondary to pressure injury, leakage of toxic contents, and electrolysis of water. However, studies have consistently shown that the primary mechanism of tissue injury is the electrolysis and production of hydroxide ions. 18 , 33 The electrical potential of BBs induces an isothermic water hydrolysis reaction at the junction of the BB negative pole and generation of hydroxide ions caused by the current created through the adjacent tissue, which essentially “connects the circuit” between the two poles of the BB. The accumulation of hydroxide ions rapidly increases the surrounding tissue environment to a local tissue pH of 12 to 13. This highly alkaline environment then creates an ensuing liquefactive necrosis which can results in deep tissue caustic injury. The positive pole induces a focal acidic environment and coagulative necrosis, limiting depth of tissue injury. Lithium cells contain only a mildly irritating organic electrolyte and do not contain an alkaline electrolyte. Although initially considered, leakage of alkaline electrolyte, pressure necrosis, and systemic heavy metal or lithium poisoning are no longer considered to be significant factors in BB injury. 11 , 18
BBs can induce initial visible injury in as early as 15 minutes if lodged in the esophagus and serious injury can occur in as little as 2 hours. 18 , 34 When comparing chemistries, cadaveric porcine esophageal studies revealed that the most rapid induction of injury occurred with lithium BBs followed by alkaline and silver oxide which required 2 to 4 hours longer to induce the same amount of damage. Though lithium BBs tend to have higher voltage, alkaline and silver oxide BB also created environments that reached a pH of 12, although at a slower rate. Interestingly, the aqueous, saliva‐rich environment of the esophagus appears to block any oxygen from entering the zinc air BB, reducing the chance of esophageal injury. 18
Even after BB removal, tissue injury may progress if the site is not irrigated and the alkaline tissue environment is not neutralized. Jatana et al conducted several studies that demonstrated pH neutralization decreases tissue damage and halts eschar formation. In an initial experiment using the porcine model, irrigation of the esophagus with an impacted BB every 5 minutes with acidic liquids such as cola, lemon, and orange juice demonstrated limited tissue damage to various degrees. 18 Then, using both in vitro cadaveric and in vivo porcine models, piglets were randomized to receive 10 mL of honey, sucralfate (Carafate), or saline 10 minutes after BB placement in the esophagus and every 10 minutes thereafter. Honey is a palatable, viscous weak acid found in most households and sucralfate is a cherry‐flavored weakly acidic suspension with mucosal protective effects found in hospitals as it is used to treat ulcers. The honey and sucralfate appeared to form a physical barrier between the BB and surrounding tissue, neutralized the esophageal tissue, and reduced esophageal burns. One week after ingestion, half of the saline control group developed esophageal perforations whereas none of the piglets treated with honey and sucralfate developed perforations. 35 Irrigation of the alkaline tissue with 50 to 150 mL of 0.25% sterile acetic acid after BB removal was proposed to prevent delayed injury. A recent study using a cadaveric goat model also found a statistically significant reduction in mucosal injury with the application of honey compared with BBs alone. Progression of the injury was further diminished with the application of acetic acid after BB removal. 36 Despite a longstanding belief that neutralization causes thermal injury and thus should be avoided, porcine and goat experiments demonstrated minimal increase in esophageal temperature (0°C‐3°C) and no thermal injury. 35 , 36
This neutralization strategy was then applied clinically in a series of six pediatric patients who received 0.25% sterile acetic acid irrigation following BB removal. All patients were reported to have improved mucosal appearance, and none developed esophageal perforation or stricture. This positive outcome was thought to be secondary to immediate pH neutralization and prevention of alkaline‐induced liquefactive necrosis 19 As a result of these experiments, recent changes to the NCPC treatment algorithm guidelines were made. 37
In one notable case, a 15‐month‐old boy ingested two 3‐V lithium BBs that became lodged in the upper esophagus. The authors describe a “macaroon sign” on radiographic imaging with the two BBs parallel to one another with positive poles facing each other. The negative poles were thus facing outwards toward the esophageal tissue creating a circumferential injury. Eight hours after ingestion, the BBs were removed endoscopically and 100 mL of 0.25% acetic acid was used to neutralize the tissue. The patient subsequently recovered with a significantly better clinical outcome than predicted without development of stricture which the authors attribute to neutralization. 38
2. CLINICAL CONSIDERATIONS
2.1. Presentation
When BB ingestion is known, it is important to obtain a thorough history including information regarding battery type, charge state, number of batteries ingested, time of ingestion, magnet co‐ingestion, and any history of esophageal pathology or previous surgery. Unfortunately, many BB ingestions are unwitnessed and this information is unknown. 15 Furthermore, symptoms associated with BB ingestions are often vague and present similarly to common viral respiratory or gastrointestinal illnesses. 39 , 40 Finally, a majority of severe outcomes occur with unwitnessed ingestion and patients are often asymptomatic until a complication occurs. 24 , 41 Thus, the clinician is often presented with a challenging scenario and must rely on astute clinical investigation and radiographic imaging to obtain the diagnosis.
The frequency of symptoms appears to vary by study, with one study reporting that the most common symptoms associated with BB ingestion are dysphagia, fever, and cough 40 and another reporting vomiting, difficulty feeding, and cough. 39 Infants and toddlers may also present with irritability, anorexia, dyspnea, and drooling, whereas older children may be able to localize symptoms such as throat, chest, or abdominal pain or may be able to give FB ingestion history. 40 However, these symptoms are not obligatory, can vary by age, and are nonspecific. 26 , 39 , 40 The NCPC guidelines suggest consideration of BB ingestion with airway obstruction or wheezing, drooling, vomiting, chest discomfort, difficulty swallowing, decreased appetite, refusal to eat, coughing, choking, or gagging with eating or drinking. 37 Patients may also be asymptomatic and clinically stable. Thus, clinicians must maintain a high index of suspicion.
Although rare, BBs can also be aspirated into the tracheobronchial tree. Only three cases have been reported in the literature and included symptoms such as cough, vomiting, diarrhea, fever, shortness of breath, and respiratory distress. 42 , 43 , 44 In one case, a 4‐year‐old boy was initially diagnosed with viral illness and subsequently developed right lobar pneumonia. Several days later, endoscopic removal revealed an 8‐mm corroded BB in the right mainstem bronchus. 44 Recent in vitro porcine studies demonstrated tracheobronchial cartilage damage within 4 hours and significant necrosis of surrounding tissues in 12 hours, highlighting the need for prompt diagnosis and treatment. 45
In addition, smaller BBs have the unique ability to be inserted into a nasal cavity or ear canal which may be suspected in patients with fever or blood‐tinged, purulent, or foul‐smelling discharge. Patients with nasal cavity BB insertions may also present with rhinorrhea, nasal obstruction, epistaxis, facial swelling or periorbital cellulitis, and patients with ear canal BBs may present with ear pain or similarly to otitis externa. Nasal or ear canal BB insertions can lead to significant morbidity such as nasal septal perforations (Figure 3) or tympanic membrane perforations via a similar mechanism to BB ingestion. 33
FIGURE 3.
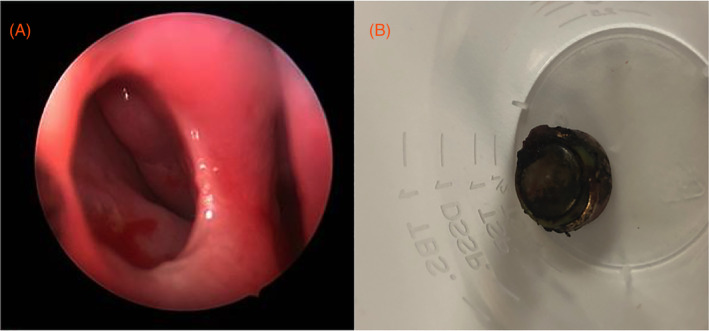
Nasal septal perforation from nasal cavity button battery (BB) insertion in a 4‐year‐old child. Upon endoscopic examination following removal, a nasal septal perforation was identified, A. The BB was nonlithium alkaline LR44, B. Reproduced with permission from K. R. Jatana
2.2. Diagnosis
2.2.1. Imaging
Diagnosis should be obtained without delay to expedite treatment and minimize morbidity and mortality. As previously mentioned, radiographic imaging is an essential tool in the prompt diagnosis of BB ingestion given that BB ingestions are often unwitnessed and present with vague symptoms. If there is a known BB ingestion, X‐ray should be obtained in all patients except those who are greater than 12 years old, asymptomatic, and the BB is known to be smaller than 12 mm. 37 Chest XR with both anterior‐posterior (AP) and lateral views should be obtained immediately in all other patients with suspected or known BB ingestion to determine if present and the anatomic location. As BBs are round, opaque FBs with a similar radiographic appearance to coins, providers should use magnification to “zoom in” and assess for the classic “double ring” or “halo” sign in the AP view and “step‐off” in the lateral view (Figure 4). 46 Notably, the AP view is most reliable as some slimmer BBs on lateral view do not have a classic “step‐off” appearance, or the film may not be precisely perpendicular to the plane of the BB. 11
FIGURE 4.
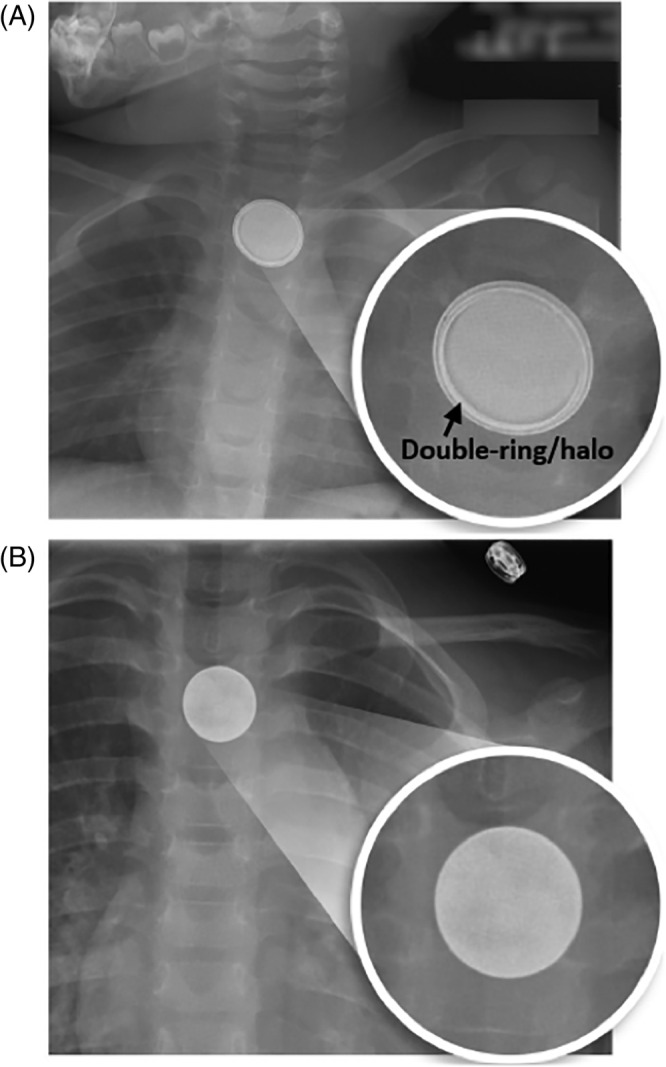
On radiographs, button batteries have a double‐ring or halo appearance, A. In contrast, coins have a homogenous appearance, B. Reproduced with permission from K. R. Jatana
The negative pole yields the most severe tissue damage and thus, identifying its orientation can be important to predict which structures are at risk for injury and development of complications. The 3N's is a simple mnemonic that has been proposed to remind clinicians where to anticipate the most damage, “Negative‐Narrow‐Necrotic” (Figure 5) referring to the negative BB pole orientation identified as the narrowest side on lateral chest XR causes the most severe necrotic injury. 11 , 15 , 37 If unknown, the BB diameter can also be determined radiographically after factoring out magnification which overestimates diameter. 37 A retrospective review of 139 patients from a pediatric tertiary care center from 2017 to 2019 with suspected coin vs BB ingestion revealed that combined history and radiologic diagnosis yielded a sensitivity of near 100%. For diagnosis with radiology alone, the negative predictive value and accuracy were 97% and 81%, respectively. 47 If metallic nasal cavity or ear canal FBs is present, XR imaging can be obtained to further elucidate if the metallic BB is present.
FIGURE 5.
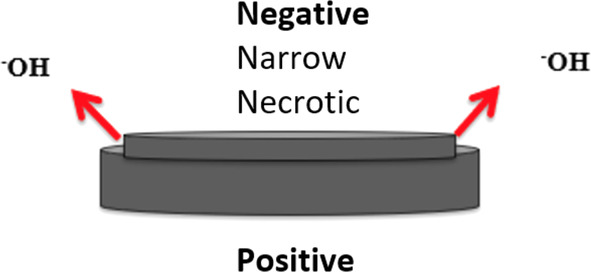
The narrow side of the button battery is the negative pole. It produces hydroxide ions and thus causes the most severe tissue damage. This can be remembered by the 3N's mnemonic: “Negative‐Narrow‐Necrotic”
2.3. Potential role of novel metal detector
Given the extremely high prevalence of pediatric patients who present with nonspecific symptoms of viral illness, there appears to be a role for a universal triage screening tool to minimize the amount of XRs obtained in the health care system. A specialized handheld metal detector device used for detection of BBs would increase efficiency and safety while minimizing radiation exposure (Figure 6). At this time, readily available metal detectors do not contain the sensitivity or specificity to perform this function. A novel device that could accurately screen children for BB or coin ingestion in the primary care, urgent care, or emergency room setting is currently under development and seeking an industry partner.
FIGURE 6.
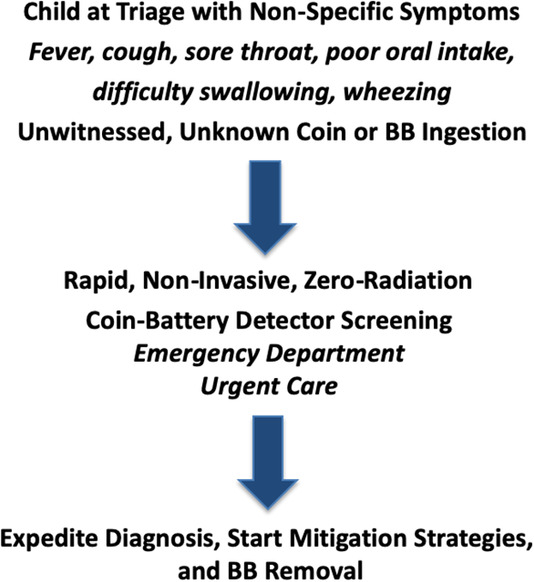
Coin‐battery detector (patent pending) that could be developed for quicker identification and management of battery ingestions
2.4. Complications
It is important to predict, promptly detect, and treat potential severe complications that may be associated with BB ingestions. Reported complications include esophageal stenosis or perforation, mediastinitis, tracheoesophageal fistula (Figure 7), vocal cord paresis and paralysis, spondylodiscitis, intestinal perforation with peritonitis, cardiovascular and respiratory failure, pneumothorax, pneumoperitoneum, anterior spinal artery syndrome with bilateral lower extremity paralysis, vascular fistula leading to hemorrhage, and death. 24 , 26 , 48 According to the NCPC, 251 nonfatal BB ingestion cases with severe esophageal or airway injury have been reported. 49 Importantly, complications often present in a delayed fashion with bleeding events reported weeks after BB removal. 1 , 24 , 50 Other complications such as esophageal stricture and perforation, spondylodiscitis, and tracheoesophageal fistula have been reported to present weeks to months later with some cases as late as 6 to 8 months following BB ingestion. 24 , 49 In a recent retrospective review of 189 patients, 2% developed acute esophageal perforation <24 hours after BB ingestion with the earliest reported between 11 and 17 hours. The authors report very low likelihood of esophageal perforation within 12 hours, and 66.4% evident by 9 days. 51 Patients with severe complications may also require gastrostomy tube placement, tracheostomy placement, parenteral nutrition, intubation, and extracorporeal membrane oxygenation. 24 , 48
FIGURE 7.
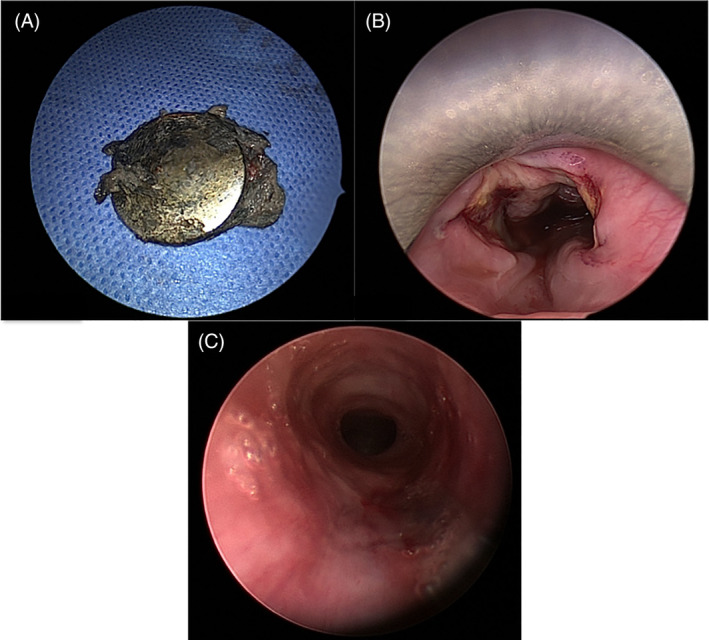
Tracheoesophageal fistula in 17‐month‐old girl following button battery (BB) ingestion. The corroded BB was identified, A. Circumferential severe esophageal injury was identified on rigid esophagoscopy, B. Bronchoscopy revealed a posterior tracheal wall injury consistent with a tracheoesophageal fistula, C. Reproduced with permission from K. R. Jatana
Of the 65 reported BB ingestion fatalities, a majority occurs secondary to vascular‐esophageal fistula resulting in massive hemorrhage and exsanguination. Many children with reported fatalities initially had very little tissue damage reported upon removal but developed catastrophic consequences likely due to the persistent alkaline environment inducing further liquefactive necrosis. 26 A key presenting symptom in these patients is hematemesis and clinicians must evaluate this further in patients with a history of BB ingestion at risk for delayed aortoesophageal fistula (AEF). AEF is nearly always fatal with only six reported cases of survival after BB ingestion, all of whom were 3 years of age or younger, had hematemesis after removal, and for the most part, had unknown duration of BB exposure. 52 , 53 , 54 , 55 , 56 In one large retrospective study, the cause of death was unknown in over half of the fatalities associated with BB ingestion with the remaining deaths attributed to arterial fistulization and bronchopneumonia. 21 Fatalities related to gastric BBs are exceedingly rare, and likely related to esophageal injury in transit. 57
As discussed, symptoms of BB ingestion are nonspecific and can easily be attributed to viral or bacterial infections. Unfortunately, several patients with BB ingestion‐related fatalities were initially misdiagnosed. 26 Reported symptoms that could indicate development of severe complications in addition to those mentioned earlier include hematemesis, melena, sialorrhea, stridor, and aphasia. 24 In addition, several factors have been shown to predict long‐term complications of BB ingestion; these include the esophageal location and orientation of the BB negative pole, the anatomic location of the most severe esophageal injury, the estimated duration of impaction, and the properties of the specific battery. 58 Clinicians should utilize these predictive factors to stratify their management and follow‐up plans.
3. MANAGEMENT
3.1. Guidelines
The latest NCPC guidelines for BB ingestion can be reviewed at: www.poison.org/battery/guideline. 37 Recent additions regarding preremoval mitigation and intraoperative neutralization strategies have been added to the guidelines to reflect the most updated literature supporting their use. 18 , 19 , 35 Table 3 summarizes considerations before, during, and after removal for BB ingestion. Figure 8 demonstrates the comprehensive NCPC triage and treatment algorithm for BB ingestion.
TABLE 3.
Considerations for esophageal button batteries (BBs): before removal, during removal in the operating room, and after removal
| Pre‐removal |
At home and during transport: 10 mL (two teaspoons) honey every 10 min until arrive at hospital. Avoid honey in children <1‐year‐old. In hospital or clinical setting: 10 mL (two teaspoons) sucralfate (Carafate) every 10 min until BB removal can occur. This can be initiated even before X‐ray confirmation for witnessed or highly suspected ingestions. Up to six doses in prehospital setting and three additional doses in clinical setting are recommended by National Capital Poison Center (NCPC) guidelines; providers should use clinical judgment for giving additional doses if going to be further delay (ie, prolonged transport to a different facility). Warning: These are mitigation strategies and not a substitute for prompt esophageal BB removal. |
| Removal |
Esophageal BB is an acute surgical emergency, proceed to operating room regardless of nil per os (NPO) status. Anesthesia: Rapid sequence induction. Endoscopic approach with direct visualization is preferred with either flexible or rigid esophagoscopy. Consider direct laryngoscopy and bronchoscopy to evaluate for laryngotracheal airway injury (existing or developing trachea‐esophageal fistula), especially in cases where negative pole faces anterior direction (BB step‐off anterior). Consider general potential acute complications such as esophageal perforation, tracheoesophageal fistula, vocal cord paresis or paralysis, proximity to major vascular structures (arterial fistula). If no visible esophageal perforation exists, perform endoscopic irrigation of site of tissue injury using 50‐150 mL of 0.25% sterile acetic acid while simultaneously suctioning excess irrigation. If suspect perforation or severe circumferential injury present, consider nasogastric tube placement while in the operating room (OR). |
| Post‐removal |
Remember tissue injury may progress after BB removal. Consider esophagram to rule out perforation prior to starting oral intake. Consider contrast imaging of chest (MRI, CTA) if severe injury exists and to assess for proximity to major vascular structures (i.e. aorta, etc) Monitor for potential delayed complications: esophageal perforation, tracheoesophageal fistula, aortoesophageal fistula, vocal cord paresis or paralysis, mediastinitis, spondylodiscitis or esophageal stricture. These BB complications can sometimes present days to weeks later. Consider need for serial imaging, endoscopy, or stool guaiac tests. |
FIGURE 8.
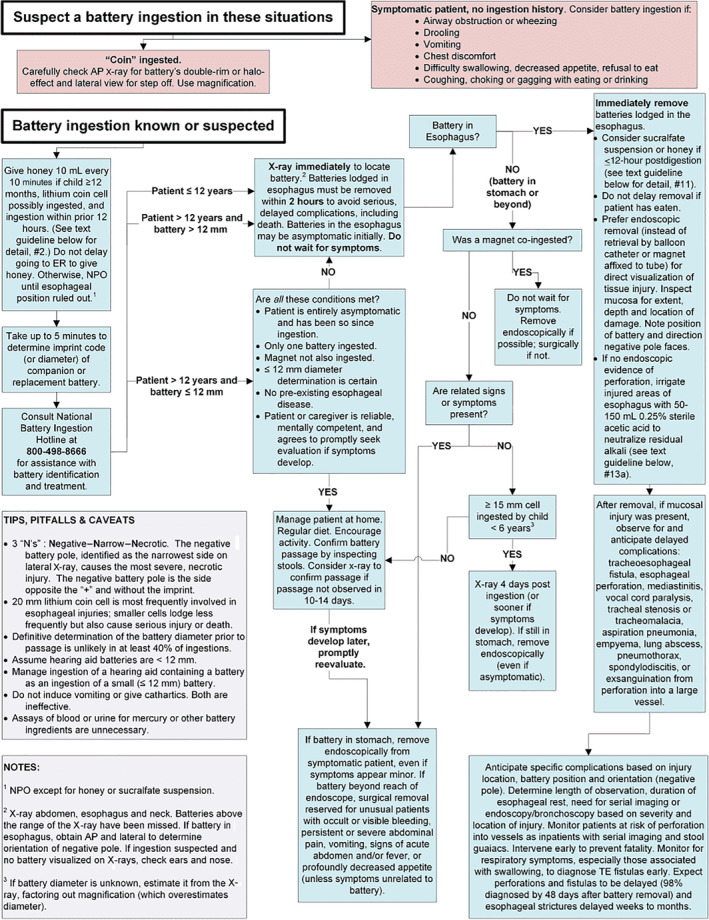
National Capital Poison Center Ingestion Button Battery Triage and Treatment guideline. Reproduced with permission from T. Litovitz
3.2. Time to removal
BB ingestion can cause a rapid, destructive injury requiring the clinician to act quickly for diagnosis and intervention. Given that serious caustic injury can occur in as quickly as 2 hours, there exists only a small window for a child to present to the ED or Urgent Care, undergo diagnosis with XR, be transported to the operating room (OR), and undergo BB removal. A Clinical Pediatric Emergency Medicine review currently in press discusses BB ingestion and encourages prompt triage and disposition by ED providers to expedite care. 2 Russell et al demonstrated that the use of a trauma 1 activation led to significantly shorter times to evaluation and BB removal for patients with suspected BB ingestion. The mean time from facility arrival to OR was 33 minutes vs 183 minutes for the trauma 1 activation group vs the standard emergency room triage group. 59 The American College of Surgeons has also investigated time from XR diagnosis of esophageal BB cases to OR through the National Surgical Quality Improvement Program Pediatric (ACS‐NSQIP P) Process Measure. For participating centers, these national data can be used for centers to compare to other similar institutions and make center‐specific quality improvements.
3.3. Initial management
As with any pediatric FB case, the patient must first be stabilized appropriately. However, the emphasis in BB ingestion cases should be on mitigating tissue damage and prompt removal. Patient should remain nil per os (NPO) until esophageal position is ruled out via XR other than consumption of honey or sucralfate if indicated. When possible, knowing the imprint code or diameter from a companion or replacement BB, battery packaging, or product instructions can be helpful. A U.S. penny (19 mm) or nickel (21 mm) can be used as a reference to estimate diameter. Hearing aid BBs can be assumed to be <12 mm. The NBIH should also be consulted at 1‐800‐498‐8666 for assistance with BB identification and treatment. Vomiting should not be induced and the use of ipecac, chelation, cathartics, polyethylene glycol electrolyte solution, or laxatives are not recommended. Serum assays for BB ingredients or mercury are not recommended.
Patients who are asymptomatic, >12 years of age with known isolated BB ingestion of ≤12 mm diameter, have no preexisting esophageal disease and have a reliable caregiver can be observed at home with regular diet and encouraged activity, with consideration of XR if BB passage has not been confirmed by stool inspection in 10 to 14 days. In all other patients, immediate XR of the abdomen, esophagus, and neck should be obtained to locate the BB. Notably, BBs above the range of the XR have been missed in the past. AP and lateral views should be closely viewed, assessing for the classic “double rim,” “halo,” and “step‐off” signs and the orientation of the negative pole (Negative‐Narrow‐Necrotic). XR should not be delayed until after the onset of symptoms as injury can occur in as quickly as 2 hours. A multidisciplinary approach is often required and involving the appropriate teams early is critical.
3.4. Pre‐removal mitigation strategies
If lithium BB ingestion is known or suspected within the prior 12 hours and the child is ≥12 months, 10 mL (2 teaspoons) of commercial honey should be given by mouth (PO) every 10 minutes immediately and en route to the ED for up to 6 doses. Alternatively, sucralfate (Carafate suspension, 1 g/10 mL) can be administered with the same dosing and frequency. These efforts can start after arrival until XR can be obtained. An additional three doses can be given from the time of XR confirmation of BB lodged in esophagus until sedation is given for endoscopy. Children <12 months of age should not be administered honey or sucralfate. These strategies should be avoided if the BB was in place for over 12 hours as the risk of esophageal perforation increases after this time period. 51 Transportation to the ED should not be delayed to give honey. 37
3.5. Anesthetic considerations
BB removal should occur without delay to minimize tissue damage and potential complications. Standard NPO guidelines should not be followed as the risk of ongoing BB esophageal injury is thought to significantly outweigh the risk of pulmonary aspiration. Rapid sequence induction and intubation are recommended. 32 Patients can also be stratified into severe, intermediate, or low risk based on age, BB size, location of BB impaction, symptoms, and history of esophageal pathology. This risk stratification should determine the specialization of the OR, availability of specialists, intraoperative monitoring, and postoperative level of care. 60 Blood product administration should be available if needed based on clinical concern. The anesthesia guidelines have been updated to accommodate the latest NCPC guidelines. 32 When evidence of significant hematemesis or hemoptysis is present preoperatively, early involvement of cardio‐thoracic surgery and cardiopulmonary bypass/ECMO (extracorporeal membrane oxygenation) team may be life‐saving. In these cases, doing the BB removal in a specialized cardiac OR setting with all relevant teams immediately available is an important consideration.
3.6. Removal in OR
For BB removal, emergent endoscopic approach with direct visualization is preferred to allow for tissue injury inspection and identification of position and orientation of the BB. Other methods, such as retrieval by balloon catheter, magnet affixed to tube, or fluoroscopic removal are not recommended. The BB should be removed carefully with optical graspers. 11 , 37 Both flexible and rigid esophagoscopy have been used with success and having both available may be beneficial if an initial removal attempt fails. 48 , 61
After removal, mucosa should be inspected for extent, depth, and location of tissue damage. The orientation of the negative pole should be identified to determine structures at risk for further injury and subsequent complications. Excessive manipulation of the BB, may induce esophageal perforation and should be avoided. If there is no endoscopic evidence of perforation, the area should be irrigated with 50 to 150 mL sterile 0.25% acetic acid to neutralize the alkaline environment followed by endoscopic removal of excess fluid and debris. 37 Concurrent direct laryngoscopy and bronchoscopy should be considered to evaluate for airway injury, especially when the negative pole of BB is facing anteriorly. If moderate to severe esophageal injury is present, a soft nasogastric feeding tube may be placed under direct visualization for esophageal rest and enteral nutrition while awaiting an esophagram.
3.7. Gastric BB and beyond
If the BB is found to be lodged in the esophagus, immediate removal is warranted. If the BB is found to be in the stomach or beyond and a magnet was co‐ingested, removal should be performed endoscopically ideally and if required, surgically. Certain patients with gastric BB or beyond may be observed if they are asymptomatic, >6 years of age, and the BB was <15 mm. Symptomatic patients or those with BBs that remain in the stomach 4 days postingestion should undergo removal. Ultimately, the decision requires evaluation in a case‐by‐case basis taking into account ingestion timing and risk factor stratification (potential for in‐transit esophageal injury that needs to be endoscopically assessed), such as in higher‐risk patients ≤5 years of age or with BB size ≥20 mm. 32 , 37 , 57 , 62
3.8. Nasal or ear canal BB
Prompt removal of the BB from the nasal cavity or ear canal should be performed either at bedside in the urgent care or ED setting, clinic, or in the OR under general anesthesia depending on age, cooperation, and available resources. At this time, preremoval mitigation strategies do not apply to nasal or ear canal BB.
3.9. Tracheobronchial BB
BB aspiration leading to airway FBs should be emergently removed using direct laryngoscopy and bronchoscopy in the OR. Preremoval mitigation strategies do not apply to airway BBs.
3.10. Post‐removal
If mucosal injury was present following BB removal from the esophagus, patients must be observed for delayed complications such as tracheoesophageal fistula, esophageal stricture or perforation, mediastinitis, vocal cord paralysis, tracheal stenosis or tracheomalacia, empyema, lung abscess, pneumothorax, spondylodiscitis, or exsanguination from large vessel fistula. A contrast esophagram can be obtained to rule out perforation. Based on severity and location of the injury, as well as battery position and orientation, and using previously reported predictive factors, 58 the length of observation, duration of esophageal rest, need for serial imaging or additional endoscopy can be individualized for each patient.
Patients at risk for vascular fistulas should be followed closely as inpatients with serial imaging and stool guaiac tests. If any concerns regarding possible AEF develop, early involvement of the pediatric and cardiothoracic surgery teams is essential to prevent fatality. Tracheoesophageal fistula and esophageal stricture or perforation can present weeks to months later with delayed respiratory and swallowing symptoms requiring further investigation. Awake flexible laryngoscopy should be performed for any voice changes, stridor, or aspiration with consideration of laryngeal electromyography (EMG). Contrasted computerized tomography (CT) or magnetic resonance imaging (MRI) can be used to evaluate for mediastinitis and proximity of inflammation to blood vessels. MRI may be warranted to rule out spondylodiscitis in patients who develop neck stiffness who had a posterior‐facing BB‐negative pole. Following discharge, repeat esophagram or endoscopic evaluation should be scheduled for surveillance with sooner evaluation warranted for more severe injuries. 11 , 37
4. PREVENTION
4.1. Awareness/outreach
Increasing awareness is vital for consumers and caregivers as they are unable to protect their children if they are not effectively made aware of the hazards associated with BBs. A simple and effective way to prevent BB ingestion injuries is to implore caregivers to ensure items with BBs are made inaccessible to children. In 2011, Energizer and Safe Kids Worldwide started the Battery Controlled public awareness campaign to inform caregivers of the risks of BB ingestion via media and community outreach. In February 2020, the AAP and National BBTF organized a national “Day of Action” during which medical professionals used the hashtag #ButtonUpBatteries to unify and share BB information on social media. These platforms allow for rapid and widespread dissemination of knowledge to the public and to health professionals who may care for these patients. In addition, members of National BBTF have attended numerous society meetings and conferences to educate medical professionals on this topic. 11
4.2. Reporting
To improve ease, access, and detail associated with reporting FB cases, a nonprofit 501(c)(3), Global Injury Research Collaborative (GIRC, www.globalirc.org), provides free access to injury reporting apps designed for medical professionals. In the App Store and GooglePlay as “GIRC App” can be used by various health care team members to efficiently collect pertinent FB injury information (Figure 9). In a recent survey administered to 400+ physicians who managed over 32 000 pediatric FB injuries, only 11% of BB and 4% of overall FB injury cases were reported to an existing data source. In addition, 92% of respondents stated they would contribute more to injury statistics if it were more convenient, thus highlighting the need for better reporting mechanisms. 27 With the “GIRC App,” a more modern, user‐friendly method of reporting is now accessible for free download via the App Store and Google Play. Reporting includes FB picture, dimensions, location, hazard severity, and patient follow‐up and should take medical professionals less than 2 minutes to enter relevant injury data. This unique combination of data are not captured through other injury databases anywhere in the world. Trainees, including residents and fellows can participate in this effort. Since no personal health information is requested, the information is Health Insurance Portability and Accountability Act (HIPAA) compliant and submitted reports are deidentified from user. 63
FIGURE 9.
The Global Injury Research Collaborative smartphone application (“GIRC App”) provides an efficient, secure way for providers to report their foreign body injury cases (www.globalirc.org). This centralized database can help to prevent future injuries. It is available for no charge to medical professionals, both on the App Store (iOS) and GooglePlay (Android). Search “GIRC App,” download, and register to start reporting injuries
4.3. Role of industry
Major BB industrial companies such as Energizer and Duracell have implemented several modifications to minimize the risk of BB injury in children. For example, they have added child‐resistant double packaging which requires use of scissors to remove the initial packing layer as well as warning label stickers to keep BBs away from children. 64 , 65 , 66
More recently, Duracell has released three lithium BBs (CR2016, CR2025, CR2032) with a nontoxic bitter coating designed to try to help prevent accidental ingestions in children. 66 , 67 This is an application of a taste deterrent on the BB that, to our knowledge, has not been previously reported in the medical literature. As younger children are often most vulnerable to severe complications, taste aversion provides a possible prevention tool. However, it is still unknown what injury prevention impact this will have on BB ingestions given bitterants have not always prevented ingestion of other hazards. 68 , 69 , 70
4.4. Safety standards
BB injury risks can be mitigated with universal safety standards for industry. Organizations such as the American National Standards Institute and Underwriters Laboratories (UL) have outlined standards such as UL60065 and UL4200A which require two or more simultaneous independent movements, or the use of a tool to open lithium BB compartments. 71 In 2017, the U.S. CPSC enacted a mandatory standard by updating ASTM F963‐17, the standard consumer safety specification for toy safety to include use of warning labels and instructions informing consumers of BB risks for BB‐requiring toys intended for children <14 years old as well as new testing requirements. 72 Unfortunately, these “child‐resistant” standards do not extend to the many other BB‐containing household items that pose a significant risk for injury to children.
4.5. Advocacy
Despite lobbying efforts by several pediatric otolaryngologists and members of the National BBTF, legislation that would have provided regulations for the BB industry failed to be enacted in 2012. 8 Today, medical professionals on the National BBTF continue to encourage lawmakers to reconsider formal legislation to minimize BB injuries. All providers who care for these patients are well‐suited to play a key role in the lobby process and opportunities are continually available to participate; these medical specialists can be advocates for injury prevention by reporting each BB injury case they see to the GIRC App.
4.6. Safer BB technology
Primary prevention of BB injuries by modifying the BB design to be safer would prove most effective in eliminating the BB ingestion hazard. Studies have shown that quantum tunneling composite‐coated batteries conduct energy in high pressure powered electronics but not in low pressure settings such as the pediatric esophagus. This can prevent the hydrolysis reaction and subsequent liquefactive tissue necrosis. 73 Introduction of this coating provided a promising foundation for manufacturers to explore and adopt BB design alteration to try to prevent injuries. Landsdowne Labs LLC (https://www.landsdownelabs.com) is a leader in this space, dedicated to innovating a safer BB design to reduce the hazard severity with its ChildLokTM technology. The development of a novel BB design that reduces or eliminates the severe injuries seen in children is critical for a long‐term solution.
4.7. Future considerations
Other potential opportunities for BB injury prevention include the development of similar standards for nonlithium as exist for lithium BB. Active warning labels on all products that contain BBs, child‐resistant packaging for products. A salivary amylase‐activated dye coating could stain the lips and mouth with a designated color. Appearance of this color could prompt caregivers to seek emergent medical evaluation and could minimize duration of exposure. 11
5. CONCLUSION
BBs are commonly found in many household items and present a severe risk of injury to children upon ingestion. When lodged in the esophagus, BBs induce an alkaline reaction in as little as 2 hours that can lead to caustic injury. The mechanism is related to liquefactive necrosis and can occur even after removal resulting in delayed, severe morbidity and mortality. Diagnosis is challenging as BB ingestion is often unwitnessed and patients present with vague symptoms. Treatment involves prompt endoscopic removal. Mitigation strategies with pre‐removal use of honey and sucralfate and intraoperative use of acetic acid irrigations are new additions to the management guidelines aimed at neutralizing the pH and preventing propagation of tissue damage. Numerous prevention efforts have been implemented to minimize BB injury and are continually ongoing. It is important that medical professionals who manage these cases consistently report the relevant details of these injuries. Ultimately, reduction in esophageal BB hazard severity through innovation can help prevent morbidity and mortality in children.
CONFLICT OF INTEREST
Kris R. Jatana serves as a general product safety medical consultant for Intertek Inc. Kris R. Jatana has a patent pending coin/battery metal detector device under development and receives royalties for a patented, commercially available medical device, not related to nor discussed in this article from Marpac Inc. Kris R. Jatana is a shareholder in Zotarix LLC, Landsdowne Labs LLC, and Tivic Health Systems. Kris R. Jatana serves in leadership positions on the National Button Battery Task Force, supported by and affiliated with the American Academy of Pediatrics and American Broncho‐Esophagological Association. Kris R. Jatana and Keith Rhoades are advisory board members of the Global Injury Research Collaborative which is a U.S. IRS‐designated, 501(c)(3) nonprofit organization. This article is for educational use only, and the content is based on review of available resources to the authors on this topic. The content does not necessarily represent the opinions of any of the institutions or organizations authors are affiliated with.
Sethia R, Gibbs H, Jacobs IN, Reilly JS, Rhoades K, Jatana KR. Current management of button battery injuries. Laryngoscope Investigative Otolaryngology. 2021;6:549–563. 10.1002/lio2.535
REFERENCES
- 1. Litovitz T, Whitaker N, Clark L. Preventing battery ingestions: an analysis of 8648 cases. Pediatrics. 2010;125(6):1178‐1183. 10.1542/peds.2009-3038. [DOI] [PubMed] [Google Scholar]
- 2. Gibbs H, Rhoades K, Jatana KR. Management of pediatric button battery injuries from ingestion, aspiration, and insertion in the urgent care and emergency room setting. Clin Pediatr Emerg Med. In Press. 2020;21(2). 10.1016/j.cpem.2020.100775. [DOI] [Google Scholar]
- 3. allaboutbatteries.com . History of Battery Invention and Development. 2011. http://www.allaboutbatteries.com/history-of-batteries.html. Accessed October 20, 2020.
- 4. Poison Control Center . Battery Ingestion Hotline. 2020. https://www.batteryingestionhotline.com/. Accessed October 19, 2020.
- 5. Consumer Product Safety Commission . CPSC Issues Warning On Button Batteries. 1983. https://www.cpsc.gov/Newsroom/News‐Releases/1983/CPSC‐Issues‐Warning‐On‐Button‐Batteries/. Accessed October 21, 2020.
- 6. National Capital Poison Center . Button Battery Ingestion Statistics. 2020. https://www.poison.org/battery/stats. Accessed October 20, 2020.
- 7. Midgett J. U.S. Consumer Product Safety Commission Log of Meeting. 2020. https://www.cpsc.gov/s3fs-public/pdfs/foia_buttonbatt03172011.pdf. Accessed October 20, 2020.
- 8. Congress 112th. S. 1165—112th Congress: Button Cell Battery Safety Act of 2011. https://www.govtrack.us/congress/bills/112/s1165. 2011. Accessed October 21, 2020.
- 9. Centers for Disease Control and Prevention . Injuries from Batteries Among Children Aged <13 Years—United States, 1995–2010. Morbidity and Mortality Weekly Report (MMWR). http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6134a1.htm#tab2. Accessed October 20, 2020. [PubMed]
- 10. The American Academy of Pediatrics . Button Battery Task Force. https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Pages/Button-Battery.aspx. Accessed October 20, 2020.
- 11. Jatana KR, Litovitz T, Reilly JS, Koltai PJ, Rider G, Jacobs IN. Pediatric button battery injuries: 2013 task force update. Int J Pediatr Otorhinolaryngol. 2013;77(9):1392‐1399. 10.1016/j.ijporl.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 12. Jatana KR, Chao S, Jacobs IN, Litovitz T. Button battery safety: industry and academic partnerships to drive change. Otolaryngol Clin North Am. 2019;52(1):149‐161. 10.1016/j.otc.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 13. Battery Association of Japan . The History of the Battery. 2016. http://www.baj.or.jp/e/knowledge/history02.html. Accessed October 20, 2020.
- 14. I.E. Commission . International Standard IEC 60086‐2. 2001. http://www.iec.ch. Accessed October 20, 2020.
- 15. Litovitz T, Whitaker N, Clark L, White NC, Marsolek M. Emerging battery‐ingestion hazard: clinical implications. Pediatrics. 2010;125(6):1168‐1177. 10.1542/peds.2009-3037. [DOI] [PubMed] [Google Scholar]
- 16. Blatnik DS, Toohill RJ, Lehman RH. Fatal complication from an alkaline battery foreign body in the esophagus. Ann Otol Rhinol Laryngol. 1977;86:611‐615. 10.1177/000348947708600508. [DOI] [PubMed] [Google Scholar]
- 17. Shabino CL, Feinberg AN. Esophageal perforation secondary to alkaline battery ingestion. J Am Coll Emerg Physicians. 1979;8:360‐362. 10.1016/S0361-1124(79)80259-2. [DOI] [PubMed] [Google Scholar]
- 18. Jatana KR, Rhoades K, Milkovich S, Jacobs IN. Basic mechanism of button battery ingestion injuries and novel mitigation strategies after diagnosis and removal. Laryngoscope. 2017;127(6):1276‐1282. 10.1002/lary.26362. [DOI] [PubMed] [Google Scholar]
- 19. Jatana KR, Barron CL, Jacobs IN. Initial clinical application of tissue pH neutralization after esophageal button battery removal in children. Laryngoscope. 2019;129(8):1772‐1776. 10.1002/lary.27904. [DOI] [PubMed] [Google Scholar]
- 20. Gurevich Y, Sahn B, Weinstein T. Foreign body ingestion in pediatric patients. Curr Opin Pediatr. 2018;30:677‐682. 10.1097/MOP.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 21. Varga Á, Kovács T, Saxena AK. Analysis of complications after button battery ingestion in children. Pediatr Emerg Care. 2018;34:443‐446. 10.1097/PEC.0000000000001413. [DOI] [PubMed] [Google Scholar]
- 22. Orsagh‐Yentis D, McAdams RJ, Roberts KJ, McKenzie LB. Foreign‐body ingestions of young children treated in US emergency departments: 1995–2015. Pediatrics. 2019;143:e20181988. 10.1542/peds.2018-1988. [DOI] [PubMed] [Google Scholar]
- 23. Sharpe SJ, Rochette LM, Smith GA. Pediatric battery‐related emergency department visits in the United States, 1990‐2009. Pediatrics. 2012;129:1111‐1117. 10.1542/peds.2011-0012. [DOI] [PubMed] [Google Scholar]
- 24. Labadie M, O'Mahony E, Capaldo L, et al. Severity of button batteries ingestions: data from French Poison Control Centres between 1999 and 2015. Eur J Emerg Med. 2018;25(4):e1‐e8. 10.1097/MEJ.0000000000000528. [DOI] [PubMed] [Google Scholar]
- 25. Bolton SM, Saker M, Bass LM. Button battery and magnet ingestions in the pediatric patient. Curr Opin Pediatr. 2018;30:653‐659. 10.1097/MOP.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 26. National Capital Poison Center . Fatal Button Battery Ingestions. 2020. https://www.poison.org/battery/fatalcases. Accessed October 18, 2020.
- 27. Rhoades K. Data driven change. In: Paper Presented at: American College of Surgeons: Clinical Congress; 2019; San Francisco, CA.
- 28. Gibbs H, Rhoades K, Reilly J, et al. Pediatric otolaryngology reporting practices from aspiration or ingestion cases: Data and collaboration to drive change. In: Poster Presentation Presented at American Society of Pediatric Otolaryngology Meeting; 2020; Atlanta, GA (Virtual). [Google Scholar]
- 29. Pizzol A, Rigazio C, Calvo PL, et al. Foreign‐body ingestions in children during COVID‐19 pandemic in a pediatric referral center. JPGN Rep. 2020;1:e018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.United States Consumer Product Safety Commission. Hospital emergency room treatment for some product‐related injuries rose during the pandemic, even as overall ER visits dropped. https://www.cpsc.gov/Newsroom/News‐Releases/2021/Hospital‐Emergency‐Room‐Treatment‐for‐Some‐Product‐Related‐Injuries‐Rose‐During‐the‐Pandemic‐Even‐as‐Overall‐ER‐Visits‐Dropped. Accessed March 15, 2021.
- 31. Thomson M, Sharma S. The hazards of button battery ingestion. Arch Dis Child. 2015;100:1010‐1011. 10.1136/archdischild-2015-308313. [DOI] [PubMed] [Google Scholar]
- 32. Hoagland MA, Ing RJ, Jatana KR, Jacobs IN, Chatterjee D. Anesthetic implications of the new guidelines for button battery ingestion in children. Anesth Analg. 2020;130(3):665‐672. 10.1213/ANE.0000000000004029. [DOI] [PubMed] [Google Scholar]
- 33. Sancaktar ME, Bayraktar C, Bakırtaş M. Injury mechanism of button batteries in the nasal cavity and possible mitigation strategies during impaction. Laryngoscope. 2020;130:2487‐2493. 10.1002/lary.28913. [DOI] [PubMed] [Google Scholar]
- 34. Yamashlta M, Saito S, Koyama K, Hattori H, Ogata T. Esophageal electrochemical burn by button‐type alkaline batteries in dogs. Vet Hum Toxicol. 1987.29(3):226–230. [PubMed] [Google Scholar]
- 35. Anfang RR, Jatana KR, Linn RL, Rhoades K, Fry J, Jacobs IN. pH‐neutralizing esophageal irrigations as a novel mitigation strategy for button battery injury. Laryngoscope. 2019;129(1):49‐57. 10.1002/lary.27312. [DOI] [PubMed] [Google Scholar]
- 36. Gyawali BR, Guragain R, Gyawali DR. Role of honey and acetic acid in mitigating the effects of button battery in esophageal mucosa: a cadaveric animal model experimental study. Indian J Otolaryngol Head Neck Surg. 2021. 10.1007/s12070-021-02382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. National Capital Poison Center . National Capital Poison Center Button Battery Ingestion Triage and Treatment Guideline. 2020. https://www.poison.org/battery/guideline. Accessed October 20, 2020.
- 38. Littlehales E, Levi E, Mills N, Metcalfe R, Hamill J. Double button battery ingestion—the “macaroon” sign. J Pediatr Surg Case Rep. 2018;36:36‐39. 10.1016/j.epsc.2018.06.013. [DOI] [Google Scholar]
- 39. Ettyreddy AR, Georg MW, Chi DH, Gaines BA, Simons JP. Button battery injuries in the pediatric aerodigestive tract. Ear Nose Throat J. 2015;19(12):486‐493. 10.1177/014556131509401207. [DOI] [PubMed] [Google Scholar]
- 40. Buttazzoni E, Gregori D, Paoli B, et al. Symptoms associated with button batteries injuries in children: an epidemiological review. Int J Pediatr Otorhinolaryngol. 2015;79(12):2200‐2207. 10.1016/j.ijporl.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 41. Eliason MJ, Ricca RL, Gallagher TQ. Button battery ingestion in children. Curr Opin Otolaryngol Head Neck Surg. 2017;25:520‐526. 10.1097/MOO.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 42. Moral L, Serna JV, Castillo B. Aspiración de pila de botón: caso único en la literatura médica. Arch Bronconeumol. 2010;46:153‐154. 10.1016/j.arbres.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 43. Acosta Díaz HG, Trinidad Ruíz G, Pantoja Hernández CG, Samaniego B, Pando J, Rejas E. Lesión bronquial por aspiración de una pila alcalina (pila de botón). An Pediatr. 2013;79:267‐268. 10.1016/j.anpedi.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 44. McLarty JD, Krishnan M, Rowe MR. Disk battery aspiration in a young child: a scarcely reported phenomenon. Arch Otolaryngol Head Neck Surg. 2012;138:680‐682. 10.1001/archoto.2012.1097. [DOI] [PubMed] [Google Scholar]
- 45. Voelker J, Voelker C, Engert J, Schendzielorz P, Hagen R, Rak K. Severe tracheobronchial harm due to lithium button battery aspiration: an in vitro study of the pathomechanism and injury pattern. Int J Pediatr Otorhinolaryngol. 2020;139:110431. 10.1016/j.ijporl.2020.110431. [DOI] [PubMed] [Google Scholar]
- 46. Jatana KR. Button Battery Injuries in Children: A Growing Risk. Everything Matters in Patient Care. Columbus, OH: Nationwide Children's Hospital; 2013. [Google Scholar]
- 47. Torrecillas V, Meier JD. History and radiographic findings as predictors for esophageal coins versus button batteries. Int J Pediatr Otorhinolaryngol. 2020;137:110208. 10.1016/j.ijporl.2020.110208. [DOI] [PubMed] [Google Scholar]
- 48. Krom H, Visser M, Hulst JM, et al. Serious complications after button battery ingestion in children. Eur J Pediatr. 2018;177:1063‐1070. 10.1007/s00431-018-3154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. National Capital Poison Center . Nonfatal Button Battery Ingestions with Severe Esophageal or Airway Injury: 248 Reported Cases. 2020. http://www.poison.org/battery/severecases. Accessed October 21, 2020.
- 50. Wright K, Parkins K, Jahn H, Rowlands R, Davies F. Catastrophic haemorrhage from button battery ingestion in children: a growing problem. Acta Paediatr Int J Paediatr. 2017;106:1391‐1393. 10.1111/apa.13934. [DOI] [PubMed] [Google Scholar]
- 51. Soto PH, Reid NE, Litovitz TL. Time to perforation for button batteries lodged in the esophagus. Am J Emerg Med. 2019;37:805‐809. 10.1016/j.ajem.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 52. Granata A, Gandolfo C, Acierno C, Piazza M, Burgio G, Traina M. Button battery removed from the stomach resulting in a missed aortoesophageal fistula—a multidisciplinary approach to rescuing a very young patient: a case report 11 medical and health sciences 1103 clinical sciences. J Med Case Reports. 2018;12:318. 10.1186/s13256-018-1818-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mahajan S, Jaswal V, Thingnam SKS, Dogra N. Successful surgical management of an aorto‐oesophageal fistula caused by button battery ingestion. Eur J Cardiothoracic Surg. 2019;55:790‐791. 10.1093/ejcts/ezy302. [DOI] [PubMed] [Google Scholar]
- 54. Spiers A, Jamil S, Whan E, Forbes D, Gollow I, Andrews D. Survival of patient after aorto‐oesophageal fistula following button battery ingestion. ANZ J Surg. 2012;82:186‐187. 10.1111/j.1445-2197.2011.05984.x. [DOI] [PubMed] [Google Scholar]
- 55. Bartkevics M, Stankovic Z, Schibli S, et al. A near miss and salvage management of aortoesophageal fistula secondary to cell battery ingestion. World J Pediatr Congenit Hear Surg. 2020;11:120‐122. 10.1177/2150135119880549. [DOI] [PubMed] [Google Scholar]
- 56. Duell M. Girl, 2, is Left Fighting for Her Life After Accidentally Swallowing a 10‐p Sized “Button” Battery from Inside a Car Key Which Burned Through Her Stomach and Arteries. 2017. https://www.dailymail.co.uk/news/article‐4400750/Derby‐girl‐2‐accidentally‐swallows‐button‐battery.html. Accessed October 21, 2020.
- 57. Lerner DG, Brumbaugh D, Lightdale JR, Jatana KR, Jacobs IN, Mamula P. Mitigating risks of swallowed button batteries: new strategies before and after removal. J Pediatr Gastroenterol Nutr. 2020;70(5):542‐546. 10.1097/MPG.0000000000002649. [DOI] [PubMed] [Google Scholar]
- 58. Eliason MJ, Melzer JM, Winters JR, Gallagher TQ. Identifying predictive factors for long‐term complications following button battery impactions: a case series and literature review. Int J Pediatr Otorhinolaryngol. 2016;87:198‐202. 10.1016/j.ijporl.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 59. Russell RT, Griffin RL, Weinstein E, Billmire DF. Esophageal button battery ingestions: decreasing time to operative intervention by level i trauma activation. J Pediatr Surg. 2014;49:1360‐1362. 10.1016/j.jpedsurg.2014.01.050. [DOI] [PubMed] [Google Scholar]
- 60. Ing RJ, Hoagland M, Mayes L, Twite M. The anesthetic management of button battery ingestion in children. Can J Anesth. 2018;65:309‐318. 10.1007/s12630-017-1023-9. [DOI] [PubMed] [Google Scholar]
- 61. Gmeiner D, von Rahden BHA, Meco C, Hutter J, Oberascher G, Stein HJ. Flexible versus rigid endoscopy for treatment of foreign body impaction in the esophagus. Surgical Endoscopy. 2007;21(11):2026‐2029. 10.1007/s00464-007-9252-6. [DOI] [PubMed] [Google Scholar]
- 62. Khalaf RT, Ruan W, Orkin S, et al. Gastric injury secondary to button battery ingestions: a retrospective multicenter review. Gastrointest Endosc. 2020;92:276‐283. 10.1016/j.gie.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jatana KR, Rhoades K, Gibbs H, et al Importance of global foreign body injury reporting: Chevalier Jackson's legacy carried to smartphone app. In: Oral Presentation Presented at American Broncho‐Esophagological Association Meeting; 2020; Atlanta, GA (Virtual).
- 64. Spinner J. Energizer charges into child‐resistant packaging. Packaging Digest . 2010. www.packagingdigest.com/packaging-design/energizer-charges-child-resistant-packaging. Accessed October 22, 2020.
- 65. Energizer . Coin Lithium Battery Safety. 2020. http://www.energizer.com/Responsibility/coin-lithium-battery-safety/preventing-coin-lithium-battery-injury. Accessed October 19, 2020.
- 66. Duracell Inc . Lithium Coin Battery Safety—Duracell. 2016. https://www.duracell-me.com/technology/lithium-coin-battery-safety/. Accessed October 21, 2020.
- 67.Duracell Inc. 2032 lithium coin battery with bitter coating. 2020. https://www.duracell.com/en-us/product/cr-2032-lithium-coin-button-battery/. Accessed March 20, 2021.
- 68. White NC, Litovitz T, Benson BE, Horowitz BZ, Marr‐Lyon L, White MK. The impact of bittering agents on pediatric ingestions of antifreeze. Clin Pediatr (Phila). 2009;48:913‐921. 10.1177/0009922809339522. [DOI] [PubMed] [Google Scholar]
- 69. White NC, Litovitz T, White MK, et al. The impact of bittering agents on suicidal ingestions of antifreeze. Clin Toxicol. 2008;46:507‐514. 10.1080/15563650802119700. [DOI] [PubMed] [Google Scholar]
- 70. Gould Soloway RA Taste Changes Don't Stop Poisonings. 2020. https://www.poison.org/articles/2009-oct/taste-changes-dont-stop-poisonings. Accessed October 20, 2020.
- 71. UL . Standard for Safety for Products Incorporating Button or Coin Cell Batteries of Lithium Technologies. 2020. https://standardscatalog.ul.com/standards/en/standard_4200a_1. Accessed October 20, 2020.
- 72. Consumer Product Safety Commission . ASTM F 963–17 Requirements. https://www.cpsc.gov/Business–Manufacturing/Business-Education/Toy-Safety/ASTM-F-963-Chart.
- 73. Laulicht B, Traverso G, Deshpande V, Langer R, Karp JM. Simple battery armor to protect against gastrointestinal injury from accidental ingestion. Proc Natl Acad Sci USA. 2014;111:16490‐16495. 10.1073/pnas.1418423111. [DOI] [PMC free article] [PubMed] [Google Scholar]


