Abstract
Objectives
To assess outcomes following cochlear implantation (CI) in patients with hearing loss secondary to primary or secondary autoimmune inner ear disease (AIED).
Methods
A systematic review and narrative synthesis was completed according to PRISMA guidelines. Databases searched included MEDLINE, PubMed, EMBASE, Web of Science, Cochrane Collection, and ClinicalTrials.gov. No limits were placed on year of publication or language.
Results
A total of 551 studies were identified, of which 29 were included after removal of duplicates, and screening the title, abstract, and full text. All except one study were OCEBM grade IV. 114 of 115 patients displayed improvement in hearing following cochlear implantation. With implant use, roughly a third of these patients had hearing that improved over time, a third improved and plateaued, and a third remained stable. There was no additional risk of perioperative complications found in AIED patients compared what is generally accepted in general cochlear implantation, although two episodes of device failure after 6 months were noted, and four patients with secondary AIED displayed poor initial audiological outcomes.
Conclusion
CI in both primary and secondary AIED provides marked improvement in hearing. Early CI may be a valid management option, provide long‐lasting hearing in patients and reduce the side effects of long‐term systemic immunosuppressants. However, patients should be counseled residual hearing may be lost if there is cochlear ossification or fibrosis which may make implant insertion more traumatic.
Level of Evidence
NA.
Keywords: autoimmune inner ear disease, Cochlear implants, sensorineural hearing loss, systematic review
Short abstract
Autoimmune inner ear disease is characterised by bilateral, assymetrical, progressive (or fluctuating) hearing loss that develops over weeks to months and is steroid responsive. Cochlear implantation has been suggested as a useful intervention in this group when medical management fails, however there is limited data in the literature. This review aims to systematically review all available evidence of post‐cochlear implantation hearing outcomes in patients with autoimmune inner ear disease to assess its benefit.
1. INTRODUCTION
Autoimmune inner ear disease (AIED) is a rare disease that can lead to profound bilateral SNHL. 1 , 2 As well as being very uncommon, comprising <1% of all hearing loss or dizziness, 3 the diagnosis of AIED may be difficult due to its masked clinical presentation by its underlying etiology. AIED can be categorized into primary or secondary causes. Where the autoimmune process is limited to the cochlea or vestibular system, this condition is termed primary AIED. It is estimated that up to a third of all AIED is secondary AIED, that is, hearing loss as a consequences of a wider systemic autoimmune disease. 2 , 3 , 4 This includes an extensive differential list that includes, but is not limited to, Cogan's syndrome, 5 Vogt Koyanagi Harada (VKH) syndrome, 6 granulomatosis with polyangiitis, 7 systemic lupus erythematosus (SLE), 8 polyarteritis nodosa (PAN), 9 relapsing polychondritis, inflammatory bowel disease (IBD), rheumatoid arthritis (RA), 10 and Sjögren's syndrome. 11
1.1. Diagnosis
Although several autoantibodies have been postulated, some of which may predict response to steroid treatment, no specific diagnostic marker for AIED has been identified. 3 , 12 The mainstay of diagnosis therefore is through clinical history, examination, and characteristic response to steroids and immunosuppressants. 1 This clinical presentation was first noted by McCabe in 1972 13 when he noted that AIED patients tended to display a bilateral and asymmetrical hearing loss that was progressive or fluctuating, occurring over weeks to months, and responsive to steroids. When diagnosing AIED, it is critical to rule out systemic autoimmune causes before a diagnosis of primary AIED is made, as this may affect treatment and prognosis. Blood tests, therefore, should screen for causes of secondary AIED and may include a full blood count (FBC), erythrocyte sedimentation rate (ESR), anti‐double stranded DNA (dsDNA), rheumatoid factor (RF), Anti‐Neutrophil Cytoplasmic Antibodies (ANCA), C3 and C4 complement levels, and Human Immunodeficiency Virus (HIV) testing. 2
1.2. Pathophysiology
There are various theories as to the pathophysiology underlying AIED. Currently, the favored theory is that of humoral and cell‐mediated self‐targeting of antigens within the inner ear. 2 , 12 , 14 These antigens may have been introduced as a result of systemic, or direct damage to the cochlea leading to a type 1 T helper (Th1) cell response and subsequent tissue damage via autoantibody formation and/or immune‐complex deposition. 14 This is supported by studies in rats, whereby labyrinthitis was induced experimentally after introducing a systemic inner ear antigen. 15
1.3. Current treatment
The mainstay of treatment is pharmacological: oral steroids, intratympanic (IT) steroids, and methotrexate (MTX) seem to be most widely used. Other treatments such as azathioprine (AZA) and plasmapheresis have also been trialled. 1 , 12 More recently, various biologics both systemically and intratympanically have been tested. There is little consensus as to the most effective treatment. 16 In some cases, however, the progressive nature of the AIED results in the need for hearing aids and/or cochlear implantation due to failure of medical therapy. 12
1.4. Risks of cochlear implantation
It is thought that CI confers good patient benefit, 1 , 2 however given the scarcity of AIED cases, data for CI in this group is lacking. There are no additional risks universally accredited to AIED beyond what is already accepted for cochlear implantation in the current literature. 17 Importantly, some patients with AIED have been noted to develop ossification of the cochlea 8 which could affect the surgical placement of CI electrodes, that is, partial, difficult or more traumatic insertion, which could further result in more frequent loss of residual hearing. 18 Hearing loss in AIED may also fluctuate, making diagnosis and hearing rehabilitation more challenging.
1.5. Objectives
The aim of this review was to compile documented cases of CI in AIED patients, to assess the pre‐ and post‐operative hearing outcomes, note any significant perioperative complications, and to ultimately evaluate the benefit of this intervention for this challenging patient group.
Population: Children or adults with systemic or inner ear autoimmune hearing loss.
Intervention: Cochlear implantation.
Comparison: No formal comparison, may demonstrate intra‐subject change pre and post‐operatively or report outcomes compared to non‐AIED patients.
Outcomes: Pre‐ vs post‐implantation audiometric outcomes with cochlear implant usage (where pre‐implantation outcomes were not available, only post‐implantation audiometric outcomes were included). Complications associated with perioperative period in patients receiving cochlear implantation.
2. MATERIALS AND METHODS
The study protocol was registered in the PROSPERO prospective database of systematic reviews (CRD42021229196).
2.1. Study inclusion criteria
Clinical studies of cochlear implantation in patients with hearing loss secondary to primary or secondary autoimmune inner ear disease (AIED), where hearing outcomes were reported at 3 months (or later) post‐implantation. Studies of any experimental or observational design in humans were included. Animal and human studies without a report of postoperative audiometric outcomes or where the abstract or full text was unavailable were excluded. Diabetes and multiple sclerosis were not included in the search strategy, as the effects are likely not due to primary autoimmune disease in the inner ear.
2.2. Search strategy
JL performed the searches, which was rechecked by a clinical librarian. In total, 2 reviewers (JL/KB) independently screened the abstracts. The following databases were searched: MEDLINE, PubMed, EMBASE, Web of Science, Cochrane Collection, and ClinicalTrials.gov.
The search terms used can be found in Appendix 1.
No limit was placed on language or year of publication.
2.3. Selection of studies
Searches were performed by JL. Two reviewers (JL/KB) independently screened all the records by title and abstract identified from the database searches. Studies describing cochlear implantation in patients with systemic or inner ear autoimmune hearing loss were assessed against the inclusion and exclusion criteria, with any disagreement resolved by discussion with a third reviewer (JM).
Studies without accessible full text after screening the title and abstract were gathered by contacting the respective study authors. If they remained unavailable or the author did not reply, the study was excluded. Studies were excluded if they did not report post‐intervention audiometric outcomes 3 months (or later) post‐procedure. Potentially relevant studies identified from the initial searches and abstract screening then underwent full‐text screening by the two independent reviewers before data extraction. Conflicts on the selection were resolved by discussion between the reviewers.
2.4. Data extraction
Data were extracted by the first reviewer (JL) and then checked by a second reviewer (KB). Extracted data were arranged in a spreadsheet (Excel, Microsoft Corp., Redmond, Washington).
2.5. Risk of biased quality scoring
Two reviewers independently assessed the risk of bias using the Brazzelli risk of bias tool for non‐randomized studies. 19 Studies were also graded according to the Oxford Centre for Evidence‐Based Medicine (OCEBM) grading system. 20 Discrepancies between the reviewers were resolved by discussion.
3. RESULTS
Searches were first performed on the 30th of December 2020, and re‐checked on the 16th of January 2021. A total of 551 records were identified, of which 309 remained after removing duplicates (Figure 1). A further 250 studies were excluded by abstract and title screening, and 30 full text articles were excluded due to the following reasons; no audiometry after 3 months stated (n = 19), no access to full text from author (n = 4), poster or oral presentation (n = 2), data pooled with other non‐autoimmune group results (n = 2), no cochlear implantation (n = 2), no autoimmune disease (n = 1).
FIGURE 1.
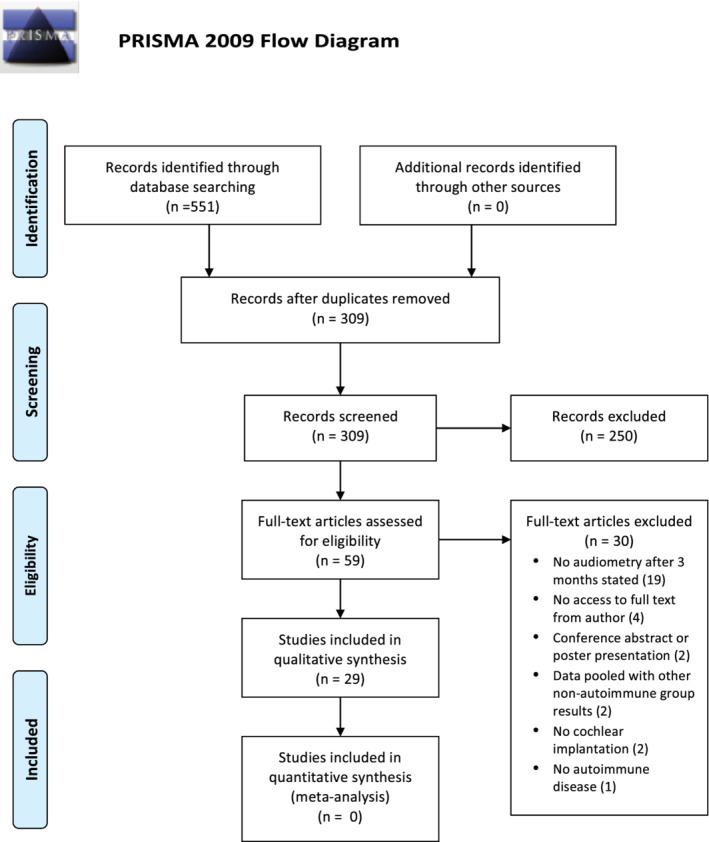
PRISMA (2009) flow diagram
Studies took place between 1996 and 2021, consisting of 20 single case reports, 4 case series, 3 cohort studies, 1 case‐control study, and 1 chart review.
There were a total of 115 patients of which there was a female preponderance (77 females, 38 males). Ages ranged from 4 to 84 years at the time of implantation, and the time from symptoms to cochlear implantation ranged widely from 1 to 120 months. A minority of patients had primary AIED (38) compared to secondary AIED (77) such as Cogan's syndrome (n = 42), relapsing polychondritis (n = 6), ANCA‐associated vasculitis (n = 4), rheumatoid arthritis (n = 3), granulomatosis with polyangiitis (n = 3), inflammatory bowel disease (n = 2), Vogt‐Koyanagi‐Harada syndrome (n = 2), polyarteritis nodosa (n = 2), unspecified vasculitis (n = 2), eosinophilic granulomatosis with polyangiitis (n = 1), Beçet's disease (n = 1) cerebral vasculitis (n = 1), Sjögren's syndrome (n = 1), primary sclerosing cholangitis (n = 1), neurosarcoidosis (n = 1), systemic psoriasis (n = 1), systemic lupus erythematosus (n = 1), Sweet's disease (n = 1), chronic demyelinating inflammatory polyneuropathy (n = 1), and systemic sclerosis (n = 1). Diagnosis was mostly clinical, however one study 21 conducted genetic tests to rule out other causes (Muckle‐Wells syndrome). Common presenting symptoms included vestibular symptoms (26% of patients reporting dizziness, vertigo, or unsteadiness) and tinnitus (18%). This was much lower than estimated by Vambutas et al, Mijovic et al, and Bovo et al, who estimated half of all AIED patients display vestibular symptoms and a quarter to half displayed tinnitus, 2 , 3 , 12 , 22 however this may just be due to an omission of reporting in studies. Other symptoms appear more related to the systemic autoimmune condition such as keratitis in Cogan's syndrome, 23 or sclerodactyly in systemic sclerosis. 24
Apart from three studies, 11 , 25 , 26 details on implant type were given. A minimum of 9 patients were recorded to have bilateral cochlear implants, however this number may be an under‐representation as some studies did not disclose if there was unilateral or bilateral implantation. Follow‐up durations after surgery varied between 3 months ‐ 16 years. Study characteristics are summarized in Table 1.
TABLE 1.
Study characteristics
| Authors | Year | Country | Number of patients | Population | Autoimmune disease | Study type | OCEBM* Grade |
|---|---|---|---|---|---|---|---|
| Abou‐Elhmd et al 7 | 1996 | UK | 1 | Adult | GPA | Retrospective Case report | IV |
| Aftab et al 8 | 2010 | US | 10 | Adult | Primary AIED (8), Lupus (1), Psoriasis (1) | Retrospective chart review | III |
| AlHelali et al 6 | 2019 | Saudi Arabia | 1 | Adult | Vogt‐Koyanagi‐Harada syndrome | Retrospective Case report | IV |
| Aschendorff et al 24 | 2004 | Germany | 6 | Adult | Cogan's syndrome | Retrospective Cohort study | IV |
| Bacciu et al 25 | 2015 | Italy | 12 | Adults | Cogan's syndrome | Retrospective case series | IV |
| Bovo et al 26 | 2011 | Italy | 3 | Adults | Cogan's syndrome | Retrospective case series | IV |
| Cacco et al 27 | 2021 | Italy | 1 | Adult | eGPA | Retrospective case report | IV |
| Canzi et al 9 | 2019 | Italy | 1 | Adult | Polyarteritis nodosa | Retrospective case report | IV |
| Cassis et al 28 | 2018 | US | 1 | Adult | Cogan's syndrome | Retrospective case report | IV |
| Cheng et al 29 | 2010 | Australia | 1 | Adult | Sweets disease | Retrospective case report | IV |
| Dhanjal et al 30 | 2014 | UK | 1 | Adult | Neurosarcoidosis | Retrospective case report | IV |
| Im et al 31 | 2008 | South Korea | 1 | Adult | Cogan's syndrome | Retrospective case report | IV |
| Kamakura et al 32 | 2017 | US | 1 | Adult | Cogan's syndrome | Retrospective case report | IV |
| Kawamura et al 32 | 2010 | Japan | 1 | Adult | Cogan's syndrome | Retrospective case report | IV |
| Kontorinis et al 22 | 2010 | Germany | 4 | Mixed | Cogan's syndrome | Retrospective case series | IV |
| Low et al 21 | 2019 | Singapore | 1 | Adult | Cogan's syndrome | Retrospective case report | IV |
| Low et al 33 | 2000 | Singapore | 1 | Adult | Cogan's syndrome | Retrospective case report | IV |
| Malik et al 11 | 2012 | US | 26 | Adults | Primary IED (16), Cogan's syndrome (2), RP (3), Sjögren (1), RA (1), PSC (1), GPA (1), cerebral Vasculitis (1) | Retrospective cohort study | IV |
| Mowry et al 34 | 2017 | US | 1 | Adult | Chronic demyelinating inflammatory polyneuropathy | Retrospective case report | IV |
| Patrizia et al 35 | 2011 | Italy | 1 | Adult | RP | Retrospective case report | IV |
| Psillas et al 36 | 2007 | Greece | 1 | Adult | Polyarteritis nodosa | Retrospective case report | IV |
| Quaranta et al 37 | 2002 | Italy | 5 | Adults | Cogan's syndrome (2), vasculitis (unspecified) (2), Beçet's disease (1) | Retrospective cohort study | IV |
| Salahaldin et al 38 | 2010 | Qatar | 1 | Child | Primary AIED | Retrospective case report | IV |
| Santarelli et al 39 | 2006 | Italy | 1 | Adult | Systemic sclerosis | Retrospective case report | IV |
| Seo et al 40 | 2012 | South Korea | 1 | Adult | RP | Retrospective case report | IV |
| Sweetow et al 41 | 2005 | US | 1 | Child | RA | Retrospective case report | IV |
| Sydlowski et al 42 | 2014 | US | 1 | Adult | Vogt‐Koyanagi‐Harada syndrome | Retrospective case report | IV |
| Wang et al 10 | 2010 | Canada | 25 | Adult | Primary AIED (13), Cogan's syndrome (7), RP (1), RA (1), GPA (1), 1 UC (1) Crohns disease (1) | Retrospective case control | IV |
| Watanabe et al 23 | 2018 | Japan | 4 | Adult | ANCA‐associated vasculitis | Retrospective case series | IV |
Abbreviations: ANCA, antineutrophil cytoplasmic antibody; GPA, granulomatosis with polyangiitis; eGPA, eosinophilic granulomatosis with polyangiitis; PSC, primary sclerosing cholangitis; RA, rheumatoid arthritis; RP, relapsing polychondritis; UC, ulcerative colitis.
3.1. Quality of studies
All studies were retrospective and tended to have a small population size. Owing to the rare nature of AIED, the majority of the studies were single case reports or uncontrolled case series, and therefore Oxford Centre for Evidence‐Based Medicine (OCEBM) grade IV, with the exception of one retrospective cohort study with randomised controls that was OCEBM grade III. 8 There was significant heterogeneity between the various studies' reporting of pre‐ and post‐operative audiometric evaluations, surgical technique, and follow‐up management, which prevented meta‐analyses. A tabular representation of the Brazzelli risk of bias is presented in Table 2. The majority of the studies had a high risk of bias in selecting representative samples, lack of clarifying inclusion and exclusion criterias, and method of patient selection and data collection (mostly restrospective and nonconsecutive patients). Most studies did not disclose the center's facilities or expertise in conducting cochlear implantation. All studies considered important outcomes and objective outcome measures (as required in the inclusion criteria).
TABLE 2.
Tabular representation of Brazzelli 19 risk of bias tool
| Authors | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abou‐Elhmd et al, 1996 | ||||||||||||||||||
| Aftab et al, 2010 | ||||||||||||||||||
| AlHelali et al, 2019 | ||||||||||||||||||
| Aschendorff et al, 2004 | ||||||||||||||||||
| Bacciu et al, 2015 | ||||||||||||||||||
| Bovo et al, 2011 | ||||||||||||||||||
| Cacco et al, 2021 | ||||||||||||||||||
| Canzi et al, 2019 | ||||||||||||||||||
| Cassis et al, 2018 | ||||||||||||||||||
| Cheng et al, 2010 | ||||||||||||||||||
| Dhanjal et al, 2014 | ||||||||||||||||||
| Im et al, 2008 | ||||||||||||||||||
| Kamakura et al, 2017 | ||||||||||||||||||
| Kawamura et al, 2010 | ||||||||||||||||||
| Kontorinis et al, 2010 | ||||||||||||||||||
| Low et al, 2019 | ||||||||||||||||||
| Low et al, 2000 | ||||||||||||||||||
| Malik et al, 2012 | ||||||||||||||||||
| Mowry et al, 2017 | ||||||||||||||||||
| Patrizia et al, 2011 | ||||||||||||||||||
| Psillas et al, 2007 | ||||||||||||||||||
| Quaranta et al, 2002 | ||||||||||||||||||
| Salahaldin et al, 2010 | ||||||||||||||||||
| Santarelli et al, 2006 | ||||||||||||||||||
| Seo et al, 2012 | ||||||||||||||||||
| Sweetow et al, 2005 | ||||||||||||||||||
| Sydlowski et al, 2014 | ||||||||||||||||||
| Wang et al, 2010 | ||||||||||||||||||
| Watanabe et al, 2018 |
Note: Green = Yes (low risk of bias); Red = No (high risk of bias); Yellow = unclear (unclear risk of bias); Gray = Not applicable.
1. Were participants a representative sample selected from a relevant patient population?
2. Were the inclusion/exclusion criteria of participants clearly described?
3. Were participants entering the study at a similar point in their disease progression?
4. Was selection of patients consecutive?
5. Was data collection undertaken prospectively?
6. Were the groups comparable on demographic characteristics and clinical features?
7. Was the intervention (and comparison) clearly defined?
8. Was the intervention undertaken by someone experienced at performing the procedure?
9. Were the staff, place, and facilities where the patients were treated appropriate for performing the procedure?
10. Were any of the important outcomes considered (ie, on clinical effectiveness, cost‐effectiveness, or learning curves)?
11. Were objective outcome measures used, including satisfaction scale?
12. Was the assessment of main outcomes blind?
13. Was follow‐up long enough (≥1 year) to detect important effects on outcomes of interest?
14. Was information provided on non‐respondents, dropouts?
15. Were the characteristics of withdrawals/dropouts similar to those that completed the study and therefore unlikely to cause bias?
16. Was length of follow‐up similar between comparison groups.
17. Were the important prognostic factors identified?
18. Were the analyses adjusted for confounding factors?
3.2. Audiological outcomes
Hearing outcomes (Table 3) were mostly positive across the studies with the exception of 4 patients: case 3 (Cogan's syndrome, Cochlear Nucleus 24k, unknown if full insertion) in Bovo et al, 27 and cases 1, 2 and 4 (3 ANCA‐associated vasculitis patients, unknown CI device or whether full insertion) in Watanabe et al. 26 Reported outcome measures were heterogeneous throughout, with over 20 different audiometric outcome measures being used across the various studies; some even using different combinations within the same study pre‐ and post‐operatively. All studies revealed pre‐operative hearing assessments, of which 13 specifically mentioned pure tone audiometry (PTA), all showing severe to profound hearing loss or anacusis. Three studies additionally also reported otoacoustic emission testing pre‐operatively, all reporting no response, which would suggest that the diseases primarily affect the cochlea and not the auditory nerve. 6 , 28 , 29 With the exception of Aschendorff (who did not report post‐op outcomes in 3 of 4 cases), 30 all studies gave post‐operative audiometric data for each individual case or as an average. Multiple heterogeneous outcome measures were used (see Table 3 for list).
TABLE 3.
Audiological outcomes
| Authors | Patients (implant) | Preoperative data | Postoperative data | Overall benefit (subjective assessment) | Follow‐up (months) |
|---|---|---|---|---|---|
| Abou‐Elhmd et al, 1996 | 1(1) |
Right: Initially SNHL of 30‐50 dB, then no PTA response over 22 months Left: Initially mixed hearing loss of 80‐90 dB then no PTA response |
9 months post‐op: Right (implanted ear): PTA: 40 dB hearing loss‐ BKB: 20% Gap detection test: 65 (71%). VCV testing: Correctly identified 27.1% of consonants. CDT score: 74 words/3 minutes |
Positive response from no hearing | 9 |
| Aftab et al, 2010 | 10(12) |
Mean preoperative PTA: 90 ± 13 dB Mean SRT 77.9 ± 38 dB Mean ST (short term <12mo) SRT: 24 ± 7 dB Words scores 11% ± 17% Sentence score was 11% ± 15% Sentence scores were determined by hearing in noise testing, except in 3 patients where the CID Everyday Sentence test was used. |
<12 months post‐op Word score: 74% ± 15% Sentences score: 94% ± 6% ≥12 months post‐op Word score: 87% ±11% Sentences score: 96% ±4% |
Good improvement in word scores at short term (<12 months) follow‐up, which improved in the long‐term (>12 months) | Not stated. ≥12 |
| AlHelali et al, 2019 | 1(2) | SRT: 45 dB SPL (sound field) in the better earSDS: 0% at 100dBSPLOAE: Absent response bilaterallyPTA: profound to no hearing bilaterally. |
5 years post‐op SRT: 25 dB HL bilaterally SDS: 84% (Right), 72% (Left) without visual cues. SDS: 100% with visual cues. CAP: 8 Speech intelligibility rating: 5 |
Excellent lasting response | 60 |
| Aschendorff et al, 2004 | 6(6) | 4 patients with bilateral deafness2 patients with unilateral deafness and contralateral residual hearing |
5‐9 years post‐op Results available for 3 cases only Case 1: Freiburger Numbers: 100%, Freiburg monosyllable: 80%, Oldenburg sentence test: 90% Case 2: Freiburger Numbers: 80%, Freiburg monosyllable: 20%, Oldenburg sentence test: 87% Case 3: Freiburger Numbers: 75%, Freiburg monosyllable 25%,, Göttingen sentence test: 39% All language tests were performed in listening mode with CI at 70 dB SPL |
All cases with reported outcomes showed good to excellent response compared to pre‐op, however the authors did not present half of the study populations (n = 3) audiometry | 60‐108 |
| Bacciu et al, 2015 | 12(X) |
All patients exhibited either complete deafness or a bilateral profound SNHL. Mean WRS: 9.7% (range 0‐30%) Mean SRS: 10.9% (range 0‐48%) |
12 months post‐op Mean WRS: 91.4% (range 75‐100%) Mean SRS: 93.1% (range 76‐100%). 5 years post‐op Mean WRS: 94% (range 85‐100%) Mean SRS: 96.3% (range 90‐100%). |
Excellent lasting response that improved from 1‐5 years | 94.7 (64‐158) |
| Bovo et al, 2011 | 3(5) |
Case 1: Profound bilateral deafness permitting only detection of words. Case 2:40% word recognition in closed set word identificationCase 3: Sudden hearing loss in high frequencies, closed set word identification of 50% |
Case 1 ‐ 3 months post‐op: WRS(open set): 80‐90% ‐ 6 months post‐op: able to use the telephone with family members ‐ Electrodes 1‐4 became faulty secondary to increased electrical impedance, and closed set WRS fell to 80%, while the aided threshold corresponded to 30 dB for the frequencies between 0.25 and 4 KHz. Case 2 ‐ 3 months post‐op: open set WRS 90% ‐ 28 months post‐op: no significant variation in electrical impedance of any of the electrode and good functional results unchanged. Case 3 ‐ 3 months post‐op: aided threshold of 30 dB from 0.25 to 4 kHz. ‐ Only reached a closed set word identification performance up until follow up at 42 months |
Good response in Case 1 and 2, however case 3 does not display any benefit, and case 1 may decline in the future due to increasing electrical impedance | 31.3(24‐42) |
| Cacco et al, 2021 | 1(1) |
Right: PTA (Profound SNHL) 80 dB 125 Hz, 90 dB 250 Hz, 95 dB 500 Hz, 95 dB 1 kHz, no response in higher frequencies. Left: PTA: (severe SNHL with hearing remnants) No response except 100 dB at 250 Hz and 120 dB at 500 Hz. ‐ SRT: No response ‐ WRS: was 0% at 100 dB nHL. ‐ ABR: demonstrated a destructured path and absence of recognizable waves. |
18 months post‐op ‐ PTA: 50 dB 125 Hz, 40 dB 250 Hz, 35 dB 500 Hz, 30 dB 1 kHz, 30 dB 2 kHz, 40 dB 4 kHz, 40 dB 8 kHz. ‐ WRS 50% at 60 dB nHL |
Good response | Not stated. ≥18 |
| Canzi et al, 2019 | 1(2) |
Right: Severe hearing loss with PTA in the 0.5‐2 kHz frequency range of 85 dB HL, without effective discrimination at speech audiometry Left: No response |
18 months post‐op Right: PTA of 40 dB HL ‐ SRS in quiet: 80% at 70 dB HL Left: PTA: 60 dB HL Binaural: SRS in quiet: 90% at 70 dB HL |
Good response | 18 |
| Cassis et al, 2018 | 1(2) | Profound bilateral hearing loss with 0% speech discrimination bilaterally |
5 months post‐activation ‐WRS: 76% |
Good response | Not stated. ≥5 |
| Cheng et al, 2010 | 1(1) | PTA: near‐symmetrical, severe to profound bilateral SNHL with no speech perception |
3 months post‐op Speech perception CUNY sentence test: ‐ In quiet (65 dB SPL):99% ‐ In noise: 41% Right PTA: Aided average across four frequencies (0.5, 1, 2 and 4 kHz) was 23.75 dB |
Good response | Not stated. ≥3 |
| Dhanjal et al, 2014 | 1(1) |
Bilateral: Profound bilateral sensorineural hearing loss ‐ ABR: no response ‐ Amplification aids provided no improvement in his symptoms. Right: Three thresholds at 115 dB in the mid frequencies on the right. ‐ CUNY speech perception tests*: 2.8% with sound and lip reading. Left: one recordable threshold at 1 kHz *measured at 70 dB(A) in quiet |
4 months post‐op BKB sentence testing with implant and lip reading: 79% |
Good response | 4 |
| Im et al, 2008 | 1(1) | Total bilateral deafness |
1 year post‐activation Mean open‐set word tests: 91% Mean everyday SRS: 96% |
Excellent response | 12 |
| Kamakura et al, 2017 | 1(2) |
Right: PTA: 90 dB, Severe to profound SNHL ‐ 0% speech discrimination Left: No response |
1 year post‐op Bilateral: Sound awareness threshold: approximately 30 dB. ‐ WRS (CNC list): 56% Left: WRS 50% Right: WRS 60% |
Good response | 24 |
| Kawamura et al, 2010 | 1(1) |
Bilateral: Profound SNHL ‐ Speech audiometry: no response. ‐ Distortion product OAE: no response |
12‐month post‐op Good perception scores: ‐ Monosyllable: 80% ‐ Word: 78% ‐ Sentence: 79% |
Good response | 12 |
| Kontorinis et al, 2010 | 4(6) |
Case 1: Right PTA* 83, AEP 90. Case 2: Right PTA* 100, AEP 100 // Left PTA* 77 AEP 80. Case 3: Left PTA* 93, AEP 90 Case 4: Right PTA* 100 // Left PTA* 100 *Mean hearing threshold of 0.5, 1, 2 and 4 kHz |
Case 1: 12 months speech tracking 86.6, MS 90%, N 100%, HSMs 84.94, HSM (10) 3.66. ‐ 16 years post‐op ST 78.6w/m, MS 90%, N 100%, HSMs100%, HSM(10)39.67 Case 2: 12 months ST 74w/m, MS 85%, N 100%, HSMs 99.06 seconds, HSM (10) 44.33. ‐ 12 years post‐op ST70.6w/m, MS 95%, N 100%, HSMs 100, HSM(10)34.9. Case 3: 12 months ST 30.6w/m, MS 70%, N 100%, HSMs 86.8. ‐ 8 years post‐op ST 42.8w/m, MS 65%, N 100%, HSMs 87.7, HSM (10) 2.8 Case 4:12 months MS 70%, N 100%, HSMs 99.1, HSM (10) 31.13. All cases: ‐ Mean HSMs 12 months post‐op: 95.05% ‐ Mean HSMs final latest follow‐up: 96.7% ‐ Freiburg Monosyllabic Word Test: 100% across all time periods ‐ All patients enjoyed high levels of speech recognition and were able to use the telephone without any difficulties. ‐ Bilateral CI (case 2 and 4), and bimodal CI (case 1) had better scores in noisy surroundings and satisfactory sound orientation. |
Excellent response from all cases | 111(12‐192) |
| Low et al, 2019 | 1(2) | Bilateral profound hearing loss |
<3 months post‐op: Speech test: 90% 3 months post‐op: right ear reduced hearing with otalgia 3 years post op: speech test: 83% (Right), 0% (Left) |
Very good response initially, but declined to just be painful over time. | 36 |
| Low et al, 2000 | 1(1) |
Profound hearing loss AB word list: 0% BKB sentences (closed‐set): 0% |
3 months post‐op ‐ AB word list: 31% ‐ BKB sentences (closed‐set): 72% |
Good response | Not stated. ≥3 |
| Malik et al, 2012 | 26(X) |
CNC‐W: 10 CNC‐P: 20 HINT‐Q: 15 |
6 to 11 months post‐op HINT‐Q (mean ± SD): Primary AIED 14.8 ± 23.4, Secondary AIED 75.7 ± 24.9) CNC‐W(mean ± SD): Primary AIED 9.1 ± 12.1, Secondary 54.4 ± 25.5 CNC‐P(mean ± SD): Primary 19.4 ± 21.0 and Secondary 71.7 ± 17.9. 12 to 17 months post‐op HINT‐Q: Primary AIED scores higher than secondary by average of 15.52, otherwise hearing remained generally stable. |
Good response in Secondary AIED <12 months with minimal to no improvement in Primary AIED; However, good response in Primary AIED >12 months | Not stated. <24 |
| Mowry et al, 2017 | 1(1) |
PTA: No response AzBio: 0% ABR: No response |
6 months post‐activation: AzBio: 21% 1 year post‐activation: AzBio: 40% 18 months post‐activation: AzBio: 35%, Ling sounds: 67% |
Poor to moderate response | 18 |
| Patrizia et al, 2011 | 1(1) | Rapidly progressive bilateral SNHL |
4 years post‐op: 100% bisyllabic word and sentences recognition in quiet and at SNR +10. 13 years post‐op: Words and sentences in quiet 100%, SNR +10 words 70%, sentences 80%. CAP = 6 able to understand conversation without speech reading. |
Excellent lasting response | 156 |
| Psillas et al, 2007 | 1(1) |
PTA: No response BAER: No recordable residual hearing. Audiometric scoring for conversation, word recognition and telephone tracking 0% |
3 months post‐op Conversation 100% WRS 96% Telephone tracking: 98% |
Excellent provisional response | 6 |
| Quaranta et al, 2002 | 5(X) |
Case 1: Anacusis, SDS 0% Case 2: PTA 100 dB SDS 0% Case 3 Anacusis, SDS 0% Case 4 Anacusis SDS 0% Case 5 PTA 500 dB, SDS 10%. |
Case 1: ‐ 2‐syllable word recognition*: 3 months: 45, 1 year: 70, 2 years: 75—Sentences: 3 months: 65, 1 year: 100, 2 years: 70 ‐ Speech tracking: 3 months: 17, 1 year: 46, 2 years: 26. Case 2: ‐ 2‐syllable word recognition: 3 months: 50, 1 year: 70, 2 years: 90 ‐ Sentences: 3 months: 30, 1 year: 100, 2 years: 100 ‐ Speech Tracking: 3 months: 23, 1 year: 50, 2 years: 68 Case 3: ‐ 2 syllable word recognition: 3 months: 60, 1 year: 90, 2 years: 70 ‐ Sentences: 3 months:90, 1 year: 100, 2 years: 90 ‐ Speech tracking: 3 months: 27, 1 year: 45, 2 years: 46 Case 4: ‐ 2 syllable word recognition: 3 months: 90, 1 year: 100 2 years: 80 ‐ Sentences: 3 months: 90, 1 year: 100, 2 years: 95 ‐ Speech tracking: 3 months: 33, 1 year: 36, 2 years: 46 Case 5: ‐ 2 syllable word recognition: 3 months: 60, 1 year: 90, 2 years: 80 ‐ Sentences: 3 months: 70, 1 year: 100, 2 years: 90 ‐ Speech tracking: 3 months: 25, 1 year: 45, 2 years: 47. Average results (3 months;1 year; 2 years): ‐ Open set 2‐syllable word recognition (61;84;79) ‐ Sentence scores (69;100;89) ‐ Speech Tracking (25;44.5;46.6) *Number of words correctly repeated in 1 minute |
Moderate to excellent response that generally improves and plateaus over the 2 years | 24 |
| Salahaldin et al, 2010 | 1(2) | ABR: normal. No clear response to maximum stimulation of 90 dB nHL indicating bilateral profound sensorineural hearing loss at birth |
1 year post‐op Right: ‐ FF testing: 45, 40, 25, 35, 40 dB at 0.25, 0.5, 1, 2, and 4 kHz. ‐ DS score: 50% at 90 dB level 5 years post‐op Left: ‐ FF testing: 10, 15, 15, 20, 25 dB at 0.25, 0.5, 1, 2, and 4 kHz ‐ DS score: 100% at 70 dB level |
Excellent response from left ear, moderate response from right | 60 |
| Santarelli et al, 2006 | 1(1) |
Bilateral: hearing loss that worsened with higher frequencies. Right: 35 dB 125 Hz, 45 dB 250 Hz, 80 dB 500 Hz, 95 dB 1 kHz, 95 dB 2 kHz. Left: 30 dB 125 Hz, 35 dB 250 Hz, 32 dB 500 Hz, 45 dB 1 kHz, 85 dB 2 kHz and no response at higher frequencies. ‐ Disyllabic words 100%. ‐ Trisyllabic words 15% ‐ Sentences 20% ‐ TIPI1 50% ‐ Vowel identification 70%, consonant identification 10%, ABR: Not detectable |
3 months post‐activation ‐ Disyllabic words 75 ± 20% ‐ Trisyllabic words 89 ± 12% ‐ Sentences 96 ± 7% ‐ TIPI1 95% |
Good to very good initial response | Not stated. ≥3 |
| Seo et al, 2012 | 1(1) |
ABR: No response DPOE: No response CAP score: 0 ‐ MS word DS 0% without lip reading ‐ Sentence DS: 17% |
Aided audiogram showed a 40 dB threshold through all frequencies. 4 months post‐op ‐ CAP score: 5 ‐ MS word DS: 90% with lip reading, 40% without it. ‐ Sentence DS: 92% |
Good to very good initial response | 4 |
| Sweetow et al, 2005 | 1 |
Right: profound hearing loss, WRS 0% Left: severe to profound loss, WRS 0% Acoustic reflexes and OAE: Absent. Mum reported decline in expressive speech intelligibility |
4 months post‐op ‐ WIPI: 100% ‐ open set PBK‐50s: 82% at 55 dB HL without visual cues. 14 months post‐op: WRS 92% |
Excellent response | 24 |
| Sydlowski et al, 2014 | 1(2) |
Right: PTA moderate to severe: 55 dB (250 Hz), 60 dB 500 Hz), 65 dB (1 kHz), 55 dB (2 kHz), 60 dB (3 kHz), 70 dB (4 kHz) 85 dB (6 kHz + 8 kHz) Left: PTA No response. |
6 months post‐activation Right: CNC‐P: 93%, CNC‐W: 80%, AzBio (quiet): 99%, AzBio (+10 dB SNR) 80%, BKB‐SIN SNR50: 6.5, SNR loss 9, Degree: moderate Left: CNC‐P: 92%, CNC‐W: 84%, AzBio (quiet): 97%, AzBio (+10 dB SNR) 81%, BKB‐SIN SNR50: 6.5, SNR loss: 9, Degree: moderate Bilateral: CNC‐P: 96%, CNC‐W: 88%, AzBio (quiet): 99%, AzBio (+10 dB SNR) 88%, BKB‐SIN SNR50: 1.5, SNR loss: 4, Degree: mild |
Very good to excellent response | 12 |
| Wang et al, 2010 | 25(27) | Open set sentence score (mean ± SD, %) 7 ± 12.3 |
Open set sentence score (mean ± SD): ‐ 6 months: 92.8 ± 12.1 ‐ 1 year: 97.3 ± 5.3 ‐ >2 years = 96.4 ± 4.9 |
Excellent lasting response | Not stated. ≥24 |
| Watanabe et al, 2018 | 4(4) |
Case 1: ‐ Right: No response. ‐ Left PTA 90 dB (500 Hz) 65 dB (1 kHz), 70 dB (2 kHz), 85 dB(4 kHz). Case 2: Bilateral total deafness Case 3: ‐ Right PTA 50 dB (125 Hz), 60Db (250 Hz), 70 dB (500 Hz), 75 dB(1 kHz), 80 dB(2 kHz), 90 dB (4 kHz), No response (8 kHz). ‐ Left PTA 45 dB (125 Hz), 55Db (250 Hz), 60 dB (500 Hz), 65 dB(1 kHz), 80 dB(2 kHz), 85 dB (4 kHz), 100 dB (8 kHz). Case 4: Bilateral total deafness |
Case 1: ‐ Word recognition: 8% (60% with auditory and visual data) ‐ Sentence recognition: 3% (52% with auditory and visual data) Case 2:18 months post‐op: (poor response) ‐ MS recognition: 18% ‐ Word recognition: 40% ‐ Sentence recognition: 40% Case 3: (good response) ‐ MS recognition: 90% ‐ Word recognition: 100% ‐ Sentence recognition: 100% Case 4: (poor response) ‐ MS recognition: 0% ‐ Word recognition: 0% ‐ Sentence recognition: 0% |
Poor response in Case 1, 2, 4. Good response in Case 3; however poor reporting of follow‐up times, and therefore this may have improved over time, or been as a result of deterioration over time |
Case 1: Unknown Case 2: 18 Case 3: Unknown Case 4: <3 |
Abbreviations: AB, Arthur Boothroyd isophonemic monosyllabic word test; ABR, Auditory Brainstem Response test; AEP, Auditory Evoked Potential; AzBio, Arizona state university sentences; BAER, Brainstem Autiroy Evoked Response; BKB, Bamford‐Kowal‐Bench sentence testing; CAP, Categories of Auditory Performance; CDT, Connected Discourse Tracking; CNC, Consonant Nucleus Consonant scores; CNC‐W, CNC Word; CNC P, CNC Phonemes; CUNY, City University of New York; DPOE, Distortion Product Otoacoustic Emissions; DS, discrimination score; FF, free field testing; HINT‐Q, hearing in noise sentence test presented in quiet; HSM, Hochmair‐Schulz‐Moser sentence test; HSMs, HSM test in quiet; HSM 10, HSM test at 65 dB with 55 dB surrounding noise; MS, Monosyllabic; N, numbers; nHL, Normal Hearing Level; OAE, Otoacoustic emissions; PBK, Phonetically Balanced Kindergarten (word recognition test); PTA, Pure Tone Audiometry; SAT, Speech Awareness Threshold; SDS, Speech Discrimination Score; SIN, Speech In Noise; SNHL, Sensorineural Hearing Loss; SNR, Signal to Noise Ratio; SPL, Sound Pressure Level; SRS, Sentence Recognition Score; SRT, Speech Recognition Threshold; ST, Speech Tracking; TIPI1, Test di Identificazione Parole Infantili 1 (childhood word identification test‐1); VCV, Vowel‐Consonant‐Vowel; WIPI, Word Intelligibility by Picture Identification Test; w/m, words per minute; WRS, Word Recognition Score.
No studies reported any standardized measures of patient reported outcomes. Aftab et al 8 conducted the only study with a randomized control group, and furthermore conducted the only statistical analysis. This revealed no difference in postoperative audiometric outcomes between patients with or without AIED after CI.
3.3. Surgical outcomes
Four patients of 115 were reported to have had immediate complications; Wang 10 mentioned one intraoperative CSF leak (unspecified etiology of AIED as in a mixed group) which was successfully repaired with fascia, and a further patient (case 3, unknown etiology of AIED) that developed minor wound dehiscence that required topical antibiotic cover. Kontorinis 25 similarly reported a case (Cogan's syndrome) with recurrent skin infections that was treated with antibiotics, and Low 31 reported a patient (Cogan's syndrome) with scalp pressure sore from the dressing that healed conservatively. Other reports not within the immediate post‐operative period (>6 months, or time not reported) include: CI failure (n = 2, one of which had Cogan's syndrome, and the other was not specified in a mixed group), 10 , 30 facial tactile sensations (n = 1, Cogan's syndrome), 27 and worsening facial pain with reduced hearing bilaterally (n = 1, Cogan's syndrome). 21 The remainder of the studies did not state any surgical complications, and Bacciu 23 explicitly stated that none of their patients suffered from complications from their flap or systemic disease.
3.4. Inner ear ossification
In Aftab's 12 implanted ears, 6 showed intraluminal fibrosis and neo‐osteogenesis (of mixed aetiology). 8 Bacciu noted that this ossification may not be identified on pre‐operative imaging, with 3 cases having clear imaging but findings of intraoperative osteogenesis 23 (all patients had Cogan's syndrome). Of the 14 studies (43 patients) that mentioned intra‐operative findings, 53.5% (23 patients) were found to have unilateral or bilateral fibrosis or osteogenesis of a section of the cochlea (14 Cogan's syndrome, 1 Vogt‐Koyanagi‐Harada syndrome, 1 neurosarcoidosis, 1 PAN, 6 not specified), 6 , 8 , 9 , 23 , 28 , 30 , 31 , 32 , 33 , 34 , 35 10 of which required a drill out (7 Cogan's syndrome, 1 PAN, 2 unknown). 8 , 9 , 23 , 28 , 31 In 6 patients, electrodes were still unable to be placed within the scala tympani (ST) and therefore the scala vestibuli (SV) was used (4 Cogan's syndrome, 1 Vogt‐Koyanagi‐Harada syndrome, 1 neurosarcoidosis). 6 , 23 , 30 , 32 Despite findings that SV insertion is traumatic to the cochlea and has a higher risk of loss of residual hearing, 18 all studies with implantation into the SV reported good or excellent hearing outcomes post CI, although Aschendorff et al 30 did not fully disclose the data for all of their patients, and so it is not known if the three reported include those with electrodes in the SV.
3.5. Statistical analysis
After discussion with the University Hospital Birmingham's statistician, statistical analysis was not thought to be beneficial or possible given the heterogeneity of the methodology, reporting outcomes, and results (some studies pooling averages as opposed to giving individual scores).
4. DISCUSSION
Of the 115 patients, 114 showed improvement in hearing which was demonstrated across a variety of audiometric outcomes (see Table 3) compared to baseline after cochlear implantation. Poor outcomes were noted in only 4 cases who also happened to have secondary AIED (3 ANCA‐associated vasculitis, 1 Cogan's syndrome) 26 , 36 ; however, it may be relevant to note that 3 of these had chronic otitis media which can cause difficulties in cochlear implantation. 37 Additionally, the hearing assessments conducted in these cases were in the early post‐implant period (1 case less than 3 months) or not mentioned (2 cases). Despite the heterogeneity of the studies, the primary outcome of this systematic review was achieved and revealed that post‐CI outcomes in AIED are largely positive.
4.1. Clinical and research findings
Interestingly, although it is commonly quoted that up to 30% of patients have secondary AIED, 2 , 3 , 38 our study found the converse, with only 33% of patients having primary AIED, with the remaining majority having secondary causes. This difference may be due to a number of reasons. Firstly, the data from these older studies may be outdated. Alternatively, secondary AIED might progress more often to needing a CI, so that we are selecting a more severe subset of the total sample.
Currently there are different schools of thought surrounding optimum time for cochlear implantation in AIED; for example, Cacco and Aftab conclude that earlier cochlear implantation can be beneficial to reduce the morbidities of long‐term immunosuppressant in attempts to preserve hearings. 8 , 39 In reality, the optimum time will likely differ on a case‐by‐case basis. We found a range of 1 month to 10 years from deafness to cochlear implantation, although the majority seemed to take place within 2 years. Time to implantation did not seem to worsen post‐operative outcomes. Malik et al found a difference between subgroups, with some subtypes of secondary AIED (namely Cogan's syndrome and relapsing polychondritis) progressing to deafness quicker than primary AIED (P < .001), but interestingly other causes of secondary AIED had a slower decline when compared to primary AIED. 11 This may affect the clinician's decision‐making surrounding the optimum time frame in preoperative counseling of patients with different types of AIED. We have not been able to carry out subgroup analyses in our study to support or challenge this claim as some of the studies had mixed primary and secondary AIED populations, but reported their information as a pooled average of both groups.
Intra‐operatively, a variety of CIs were used. Of the studies that reported electrode insertions, all were fully inserted except for four years in whom partial insertion was achieved (1 PAN, 1 Primary AIED, 1 Relapsing Polychondritis, 1 unspecified). 9 , 10 , 40 , 41 Although it is thought that full insertion of electrodes show better hearing outcomes post‐operatively, 8 , 11 overall all patients receiving partial insertion in this group still received significant improvement in hearing, with improvement of hearing thresholds from a severe or profound level to a mild‐moderate hearing loss on aided audiometry. Salahaldin 41 noted an excellent response post‐operatively from the partially inserted left ear (speech discrimination score [SDS] of 100% at 70 dB at 5 years), which superseded the fully inserted right ear (SDS of just 50% at 90 dB at 1 year).
Theoretically, osteoneogenesis inside the cochlea could lead to an increase in electrical impedance over time, resulting in reduced CI efficiency and function. However, of the 85 patients (10 studies) in this review that were reported with consecutive audiometric data post‐operatively (or compared short term with long term follow‐up data), 30% 8 , 23 , 25 , 35 (26 patients) showed improvement in CI outcomes over a few years, 33% 11 , 27 , 42 (28 patients) reported patients with a “generally stable” hearing level over time, 35% 10 , 35 , 36 (30 patients) reported initial improvement up to 1 year and then plateauing or mild worsening of hearing thereafter, and 1.2% (1 patient) showed good initial response but complete deterioration due to pain after 18 months. 21 In one study, 11 a further sub‐group analysis suggested that cochlear implantation may initially show poor results in primary AIED, but then improve after 12 months; however this studies length of follow‐up (<2 years) may not be sufficient as symptomatic osteoneogenesis may be a lengthier process. That said, it is encouraging to note maintained hearing even up to 16 years post‐implant. 25
In general, perioperative complications were rare, with only 3.5% (n = 4) of cases being reported within 6 months. Considering the fact that the vast majority of patients took systemic steroids or immunosuppresants (Table 4), it is reassuring that this percentage for wound complication in AIED is not higher than that seen in overall CI cases (1‐8%). 43 Longer‐term complications did develop as mentioned in the results section. Patients should therefore be counseled that in rare occasions, facial pain or device failure may develop, and that residual hearing may be lost should insertion into the scala vestibuli be required.
TABLE 4.
Patient characteristics and operative details
| Authors | Sex | Average age at implantation (range) | Duration to implantation (range) | Medical treatment | Full or partial insertion | Implant type |
|---|---|---|---|---|---|---|
| Abou‐Elhmd et al, 1996 | 1 male | 71 | Over 26 months | Prednisolone, cyclophosphamide | Not stated | Digisonic 15 |
| Aftab et al, 2010 |
4 males 6 females |
49.6(31‐77) | 14(1‐96) months |
Steroids: All except 2 AIED patients (range: 9 days to 10 years). MTX + steroids: 3 patients |
Full |
Nucleus 24 system: 9 patients Med‐El Combi 40+: 1 patient |
| AlHelali et al, 2019 | 1 female | 30 | Over 2 years | Prednisolone, atropine eye drops, mycophenolate mofetil | Full | MED‐EL CONCERTO |
| Aschendorff et al, 2004 | 6 females | 31.5 | 4.2(0.1‐11) years | Not stated | Full |
Nucleus CI22M: 1 (+1 re‐implant due to CI22 failure), Nucleus CI22: 2 Nucleus CI24RCS: 3 |
| Bacciu et al, 2014 |
4 males 8 females |
34.1(16‐52) | 19 (6‐48) months | All but one had preoperative steroid and immunosuppressive therapy. | Full |
Nucleus 24M device: 4 Nucleus 22M device: 1 Nucleus Contour model: 2 MXM Digisonic device: 5 |
| Bovo et al, 2011 | 3 females | 32.3(18‐48) | Not stated |
Case 1: Not stated Case 2: Steroid, cyclophosphamides, MTX Case 3: “Prompt immunosuppresion” |
Not stated |
Case 1: Cochlear Nucleus 24 Case 2: MED‐EL Sonata TI100 Case 3: Cochlear Nucleus 24k |
| Cacco et al, 2021 | 1 female | 35 | 2 months | Corticosteroids and MTX | Not stated | HiFocus Advantage |
| Canzi et al, 2019 | 1 female | 53 | 1.5 months | Prednisolone, MTX | Partial | Digisonic SP |
| Cassis et al, 2018 | 1 female | 24 | 7 weeks | High dose steroid, MTX | Full | HiRes ultra device with mid‐scala electrode |
| Cheng et al, 2010 | 1 female | 63 | Not stated |
Oral prednisolone, pulsed MP, mycophenolate, IT dexamethasone into right ear. Trial of cyclosporin |
Not stated | Nucleus CI‐24RE(ST) implant |
| Dhanjal et al, 2014 | 1 male | 40 | 4 years | Prednisolone | Full | Nucleus CI422 electrode |
| Im et al, 2008 | 1 female | 25 | 7 months | Oral steroids, MTX | Full | Combi 40 device |
| Kamakura et al, 2017 | 1 male | 63 | Around 3 years | Oral steroids | Full | HiRes 90K receiver stimulator with HiFocus Helix electrodes (perimodiolar) |
| Kawamura et al, 2010 | 1 female | 57 | Around 3 years | Corticosteroids, MTX | Full | Nucleus CI24R device |
| Kontorinis et al, 2010 | 4 females | 24.4(9.7‐35.8) | 46.3(11‐93) months | Case 4: Systemic corticosteroids and MTX | Not stated | Not stated |
| Low et al, 2019 | 1 female | 23 | 4 months | Oral & IT steroids, hyperbaric oxygen, cyclophosphamide | Not stated | HiRes 90K HiFocus Mid‐Scala |
| Low et al, 2000 | 1 male | 35 | 10 years | Oral steroids | Full | Nucleus 22 |
| Malik et al, 2012 |
13 males 13 females |
54.53 (24‐84) | 12.4(1‐53.73) months |
Oral steroids: 7 Oral and IT steroids: 8 Immunosuppressants, for example, MTX, cyclophosphamide or mycophenolate mofetil: 9 |
Full except 2 | Not stated |
| Mowry et al, 2017 | 1 female | 49 | 15 months | Steroids, IVIg, plasmapheresis | Not stated | Nucleus 24 RE with Contour Advance electrode |
| Patrizia et al, 2011 | 1 female | 29 | 12 months | Steroids and AZA (initially diagnosed as having Cogan's) | not stated | Clarion 1.2 |
| Psillas et al, 2007 | 1 male | 71 | 57 months | Corticotherapy | Full | Nucleus 3G |
| Quaranta et al, 2002 |
3 males 2 females |
33.6(22) | 13(6–24) months | Prednisolone in one case, and prednisolone with cyclosporin in 2 cases | Not stated |
Cases 1, 2, 4 and 5: Nucleus 24 Case 3: Nucleus 22 |
| Salahaldin et al, 2010 | 1 male | 2 months | 10 years | Prednisolone, MTX |
Partial (left) Full (right) |
MedEL C40+ device (left) MedEl pulser (right) |
| Santarelli et al, 2006 | 1 female | 18 | 4 years | Not stated | Not stated | Nucleus Esprit 3G |
| Seo et al, 2012 | 1 male | 34 | 4.5 years | Prednisolone, MTX, plasmapheresis, | Partial | Clarion HiRes90k |
| Sweetow et al, 2005 | 1 female | 4 | 6 months | Prednisolone | Not stated | Nucleus 24C |
| Sydlowski et al, 2014 | 1 female | 26 | 6 months | Oral prednisolone, IT steroids | Not stated | Nucleus Freedom Contour Advance CI24RE(CA) |
| Wang et al, 2010 |
7 males 18 females |
45.8(23‐73) | Not stated | Corticosteroids in some | 24 patients full, 1 partial |
Clarion C90K: 7 Nucleus 22M: 6 Med‐El Pulsar: 2 Nucleus Contour: 2 Clarion 1.2 enhanced bipolar: 2 Clarion 1.2 standard: 2 Clarion HiFocus: 1 Clarion II: 1 Nucleus 24M: 1 Nucleus Freedom: 1 |
| Watanabe et al, 2018 | 4 females |
Case 1: 71 Case 2: 35 Case 3: 49 Case 4: 67 |
Case 1: 22 Case 2: 4 Case 3: 89 Case 4: 8 (months) |
Case 1: AZA, prednisolone Case 2: MP, Tacrolimus Case 3: Cyclophosphamide, prednisolone Case 4: Steroid, MTX |
Not stated throughout | Not stated |
Abbreviations: AZA, Azathioprine; IT, Intratympanic; IVIg, Intravenous Immunoglobulin; MP, Methylprednisolone; MTX, Methotrexate; ST, Scala Tympani; SV, Scala Vestibuli.
4.2. Limitations of this study
There are several limitations to this systematic review. Firstly, we report pooled results from a range of single case or small sized studies. This is compounded by the heterogeneity between and within studies for follow‐up duration (range 0‐180 months), type of audiological outcome (Table 5), reporting of intra‐operative technique and findings, and post‐operative complications and treatment response. As highlighted in Gaylor's meta‐analysis of CIs in 2013, 44 longer follow‐up durations are essential for properly assessing hearing outcomes. This heterogeneity therefore precluded subgroup comparisons such as hearing outcomes in bilateral CIs vs unilateral CI, or primary vs secondary AIED. Furthermore, given the relatively small sample size (115 patients), our findings may not accurately reflect true values for AIED. For example, only one study explicitly reported a considerable improvement in quality of life after CI, 45 however given the vast majority of patients obtaining improvement in hearing post‐operatively, the true impact to quality of life is likely to be much greater.
TABLE 5.
Reported outcomes per study
| Reported outcomes | Study |
|---|---|
| Arthur Boothroyd isophonemic monosyllabic word test (AB) | Low (2000) |
| Arizona State University sentences (AzBio) | Sydlowski (2014), Mowry (2017) |
| Bamford‐Kowal‐Bench sentence testing (BKB) | Abou‐Elhmd (1996), Dhanjal (2014), Low (2000), Sydlowski (2014) |
| Categories of Auditory Performance (CAP) | AlHelali (2019), Patrizia (2011), Seo (2012) |
| Connected Discourse Tracking (CDT) | Abou‐Elhmd (1996) |
| Consonant Nucleus Consonant scores (CNC) | Malik (2012), Sydlowski (2014), Kamakura (2017) |
| City University of New York sentence tests (CUNY) | Cheng (2010) |
| Discrimination tests (discrimination scores, word discrimination and speech discrimination) | AlHelali (2019), Seo (2012), Salahaldin (2010) |
| Free Field testing (FF) | Salahaldin (2010) |
| Hearing in noise sentence test presented in quiet (HINT‐Q) | Malik (2012) |
| Hochmair‐Schulz‐Moser sentence test (HSM, including HSMs, HSM 10) | Kontorinis (2010) |
| Phonetically Balanced Kindergarten (PBK, word recognition test) | Sweetow (2005) |
| Pure Tone Audiogram (PTA) | Abou‐Elhmd (1996), Canzi (2019), Cacco (2021), Cheng (2010) |
| Speech In Noise (SIN) | Sydlowski (2014) |
| Sentence Recognition Score (SRS) | Canzi (2019), Bacciu (2015), Im (2008) |
| Speech intelligibility | AlHelali (2019) |
| Speech Tracking (ST) | Kontorinis (2010), Quaranta (2002) |
| Test di Identificazione Parole Infantili 1 (childhood word identification test‐1, TIPI1) | Santarelli (2006) |
| Vowel‐Consonant‐Vowel identification (VCV) | Abou‐Elhmd (1996) |
| Word Intelligibility by Picture Identification Test (WIPI) | Sweetow (2005) |
| Word Recognition Score (WRS) | Bovo (2011), Sweetow (2005), Cacco (2021), Kamakura (2017), Bacciu (2015), Cassis (2018), Psillas (2007) |
Further research is required into the long‐term effects of CI in AIED patients, and particularly among the different etiologies. Future publications should be mindful in reporting data as individual patient level where possible as opposed to averages to allow for subgroup analyses, and should consider extended follow‐up durations to monitor for deterioration in hearing and to widen our understanding of long‐term prognosis. Although difficult to organize, internationally, pre‐ and post‐audiometric outcomes should be standardized at least within single centers to reduce heterogeneity between studies, and therefore improve our understanding of CI efficacy over time.
5. CONCLUSION
Cochlear implantation in autoimmune inner ear disease provides marked improvement in hearing for the majority of patients, which is maintained long term. Benefit is reported in both primary and secondary AIED, however the latter subgroup may be at a higher risk of poor response. Surgically, despite patients often taking concurrent steroids and the potential presence of cochlea ossification, complication rates are comparable to implantation in non‐autoimmune hearing loss patients, and appear to be stable. Early CI may therefore be a valid management option in AIED, as it can provide excellent long lasting hearing to patients.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
The authors thank Jennifer Manders, the librarian at University Hospitals Birmingham UK, for her assistance in optimizing the search strategy for this systematic review, and Peter Nightingale, statistician at University Hospitals Birmingham UK, for his advice and expertise in meta‐analysis.
Appendix A. Search strategy used for MEDLINE/pubmed and EMBASE. The same search terms were used for other databases
1 “Cochlear implant*”.mp.
2 Cochlear Implantation/ or Cochlear Implants/
3 1 or 2
4 exp Vasculitis/
5 Vasculitis.mp.
6 “Giant cell arteritis”.mp.
7 “temporal arteritis”.mp.
8 “Wegener's granulomatosis”.mp.
9 “Granulomatosis with polyangiitis”.mp.
10 “Henoch‐Schönlein purpura”.mp.
11 “Kawasaki disease”.mp.
12 “Microscopic polyangiitis”.mp.
13 “Polyarteritis nodosa”.mp.
14 “Polymyalgia rheumatic”.mp.
15 “Takayasu arteritis”.mp.
16 “Behçet's disease”.mp.
17 “Buerger's disease”.mp.
18 “Cogan's syndrome”.mp.
19 (“Primary angiitis” adj3 “central nervous system”).mp.
20 Autoimmune.mp.
21 “Addison's disease”.mp.
22 (“Immune‐mediated” or “Immune mediated”).mp.
23 “Rheumatoid arthritis”.mp.
24 “Psoria* arthritis”.mp.
25 IMIED.mp.
26 “Coeliac disease”.mp.
27 “Inflammatory bowel disease”.mp.
28 “Graves' disease”.mp.
29 “Pernicious an?emia”.mp.
30 exp Autoimmune Diseases/
31 Immune.mp.
32 “Vogt‐Koyanagi‐Harada syndrome”.mp.
33 Sarcoidosis.mp.
34 “Relapsing polychondritis”.mp.
35 Thyroiditis.mp.
36 Connective Tissue Disease.mp.
37 Sjogren*.mp.
38 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37
36 3 and 38
Lee J, Biggs K, Muzaffar J, Bance M, Monksfield P. Hearing loss in inner ear and systemic autoimmune disease: A systematic review of post‐cochlear implantation outcomes. Laryngoscope Investigative Otolaryngology. 2021;6:469–487. 10.1002/lio2.563
BIBLIOGRAPHY
- 1. Penêda JF, Lima NB, Monteiro F, Silva JV, Gama R, Condé A. Immune‐mediated inner ear disease: diagnostic and therapeutic approaches. Acta Otorrinolaringol Esp. 2019;70(2):97‐104. 10.1016/j.otorri.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 2. Mijovic T, Zeitouni A, Colmegna I. Autoimmune sensorineural hearing loss: the otology‐rheumatology interface. Rheumatology (Oxford). 2013;52(5):780‐789. 10.1093/rheumatology/ket009. [DOI] [PubMed] [Google Scholar]
- 3. Bovo R, Aimoni C, Martini A. Immune‐mediated inner ear disease. Acta Otolaryngol. 2006;126(10):1012‐1021. 10.1080/00016480600606723. [DOI] [PubMed] [Google Scholar]
- 4. Ciorba A, Corazzi V, Bianchini C, et al. Autoimmune inner ear disease (AIED): a diagnostic challenge. Int J Immunopathol Pharmacol. 2018;32:205873841880868. 10.1177/2058738418808680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cogan DG. Syndrome of nonsyphilitic interstitial keratitis and vestibuloauditory symptoms. Arch Ophthalmol. 1945;33(2):144‐149. 10.1001/archopht.1945.00890140064007. [DOI] [PubMed] [Google Scholar]
- 6. AlHelali N, Hajr E, Almuhawas F, Hagr A. Bilateral Cochlear implantation in Vogt‐Koyanagi‐Harada syndrome: a case report. Otol Neurotol. 2019;40(7):e694‐e697. 10.1097/MAO.0000000000002262. [DOI] [PubMed] [Google Scholar]
- 7. Abou‐Elhmd KA, Hawthorne MR, Flood LM. Cochlear implantation in a case of Wegener's granulomatosis. J Laryngol Otol. 1996;110(10):958‐961. 10.1017/s0022215100135455. [DOI] [PubMed] [Google Scholar]
- 8. Aftab S, Semaan MT, Murray GS, Megerian CA. Cochlear implantation outcomes in patients with autoimmune and immune‐mediated inner ear disease. Otol Neurotol. 2010;31(8):1337‐1342. 10.1097/MAO.0b013e3181f0c699. [DOI] [PubMed] [Google Scholar]
- 9. Canzi P, Aprile F, Manfrin M, et al. “Emergency” Cochlear implantation in labyrinthitis ossificans secondary to polyarteritis nodosa: how to face a rare entity. J Int Adv Otol. 2019;15(1):156‐159. 10.5152/iao.2018.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang JR, Yuen HW, Shipp DB, et al. Cochlear implantation in patients with autoimmune inner ear disease including Cogan syndrome: a comparison with age‐ and sex‐matched controls. Laryngoscope. 2010;120(12):2478‐2483. 10.1002/lary.21060. [DOI] [PubMed] [Google Scholar]
- 11. Malik MU, Pandian V, Masood H, et al. Spectrum of immune‐mediated inner ear disease and cochlear implant results. Laryngoscope. 2012;122(11):2557‐2562. 10.1002/lary.23604. [DOI] [PubMed] [Google Scholar]
- 12. Vambutas A, Pathak S. AAO: autoimmune and autoinflammatory (disease) in otology: what is new in immune‐mediated hearing loss. Laryngosc Investig Otolaryngol. 2016;1(5):110‐115. 10.1002/lio2.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCabe BF. Autoimmune sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1979;88(5):585‐589. 10.1177/000348947908800501. [DOI] [PubMed] [Google Scholar]
- 14. Goodall AF, Siddiq MA. Current understanding of the pathogenesis of autoimmune inner ear disease: a review. Clin Otolaryngol. 2015;40(5):412‐419. 10.1111/coa.12432. [DOI] [PubMed] [Google Scholar]
- 15. Ikezono T, Tomiyama S, Pawankar R, Jinnouchi K, Suzuki Y, Yagi T. Passive transfer of experimental autoimmune labyrinthitis. Audiol Neurotol. 2000;5(5):292‐299. 10.1159/000013893. [DOI] [PubMed] [Google Scholar]
- 16. Breslin NK, Varadarajan VV, Sobel ES, Haberman RS. Autoimmune inner ear disease: a systematic review of management. Laryngosc Investig Otolaryngol. 2020;5(6):1217‐1226. 10.1002/lio2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Connell BP, Hunter JB, Wanna GB. The importance of electrode location in cochlear implantation. Laryngosc Investig Otolaryngol. 2016;1(6):169‐174. 10.1002/lio2.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adunka O, Kiefer J, Unkelbach MH, Radeloff A, Gstoettner W. Evaluating cochlear implant trauma to the scala vestibuli: scala vestibuli cochlear implantations. Clin Otolaryngol. 2005;30(2):121‐127. 10.1111/j.1365-2273.2004.00935.x. [DOI] [PubMed] [Google Scholar]
- 19. Brazzelli M, Cruickshank M, Tassie E, et al. Collagenase clostridium histolyticum for the treatment of Dupuytren's contracture: systematic review and economic evaluation. Health Technol Assess. 2015;19(90):1‐202. 10.3310/hta19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. OCEBM Levels of Evidence Working Group . Howick J, Chalmers I, Glasziou P, et al. Levels of evidence—Centre for Evidence‐Based Medicine (CEBM). University of Oxford: University of Oxford: 2011. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence. [Accessed January 23, 2021]. [Google Scholar]
- 21. Low WK, See JY, Ng WN, Fong A, Chew SH. An unusual case of post‐cochlear implant performance degradation in a patient with suspected Cogan's syndrome. Cochlear Implants Int. 2019;20(2):94‐99. 10.1080/14670100.2018.1548077. [DOI] [PubMed] [Google Scholar]
- 22. Bovo R, Ciorba A, Martini A. The diagnosis of autoimmune inner ear disease: evidence and critical pitfalls. Eur Arch Otorhinolaryngol. 2009;266(1):37‐40. 10.1007/s00405-008-0801-y. [DOI] [PubMed] [Google Scholar]
- 23. Bacciu A, Pasanisi E, Di Lella F, Guida M, Bacciu S, Vincenti V. Cochlear implantation in patients with Cogan syndrome: long‐term results. Eur Arch Otorhinolaryngol. 2015;272(11):3201‐3207. 10.1007/s00405-014-3376-9. [DOI] [PubMed] [Google Scholar]
- 24. Santarelli R, Scimemi P, Dal Monte E, Genovese E, Arslan E. Auditory neuropathy in systemic sclerosis: a speech perception and evoked potential study before and after cochlear implantation. Eur Arch Otorhinolaryngol. 2006;263(9):809‐815. 10.1007/s00405-006-0075-1. [DOI] [PubMed] [Google Scholar]
- 25. Kontorinis G, Giourgas A, Neuburger J, Lesinski‐Schiedat A, Lenarz T. Long‐term evaluation of Cochlear implantation in Cogan syndrome. ORL J Otorhinolaryngol Relat Spec. 2010;72(5):275‐279. 10.1159/000318870. [DOI] [PubMed] [Google Scholar]
- 26. Watanabe T, Yoshida H, Kishibe K, et al. Cochlear implantation in patients with bilateral deafness caused by otitis media with ANCA‐associated vasculitis (OMAAV): a report of four cases. Auris Nasus Larynx. 2018;45(5):922‐928. 10.1016/j.anl.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 27. Bovo R, Ciorba A, Trevisi P, et al. Cochlear implant in Cogan syndrome. Acta Otolaryngol. 2011;131(5):494‐497. 10.3109/00016489.2010.535214. [DOI] [PubMed] [Google Scholar]
- 28. Kawamura S, Sakamoto T, Kashio A, et al. Cochlear implantation in a patient with atypical Cogan's syndrome complicated with hypertrophic cranial pachymeningitis. Auris Nasus Larynx. 2010;37(6):737‐741. 10.1016/j.anl.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 29. Sweetow RW, Rosbe KW, Philliposian C, Miller MT. Considerations for cochlear implantation of children with sudden, fluctuating hearing loss. J Am Acad Audiol. 2005;16(10):770‐780. 10.3766/jaaa.16.10.2. [DOI] [PubMed] [Google Scholar]
- 30. Aschendorff A, Lohnstein P, Schipper J, Klenzner T. Obliterated cochlea in Cogan's syndrome – implications for cochlear implant surgery. Laryngo‐ Rhino‐ Otol. 2004;83(12):836‐839. 10.1055/s-2004-826004. [DOI] [PubMed] [Google Scholar]
- 31. Low WK, Burgess R, Teoh CK. Cochlear implantation in a patient with Cogan's syndrome, chronic ear disease and on steroid therapy. Adv Otorhinolaryngol. 2000;57:157‐159. [DOI] [PubMed] [Google Scholar]
- 32. Dhanjal H, Rainsbury J, Irving RM. Bilateral sensorineural hearing loss and labyrinthitis ossificans secondary to neurosarcoidosis. Cochlear Implants Int. 2014;15(6):337‐340. 10.1179/1754762814Y.0000000073. [DOI] [PubMed] [Google Scholar]
- 33. Kamakura T, Lee DJ, Herrmann BS, Nadol JB. Histopathology of the human inner ear in the Cogan syndrome with Cochlear implantation. Audiol Neurootol. 2017;22(2):116‐123. 10.1159/000477534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cassis AM. Cochlear ossification in a patient with Cogan's syndrome undergoing bilateral Cochlear implantation. Case Rep Otolaryngol. 2018;2018:7395460‐7395462. 10.1155/2018/7395460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Quaranta N, Bartoli R, Giagnotti F, Di Cuonzo F, Quaranta A. Cochlear implants in systemic autoimmune vasculitis syndromes. Acta Otolaryngol Suppl. 2002;548:44‐48. 10.1080/00016480260094974. [DOI] [PubMed] [Google Scholar]
- 36. Mowry SE, King S. Cochlear implantation in chronic demyelinating inflammatory polyneuropathy. Cochlear Implants Int. 2017;18(2):116‐120. 10.1080/14670100.2016.1264115. [DOI] [PubMed] [Google Scholar]
- 37. Vincenti V, Pasanisi E, Bacciu A, Bacciu S, Zini C. Cochlear implantation in chronic otitis media and previous middle ear surgery: 20 years of experience. Acta Otorhinolaryngol Ital. 2014;34(4):272‐277. [PMC free article] [PubMed] [Google Scholar]
- 38. Hughes GB, Kinney SE, Barna BP, Calabrese LH. Practical versus theoretical management of autoimmune inner ear disease. Laryngoscope. 1984;94(6):758‐767. 10.1288/00005537-198406000-00006. [DOI] [PubMed] [Google Scholar]
- 39. Cacco T, Castello E, Canevari FRM, et al. Cochlear implantation as a treatment for sudden autoimmune sensorineural hearing loss in a patient affected by eosinophilic granulomatosis with polyangiitis: a case report and a review of literature. Ann Otol Rhinol Laryngol. 2021;130(1):112‐115. 10.1177/0003489420938827. [DOI] [PubMed] [Google Scholar]
- 40. Seo YJ, Choi JY, Kim SH, Kim T‐J. Cochlear implantation in a bilateral sensorineural hearing loss patient with relapsing polychondritis. Rheumatol Int. 2012;32(2):479‐482. 10.1007/s00296-009-1259-y. [DOI] [PubMed] [Google Scholar]
- 41. Salahaldin AH, Hadi KA, Fakhro R, Bener A. Post binaural cochlear implant fluctuating hearing loss – a case study. Curr Paediatr Res. 2010;14(1):33‐38. [Google Scholar]
- 42. Patrizia M, Giuseppe A, Marika V, Roberto F. Deterioration of hearing in a cochlear implantee with relapsing polychondritis. Acta Otolaryngol. 2011;131(6):675‐678. 10.3109/00016489.2010.545077. [DOI] [PubMed] [Google Scholar]
- 43. Vijendren A, Borsetto D, Barker EJ, et al. A systematic review on prevention and management of wound infections from cochlear implantation. Clin Otolaryngol. 2019;44(6):1059‐1070. 10.1111/coa.13444. [DOI] [PubMed] [Google Scholar]
- 44. Gaylor JM, Raman G, Chung M, et al. Cochlear implantation in adults: a systematic review and meta‐analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(3):265‐272. 10.1001/jamaoto.2013.1744. [DOI] [PubMed] [Google Scholar]
- 45. Cheng S, da Cruz M. Sweet's disease and profound, bilateral, sensorineural hearing loss. J Laryngol Otol. 2010;124(1):105‐107. 10.1017/S002221510999137X. [DOI] [PubMed] [Google Scholar]


