Abstract
The criteria and candidacy for pediatric cochlear implantation (CI) has significantly transformed over the past few decades and continues to evolve with technological advancements, and recognition of benefit in more diverse populations. Prolonged auditory deprivation among patients with profound sensorineural hearing loss has been shown to cause widespread degeneration in the central auditory system. Thus, there is increasing evidence advocating for earlier implantation within a critical neuroplastic window. However, there is a lack of consensus on this optimal age of implantation. Historically, there were concerns regarding surgical feasibility and safety, anesthesia risk, and logistical considerations in very young infants <12 months. Recent literature has investigated surgical safety and anesthesia risk as well speech and language outcomes with early implantation, resulting in the long‐awaited reduction in approved age by the FDA (<9 months for certain devices). This article reviews logistical considerations, surgical safety, anesthesia risk, and language developmental outcomes associated with early CI (<12 months).
Keywords: anesthesia risk, infant, language and speech development, pediatric cochlear implantation, surgical safety
1. INTRODUCTION
Cochlear implantation is a well‐established and effective treatment for children with sensorineural hearing loss (SNHL). The implanted internal device bypasses the organ of Corti in the cochlea and electrically stimulates auditory neurons through an array placed in the scala tympani. Since approval of the original single channel cochlear implant (CI) by the United States Food and Drug Administration (FDA), the device has rapidly evolved into several commercially available multichannel systems. 1 The FDA first approved the multichannel CI for the pediatric population in 1990. Alongside advancements in engineering and speech processing technology, the audiologic criteria expanded; the age limit was reduced to 12 months in 2000, and most recently to 9 months of age in 2020 for certain devices, for children with bilateral profound sensorineural hearing loss. The evolution of pediatric CI candidacy was primarily driven by the increasing evidence demonstrating that prolonged auditory deprivation may permanently affect the development of higher auditory centers in the central nervous system as well as communication skills. 2 This article will review evidence regarding earlier implantation in children as well as safety and logistical considerations of implanting the very young (<12 months) pediatric population.
2. THE ROLE OF NEUROPLASTICITY
Neuroplasticity, the ability of neurons or neural networks to change or reorganize, underpins the success of early implantation. Although age of implantation was long suspected to serve as a predictor for pediatric CI outcomes, Nikolopoulos and colleagues were among the first to provide robust evidence that prelingually deaf children should receive a CI as early as possible to facilitate the development of speech perception and speech intelligibility. 3 The authors conducted a prospective study demonstrating that increasing age of implantation negatively correlated with speech outcome measures. Several studies have supported the hypothesis that there is a critical or sensitive period during which the auditory system is most responsive to stimulation. 4 , 5 , 6 It is well accepted that a time‐dependent series of functions occurs in the developing auditory system based on responsive adaptations to external stimuli. 7 , 8 Maturation of the central auditory pathways is thus dependent on auditory input, and auditory deprivation has been shown to cause widespread degeneration in the central auditory system. 9 , 10 , 11 , 12 Auditory deprivation can also affect the ability to process information beyond the auditory system including central neurological and higher order neurocognitive functioning. 13 Neural prostheses such as CI have the potential to reduce the effects of sensory loss and help restore components of sensory functioning. 13 Central neuronal degeneration would presumably affect the efficacy of a CI, which depends on electrical stimulation of peripheral cochlear nerve endings; the ideal time to implant a deaf child would thus be prior to the effects of auditory deprivation on the developing central nervous system. 14 , 15
In 2002, Sharma et al investigated the consequences of implantation at different ages on the maturation of the central auditory system using the latency of the P1 cortical auditory evoked potential (CAEP). 16 The P1 latency was compared between implanted children and age‐matched normal hearing peers. The study revealed that implanted children with the longest period of auditory deprivation (≥7 years) had abnormal cortical response latencies to speech. However, children with shorter duration of auditory deprivation (< 3.5 years) demonstrated age‐appropriate latency responses within 6 months after device activation and onset of electrical stimulation. The authors concluded that in the absence of normal stimulation, there is a critical period of approximately 3.5 years during which the human central auditory system remains maximally plastic. After age 7, however, neuroplasticity appears to be greatly reduced. 16 In 2015, Sharma et al demonstrated a similar critical period with regards to the higher‐order auditory cortices as measured by the N1 CAEP, which is more dominant in older hearing children and adults. 8 The age of implantation as a cut‐off for the development of the N1 component was approximately 7 years, which further validates previous research using the P1 CAEP. The absence of the N1 component in the majority of late implanted was markedly different compared to normal hearing and early implanted children indicating abnormal higher‐level auditory cortical development. Thus, the N1 CAEP may be a valuable marker to examine long‐term development of central auditory pathways in pediatric cochlear implant recipients. 8
Sharma et al also discussed the phenomenon of cross modal plasticity, which occurs through visual and somatosensory cortical re‐organization. 8 Cross‐modal plasticity refers to recruitment of cortical resources from other intact sensory systems by the deprived modality (eg, auditory signal); this has been demonstrated in brain imaging studies and may influence speech perception performance in children with cochlear implants. 8 Cross‐modal plasticity has been well described in the context of pediatric bilateral SNHL as well as single‐sided deafness. 17 Like the N1 CAEP, changes in cortical plasticity may play a role in prediction of clinical outcomes following cochlear implantation. Despite the abundant evidence supporting earlier implantation before the critical neuroplastic period closes, studies remain unable to determine the optimum lowest age of implantation. The inner ear is fully formed by 24 weeks, and the normal 26‐week‐old fetus has the capability to perceive sound 18 ; thus, cochleae are of sufficient size to accommodate a full length electrode array right at birth.
3. ACHIEVING EARLY IMPLANTATION
Widespread adoption of universal newborn hearing screening (UNHS) starting in the 1990s has allowed for earlier identification of hearing loss in infants, enabling the possibility of earlier intervention. This successfully lowered the average age of diagnosed hearing loss from 24‐30 months to 2‐3 months. 19 The Early Hearing Detection and Intervention (EHDI) goals of “1‐3‐6” guiding newborn hearing screening (completed by 1 month of age), diagnostic audiology and audiologic interventions (by 3 months), and enrollment in early intervention services (by 6 months) is described in the most recent 2019 Position Statement of the Joint Committee on Infant Hearing (JCIH). 20 While traditional EHDI goals have been “1‐3‐6,” achieving these benchmarks even earlier (such as “1‐2‐3”) is ideal. A functional EHDI framework that acknowledges the urgency in the management of pediatric hearing loss is critical to setting the timeline for all early hearing interventions, including early cochlear implantation. For children with bilateral profound SNHL, CI evaluation must occur immediately after hearing loss is identified.
The comprehensive evaluation of candidacy in young children necessitates an experienced multidisciplinary team. For patients <6 months, audiologic confirmation of hearing loss relies on objective measures such as otoacoustic emissions and immittance audiometry (tympanometry and acoustic reflexes), along with electrophysiologic studies such as Auditory Brainstem Response (ABR) or Auditory Steady‐State Response (ASSR) testing to determine frequency‐specific thresholds and type of hearing loss. Behavioral audiometry is usually performed to confirm candidacy of bilateral severe to profound SNHL for CI in children. Aided testing and speech audiometry with best fit hearing aids are performed to demonstrate hearing aid benefit (or lack thereof). A number of studies have demonstrated how initial ABR thresholds can help determine the likelihood of CI by predicting speech perception using an Equivalent Hearing Loss methodology. 21 , 22 , 23 These authors suggest PTA > 65 dB is 75% likely, PTA > 80 dB is 80% likely, and PTA > 96 dB is 95% likely to benefit from CI over hearing aid. Hang et al described how a bilateral no‐response ABR led to 97% of children ultimately having CI recommended. 24 Thus, a hearing aid trial should not delay surgery in a child with bilateral profound SNHL.
Medical evaluation is performed by an otolaryngologist with expertise in pediatric hearing loss. Identification of etiology is important to counsel families on expected outcomes, determine timing of surgery, and ascertain co‐morbidities that could impact surgical or anesthetic risk. This is also critical in developing and interpreting literature regarding pediatric cochlear implantation, as outcomes vary widely based on etiology. A thorough history is obtained, including investigating family history of hearing loss, vision, renal, cardiac, or other conditions that may suggest genetic etiologies. Perinatal or other acquired exposures from known risk factors such as prematurity, hyperbilirubinemia, neonatal infections, ototoxic exposures, hypoxemia and trauma are ascertained. A physical examination is completed, focusing on periauricular anomalies, facial dysmorphisms, and ocular, palate or head and neck abnormalities that would suggest syndromic etiology. Selective diagnostic testing is typically initiated, which may involve Connexin and cytomegalovirus (CMV) screening. Next generation sequencing panel testing can be offered to families if initial genetic testing is not diagnostic. High‐resolution imaging of the temporal bone with multi‐planar image reformation is essential. Computed tomography (CT) and magnetic resonance imaging (MRI) are utilized to assess cochlear morphology and presence of the cochlear nerve, with MRI providing superior evaluation of the neural elements. 25 CT may be sufficient if clinical history (eg, progressive loss) or an established etiology (ie, Connexin) suggests normal nerve anatomy. MRI and CT scans are both often done in cases of abnormal facial nerve anatomy, meningitis, severe inner ear malformations, or syndromic patients with complicated anatomy (eg, CHARGE syndrome). Middle ear status should be actively assessed, and management of otitis media done accordingly. Pneumococcal vaccination status is assessed and ensured to be up to date in accordance with recommendations by the Centers for Disease Control (CDC), the American Academy of Pediatrics (AAP), and the American Academy of Otolaryngology‐Head and Neck Surgery (AAO‐HNS). 26
Early evaluation by a pediatric speech‐language pathologist proficient in hearing loss is performed to assess emerging speech and auditory skills. 27 Counseling a patient's family from diagnosis to early surgical intervention requires a tailored approach as each family may move at a different pace. Discussions must include etiology and management, managing expectations about benefit of CI, and emphasizing the importance of postoperative audiologic and speech‐language habilitation, and promoting listening strategies and narration techniques important in post‐CI habilitation. 28 A social work evaluation should be considered if any anticipated barriers to obtaining care or compliance with the postoperative habilitation is perceived.
4. SAFETY AND FEASIBILITY
Surgical and anesthetic safety in very young children were initially perceived as an obstacle to CI in this population; recent literature has investigated and described both safety and logistical considerations in implanting children <12 months of age, resulting in the long‐awaited reduction in approved age by the FDA.
4.1. Surgical safety
Compared to older children, CI surgery in young children potentially poses a greater challenge to pediatric otologists due to an underdeveloped mastoid tip, greater bone marrow content, thinner calvarium, relatively superficial course of the extratemporal facial nerve, and more delicate soft tissues. 29 , 30 Thus, minor alterations to surgical technique are considered in infants <12 months, with meticulous attention to soft tissue handling and mastoidectomy drilling (Figures 1 and 2). 29 The mastoid antrum and the facial recess are adult sized at birth; however, the mastoid bone undergoes rapid growth in the first year of life. 31 , 32 Infants <12 months have significant bone marrow in the lateral one‐third of the mastoid where pneumatization has not yet occurred (Figure 3). 30 Poor mastoid tip development places the facial nerve at higher risk of injury from infiltration with local anesthesia, and bony and soft‐tissue dissection. Early use of a diamond burr during the mastoidectomy can ablate bone marrow and provide hemostasis. Hemostasis is critical, as there is a smaller circulating blood volume in infants; hypovolemic sequelae can be seen with blood loss >10% of total blood volume (ie, ~80 mL in a 10 kg infant). Modern, slimmer stimulator‐receivers no longer necessitate drilling of a well to recess the device. However, drilling a channel for the electrode in the mastoid may reduce trauma to the array in infants with thin soft tissues. Device fixation has been described in an attempt to reduce the risk of implant migration, although many surgeons now prefer securing the stimulator‐receiver in a tight periosteal pocket (Figures 4 and 5). 33 Families are counseled of a more prominent appearance of the internal devices, due to the small, curved skulls as well as thinner skin and subcutaneous tissue.
FIGURE 1.
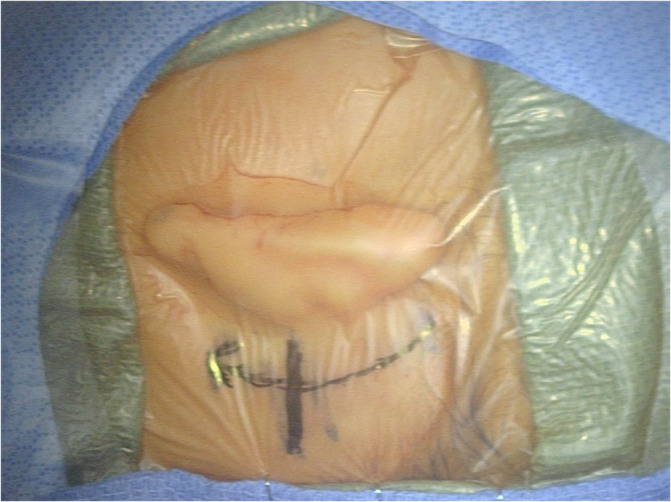
Postauricular incision marked out. Marked level of bony external auditory canal is approximately 1/3 superior from the palpated mastoid tip
FIGURE 2.
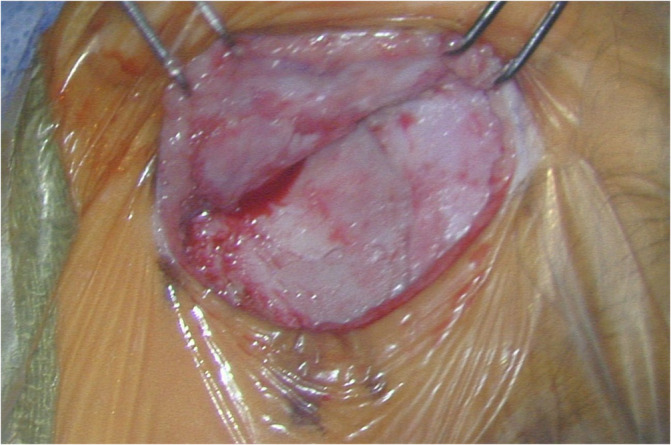
Offset periosteal and skin incisions. View is angled superiorly
FIGURE 3.
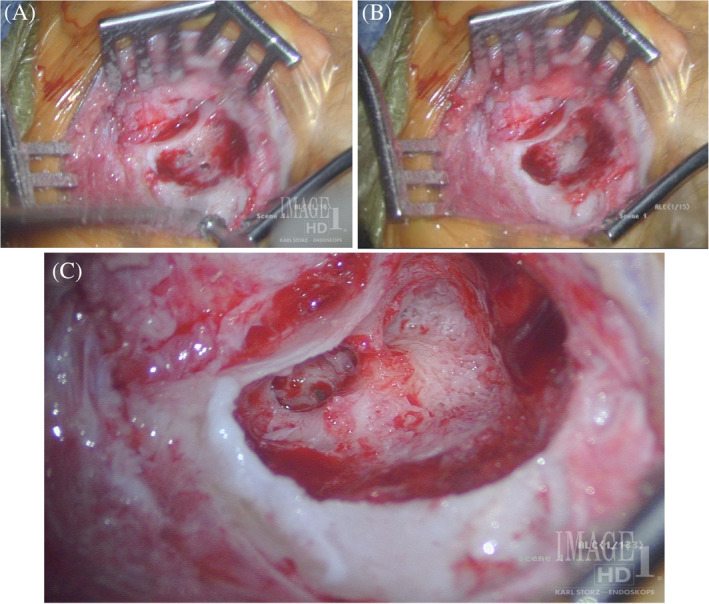
A, Non‐pneumatized marrow space in lateral mastoid and Koerner's septum. B, Koerner's septum opened and lateral semicircular canal and antrum level visible. C, Koerner's septum opened, revealing border of pneumatized air cell space with lateral marrow bone. Round window visible in facial recess. Note the relatively high mastoid tip
FIGURE 4.
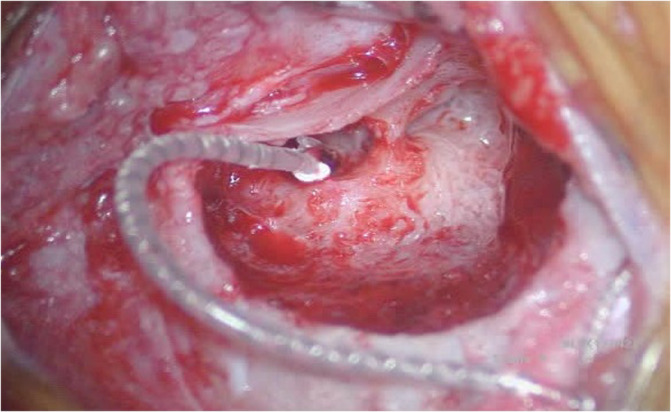
Cochlear implant electrode array after round window insertion
FIGURE 5.
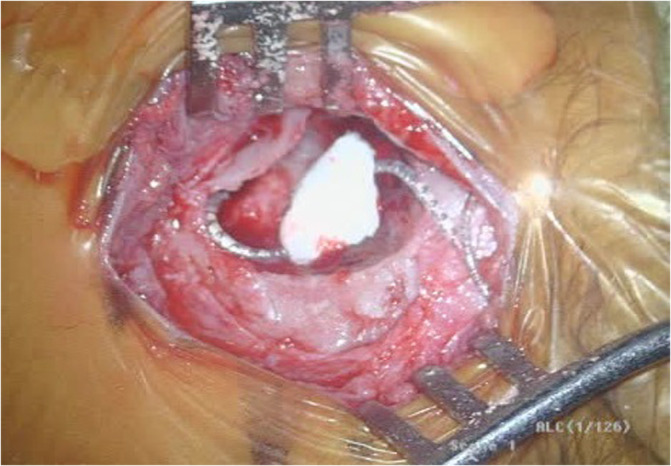
Coiled array in small mastoid, held in place with absorbable gelatin sponge
Surgical complications may either be encountered intraoperatively or postoperatively. 34 Major complications include device failure/extrusion, severe soft tissue infections, cerebrospinal fluid (CSF) leak, cholesteatoma, and any issues that require surgical intervention; minor surgical complications include skin infection, hematoma, and other problems that can be managed with conservative management and do not threaten the device. 35 , 36 , 37 Despite distinct anatomic and physiologic factors, the majority of studies reveal that CI surgery in young infants is both safe and surgically feasible. In a prospective study examining 300 CI recipients ages 1‐5 years with an average follow up duration of 4 months, Bhatia et al reported an overall rate of 2.35% and 16% for major and minor complications, respectively. The authors described a trend towards increased complications in recipients younger than 2.5 years of age (29%), although this did not reach significance (P = .12). 36
Studies that investigated post‐operative complications specifically in children <12 months also demonstrate the overall safety of early implantation. In a large retrospective review of institutional and national data, O'Connell et al demonstrated no significant differences in either surgical or medical/anesthesia‐related complications after CI between patients <12 months and those 12‐18 months. 38 The incidence of a 30‐day post‐operative surgical complication using the American College of Surgeons National Surgical Quality Improvement Program (ACS‐NSQIP) pediatric database was 3.6% for infants <12 months compared with 3.2% for infants 12‐18 months (P = 1.0). 38 In a similar large retrospective review of 1278 patients from the ACS‐NSQIP database, Kalejaiye et al showed no significant differences in complication rates (2.73% vs 1.48%, P = .96) when comparing CI recipients <12 months (n = 73) to those >12 months. 39 Patients <12 months were noted to have longer mean operative times (191 minutes vs 160 minutes, P = .0015), which may be attributed to smaller skull size and less developed mastoid cavity with more bone marrow. Due to thinner skin flaps in very young infants, there have been concerns about increased risks of soft tissue complications including skin breakdown. 37 However, Kalejaiye et al reported a soft tissue complication rate of 1.33%, which is comparable to other reported rates in the literature. 35 , 39 , 40 , 41
4.2. Anesthesia safety
The majority of CIs are performed on a non‐emergent basis and on infants with relatively good physical status based on the American Society of Anesthesiology (ASA index), which reduces the overall anesthetic risk. 42 , 43 Hoff et al retrospectively examined the safety and effectiveness among 39 young children implanted <12 months and reported no major anesthetic complications, which is consistent with several other studies. 30 , 34 , 35 , 41 , 44 , 45 , 46 , 47 Medical or anesthesia‐related complications after CI in infants can include re‐intubation, respiratory failure, seizure, stroke, cardiac arrest, sepsis, and death. 38 , 39
Innate physiologic differences in infants that increase the risk of anesthesia include greater oxygen demand and less oxygen reserve, underdevelopment of the sympathetic response, decreased cardiovascular reserve to compensate for hypotension, and hypercarbia that can result in apneic response instead of hyperventilation. 30 , 48 The incidence of anesthesia‐related complications has declined over the past several decades, but the incidence of morbidity and mortality in infants <12 months is still higher than for children >12 months and adults. 42 , 43 , 49 , 50 Cohen et al demonstrated that the greatest anesthesia risk is during the first month of life, although this may be confounded by more invasive and complex surgical interventions in these infants. 49 Keenan et al showed that the risk of cardiac arrest among infants <12 months was 19.7 per 10 000 anesthetics with general non‐pediatric anesthesiologists compared to none for pediatric anesthesiologists. 50 The authors acknowledge that these statistics should be interpreted cautiously but emphasize that the safety and use of general anesthesia in infants <12 months is improved with the involvement of pediatric anesthesiologists. 30 , 42 , 50
There has been significant public health debate regarding the risk of general anesthesia among the pediatric population due to concerns for neurodevelopmental sequelae. 51 However, a prospective, randomized‐controlled trial, commonly referred to as the general anesthesia (GAS) trial, showed no association between a short exposure of general anesthesia (<1 hour) and neurocognitive deficits. 52 , 53 These findings are supported by two cohort studies, known as Pediatric Anesthesia Neurodevelopment Assessment (PANDA) and Mayo Anesthesia Safety in Kids (MASK), that analyzed similar outcomes. 54 , 55 Of note, the FDA amended its initial 2016 warning on the use of general anesthetics or sedatives in young children (<3 years) to suggest that repeated or prolonged exposure (≥3 hours) may be associated with neurocognitive deficits, which is primarily based on preclinical studies. 51 , 52 Further research is warranted to determine the impact on neurocognitive outcomes for patients with multiple or prolonged anesthesia exposure. 52
5. LANGUAGE AND SPEECH OUTCOMES
The role for earlier CI is generally accepted due to improved developmental outcomes compared to older counterparts. 47 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 Here, we highlight and review studies that focused on children implanted <12 months and the associated impact on language development, speech perception, and speech production outcomes.
5.1. Language development
Karltorp et al demonstrated that children implanted between 5 and 11 months reached age‐equivalent levels of language understanding and improved vocabulary outcomes earlier than children implanted between 12 and 29 months. 64 Nicholas et al also analyzed standard scores on receptive vocabulary (Peabody Picture Vocabulary Test, PPVT) and expressive and receptive language (Auditory Comprehension and Expression Communication Scales of the Pre‐School Language Scale) at 4.5 years of age. The authors showed that children implanted between 6 and 11 months (n = 27) achieved higher scores on all measures compared to those implants between 12 and 18 months (n = 42). 65 The preponderance of other studies demonstrated similar results regarding improved language trajectories among infants implanted <12 months. 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73
In a retrospective study, however, Leigh et al showed no significant difference in the rate of receptive language growth between those children implanted <12 months (0.90) compared to those implanted between 13 and 24 months (0.92). 74 These results were similar to previous studies suggesting a limited advantage of performing CI < 12 months compared to those implanted between 13 and 24 months. 42 , 75 , 76 High maternal educational level is associated with language development, which has been previously cited as a confounding factor that can influence hearing outcomes. 67 , 70 , 74 , 77 Another inherent limitation with some of these results is that direct comparisons between groups may be confounded by differences in the length of device experience. For example, if language assessments are completed at 36 months, patients implanted <12 months have more time to develop language compared to infants implanted at a later age. 46 The majority of studies suggest that early implantation <12 months may reduce the adverse effects of auditory deprivation and lead to improved language development, including better vocabulary and linguistic outcomes. 64 , 66 In a recent systematic review, Bruijnzeel et al confirmed these findings and demonstrated that children implanted <12 months led to improved spoken language acquisition and speech recognition compared to those implanted between 12 and 24 months. 78
5.2. Speech production
Similar to language development, early implantation can result in improved language expression, vocal development, and speech production/intelligibility compared to implantation later in childhood. 35 , 79 , 80 , 81 Recent studies suggest additional benefit with implantation <12 months but there is less robust data directly comparing speech production between respective cohorts. This is likely attributed to the inability to accurately test articulation and rate speech intelligibility until children are older. 46
Leigh et al showed that children implanted <12 months (n = 16) had significantly higher speech production scores than those implanted between 13 and 24 months (n = 16) using the Diagnostic Evaluation of Articulation and Phonology (DEAP) at least 2 years post‐implantation. 74 Colletti et al also demonstrated that 5 years after CI activation, 100% of children implanted <12 months had speech intelligible to the average listeners, while 67% implanted between 12 and 23 months, and 61% implanted between 24 and 35 months had developed similar intelligible speech. 69 Early implantation has also been shown to lead early onset of babbling, which is a critical landmark in prelexical development. 41 , 82
5.3. Speech perception
Speech perception abilities may improve with earlier implantation <12 months of age, but this literature is less clear of an advantage. Much early literature was limited or did not have direct comparisons to older implant cohorts. 34 , 35 , 45 Challenges in testing infants includes using checklists or waiting until an appropriate age for open‐set word testing. 46 Some studies suggested that speech perception outcomes may not be significantly associated with earlier age of implantation. 64 , 66 , 74 , 83 However, in a long‐term (10 years of CI use) prospective cohort study, children implanted <12 months had significantly better auditory results based on both Category of Auditory Performance (CAP) and Infant‐Toddler Meaningful Auditory Integration Scale (IT‐MAIS) scores compared to children implanted between 12 and 36 months. 69
Colletti et al also showed that infants implanted <12 months had significantly better grammar skills (P < .001) and non‐auditory cognitive performances compared to children implanted later using the Griffiths Mental Development Test (GMDS) and Leiter International Performance Scale‐Revised (LIPS‐R). Despite a small sample size, the improved outcomes on the GMDS and LIPS‐R suggest that early auditory stimulation may significantly impact the development of complex cognitive functions due to increased sensory input integration. 69
In a compelling, prospective multicenter study, Dettman et al overcame some of the limitations of previous studies by using standardized measures of communication. The authors analyzed speech perception, speech production and language skills among children implanted between 6 and 12 months (n = 151), 13 and 18 months (n = 61), 19 and 24 months (n = 66), 24 and 42 months (n = 82), and 43 and 60 months (n = 43). 46 There was a significant relationship between age of implantation and speech perception; the mean open‐set word, phoneme, sentence scores were significantly better for those implanted before 24 months of age compared to the older groups but not specifically for those implanted <12 or 18 months of age. Importantly, however, children implanted between 6 and 12 months had significantly higher speech production outcomes (DEAP) and overall language standard scores (Clinical Evaluation of Language Fundamentals, CELF; Preschool Language Scale, PLS; PPVT) compared to older groups. A greater percentage of children in this cohort also demonstrated language performance within the normative range by school entry. This robust study highlights the role for CIs < 12 months and its positive impact on language development, speech productive and cognitive skills. 46
5.4. Neurocognitive and executive function
Recently, novel information has emerged on the impact of auditory deprivation on neurocognitive abilities, beyond listening and spoken language. Executive function (EF) is a term for a set of functions involved in “the cognitive control and oversight processes needed to undertake planned goal‐directed activities.” EF consists of multiple components such as working memory, controlled attention and goal direction, self‐monitoring and inhibition, organization, and cognitive flexibility. 13 , 84 Nicastri et al found that children implanted under 2 years of age generally did well on EF domains of flexibility and inhibitory control, and that children implanted under 12 months of age performed better. 85
6. COST EFFECTIVENESS
CIs are known to be a highly cost‐effective surgical intervention regardless of age. 86 , 87 Cheng et al conducted a cost‐utility analysis and demonstrated that the lifetime net expected savings for children receiving a CI was $53 198. 88 In a prospective longitudinal assessment, Semenov et al highlighted that early CI (<18 months of age) was associated with greater and longer quality of life improvement, increased net societal savings, and improved economic outcomes. 89 Earlier implantation may also provide cost benefit with better employment opportunities and decreased psychosocial and educational support systems over the long term. 90 Colletti et al showed that the net savings to society for infants implanted <12 months was approximately 21 000€ when compared to those implanted between 12 and 23 months. 86 This significant net savings to society for children implanted <12 months further supports the role for early implantation.
7. ADDITIONAL FACTORS
The decision to proceed with CI is a shared‐decision making process and should be determined on a patient‐specific basis. There may be justifiable reasons to perform surgery after 9‐12 months. Limitations can include family indecision, progressive hearing loss, or complex medical conditions increasing the risk and morbidity of early implantation. While early CI clearly improves language development, age of implantation is just one of a multitude of variables that influence outcomes. 73 Duchesne et al analyzed 49 peer‐reviewed studies that examined age at implantation as a variable and concluded that age at implantation only has a moderate influence on the central components of language. 73 Other factors including pre‐operative residual hearing, developmental delay, and mode of communication can significantly impact language outcomes. Family characteristics like socioeconomic status, education level, participation in the intervention programs, and parent‐child interactions have also been reported to influence language development among infants with CIs. 21 , 79 Despite many of these additional and dynamic variables, the age at implantation is one factor that otolaryngologists can influence by helping families navigate this process in order to promote early implantation when feasible.
8. CONCLUSION
Due to recent technological advancements and increasing evidence supporting early implantation to limit auditory deprivation, the indications and criteria for pediatric CI have now evolved to allow for implantation as young as 9 months with certain devices. Historically, surgical safety, anesthesia‐related risk, and other logistical considerations have been viewed as potential deterrents for CI in very young infants (<12 months). Recent studies demonstrate that early CI (<12 months) is both safe and surgically feasible with minimal post‐operative complications when compared to older cohorts. There are an increasing number studies supporting early implantation based on improved speech and language development skills. Lastly, age at implantation is one of many factors that influences patient outcomes, but it is critical that the otolaryngologist guides the multidisciplinary team to help parents make informed decisions about interventions and management in a timely fashion.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
A. N. N., V. V. V., and P. S. M. conceived and designed the article's objective. All authors performed the data acquisition and drafting of the article. All authors were involved in interpretation of the data, provided critical revisions, and approved this version to be published.
Naik AN, Varadarajan VV, Malhotra PS. Early pediatric Cochlear implantation: An update. Laryngoscope Investigative Otolaryngology. 2021;6:512–521. 10.1002/lio2.574
BIBLIOGRAPHY
- 1. Fretz RJ, Fravel RP. Design and function: a physical and electrical description of the 3m house cochlear implant system. Ear Hear. 1985;6(3):14S‐19S. 10.1097/00003446-198505001-00004. [DOI] [PubMed] [Google Scholar]
- 2. Moore DR. Postnatal development of the mammalian central auditory system and the neural consequences of auditory deprivation. Acta Otolaryngol. 1985;99(S421):19‐30. 10.3109/00016488509121753. [DOI] [PubMed] [Google Scholar]
- 3. Nikolopoulos TP, O'Donoghue GM, Archbold S. Age at implantation: its importance in pediatric cochlear implantation. Laryngoscope. 1999;109(4):595‐599. 10.1097/00005537-199904000-00014. [DOI] [PubMed] [Google Scholar]
- 4. Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16(8):1412‐1425. 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- 5. Kral A. Auditory critical periods: a review from system's perspective. Neuroscience. 2013;247:117‐133. 10.1016/j.neuroscience.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 6. Glennon E, Svirsky MA, Froemke RC. Auditory cortical plasticity in cochlear implant users. Curr Opin Neurobiol. 2020;60:108‐114. 10.1016/j.conb.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruben RJ. A time frame of critical/sensitive periods of language development. Acta Otolaryngol. 1997;117(2):202‐205. 10.3109/00016489709117769. [DOI] [PubMed] [Google Scholar]
- 8. Sharma A, Campbell J, Cardon G. Developmental and cross‐modal plasticity in deafness: evidence from the P1 and N1 event related potentials in cochlear implanted children. Int J Psychophysiol. 2015;95(2):135‐144. 10.1016/j.ijpsycho.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hardie NA, Shepherd RK. Sensorineural hearing loss during development: morphological and physiological response of the cochlea and auditory brainstem. Hear Res. 1999;128(1‐2):147‐165. 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- 10. Nordeen KW, Killackey HP, Kitzes LM. Ascending projections to the inferior colliculus following unilateral cochlear ablation in the neonatal gerbil, Meriones unguiculatus . J Comp Neurol. 1983;214(2):144‐153. 10.1002/cne.902140204. [DOI] [PubMed] [Google Scholar]
- 11. Moore DR. Auditory brainstem of the ferret: long survival following cochlear removal progressively changes projections from the cochlear nucleus to the inferior colliculus. J Comp Neurol. 1994;339(2):301‐310. 10.1002/cne.903390209. [DOI] [PubMed] [Google Scholar]
- 12. Kral A, Dorman MF, Wilson BS. Neuronal development of hearing and language: cochlear implants and critical periods. Annu Rev Neurosci. 2019;42(1):47‐65. 10.1146/annurev-neuro-080317-061513. [DOI] [PubMed] [Google Scholar]
- 13. Kral A, Kronenberger WG, Pisoni DB, O'Donoghue GM. Neurocognitive factors in sensory restoration of early deafness: a connectome model. Lancet Neurol. 2016;15(6):610‐621. 10.1016/S1474-4422(16)00034-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ponton CW, Moore JK, Eggermont JJ. Prolonged deafness limits auditory system developmental plasticity: evidence from an evoked potentials study in children with cochlear implants. Scand Audiol Suppl. 1999;51:13‐22. [PubMed] [Google Scholar]
- 15. Kral A, Sharma A. Developmental neuroplasticity after cochlear implantation. Trends Neurosci. 2012;35(2):111‐122. 10.1016/j.tins.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002;23(6):532‐539. 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 17. Glick H, Sharma A. Cross‐modal plasticity in developmental and age‐related hearing loss: clinical implications. Hear Res. 2017;343:191‐201. 10.1016/j.heares.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Querleu D, Renard X, Versyp F, Paris‐Delrue L, Crèpin G. Fetal hearing. Eur J Obstet Gynecol Reprod Biol. 1988;28(3):191‐212. 10.1016/0028-2243(88)90030-5. [DOI] [PubMed] [Google Scholar]
- 19. Harrison M, Roush J, Wallace J. Trends in age of identification and intervention in infants with hearing loss. Ear Hear. 2003;24(1):89‐95. 10.1097/01.AUD.0000051749.40991.1F. [DOI] [PubMed] [Google Scholar]
- 20. Evelyn C. Year 2000 position statement. Am J Audiol. 2000;9(1):9‐29. 10.1044/1059-0889(2000/005). [DOI] [PubMed] [Google Scholar]
- 21. Leigh JR, Dettman SJ, Dowell RC. Evidence‐based guidelines for recommending cochlear implantation for young children: audiological criteria and optimizing age at implantation. Int J Audiol. 2016;55(suppl 2):S9‐S18. 10.3109/14992027.2016.1157268. [DOI] [PubMed] [Google Scholar]
- 22. Lovett R, Vickers D, Hearing AS‐E and, 2015. undefined. Bilateral cochlear implantation for hearing‐impaired children: Criterion of candidacy derived from an observational study. journals.lww.com. https://journals.lww.com/ear‐hearing/fulltext/2015/01000/Bilateral_Cochlear_Implantation_for.3.aspx?casa_token=7SJJNkP_CmoAAAAA:qtpLh5OMBra9DJqumN6xkn4‐CKWG6u5ROVKWD4wF4E7lETb4Gi3WnpqWoCSUkoVOdKTDEKbF0dZgfe3ph8EcgQ. Accessed December 7, 2020. [DOI] [PubMed]
- 23. hearing LD‐E and , 2006. undefined. Effects of stimulus level on the speech perception abilities of children using cochlear implants or digital hearing aids. journals.lww.com. https://journals.lww.com/ear-hearing/Fulltext/2006/10000/Criteria_of_Candidacy_for_Unilateral_Cochlear.5.aspx?casa_token=_cdR5F0vr00AAAAA:-8hjGR1_ewamB-8oUHgKenmUGcHCV7xBuPFzmya_IbgLZvNHQI1G-H4GaS_E44OhfuxUy-vsKbJoPXhqbvUktA. Accessed December 7, 2020. [DOI] [PubMed]
- 24. Hang A, Roush P, Teagle H, … CZ‐E and, 2015 undefined. Is “no response” on diagnostic auditory brainstem response testing an indication for cochlear implantation in children? journals.lww.com. https://journals.lww.com/ear-hearing/FullText/2015/01000/Is__No_Response__on_Diagnostic_Auditory_Brainstem.2.aspx?casa_token=t24ObnouH0cAAAAA:LS0vo5ovuOSLiscOXQ71u2VUwH-0Y_dtduqTVklWutu-jz2CX3gK6jy9FLq8qTVNq_CRT8P-sse3kz92qB3c1Q. Accessed December 7, 2020. [DOI] [PubMed]
- 25. Roche JP, Huang BY, Castillo M, Bassim MK, Adunka OF, Buchman CA. Imaging characteristics of children with auditory neuropathy spectrum disorder. Otol Neurotol. 2010;31(5):780‐788. 10.1097/MAO.0b013e3181d8d528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rubin LG, Papsin B. Cochlear implants in children: surgical site infections and prevention and treatment of acute otitis media and meningitis. Pediatrics. 2010;126(2):381‐391. 10.1542/peds.2010-1427. [DOI] [PubMed] [Google Scholar]
- 27. Bagatto M, Moodie S, … RS‐T in, 2011 undefined. A critical review of audiological outcome measures for infants and children. journals.sagepub.com. 2011;15(1):23–33. doi: 10.1177/1084713811412056 [DOI] [PMC free article] [PubMed]
- 28. Beer J, Kronenberger WG, Pisoni DB. Executive function in everyday life: implications for young cochlear implant users. Cochlear Implants Int. 2011;12(suppl 1). S89–S91. 10.1179/146701011x13001035752570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lesinski‐Schiedat A, Illg A, Heermann R, Bertram B, Lenarz T. Paediatric cochlear implantation in the first and in the second year of life: a comparative study. Cochlear Implants Int. 2004;5(4):146‐159. 10.1179/cim.2004.5.4.146. [DOI] [PubMed] [Google Scholar]
- 30. James AL, Papsin BC. Cochlear implant surgery at 12 months of age or younger. Laryngoscope. 2004;114(12):2191‐2195. 10.1097/01.mlg.0000149456.75758.4c. [DOI] [PubMed] [Google Scholar]
- 31. Cinamon U. The growth rate and size of the mastoid air cell system and mastoid bone: a review and reference. Eur Arch Oto‐Rhino‐Laryngol. 2009;266(6):781‐786. 10.1007/s00405-009-0941-8. [DOI] [PubMed] [Google Scholar]
- 32. Zhao P, Ding H, Lv H, et al. Growth pattern of temporal bone pneumatization: a computed tomography study with consecutive age groups. Surg Radiol Anat. 2019;41(2):221‐225. 10.1007/s00276-018-2113-2. [DOI] [PubMed] [Google Scholar]
- 33. Sweeney AD, Carlson ML, Valenzuela CV, et al. 228 cases of cochlear implant receiver‐stimulator placement in a tight subperiosteal pocket without fixation. Otolaryngology – Head and Neck Surg (United States). 2015;152:712‐717. 10.1177/0194599814567111. [DOI] [PubMed] [Google Scholar]
- 34. Valencia DM, Rimell FL, Friedman BJ, Oblander MR, Helmbrecht J. Cochlear implantation in infants less than 12 months of age. Int J Pediatr Otorhinolaryngol. 2008;72(6):767‐773. 10.1016/j.ijporl.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 35. Roland JT, Cosetti M, Wang KH, Immerman S, Waltzman SB. Cochlear implantation in the very young child: long‐term safety and efficacy. Laryngoscope. 2009;119(11):2205‐2210. 10.1002/lary.20489. [DOI] [PubMed] [Google Scholar]
- 36. Bhatia K, Gibbin KP, Nikolopoulos TP, O'Donoghue GM. Surgical complications and their management in a series of 300 consecutive pediatric cochlear implantations. Otol Neurotol. 2004;25(5):730‐739. 10.1097/00129492-200409000-00015. [DOI] [PubMed] [Google Scholar]
- 37. Das Purkayastha PK, Jewell S, James AL, Gordon K, Papsin B. Soft tissue complications after pediatric cochlear implantation in children younger than 12 months. Otol Neurotol. 2011;32(5):780‐783. 10.1097/MAO.0b013e318214ea88. [DOI] [PubMed] [Google Scholar]
- 38. O'Connell BP, Holcomb MA, Morrison D, Meyer TA, White DR. Safety of cochlear implantation before 12 months of age: Medical University of South Carolina and Pediatric American College of Surgeons‐National Surgical Quality improvement program outcomes. Laryngoscope. 2016;126(3):707‐712. 10.1002/lary.25570. [DOI] [PubMed] [Google Scholar]
- 39. Kalejaiye A, Ansari G, Ortega G, Davidson M, Kim HJ. Low surgical complication rates in cochlear implantation for young children less than 1 year of age. Laryngoscope. 2017;127(3):720‐724. 10.1002/lary.26135. [DOI] [PubMed] [Google Scholar]
- 40. McJunkin J, Jeyakumar A. Complications in pediatric cochlear implants. Am J Otolaryngol – Head Neck Med Surg. 2010;31(2):110‐113. 10.1016/j.amjoto.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 41. Colletti V, Carner M, Miorelli V, Guida M, Colletti L, Fiorino FG. Cochlear implantation at under 12 months: report on 10 patients. Laryngoscope. 2005;115(3):445‐449. 10.1097/01.mlg.0000157838.61497.e7. [DOI] [PubMed] [Google Scholar]
- 42. Holt RF, Svirsky MA. An exploratory look at pediatric cochlear implantation: is earliest always best? Ear Hear. 2008;29(4):492‐511. 10.1097/AUD.0b013e31816c409f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morray JP, Geiduschek JM, Ramamoorthy C, et al. Anesthesia‐related cardiac arrest in children: initial findings of the pediatric perioperative cardiac arrest (POCA) registry. Anesthesiology. 2000;93(1):6‐14. 10.1097/00000542-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 44. Hoff S, Ryan M, Thomas D, et al. Safety and effectiveness of Cochlear implantation of young children, including those with complicating conditions. Otol Neurotol. 2019;40(4):454‐463. 10.1097/MAO.0000000000002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Waltzman SB, Roland JT. Cochlear implantation in children younger than 12 months. Pediatrics. 2005;116(4):e487–e493. 10.1542/peds.2005-0282. [DOI] [PubMed] [Google Scholar]
- 46. Dettman SJ, Dowell RC, Choo D, et al. Long‐term communication outcomes for children receiving Cochlear implants younger than 12 months: a multicenter study. Otol Neurotol. 2016;37(2):e82‐e95. 10.1097/MAO.0000000000000915. [DOI] [PubMed] [Google Scholar]
- 47. Miyamoto RT, Hay‐McCutcheon MJ, Kirk KI, Houston DM, Bergeson‐Dana T. Language skills of profoundly deaf children who received cochlear implants under 12 months of age: a preliminary study. Acta Otolaryngol. 2008;128(4):373‐377. 10.1080/00016480701785012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Battersby E. In: Adams DA, Cinnamond MJE, eds. Scott‐Brown's Otolaryngology, Paediatric Anaesthesia. Oxford: Butterworth‐Heinemann; 1997. [Google Scholar]
- 49. Cohen MM, Cameron CB, Duncan PG. Pediatric anesthesia morbidity and mortality in the perioperative period. Anesth Analg. 1990;70(2):160‐167. 10.1213/00000539-199002000-00005. [DOI] [PubMed] [Google Scholar]
- 50. Keenan RL, Shapiro JH, Dawson K. Frequency of anesthetic cardiac arrests in infants: effect of pediatric anesthesiologists. J Clin Anesth. 1991;3(6):433‐437. 10.1016/0952-8180(91)90088-5. [DOI] [PubMed] [Google Scholar]
- 51. Vutskits L, Culley DJ. GAS, PANDA, and MASK: no evidence of clinical anesthetic neurotoxicity! Anesthesiology. 2019;131(4):762‐764. 10.1097/ALN.0000000000002863. [DOI] [PubMed] [Google Scholar]
- 52. McCann ME, Berde C, Soriano S, et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake‐regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet. 2019;393(10172):664‐677. 10.1016/S0140-6736(18)32485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Davidson AJ, Disma N, De Graaff JC, et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake‐regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387(10015):239‐250. 10.1016/S0140-6736(15)00608-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Warner DO, Zaccariello MJ, Katusic SK, et al. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: the mayo anesthesia safety in kids (MASK) study. Anesthesiology. 2018;129(1):89‐105. 10.1097/ALN.0000000000002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun LS, Li G, Miller TLK, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA – J Am Med Assoc. 2016;315(21):2312‐2320. 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Svirsky MA, Teoh SW, Neuburger H. Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiol Neurootol. 2004;9:224‐233. 10.1159/000078392. [DOI] [PubMed] [Google Scholar]
- 57. Tomblin JB, Barker BA, Spencer LJ, Zhang X, Gantz BJ. The effect of age at cochlear implant initial stimulation on expressive language growth in infants and toddlers. J Speech Lang Hear Res. 2005;48(4):853‐867. 10.1044/1092-4388(2005/059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Connor CMD, Craig HK, Raudenbush SW, Heavner K, Zwolan TA. The age at which young deaf children receive cochlear implants and their vocabulary and speech‐production growth: is there an added value for early implantation? Ear Hear. 2006;27(6):628‐644. 10.1097/01.aud.0000240640.59205.42. [DOI] [PubMed] [Google Scholar]
- 59. Zwolan TA, Ashbaugh CM, Alarfaj A, et al. Pediatric cochlear implant patient performance as a function of age at implantation. Otol Neurotol. 2004;25(2):112‐120. 10.1097/00129492-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 60. Dunn CC, Walker EA, Oleson J, et al. Longitudinal speech perception and language performance in pediatric cochlear implant users: the effect of age at implantation. Ear Hear. 2014;35(2):148‐160. 10.1097/AUD.0b013e3182a4a8f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baumgartner WD, Pok SM, Egelierler B, Franz P, Gstoettner W, Hamzavi J. The role of age in pediatric cochlear implantation. Int J Pediatr Otorhinolaryngol. 2002;62(3):223‐228. 10.1016/S0165-5876(01)00621-8. [DOI] [PubMed] [Google Scholar]
- 62. Hammes DM, Novak MA, Rotz LA, Willis M, Edmondson DM, Thomas JF. Early identification and cochlear implantation: critical factors for spoken language development. Ann Otol Rhinol Laryngol. 2002;111:74‐78. 10.1177/00034894021110s516. [DOI] [PubMed] [Google Scholar]
- 63. Manrique M, Cervera‐Paz FJ, Huarte A, Molina M. Advantages of cochlear implantation in prelingual deaf children before 2 years of age when compared with later implantation. Laryngoscope. 2004;114(8 I):1462‐1469. 10.1097/00005537-200408000-00027. [DOI] [PubMed] [Google Scholar]
- 64. Karltorp E, Eklöf M, Östlund E, Asp F, Tideholm B, Löfkvist U. Cochlear implants before 9 months of age led to more natural spoken language development without increased surgical risks. Acta Paediatr Int J Paediatr. 2020;109(2):332‐341. 10.1111/apa.14954. [DOI] [PubMed] [Google Scholar]
- 65. Nicholas JG, Geers AE. Spoken language benefits of extending cochlear implant candidacy below 12 months of age. Otol Neurotol. 2013;34(3):532‐538. 10.1097/MAO.0b013e318281e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Houston DM, Miyamoto RT. Effects of early auditory experience on word learning and speech perception in deaf children with cochlear implants: implications for sensitive periods of language development. Otol Neurotol. 2010;31:1248‐1253. 10.1097/MAO.0b013e3181f1cc6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ching TYC, Dillon H, Day J, et al. Early language outcomes of children with cochlear implants: interim findings of the NAL study on longitudinal outcomes of children with hearing impairment. Cochlear Implants Int. 2009;10(suppl 1):28‐32. 10.1002/cii.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. May‐Mederake B. Early intervention and assessment of speech and language development in young children with cochlear implants. Int J Pediatr Otorhinolaryngol. 2012;76(7):939‐946. 10.1016/j.ijporl.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 69. Colletti L, Mandalà M, Zoccante L, Shannon RV, Colletti V. Infants versus older children fitted with cochlear implants: performance over 10 years. Int J Pediatr Otorhinolaryngol. 2011;75(4):504‐509. 10.1016/j.ijporl.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 70. Cuda D, Murri A, Guerzoni L, Fabrizi E, Mariani V. Pre‐school children have better spoken language when early implanted. Int J Pediatr Otorhinolaryngol. 2014;78(8):1327‐1331. 10.1016/j.ijporl.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 71. Dettman S, Sadeghi‐Barzalighi A, Ambett R, Dowell R, Trotter M, Briggs R. Cochlear implants in forty‐eight children with cochlear and/or vestibular abnormality. Audiol Neurotol. 2011;16(4):222‐232. 10.1159/000320608. [DOI] [PubMed] [Google Scholar]
- 72. Levine D, Strother‐Garcia K, Golinkoff RM, Hirsh‐Pasek K. Language development in the first year of life: what deaf children might be missing before cochlear implantation. Otol Neurotol. 2016;37:e56‐e62. 10.1097/MAO.0000000000000908. [DOI] [PubMed] [Google Scholar]
- 73. Duchesne L, Marschark M. Effects of age at cochlear implantation on vocabulary and grammar: a review of the evidence. Am J Speech‐Lang Pathol. 2019;28(4):1673‐1691. 10.1044/2019_AJSLP-18-0161. [DOI] [PubMed] [Google Scholar]
- 74. Leigh J, Dettman S, Dowell R, Briggs R. Communication development in children who receive a cochlear implant by 12 months of age. Otol Neurotol. 2013;34(3):443‐450. 10.1097/MAO.0b013e3182814d2c. [DOI] [PubMed] [Google Scholar]
- 75. Vlastarakos PV, Proikas K, Papacharalampous G, Exadaktylou I, Mochloulis G, Nikolopoulos TP. Cochlear implantation under the first year of age‐the outcomes. A critical systematic review and meta‐analysis. Int J Pediatr Otorhinolaryngol. 2010;74(2):119‐126. 10.1016/j.ijporl.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 76. Black J, Hickson L, Black B, Khan A. Paediatric cochlear implantation: adverse prognostic factors and trends from a review of 174 cases. Cochlear Implants Int. 2014;15(2):62‐77. 10.1179/1754762813Y.0000000045. [DOI] [PubMed] [Google Scholar]
- 77. Yoshinaga‐Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early‐ and later‐identified children with hearing loss. Pediatrics. 1998;102(5):1161‐1171. 10.1542/peds.102.5.1161. [DOI] [PubMed] [Google Scholar]
- 78. Bruijnzeel H, Ziylan F, Stegeman I, Topsakal V, Grolman W. A systematic review to define the speech and language benefit of early (<12 months) pediatric Cochlear implantation. Audiol Neurotol. 2016;21(2):113‐126. 10.1159/000443363. [DOI] [PubMed] [Google Scholar]
- 79. Niparko JK, Tobey EA, Thal DJ, et al. Spoken language development in children following cochlear implantation. JAMA – J Am Med Assoc. 2010;303(15):1498‐1506. 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ertmer DJ, Jung J. Prelinguistic vocal development in young cochlear implant recipients and typically developing infants: year 1 of robust hearing experience. J Deaf Stud Deaf Educ. 2012;17(1):116‐132. 10.1093/deafed/enr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Habib MG, Waltzman SB, Tajudeen B, Svirsky MA. Speech production intelligibility of early implanted pediatric cochlear implant users. Int J Pediatr Otorhinolaryngol. 2010;74(8):855‐859. 10.1016/j.ijporl.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Schauwers K, Gillis S, Daemers K, De Beukelaer C, Govaerts PJ. Cochlear implantation between 5 and 20 months of age: the onset of babbling and the audiologic outcome. Otol Neurotol. 2004;25(3):263‐270. 10.1097/00129492-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 83. Tajudeen BA, Waltzman SB, Jethanamest D, Svirsky MA. Speech perception in congenitally deaf children receiving cochlear implants in the first year of life. Otol Neurotol. 2010;31:1254‐1260. 10.1097/MAO.0b013e3181f2f475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kronenberger WG. Executive functioning and language development in children with cochlear implants. Cochlear Implants Int. 2019;20(suppl 1):2‐5. [PMC free article] [PubMed] [Google Scholar]
- 85. Nicastri M, Giallini I, Amicucci M, et al. Variables influencing executive functioning in preschool hearing‐impaired children implanted within 24 months of age: an observational cohort study. Eur Arch Otorhinolaryngol. 2020. 10.1007/s00405-020-06343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Colletti L, Mandalà M, Shannon RV, Colletti V. Estimated net saving to society from cochlear implantation in infants: a preliminary analysis. Laryngoscope. 2011;121(11):2455‐2460. 10.1002/lary.22131. [DOI] [PubMed] [Google Scholar]
- 87. O'Neill C, O'Donoghue GM, Archbold SM, Normand C. A cost‐utility analysis of pediatric cochlear implantation. Laryngoscope. 2000;110(1):156‐160. 10.1097/00005537-200001000-00028. [DOI] [PubMed] [Google Scholar]
- 88. Cheng AK, Rubin HR, Powe NR, Mellon NK, Francis HW, Niparko JK. Cost‐utility analysis of the cochlear implant in children. JAMA. 2000;284(7):850‐856. 10.1001/jama.284.7.850. [DOI] [PubMed] [Google Scholar]
- 89. Semenov YR, Yeh ST, Seshamani M, et al. Age‐dependent cost‐utility of pediatric cochlear implantation. Ear Hear. 2013;34(4):402‐412. 10.1097/AUD.0b013e3182772c66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dettman SJ, Pinder D, Briggs RJS, Dowell RC, Leigh JR. Communication development in children who receive the cochlear implant younger than 12 months: risks versus benefits. Ear Hear. 2007;28(suppl 2): 11S–18S. 10.1097/AUD.0b013e31803153f8. [DOI] [PubMed] [Google Scholar]


