Abstract
Objective
To evaluate salivary gland chemodenervation with botulinum toxin in chronic parotid sialadenitis.
Methods
Patients who underwent parotid gland chemodenervation for chronic sialadenitis due to duct stenosis refractory to siaendoscopy were reviewed (case series). Additionally, a systematic review of the literature on botulinum toxin injection for chronic parotid sialadenitis was performed. Inclusion criteria included studies containing original data on botulinum toxin injections in patients with chronic sialadenitis symptoms.
Results
Sialadenitis symptoms from 10 patients with 13 affected parotid glands were examined. All had duct stenosis diagnosed on sialendoscopy, refractory sialadenitis symptoms, and received parotid onabotulinum toxin injection(s) (median dose 65U). Of patients with 3‐month follow‐up, 78% reported significant improvement in symptoms. Mean Chronic Obstructive Sialadenitis Symptoms (COSS) Score improved at 3 months post‐injection (47‐25.9, P = .039) with significant reduction in gland pain frequency and gland swelling severity. No patients had a facial nerve paralysis or increased xerostomia. With the systematic review, 518 abstracts were reviewed and 11 studies met inclusion criteria and included case series or case reports with a total of 40 patients treated with botulinum toxin for chronic parotitis. Thirty‐four out of a total of 35 patients in the studies (97%) reported complete (9, 26%) or partial (25, 71%) improvement in sialadenitis symptoms with minimal complications.
Conclusion
Parotid gland chemodenervation with botulinum toxin is a minimally invasive treatment option for symptomatic chronic sialadenitis refractory to medical treatment or sialendoscopy. Botulinum toxin injections alleviate gland pain and swelling associated with salivary obstruction and provide an alternative to parotidectomy for recurrent sialadenitis.
Level of evidence: 4.
Keywords: botulinum toxin, Chronic Obstructive Sialadenitis Symptoms (COSS) Questionnaire, chronic sialadenitis, salivary duct stenosis, sialendoscopy
Parotid gland chemodenervation with botulinum toxin is a minimally invasive treatment option for symptomatic chronic parotid sialadenitis refractory to medical treatment or sialendoscopy. Botulinum toxin injections alleviate obstructive symptoms of gland pain and swelling and provide an alternative to parotidectomy for recurrent sialadenitis.
1. INTRODUCTION
Chronic sialadenitis is characterized by recurrent inflammation of one or more of the major salivary glands. Patients typically note recurrent pain, tenderness, and localized swelling of the affected gland sometimes triggered by meals. 1 , 2 It is caused by a variety of conditions that cause chronic obstruction of the outflow tract, including sialolithiasis, radioactive iodine therapy, autoimmune diseases such as Sjogren's syndrome, recurrent infections, or idiopathic causes. Regardless of underlying condition, the recurrent inflammation of chronic sialadenitis leads to salivary stasis, glandular inflammation, and acute infections in some cases. 2 First line treatment is typically conservative including increasing hydration, use of sialagogues and gland massage to stimulate salivary flow and antibiotics for acute infections. In cases where medical therapy fails, sialendoscopy‐assisted salivary duct surgery or gland removal are typically offered.
In patients with chronic sialadenitis without sialolithiasis, the effectiveness of medical and surgical treatment varies. Salivary duct stenosis is the second most common cause of chronic obstructive sialadenitis after sialolithiasis, and more commonly effects the parotid glands. Many of these patients report frustration with their symptoms and have failed conservative treatment. 3 Sialendoscopy‐assisted salivary duct surgery (SASDS) is a minimally invasive technique that allows direct visualization of the major salivary ducts and diagnoses and treats obstructive pathology within the ductal system. However, 50% or more of patients with sialadenitis without sialolithiasis have presumed salivary duct stenoses and exhibit persistent or recurrent symptoms even after sialendoscopy. 3 , 4 , 5 Additionally, not all centers have access to sialendoscopy equipment or techniques. In cases of disease refractory to medical or sialendoscopic management, gland removal may be required and is associated with potential risks of long‐term xerostomia, cosmetic defects, and nerve injury. 6 , 7 , 8
Thus, there is a great need to find alternative, minimally invasive treatment options to alleviate chronic sialadenitis symptoms as an alternative to gland removal. Botulinum toxin is a known chemodenervating agent affecting cholinergic neurotransmission. Botulinum toxin prevents release of acetylcholine at the neuromuscular junction and thus has an inhibitory action at cholinergic receptors. Through its anti‐cholinergic action, botulinum toxin reduces salivary flow by depressing secretory activation of the salivary glands. 9 It was first described as a treatment for chronic sialorrhea in patients with amyotrophic lateral sclerosis in 1997. 10 In addition to sialorrhea, botulinum toxin use has expanded to a variety of indications in the salivary glands, including Frey syndrome, salivary fistulae, and sialoceles. 9 In chronic sialadenitis, chemodenervation can reduce salivary production and potentially ameliorate salivary stasis and gland swelling related to dysfunctional salivary flow in the ducts.
The objective of this study is to evaluate the effectiveness of botulinum toxin injections for the treatment of chronic parotid sialadenitis with a case series and systematic review of the literature. We examine sialadenitis cases refractory to medical management and/or refractory to sialendoscopic‐guided surgery.
2. METHODS
2.1. Case series
This study was reviewed and approved by the University of California, San Francisco Institutional Review Board (IRB 13‐11170). Subjects were consented prospectively for collection of data regarding their salivary gland disease. A retrospective review was performed on all patients with chronic sialadenitis with recurrent symptoms after sialendoscopy who were then treated with botulinum toxin injections to the affected salivary gland between 2015 and 2019. Demographic data, clinical data, imaging and operative findings, treatment details, and response to treatment were reviewed. Patients followed were asked to complete patient‐reported symptom surveys and the UCSF Chronic Obstructive Sialadenitis Symptoms Score (COSS) at clinic visits and online from home at 2 to 3 months and 9 to 12 months after treatment. 1 , 3 , 4 The COSS score is based on a 20‐item survey specific to the affected gland and has been used in prospective studies to measure symptom score changes after treatment and surgery. When available, the COSS symptom scores were reviewed at baseline and after treatment with botulinum. All patients treated had chronic parotid sialadenitis. Patients were excluded if they did not undergo sialendoscopy prior to botulinum toxin treatment. Statistical analysis of mean COSS scores and sub‐scores were performed with one‐way analysis of variance (ANOVA) with repeated measures (3 time‐points). The cutoff value for statistical significance was P < .05. All reported P values are two tailed. Statistical analysis was performed using Stata (StataCorp, College Station, Texas).
2.2. Sialendoscopy techniques
Sialendoscopy was performed using 1.1 to 1.3 mm sialendoscopes after adequate dilation of the Stensen's duct papilla. Degree and location of stenosis was examined. Complete stenosis was defined when a lumen could not be identified or navigated with a guidewire. Proximal stenosis was defined as located posterior to the masseter muscle or proximal to the hilum of the gland at the primary branchpoint of the main duct. Stenosis was managed with dilation with the sialendoscope or balloon dilation. Most cases were managed with triamcinolone irrigation and stent placement was dependent on the degree of trauma in the duct after dilation. Patients with recurrence of moderate to severe symptoms within 6 months of sialendoscopy were offered botulinum toxin.
2.3. Ultrasound‐guided injection technique
Botulinum toxin injections were performed using an ultrasound‐guided technique. Onabotulinum toxin A (Botox, Allergan, Dublin, Ireland) was diluted 100U per 2 mL. The solution was drawn into 1cc syringes and 27G 1.5inch needles were used for injection. The ultrasound probe was used to localize the parenchyma of the parotid gland and ensure injections were placed within the most prominent areas of gland parenchyma just anterior to the tragus and inferior to the earlobe to treat the parotid tail. Two injections were performed in the longitudinal plane of the ultrasound probe into the parotid body and tail parenchyma (Figure 1A,B). The needle is seen on ultrasound (Figure 1C) and confirmed to be within the gland parenchyma. The botulinum toxin solution was injected as the needle was withdrawn from the gland to allow for diffusion of the medication into the parenchyma. Dose delivered to the parotid gland depended on disease severity and whether the patient had bilateral symptoms. The most severely affected gland was treated with 70 to 100 U with remaining dose (total 100 U/bottle) to the other affected gland if present.
FIGURE 1.
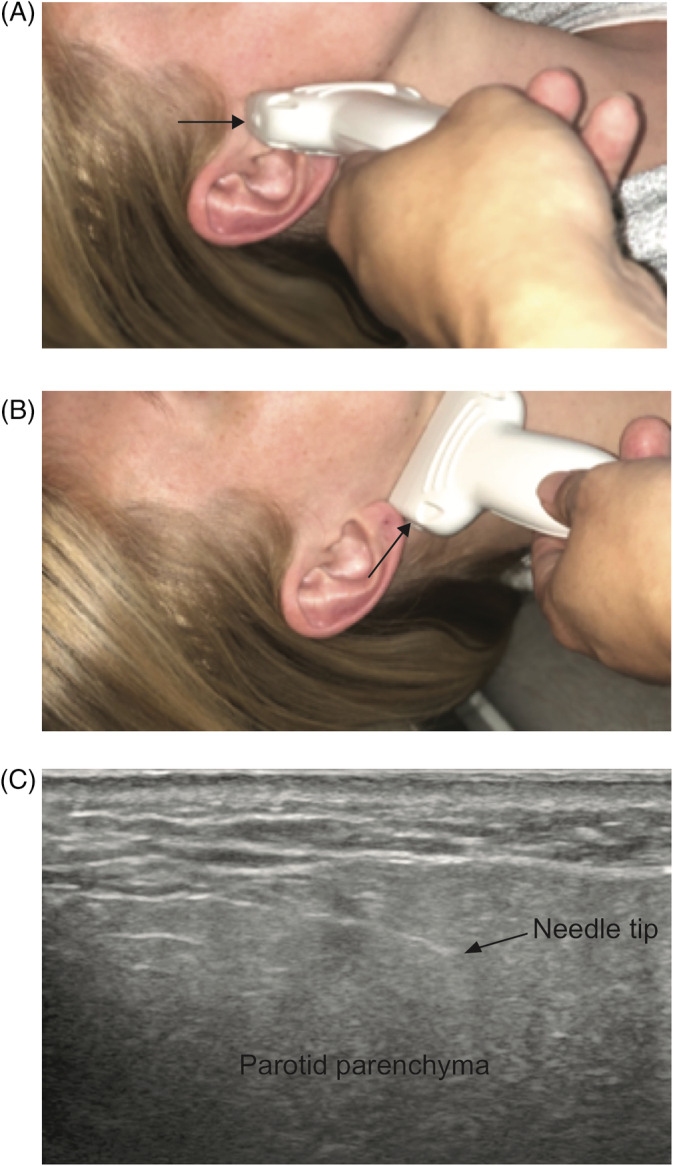
Ultrasound‐guided parotid botulinum injections. A, Needle placement in the longitudinal plane. B, Injection in the tail of the parotid. C, Ultrasound imaging of the parotid with needle visualized within gland parenchyma
2.4. Systematic review search strategy
A systematic review was performed following the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) guidelines using PubMed‐MEDLINE, Embase, and Web of Science to search the literature from January 1, 2001 to December 1, 2019, as well as the conference proceedings of the Triological society annual and combined sections meetings from 2014 to 2019. The search strategy was developed in PubMed using Medical Subject Headings (MeSH) search terms and translated into searches tailored to Embase and Web of Science databases with the assistance of a research librarian. We additionally searched the references for each article which met study inclusion criteria to identify additional articles of possible interest. Search criteria per MeSH terms (Medical Subject Headings) included “Botulinum toxins, botulin, botox, botulinum, sialadenitis, sialoadenitis, salivary gland adenitis, salivary gland inflammation, parotitis, salivary gland, salivary duct, salivary stenosis” and their combinations. After initial query with the search terms, articles were filtered for full‐text availability, and human subject research, and subsequent abstracts were reviewed for selection. Further selection of studies was obtained from bibliography searches of selected articles. Two study investigators (MPS and JLC) independently reviewed study abstracts to identify relevant articles for inclusion. In the case of disagreement, consensus was obtained by review and discussion between the authors.
2.5. Systematic review selection criteria
All human studies with original data were included. Specifically, studies of pediatric and adult patients with symptoms of chronic sialadenitis including recurrent or chronic pain and/or swelling of the salivary glands who underwent botulinum toxin treatments for their condition were included. Studies that looked at botulinum toxin injections for any other reason such as sialorrhea, Frey syndrome, sialoceles, and cosmetic concerns were excluded. Studies of any nonhuman, radiologic only, anatomic, and histologic studies were excluded. Abstracts in non‐English language were translated using an online translation tool for review for eligibility for inclusion. Articles with unobtainable full text, review articles, and studies without outcome measures were further excluded. The PRISMA standards were followed for the selection and review of articles in this systematic review and the full list of references were screened for potentially relevant articles (Figure 2). The articles were reviewed in full to assess for study objective and to determine the level of evidence in accordance with the Oxford Center for evidence‐based medicine guidelines. 11 A risk of bias assessment was performed for each study included using the National Institutes of Health (NIH) quality assessment tool for the appropriate study type. 12 Mean dose of botulinum toxin injections, number of injections and interval of injections were collected for each study. Outcome measures included reporting of symptom improvement after botulinum toxin injections. Mean equivalent dose of onabotulinum toxin was calculated using published equivalent dosing: 1:1 for onabotulinum toxin‐A to incobotulinum toxin‐A and 1:3 for onabotulinum toxin‐A to abobotulinum toxin‐A. 13 Since only a subset of articles described use of sialendoscopy prior to botulinum therapy, we included all studies that involved botulinum for chronic sialadenitis. One study described treatment of submandibular glands and that portion was excluded from the analysis.
FIGURE 2.
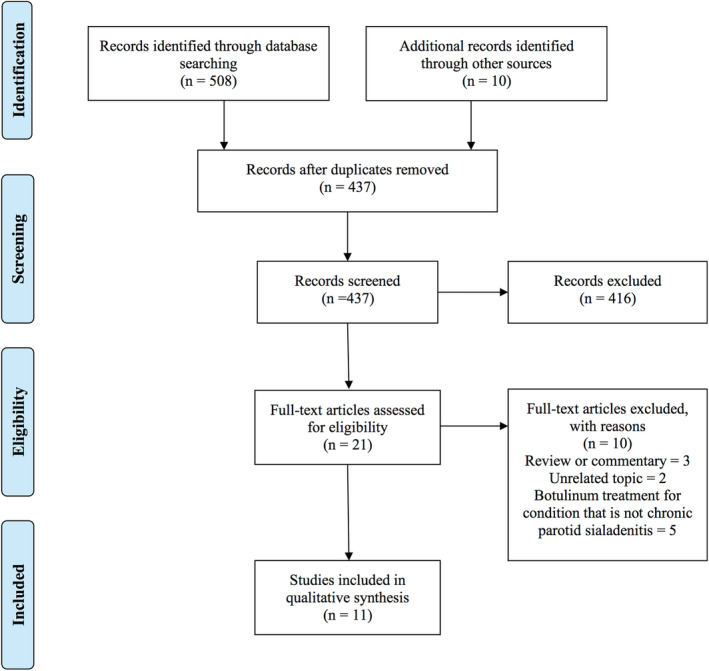
Systematic review article selection flowchart
3. RESULTS
3.1. Case series
Ten patients with 13 affected parotid glands had recurrent sialadenitis symptoms after sialendoscopic‐guided surgery and were subsequently treated with botulinum toxin. Three patients were excluded from the study as they did not undergo sialendoscopy and did not have follow up after botulinum toxin treatment. Demographic and baseline data are reviewed in Table 1. The mean age was 52 years (range 27‐68), and female predominance (80%). Five patients (50%) had suspected or confirmed autoimmune disease. Intraoperative sialendoscopy findings showed 8 (61%) parotid glands with partial stenosis and 4 (30%) glands with complete stenosis (Table 2). The majority of glands displayed proximal stenosis and 4 (30%) exhibited generalized or multifocal stenoses. In addition to sialendoscopic dilation and saline irrigation of the ducts, 53% of glands were treated with additional balloon or rigid instrument dilation and most with steroid irrigation.
TABLE 1.
Case series demographic information
| Patients (n) | 10 |
| Affected parotid glands (n) | 13 |
| Age, mean years [range] | 52 [27‐68] |
| Sex (M:F) | 2:8 |
| Autoimmune disorder (n, %) | 5 (50%) |
| Pre‐treatment COSS | |
| Mean score [range] | 47 [28.5‐75.5] |
| 2‐3 months post‐Botox COSS | |
| Mean score [range] | 25.9 [18.5‐53]* |
| 9‐12 months post‐Botox COSS | |
| Mean score [range] | 26.1 [18.5‐51.5] |
| Presenting symptom (n, % of glands) | |
| Swelling only | 5 (38%) |
| Swelling and pain | 7 (54%) |
| Itching | 1 (8%) |
Abbreviations: COSS, Chronic Obstructive Sialadenitis Symptoms Score; M:F, male to female ratio; n, number.
*P < .05 denotes significant difference from baseline.
TABLE 2.
Intraoperative findings on sialendoscopy
| n (%) | |
|---|---|
| Degree of stenosis | |
| Partial | 8 (61%) |
| Complete | 4 (30%) |
| None reported | 1 (9%) |
| Location of stenosis | |
| Proximal | 9 (70%) |
| Generalized/multifocal | 4 (30%) |
| Sialendoscopic treatment | |
| Balloon or catheter dilation | 7 (53%) |
| Steroid irrigation | 11 (84%) |
| Stent placement | 4 (30%) |
Ten patients with 13 affected parotid glands displayed recurrent obstructive sialadenitis symptoms after sialendoscopy and received ultrasound‐guided onabotulinum toxin A injections at an average of six months after sialendoscopy. Injection details are reviewed in Table 3. The majority of patients underwent 1 or 2 injections to the symptomatic gland(s). For those with repeated injections, the median time between injections was 3 months. The median dose of onabotulinum toxin A to the most symptomatic gland was 65 U, with a range of 40 to 100 U. Additional lower doses were injected when multiple glands were symptomatic during the same visit. In these cases (N = 3), the median dose to the second gland was 40 U (range 25‐50 U). Nine patients had at least 3 months of follow‐up. Of those, 7 (7/9, 77%), 2 reported complete, and 5 reported partial subjective improvement in symptoms at 3 months and beyond. For the two patients who did not report improvement, one patient presented with the main complaint of gland itching with eating and minimal gland swelling, whereas the other had chronic gland enlargement with mild obstructive symptoms.
TABLE 3.
Onabotulinum toxin A treatment details
| Onabotulinum units per treatment, a median [range] | 65 U [40‐100U] |
| Number of treatments, n (%) | |
| 1 | 4 (40%) |
| 2 | 4 (40%) |
| 3 | 1 (10%) |
| 4 | 1 (10%) |
| Time between treatments, median months [range] | 3 [2‐28] |
| Patients with > 3 months follow‐up, n (%) | 9 (90%) |
| Patients with > 9 months follow‐up, n (%) | 8 (80%) |
| Follow‐up time, mean months [range] | 13 [1‐36] |
Onabotulinum toxin dose to most symptomatic gland.
The patients were asked to fill out UCSF COSS questionnaire at baseline and after botulinum toxin treatment. Five patients with eight affected glands had pre‐ and post‐botulinum injection COSS scores available for review. Five patients with 8 affected glands had symptom data at 2 to 3 months; three patients with four glands had data at 9 to 12 months post injection. The mean preoperative COSS score was 47 (range 28.5‐75.5) suggesting severe disease (Table 1). Mean COSS sialadenitis symptom score decreased at 2 to 3 months after the first botulinum toxin injection (25.9, P = .039). The mean COSS score remained low at 9 to 12 months in three patients, but was not statistically significant (26.1, P = .114).
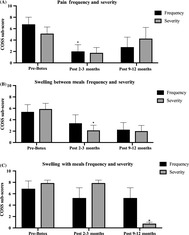
Figure 3 shows salivary gland pain frequency and severity sub‐scores (A), gland swelling between meals (B) and with meals (C) over time. Two patients in this cohort underwent repeat injections between 3 and 9 months. Gland pain frequency and severity of gland swelling between meals scores both declined significantly at 2‐3 months after injection compared to baseline (P = .041 and P = .032, respectively). The scores remained at similar levels at the 9‐12 month period, but was not significant compared to baseline. Severity of gland swelling with meals declined at 9‐12 months compared to baseline (P < .0001) and compared to the 2 to 3 month mark (P < .0001). Frequency of gland swelling with meals scores were not statistically different across time points likely due to limited numbers in follow‐up data.
FIGURE 3.
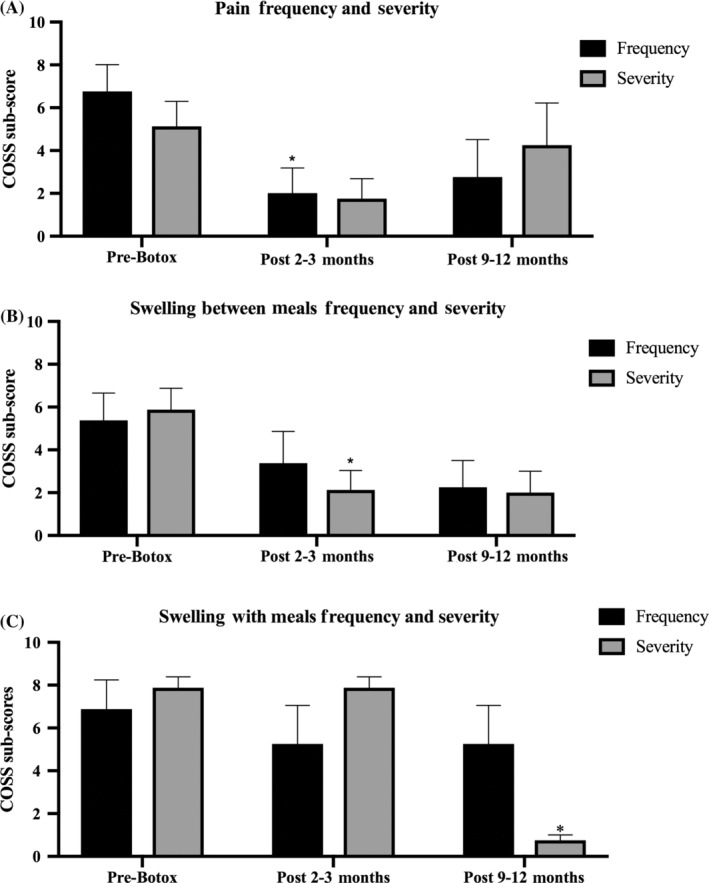
Gland pain and swelling after onabotulinum toxin injection (five patients, eight glands). A, Mean gland pain swelling and frequency scores. B, Gland swelling severity. C, Severity of gland swelling with meals. *P < .05
During follow‐up, there was no noted increase or change in xerostomia symptom scores 2 to 3 months after botulinum toxin injection and no instances of facial nerve paralysis. No patients in the cohort have undergone subsequent parotidectomy within the follow‐up period (mean 13 months, range 1‐36 months).
3.2. Systematic review of the literature
For the systematic review, a total of 518 abstracts were reviewed in our initial inquiry. After selection and review, 11 articles fulfilled eligibility. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 We found four non‐English abstracts that were translated and did not meet inclusion criteria. All studies were case reports and retrospective case series (level 4 evidence). Using the appropriate NIH quality assessment tool, a risk of bias assessment was performed for each study based on study type. 12 We found four studies to be of “fair” quality and seven studies to be of “poor” quality (Table S1). Mean dose of botulinum toxin injections, number of injections and interval of injections were evaluated. Outcome measures included presence or absence and complete or partial (if available) symptom improvement after botulinum toxin injections.
A total of 40 patients were included in the 11 studies (Table 4), with 35 patients with outcome data. Among patients with age data available, mean collective age was 46.5 years, and ranged 8 to 71 years. Among 19 patients with available data, 13 were female and 6 were male. All patients complained of symptoms related to chronic parotid sialadenitis including gland pain, gland swelling, and/or recurrent infections. Etiologies for reported disease included chronic sialadenitis, 15 , 19 , 22 , 23 Sjogren's syndrome, 18 , 23 cystic fibrosis, 24 recurrent parotitis, 16 , 17 and duct stenosis due to masseteric hypertrophy. 20 Treatments that were tried prior to botulinum toxin injection included conservative management, medical treatment of Sjogren's syndrome, sialography with dilation, and sialendoscopy. Based on prior treatment data, 27/40 (68%) patients had undergone prior sialendoscopy. 19 , 20 , 22 , 23
TABLE 4.
Summary of studies in systematic review
| Article | Mean age years (range) | Cases N (M:F) | Treatment before injection | Technique | Mean dose per injection (range) a | Mean number of injections (range) | Mean interval of injections (range) | Mean follow‐up (range) | Outcomes | Safety/adverse effects |
|---|---|---|---|---|---|---|---|---|---|---|
| Gutinas‐Lichius & Jungelhulsin, 2002 | 60 | 1 (M) | Medical management | NS | 100U | 1 | NA | 12 months | Complete resolution of symptoms | No side effects reported |
| Ellies et al, 2004 | NS | 2 | NS | Ultrasound‐guided | 22.5U | 1 | NA | 12.5 months | Complete resolution of symptoms | One patient reported thickened saliva, no severe side effects reported |
| Cappaccio et al, 2008 | 48 (37‐59) | 6 (3M:3F) | NS | Ultrasound‐guided | 60U (45‐90) | 2 (1‐3) | 4.8 months (4‐7) | 2 months (6‐43) | 4 significant recovery, 2 partial recovery | Pain at injection site, no major side effects reported |
| Kruegel et al, 2010 | 58 | 1 (M) | Dilation under sialography x 2, percutaneous radiotherapy 7.5Gy x 1 | Ultrasound‐guided | 28.5U (22.5‐30) | 4 | 3.3 months (2‐4) | 11 months | Complete resolution in symptoms | No side effects reported |
| Daniel & Diamond, 2011 | 8 | 1 (M) | Medical therapy for Sjogren's | NS | 20U | 1 | NA | 12 months | Complete resolution in symptoms | No side effects reported |
| Gillespie et al, 2011 b | NS | 5 | Sialendoscopy, dilation/stenting all cases | Ultrasound‐guided | 100U | 1 | NA | 8 months (1‐24) | Outcomes of botulinum toxin cohort are not specified | No botox‐related side effects reported |
| Reddy et al, 2012 b | 50 (49‐51) | 2 (F) | Sialendoscopy in both cases | Ultrasound‐guided | 75U | 1.5 (1‐2) | 3 months | 12 months, 16 months | Complete resolution in symptoms | No side effects reported |
| O'Neil et al, 2016 | 65 | 1 (F) | Medical therapy for Sjogren's | Ultrasound‐guided | 50U | NS | 3‐4 months | 36 months | Complete resolution in symptoms | No side effects reported |
| El Koury et al, 2016 | 19 | 1 (F) | Medical therapy for cystic fibrosis | NS | 37.5U | 1 | NA | 6 months | Complete resolution in symptoms | No side effects reported |
| Trapeau et al, 2017 b | 64 (48‐71) | 6 (F) | Sialendoscopy with balloon catheter dilation | NS | 50U | 3.6 (1‐9) | 5.7 months (3‐10) | 18.6 months (6‐39) | 4 significant improvement, 1 partial improvement, 1 with no improvement (underwent parotidectomy) |
One patient reported local infection at injection site, two patients reported xerostomia One subsequent parotidectomy |
| Graillon et al, 2019 b | NS | 14 | Sialendoscopy in all cases | NS | 65U | 4.1 | 3 months | 17 months (3‐48) | Significant improvement in symptoms in entire cohort |
Two patients reported xerostomia No subsequent gland excisions |
Abbreviations: M:F, male:female ratio; N, number; NA, not applicable; NS, not specific.
Mean equivalent dose of onabotulinum toxin A injection per gland.
Studies in which patient underwent sialendoscopy prior to botulinum toxin treatment.
The mean equivalent dose of onabotulinum toxin A injected per gland ranged from 22.5U to 100U. The mean number of injections for the studies was 1.8, with a range of 1 to 9 injections. The mean interval between injections ranged from 2 to 10 months. The mean follow‐up across all studies was 13.6 months, with a range of 1 to 43 months.
Outcome measurements were available in 35 patients. The majority of studies focused on reduction or resolution of symptom severity and episodes. Seven studies with nine patients total reported complete resolution of salivary gland symptoms (9/35, 26%). Twenty‐five patients had either partial or significant improvement in symptoms (25/35, 71%). Only one patient out of the cohort had no improvement (1/35, 3%).
Among subjects in four studies who had undergone prior sialendoscopy, 19 , 20 , 22 , 23 22 patients had outcome measures available. Two patients had complete improvement in symptoms (2/22, 9%), 19 had significant or partial improvement (19/22, 86%), and one had no improvement (1/22, 5%) at an average of seven months after treatment. Five patients who had undergone prior sialendoscopy had no outcome data reported.
The majority of studies reported no side effects from the botulinum toxin injections. Out of 40 patients, five patients reported dry mouth or thickened saliva (12%), one patient reported pain at the injection site (2%), and one patient reported injection site infection (2%). Subsequent parotidectomy for persistent symptoms was reported in one patient (2%). 22 There were no reported cases of facial paralysis.
Adding our case series to the review cohort with outcome measures available, a total 41 out of 44 patients showed (93%) some degree of symptom improvement (11/44 complete improvement and 30/44 partial improvement) after botulinum toxin injections (mean time 13.5 months, range 1‐43 months).
4. DISCUSSION
Chronic parotid sialadenitis due to salivary duct stenosis is associated with significant impact on quality of life and can be recurrent even after sialendoscopic guided surgery or medical management. The advent of sialendoscopy and sialendoscopic‐assisted approaches introduced an effective, minimally‐invasive treatment option for patients with chronic sialadenitis due to sialolithiasis and salivary duct stenoses. Sialendoscopy helps diagnose and target the treatment of distal and proximal duct stenoses by guiding duct dilation with irrigation, rigid or balloon dilation and approaches for duct repair. 4 , 25 Salivary duct stenosis has been associated with radioactive iodine induced sialadenitis, autoimmune diseases, masseter muscle hypertrophy, and idiopathic causes. 8 Symptoms of sialadenitis in patients with duct stenoses improve after sialendoscopic‐guided surgeries; however, symptom recurrence is reported in up to 50% of patients leading to frustration with their symptoms and a need for alternative treatment options prior to sialadenectomy. 1 , 3 , 5 , 26
This study shows that botulinum toxin therapy for chronic parotid sialadenitis due to duct stenosis is a safe and effective treatment option with the potential to alleviate symptoms in the first year after treatment. Botulinum toxin injections to the parotid glands can be repeated for recurrent symptoms. Our case series showed improved symptom scores for gland pain frequency and gland swelling severity between meals at 3 months after botulinum injection, with some rebound in some symptoms at 9 to 12 months. Nearly all of the patient cohort in the systematic review reported complete or partial symptom improvement.
Botulinum toxin for chronic obstructive sialadenitis has been described in various case reports and case series. In 2002, Gutinas‐Lichius and Jungehulsing published the earliest case report on a patient with chronic parotid sialectasis and obstructive sialadenitis. The patient underwent a single botulinum toxin injection of 200 U of Dysport and then kept a weekly journal of salivary gland associated symptoms. This patient saw a complete resolution in symptoms that was maintained for 30 weeks. 14 Expert panels often discuss the use of botulinum toxin as a treatment for severe and recurrent sialadenitis cases due to salivary duct stenosis. However, little data exist on treatment protocols, dosing, and outcomes after botulinum toxin injections for refractory obstructive sialadenitis.
The use of botulinum toxin for disorders of salivary hypersecretion has been studied in both pediatric and adult patients. For pediatric sialorrhea, botulinum toxin was found to be equally efficacious or superior to anticholinergic agents with fewer side effects. 27 , 28 In a meta‐analysis of 8 studies on pediatric sialorrhea, maximal effect was seen between 2 and 8 weeks, and effects lasted for 3 to 6 months. The most commonly reported side effect was xerostomia along with transient dysphagia, thick saliva, altered salivary flow, and an initial increase in saliva. 29 A recent randomized control trial evaluating botulinum toxin for chronic sialorrhea in adults, patients were randomized to receive a placebo or incobotulinumtoxin A of 75 or 100 U for four glands. Both dosage groups showed a significant reduction in stimulated salivary flow rate compared to placebo. The most frequent treatment‐related side effects were dry mouth (2.7%‐5.4%) and dysphagia (0%‐2.7%). Higher dosages did not correlate with the higher rates of side effects. 30 Less evidence exists for the benefit of botolinum toxin in sialoceles, salivary fistulae and Frey syndrome with case reports describing variable dosing and success rates. 9
Chronic sialadenitis symptoms secondary to salivary duct stenosis are related to the retention or stasis of saliva within the gland presumably due to diseased or narrow salivary ducts. 8 , 25 Botulinum toxin works by functionally silencing the salivary gland and decreasing output, therefore preventing the sequalae of salivary stasis. Therefore, severity and frequency of gland swelling should be reduced as long as the botulinum toxin effect lasts. Ellies et al hypothesized that temporarily silencing the gland might promote regeneration of the gland tissue, and as a result may have a longer lasting effect on symptoms. 15 Other explanations include promotion of gland atrophy which is associated with overall reduced salivary production over time. Additionally, botulinum toxin is hypothesized to have anti‐inflammatory and anti‐hyperalgesia effects as evidenced by its effectiveness in treating pain in a number of conditions such as migraine headache, neuropathic pain, bladder pain and lower back pain. 31 This has important implications in treating episodic gland pain and swelling. The results of this study demonstrate botulinum toxin can be beneficial for chronic sialadenitis symptoms, especially if refractory to sialendoscopic therapies which attempt to treat duct stenosis by improving patency. We found that the majority of patients across studies had at least partial improvement in their sialadenitis symptoms with botulinum toxin injections. Only one patient in the review had subsequent parotidectomy for their recurrent symptoms. These data suggest that botulinum toxin injection may reduce the necessity of parotidectomy in patients with recurrent symptoms despite maximal medical management and/or failure of sialendoscopic‐assisted therapy. This is particularly important as not all centers have access to sialendoscopy equipment or techniques.
Across studies, the mean dose utilized was around 60 U per gland; however, the range of dosing was variable. The timing of injections varied as well, with the majority of patients undergoing one to two injections, and some patients requiring multiple injections. It was unclear if higher and repeated doses are associated with better results. Few complications related to salivary gland botulinum injections are reported. In our case series, we had no increase in xerostomia sub‐scores and no facial nerve paralysis. In the systematic review, the most common side effect reported was dry mouth in 12%. There were no reported cases of facial paralysis. This corroborates the low incidence of side effects reported in prior trials evaluating botulinum toxin for chronic sialorrhea in large numbers of children and adults. 9 , 28
Ultrasound is a noninvasive adjunct to botulinum toxin injections that allows for real‐time imaging of glandular tissues and surrounding structures and muscles. Small randomized studies in the neurology literature suggest that ultrasound‐guided botulinum injections in all areas of the body can improve therapeutic efficacy and reduce adverse effects of botulinum toxin treatment when compared to conventional treatment. 32 With injections in the head and neck, ultrasound aids with preventing accidental injection into unintended targets including the facial musculature during parotid injections and the pharyngeal musculature during submandibular injections.
This study has multiple limitations. The case series is limited by its small sample size with a variable cohort, the lack of a control group and limited follow‐up data with symptom score reporting. The follow‐up timing was variable and typically collected when patients returned for repeated clinic visits for recurrent symptoms thus biasing the symptom scores both for timepoints with more severe symptoms and for patients who benefitted from botulinum toxin and sought repeated treatment. This study demonstrates potential methods for querying symptoms after botulinum injections and highlights the challenges in capturing symptom changes due to the recurrent and intermittent nature of sialadenitis symptoms. There is a targeted need for more granular and routine symptom data to understand the maximum potential outcomes of botulinum toxin therapy.
The main limitation in the systematic review is the small number of studies and low level of evidence of the studies included in the review with a high potential for bias toward reporting cases that demonstrate improvement. The ability to perform a meta‐analysis was limited by the non‐availability of translatable outcome data. Furthermore, all the studies in the review represented small case series or case reports with a large degree of heterogeneity among the patient populations, botulinum toxin treatment details, and outcome reporting with nonuniform criteria for measuring symptom improvement. Our study limitations highlight the weaknesses of the literature on the use of botulinum toxin as a treatment for chronic parotid sialadenitis and demonstrate that this is an area of needed research for obstructive salivary gland diseases.
Further controlled studies are needed to examine the dosing, frequency of injections, and long‐term results of botulinum toxin injections. A defined paradigm with routine, high‐dose injections may have the ability to induce permanent gland atrophy and prevent the need for gland removal. Future studies need to include more granular evaluation of symptoms to better understand the optimal timing of injections, level, and length of effective symptom benefit from botulinum toxin injections. Other routes of delivery of botulinum toxin may be more effective. The potential for intraductal botulinum toxin infusion has been described in a study of two patients and is a possible future direction. 33 Finally, there is a need for standardized outcome measures for salivary gland disease so outcomes across centers and studies can be adequately compared.
5. CONCLUSION
Parotid gland chemodenervation is a minimally invasive treatment option for symptomatic chronic sialadenitis refractory to sialendoscopy and/or medical management. Salivary chemodenervation with botulinum toxin injections alleviates obstructive symptoms of gland pain and swelling and provides an alternative to parotidectomy for recurrent sialadenitis.
DISCLOSURE OF INTERESTS
Dr. William Ryan is a Scientific Advisory Board Member of Medtronic and Olympus, and a consultant for Ziteo. The remaining authors have no disclosures.
Supporting information
Table S1. Detailed risk of bias assessment using the National Institutes of Health Quality Assessment Tool for before‐after (pre‐post) studies with no control group
Strohl MP, Chang C‐F, Ryan WR, Chang JL. Botulinum toxin for chronic parotid sialadenitis: A case series and systematic review. Laryngoscope Investigative Otolaryngology. 2021;6:404–413. 10.1002/lio2.558
This study was presented as an oral presentation at the Triological Society Combined Sections Meeting in Coronado, CA, January 25, 2020 (#320).
REFERENCES
- 1. Aubin‐Pouliot A, Delagnes EA, Eisele DW, Chang JL, Ryan WR. The Chronic Obstructive Sialadenitis Symptoms Questionnaire to assess sialendoscopy‐assisted surgery. Laryngoscope. 2016;126:93‐99. [DOI] [PubMed] [Google Scholar]
- 2. Gillespie MB, O'Connell BP, Rawl JW, McLaughlin CW, Carroll WW, Nguyen SA. Clinical and quality‐of‐life outcomes following gland‐preserving surgery for chronic sialadenitis. Laryngoscope. 2015;125:1340‐1344. [DOI] [PubMed] [Google Scholar]
- 3. Delagnes EA, Aubin‐Pouliot A, Zheng M, Chang JL, Ryan WR. Sialadenitis without sialolithiasis: prospective outcomes after sialendoscopy‐assisted salivary duct surgery. Laryngoscope. 2017;127:1073‐1079. [DOI] [PubMed] [Google Scholar]
- 4. Delagnes EA, Zheng M, Aubin‐Pouliot A, Chang JL, Ryan WR. Salivary duct stenosis: short‐term symptom outcomes after sialendoscopy‐assisted salivary duct surgery. Laryngoscope. 2017;127:2770‐2776. [DOI] [PubMed] [Google Scholar]
- 5. Koch M, Kunzel J, Iro H, Psychogios G, Zenk J. Long‐term results and subjective outcome after gland‐preserving treatment in parotid duct stenosis. Laryngoscope. 2014;124:1813‐1818. [DOI] [PubMed] [Google Scholar]
- 6. Guntinas‐Lichius O, Klussmann JP, Wittekindt C, Stennert E. Parotidectomy for benign parotid disease at a university teaching hospital: outcome of 963 operations. Laryngoscope. 2006;116:534‐540. [DOI] [PubMed] [Google Scholar]
- 7. Koch M, Zenk J, Iro H. Long‐term results of morbidity after parotid gland surgery in benign disease. Laryngoscope. 2010;120:724‐730. [DOI] [PubMed] [Google Scholar]
- 8. Baurmash HD. Chronic recurrent parotitis: a closer look at its origin, diagnosis, and management. J Oral Maxillofac Surg. 2004;62:1010‐1018. [DOI] [PubMed] [Google Scholar]
- 9. Lovato A, Restivo DA, Ottaviano G, Marioni G, Marchese‐Ragona R. Botulinum toxin therapy: functional silencing of salivary disorders. Acta Otorhinolaryngol Italica. 2017;37:168‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bushara KO. Sialorrhea in amyotrophic lateral sclerosis: a hypothesis of a new treatment—botulinum toxin A injections of the parotid glands. Med Hypotheses. 1997;48:337‐339. [DOI] [PubMed] [Google Scholar]
- 11. The Oxford Center for Evidence‐Based Medicine: Levels of Evidence . https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009. Accessed December 5, 2020.
- 12. Quality Assessment Tool for Before‐After (Pre‐Post) Studies with No Control Group ; 2014. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed December 5, 2020.
- 13. Scaglione F. Conversion ratio between Botox®, Dysport®, and Xeomin® in clinical practice. Toxins. 2016;8:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guntinas‐Lichius O, Jungehulsing M. Treatment of chronic parotid sialectasis with botulinum toxin A. Laryngoscope. 2002;112:586‐587. [DOI] [PubMed] [Google Scholar]
- 15. Ellies M, Gottstein U, Rohrbach‐Volland S, Arglebe C, Laskawi R. Reduction of salivary flow with botulinum toxin: extended report on 33 patients with drooling, salivary fistulas, and sialadenitis. Laryngoscope. 2004;114:1856‐1860. [DOI] [PubMed] [Google Scholar]
- 16. Capaccio P, Torretta S, Osio M, et al. Botulinum toxin therapy: a tempting tool in the management of salivary secretory disorders. Am J Otolaryngol. 2008;29:333‐338. [DOI] [PubMed] [Google Scholar]
- 17. Kruegel J, Winterhoff J, Koehler S, Matthes P, Laskawi R. Botulinum toxin: a noninvasive option for the symptomatic treatment of salivary gland stenosis—a case report. Head Neck. 2010;32:959‐963. [DOI] [PubMed] [Google Scholar]
- 18. Daniel SJ, Diamond M. Botulinum toxin injection: a novel treatment for recurrent cystic parotitis Sjogren syndrome. Otolaryngol Head Neck Surg. 2011;145:180‐181. [DOI] [PubMed] [Google Scholar]
- 19. Gillespie MB, Intaphan J, Nguyen SA. Endoscopic‐assisted management of chronic sialadenitis. Head Neck. 2011;33:1346‐1351. [DOI] [PubMed] [Google Scholar]
- 20. Reddy R, White DR, Gillespie MB. Obstructive parotitis secondary to an acute masseteric bend. ORL. 2012;74:12‐15. [DOI] [PubMed] [Google Scholar]
- 21. O'Neil LM, Palme CE, Riffat F, Mahant N. Botulinum toxin for the management of Sjogren syndrome‐associated recurrent parotitis. J Oral Maxillofac Surg. 2016;74:2428‐2430. [DOI] [PubMed] [Google Scholar]
- 22. Trapeau C, Foletti JM, Collet C, Guyot L, Chossegros C. Clinical efficacy of botulinum toxin in salivary duct stenosis: a preliminary study of six cases. J Stomatol Oral Maxillofac Surg. 2017;118:349‐352. [DOI] [PubMed] [Google Scholar]
- 23. Graillon N, Le Roux MK, Chossegros C, Haen P, Lutz JC, Foletti JM. Botulinum toxin for ductal stenosis and fistulas of the main salivary glands. Int J Oral Maxillofac Surg. 2019;48:1411‐1414. [DOI] [PubMed] [Google Scholar]
- 24. El Khoury J, Haber E, Nasr M, Hokayem N. Botulinum neurotoxin A for parotid enlargement in cystic fibrosis: the first case report. J Oral Maxillofac Surg. 2016;74:1771‐1773. [DOI] [PubMed] [Google Scholar]
- 25. Koch M, Zenk J, Iro H. Algorithms for treatment of salivary gland obstructions. Otolaryngol Clin N Am. 2009;42:1173‐1192. [DOI] [PubMed] [Google Scholar]
- 26. Plonowska KA, Gurman ZR, Humphrey A, Chang JL, Ryan WR. One‐year outcomes of sialendoscopic‐assisted salivary duct surgery for sialadenitis without sialolithiasis. Laryngoscope. 2019;129:890‐896. [DOI] [PubMed] [Google Scholar]
- 27. Hughes A, Choi S. Does intraglandular injection of botulinum toxin improve pediatric sialorrhea? Laryngoscope. 2018;128:1513‐1514. [DOI] [PubMed] [Google Scholar]
- 28. Jongerius PH, van den Hoogen FJ, van Limbeek J, Gabreels FJ, van Hulst K, Rotteveel JJ. Effect of botulinum toxin in the treatment of drooling: a controlled clinical trial. Pediatrics. 2004;114:620‐627. [DOI] [PubMed] [Google Scholar]
- 29. Porte M, Chaleat‐Valayer E, Patte K, D'Anjou MC, Boulay C, Laffont I. Relevance of intraglandular injections of Botulinum toxin for the treatment of sialorrhea in children with cerebral palsy: a review. Eur J Paediatr Neurol. 2014;18:649‐657. [DOI] [PubMed] [Google Scholar]
- 30. Chan KH, Liang C, Wilson P, Higgins D, Allen GC. Long‐term safety and efficacy data on botulinum toxin type A: an injection for sialorrhea. JAMA Otolaryngol Head Neck Surg. 2013;139:134‐138. [DOI] [PubMed] [Google Scholar]
- 31. Luvisetto S, Gazerani P, Cianchetti C, Pavone F. Botulinum toxin type a as a therapeutic agent against headache and related disorders. Toxins. 2015;7:3818‐3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walter U, Dressler D. Ultrasound‐guided botulinum toxin injections in neurology: technique, indications and future perspectives. Expert Rev Neurother. 2014;14:923‐936. [DOI] [PubMed] [Google Scholar]
- 33. Schwalje AT, Hoffman HT. Intraductal salivary gland infusion with botulinum toxin. Laryngosc Investig Otolaryngol. 2019;4:520‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Detailed risk of bias assessment using the National Institutes of Health Quality Assessment Tool for before‐after (pre‐post) studies with no control group


