Abstract
Older adults with diabetes are heterogeneous in their medical, functional and cognitive status, requiring careful individualization of treatment regimens. However, in the absence of detailed information from clinical trials involving older people with varying characteristics, there is a lack of evidence-based guidance which is a significant limitation of current approaches to care. It is important to recognize that older people with diabetes may vary in their profiles according to age category, functional health, presence of frailty and comorbidity profiles. In addition, all older adults with diabetes require an individualized approach to care, ranging from robust individuals to those residing in care homes with limited life expectancy, those requiring palliative care, or end-of-life management. With this background, an International Workshop of experts in the field from multidisciplinary backgrounds set about the important task of identifying key research gaps in areas which may influence large numbers of older adults with diabetes, and in turn, enhance our scientific understanding and their clinical outcomes.
Background and Rationale
Over the past decade, several diabetes organizations and societies have published position statements, guidelines, and consensus reports to guide the management of older adults with diabetes with consideration of these unique challenges(1–11). In addition, there have been special collections of published articles with a major focus on diabetes and older adults where the emerging science, the complexity of management, and goals of care have been discussed(12) in varying situations such as the presence of cognitive dysfunction(13) or the management of inpatient hyperglycemia(14). This literature supports the view that many factors necessitate different approaches to diabetes care in older adults compared with younger adults.
Older adults are a heterogeneous population and are frequently defined based on chronological age, functional status, or presence of comorbid conditions. This variability in definitions is seen in the studies in current literature focusing on older adults. In this regard, we may have to define “older adults” in context of the purpose of the study or review. In the past decade, various guidelines and consensus reports have provided clinical recommendations based on presence of severe comorbidities, cognitive status and functionality, avoiding chronological age as the defining factor. The three groups of older adults with diabetes are usually defined for the purposes of allocating recommendations are: (1) individuals in good health with little or no cognitive or functional impairment and long-life expectancy (e.g. >10–15 years); (2) those with some comorbidities and mild disability; and (3) those with high comorbidity and/or disability, and shorter life expectancy (e.g. <5 years).
The recommendations from these guidelines provide important information for clinicians providing care for older adults with diabetes. However, older adults, particularly those with evidence of functional loss, frailty, and cognitive impairment, remain under-represented in clinical trials leading to management guidelines that rely on expert opinion only. In addition, research and clinical guidance on the care of older adults with type 1 diabetes are almost entirely lacking. Thus, there is an increasing and urgent need to develop evidence-based treatment recommendations for this growing population that has unique and often unmet needs.
Search strategy and selection criteria:
An International Geriatric Diabetes workshop was held in Boston on September 23–24, 2019 to address the need for evidence-based recommendations in management of older adults with diabetes. The workshop organizers selected topics that were deemed most important in clinical practice, and had significant knowledge gaps. The participants included authors of this paper, clinicians, leading researchers, policy leaders, patient representatives, and industry partners focusing on the care of older adults with diabetes. Each speaker, selected for their expertise in the given topic, performed literature searches for relevant articles, and also provided their own recommendations based on their extensive expertise. Speakers discussed current evidence-based recommendations in the diagnosis, prevention, and management of diabetes in older adults, and then identified important evidence-gaps, research questions, and possible strategies to fill the gaps in each topic area. Figure 1 shows the schematic representation of the topics covered in the workshop. Each presentation was followed by a group discussion and was summarized by the workshop chairs. A summary of the discussion in each topic area is presented in this paper.
Figure 1.
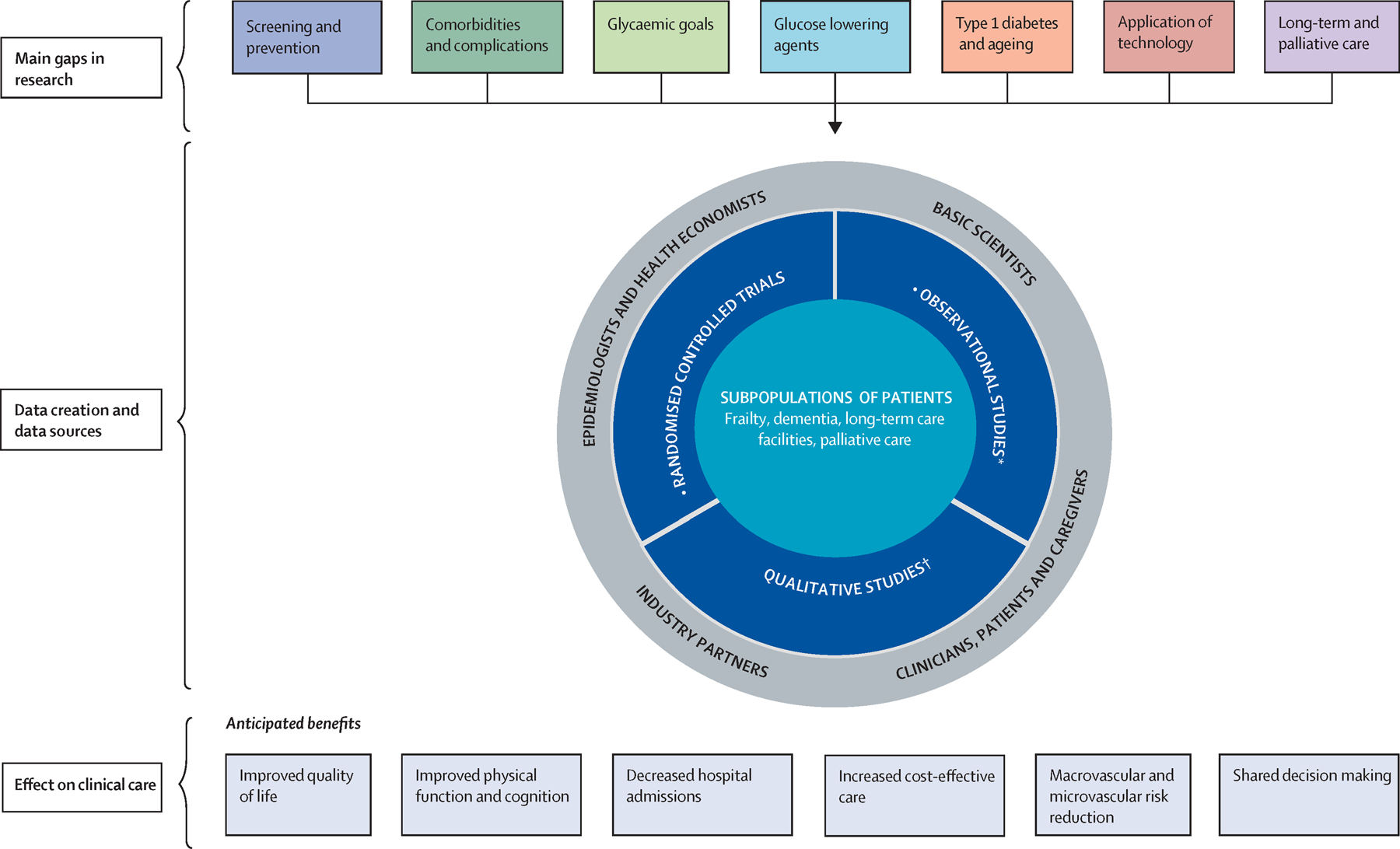
Research roadmap for diabetes in older people, with a focus on establishing the most important outcomes and a focus on registry-based real-world populations Modified from Sinclair and colleagues.15
*With a focus on establishing the outcomes most important to older adults. With a focus on registry-based real-wold populations.
Screening and Prevention of Diabetes in Older Adults
Current Evidence
Several international organizations have developed criteria for screening for diabetes. Although large data sets have found that mean glycemia increases with age(15–18), most of the guidelines do not have age-specific criteria or recommendations for screening frequency based on age(11, 19, 20),(21). In addition, data suggest that if only an FPG or an HbA1c is done to screen, a substantial number of patients with IGT or diabetes will be missed(22, 23). If an OGTT is performed, it may diagnose diabetes in some individuals who were otherwise diagnosed as having IGT or normal glucose levels by other screening methods. However, how such a diagnosis or its treatment would alter clinically meaningful outcomes in an older population is unclear.
Several interventions have been shown to be effective at preventing diabetes. The Diabetes Prevention Program (DPP) investigated the effects of life-style modification, metformin, or placebo in the prevention of diabetes in individuals at high risk for diabetes development (IGT plus impaired fasting glucose in overweight/obese subjects), and found that a lifestyle intervention aimed at weight loss and increased physical activity was highly effective (58% reduction) and that metformin was less effective (31% reduction) in preventing progression to diabetes(24),(25). Of note, the DPP lifestyle intervention was relatively more effective in the sub-cohort ≥age 60 at baseline, with a 71% reduction in diabetes development than in the younger groups (48–59% reduction). In other studies, acarbose(26), rosiglitazone(27) and pioglitazone(28) were found to be effective in preventing diabetes in older adults. However, no studies have shown that preventing diabetes in older people alters clinically important outcomes. All diabetes organizations have acknowledged the long-time period required for the development of complications and suggested that screening is not necessary when life expectancy is short enough that benefits are not likely.
Research Gaps
Whether age-specific criteria (regarding screening methods, frequency, and glycemic levels) should guide screening decisions in older adults is unclear. No studies have demonstrated that screening for diabetes and subsequent treatment alters clinically meaningful outcomes. In addition, there is no universal agreement regarding which outcomes should be measured. As there are not enough older people with multiple comorbidities in screening studies, there is also a lack of understanding regarding possible implications of those comorbidities and related treatments on the screening tests and the risk of diabetes. It is unclear if an OGTT should be done in patients who have prediabetes by HbA1c or FPG to identify further cases of diabetes, or if an OGTT should be done in patients who have normal screening values for these parameters. The cost implications of such a program need to be considered carefully, particularly since the benefits have not been defined.
Comorbidities and Complications
Current Evidence
One of the great clinical challenges of managing diabetes in older adults is that the disease is frequently accompanied by multiple comorbidities(29, 30). Based on concepts such as competing mortality and lag time to benefit(31), classifying older patients by comorbidities has been viewed as an important way to individualize the intensity and approach to diabetes management. There have been few studies to classify older patients by comorbid conditions, but these few studies have been the basis for the current three-tiered management system adopted by several diabetes care guidelines.
In one study(29), older adults with diabetes (age 57–85 years) were classified into three distinct subgroups according to the presence of 14 highly prevalent comorbid conditions using latent class analysis to identify those more or less likely to benefit from intensive glycemic control. While relying on a particular pattern of comorbid conditions alone may not be sufficient to guide all treatment decisions, a key finding was that those with cardiovascular disease and six or more comorbid conditions were a subgroup unlikely to benefit from intensified therapy. In another study(30), the presence of certain health status characteristics was shown to influence the ability of an individual to self-manage his or her diabetes and thus increased the risk of worse clinical outcomes. Separation into three groups based on health status characteristics, including comorbid profiles, appeared to be related to mortality risk as previously shown(29,31). Data from the US Health and Retirement study also found that this approach of separation into three groups was of value in determining how clinically complex individuals with diabetes can be effectively managed and what research is necessary in this area(32, 33).
Two further studies provide additional insight into comorbidity profiling and outcomes in older adults with diabetes(32,33). One study using a diabetes simulation model showed that a combination of multiple comorbid illnesses and functional impairments was a more important predictor of limited life expectancy and diminishing benefits of intensive glucose control, than age alone(32). Another 5-year observational study characterized participants with type 2 diabetes into different comorbidity groups based on the use of a patient-reported measure (Total Illness Burden Index) and observed the occurrence of cardiovascular events according to levels of glycemia measured by HbA1c(34). Those with an HbA1c at baseline of 7.0%(53 mmol/mol) or less with low to moderate comorbid levels had significantly fewer cardiovascular events compared with those in the high comorbid group. Of note, the latter study included a relatively young group of older adults, and causal inferences were not possible. However, together these studies suggest that the comorbidity profile should be considered when tailoring glycemic goals and glucose-lowering therapies in older adults with type 2 diabetes.
Research Gaps
There are many important knowledge gaps related to comorbidities and diabetes. At present, we do not have a reliable comorbidity classification system that identifies clinically distinct subgroups of older patients. In addition, not all comorbidities are the same, and the studies to date have not accounted for disease severity. Thus, clusters and counts are difficult to operationalize in clinical practice, since types and severity of the comorbid conditions likely matter more than an overall count. Newer classification systems also need to consider additional independent variables, such as frailty and sarcopenia, and should be externally validated beyond the original development data set. An alternative to classifying patients based on clusters of baseline comorbidities is to classify patients based on risk prediction models for premature mortality and adverse drug events such as hypoglycemia(35). Apart from improving the approach to patient classification, evidence is needed regarding optimal targets for glycemic control and drug selection for the distinct classes of patients. Even after establishing a classification system, numerous research questions regarding implementation will remain.
Glycemic Goals
Current Evidence
In older adults with diabetes, a multidimensional and individual treatment and management approach is needed(36). Microvascular complications develop over time, and for many older patients with a limited life expectancy, intensive glycemic treatment will offer no net benefits. Factors such as functional status, comorbidities, life expectancy, social factors, and patient preferences need to be considered. These will also determine the appropriate target ranges for glycemia. Indeed, many clinical practice guidelines have recognized the need for age-specific guidelines and have proposed treatment goals that align with older patients’ unique characteristics and goals of care.
Several observational studies show a J-shaped relationship between HbA1c and mortality in older adults(37),(38),(39). Threshold values for HbA1c at which mortality is increased are generally <6 – 6.5% (42–48 mmol/mol) and >7.5–10% (58–86 mmol/mol). In addition, the mortality risks associated with lower HbA1c values are amplified in patients taking medications associated with hypoglycemia (e.g., insulin)(40). In fact, secondary analysis of trial data in ACCORD shows greater mortality risk for older adults even with high HbA1c if they were on intensive therapy(41).
Owing to the dearth of acceptable, high-quality data in the older population with diabetes, guideline-directed glycemic targets in older adults(42),(2),(1),(6),(11) are generally expert opinion-based. They are generally designed to balance the risks associated with acute hyperglycemia and microvascular disease, while taking into account comorbidities, life expectancy, presence or absence of complications, polypharmacy, hypoglycemia and other adverse events, treatment burden, and social determinants of health. However, new information is emerging that may well influence future recommendations in glycemic control in older adults. A cross-sectional study(43) has shown an association between diabetes and disability, partially explained by glycemic control (higher risk for those with HbA1c ≥8% (64 mmol/mol). Several longitudinal studies have also shown a relationship between diabetes and increased frailty and mobility disorders(44),(45), as well as worse cognitive outcomes(46) in those with poor glycemic control.
As described in the section on comorbidities, three groups of older adults with diabetes are usually defined for the purposes of allocating recommendations for glycemic targets and thresholds: (1) individuals in good health with little or no functional impairment and long life expectancy (e.g., >10–15 years); (2) those with some comorbidities and mild disability; and (3) those with high comorbidity and/or disability, and shorter life expectancy (e.g., <5 years). In general and even in the absence of direct data,, recommended targets for group 1 are HbA1c levels of 7–7.5% (53 – 58 mmol/mol), with group 2 at 7.5–8% (58 – 64 mmol/mol), and group 3 having levels 8 – 8.5% (64 – 69 mmol/mol). Lower targets are deemed appropriate in some patients based on shared decision-making, while considering the safety and tolerability of therapy.
Unfortunately, many older adults with diabetes may be over-treated. For example, about 50% of patients >65 years with diabetes have an HbA1c <7% (53 mmol/mol)(47),(48) while using insulin or sulfonylurea, regardless of their functional status. This creates a potential for harm, particularly with the accompanying burdens of the treatment (e.g., polypharmacy, monitoring, adverse events, and costs) and increased risk of hypoglycemia with insulin and sulfonylurea in the older population(49),(50). Patient preferences(1, 2, 6, 42), care-giver perspectives(when appropriate(9)), and treatment adherence(51) are important aspects of diabetes management in all adults. However, in older adults they are essential in determining appropriate HbA1c targets and in identifying specific treatments to achieve those targets.
Research Gaps
The heterogeneity of older patients with diabetes raises the issue of HbA1c targets that take into account the personal characteristics and the care environment and the agreed-upon benefits to be achieved. Further complicating this is the fact that many older people with diabetes have conditions that alter red blood cell (RBC) life span such as anemia, making the HbA1c a less reliable measure of glucose control(10). Thus, an alternative to HbA1c as a glycemic target, especially in populations with high prevalence of these conditions, such as those residing in subacute care facilities or nursing homes, is needed(9). One such approach may be related to the use of fasting blood glucose variability which in one study of older adults (mean age 60 years) was shown to be an effective predictor of ischemic stroke(52).
One approach to HbA1c goal-setting is to consider establishing a target range, for example 7–8% (53–64 mmol/mol), and then tracking how often the measured levels reside within that range. This method avoids looking only at the absolute levels, which are subject to both biologic and measurement variability, and focuses attention on upper and lower bounds that are appropriate for an individual to balance risks and benefits. This may have the advantage of moving the locus of glycemic control from a dichotomous paradigm (i.e., good vs poor control) to a more continuous and comprehensive one.
The evidence of benefit to older adults by achieving and maintaining lower HbA1c levels (e.g., <7% [53 mmol/mol]) is limited, and there is concern about risk and lag time to accruing benefits as part of shared decision-making with patients(31).
The impact of maintaining target HbA1c levels on other outcomes, such as functional deterioration, cognitive impairment, falls, institutionalization, hospitalization, and premature death, has not been studied in any detail. Similarly, the impact of glycemic targets on quality of life measures, diabetes-related distress, or disease burden indices is not well studied. There are scarce data from interventional studies assessing the effectiveness of achieving different glycemic targets to improve or maintain functional status in older adults(53, 54).
Last, there is a need to examine clinical decision support tools (e.g., shared decision-making), electronic health record tools (e.g., decision aids and clinical reminders), quality measures to track potential overtreatment, and real-life observational studies to assess the relationship of HbA1c to micro- and macro-vascular disease risk, functional status, and quality of life.
Glucose-lowering Agents in Older Adults
Current Evidence
Most clinical guidelines for the treatment of diabetes now recommend personalizing therapy through a shared decision making approach. In the majority of cases, these guidelines have relied on data extrapolated from trials in younger, generally healthier individuals or are based on expert consensus opinion. Minimizing hypoglycemia is often a key goal when guidelines are tailored for older people, and guidance often precludes the use of glyburide (glibenclamide) because of its long half-life and propensity for provoking hypoglycemia, although great caution is recommended in the use of any sulfonylurea where there are additional risk factors for hypoglycemia. Similarly, complex insulin regimes are not advised because of the excess risk of unwanted hypoglycemia, particularly in those with a shortened life expectancy or who have extensive comorbidity profiles(9).
Following advice issued in December 2008 by the U.S. Food and Drug Administration (FDA) to the pharmaceutical industry, all new agents for the treatment of diabetes have had to undergo long-term CardioVascular Outcomes Trials (CVOT) to demonstrate safety. A detailed review of this area by an expert review group is available (55) and the results of subgroup analyses relating to event rate by age of various cardiovascular outcome trials has also been recently reported (56). All manufacturers of dipeptidyl peptidase 4 inhibitors (DPP-4is), glucagon-like peptide-1 receptor agonists (GLP-1RAs)(57–63) and sodium–glucose cotransporter-2 inhibitors (SGLT-2is), (64),(65, 66) have carried out many CVOTs. Overall cardiovascular safety has been shown across all three classes using the composite Major Adverse Cardiovascular Events (MACE) (cardiovascular death, non-fatal myocardial infarction, non-fatal stroke). SGLT-2is also appear to have a more marked effect on preventing hospitalization for heart failure and progression of kidney disease(67–69). There are some results of interest for older age group. For example, in the LEADER trial with Liraglutide, those age ≥50 and CVD at baseline had a reduction in primary outcome (n= 7,598; HR 0.83); whereas those ≥60, but no established CVD, had a significantly adverse outcome (n=1,742; HR 1.20, p: 0.04), except in a small subgroup of those age ≥75 years(61, 67). A similar trend was seen in the HARMONY trial with Albiglutide(57), comparing participants’ age <65 to those ages 65–75 and a smaller number ≥75 years. In the EMPA-REG trial with Empagliflozin, all with established CVD, those age <65 at baseline had no significant reduction in the primary outcome (n=3,793, HR 1.04); whereas those age ≥65 did (n=3,127, HR 0.71). Overall, the limited data in the older adults suggest there is a need for further studies to compare the differences in the outcomes. A comprehensive, evidenced-based review of diabetes care in older people has recently been published(68).
Research Gaps
A major limitation of most of the RCTs in general is that the mean age of participants varied between 62 and 66 years, and a smaller proportion of adults ≥65 years was recruited. This is of much relevance in view of the increasing population in age group >65 years, developing CVD including HF, as well as advancing renal complications, where the outcomes with the newer agents are not clear. Additional safety data are needed in this age group. Another limitation is the uncertainty regarding the potentially adverse renal effects with SGLT2i in the long-term (>3 years) in older population (69). Other questions in regards to an older population include the need for trials dedicated to older age group and trials in those who are frail with multiple comorbidities. A recent guidance from the Food and Drug Administration (FDA) will go a long way in improving enrollment of the number of participants >65 years, and those with comorbidities like CKD and CVD in trials of drugs for glycemic control(70). Finally, significant questions remain about the optimal choice of drugs for T2D in older adults, optimal combination therapy (which is usually required), and the optimal sequence of their use.
Older adults with T1D
Current Evidence
People with type 1 diabetes are living longer in Western societies(71). While the survival gap between people with and without diabetes remains(72), life-expectancy for people with type 1 diabetes is getting closer to the population average(73). Studies of people with type 1 diabetes who survive into older age have identified a number of protective factors, including: a family history of longevity; elevated HDL; good glycemic control (HbA1c <7.8%, 62 mmol/mol); non-smoking status; and low alcohol consumption(74). Survival may be related to genetic factors and residual beta-cell function(75). It is also important to note that the development of type 1 diabetes is not restricted to children and adolescents and continues throughout adult life, extending into old age(76),(77). Hence, the population of older people living with type 1 diabetes is a heterogeneous group, with varying diabetes duration and potential underlying pathology.
There are multiple hazards for older people with type 1 diabetes, including: hypoglycemia; cognitive function, and comorbidity. Hypoglycemia is an important hazard, increasing the risk of falls, fractures, and hospitalization. The incidence of hypoglycemia (including severe hypoglycemia) in older people with type 1 diabetes is increased(78), and hypoglycemia awareness can become impeded in older age(79). Cognitive impairment is another important hazard that could lead to insulin errors and hypoglycemia. Studies have reported higher risks for cognitive dysfunction in older people with type 1 diabetes compared to people without diabetes(80, 81). Comorbidities are also more prevalent in older people with type 1 diabetes, with negative impacts on mental and physical function. Collectively these comorbidities can increase care complexity and contribute to frailty. There have been no conclusive clinical trials assessing optimal metabolic targets in older people with type 1 diabetes, although some observational studies have shown lower diabetes complications in older people with more intensive control of cardiovascular risk (cholesterol and blood pressure)(82).
Research Gaps
Older age is associated with changes that mediate metabolic processes which may affect glucose regulation and increase the risk of hypoglycemia. These include reduced activity and nutrition and alterations in the fat to muscle ratio. These age-related changes may affect insulin sensitivity, so we need to understand how to compensate for them clinically and provide better self-management support.
Older people’s experiences of aging and how it affects their diabetes is not well understood. Such an insight would be important in understanding the issues older people with type 1 diabetes face. In addition, as older people become less independent, their reliance on formal and informal caregivers increases, so identifying the perspectives of relatives and caregivers also need to be considered(83). In the same context, there is a need for care models and education of providers in different settings, such as long-term care facilities where it is assumed that older adults have T2D, which leads to mistakes such as stopping insulin because of the fear of hypoglycemia.
There is also limited knowledge about the role of newer technologies used by older adults with type 1 diabetes such as insulin pumps or CGM in these facilities. Finally, while international expert-based clinical guidelines are now available(84), there are still significant evidence gaps in relation to optimal approaches for the clinical assessments of age related risks and for effective clinical intervention to minimize risk and promote physical and mental function. It is also important to recognize that changing care approaches can be more challenging for older people as they may have to adjust long-learned behavioral patterns; hence, we need to identify new approaches for self-management support in helping people with type 1 diabetes prepare for and adjust to older age.
Technology Use in Older Adults
Current Evidence
Despite good evidence in younger individuals with type 1 diabetes, with respect to the efficacy and safety of insulin pump use, limited data exist for those over the age of 65, as many of the randomized controlled trials excluded older people(85, 86). More recent studies using sensor augmented pump therapy and automated insulin delivery, specifically the hybrid closed loop G670 system, included individuals over the age of 60 and reported a similar improvement in glucose indices as for the entire cohort(87, 88). Real life data for 1,946 individuals ≥60 years of age using the hybrid closed loop demonstrated similar improvements in glucose indices when compared with the younger cohort after initiating auto mode(89). The Opt2mize trial tested the effect of insulin pump therapy vs MDI (multiple daily injections) in individuals aged 35–75 years with type 2 diabetes inadequately controlled on MDI, and demonstrated an improvement in HbA1c that was independent of diabetes duration and cognitive score using the MoCA (Montreal Cognitive Assessment) (90).
Good evidence exists in individuals with type 1 diabetes with respect to the efficacy of continuous glucose monitoring (CGM) in improving glucose control and reducing hypoglycemia rates(91–93); however, these studies have generally excluded older individuals. In the more recent DIAMOND trial of CGM, 20% of individuals were over the age of 60. Over the 24 weeks of the trial, a 0.6% (6.6 mmol/mol) reduction in HbA1c was noted with no significant interaction of the effect according to age (94). Another trial conducted in individuals ≥ 60 years with type 1 and type 2 diabetes using multiple daily injections demonstrated an HbA1c reduction of 0.4% (4.4 mmol/mol)(95). The Wireless Innovations for Seniors With Diabetes Mellitus (WISDM) trial randomized 200 individuals with type 1 diabetes over the age of 60 years to CGM vs. finger-stick glucose monitoring. Over 6 months, CGM was associated with significant reductions in time spent in the hypoglycemic range (< 70 mg/dL (3.9 mmol/L) and < 54 mg/dL (3.0 mmol/L)) and in severe hypoglycemic events. The group randomized to CGM also had significantly greater time-in-range (70–180 mg/dl (3.9–10.0 mmol/L)) and decreases in hyperglycemia and HbA1c (Pratley et al, in press). Although the vast majority of studies assessing the use of CGM have been conducted in type 1 diabetes, trials of multiple daily injections in individuals with type 2 diabetes, including ~50% over the age of 60, demonstrated a reduction in HbA1C ranging from 0.3% to 0.5%(3.3 mmol/mol to 5.5 mmol/mol)(94, 96).
There is increasing evidence for the role of mobile phone applications for the management of diabetes. A recent review of RCT that included individuals ≥55 years with type 2 diabetes reported an improvement in various outcome measures including HbA1c, body weight, physical activity, blood pressure, and lipid profiles(97).
Research Gaps
It is not clear if the existing data with respect to the use of insulin pumps and CGM in aging populations may be generalizable to individuals with cognitive/functional deficits. It is also not clear how the ability of the individual to utilize technology may be assessed. As both insulin scheduling and coping with hypoglycemia become a challenge in older adults with cognitive/functional deficits, it well may be that adapting existing technological tools (insulin pump, CGM) by creating a platform that also includes diabetes-related cognitive rehabilitation modules such as reminders, alerts, and caregiver/case manager interaction modules may benefit this population. In this context, it is worthwhile mentioning the ongoing technological advances in glucose management in older adults (TANGO) study that will assess the effectiveness of CGM enhanced by a diabetes management platform in the care of individuals with type 1 diabetes over the age of 65. We need more data to understand how caregivers can facilitate the use of technology used by older adults. It is important to integrate the perspectives of older adults to improve and ease adaptation of these technologies (e.g., larger buttons, better contrast, etc.) in this population.
Other interesting alternatives to pump therapy that may have a role in aging populations with diabetes are Bluetooth-enabled insulin delivery devices that enable tracking of insulin dosing through a smart phone app. There are several devices on the market. Some use reusable pens with a smart phone app tracking and advisor application. Some are caps that are put on disposable insulin pens and track insulin dosing. This technology, combined with a bolus advisor, alert system, case management, and the addition of other technology-based health behavior change techniques, could potentially aid in the unmet needs of older people with cognitive deficits who need a basal bolus regimen along with support for insulin dosing to prevent hypoglycemia.
There is a lack of long term studies on the role of mobile phone applications in the management of diabetes, and very few of the current studies have included individuals over the age of 70 or individuals with cognitive/functional impairment(97). And finally, studies on the development, design, and efficacy of educational/training programs for the use of technologies in older patients and their caregivers, including cost-effective assessments, are needed.
Diabetes in Long Term Care (LTC) and Palliative care
Current Evidence
It has been recognized for some time that for the residents of the LTC facilities, there is little evidence of structured diabetes care or clear oversight on the safety and efficacy of different treatment regimens(98). Together, a timely position statement of diabetes in long-term care and skilled nursing facilities(4) and a recent comprehensive review of this area(94) have provided priority lists of actions that if undertaken are likely to lead to an improvement in the quality of care provided in these settings. Older adults residing in LTC are not homogenous in their clinical and functional characteristics. In addition, they frequently need bidirectional care transitions between the hospital, the care home, skilled nursing facility for rehabilitation, assisted living, or their home. LTC residents have a high prevalence of diabetes and multimorbid states, high admission rates to hospitals because of metabolic decompensation or infection, and poor quality of life(9, 99). Some of these adverse outcomes are likely owing to poor diabetes training and expertise of care staff combined with little implementation of diabetes care protocols, the lack of agreement on glycemic targets, and inconsistent and often unsafe insulin administration regimes(100).
By the end of 2018, ten intervention studies with focus on diabetes care in the LTC facilities had been conducted. The study topics included staff education(101, 102), retrospective review using guideline adherence(103) or comparative effectiveness of basal insulin approaches(104), diabetes medication withdrawal(105), teleconsultation between an endocrinologist and care staff (106), and four randomized clinical trials which included resident education(99), use of sliding scale insulin vs. basal-bolus insulin(107), and the effect of linagliptin (a DPP4-inhibitor) or other oral agents on hypoglycemia risk and glycemic control (compared with insulin glargine)(108, 109). These studies have in general been short term (<6 months) and with small sample size, and in some cases have lacked objectivity and consistency in outcome assessment. None have had a major influence on clinical practice but offer some important support for good clinical practice in promoting staff education and in reducing hypoglycemia risk by avoiding sliding scale insulin methods and over-intensification of treatment approaches.
Research Gaps
There are significant shortfalls in our knowledge of care home residents with diabetes, those with diabetes in other long-term aged facilities, and particularly those with diabetes in palliative care settings. The expression ‘end of life care’ may be applied to this latter group where there is required new momentum to undertake both descriptive and intervention studies for this neglected area of study and to assess the value of implementing clinical guidelines(110, 111).
There is a paucity of controlled clinical studies of intervention in the area of LTC and during palliative care. A study of doctors and nurses working in the palliative care setting suggested the use of a range of practices and BG testing frequencies based on experience and not according to robust evidence(112). There is moderate evidence describing the characteristics of older people with diabetes residing in long-term care or care homes(5) but limited descriptive information for end of life adults with diabetes(2). The value of a multidimensional approach (nutritional planning, exercises, staff education, hypoglycemia risk management, and medication review) to improving key outcomes such as reduction in hospitalization rate, hypoglycemia rate, infection rate, maintenance of physical function, and quality of life is also not well understood. Studies are also needed to understand variations in care provided in different residential settings and how they impact outcomes. In long-term care facilities, few if any studies have addressed concerns regarding the ratio of patients: staff and temporary staffing, and the role of the clinical pharmacist in diabetes management. Data are also needed in regards to stakeholders and their socioeconomic burden in order to inform changes in care practices for stakeholders.
Critical Studies to Guide Implementation
Current Evidence
In the absence of an adequate evidence base, guidelines are almost exclusively based on expert opinion and extrapolated from trials in younger or healthier populations(1–11). Thus, additional studies are urgently needed to 1) identify which older adults with diabetes would benefit from which diabetes interventions and 2) determine which outcomes are most important for subpopulations of older adults with diabetes.
Research Gaps
Since relatively little is known about diabetes in older adults, a wide variety of studies are needed across the spectrum of older adults with diabetes. Different types of studies, including observational, qualitative, and intervention studies must be conducted to provide complementary insights. Future research should also explicitly focus on the wide spectrum of older adults, from healthier young-old adults as well as frail, cognitively impaired old-old adults residing in nursing homes.
There are many critical knowledge gaps in geriatric diabetes. We outline three specific gaps below. First, it is currently unclear when older adults experience symptoms of hyperglycemia. For many older adults with limited life expectancy, the goal of glycemic treatment is focused on avoiding symptomatic hyperglycemia. However, it is uncertain what levels of hyperglycemia and for what period of time lead to specific symptoms. For example, does a 30-minute glycemic excursion to glucose level >350 mg/dL (19.4 mmol/L) lead to fatigue? Does a 1-day glycemic excursion to glucose level >200 mg/dL (11.1 mmol/L) lead to worsening urinary incontinence or infection risk? Patient characteristics may also be important factors in whether hyperglycemia leads to symptoms or poor outcomes. Since the goal of glycemic treatment in many older adults with limited life expectancy is to avoid symptomatic hyperglycemia, determining when hyperglycemia results in symptoms could inform the clinical care of many older adults with diabetes.
Second, the prevalence and impact of hypoglycemia is poorly understood in this population. For example, how frequently does hypoglycemia lead to falls and fractures in older adults? How often do older adults experience hypoglycemia during sleep? What factors lead to hypoglycemia in older adults? Skipped meals? Cognitive or visual impairment leading to dosing errors? Does hypoglycemia accelerate cognitive decline? A more granular understanding of hypoglycemia and its impact on older adults could provide pivotal information that could inform glycemic treatment decisions and highlight potential targeted interventions to decrease risk.
Third, it is currently unclear how best to care for older adults approaching the end of life and receiving palliative care. Although there is a general recognition that decreased oral intake often leads to less hyperglycemia, lessening the need for glucose lowering medications, the appropriate timing and extent of deintensification is unknown. This results in a substantial burden of both hyperglycemia and hypoglycemia in these patients(113).
Conclusion
With the increasing number of older adults with diabetes around the world(114), and the emerging recognition that goals of care may vary according to the health profiles of these individuals, we feel it is timely to emphasize the importance of further research. This will provide a more robust platform to develop evidence-based recommendation to improve outcomes of interest in this population. We hope that this paper will be of interest and utility for future investigators in diabetes and aging.
Critical Knowledge Gaps
Age specific criteria for the diagnosis of impaired glucose tolerance (IGT), pre-diabetes, and diabetes
Best method to screen for diabetes in older adults and the role of the oral glucose tolerance test (OGTT) in varied settings
Impact of intervention on clinically meaningful outcomes post-screening
Important Research Questions
Should screening criteria (methods, glycemic levels, and frequency) for pre-diabetes and diabetes be age specific?
What are the implications for screening for diabetes on interventions and clinically relevant outcomes in older adults?
Can existing large population or cohort-based data sets be used to establish new criteria for IGT pre-diabetes, and diabetes in older people?
Should older people be screened with both a hemoglobin A1c (HbA1c) and fasting plasma glucose (FPG) test instead of one or the other?
Is an OGTT needed in older people who have FPG and/or HbA1c levels in normal or prediabetes ranges?
Critical Knowledge Gaps
Development of a reliable comorbidity classification system that identifies clinically distinct phenotypic subgroups of older patients.
Inclusion of adequate-sized subgroups of older patients with diabetes and prediabetes in major randomized controlled trials of diabetes prevention, treatment and care.
How glycemic control-outcome relationships differ in older age compared with younger age persons with pre-diabetes and diabetes.
How the effects (glucose lowering, side-effects, and long-term consequences) of diabetes medications differ by age.
Important Research Questions
How do we identify the important subgroups of older patients with diabetes?
What specific glycemic goals should be recommended by subgroup?
What is the optimal study design for acquiring the evidence for treatment selection by subgroup?
How do we incorporate health status into clinical decisions in busy practice?
How do we communicate with older patients about changing health status and goals?
How do we acquire the evidence to demonstrate whether or not personalizing diabetes care can improve outcomes for older patients?
Critical Knowledge Gaps
Individual glycemic goals to optimize functional, cognitive, and quality of life outcomes as well as use of resources (e.g., hospitalization, long-term care, institutionalization).
Risks and effectiveness of different HbA1c thresholds in older populations.
Role of HbA1c target ranges (e.g., 7–8% or 53–64 mmol/mol) and time-in-range as glycemic goals, balancing risks and benefits, in older adults.
Effectiveness of clinical decision support tools (e.g., shared decision making), electronic medical record tools (e.g., decision aids and clinical reminders), and quality measures to guide clinical care.
Important Research Questions
Develop real life observational studies to assess the relationship of HbA1c to microvascular and macrovascular disease risk, mortality, functional status, and quality of life in older adults.
What are the appropriate HbA1c targets to minimize different outcomes (e.g., functionality and quality-of-life) in older adults with different categories of risk (e.g., young-old or old-old age, frailty, mild or severe disability, impaired cognition)?
Is it feasible and useful to implement alternative measures of glycemic control to HbA1c?
Are current technologies to improve glycemic control, such as CGM, cost-effective, and do they promote the empowerment of older adults with diabetes?
Critical Knowledge Gaps
Lack of focus on participants who are older and frail with diabetes in randomized control trials (RCTs) and clinical development programs for medications
Data on the safety and efficacy of glucose-lowering drugs in older populations
Identifying the most important outcomes with different classes of medications for older individuals with diabetes by patient type, complications and comorbidities
Defining an optimal treatment approach in different settings such as home, rehabilitation facilities, hospitals, or long-term care
Important Research Questions
The value of long-term RCTs using newer agents in older populations focusing on outcomes of interest in older age and relative cost-effectiveness.
Results of pooled analyses of the efficacy and safety of specific drug classes within clinical development programs, cardiovascular outcome trials (CVOT), and real-world evidence from databases around the world.
What is the optimal sequence of drugs for type 2 diabetes management in older adults with different categories of risk (e.g., young-old or old-old age, different racial groups, frailty, mild or severe disability, impaired cognition, various patient preferences)?
What are optimal combinations of drugs in specific populations of older adults, including those with cognitive and physical decline? What is the add-on value of new classes of glucose-lowering medications?
What are the optimal glucose-lowering treatments in older patients with comorbidities including atherosclerotic cardiovascular disease (ASCVD), heart failure and chronic kidney disease (CKD)?
What are the effects of SGLT2 inhibitors on mechanisms of reducing progression of heart failure and on long-term renal outcomes?
What is the optimal approach in different settings such as hospitalized older adults or long-term care residents?
Critical Knowledge Gaps
National and global data sources for the epidemiological profiling of type 1 diabetes
Impact of aging on metabolic regulation and self-management behavior
Risk for and of hypoglycemia and preventive interventions
Interaction between physical and mental function, with diabetes self-management.
Important Research Questions
What age related factors mediate diabetes outcomes and adverse treatment events in older people with type 1 diabetes?
What are the experiences of older adults living with type 1 diabetes (age-related changes and challenges)?
What are the optimal methods for risk assessment and minimization in older people with type 1 diabetes, considering metabolic objectives, self-management support and hypoglycemia prevention?
What are the needs of formal (e.g., visiting nurses) and informal (e.g., family members) care-providers in supporting older people with type 1 diabetes?
Critical Knowledge Gaps
Data regarding efficacy (outcomes, glucose control, hypoglycemia events) and safety of pump therapy, sensor augmented pump therapy/hybrid closed loop and CGM in older people with diabetes as a whole or in those with multimorbidity, including cognitive and functional decline.
Data relating to the use of home-based technological support or mobile, smartphone-based support in effective diabetes management of older adults
Important Research Questions
Which older adults are likely to benefit and which are likely to be harmed by use of varied technologies, and which tools may be used in order to identify the latter?
What diabetes lifestyle and/or management apps can be used successfully in older populations?
How can technology be harnessed for older people with special needs with cognitive or functional deficits?
How can we create a platform that includes additions to the existing technological tools (insulin pump, CGM) for diabetes related to cognitive rehabilitation modules, such as reminders, alerts and caregiver/case manager interaction modules, and physical activity monitoring and promotion tools?
Critical Knowledge Gaps
Roadmap for incorporating current evidence, patient preference and values, and prognosis into the therapeutic plan.
Data on target range of HbA1c, frequency of glucose monitoring
Best approach to select oral glucose-lowering regime or insulin scheme
Best nutritional and exercise-based approaches to maintaining physical function
Value of implementing End of Life Care guidance and approaches as a basis of planning palliative care
New Research Questions
What is the best approach to select individualized glycemic goals and what is the best measure of glycemic control (HbA1c versus glucose monitoring by glucometers or CGM) for residents with diabetes and those at end of life?
What is the best approach to use glucose-lowering regimes that reduce the risk of hypoglycemia, meet acceptable targets of glycemia, and improve outcomes of interest to this population?
Is there an average glucose, glucose excursion, or HbA1c level above which clinical outcomes can worsen in patients receiving palliative care?
What is the best nutrition and exercise-based approached in LTC and at end of life care?
What outcome indicators can be universally used to assess the standards of diabetes care within care homes and longer-term aged care facilities?
Is there a need for open real life and observational studies to acquire more data on the impact of diabetes on residents from care homes and at end of life?
Critical Knowledge Gap
Knowledge of the level and duration of hyperglycemia leading to symptoms.
Frequency of hypoglycemia in various populations of older adults (i.e., community-dwelling vs nursing home; cognitively intact vs cognitively impaired and across the spectrum of frailty).
When does hyperglycemia lead to geriatric outcomes such as falls, physical functional decline, and cognitive decline?
Best approach for the deintensification of glycemic treatment near the end of life?
Important Research to be Undertaken
A prospective study to correlate symptoms to glycemic levels using app-based tracking of symptoms, conducted across different subsets of older adults (i.e., community-dwelling vs nursing home; cognitively intact vs cognitively impaired and across the spectrum of frailty).
An observational study that examines the current state of glycemic control across those in long-term care facilities and other settings.
Qualitative studies to examine what outcomes are important to older adults with diabetes, ultimately informing consensus on outcome measures for future studies.
An interventional study aimed at different patient subtypes (i.e., those living at home, assisted living, long-term care facilities hospice), that would include qualitative feedback on symptoms and ultimately would examine longer term complications where appropriate (CVA, CVD, CKD).
As evidence accumulates, studies will be needed to identify optimal glycemic targets and treatments in different patient subgroups- those with Type 1 diabetes, dementia, and moderate to severe frailty.
Observational measures across large care networks identifying variation in care and staff education regarding appropriate medication review, nutrition and exercise interventions and individualized care plans, to disseminate knowledge to the care settings these individuals use.
Identify potential cost savings (i.e. reduced hospitalizations) to accelerate adoption
Acknowledgements
The authors acknowledge:
Dr. Marcel Salive, MD, MPH, Health Scientist Administrator, DGCG, NIA for his contribution regarding the NIA perspective during the workshop.
Christine Slyne for her invaluable assistance organizing the workshop and developing this manuscript.
Dr. Salive and Christine provided consent for their work to be acknowledged on this manuscript.
Funding Sources
The manuscript is based on the discussion at the workshop which was funded by unrestricted grants from Abbott, Dexcom, and Eli Lilly. The funding sources had no role in the drafting or editing of this manuscript, or the decision to submit for publication. No authors were paid to write this manuscript by a pharmaceutical company or other agency. Dr. Munshi had final responsibility for the decision to submit this manuscript for publication.
Footnotes
Declaration of Interest
MNM reports other from Sanofi, other from Lilly, outside the submitted work; GSM reports other from Merck, other from Abbott, other from Novo Nordisk, outside the submitted work. LRM has nothing to disclose. KC reports other from Helmsley Charitable Trust, other from Abbott, other from Adocia, other from Ascensia, other from Apple Pickers Foundation, other from AstraZeneca, other from Becton Dickinson (BD), other from Beta Bionics, other from BigFoot BioMedical, other from Boehringer Ingelheim, other from Boston Consulting Group, other from BrightInsight, a Flex Company, other from Cellnovo, other from CeQur, other from Dexcom, other from Ella Fitzgerald Charitable Foundation, other from Glooko, other from Insulet, other from Intarcia, other from Johnson & Johnson/Janssen, other from Lexicon, other from Lilly, other from Livongo Health, other from MannKind, other from Medtronic, other from Merck, other from Novo Nordisk, other from Onduo, other from Profil (EU & US), other from Prosciento, other from Qualcomm, other from Roche, other from Sanofi, other from Sensionics, other from Tandem, other from WellDoc, other from Xeris, outside the submitted work; PRC reports grants from Department of Veterans Affairs, grants from National Institutes of Health, during the conduct of the study. TCY reports grants from Medtronic, other from MSD, other from Medtronic, other from Sanofi, other from Astra Zeneca, other from Lilly, outside the submitted work. AF has nothing to disclose. OPG reports personal fees from Sanofi, grants and personal fees from Amarin, personal fees from Boehrnger-Ingelheim/Eli Lilly, outside the submitted work. CRK reports personal fees from CohBar, personal fees from Kaleido Bioscience, outside the submitted work. EH reports grants from NIH (NIDDK and NIA), during the conduct of the study. LML reports personal fees from Astra Zeneca, personal fees from Boehringer Ingelheim, personal fees from ConvaTec, personal fees from Dexcom, personal fees from Eli Lilly, personal fees from Insulet, personal fees from Insulogic, personal fees from Janssen Pharmaceuticals, personal fees from Johnson & Johnson, personal fees from Laxmi, personal fees from LifeScan, personal fees from MannKind, personal fees from Medtronic, personal fees from Menarini Diagnostics, personal fees from Merck, personal fees from Novo Nordisk, personal fees from Roche Diagnostics, personal fees from Sanofi, outside the submitted work. CGL has nothing to disclose. SL has nothing to disclose. DMN has nothing to disclose. NP discloses that she is a speaker for Eli Lilly. RP reports speaker and consulting fees from AstraZeneca; consulting fees from Boehringer-Ingelheim; consulting fees from Eisai, Inc.; consulting fees from GlaxoSmithKline; consulting fees from Glytec, LLC; consulting fees from Janssen; grants from Lexicon Pharmaceuticals; grants and consulting fees from Ligand Pharmaceuticals, Inc;, grants and consulting fees from Lilly; grants and consulting fees from Merck; consulting fees from Mundipharma; grants, speaker fees and consulting fees from Novo Nordisk; consulting fees from Pfizer; grants and consulting fees from Sanofi; grants, speaker fees and consulting fees from Takeda; personal consulting fees from Sanofi US Services, Inc., outside the submitted work. Except for consulting fees in February 2018 and June 2018 from Sanofi US Services, Inc., Dr. Richard Pratley’s services were paid for directly to AdventHealth, a nonprofit organization. RG reports and discloses advisory boards of Onduo, Form Health, Vida Health, Lark and Health Reveal. AJS has nothing to disclose.
Contributor Information
Medha N Munshi, Harvard Medical School, Joslin Diabetes Center and Beth Israel Deaconess Medical Center, 110 Francis Street, LMOB 1B, Boston, MA 02215.
Graydon S Meneilly, University of British Columbia, 2775 Laurel Street, Vancouver, BC, Canada V5Z 1M9.
Leocadio Rodríguez Mañas, Hospital Universitario de Getafe, Carretera de Toledo, Km 12.5; 28905-Getafe, Spain.
Kelly L Close, The diaTribe Foundation, president, Close Concerns, 804 Haight Street, San Francisco, CA 94117, USA.
Paul R Conlin, Harvard Medical School, VA Boston Healthcare System, 1400 VFW Parkway, West Roxbury, MA 02132.
Tali Cukierman-Yaffe, Division of Endocrinology, Diabetes and Metabolism, Gertner Institute, Sheba Medical Center, Epidemiology Department, Sackler School of Medicine, Herczeg Institute on Aging, Tel-Aviv University, Derech Sheba 2, Ramat Gan, Israel.
Angus Forbes, King’s College London, James Clerk Maxwell Building, Waterloo Road, London, SE1 8WA.
Om P Ganda, Joslin Diabetes Center and Harvard Medical School, One Joslin Place, Boston, MA 02215.
C Ronald Kahn, Harvard Medical School, Joslin Diabetes Center, One Joslin Place, Boston, MA 02215.
Elbert Huang, Center for Chronic Disease Research and Policy (CDRP), Section of General Internal Medicine, The University of Chicago, 5841 S. Maryland Avenue, MC 2007, Room B214, Chicago, Illinois 60637.
Lori M Laffel, Behavioral and Outcomes Research, Joslin Diabetes Center, Professor of Pediatrics, Harvard Medical School, One Joslin Place, Boston, MA 02215.
Christine G Lee, Division of Diabetes, Endocrinology and Metabolic Diseases, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, 6707 Democracy Boulevard, Rm. 7217, Bethesda, MD 20892-5460.
Sei Lee, University of California-San Francisco, Geriatrics and Extended Care, San Francisco Veterans Affairs Health Care System, Building 1, Room 220F, Box 181G, 4150 Clement Street, San Francisco, CA 94121.
David M Nathan, Harvard Medical School, Director, Diabetes Research Center and Clinical Research Center, Massachusetts General Hospital, 50 Staniford Street, Suite 340, Boston, MA 02114.
Naushira Pandya, Department of Geriatrics, Project Director, NSU South Florida GWEP, Kiran C. Patel College of Osteopathic Medicine, Nova Southeastern University, Program Director, Geriatric Medicine Fellowship, Aventura Hospital and KPCOM, 3200 S. University Dr. FT. Lauderdale, FL 33328.
Richard Pratley, AdventHealth Samuel E. Crockett Chair in Diabetes Research, Medical Director, AdventHealth Diabetes Institute, Senior Investigator and Diabetes Program Lead, AdventHealth Translational Research Institute, N Orange Ave Suite 502, Orlando, FL 32804.
Robert Gabbay, Joslin Diabetes Center, Harvard Medical School, One Joslin Place, Boston, MA USA.
Alan J Sinclair, Diabetes Frail Ltd and King’s College, London, Oakmoore Court, Kingswood Road, Droitwich WR9 0QH, UK.
References
- 1.Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, et al. Diabetes in older adults. Diabetes Care. 2012;35(12):2650–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinclair A, Morley JE, Rodriguez-Manas L, Paolisso G, Bayer T, Zeyfang A, et al. Diabetes mellitus in older people: position statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J Am Med Dir Assoc. 2012;13(6):497–502. [DOI] [PubMed] [Google Scholar]
- 3.Benetos A, Novella JL, Guerci B, Blickle JF, Boivin JM, Cuny P, et al. Pragmatic diabetes management in nursing homes: individual care plan. J Am Med Dir Assoc. 2013;14(11):791–800. [DOI] [PubMed] [Google Scholar]
- 4.Mallery LH, Ransom T, Steeves B, Cook B, Dunbar P, Moorhouse P. Evidence-informed guidelines for treating frail older adults with type 2 diabetes: from the Diabetes Care Program of Nova Scotia (DCPNS) and the Palliative and Therapeutic Harmonization (PATH) program. J Am Med Dir Assoc. 2013;14(11):801–8. [DOI] [PubMed] [Google Scholar]
- 5.meneilly GS, Knip A, Tessier D. Canandian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Diabetes in the elderly. Canadian Journal of Diabetes. 2013;37 (suppl 1):S184–90. [DOI] [PubMed] [Google Scholar]
- 6.IDF working group: Cho N, Colagiuri S, Distiller L, Dunning T, Gadsby R, Goel A, Munshi M, Sinclair A, Sinay I. Managing Older People with Type 2 Diabetes. Global Guideline 2013. Available from: https://www.ifa-fiv.org/wp-content/uploads/.../IDF-Guideline-for-Older-People.pdf.
- 7.Dunning T, Duggan N, Savage S, Martin P. Diabetes and end of life: ethical and methodological issues in gathering evidence to guide care. Scand J Caring Sci. 2013;27(1):203–11. [DOI] [PubMed] [Google Scholar]
- 8.Sinclair AJ, Abdelhafiz A, Dunning T, Izquierdo M, Rodriguez Manas L, Bourdel-Marchasson I, et al. An International Position Statement on the Management of Frailty in Diabetes Mellitus: Summary of Recommendations 2017. J Frailty Aging. 2018;7(1):10–20. [DOI] [PubMed] [Google Scholar]
- 9.Munshi MN, Florez H, Huang ES, Kalyani RR, Mupanomunda M, Pandya N, et al. Management of Diabetes in Long-term Care and Skilled Nursing Facilities: A Position Statement of the American Diabetes Association. Diabetes Care. 2016;39(2):308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes A. 12. Older Adults: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S139–S47. [DOI] [PubMed] [Google Scholar]
- 11.LeRoith D, Biessels GJ, Braithwaite SS, Casanueva FF, Draznin B, Halter JB, et al. Treatment of Diabetes in Older Adults: An Endocrine Society* Clinical Practice Guideline. J Clin Endocrinol Metab. 2019;104(5):1520–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalyani RR, Golden SH, Cefalu WT. Diabetes and Aging: Unique Considerations and Goals of Care. Diabetes Care. 2017;40(4):440–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munshi MN. Cognitive Dysfunction in Older Adults With Diabetes: What a Clinician Needs to Know. Diabetes Care. 2017;40(4):461–7. [DOI] [PubMed] [Google Scholar]
- 14.Umpierrez GE, Pasquel FJ. Management of Inpatient Hyperglycemia and Diabetes in Older Adults. Diabetes Care. 2017;40(4):509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pani LN, Korenda L, Meigs JB, Driver C, Chamany S, Fox CS, et al. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001–2004. Diabetes Care. 2008;31(10):1991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menke A, Casagrande S, Cowie CC. Contributions of A1c, fasting plasma glucose, and 2-hour plasma glucose to prediabetes prevalence: NHANES 2011–2014. Ann Epidemiol. 2018;28(10):681–5 e2. [DOI] [PubMed] [Google Scholar]
- 17.Edelstein SL, Knowler WC, Bain RP, Andres R, Barrett-Connor EL, Dowse GK, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997;46(4):701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schottker B, Raum E, Rothenbacher D, Muller H, Brenner H. Prognostic value of haemoglobin A1c and fasting plasma glucose for incident diabetes and implications for screening. Eur J Epidemiol. 2011;26(10):779–87. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13–S28. [DOI] [PubMed] [Google Scholar]
- 20.Diabetes Canada Clinical Practice Guidelines Expert C, Ekoe JM, Goldenberg R, Katz P. Screening for Diabetes in Adults. Can J Diabetes. 2018;42 Suppl 1:S16–S9. [DOI] [PubMed] [Google Scholar]
- 21.Aschner P New IDF clinical practice recommendations for managing type 2 diabetes in primary care. Diabetes Res Clin Pract. 2017;132:169–70. [DOI] [PubMed] [Google Scholar]
- 22.Lipska KJ, De Rekeneire N, Van Ness PH, Johnson KC, Kanaya A, Koster A, et al. Identifying dysglycemic states in older adults: implications of the emerging use of hemoglobin A1c. J Clin Endocrinol Metab. 2010;95(12):5289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipska KJ, Wang Y, Kosiborod M, Masoudi FA, Havranek EP, Krumholz HM, et al. Discontinuation of antihyperglycemic therapy and clinical outcomes after acute myocardial infarction in older patients with diabetes. Circ Cardiovasc Qual Outcomes. 2010;3(3):236–42. [DOI] [PubMed] [Google Scholar]
- 24.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diabetes Prevention Program Research G, Crandall J, Schade D, Ma Y, Fujimoto WY, Barrett-Connor E, et al. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci. 2006;61(10):1075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359(9323):2072–7. [DOI] [PubMed] [Google Scholar]
- 27.Investigators DT, Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368(9541):1096–105. [DOI] [PubMed] [Google Scholar]
- 28.Inzucchi SE, Viscoli CM, Young LH, Furie KL, Gorman M, Lovejoy AM, et al. Pioglitazone Prevents Diabetes in Patients With Insulin Resistance and Cerebrovascular Disease. Diabetes Care. 2016;39(10):1684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–63. [DOI] [PubMed] [Google Scholar]
- 30.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–24. [DOI] [PubMed] [Google Scholar]
- 31.Lee SJ, Leipzig RM, Walter LC. Incorporating lag time to benefit into prevention decisions for older adults. JAMA. 2013;310(24):2609–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blaum C, Cigolle CT, Boyd C, Wolff JL, Tian Z, Langa KM, et al. Clinical complexity in middle-aged and older adults with diabetes: the Health and Retirement Study. Med Care. 2010;48(4):327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cigolle CT, Kabeto MU, Lee PG, Blaum CS. Clinical complexity and mortality in middle-aged and older adults with diabetes. J Gerontol A Biol Sci Med Sci. 2012;67(12):1313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenfield S, Billimek J, Pellegrini F, Franciosi M, De Berardis G, Nicolucci A, et al. Comorbidity affects the relationship between glycemic control and cardiovascular outcomes in diabetes: a cohort study. Ann Intern Med. 2009;151(12):854–60. [DOI] [PubMed] [Google Scholar]
- 35.Karter AJ, Warton EM, Lipska KJ, Ralston JD, Moffet HH, Jackson GG, et al. Development and Validation of a Tool to Identify Patients With Type 2 Diabetes at High Risk of Hypoglycemia-Related Emergency Department or Hospital Use. JAMA Intern Med. 2017;177(10):1461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375(9713):481–9. [DOI] [PubMed] [Google Scholar]
- 37.Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care. 2011;34(6):1329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Twito O, Ahron E, Jaffe A, Afek S, Cohen E, Granek-Catarivas M, et al. New-onset diabetes in elderly subjects: association between HbA1c levels, mortality, and coronary revascularization. Diabetes Care. 2013;36(11):3425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forbes A, Murrells T, Mulnier H, Sinclair AJ. Mean HbA1c, HbA1c variability, and mortality in people with diabetes aged 70 years and older: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(6):476–86. [DOI] [PubMed] [Google Scholar]
- 40.Currie CJ, Holden SE, Jenkins-Jones S, Morgan CL, Voss B, Rajpathak SN, et al. Impact of differing glucose-lowering regimens on the pattern of association between glucose control and survival. Diabetes Obes Metab. 2018;20(4):821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller ME, Williamson JD, Gerstein HC, Byington RP, Cushman WC, Ginsberg HN, et al. Effects of randomization to intensive glucose control on adverse events, cardiovascular disease, and mortality in older versus younger adults in the ACCORD Trial. Diabetes Care. 2014;37(3):634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinclair AJ, Paolisso G, Castro M, Bourdel-Marchasson I, Gadsby R, Rodriguez Manas L. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab. 2011;37 Suppl 3:S27–38. [DOI] [PubMed] [Google Scholar]
- 43.Kalyani RR, Saudek CD, Brancati FL, Selvin E. Association of diabetes, comorbidities, and A1C with functional disability in older adults: results from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Diabetes Care. 2010;33(5):1055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Esquinas E, Graciani A, Guallar-Castillon P, Lopez-Garcia E, Rodriguez-Manas L, Rodriguez-Artalejo F. Diabetes and risk of frailty and its potential mechanisms: a prospective cohort study of older adults. J Am Med Dir Assoc. 2015;16(9):748–54. [DOI] [PubMed] [Google Scholar]
- 45.Kalyani RR, Tian J, Xue QL, Walston J, Cappola AR, Fried LP, et al. Hyperglycemia and incidence of frailty and lower extremity mobility limitations in older women. J Am Geriatr Soc. 2012;60(9):1701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rawlings AM, Sharrett AR, Albert MS, Coresh J, Windham BG, Power MC, et al. The Association of Late-Life Diabetes Status and Hyperglycemia With Incident Mild Cognitive Impairment and Dementia: The ARIC Study. Diabetes Care. 2019;42(7):1248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175(3):356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Formiga F, Franch-Nadal J, Rodriguez L, Avila L, Fuster E. Inadequate Glycaemic Control and Therapeutic Management of Adults over 65 Years Old with Type 2 Diabetes Mellitus in Spain. J Nutr Health Aging. 2017;21(10):1365–70. [DOI] [PubMed] [Google Scholar]
- 49.Munshi M, Slyne C, Segal AR, Saul N, Lyons C, Weinger K. Simplification of Insulin Regimen in Older Adults and Risk of Hypoglycemia. JAMA Internal Medicine. 2016;Online First. [DOI] [PubMed]
- 50.Lipska KJ, Ross JS, Wang Y, Inzucchi SE, Minges K, Karter AJ, et al. National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med. 2014;174(7):1116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ligthart SA, van den Eerenbeemt KD, Pols J, van Bussel EF, Richard E, Moll van Charante EP. Perspectives of older people engaging in nurse-led cardiovascular prevention programmes: a qualitative study in primary care in the Netherlands. Br J Gen Pract. 2015;65(630):e41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin CC, Yang CP, Li CI, Liu CS, Chen CC, Lin WY, et al. Visit-to-visit variability of fasting plasma glucose as predictor of ischemic stroke: competing risk analysis in a national cohort of Taiwan Diabetes Study. BMC Med. 2014;12:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinclair A, Morley J. Frailty and diabetes. Lancet. 2013;382(9902):1386–7. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Manas L, Laosa O, Vellas B, Paolisso G, Topinkova E, Oliva-Moreno J, et al. Effectiveness of a multimodal intervention in functionally impaired older people with type 2 diabetes mellitus. J Cachexia Sarcopenia Muscle. 2019;10(4):721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cefalu WT, Kaul S, Gerstein HC, Holman RR, Zinman B, Skyler JS, et al. Cardiovascular Outcomes Trials in Type 2 Diabetes: Where Do We Go From Here? Reflections From a Diabetes Care Editors’ Expert Forum. Diabetes Care. 2018;41(1):14–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schernthaner G, Schernthaner-Reiter MH. Diabetes in the older patient: heterogeneity requires individualisation of therapeutic strategies. Diabetologia. 2018;61(7):1503–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hernandez AF, Green JB, Janmohamed S, D’Agostino RB Sr., Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29. [DOI] [PubMed] [Google Scholar]
- 58.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377(13):1228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2019;381(9):841–51. [DOI] [PubMed] [Google Scholar]
- 60.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375(19):1834–44. [DOI] [PubMed] [Google Scholar]
- 61.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375(4):311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pfeffer MA, Claggett B, Probstfield JL. Lixisenatide in Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2016;374(11):1095–6. [DOI] [PubMed] [Google Scholar]
- 63.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30. [DOI] [PubMed] [Google Scholar]
- 64.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377(7):644–57. [DOI] [PubMed] [Google Scholar]
- 65.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380(4):347–57. [DOI] [PubMed] [Google Scholar]
- 66.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117–28. [DOI] [PubMed] [Google Scholar]
- 67.Gilbert MP, Bain SC, Franek E, Jodar-Gimeno E, Nauck MA, Pratley R, et al. Effect of Liraglutide on Cardiovascular Outcomes in Elderly Patients: A Post Hoc Analysis of a Randomized Controlled Trial. Ann Intern Med. 2019;170(6):423–6. [DOI] [PubMed] [Google Scholar]
- 68.Sinclair AJ, Abdelhafiz AH, Forbes A, Munshi M. Evidence-based diabetes care for older people with Type 2 diabetes: a critical review. Diabet Med. 2019;36(4):399–413. [DOI] [PubMed] [Google Scholar]
- 69.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380(24):2295–306. [DOI] [PubMed] [Google Scholar]
- 70.Administration FaD. Type 2 Diabetes Mellitus: Evaluating the Safety of New Drugs for Improving Glycemic Control. Guidance for Industry. In: Services USDoHaH, editor. Center for Drug Evaluation and Research (CDER): U.S. Department of Health and Human Services; 2020. [Google Scholar]
- 71.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55(5):1463–9. [DOI] [PubMed] [Google Scholar]
- 72.Livingstone SJ, Levin D, Looker HC, Lindsay RS, Wild SH, Joss N, et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008–2010. JAMA. 2015;313(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53(11):2312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bain SC, Gill GV, Dyer PH, Jones AF, Murphy M, Jones KE, et al. Characteristics of Type 1 diabetes of over 50 years duration (the Golden Years Cohort). Diabet Med. 2003;20(10):808–11. [DOI] [PubMed] [Google Scholar]
- 75.Oram RA, McDonald TJ, Shields BM, Hudson MM, Shepherd MH, Hammersley S, et al. Most people with long-duration type 1 diabetes in a large population-based study are insulin microsecretors. Diabetes Care. 2015;38(2):323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rogers MAM, Kim C, Banerjee T, Lee JM. Fluctuations in the incidence of type 1 diabetes in the United States from 2001 to 2015: a longitudinal study. BMC Med. 2017;15(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thunander M, Petersson C, Jonzon K, Fornander J, Ossiansson B, Torn C, et al. Incidence of type 1 and type 2 diabetes in adults and children in Kronoberg, Sweden. Diabetes Res Clin Pract. 2008;82(2):247–55. [DOI] [PubMed] [Google Scholar]
- 78.Schutt M, Fach EM, Seufert J, Kerner W, Lang W, Zeyfang A, et al. Multiple complications and frequent severe hypoglycaemia in ‘elderly’ and ‘old’ patients with Type 1 diabetes. Diabet Med. 2012;29(8):e176–9. [DOI] [PubMed] [Google Scholar]
- 79.Weinstock RS, DuBose SN, Bergenstal RM, Chaytor NS, Peterson C, Olson BA, et al. Risk Factors Associated With Severe Hypoglycemia in Older Adults With Type 1 Diabetes. Diabetes Care. 2015. [DOI] [PubMed]
- 80.Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care. 2005;28(3):726–35. [DOI] [PubMed] [Google Scholar]
- 81.Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29(4):494–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weinstock RS, Schutz-Fuhrmann I, Connor CG, Hermann JM, Maahs DM, Schutt M, et al. Type 1 diabetes in older adults: Comparing treatments and chronic complications in the United States T1D Exchange and the German/Austrian DPV registries. Diabetes Res Clin Pract. 2016;122:28–37. [DOI] [PubMed] [Google Scholar]
- 83.Litchman ML, Wawrzynski SE, Allen NA, Tracy EL, Kelly CS, Helgeson VS, et al. Yours, Mine, and Ours: A Qualitative Analysis of the Impact of Type 1 Diabetes Management in Older Adult Married Couples. Diabetes Spectr. 2019;32(3):239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sinclair AJ, Dunning T, Dhatariya K, an International Group of E. Clinical guidelines for type 1 diabetes mellitus with an emphasis on older adults: an Executive Summary. Diabet Med. 2020;37(1):53–70. [DOI] [PubMed] [Google Scholar]
- 85.Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010(1):CD005103. [DOI] [PubMed] [Google Scholar]
- 86.Beck RW, Riddlesworth TD, Ruedy KJ, Kollman C, Ahmann AJ, Bergenstal RM, et al. Effect of initiating use of an insulin pump in adults with type 1 diabetes using multiple daily insulin injections and continuous glucose monitoring (DIAMOND): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(9):700–8. [DOI] [PubMed] [Google Scholar]
- 87.Bergenstal RM, Garg S, Weinzimer SA, Buckingham BA, Bode BW, Tamborlane WV, et al. Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients With Type 1 Diabetes. JAMA. 2016;316(13):1407–8. [DOI] [PubMed] [Google Scholar]
- 88.Garg SK, Weinzimer SA, Tamborlane WV, Buckingham BA, Bode BW, Bailey TS, et al. Glucose Outcomes with the In-Home Use of a Hybrid Closed-Loop Insulin Delivery System in Adolescents and Adults with Type 1 Diabetes. Diabetes Technol Ther. 2017;19(3):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stone MP, Agrawal P, Chen X, Liu M, Shin J, Cordero TL, et al. Retrospective Analysis of 3-Month Real-World Glucose Data After the MiniMed 670G System Commercial Launch. Diabetes Technol Ther. 2018;20(10):689–92. [DOI] [PubMed] [Google Scholar]
- 90.Aronson R, Cohen O, Conget I, Runzis S, Castaneda J, de Portu S, et al. OpT2mise: a randomized controlled trial to compare insulin pump therapy with multiple daily injections in the treatment of type 2 diabetes-research design and methods. Diabetes Technol Ther. 2014;16(7):414–20. [DOI] [PubMed] [Google Scholar]
- 91.Heinemann L, Freckmann G, Ehrmann D, Faber-Heinemann G, Guerra S, Waldenmaier D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391(10128):1367–77. [DOI] [PubMed] [Google Scholar]
- 92.Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lind M, Polonsky W, Hirsch IB, Heise T, Bolinder J, Dahlqvist S, et al. Continuous Glucose Monitoring vs Conventional Therapy for Glycemic Control in Adults With Type 1 Diabetes Treated With Multiple Daily Insulin Injections: The GOLD Randomized Clinical Trial. JAMA. 2017;317(4):379–87. [DOI] [PubMed] [Google Scholar]
- 94.Beck RW, Riddlesworth TD. Continuous Glucose Monitoring Versus Usual Care in Patients With Type 2 Diabetes Receiving Multiple Daily Insulin Injections. Ann Intern Med. 2018;168(7):526–7. [DOI] [PubMed] [Google Scholar]
- 95.Ruedy KJ, Parkin CG, Riddlesworth TD, Graham C, Group DS. Continuous Glucose Monitoring in Older Adults With Type 1 and Type 2 Diabetes Using Multiple Daily Injections of Insulin: Results From the DIAMOND Trial. J Diabetes Sci Technol. 2017;11(6):1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yaron M, Roitman E, Aharon-Hananel G, Landau Z, Ganz T, Yanuv I, et al. Effect of Flash Glucose Monitoring Technology on Glycemic Control and Treatment Satisfaction in Patients With Type 2 Diabetes. Diabetes Care. 2019;42(7):1178–84. [DOI] [PubMed] [Google Scholar]
- 97.Sinclair AJAA, Cukierman-Yaffe T. mHealth Applications, Older People and Type 2 Diabetes–a Detailed Review using Systematic Methodology. Journal of Diabetes Research and Therapeutics. 2019;5(1). [Google Scholar]
- 98.Sinclair AJ, Allard I, Bayer A. Observations of diabetes care in long-term institutional settings with measures of cognitive function and dependency. Diabetes Care. 1997;20(5):778–84. [DOI] [PubMed] [Google Scholar]
- 99.Sinclair aj GA, Gadsby R, Bourdel-Marchasson I, Bayer AJ. Diabetes in care homes: a cluster randomised controlled trial of resident education. British Journal of Vascular Disease. 2012;12:238–42. [Google Scholar]
- 100.Sinclair AJ, Gadsby R, Abdelhafiz AH, Kennedy M. Failing to meet the needs of generations of care home residents with diabetes: a review of the literature and a call for action. Diabet Med. 2018. [DOI] [PubMed]
- 101.Deakin TA, Littley MD. Diabetes care in residential homes: staff training makes a difference. J Hum Nutr Diet. 2001;14(6):443–7. [DOI] [PubMed] [Google Scholar]
- 102.Boyle PJ, O’Neil KW, Berry CA, Stowell SA, Miller SC. Improving diabetes care and patient outcomes in skilled-care communities: successes and lessons from a quality improvement initiative. J Am Med Dir Assoc. 2013;14(5):340–4. [DOI] [PubMed] [Google Scholar]
- 103.Horning KK, Hoehns JD, Doucette WR. Adherence to clinical practice guidelines for 7 chronic conditions in long-term-care patients who received pharmacist disease management services versus traditional drug regimen review. J Manag Care Pharm. 2007;13(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Davis KL, Wei W, Meyers JL, Kilpatrick BS, Pandya N. Use of basal insulin and the associated clinical outcomes among elderly nursing home residents with type 2 diabetes mellitus: a retrospective chart review study. Clin Interv Aging. 2014;9:1815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sjoblom P, Anders Tengblad, Lofgren UB, Lannering C, Anderberg N, Rosenqvist U, et al. Can diabetes medication be reduced in elderly patients? An observational study of diabetes drug withdrawal in nursing home patients with tight glycaemic control. Diabetes Res Clin Pract. 2008;82(2):197–202. [DOI] [PubMed] [Google Scholar]
- 106.Dy P, Morin PC, Weinstock RS. Use of telemedicine to improve glycemic management in a skilled nursing facility: a pilot study. Telemed J E Health. 2013;19(8):643–5. [DOI] [PubMed] [Google Scholar]
- 107.Dharmarajan TS, Mahajan D, Zambrano A, Agarwal B, Fischer R, Sheikh Z, et al. Sliding Scale Insulin vs Basal-Bolus Insulin Therapy in Long-Term Care: A 21-Day Randomized Controlled Trial Comparing Efficacy, Safety and Feasibility. J Am Med Dir Assoc. 2016;17(3):206–13. [DOI] [PubMed] [Google Scholar]
- 108.Pasquel FJ, Powell W, Peng L, Johnson TM, Sadeghi-Yarandi S, Newton C, et al. A randomized controlled trial comparing treatment with oral agents and basal insulin in elderly patients with type 2 diabetes in long-term care facilities. BMJ Open Diabetes Res Care. 2015;3(1):e000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Umpierrez GE, Cardona S, Chachkhiani D, Fayfman M, Saiyed S, Wang H, et al. A Randomized Controlled Study Comparing a DPP4 Inhibitor (Linagliptin) and Basal Insulin (Glargine) in Patients With Type 2 Diabetes in Long-term Care and Skilled Nursing Facilities: Linagliptin-LTC Trial. J Am Med Dir Assoc. 2018;19(5):399–404 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dunning T, Duggan N, Savage S. The McKellar Guidelines for Managing Older People with Diabetes in Residential and Other Care Settings. Geelong, Australia: Center for Nursing adn Allied Health, Deakin University and Barwon Healh; 2014. [Google Scholar]
- 111.Dunning T, Martin P. Palliative and end of life care of people with diabetes: Issues, challenges and strategies. Diabetes Res Clin Pract. 2018;143:454–63. [DOI] [PubMed] [Google Scholar]
- 112.Quinn K, Hudson P, Dunning T. Diabetes management in patients receiving palliative care. J Pain Symptom Manage. 2006;32(3):275–86. [DOI] [PubMed] [Google Scholar]
- 113.Petrillo LA, Gan S, Jing B, Lang-Brown S, Boscardin WJ, Lee SJ. Hypoglycemia in Hospice Patients With Type 2 Diabetes in a National Sample of Nursing Homes. JAMA Intern Med. 2018;178(5):713–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sinclair A SP, Kaundal A, Karuranga S, Malanda B, Williams R. Diabetes and global ageing among 65–99-year-old adults: findings from the international diabetes federation diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020. [DOI] [PubMed]


