Abstract
The parasitic filarioid Onchocerca lupi causes ocular disease characterized by conjunctivitis and nodular lesions. This nematode was first described in 1967 in a wolf from Georgia, and since then cases of infection from dogs and cats with ocular onchocercosis and sporadically from humans also with subcutaneous and cervical lesions caused by O. lupi have been reported from the Middle East, Europe, and North America. Due to its zoonotic potential, this parasitic infection has gained attention in the past 20 years. Phylogenetic studies have highlighted the recent divergence of O. lupi from other Onchocerca spp. and the importance of domestication in the evolutionary history of this worm. Moreover, the finding of an O. lupi genotype associated with subclinical and mild infection in the Iberian Peninsula, raises important questions about the pathogenicity of this presently enigmatic parasite.
Keywords: canine ocular onchocercosis, helminthiasis, Onchocerca lupi, zoonosis
Introduction
Onchocerca lupi is a filarioid nematode which parasitizes mainly dogs, but also cats, in Europe and North America [1,2]. In the last decade, this worm has gained the attention of the scientific community due to its zoonotic potential [3]. Human cases of ocular onchocercosis in the United States, Germany, Turkey, Tunisia and Iran have renewed the interest in the biology of this parasite and its yet unknown vector, as well as its epidemiology, with coyotes suggested as reservoirs of the nematode [4].
Life Cycle
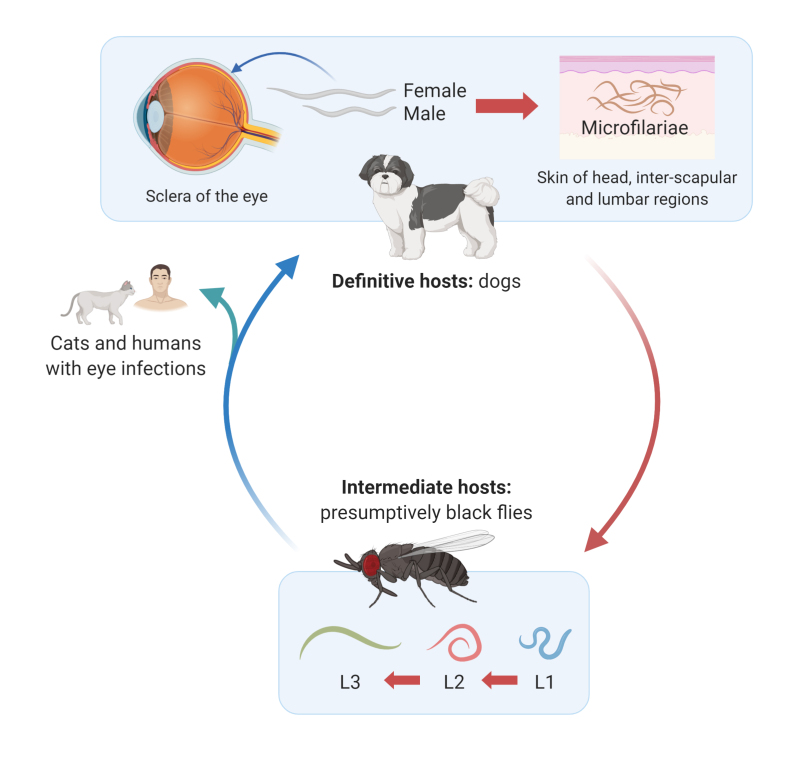
The life cycle of O. lupi involves canids as definitive hosts and unknown arthropod species as intermediate hosts (Figure 1). In the definitive hosts, male and female adult stages usually develop in the connective tissue of the subconjunctiva, conjunctiva, eyelids, and nictitating membrane sitting on top of the sclera until reaching sexual maturity [5,6]. Aberrant migration to the laryngeal soft tissue in dogs [7] and spinal cord in humans [8,9] has also been reported. Although experimental infection of O. lupi in dogs has not been carried out to date, the lifespan of adult worms in dogs has been estimated to be between 3 [10] and 8 years [11]. Additionally, cats [12,13] can also be infected with this nematode, along with dogs and humans [14-18].
Figure 1.
Life cycle of Onchocerca lupi. Dogs act as definitive hosts of the parasite and female and male helminth adults develop in the top of the sclera of the eye, leading to conjunctivitis, scleral nodules, and even loss of eyesight and irreversible ocular damage. Cases have been reported among cats and humans with periocular pathology. Adult females residing in the eye release microfilariae into the skin of the head, interscapular, and lumbar regions of canids. Thereafter, microfilariae are ingested by intermediate hosts, presumptively black flies of the genus Simulium, in which larval development from L1 to L3 will occur. This figure was created using www.biorender.com.
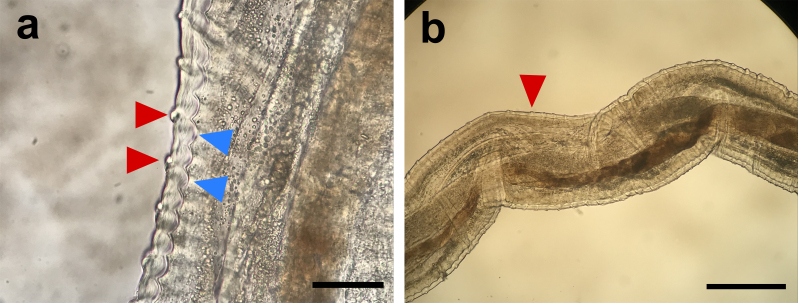
Adults of O. lupi are white, measure 4-12 cm in length, are slender with rounded anterior ends and have atrophic musculature, prominent hypodermis and lateral chords, a small digestive system and a multilayer cuticle [6,12]. Their cuticle is 4-5 µm thick with characteristic annular ridges 2-3 µm high and wide and evenly spaced at 25-30 µm intervals in its outer layer with transverse striations 14 µm wide and 1 µm thick in its inner layer (Figure 2) [5,12,19]. Males are 4.3-5 cm long by 0.1-0.2 mm in diameter with two unequal spicules [20]. Females are usually longer than males, measuring up to 16.5 cm [20,21], and containing two uteri. However, their exact length is currently unknown due to the difficulty in removing complete adult females from tissues [5,19-21]. Males and females copulate, and the latter develop microfilariae inside their uterus, which are released to subcutaneous tissues, especially in the head, inter-scapular, and lumbar regions of dogs [22]. Microfilariae are straight, measure between 81-115 µm and have a bluntly rounded anterior end [6]. In addition, their posterior portion ends sharply with a bent tail [6].
Figure 2.
Onchocerca lupi female observed under light microscopy. a. Transverse striations and annular ridges of the cuticle are marked with red and blue triangles, respectively (Bar=25 µm). b. Transverse striations of the cuticle (Bar=0.4 mm).
Microfilariae develop further to the infective L3 stage after entering their intermediate host. Black flies of the genus Simulium are involved as intermediate hosts of other Onchocerca spp. Simulium damnosum, Simulium neavei, and Simulium ochraceum are the most widespread hosts of Onchocerca volvulus, the etiological agent of river blindness in humans [23]. Moreover, S. damnosum sensu lato and Simulium ornatum are the hosts of Onchocerca ochengi [24] and Onchocerca gutturosa [25], respectively, both species producing skin nodules in cattle. The arthropod species involved in the life cycle of O. lupi have not been confirmed so far. Black flies of the species Simulium velutinum, Simulium reptans, and Simulium pseudequinum which fed on an O. lupi-infected dog from Greece did not demonstrate progression of nematode larval development [26]. However, O. lupi DNA has been found in Simulium tribulatum black flies from the US [27]. These findings warrant further studies, since detection of DNA does not confirm an active role of the black fly in the life cycle of this nematode. The recent finding of Onchocerca sp. from the sand fly Psychodopygus carrerai suggests that insect species other than simuliids may acquire microfilariae of this onchocercids [28].
Definitive hosts become infected when the intermediate hosts presumably inject L3 into their subcutaneous tissues while feeding. However, the exact mechanisms have not been elucidated as experimental infections of dogs and flies with O. lupi are difficult to perform. Therefore, the incubation time, as well as prepatent period remain unknown, although a person from Turkey bitten by a fly in the upper lid was reported to develop ocular signs after 30 days [29].
Epidemiology and Distribution
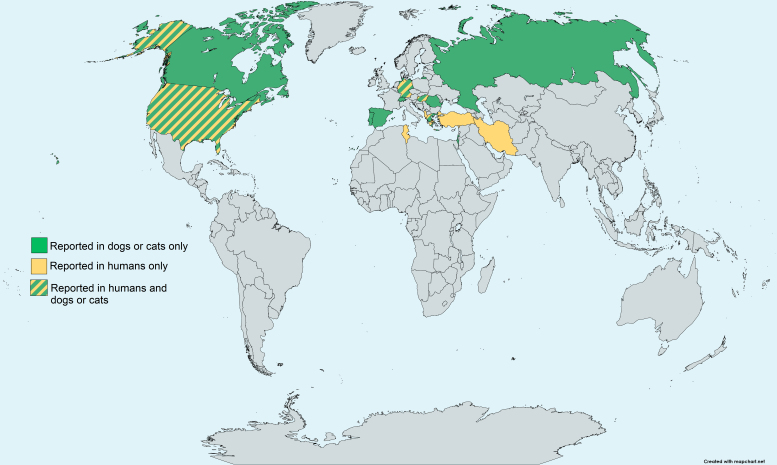
The first case of O. lupi infection was reported in a wolf (Canis lupus) from the Caucasian republic of Georgia in 1967 [20]. Although canine ocular onchocercosis caused by Onchocerca spp. was reported in dogs from the US during the 1990s [30,31], there were no scientific publications that described O. lupi for more than three decades thereafter. In 2001, ocular onchocercosis was reported in four dogs from Hungary [32]. The specimens collected from the animals were morphologically similar to the specimens described in the US [30,31] and were molecularly confirmed as O. lupi [6]. Since then, O. lupi has been detected in domestic dogs (Canis lupus familiaris), domestic cats (Felis catus), and humans in North America, Europe, Asia, and Africa (Figure 3). Nevertheless, O. lupi might be circulating in additional geographical locations, and the increased movement of infected animals between countries might promote the spread of the parasite to new previously unreported regions. This situation has been described in dogs imported to Canada from the US [10] as well as a case imported to Italy from southern Portugal [33]. Therefore, the actual global presence of infection with this nematode might be underestimated.
Figure 3.
Global distribution of O. lupi. Reports of O. lupi infection in domestic dogs, cats and humans. In the Americas, infection has been detected in Canada and the US. In Europe, O. lupi has been reported in Albania, Georgia, Germany, Greece, Hungary, Portugal, Romania, Russia, Spain, Switzerland, and Turkey. In Asia, reports of infection have been from Iran and Israel.
Infection with O. lupi has been studied in dogs from Europe and the US [2,34]. O. lupi microfilariae were previously detected in the interocular region from 2/23 and 7/84 dogs in studies from Greece and Portugal, respectively [34], and none of the O. lupi-positive dogs showed clinical signs. Furthermore, 4.8% (5/104) of dogs in a study from southern Spain were positive for O. lupi microfilariae, with only one of the infected dogs showing neurological abnormalities, possibly unrelated to O. lupi-infection [2]. No granulomatous nodules were detected using imaging tests in the latter case [2] and neurological signs due to O. lupi infection have not been reported in dogs to date.
In the US, O. lupi is considered endemic in the states of New Mexico and Arizona [4,35], although it has been found in rescued dogs from other southwestern states and dogs with a travel history to New Mexico [36]. Interestingly, recurrence of clinical signs is a common finding in dogs [35,36], as opposed to observations made from cases in Greece, in which treated dogs did not show any further signs after a year of follow up [37]. This might suggest ineffective medical or surgical treatment, or possibly that the parasite circulation is maintained among canid communities, enabling constant infections and reinfections in dogs. For example, the detection of O. lupi in 5.2% of 707 analyzed skin samples collected from coyotes (Canis latrans) in eight counties of Arizona and New Mexico [4] suggests that this wild canid has a role in the maintenance of the infection in some areas. This highlights the difficulty in controlling the nematode in locations where both domestic and wild canids are infected by O. lupi.
Clinical cases of O. lupi infection in domestic cats have been reported in Portugal [13] and the US (ie, Utah) [12]. A survey of 155 apparently healthy stray cats from Portugal found that only one cat was positive for O. lupi [13]. These findings suggest that although cats can be competent hosts of the parasite, their role either as definitive hosts and reservoirs of the nematode might be less important than that of canids.
Twelve cases of human O. lupi infection have been confirmed in the US [15], Germany [18], Turkey [16], Tunisia [38], and Iran [17] between 2011 and 2015. Prior to this time, three cases of human ocular onchocercosis from Ukraine [39], Albania [40], and Hungary [41] were suspected based on their ocular presentation and the morphology of extracted worms. In 2011, O. lupi was confirmed as a zoonotic agent when worms extracted from ocular nodular lesions of an 18-year-old woman from Turkey were identified as O. lupi based both on the morphology of the adult worms and on the gene sequence identity compared with sequences available in Genbank [14]. Interestingly, spinal onchocercomas have been reported exclusively in humans [8,9], whereas this clinical presentation has not been detected in dogs. Moreover, most cases in humans have been reported in 15- to 28-year-old young adults [14,16-18], without gender or occupation predilection. Importantly, most cases from the US report travel history to endemic states or indicate outdoor activities near lakes or rivers.
Biology and Evolution
O. lupi has an obligate symbiotic relationship with Wolbachia bacteria [42], as do most filarial nematodes including other Onchocerca spp. [43]. These bacteria have been observed enclosed in cytoplasmic double-membrane vacuoles from the lateral chords of female and male adults and in microfilariae [42]. Molecular analysis of the 16S rRNA gene of Wolbachia demonstrated that O. lupi endosymbionts belong to supergroup C of Wolbachia pipientis and are not identical with those from other Onchocerca spp. [42]. Based on the Wolbachia surface protein (wsp) and the bacterial cell-cycle ftsZ gene sequences, it was also confirmed that O. lupi-associated Wolbachia diverged early from other Onchocerca spp.-Wolbachia [44] and that O. lupi-derived Wolbachia have coevolved with its nematode host [45]. The elucidation of this symbiotic relationship emphasizes the potential implication of Wolbachia in the pathology and treatment of the infection [42] and assisted in the analysis of this worm’s evolution.
The divergence of the genus Onchocerca has been estimated to occur in the past 2.5 million years [46] and independent studies have pointed out that O. lupi has a recent evolutionary history [44,45]. Indeed, initial phylogenetic studies using a fragment of the cytochrome oxidase subunit 1 gene (cox1) suggested that O. lupi was clearly separated from O. volvulus and O. ochengi, O. gutturosa and O. gibsoni, all infecting bovines. This finding confirms previous observations that O. lupi has both primitive and evolved morphological traits [44,45]. Furthermore, analyses using seven concatenated O. lupi markers indicated that this dog parasite is located in a cluster composed of human and cattle-derived Onchocerca spp. and separated from a second group containing wild animal and horse-derived Onchocerca spp. [45]. This suggests that the adaptation of O. lupi to dogs and cats might be related to their domestication [45], occurring between 4,000 and 15,000 years ago [47,48]. In addition, the presence of genetically identical O. lupi in cats and dogs might be related to the recent evolutionary appearance of these hosts species or to the inability of this worm to diverge in different parasite species, as shown in cophylogenetic studies [45]. Altogether, these data suggest that species divergence in the clade containing Onchocerca spp. of dogs, cats, and humans, including O. lupi, is more recent than the divergence of the clade containing Onchocerca spp. from wild animals and horses. The process of domestication of dogs and cats might have contributed to host switching events that led to speciation within this clade [45].
O. lupi has two different genotypes, genotype 1 includes worms from dog, cat, and human hosts from the Americas and Europe/Asia, and genotype 2 comprises nematodes from dogs and cats of the Iberian Peninsula [49]. Due to the sequence homogeneity within genotype 1, which includes nematodes from the US, Greece, Israel, Germany, Hungary, and Turkey, it has been hypothesized that O. lupi was imported to the US from Europe [36] as a recent event [35]. Interestingly, it was suggested that genotype 2 worms induce a subclinical to mild infection in its hosts [49], since infected dogs and cats from Spain and Portugal were reported to show minimal clinical manifestations compared to genotype 1 infections [2,13,34]. This differential pathology observed in separate genotypes has also been described for Trichinella spp. [50], and warrants more research focusing on epidemiological and intrinsic biological factors that underlie this observation.
Pathology
Onchocercosis is a disease inducing a wide range of clinical manifestations and affecting many different host species, yet little is reported on the pathogenic process which develops following the parasite’s establishment in the host, except for the infections induced by O. volvulus and O. ochengi [34]. O. lupi has been reported in several anatomical locations, mostly in subconjunctival and subcutaneous tissues from dogs [51], cats [12], and humans [29,52]. Less frequently, O. lupi has been detected in the cervical spinal cord in humans [8,9,15]. Ocular infections in canid and felid hosts may be characterized by acute or chronic ocular clinical signs [3]. The prepatent period of acute infections is unknown for O. lupi, but it is estimated to be at least several months based on what is known from other Onchocerca spp. [5]. Acute manifestations can include: periorbital swelling, lacrimation, conjunctivitis, protrusion of the nictitating membrane, exophthalmia, chemosis, photophobia, diffuse corneal stromal edema, scleral indentation, retinal detachment, and less commonly, blepharitis, corneal ulcers, anterior and posterior uveitis, and blindness [3,5,35,53]. Ocular pathology may lead to a decrease in visual acuity and, in severe instances, to total destruction of the eye and phthisis bulbi [49]. In some cases, portions of the gravid worm have been observed on the surface of the conjunctiva or beneath it, and in ocular and periocular tissues. Moreover, single or multiple granulomatous nodules or cyst-like formations affecting one or both eyes [32] were described as the most common presentation during chronic ocular disease within the retrobulbar spaces, orbital fascia, conjunctiva, nictitating membranes, or on top of the sclera [3]. In addition, a case of aberrant migration of O. lupi lodging in a single sessile nodule in the larynx was reported in a dog from Portugal [7]. In this report, a severe reduction of glottal and tracheal diameter led to dyspnea, tachypnoea, and cyanosis [7]. This parasitic infection in dogs usually does not manifest cutaneous signs, but dermatitis due to microfilariae cannot be excluded and should be distinguished from scabies, demodicosis, and other skin pathologies [5].
In humans, ocular onchocercosis is usually displayed as a single conjunctival nodule with mild conjunctival hyperemia and discomfort and no effect on vision acuity (Table 1) [17]. However, subcutaneous manifestations have also been reported in humans, ranging from erythematous swollen areas to non-erythematous and non-pruritic granulomatous cysts [52]. Spinal involvement has been detected in three cases all occurring in the US in children younger than 13 years old. Cervical mass lesions localized intra- or extra-durally have been described between the vertebral neural foramina of C2 and C4. Common symptoms displayed in these patients include a limited range of motion of the neck due to moderate to severe spinal cord compression, dysphagia, and headache [15,52]. Interestingly, nongravid female worms have been detected in all zoonotic onchocercosis cases, except in those reports with spinal involvement, which have occurred mostly in children [8,9,15]. This can be explained by the recovery of O. lupi worms from lesions before they reach sexual maturity. Alternatively, this might suggest that humans act as accidental, dead-end hosts of O. lupi in which the parasite cannot complete its life cycle. An immune response against the worms in adult patients may halt their development, as observed in human toxocarosis [54]. However, further research is required to elucidate the involvement of humans in the life cycle of O. lupi.
Table 1. Summary of confirmed O. lupi infections reported in humans.
| Case | Age (years)/Sex of patient | Geographical location | Year | Anatomical location of the lesion | Parasite sex | Diagnosis | Method of confirmation | Treatment | Reference |
| 1 | 8/NR | Tunisia | 2005† | Right eye | NGF | Ophthalmoscopy | Morphology | Surgical excision | [38] |
| 2 | 18/F | Turkey | 2011 | Left eye | NGF | Biomicroscopy + Imaging | Morphology + Molecular | Surgical excision | [29] |
| 3 | 26/M | Turkey | 2012 | Right eye | NGF | Ophthalmoscopy | Morphology + Molecular | Surgical excision | [38] |
| 4 | 2/F | Arizona, USA | 2013 | Upper cervical spinal cord | GF | Imaging | Morphology | Surgical excision, albendazole, ivermectin | [15] |
| 5 | 20/M | Qom, Iran | 2013 | Left eye | NGF | Biomicroscopy + Imaging | Morphology + Molecular | Surgical excision | [17] |
| 6 | 28/M | Turkey | 2013 | Right eye | NGF | Biomicroscopy | Morphology + Molecular | Surgical excision | [16] |
| 7 | 10/F | New Mexico, USA | 2013 | Right posterior-parietal scalp | NGF | Macroscopic observation | Morphology + Molecular | Surgical excision | [52] |
| 8 | 50/F | Arizona, USA** | 2014 | Subcutaneous forearm | NGF | Imaging | Morphology + Molecular | Surgical excision, doxycycline, ivermectin | [52] |
| 9 | 28/M | Erlangen, Germany* | 2014 | Right eye | NR | Ophthalmoscopy | Molecular | Surgical excision | [18] |
| 10 | 5/F | New Mexico, USA | 2014 | Upper cervical spinal cord | GF | Imaging | Morphology | Surgical excision, doxycycline, ivermectin | [9] |
| 11 | 10/M | Texas, USA | 2014 | Left eye | NGF | Imaging | Morphology + Molecular | Surgical excision, doxycycline, ivermectin | [52] |
| 12 | 13/M | Arizona, USA | 2015 | Upper cervical spinal cord | NGF | Imaging | Morphology | Surgical excision, doxycycline, ivermectin | [8] |
GF: gravid female, NGF: Nongravid female, NR: not reported, *: travel history to Tunisia and Turkey, **: travel history to Jamaica and Utah, USA, †Redescribed in 2012.
Diagnosis
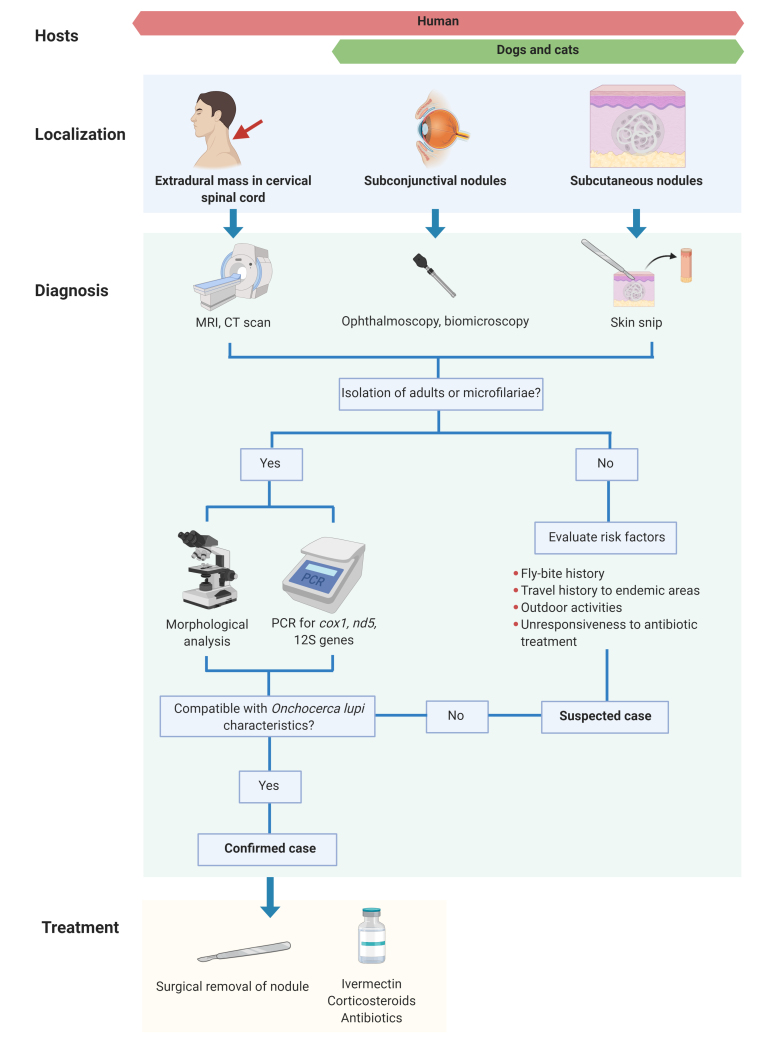
The diagnosis of O. lupi infection is difficult to establish, due to the variety of its anatomical locations and manifestations that it induces in its hosts (Figure 4) [3]. For canids and felids, skin snips are the most employed strategies and the most sensitive procedures for diagnosis of subclinical infection [1]. Briefly, skin snips consist of sampling a 0.2 cm3 piece of tissue surrounding the interocular cutaneous region of the head, ear tip, interscapular, and lumbar regions using a scalpel. This superficial biopsy is stored in saline solution for approximately 12 hours to enable the release of microfilariae into the liquid. The liquid solution is then centrifuged at 1500 rpm for 3 minutes, the sediment is recovered and viewed under microscope for the presence of microfilariae [2,34] (Figure 4). Skin snips are commonly used for the diagnosis of O. lupi because of the high sensitivity of this sampling technique in cases with no clinical signs, the ease of identifying worms and the simple accessibility to this method in most veterinary clinics. However, taking skin snips can be time consuming and false negative results may occur when microfilariae are not concentrated or not present in some anatomical locations [22,55].
Figure 4.
Diagnostic and treatment protocols of O. lupi infection in dogs, cats, and humans. This figure was created using www.biorender.com.
The presence of O. lupi in any anatomical location can be confirmed using imaging tools, such as magnetic resonance imaging, computerized tomography, and ultrasound scans for detecting ocular, subcutaneous, or cervical spinal cord infections in dogs [56] and humans [15]. These methods analyze the anatomic location of the nodules or cysts in the hosts and determine if local tissue damage is present due to helminth migration or a host inflammatory response [56]. In addition, these imaging tools are minimally invasive, relatively cost-effective and helpful in getting a confirmatory diagnosis [56]. Moreover, ophthalmic examination using fundoscopy [18,57] and biomicroscopy [16] is useful as a preliminary step in evaluating ocular involvement of O. lupi in dogs and humans. In addition, in vivo confocal microscopy has been applied in canids for diagnosing the parasite and for treatment follow-up [58]. These methods are fast and non-invasive but cannot accurately identify the specific agent involved in the observed lesions [57]. Still, for the identification of the etiological agent, invasive strategies such as nodule excision and worm extraction are needed [56], given the fact that no serological diagnostic test is commercially available for the diagnosis of O. lupi infection.
Preliminary serological tests have been developed for dogs. An enzyme-linked immunosorbent assay (ELISA) using an Og4C3 monoclonal antibody [59] as well as a western blot against the protein paramyosin of O. lupi [55] have shown promising results as preliminary approaches for detecting infection. Serological diagnosis is minimally invasive and highly specific if appropriate antigens are targeted, and it’s useful for large scale epidemiological surveys [58]. However, the ELISA developed for detecting O. lupi paramyosin has a sensitivity of 50%, its potential cross-reactions with closely related parasites have not been determined, and its clinical usefulness has not been assessed [59].
Morphological and molecular analyses are recommended for the confirmative identification of O. lupi in lesions. The nematode observed in histopathological analysis of formalin-fixed paraffin embedded (FFPE) samples or extracted from nodular lesions can be morphologically distinguished from other Onchocerca spp. by carefully observing the inner and outer layer features of its cuticle. Some of these characteristics include the presence of rounded, equally sized, and evenly spaced annular ridges in the external cuticle with two striae between adjacent annular ridges [19,60] and the ratio of body diameter to the distance between ridges which should be between 6:1 and 10:1 [6]. However, these characteristics are usually difficult to ascertain, require expertise from the observer, and thus, more specific assays are needed to accomplish the identification of collected nematodes.
Polymerase chain reaction (PCR) assays have been used for the sensitive and specific identification of O. lupi by amplifying the cox1 [2], 12S ribosome subunit [18] and NADH dehydrogenase subunit 5 [16] genes from formalin-fixed paraffin embedded (FFPE) nodule biopsies [12], portions of adult worms [18], or microfilariae obtained from skin snips [34]. A qPCR protocol targeting a 90 bp fragment of the cox1 gene of O. lupi was developed with 100% of specificity and an analytical sensitivity of 8 x 10−1 fg of adult O. lupi DNA/2μl and 3.6 x 10-1 pg of microfilariae DNA/2μl [61]. In addition, a duplex qPCR amplifying a region of the cox1 discriminated between O. lupi and O. volvulus, which highlights its potential use in O. lupi non-endemic regions due to the difficulty in morphologically distinguishing between these two species [62]. This duplex qPCR detected 10 out of 11 samples with O. lupi, and all six positive for O. volvulus [62]. Altogether, these molecular assays are useful for epidemiological surveys, detection of subclinical infections, cases with low parasitic loads, and for monitoring treatment outcomes.
Treatment
Currently there are no evidence-based protocols for treating O. lupi infections [63]. Drugs are administered based on treatments against other filarial parasites, mainly O. volvulus [63]. The primary approach has traditionally been the surgical excision of the adult-containing nodules or cysts (Figure 4). This strategy may lead to a strong host inflammatory response against released microfilariae from gravid female worms [3], as observed in O. volvulus. The extraction of helminths from the lesions in pets or humans, is usually accompanied by treatment with anthelminthic drugs, topical steroids, and antibiotics. Ivermectin is a widely used microfilaricidal employed for treating human O. volvulus-infections, and in zoonotic ocular onchocercosis cases [52] and canine infections [57]. This anthelminthic suppresses microfilariae formation and induces female worm sterilization [52], thus blocking the transmission of infection for several months. One of the major disadvantages of the use of ivermectin is a lack of well controlled studies focusing on long-term outcomes of this treatment. Studies of dogs with subconjunctival onchocercosis from Greece treated with ivermectin have not reported infection recurrence [37]. In contrast, a retrospective study of 16 ivermectin-treated dogs with ocular onchocercosis from the US showed a 67% of clinical signs recurrence, allegedly because the particular strain of O. lupi endemic in this country may represent a haplotype more difficult to control than the ones present in Europe [35]. Melarsomine has been employed in treating ocular infections in dogs as an adulticide by intramuscular injection before ivermectin for the control of uveitis and orbital disease [3,32,57]. Moreover, other drugs like oxfendazole have proved inefficient in reducing ocular lesions and skin-dwelling O. lupi microfilariae in dogs [64]. A possible explanation of this may be due to a low drug concentration that is able to penetrate the nodules and act on O. lupi adults, as knowledge about the vascularization of these lesions is absent [64].
Steroids, mainly corticosteroids, are administered to control inflammation caused by the presence of the nematode and to avoid inflammatory reactions after the elimination of adults [3]. Moreover, antibiotics like doxycycline [35,52] and oxytetracycline [42] are routinely applied to target the endosymbiont bacteria Wolbachia [42]. The need for new, accurate, and specific drugs to treat ocular onchocercosis is highlighted given the limited knowledge about treatment strategies.
Conclusions and Outlook
Onchocercosis caused by O. lupi is a zoonotic infection affecting canine, feline, and human hosts in Asia, Europe, and North America. This review highlights the presence of the parasite in wild canid reservoirs as well as clinically affected and subclinically infected cats and dogs, which together, may facilitate the spread of the parasite to different geographical locations if suitable intermediate hosts are present. Further research should be carried to identify the intermediate hosts of O. lupi, the involvement of humans in the life cycle of this nematode, to design novel diagnostic tools, and to improve treatment strategies against this zoonotic parasite.
Glossary
- PCR
polymerase chain reaction
Author Contributions
All authors have contributed to writing and editing the manuscript. AR prepared the figures. Funding from AR and FM-C is provided by the Centro de Investigación en Enfermedades Tropicales (CIET), University of Costa Rica. Funding for HS and GB is provided by internal lab funds from Prof. Gad Baneth. DO funds are provided by internal funds from Prof. Domenico Otranto.
References
- Otranto D, Giannelli A, Scotty Trumble N, Chavkin M, Kennard G, Latrofa MS, et al. Clinical case presentation and a review of the literature of canine onchocercosis by Onchocerca lupi in the United States. Parasit Vectors. 2015;8:89. doi: 10.1186/s13071-015-0699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miro G, Montoya A, Checa R, Galvez R, Minguez JJ, Marino V, et al. First detection of Onchocerca lupi infection in dogs in southern Spain. Parasit Vectors. 2016;9(1):290. Epub 2016/05/20. doi: 10.1186/s13071-016-1587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grácio AJ, Richter J, Komnenou AT, Grácio MA. Onchocerciasis caused by Onchocerca lupi: an emerging zoonotic infection. Systematic review. Parasitol Res. 2015. July;114(7):2401–13. 10.1007/s00436-015-4535-7 [DOI] [PubMed] [Google Scholar]
- Roe CC, Yaglom H, Howard A, Urbanz J, Verocai GG, Andrews L, et al. Coyotes as Reservoirs for Onchocerca lupi, United States, 2015-2018. Emerg Infect Dis. 2020. December;26(12):2989–93. 10.3201/eid2612.190136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sréter T, Széll Z. Onchocercosis: a newly recognized disease in dogs. Vet Parasitol. 2008. January;151(1):1–13. 10.1016/j.vetpar.2007.09.008 [DOI] [PubMed] [Google Scholar]
- Egyed Z, Sréter T, Széll Z, Beszteri B, Oravecz O, Márialigeti K, et al. Morphologic and genetic characterization of Onchocerca lupi infecting dogs. Vet Parasitol. 2001. December;102(4):309–19. 10.1016/S0304-4017(01)00541-6 [DOI] [PubMed] [Google Scholar]
- Alho AM, Cruz L, Coelho A, Martinho F, Mansinho M, Annoscia G, et al. Aberrant laryngeal location of Onchocerca lupi in a dog. Parasitol Int. 2016. June;65(3):218–20. 10.1016/j.parint.2015.12.010 [DOI] [PubMed] [Google Scholar]
- Chen T, Moon K, deMello DE, Feiz-Erfan I, Theodore N, Bhardwaj RD. Case report of an epidural cervical Onchocerca lupi infection in a 13-year-old boy. J Neurosurg Pediatr. 2015. August;16(2):217–21. 10.3171/2014.12.PEDS14462 [DOI] [PubMed] [Google Scholar]
- Dudley RW, Smith C, Dishop M, Mirsky D, Handler MH, Rao S. A cervical spine mass caused by Onchocerca lupi. Lancet. 2015. October;386(10001):1372. 10.1016/S0140-6736(14)62255-8 [DOI] [PubMed] [Google Scholar]
- Verocai GG, Conboy G, Lejeune M, Marron F, Hanna P, MacDonald E, et al. Onchocerca lupi Nematodes in Dogs Exported from the United States into Canada. Emerg Infect Dis. 2016;22(8):1477-9. 10.3201/eid2208.151918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodžić A, Hinney B, König S, Naucke TJ, Duscher G, Joachim A. A case of ocular infection with Onchocerca lupi in a dog from Germany. Transbound Emerg Dis. 2018. February;65(1):e214–6. 10.1111/tbed.12715 [DOI] [PubMed] [Google Scholar]
- Labelle AL, Daniels JB, Dix M, Labelle P. Onchocerca lupi causing ocular disease in two cats. Vet Ophthalmol. 2011. September;14 Suppl 1:105–10. 10.1111/j.1463-5224.2011.00911.x [DOI] [PubMed] [Google Scholar]
- Maia C, Annoscia G, Latrofa MS, Pereira A, Giannelli A, Pedroso L, et al. Onchocerca lupi Nematode in Cat, Portugal. Emerg Infect Dis. 2015;21(12):2252-4. doi: 10.3201/eid2112.150061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D, Sakru N, Testini G, Gurlu VP, Yakar K, Lia RP, et al. Case report: First evidence of human zoonotic infection by Onchocerca lupi (Spirurida, Onchocercidae). Am J Trop Med Hyg. 2011;84(1):55-8. doi: 10.4269/ajtmh.2011.10-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard ML, Ostovar GA, Chundu K, Hobohm D, Feiz-Erfan I, Mathison BA, et al. Zoonotic Onchocerca lupi infection in a 22-month-old child in Arizona: first report in the United States and a review of the literature. Am J Trop Med Hyg. 2013;88(3):601-5. doi: 10.4269/ajtmh.12-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilhan HD, Yaman A, Morishima Y, Sugiyama H, Muto M, Yamasaki H, et al. Onchocerca lupi infection in Turkey: a unique case of a rare human parasite. Acta Parasitol. 2013. September;58(3):384–8. 10.2478/s11686-013-0152-8 [DOI] [PubMed] [Google Scholar]
- Mowlavi G, Farzbod F, Kheirkhah A, Mobedi I, Bowman DD, Naddaf SR. Human ocular onchocerciasis caused by Onchocerca lupi (Spirurida, Onchocercidae) in Iran. J Helminthol. 2014. June;88(2):250–5. 10.1017/S0022149X13000060 [DOI] [PubMed] [Google Scholar]
- Bergua A, Hohberger B, Held J, Muntau B, Tannich E, Tappe D. Human case of Onchocerca lupi infection, Germany, August 2014. Euro Surveill. 2015. April;20(16):21099. 10.2807/1560-7917.ES2015.20.16.21099 [DOI] [PubMed] [Google Scholar]
- Mutafchiev Y, Dantas-Torres F, Giannelli A, Abramo F, Papadopoulos E, Cardoso L, et al. Redescription of Onchocerca lupi (Spirurida: Onchocercidae) with histopathological observations. Parasit Vectors. 2013;6(1):309. doi: 10.1186/1756-3305-6-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodonaja T. A new species of nematode, Onchocerca lupi n. sp., from Canis lupus cubanensis. Bulletin of the Academic of Science of Georgian SSR. 1967;45:715–9. [Google Scholar]
- Demiaszkiewiczl AW, Matsaberidze GV, Kvavadze ES. The female of Onchocerca lupi Rodonaja, 1967 under a scanning electron microscope. Acta Parasitologica Polonica. 1991;46(4):183–6. [Google Scholar]
- Otranto D, Dantas-Torres F, Giannelli A, Abramo F, Ignjatovic Cupina A, Petric D, et al. Cutaneous distribution and circadian rhythm of Onchocerca lupi microfilariae in dogs. PLoS Negl Trop Dis. 2013;7(12):e2585. doi: 10.1371/journal.pntd.0002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basáñez MG, Churcher TS, Grillet ME. Onchocerca-Simulium interactions and the population and evolutionary biology of Onchocerca volvulus. Adv Parasitol. 2009;68:263–313. 10.1016/S0065-308X(08)00611-8 [DOI] [PubMed] [Google Scholar]
- Makepeace BL, Tanya VN. 25 Years of the Onchocerca ochengi Model. Trends Parasitol. 2016. December;32(12):966–78. 10.1016/j.pt.2016.08.013 [DOI] [PubMed] [Google Scholar]
- Eichler DA. Studies on Onchocerca gutturosa (Neumann, 1910) and its development in Simulium ornatum (Meigen, 1818). II. Behaviour of S. ornatum in relation to the transmission of O. gutturosa. J Helminthol. 1971;45(2):259–70. 10.1017/S0022149X00007148 [DOI] [PubMed] [Google Scholar]
- Otranto D, Dantas-Torres F, Papadopoulos E, Petric D, Cupina AI, Bain O. Tracking the vector of Onchocerca lupi in a rural area of Greece. Emerg Infect Dis. 2012;18(7):1196-200.doi: 10.3201/eid1807.AD1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan HK, Bolcen S, Kubofcik J, Nutman TB, Eberhard ML, Middleton K, et al. Isolation of Onchocerca lupi in Dogs and Black Flies, California, USA. Emerg Infect Dis. 2015;21(5):789-96. doi: 10.3201/eid2105.142011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilhante AF, de Albuquerque AL, Rocha ACB, Ayres CFJ, Paiva MHS, de Avila MM, et al. First report of an Onchocercidae worm infecting Psychodopygus carrerai carrerai sandfly, a putative vector of Leishmania braziliensis in the Amazon. Sci Rep. 2020;10(1):15246. doi: 10.1038/s41598-020-72065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D, Sakru N, Testini G, Gürlü VP, Yakar K, Lia RP, et al. Case report: First evidence of human zoonotic infection by Onchocerca lupi (Spirurida, Onchocercidae). Am J Trop Med Hyg. 2011;84(1):55-8. doi: 10.4269/ajtmh.2011.10-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihel TC, Ash LR, Holshuh HJ, Santenelli S. Onchocerciasis in a California dog. Am J Trop Med Hyg. 1991. May;44(5):513–7. 10.4269/ajtmh.1991.44.513 [DOI] [PubMed] [Google Scholar]
- Gardiner CH, Dick EJ Jr, Meininger AC, Lozano-Alarcón F, Jackson P. Onchocerciasis in two dogs. J Am Vet Med Assoc. 1993. September;203(6):828–30. [PubMed] [Google Scholar]
- Széll Z, Sréter T, Erdélyi I, Varga I. Ocular onchocercosis in dogs: aberrant infection in an accidental host or lupi onchocercosis? Vet Parasitol. 2001. November;101(2):115–25. 10.1016/s0304-4017(01)00507-6 [DOI] [PubMed] [Google Scholar]
- Colella V, Lia RP, Di Paola G, Cortes H, Cardoso L, Otranto D. International dog travelling and risk for zoonotic Onchocerca lupi. Transbound Emerg Dis. 2018. August;65(4):1107–9. 10.1111/tbed.12842 [DOI] [PubMed] [Google Scholar]
- Otranto D, Dantas-Torres F, Giannelli A, Latrofa MS, Papadopoulos E, Cardoso L, et al. Zoonotic Onchocerca lupi infection in dogs, Greece and Portugal, 2011-2012. Emerg Infect Dis. 2013;19(12):2000-3. doi: 10.3201/eid1912.130264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean NJ, Newkirk K, Adema CM. Canine ocular onchocerciasis: a retrospective review of the diagnosis, treatment, and outcome of 16 cases in New Mexico (2011-2015). Vet Ophthalmol. 2017;20(4):349-56. doi: 10.1111/vop.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D, Giannelli A, Latrofa MS, Dantas-Torres F, Trumble NS, Chavkin M, et al. Canine Infections with Onchocerca lupi Nematodes, United States, 2011-2014. Emerg Infect Dis. 2015;21(5):868-71.doi: 10.3201/eid2105.141812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komnenou A, Eberhard ML, Kaldrymidou E, Tsalie E, Dessiris A. Subconjunctival filariasis due to Onchocerca sp. in dogs: report of 23 cases in Greece. Vet Ophthalmol. 2002. June;5(2):119–26. 10.1046/j.1463-5224.2002.00235.x [DOI] [PubMed] [Google Scholar]
- Otranto D, Dantas-Torres F, Cebeci Z, Yeniad B, Buyukbabani N, Boral OB, et al. Human ocular filariasis: further evidence on the zoonotic role of Onchocerca lupi. Parasit Vectors. 2012;5:84.doi: 10.1186/1756-3305-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarova NS, Miretskiĭ OI, Sonin MD. [1st case of human infection by the nematode Onchocerca Diesing, 1841 in the USSR]. Med Parazitol (Mosk). 1965. Mar-Apr;34(2):156–8. [PubMed] [Google Scholar]
- Pampiglione S, Vakalis N, Lyssimachou A, Kouppari G, Orihel TC. Subconjunctival zoonotic Onchocerca in an Albanian man. Ann Trop Med Parasitol. 2001. December;95(8):827–32. 10.1080/00034980120111163 [DOI] [PubMed] [Google Scholar]
- Sallo F, Eberhard ML, Fok E, Baska F, Hatvani I. Zoonotic intravitreal Onchocerca in Hungary. Ophthalmology. 2005. March;112(3):502–4. 10.1016/j.ophtha.2004.10.036 [DOI] [PubMed] [Google Scholar]
- Egyed Z, Sréter T, Széll Z, Nyirö G, Dobos-Kovács M, Márialigeti K, et al. Electron microscopic and molecular identification of Wolbachia endosymbionts from Onchocerca lupi: implications for therapy. Vet Parasitol. 2002. May;106(1):75–82. 10.1016/S0304-4017(02)00029-8 [DOI] [PubMed] [Google Scholar]
- Lefoulon E, Bain O, Makepeace BL, d'Haese C, Uni S, Martin C, et al. Breakdown of coevolution between symbiotic bacteria Wolbachia and their filarial hosts. Peer J. 2016;4:e1840. doi: 10.7717/peerj.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egyed Z, Sréter T, Széll Z, Nyiro G, Márialigeti K, Varga I. Molecular phylogenetic analysis of Onchocerca lupi and its Wolbachia endosymbiont. Vet Parasitol. 2002. September;108(2):153–61. 10.1016/S0304-4017(02)00186-3 [DOI] [PubMed] [Google Scholar]
- Lefoulon E, Giannelli A, Makepeace BL, Mutafchiev Y, Townson S, Uni S, et al. Whence river blindness? The domestication of mammals and host-parasite co-evolution in the nematode genus Onchocerca. Int J Parasitol. 2017. July;47(8):457–70. 10.1016/j.ijpara.2016.12.009 [DOI] [PubMed] [Google Scholar]
- Xie H, Bain O, Williams SA. Molecular phylogenetic studies on filarial parasites based on 5S ribosomal spacer sequences. Parasite. 1994. June;1(2):141–51. 10.1051/parasite/1994012141 [DOI] [PubMed] [Google Scholar]
- Frantz LA, Mullin VE, Pionnier-Capitan M, Lebrasseur O, Ollivier M, Perri A, et al. Genomic and archaeological evidence suggest a dual origin of domestic dogs. Science. 2016. June;352(6290):1228–31. 10.1126/science.aaf3161 [DOI] [PubMed] [Google Scholar]
- Driscoll CA, Macdonald DW, O'Brien SJ. From wild animals to domestic pets, an evolutionary view of domestication. Proc Natl Acad Sci U S A. 2009;106 Suppl 1:9971-8. doi: 10.1073/pnas.0901586106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas A, Salant H, Yasur-Landau D, Tsarfati H, Baneth G. First report of Onchocerca lupi from Israel and confirmation of two genotypes circulating among canine, feline and human hosts. Parasitology. 2020. December;147(14):1723–7. 10.1017/S0031182020001560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarlenga D, Thompson P, Pozio E. Trichinella species and genotypes. Res Vet Sci. 2020. December;133:289–96. 10.1016/j.rvsc.2020.08.012 [DOI] [PubMed] [Google Scholar]
- Tudor P, Turcitu M, Mateescu C, Dantas-Torres F, Tudor N, Bărbuceanu F, et al. Zoonotic ocular onchocercosis caused by Onchocerca lupi in dogs in Romania. Parasitol Res. 2016. February;115(2):859–62. 10.1007/s00436-015-4816-1 [DOI] [PubMed] [Google Scholar]
- Cantey PT, Weeks J, Edwards M, Rao S, Ostovar GA, Dehority W, et al. The Emergence of Zoonotic Onchocerca lupi Infection in the United States--A Case-Series. Clin Infect Dis. 2016;62(6):778-83. doi: 10.1093/cid/civ983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faísca P, Morales-Hojas R, Alves M, Gomes J, Botelho M, Melo M, et al. A case of canine ocular onchocercosis in Portugal. Vet Ophthalmol. 2010. March;13(2):117–21. 10.1111/j.1463-5224.2010.00763.x [DOI] [PubMed] [Google Scholar]
- Ma G, Holland CV, Wang T, Hofmann A, Fan CK, Maizels RM, et al. Human toxocariasis. Lancet Infect Dis. 2018. January;18(1):e14–24. 10.1016/S1473-3099(17)30331-6 [DOI] [PubMed] [Google Scholar]
- Campbell B, Cortes H, Annoscia G, Giannelli A, Parisi A, Latrofa MS, et al. Paramyosin of canine Onchocerca lupi: usefulness for the diagnosis of a neglected zoonotic disease. Parasit Vectors. 2016;9(1):493. doi: 10.1186/s13071-016-1783-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini D, Giannelli A, Di Paola G, Cortes H, Cardoso L, Lia RP, et al. Image diagnosis of zoonotic onchocercosis by Onchocerca lupi. Vet Parasitol. 2014. June;203(1-2):91–5. 10.1016/j.vetpar.2014.03.007 [DOI] [PubMed] [Google Scholar]
- Komnenou AT, Thomas AL, Papadopoulos E, Koutinas AF. Intraocular localization of Onchocerca lupi adult worm in a dog with anterior uveitis: A case report. Vet Ophthalmol. 2016. May;19(3):245–9. 10.1111/vop.12277 [DOI] [PubMed] [Google Scholar]
- Edelmann ML, Jager M, Espinheira F, Ledbetter EC. In vivo confocal microscopy for detection of subconjunctival Onchocerca lupi infection in a dog. Vet Ophthalmol. 2018. November;21(6):632–7. 10.1111/vop.12547 [DOI] [PubMed] [Google Scholar]
- Giannelli A, Cantacessi C, Graves P, Becker L, Campbell BE, Dantas-Torres F, et al. A preliminary investigation of serological tools for the detection of Onchocerca lupi infection in dogs. Parasitol Res. 2014. May;113(5):1989–91. 10.1007/s00436-014-3844-6 [DOI] [PubMed] [Google Scholar]
- Labelle AL, Maddox CW, Daniels JB, Lanka S, Eggett TE, Dubielzig RR, et al. Canine ocular onchocercosis in the United States is associated with Onchocerca lupi. Vet Parasitol. 2013. March;193(1-3):297–301. 10.1016/j.vetpar.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Latrofa MS, Annoscia G, Colella V, Cavalera MA, Maia C, Martin C, et al. A real-time PCR tool for the surveillance of zoonotic Onchocerca lupi in dogs, cats and potential vectors. PLoS Negl Trop Dis. 2018;12(4):e0006402. doi: 10.1371/journal.pntd.0006402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida M, Nascimento FS, Mathison BA, Bishop H, Bradbury RS, Cama VA, et al. Duplex Real-Time PCR Assay for Clinical Differentiation of. Am J Trop Med Hyg. 2020;103(4):1556-62. doi: 10.4269/ajtmh.20-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantey PT, Eberhard M, Weeks J, Swoboda S, Ostovar GA. Letter to the Editor: Onchocerca lupi infection. J Neurosurg Pediatr. 2016;17(1):118-9. doi: 10.3171/2015.6.PEDS15344. [DOI] [PubMed] [Google Scholar]
- Colella V, Maia C, Pereira A, Goncalves N, Caruso M, Martin C, et al. Evaluation of oxfendazole in the treatment of zoonotic Onchocerca lupi infection in dogs. PLoS Negl Trop Dis. 2018;12(1):e0006218. doi: 10.1371/journal.pntd.0006218. [DOI] [PMC free article] [PubMed] [Google Scholar]