Abstract
Background:
Mesenchymal stem cells (MSCs) have recently shown promise for the treatment of various types of chronic kidney disease models. However, the mechanism of this effect is still not well understood. Our study is aimed to investigate the effect of MSCs on transforming growth factor beta 1 (TGF-β1)-induced epithelial mesenchymal transition (EMT) in renal tubular epithelial cells (HK-2 cells) and the underlying mechanism related to the reciprocal balance between hepatocyte growth factor (HGF) and TGF-β1.
Methods:
Our study was performed at Ningbo University, Ningbo, Zhejiang, China between Mar 2017 and Jun 2018. HK-2 cells were initially treated with TGF-β1, then co-cultured with MSCs. The induced EMT was assessed by cellular morphology and the expressions of alpha-smooth muscle actin (α-SMA) and EMT-related proteins. MTS assay and flow cytometry were employed to detect the effect of TGF-β1 and MSCs on HK-2 cell proliferation and apoptosis. SiRNA against hepatocyte growth factor (siHGF) was transfected to decrease the expression of HGF to identify the role of HGF in MSCs inhibiting HK-2 cells EMT.
Results:
Overexpressing TGF-β1 decreased HGF expression, induced EMT, suppressed proliferation and promoted apoptosis in HK-2 cells; but when co-cultured with MSCs all the outcomes were reversed. However, after treated with siHGF, all the benefits taken from MSCs vanished.
Conclusion:
TGF-β1 was a motivating factor of kidney cell EMT and it suppressed the HGF expression. However, MSCs provided protection against EMT by increasing HGF level and decreasing TGF-β1 level. Our results also demonstrated HGF is one of the critical factor in MSCs anti- fibrosis.
Keywords: Mesenchymal stem cells, Transforming growth factor beta 1 (TGF-β1), Epithelial mesenchymal transition (EMT), Hepatocyte growth factor (HGF), Apoptosis
Introduction
A variety of animal and cell studies have shown that mesenchymal stem cells (MSCs) therapy can ameliorates renal dysfunction in chronic kidney disease (CKD)(1). MSCs are multipotent stromal cells and capable of differentiating into a variety of cell types, for example chondrocytes, osteoblasts myocytes and adipocytes (2). MSCs have the ability to modulate immune responses, attenuate extracellular matrix deposition and repair epithelial tissues (3). MSCs also can secrete a number of growth factors and cytokines including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), transforming growth factor beta 1 (TGF-β1) and hepatocyte growth factor (HGF), which are important for antiapoptotic, angiogenesis and cytoprotection (4). The protecting effects of MSCs are partially mediated by secreted factors and cytokines (5).
Interestingly, TGF and HGF play the opposite roles in the process of fibrosis. HGF is a multipotent growth factor which exerts anti-fibrotic and anti-inflammatory responses via inducing the c-Met expression (6). It improves cells growth, reduces cells apoptosis, and exerts a beneficial effect on neovascularization and tissue remodeling (7). Furthermore, the anti-fibrotic effect of HGF in renal interstitial fibrosis is achieved by inhibiting TGF-β1expression (8). TGF-β1 is a pathogenic mediator in the development of fibrosis in distinct organs (9, 10). It can induces renal tubular epithelial cells to undergo a phenotypic transformation and then cells detach from the tubular basement membrane and migrate into the interstitium (11). TGF-β1 is considered as a strong EMT inducer in different cell lines such as lung cancer cells (12), prostate cancer cells (13), osteosarcoma cells (14) and epithelial cells (15). In the past 2 decades, the potential role of TGF-β1 in the progression of nephropathy has also been recognized. Additionally, TGF-β1 is a suppressor factor in the regulation of HGF expression in many types of cells (16–18).
There is a reciprocal relationship between TGF-β1 and HGF in the progress of renal fibrosis in mice. The increased expression of TGF-β1 and the decreased expression of HGF may be responsible for renal fibrosis (19). Given the effects of TGF-β1 in the renal tubular epithelial cells EMT and the anti-fibrotic effect of HGF, we are wondering whether the protect potential of MSCs on renal fibrosis is associated with restoring the balance between HGF and TGF. In this study, HK-2 cells are induced EMT with TGF-β1 treatment. Then, we investigated the effect of MSCs on TGF-β1 induced EMT in HK-2 cells and the underlying mechanisms related to HGF.
Materials and Methods
Cell culture and transfections
All experiments between Mar 2017 and Jun 2018 were performed at Ningbo University, China in this study. The immortalized proximal tubule epithelial cell line (HK-2) was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and Human Bone Marrow Mesenchymal Stem Cells (MSCs) were purchased from Cyagen Biosciences Inc. (SuZhou, China). HK-2 cells were cultured in DMEM (HyClone, UT, USA) medium supplemented with 10% fetal bovine serum (FBS, ExCell Bio, Shanghai, China) and MSCs were cultured in Human Bone Marrow Mesenchymal Stem Cell Basal Medium supplemented with 10% Human Bone Marrow Mesenchymal Stem Cell-Qualified Fetal Bovine Serum, 1% penicillin, 1%U/mL streptomycin and 1% Glutamine (Cyagen Biosciences, SuZhou, China). All cells were cultured in a 37 °C humidified chamber with 5% CO2 (20).
SiNC (sense: 5′-UUCUCCGAACGUGUCACGUTT-3′, anti-sense: 5′-ACGUGACACGUUCGGAGAATT-3′) and siHGF (sense: 5′-GCACACCAAUGUGCUAAUATT-3′, anti-sense: 5′-UAUUAGCACAUUGGUCUGCUGCTT3-′) were purchased from GenePharma (Shanghai, China). Transfection Reagent (Promega Corporation, USA) were used to transfect siRNA according to the manufacturer’s instructions.
Co-culture of MSCs and HK-2 cells
HK-2 cells and MSCs were performed an indirect co-culture system by using transwell membranes (12mm, 0.4 μm pore; Corning, NY, USA). Briefly, HK-2 cells treated with or without TGF-β1 (10 ng/mL; R&D Systems, Minneapolis, MN) were seeded in bottom chamber. MSCs with or without transfected with siHGF for 24h were seeded in upper chamber (21). Co-cultivation was maintained for 48 h, then HK-2 cells were harvested to detect cell proliferation, apoptosis, western blot and cell morphology analysis.
Cell proliferation assay
Cell proliferation was evaluated by [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS). Cells were plated in a 96-well plate (6000 cells/well) in quintuplicate. Following incubation for 1, 2, 3, and 4 d, 20 μl of Cell Titer 96® Aqueous One Solution Reagent (PROMEGA, Madison, USA) was added to each well and the cells were incubated for another 3 h at 37 °C. The absorbance at 490 nm was read using a spectrophotometer (22).
Flow cytometric analysis of apoptosis
Cells were dissociation with trypsin (without EDTA), then washed twice with PBS and resuspended with binding buffer. Every sample incubated with 5 μl Annexin V and 10 μl PI (Multi Sciences, Hangzhou, China) following the manufacturer’s recommendation (23).
Western blot analysis
Cells were harvested and lysed with RIPA buffer (Solarbio, Beijing, China). The proteins (50 μg) were separated using 12% SDS-polyacrylamide gel and then electrophoretically transferred to 0.42 μm PVDF membranes (Millipore, Billerica, MA). The blots were blocked with 5% BSA in Tris–buffered saline (TBS, pH 7.4) for 1h, and then incubated with appropriate dilutions of specifc primary antibodies overnight at 4 °C. The following antibodies were used: HGF β (52445), α-Smooth Antibody (14968), TGF-β1 Antibody (3711), E-cadherin(3195), N-cadherin(14215), Bax (5023), Bcl-2 (15071), GAPDH Antibody (5174) (Cell Signaling Technology, MA, USA), fibronectin (ab2413) and Ki-67 (ab15580) (Abcam, Cambridge, UK). Then, the membranes were incubated with horseradish peroxidaselabeled secondary antibody (Boster, Wuhan, China). The protein bands were visualized using enhanced chemiluminescence reagent. The images were analysed with Tanon GIS version 4.1.2 software (Tanon Science and Technology Co., Ltd., Shanghai, China) to determine the integrated density (24).
Cell morphology analysis
After HK-2 cells and MSCs co-culture 48h, the morphology of HK-2 were photographed (Magnification 10×) (Motic AE31; Motic China Group Co., Ltd., Xiamen, China).
Statistical analysis
All experiments were repeated three times. The data were expressed as means ± SD. One-way analysis of variance and Fisher’s least significant difference tests were used to evaluate the differences between groups. Statistical analyses were performed with SPSS 18.0 software (Chicago, IL, USA). Differences were considered significant if P<0.05.
Results
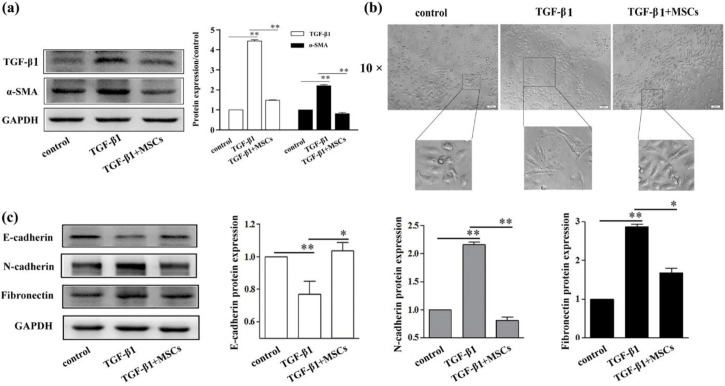
MSCs attenuated TGF-β1-stimulated EMT in HK-2 cells
To investigate the effect of MSCs on TGF-β1-stimulatejd HK-2 cell EMT, HK-2 cells were treated with TGF-β1, then co-cultured with MSCs. TGF-β1 treatment increased the expression of TGF-β1 and α-SMA (Fig. 1a). And HK-2 cell morphology changed from normal to spindle-shape (Fig. 1b), accompanying decrease of the expression of E-cadherin and the increase of the expression of fibronectin and N-cadherin in the TGF-β1 group (Fig. 1c). However, all these results were attenuate by co-culture with MSCs. The expression of TGF-β1, α-SMA fibronectin and N-cadherin were lower, while E-cadherin was higher in TGF-β1+MSCs group than TGF-β1 group (Fig. 1a and c). And TGF-β1+MSCs group had the similar cell morphology compared with control group (Fig. 1b).
Fig. 1:
MSCs attenuated TGF-β1-stimulated EMT in HK-2 cells. The expression of TGF-β1 and α-SMA were assessed by western blot analysis (a). The influence of TGF-β1 and MSCs on the morphological change of HK-2 cells (b). Western blot was used to detect the expression of E-cadherin, N-cadherin and fibronectin in HK-2 cells (c). *Significant difference (P<0.05) and **Significant difference (P<0.01). Scale bars = 100 μm
MSCs promote the HK-2 cell proliferation and suppressed apoptosis
In TGF-β1 group, we found the viability of HK-2 cells were significantly decreased compared with control group (P=0.0067), but the proliferation of TGF-β1+MSCs group was remarkable higher than TGF-β1 group (P=0.034) by MTS assay. The results meant MSCs could partially promote the down-regulated viability of cells caused by TGF-β1 treatment (Fig. 2a). Annexin V/PI dual staining was used to evaluate the effect of TGF-β1 and MSCs on cell apoptosis. In the control group, the late apoptotic rate was 13.9%, but the apoptosis rate of TGF-β1 treatment group was 39.3%. Furthermore, TGF-β1+MSCs group had apoptosis rate of 14.0%, and significantly lower than TGF-β1 group (P=0.001) (Fig. 2b). Therefore, TGF-β1 and MSCs co-treatment was less potent in inducing apoptotic than only TGF-β1 treatment.
Fig. 2:
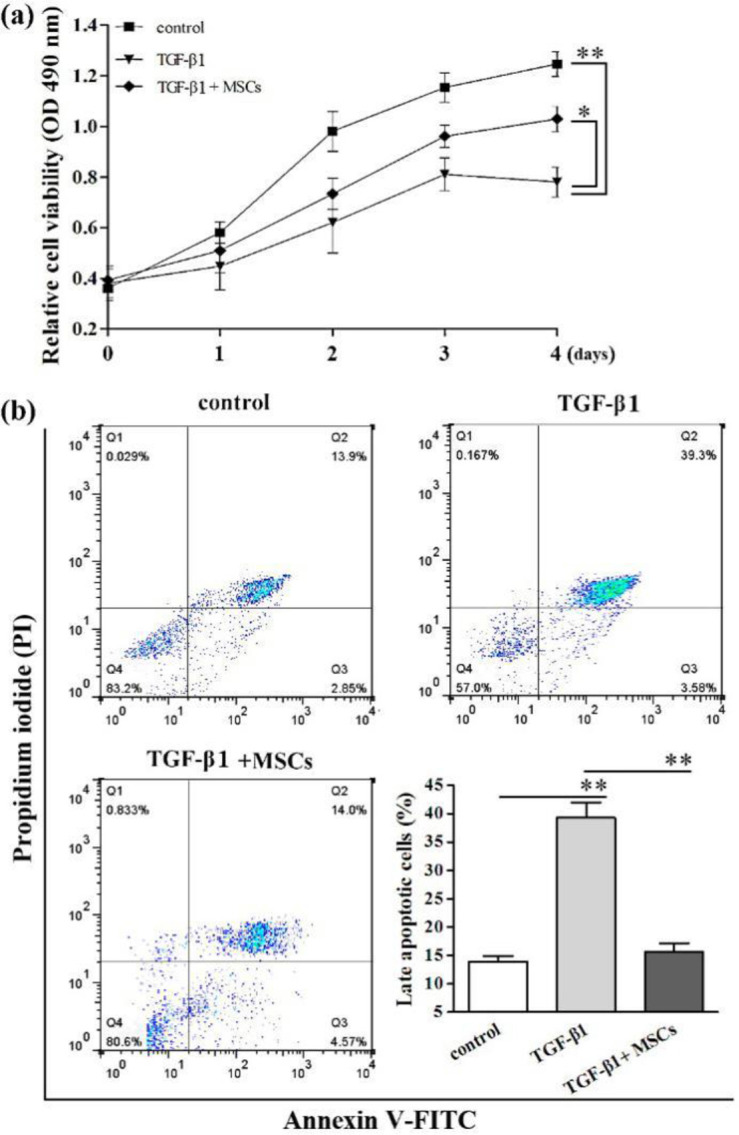
MSCs promoted the HK-2 cell proliferation and suppressed apoptosis. Cell proliferation was detected by MTS assay (a). Late apoptosis of HK-2 cells was examined by flow cytometric analysis (b). *Significant difference (P<0.05) and **Significant difference (P<0.01)
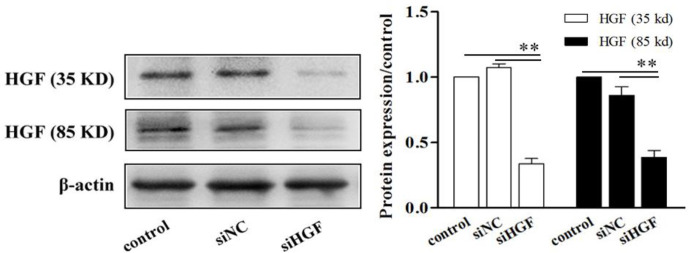
SiHGF decreased the expression of HGF
To explore whether HGF is an important factor in MSCs improving cell fibrogenesis, we down-regulated the expression of HGF by transfecting siHGF into HK-2 cells. The western blotting results confirmed that siHGF remarkable decreased the expression of HGF in HK-2 cells (P<0.01) (Fig. 3).
Fig. 3:
SiHGF decreased the expression of HGF. The expression of HGF was assessed by western blot analysis. **Significant difference (P<0.01), compared with control group
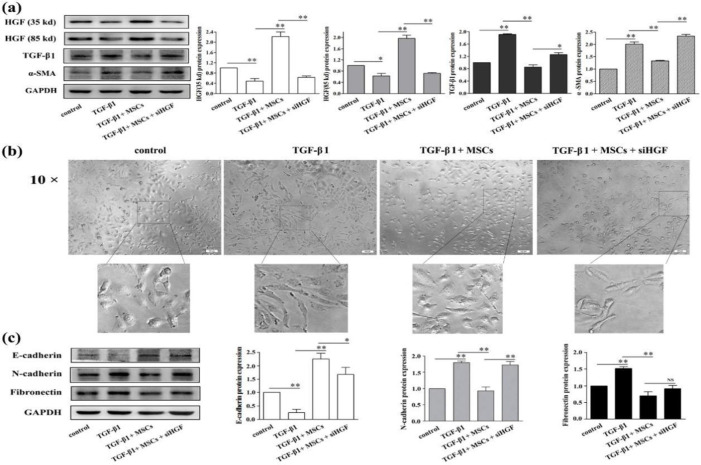
The anti-EMT effect of MSCs on HK-2 cells was blocked by the down-regulation of HGF
The expression of TGF-β1 and α-SMA in TGF-β1 group was higher, while HGF expression was lower compared with control group. However, the expression of TGF-β1 and α-SMA in TGF-β1+MSCs+siHGF group was higher and HGF expression was lower compared with TGF-β1+MSCs (Fig. 4a). Moreover, in TGF-β1 and TGF-β1+MSCs+siHGF group compared with the control and TGF-β1+MSCs group, HK-2 cells were morphologically defined by a spindle-like appearance (Fig. 4b).
Fig. 4:
The anti-EMT effect of MSCs on HK-2 cells was blocked by the down-regulation of HGF. The expression of HGF, TGF-β1 and α-SMA were assessed by western blot analysis (a). The influence of TGF-β1 and MSCs on the morphological change of HK-2 cells (b). Western blot assay for E-cadherin, N-cadherin and fibronectin expressions in HK-2 cells. *Significant difference (P<0.05) and **Significant difference (P<0.01) (C). Scale bars = 100 μm
Furthermore, HK-2 cells were further characterized by confirming EMT protein expression. The expression of E-cadherin was decreased, and the expression of fibronectin and N-cadherin was increased in TGF-β1+MSCs+siHGF group compared with TGF-β1+MSCs group, the same as in TGF-β1 group compared with control group (Fig. 4c).
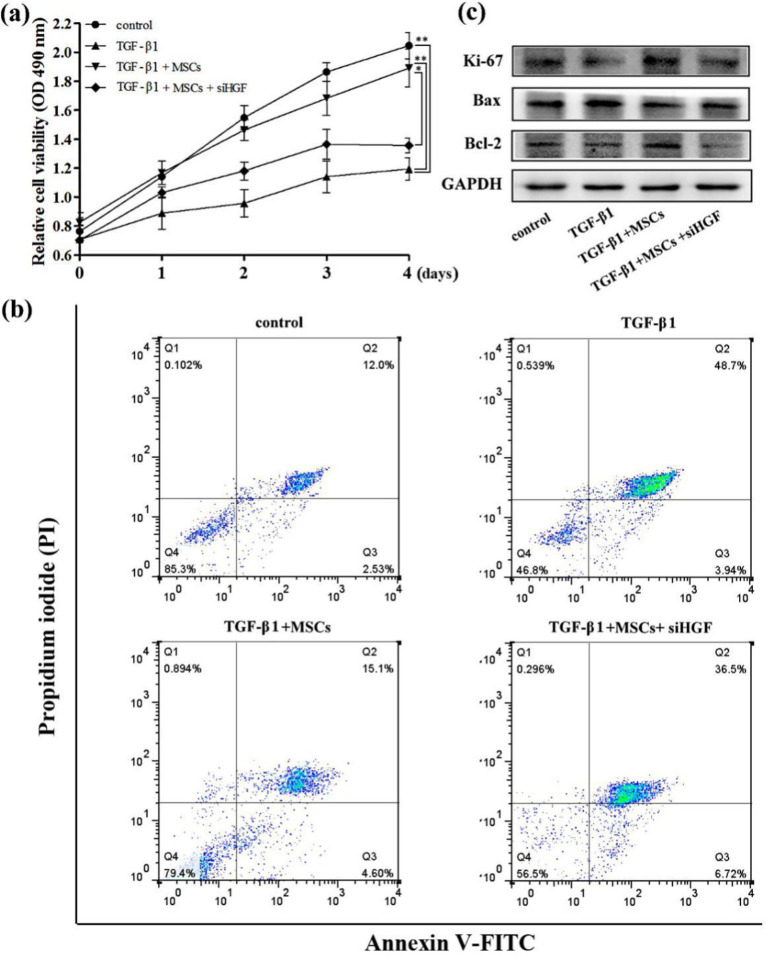
MSCs attenuated the TGF-β1 induced growth inhibition in HK-2 cells by HGF
We further evaluated the effects of HGF on MSCs attenuating the TGF-β1 induced growth inhibition in HK-2 cells. The proliferation of TGF-β1+MSCs+siHGF group was lower that TGF-β1+MSCs group. After treated with siHGF, MSCs were failed to improve the proliferation inhibition induced by TGF-β1 (Fig. 5a). The apoptosis rate of TGF-β1+MSCs+siHGF group was 36.5% and that was 15.1% in TGF-β1+MSCs group. The treatment of siHGF increased the late apoptosis rate of TGF-β1 and MSCs co-treatment cells (Fig. 5b). We also detected the expression of the proliferation-related protein Ki67 and the apoptosis-associated proteins Bax and Bcl-2, and the results were consistent with the previous experimental results (Fig. 5c).
Fig. 5:
MSCs attenuated the TGF-β1-induced growth inhibition in HK-2 cells by HGF. Cell proliferation was detected by MTS assay (a). Late apoptosis of HK-2 cells was examined by flow cytometric analysis (b). Ki67, Bax and Bcl-2 proteins expression were detected by western bolt (c)
Discussion
MSCs therapy is becoming an attractive strategy for CDK, but it is urgent need an improving understanding of how MSC act and the therapeutic potentiality (25, 26). In the present study, we sought to explore the protect potential of MSCs in culture systems of HK-2 cells and search the underlying mechanisms. We found that MSCs reversed TGF-β1-induced EMT, -suppressed proliferation and -promoted apoptosis by increasing HGF expression in HK-2 cells.
TGF-β, known for its vital role in fibrogenesis by modulating the fibroblast phenotype and function, inducing myofibroblast transdifferentiation and promoting matrix accumulation (27), are also very important to induce EMT and further contribute to the development of kidney fibrosis (28), cardiac fibrosis (29) pulmonary fibrosis (30) and liver cirrhosis (31). In our study, TGF-β1 also increased the expression of α-SMA, transformed the cell morphology to spindle-shape and decreased the expression of epithelial marker E-cadherin and increased the expression of mesenchymal markers fibronectin and N-cadherin. TGF-β1 down-regulated epithelial markers expression, up-regulated α-SMA and mesenchymal markers expression, changed the cell morphology, and thereby induced HK-2 cells fibrogenesis characterized (32). The expression of HGF also decreased by TGF-β1 treatment. These results indicated when HK-2 cells developed EMT, the TGF-β1 would be in high level, and on the contrary, the HGF was in low level. We then observed that co-culture with MSCs counter all of the performance induced by TGF-β1 treatment including growth inhibition, EMT phenotype changing and apoptosis promotion. And the expression of TGF-β1 was decreased and HGF was increased. This effect of MSCs inhibiting the process of EMT can also been seem in liver(33) and lung (34). Thus we confirmed the anti-EMT role of MSCs in renal tubular cell line and it was highly relevant with the reciprocal balance between HGF and TGF-β1.
TGF-β1 shows different effects on proliferation and apoptosis in different cell types (35). TGF-β1 potently inhibits the growth of a variety of cells, including those derived from epithelium, endothelium, the glomerular mesangium, and the lymphoid system (36). Actually, MSCs were reported to improve the proliferation of renal epithelial cells and inhibit apoptosis (37). By flow cytometry analysis following annexin V and PI staining, compared with complete media, hMSCs-conditioned media reduced cisplatin induced HK-2 cells death and thus led to significantly improved survival (38). In this study, we also discovered TGF-β1 treatment suppressed HK-2 cells proliferation and promoted apoptosis. Importantly, we also found, after co-culture with MSCs, HK-2 cells had higher viability and less apoptosis than that no co-cultured cells. Based upon these evidences we believed that MSCs can reversed the TGF-β1 induced EMT, proliferation suppression and apoptosis promotion. However, the mechanism of this process is not clear.
HGF is a cytokine that may play an important role in mitosis, anti-apoptosis and antifibrosis (39). There is a consensus that the protective and tissue reparative effects of MSCs may be achieved by the trophic factors such as HGF in lung injury (34, 40). Renal fibrosis of many types of kidney disease such as acute kidney injury and chronic aristolochic acid nephropathy could be protected by MSCs transplantation, and the mechanism probably via upregulation of HGF (41). These previous reports support our observation which MSCs exerted significant anti-EMT effects in the TGF-β1 induced EMT, however all these effects were abolished with HGF knockdown. From these results, we concluded that HGF played a critical role in the MSC anti-fibrotic in proximal tubules. Therefore, MSCs inhibited the expression of TGF-β1 by secreting HGF, thus inhibiting EMT in renal tubular epithelial cells. In addition, MSCs transfected with HGF improved cardiac function in the infarcted porcine heart by increasing angiogenesis and reducing fibrosis (42). HGF improved the therapeutic efficacy of human bone marrow MSCs via RAD51 (43). Co-cultivation with the MSCs-Lcn2 not only inhibited cisplatin-induced cytotoxicity, but prevented cisplatin-induced apoptosis, increased proliferation rate and raised expression of growth factors and the amount of antioxidants in the HK-2 and HEK293 cells (44). We believed there are beneficial effects of the treated-MSCs in cell therapy of kidney injury.
Since the current results are only validated in cell lines, future investigations will require in chronic renal injury models. MSCs with or without knocking down HGF will subcapsular injected in unilateral ureteral obstruction rat, then detect the anti-fibrosis effect of MSCs and the expression of HGF and TGF-β1 in vivo.
Conclusion
When EMT occurred in HK-2 cells, TGF-β1 and HGF showed opposite patterns. MSCs reversed TGF-β1 induced EMT by increasing HGF level and decreasing TGF-β1. These findings provided an important basis for a further exploration on understanding the action mechanisms of MSCs. Therefore, we suggest TGF-β1 is a motivate factor in renal fibrosis and MSCs can suppress the effect of TGF-β1 by secreting HGF.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
This study was funded by Ningbo Natural Science Foundation (grant no. 2012A610213). All authors read and approved the final manuscript, and have no conflict of interests.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Morigi M, Benigni A. (2013). Mesenchymal stem cells and kidney repair. Nephrol Dial Transplant, 28(4): 788–93. [DOI] [PubMed] [Google Scholar]
- 2.Uccelli A, Moretta L, Pistoia V. (2008). Mesenchymal stem cells in health and disease. Nat Rev Immunol, 8(9): 726–36. [DOI] [PubMed] [Google Scholar]
- 3.Asanuma H, Vanderbrink BA, Campbell MT, et al. (2011). Arterially delivered mesenchymal stem cells prevent obstruction-induced renal fibrosis. J Surg Res, 168(1): e51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caplan AI, Dennis JE. (2006). Mesenchymal stem cells as trophic mediators. J Cell Biochem, 98(5): 1076–84. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Z, Liu G, Meng F, et al. (2017). Paracrine effects of mesenchymal stem cells on the activation of keratocytes. Br J Ophthalmol, 101(11): 1583–90. [DOI] [PubMed] [Google Scholar]
- 6.Gallo S, Sala V, Gatti S, et al. (2015). Cellular and molecular mechanisms of HGF/Met in the cardiovascular system. Clin Sci (Lond), 129(12): 1173–93. [DOI] [PubMed] [Google Scholar]
- 7.Hu ZP, Bao Y, Chen DN, et al. (2013). Effects of recombinant adenovirus hepatocyte growth factor gene on myocardial remodeling in spontaneously hypertensive rats. J Cardiovasc Pharmacol Ther, 18(5): 476–80. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Fei S, Suo C, et al. (2018). Antifibrotic Effects of Hepatocyte Growth Factor on Endothelial-to-Mesenchymal Transition via Transforming Growth Factor-Beta1 (TGF-beta1)/Smad and Akt/mTOR/P70S6K Signaling Pathways. Ann Transplant, 23:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Border WA, Noble NA. (1994). Transforming growth factor beta in tissue fibrosis. N Engl J Med, 331(19): 1286–92. [DOI] [PubMed] [Google Scholar]
- 10.Sakai K, Jawaid S, Sasaki T, et al. (2014). Transforming growth factor-beta-independent role of connective tissue growth factor in the development of liver fibrosis. Am J Pathol, 184(10): 2611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo L, Peng W, Tao J, et al. (2016). Hydrogen Sulfide Inhibits Transforming Growth Factor-beta1-Induced EMT via Wnt/Catenin Pathway. PLoS One, 11(1): e0147018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y, Lou Z, Lee SH. (2017). Arctigenin represses TGF-beta-induced epithelial mesenchymal transition in human lung cancer cells. Biochem Biophys Res Commun, 493(2): 934–39. [DOI] [PubMed] [Google Scholar]
- 13.Shiota M, Zardan A, Takeuchi A, et al. (2012). Clusterin mediates TGF-beta-induced epithelial-mesenchymal transition and metastasis via Twist1 in prostate cancer cells. Cancer Res, 72(20): 5261–72. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Zhang K, Li Y, et al. (2017). Estrogen-related receptor alpha participates transforming growth factor-beta (TGF-beta) induced epithelial-mesenchymal transition of osteosarcoma cells. Cell Adh Migr, 11(4): 338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taiyab A, Korol A, Deschamps PA, et al. (2016). beta-Catenin/CBP-Dependent Signaling Regulates TGF-beta-Induced Epithelial to Mesenchymal Transition of Lens Epithelial Cells. Invest Ophthalmol Vis Sci, 57(13): 5736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biname F, Lassus P, Hibner U. (2008). Transforming growth factor beta controls the directional migration of hepatocyte cohorts by modulating their adhesion to fibronectin. Mol Biol Cell, 19(3): 945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chattopadhyay N, J TFH, Godbole MM, et al. (2004). Transforming growth factor beta receptor family ligands inhibit hepatocyte growth factor synthesis and secretion from astrocytoma cells. Brain Res Mol Brain Res, 121(1–2): 146–50. [DOI] [PubMed] [Google Scholar]
- 18.Mungunsukh O, Day RM. (2013). Transforming growth factor-beta1 selectively inhibits hepatocyte growth factor expression via a micro-RNA-199-dependent posttranscriptional mechanism. Mol Biol Cell, 24(13): 2088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizuno S, Matsumoto K, Kurosawa T, et al. (2000). Reciprocal balance of hepatocyte growth factor and transforming growth factor-beta 1 in renal fibrosis in mice. Kidney Int, 57(3): 937–48. [DOI] [PubMed] [Google Scholar]
- 20.Sutton MT, Fletcher D, Episalla N, et al. (2017). Mesenchymal Stem Cell Soluble Mediators and Cystic Fibrosis. J Stem Cell Res Ther, 7(9):400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng GS, Zhang YS, Zhang TT, et al. (2017). Bone marrow-derived mesenchymal stem cells modified with IGFBP-3 inhibit the proliferation of pulmonary artery smooth muscle cells. Int J Mol Med, 39(1): 223–30. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Ren Y, Zhuang H, et al. (2015). Decrease of phosphorylated proto-oncogene CREB at Ser 133 site inhibits growth and metastatic activity of renal cell cancer. Expert Opin Ther Targets, 19(7): 985–95. [DOI] [PubMed] [Google Scholar]
- 23.Liu T, Peng YF, Jia C, et al. (2015). Effect of HGF on the apoptosis of rat corpus cavernosum smooth muscle cells induced by TGF-beta1. Andrologia, 47(9): 1020–7. [DOI] [PubMed] [Google Scholar]
- 24.Guo HL, Liao XH, Liu Q, et al. (2016). Angiotensin II Type 2 Receptor Decreases Transforming Growth Factor-beta Type II Receptor Expression and Function in Human Renal Proximal Tubule Cells. PLoS One, 11(2): e0148696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aghajani Nargesi A, Lerman LO, Eirin A. (2017). Mesenchymal stem cell-derived extra-cellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res Ther, 8(1): 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleig SV, Humphreys BD. (2014). Rationale of mesenchymal stem cell therapy in kidney injury. Nephron Clin Pract, 127(1–4): 75–80. [DOI] [PubMed] [Google Scholar]
- 27.Serini G, Bochaton-Piallat ML, Ropraz P, et al. (1998). The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol, 142(3): 873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Han Z, Tao J, et al. (2017). Role of endothelial-to-mesenchymal transition induced by TGF-beta1 in transplant kidney interstitial fibrosis. J Cell Mol Med, 21(10): 2359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Wang F, Xie M, et al. (2018). Response to inhibition of TGF-beta1 might be a novel therapeutic target in the treatment of cardiac fibrosis. Int J Cardiol, 256:20. [DOI] [PubMed] [Google Scholar]
- 30.Tian XR, Tian XL, Wang HF, et al. (2016). [Regulation effect of beta-catenin pathway on TGF-beta1 induced pulmonary pro-fibrosis]. Zhonghua Yi Xue Za Zhi, 96(24): 1929–33. [DOI] [PubMed] [Google Scholar]
- 31.Kim KH, Kim HC, Hwang MY, et al. (2006). The antifibrotic effect of TGF-beta1 siRNAs in murine model of liver cirrhosis. Biochem Biophys Res Commun, 343(4): 1072–8. [DOI] [PubMed] [Google Scholar]
- 32.Zhou B, Wang Y, Zhang C, et al. (2018). Ribemansides A and B, TRPC6 Inhibitors from Ribes manshuricum That Suppress TGF-beta1-Induced Fibrogenesis in HK-2 Cells. J Nat Prod, 81(4): 913–17. [DOI] [PubMed] [Google Scholar]
- 33.Jang YO, Cho MY, Yun CO, et al. (2016). Effect of Function-Enhanced Mesenchymal Stem Cells Infected With Decorin-Expressing Adenovirus on Hepatic Fibrosis. Stem Cells Transl Med, 5(9): 1247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennelly H, Mahon BP, English K. (2016). Human mesenchymal stromal cells exert HGF dependent cytoprotective effects in a human relevant pre-clinical model of COPD. Sci Rep, 6:38207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massague J. (2012). TGFbeta signalling in context. Nat Rev Mol Cell Biol, 13(10): 616–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grande JP, Warner GM, Walker HJ, et al. (2002). TGF-beta1 is an autocrine mediator of renal tubular epithelial cell growth and collagen IV production. Exp Biol Med (Maywood), 227(3): 171–81. [DOI] [PubMed] [Google Scholar]
- 37.Roushandeh AM, Bahadori M, Roudkenar MH. (2017). Mesenchymal Stem Cell-based Therapy as a New Horizon for Kidney Injuries. Arch Med Res, 48(2): 133–46. [DOI] [PubMed] [Google Scholar]
- 38.Eliopoulos N, Zhao J, Bouchentouf M, et al. (2010). Human marrow-derived mesenchymal stromal cells decrease cisplatin renotoxicity in vitro and in vivo and enhance survival of mice post-intraperitoneal injection. Am J Physiol Renal Physiol, 299(6): F1288–98. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura T, Sakai K, Nakamura T, et al. (2011). Hepatocyte growth factor twenty years on: Much more than a growth factor. J Gastroenterol Hepatol, 26 Suppl 1:188–202. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Zheng R, Chen Q, et al. (2017). Mesenchymal stem cells microvesicles stabilize endothelial barrier function partly mediated by hepatocyte growth factor (HGF). Stem Cell Res Ther, 8(1): 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li W, Jiang H, Feng JM. (2012). Isogenic mesenchymal stem cells transplantation improves a rat model of chronic aristolochic acid nephropathy via upregulation of hepatic growth factor and downregulation of transforming growth factor beta1. Mol Cell Biochem, 368(1–2): 137–45. [DOI] [PubMed] [Google Scholar]
- 42.Lu F, Zhao X, Wu J, et al. (2013). MSCs transfected with hepatocyte growth factor or vascular endothelial growth factor improve cardiac function in the infarcted porcine heart by increasing angiogenesis and reducing fibrosis. Int J Cardiol, 167(6): 2524–32. [DOI] [PubMed] [Google Scholar]
- 43.Lee EJ, Hwang I, Lee JY, et al. (2018). Hepatocyte Growth Factor Improves the Therapeutic Efficacy of Human Bone Marrow Mesenchymal Stem Cells via RAD51. Mol Ther, 26(3): 845–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halabian R, Roudkenar MH, Jahanian-Najafabadi A, et al. (2015). Co-culture of bone marrow-derived mesenchymal stem cells overexpressing lipocalin 2 with HK-2 and HEK293 cells protects the kidney cells against cisplatin-induced injury. Cell Biol Int, 39(2): 152–63. [DOI] [PubMed] [Google Scholar]