Abstract
Background:
The COVID-19 is a pandemic viral infection with a high morbidity rate, leading to many worldwide deaths since the end of 2019. The RBD (Receptor Binding Domain) of SARS-CoV-2 through its spike utilizes several host molecules to enter host cells. One of the most important ones is the angiotensin-converting enzyme 2 (ACE2), an enzyme normally engaged in renin angiotensin pathway and is responsible for hypertension regulation. As different articles have analyzed separate compounds which can bind ACE2 as the potential virus entry blockers, and each one with a different molecular docking algorithm, in this study we compared all candidate compounds individually as well as their combinations using a unique validated software to introduce most promising ones.
Methods:
We collected and prepared a list of all available compounds which potentially can inhibit RBD binding site of the ACE2 from different studies and then reanalyzed and compared them using the Patchdock (ver. 1.3) as a suitable molecular docking algorithm for analysis of separate compounds or their combinations.
Results:
Saikosaponin A (e.g. in Bupleurum chinense), Baicalin (e.g. in several species in the genus Scutellaria), Glycyrrhizin (Glycyrrhiza glabra), MLN-4760 and Umifenovir better occupied ACE2 to inhibit viral RBD binding and are suggested as the top five inhibitors of the SARS-CoV-2 binding site of ACE2. Their combinatory effects were also inspiring concurrent ACE2 blockade.
Conclusion:
The results propose greatest compounds and their combinatory anti-SARS-CoV-2 effects in order to decrease the time and expenses required for further experimental designs.
Keywords: Compound, COVID-19, Inhibitor, SARS-CoV-2, Receptor binding domain
Introduction
The coronavirus (2019-nCoV), the causative organism of novel coronavirus pneumonia, is a positive-sense single-strand RNA virus (1). In this emergency condition, drug repurposing looks better strategy for virus inhibition or decreasing the signs of the disease (2). Therefore, according to the pathogenesis of this virus, different drugs could be suggested. The SARS-CoV-2 infection and pathogenesis are consisted of different mechanisms at different levels. The first step is the virus’ fusion to the host cells.
To develop specific SARS-CoV-2 fusion inhibitors, it is essential to study the fusion capacity of SARS-CoV-2 compared to that of previous severe acute respiratory syndrome coronavirus (SARSCoV). The SARS-CoV-2 similar to SARS-CoV uses the angiotensin-converting enzyme 2 (ACE2) as the receptor for attaching human cells. In fact, the spike of this virus embarks the first contact and binding to the host cell membrane. The spike (S) protein of SARS-CoVs is made up from S1, S2 and transmembrane subunits. However, S1 located in N-terminal of this protein interacts with the host ACE2 to initialize the fusion step (3). Despite the high similarities of these two SARS viruses, especially in their S2 subunits of spike protein, their S1 subunits which are responsible for ACE2 interaction, contain more differences. These S1 dissimilarities have led to more than 10-fold higher affinity of SARS-CoV-2 to ACE2 in comparison with that of SARS-CoV (4). The same domain of the ACE2 is engaged in S1 binding of these two SARS viruses, repurposing of any available drugs or compounds which can inhibit binding of ACE2 to SARS-CoV would be more promising for SARS-CoV-2, particularly when it has many more affinities to ACE2.
So far, the ACE2 receptor has been employed to investigate many compounds for the inhibition of SARS-CoV’s entrance, using in silico or experimental strategies. For example, 38 Chinese patent drugs were compared via docking screening for the RBD-binding site masking of ACE2 and more potent ones (hesperidin, saikosaponin A, rutin) introduced as potential viral entrance inhibitors (3). Arbidol, a broad spectrum antiviral drug, has been also reported as an entrance inhibitor via ACE2 blockade (5). Furthermore, ACE2 binding of some natural compounds were checked and reported Scutellarin as the most potent one (6). Emodin is a known compound for its anti-SARS activity via anti-ACE2/some-protease activitity (7–10). On the other hand, other hypertension modulatorsworking through ACE2 catalytic inhibition, such as N-(2-aminoethyl)-1 aziridine-ethanamine (11) and MLN-4760 (12) has been analyzed for their effects on ACE2 conformational changes and further SARS-S inhibitory effects. Therefore, in this study, we reanalyzed previously evaluated compounds by different molecular docking algorithms or experimental studies (from other related studies) for ACE2 blocking, in order to find the inhibitoriest ones for ACE2 blockade against RBD part of the S1 protein in the SARS-CoV-2.
Materials and Methods
Selection of potential ACE2 binding compounds
Considering different studies, the most effective molecules were selected as the ligands for ACE2. In more detail they were chosen from different studies: Hesperidin, saikosaponin A and rutin (3), Umifenovir (Arbidol) (5), scutellarin, glycyrrhizin, baicalin, hesperetin and nicotianamine (6), emodin (7–10), N-(2-aminoethyl)-1 aziridine-ethanamine (11), and MLN-4760 (12). Therefore, in our study, we analyzed 13 promising compounds by the molecular docking using Patchdock (ver. 1.3) (13). The structures were downloaded from ChemSpider (http://www.chemspider.com) website and optimized with Chimera 1.13.1 software (14).
Molecular Docking
The binding structure (S1 protein) of RARS-Cov-2 to ACE2 was modeled according to 6M17 (15) and for SARS-CoV was modeled according to 5WRG (16) obtained from protein data bank (PDB) for further docking analysis.
We utilized Patchdock (ver. 1.3) for our molecular docking study (13). Afterward, the candidate transformations were carried out on complementary patched structures. Then each candidate transformation was compared via the obtained scores by considering the atomic desolvation energy and geometric fit (17). The PDB coordinate file for each protein and considered ligands made the input parameters for the docking analysis. The clustering RMSD value was set to 4Å. The type of complex was changed to protein-ligand type.
Results
Identification of Top five compounds with higher affinity to ACE2
We totally analyzed 13 components by docking to ACE2. According to our docking analysis, top five compounds with higher binding affinities (docking score<about −45 kcal/mol) were identified against ACE2 (by the model of the 6M17) (Table 1). Interestingly, all of these compounds bind with higher affinity to ACE2 when comparing with SARS-CoV and SARS-CoV-2 viruses. The results of docking analysis of the compounds which may inhibit ACE2 interaction with RBD of SARSCoV-2 viruses are exposed in Fig. 1 and Table 1.
Table 1:
Docking results of the ACE2 binding compounds with RBD of SARS and SARS-CoV-2 viruses using the Patchdock (ver. 1.3) tool
| Protein Name/ID | Global Energy | Softend attractive van der wals’ energy | Softend repulsive van der wals’ energy | Atomic contact energy) (ACE) |
|---|---|---|---|---|
| SARS-COV-2 S1 | 0.21 | −35.28 | 18.64 | 9.07 |
| Saikosaponin A | −50.91 | −26.75 | 5.92 | −9.99 |
| Baicalin | −49.70 | −24.29 | 1.87 | −9.94 |
| Glycyrrhizin | −45.52 | −29.26 | 16.27 | −9.20 |
| MLN-4760 | −45.39 | −23.05 | 4.07 | −9.37 |
| Umifenovir | −44.52 | −21.33 | 7.27 | −12.16 |
| Hesperidin | −41.28 | −28.66 | 24.00 | −11.10 |
| Emodin | −38.89 | −16.13 | 3.43 | −11.33 |
| SaikosaponinB2 | −38.08 | −27.76 | 6.35 | −1.27 |
| N4-4methylpiperazinoben-zyl5isoxazolecarboxamide | −37.92 | −15.50 | 1.71 | −10.42 |
| Hesperetin | −36.44 | −18.75 | 8.25 | −9.78 |
| Rutin | −32.02 | −26.15 | 4.23 | 0.85 |
| Nicotianamine | −31.28 | −12.21 | 1.74 | −9.13 |
| N-(2-Aminoethyl)-1-aziridineethanamine | −27.29 | −9.79 | 0.84 | −8.53 |
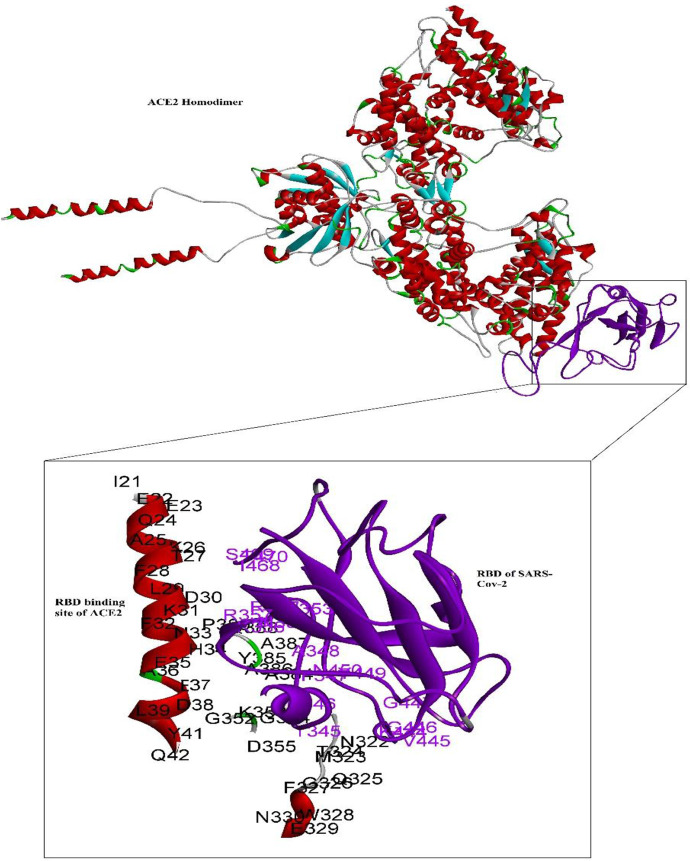
Fig. 1:
The interaction between RBD binding site of the ACE2 (red) and RBD in SARS-Vov-2 (Violet). The interacting residues are demonstrated by amino acid single letter code and residue numbers
The characteristics of interacting residues of analyzed compounds with ACE2
The detailed interactions and related residues of ACE2 with 13 investigated compounds are shown in Table 2. Interacting residues between ACE2 and RBD of SARS-CoV-2 are revealed in Table 3. Some compounds may occupy the same ACE2 regions by strong hydrogen bonds and therefore, in case of simultaneous application, may interfere each other in ACE2 binding (Table 4).
Table 2:
The interaction between ACE2 and available ACE2 binding compounds
| Ligand Name | Hydrogen Bonds | Vander waals’ Bonds | Alky | Unfavorable Bump |
|---|---|---|---|---|
| Baicalin | A396, E208, D206 | L85, Q98, E564, L392, N397, W566, TRY207, P565, V212, L91, K94, N210 | V209, L95 | K562 |
| Emodin | W566 | PRD565, E564, N394, A396, D206, K562, E208, K94, N210, V212 | V209, L95 | - |
| Glycyrrhizin | A396, N210, | *NAG905, N194, Q102, Q98, G205, R219, K562, N397, E564, W566, P565, G211, V212, L91, K94, E208, L85 | L95, H195, Y196, V209 | D206 |
| Hesperetin | E208, E564, W566, D206 | Q98, L95, K562, A396, N397, Y297 | V209 | N210 |
| Hesperidin | S563, N210 | A99, K562, N397, E396, Y207, A396, V209, P565, E564, L95, V212, L91, K94 | - | Q98, G205, E208, D206. W566 |
| MLN-4760 | R482, Y613 | E479, E495, D494, R644, E668, V670, E667, P492, H493, N674, E489, S611, R493, Y611 | K475, R673, A 673 | V 672 |
| N-(2-Aminoethyl)-1-aziridineethanamine | A396, E208 | L91, L95, Y207, N397, V209, PRD565, N210, V212, D906, W566 | - | - |
| N4-4Mhylpiperazinobenzyl5isoxazolecarboxamide | - | G205, D208, W566, V209, E564, S563, TNR92, L91, V212, UYS562 | L95 | P565 |
| Nicotianamine | - | N210, E564, W566, E208 | V212, L95, K562, A396, V209, P565 | - |
| Rutin | H493, Y613, W610, D609, R482, T608, W478, L675, N674 | A673, M474, E489, P492, A614 | K676 | S611 |
| Saikosaponin A | K562, W203, Y199 | G205, D206, N397, E208, Y207, P565, E564, L392, L95, Q98, Q102, Y196, M190 | V209, W566, A396, A99, Y202 | K187 |
| Saikosaponin B2 | K174, R671 | E171, S170, E668, TRY497, T496, D494, D637, R644, L675, M640, V672, V670, E166, S167, K689, P135, W163, N134 | C133, L664 |
N-Acetyl D-Glucosamine that interacts with mentioned amino acides
Table 3:
Interacting residues between ACE2 and RBD of the SARS-CoV-2
| Interaction | ACE2 Enzyme | Residues of the SARS-CoV-2 RBD |
|---|---|---|
| Direct | Y41, Q325, E329 | Y449 |
| Surrounding | I21, E22, E23, Q24, A25, K26, F28, L29, D30, F32, N33, H34, E35, A36, E37, L39, F40, Q42, L320, P321, N322, M323, T324, Q326, F327, W328, N330, Q354, K353, D355, P389 | T345, R346, F347, Y445, Q447, N448, Q446, Y449, N450, I468 |
Table 4:
The compounds occupying the same residue of the ACE2 by hydrogen bond
| ACE2 Residue | Compounds which make hydrogen bond |
|---|---|
| D206 | Baicalin, Hesperetin |
| E208 | Baicalin, Hesperetin, N-(2-Aminoethyl)-1-aziridineethanamine |
| N210 | Hesperetin, Glycyrrhizin |
| A396 | Baicalin, Hesperetin |
| R482 | MLN-4760, Rutin |
| Y613 | MLN-4760, Rutin |
| W566 | Emodin, Hesperetin |
The investigation of the combinatory effects of Top three compounds with ACE2
The effect of different binary combination of Top three non-synthetic compounds (Baicalin, Saikosaponin A and Glycyrrhizin) on ACE2 blockade is illustrated in Table 5. The results uncovered that the first compound may affect the affinity of the second compound in binding to ACE2.
Table 5:
The effect of permutation application of the Top three non-synthetic compounds (Baicalin, Saikosaponin A and Glycyrrhizin) required for ACE2 blockade
| First compound/s | Second compound | Global Energy for second compound | Softend attractive van der wals’ energy | Softend repulsive van der wals’ energy | Atomic contact energy) (ACE) |
|---|---|---|---|---|---|
| Saikosaponin A | Baicalin | −46.03 | −23.93 | 1.64 | −7.67 |
| Saikosaponin A | Glcyrrhizin | −45.05 | −27.35 | 11.78 | −9.23 |
| Baicalin | Saikosaponin A | −40.50 | −23.51 | 8.88 | −7.93 |
| Baicalin | Glcyrrhizin | −45.05 | −27.35 | 11.78 | −9.23 |
| Glcyrrhizin | Baicalin | −44.03 | −22.98 | 1.83 | −7.42 |
| Glcyrrhizin | Saikosaponin A | −44.03 | −44.03 | 1.83 | −7.42 |
In this interference, for example Baicalin can decrease the binding affinity of Saikosaponin from −50.91 to −40.50 albeit Saikosaponin A has not changed the affinity of Baicalin to a large extent (from −49.70 to −46.03). On the other hand, Glycyrrhizin decrease the binding affinity of each of Saikosaponin A and Baicalin to ACE2 up to about −44. However, Saikosaponin A and Baicalin did not considerably change the Glycyrrhizin binding affinity to ACE2 (from −45.52 to −45.05). The mean of binary binding affinity of Saikosaponin A and Baicalin (−43.265), Saikosaponin A and Glycyrrhizin (−44.54), and Baicalin and Glycyrrhizin (−44.54) to ACE2 were not significantly different. However, no obvious antagonistic effects were seen in combinatorial application of these compounds.
Discussion
We analyzed the effects of different ACE2-binding compounds on ACE2:S1 inhibition. Because various studies have suggested different inhibitory compounds based on their applied docking softwares, we were persuaded to analyze the top suggested ACE2-binding compoundsin different articles using a single validated software.
Separate softwares have been employed in different studies for these kinds of interaction analysis. For example, the AutoDock Vina can significantly improve the average accuracy of the binding mode predictions when this software is compared with the AutoDock. In fact, the Autodock vina consideres the pockets, cavities, conserved amino acid residues, etc. and then docks them into the predicted binding sites (6). The SwissDock (http://www.swissdock.ch) server is another suitable tool for blind docking (3). Another employed software widely used in ducking studies is Auto-Dock 4. It consists of autodock and autogrid (3). In this research, we used the Patchdock (13) because of its analysis capacity of S1 binding affinity from SARS-CoV-2 with ACE2 in the presence of other compounds. Patchdock algorithm is based on the object recognition and image segmentation techniques used in Computer Vision. This Docking type is comparable with assembling a jigsaw puzzle. The studied compounds are ranked by the binding score. For estimation of this score, Atomic Contact Energy, softened van der Waals interactions, partial electrostatics and additional estimations of the binding free energy are all involved in energy estimation (18). Therefore, Patchdock results seem to be more comprehensive for ranking different compounds affinity to a molecule. That is why in our study the estimated total energy was greater than its amount in other docking studies benefiting from the Autodock Vina (3, 6).
In our study a selection of top13 ACE2-binding compounds suggested by previous studies were analyzed. The selection consisted of those compounds which can inhibit catalytic domain of the ACE2 for probable conformational changes and decreasing subsequent ACE2:S1 interaction, as well as the ones which could potentially inhibit S1 binding site of the ACE2. According to global energies, the top five compounds among all 13 analyzed compounds were Saikosaponin A (−50.91), Baicalin (−49.70), Glycyrrhizin (−45.52), MLN-4760 (−45.39) and Umifenovir (−44.52), respectively. These compounds were previously analyzed against the RARS-COV-2 (Saikosaponin A) (3), (Baicalin and Glycyrrhizin) (6), and (Umifenovir) (5) or against the RARS-Cov (MLN-4760) (12).
Saikosaponin A, baicalin and glycyrrhizin as the top medicinal plant drugs
Saikosaponins are found in medicinal plant Bupleuri Radix. They are triterpene saponin glycosides, with different medicinal functions such as, antioxidant, antibacterial, antiviral, and anticancer, anti-inflammatory, antipyretic, and antihepatotoxic effects. They are used to diminish the signs of influenza, hepatitis, malaria, etc. To date, more than 100 saikosaponins have been identified. Saikosaponin A has been reported as a treatment for curing age-related diseases (19). However, Yan et al. were the first researchers who implemented in silico analysis and assessed the effect of Saikosaponin A on RARS-Cov-2 besides other 37 Chinese patent medicinal plant’ drugs and suggested it as one of top potential drugs used for RARS-Cov-2 treatments (3). Baicalin is a component of Chinese medicinal plant Scutellaria baicalensis georgi. Baicalin has exhibited anti-oxidative stress, anti-inflammation, and also anti-apoptosis effects. In addition, the antiviral effect of baicalin against SARS-COV has been reported in a primate cell line (6). Glycyrrhizin is another anti-SARS-CoV promising composition extracted from Chinese Medicine herb Glycyrrhiza radix. It has been previously reported as an effective anti-adsorption and anti-penetration of SARS-CoV in experimental studies. According to abovementioned issues, Baicalin and Glycyrrhizin have been analyzed as the candidates of anti-SARS-CoV-2 investigation besides other compounds, using silico tools (6). As we checked the combinatorial effect of these medicinal herb metabolites, there is also a good opportunity to benefit from their additive antiviral effects.
MLN-4760 and umifenovir as the top synthetic drugs
MLN-4760 (100 nM) cannot inhibit SARS binding to host cells (12). However, higher concentration of MLN-4760 is a good candidate for anti-SARS effects (20). Umifenovir (Arbidol), a recognized antiviral drug by virus-host cell fusion inhibition mechanism, has been entered into a clinical trial for the inhibition of SARS-CoV-2 entrance to the host cells (5). This drug has a license, for example in Russia, for the antiviral treatment or influenza infection, which can inhibit the replication of human influenza A and influenza B viruses in cell cultures (21).
Conclusion
Overall, in this study among different suggested medicinal herbs, or synthetic compounds, Saikosaponin A, Baicalin, Glycyrrhizin, MLN-4760 and Umifenovir are suggested as the top five compounds which might inhibit SARS-CoV-2 fusion to host cells via RBD-binding site of the ACE2.
Since the two of above mentioned synthetic drugs do not have any FDA approval or using license, we recommend three remaining suggested medicinal herbs, with a long history of applications, especially for their antiviral effects. Interestingly, there are no obvious antagonistic effects for combinatorial application of these top three medicinal plant compounds. Therefore, we propose separate or combinatorial formulations for achieving the highest SARS-CoV-2 inhibition.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
The authors would like to express their appreciation to Dr. M. Karbasioun, the professional editor for the great proofreading and improving the quality of the manuscript. The researchers did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Huang C, Wang Y, Li X, et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 15;395(10223): 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang M, Cao R, Zhang L. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res, 30(3): 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan Y, Shen X, Cao Y, et al. (2020). Discovery of anti-2019-nCoV agents from Chinese patent drugs via docking screening. Preprints. 2020020254 (doi: 10.20944/pre-prints202002.0254.v1 [DOI]
- 4.Xia S, Liu M, Wang C, et al. (2020). Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res, 30(4): 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C, Zhou Q, Li Y, et al. (2020). Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci,6(3): 315–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Du Q. (2020). Potential natural compounds for preventing SARS-CoV-2 (2019-nCoV) infection. Preprints. 2020010358 (doi: 10.20944/preprints202001.0358.v3 [DOI]
- 7.Chandel V, Raj S, Rathi B, Kumar D. (2020). In Silico Identification of Potent COVID-19 Main Protease Inhibitors from FDA Approved Antiviral Compounds and Active Phytochemicals through Molecular Docking: A Drug Repurposing Approach. Preprints, doi: 10.20944/preprints202003.0349.v1. [DOI]
- 8.Yang Y, Islam MS, Wang J, et al. (2020). Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci, 16(10): 1708–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarz S, Wang K, Yu W, et al. (2011). Emodin inhibits current through SARS-associated coronavirus 3a protein. Antivir Res,90(1): 64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho TY, Wu SL, Chen JC, et al. (2007). Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir Res, 74(2): 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huentelman MJ, Zubcevic J, Hernandez Prada JA, et al. (2004). Structure-based discovery of a novel angiotensin-converting enzyme 2 inhibitor. Hypertension, 44(6): 903–6. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Zhang C, Sui J, et al. (2005). Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J, 24(8): 1634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneidman-Duhovny D, Inbar Y, Nussinov R, et al. (2005). PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res, 33: W363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pettersen EF, Goddard TD, Huang CC, et al. (2004). UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem,25(13): 1605–12. [DOI] [PubMed] [Google Scholar]
- 15.Gui M, Song W, Zhou H, et al. (2017). Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Cell Res, 27(1): 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan R, Zhang Y, Li Y, et al. (2020). Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science,367(6485): 1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pradhan N, Sharma K. (2014). Understanding Ubl-rpn1 Intermolecular Interaction. J Adv Pharm Technol Res, 1(3): 1. [Google Scholar]
- 18.Zhang C, Vasmatzis G, Cornette JL, et al. (1997). Determination of atomic desolvation energies from the structures of crystallized proteins. J Mol Biol, 267(3): 707–26. [DOI] [PubMed] [Google Scholar]
- 19.Kim BM. (2018). The role of saikosaponins in therapeutic strategies for age-related diseases. Oxid Med Cell Longev, 2018: 8275256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamai L. (2020). The Yin and Yang of ACE/ACE2 Pathways: The Rationale for the Use of Renin-Angiotensin System Inhibitors in COVID-19 Patients. Cells, 9(7): 1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leneva IA, Russell RJ, Boriskin YS, et al. (2009). Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol. Antiviral Res, 81(2): 132–40. [DOI] [PubMed] [Google Scholar]