Abstract
Background:
Serum miR-497 can be used as a predictive index of the early diagnosis and poor prognosis of atherosclerosis cerebral infarction (ATCI).
Methods:
Overall, 135 ATCI patients, treated in The Second Affiliated Hospital of Nantong University, Nantong 226001, P.R.China from Apr 2012 to Jan 2015, were included in ATCI group. Whereas, 77 patients with non-atherosclerosis cerebral infarction were put in the control group. RT-qPCR was performed for detecting serum miR-497 expression, whose relationship with the patients’ clinicopathological parameters was analyzed. Receiver operating characteristic (ROC) curves were plotted to evaluate values of serum miR-497 for diagnosing ATCI patients and their 3-year and 5-year overall survival rates (OSRs). Cox regression analysis was conducted on prognostic factors of ATCI patients.
Results:
miR-497 remarkably rose in the serum of ATCI patients, and was correlated with histories of hypertension, smoking and diabetes mellitus (DM). Its areas under curves (AUCs) for diagnosing these pathological parameters were 0.803, 0.817 and 0.819, respectively. Its expression was higher in the serum of the patients with recurrence and poor prognoses. Its AUCs for predicting the two conditions were 0.924 and 0.937, respectively. The 3- and 5-year OSRs of patients with low expression were remarkably higher than those of patients with high expression.
Conclusion:
miR-497 and histories of hypertension, smoking and DM were independent prognostic factors affecting the 3-year OSR of ATCI patients. miR-497 expression rises in ATCI patients, so this miR is expected to become a serum diagnostic marker for ATCI.
Keywords: Serum microRNA-497, Atherosclerosis cerebral infarction, Prognosis, Predictive index
Introduction
Atherosclerosis cerebral infarction (ATCI) is a disease with a relatively high disability rate and mortality rate in clinical practice (1). Atherogenesis and thrombosis in the brain lead to the luminal stenosis or occlusion of cerebral vessels, thus resulting in acute insufficiency of cerebral blood supply and the ischemic necrosis of the local brain tissue (2, 3). ATCI usually causes aphasia, hemiplegia and other symptoms of focal cerebral damage (4), but clinically therapeutic schemes for it are few, because it is easy to have a relapse and results in complex adverse reactions of prognoses (5). Therefore, exploring biomarkers for the early diagnosis and prognosis of ATCI patients is of great value for improving their poor prognoses.
As non-coding single-stranded RNAs that are composed of 19–24 nucleotides, microRNAs (miRNAs) are involved in most biological processes, and their loss and disorders have a great effect on some diseases, such as cancer (6,7). They have certain clinical values for the diagnosis, prognosis and recurrence prediction of the diseases (8,9). For instance, serum miR-320b is a specific serum marker for carotid atherosclerosis cerebral infarction and vulnerable plaques (10). miR-29b and miR-424 are prognostic markers for patients with acute cerebral infarction (ACI) (11). In this study, we focused on serum miR-497. This miR is abnormally expressed in the serum of with hepatocellular carcinoma, and associated with the patients’ invasive clinicopathological features and poor prognoses (12). Serum miR-497 rises in ACI patients, and it can be used as a prognostic marker, diagnostic marker and therapeutic target for ACI (13). All these indicate the potential of miR-497 to diagnose diseases and evaluate their prognoses.
Therefore, in this study, miR-497 expression in the serum of ATCI patients was detected, and the correlation of this miR with the patients’ pathological features was analyzed, so as to discuss the clinical value of miR-497 for ATCI.
Materials and Methods
General data
Overall, 135 ATCI patients, treated in The Second Affiliated Hospital of Nantong University, Nantong 226001, P.R.China from April 2012 to January 2015, were included in ATCI group. Whereas, 77 patients with non-atherosclerosis cerebral infarction were put in the control group Inclusion criteria: Patients confirmed with ATCI by CT or MRI (14); patients aged >18 years and with the initial attack of acute ATCI; patients with the time of onset ≤72 hours; patients with complete general clinical data.
The included patients were informed of this study and signed a written informed consent form. The experimental processes were approved by the Ethics Committee of our hospital and in line with the Declaration of Helsinki.
Exclusion criteria: Those accompanied by cerebral hemorrhage or with infection and fever during hospitalization; those accompanied by renal or cardiac diseases; those complicated with severe organ dysfunction, malignant tumors or mental illness; those withdrawing from the experiment midway; those who did not cooperate in follow-ups and were lost to visits.
Sample collection
The serum (5 mL each) was collected from the patients in both groups, and centrifuged at 1500Xg (4°C, 10 min), to obtain the supernatant for carrying out follow-up experiments.
Detection of miR expression
TRIzol reagents (Simgen, Hangzhou, China, 5301100) were applied to the extraction of total RNA from the collected serum and tissues, with an UV spectrophotometer (Noted Scientific Instrument Co., Ltd, Hangzhou, China, 760 CRT) and agarose gel electrophoresis (Biolab Science and Technology Co., Ltd., Beijing, China, GL1177-VYF) used for detecting its purity, concentration and integrity. Reverse transcription kits (Kemin Biotechnology Co., Ltd., Shanghai, China, 205311) were applied to the reverse transcription of the total RNA, with the steps strictly carried out based on the manufacturer’s instructions. After that, cDNA was collected for its amplification with miRNA Realtime qRT-PCR kits (Yu Bo Biotech Co., Ltd., Shanghai, China, YB131042-25) and ABI 7500 (Ai Biological Research, Shanghai, China, 4365463). The amplification system was mirVana 5X PCR Buffer (5 μL), 50X ROX™ (0.5 μL), cDNA (1 μL), upstream and downstream primers (0.5 μL each), and Nuclease-free Water that was finally added to make up to 20 μL. The conditions were pre-denaturation (95 °C, 3 min), denaturation (95°C, 15 sec), and annealing and extension (60 °C, 30 sec), which were cycled for 40 times. There were 3 same wells for each sample. Three repeated experiments were performed. In this study, U6 was used as the internal reference and 2−ΔΔct was applied to data analysis. (Primer sequences are shown in Table 1).
Table 1:
Primer sequences
| Forward | Reverse | |
|---|---|---|
| miR-497 | 5′-AGTCCAGTTTTCCCAGGAATCCCT-3′ | 5′-ACCAGCAGCACACTGTGGTTTGT-3′ |
| U6 | 5′-TCCGATCGTGAAGCGTTC-3′ | 5′-GTGCAGGGTCCGAGGT-3′ |
Follow-ups
Through telephones, WeChat and outpatient medical records, the patients were followed up for 5 years, once every 4 months, to record their survival status. Overall survival (OS) was the time from starting treatment to the patients’ deaths or the end time of the last follow-up.
Statistical analysis
SPSS20.0 (Easy Bio System Inc., Beijing, China) was applied to statistical analysis. GraphPad Prism 6 (GraphPad Software, San Diego, USA) was used for plotting figures. Count data were expressed as the number of cases/percentage [n (%)], and compared between groups by a chi-square test. Measurement data were expressed as mean ± standard deviation (mean±SD), and compared between groups by an independent samples t test, with the comparison before and after treatment conducted by a paired t test. Receiver operating characteristic (ROC) curves were plotted to evaluate values of serum miR-497 for diagnosing ATCI patients and predicting their recurrence and prognoses. The Kaplan-Meier method was used to plot survival curves of the patients’ 3-year and 5-year OS, which was compared by a Log-rank test. Univariate and multivariate Cox regression analyses were conducted on relevant prognostic factors. P<0.05 indicated a statistically significant difference.
Results
Comparison of general information
The differences were not significant between the ATCI and control groups in gender, age, body mass index (BMI), history of hypertension, history of drinking, history of smoking, history of diabetes mellitus (DM), hyperlipidemia, cholesterol (Chol), low-density lipoprotein (LDL) and high-density lipoprotein (HDL) (Table 2).
Table 2:
Comparison of general information [n(%), mean±SD]
| Categories | ATCI group (n=135) | Control group (n=77) | t/χ2 value | P value |
|---|---|---|---|---|
| Gender | 0.974 | 0.324 | ||
| Male | 83 (61.48) | 42 (54.55) | ||
| Female | 52 (38.52) | 35 (45.45) | ||
| Age (Years) | 0.129 | 0.719 | ||
| ≥53 | 79 (58.52) | 47 (61.04) | ||
| <53 | 56 (41.48) | 30 (38.96) | ||
| BMI (kg/m2) | 0.438 | 0.507 | ||
| ≥23 | 62 (45.93) | 39 (50.65) | ||
| <23 | 73 (54.07) | 38 (49.35) | ||
| History of hypertension | 0.484 | 0.486 | ||
| Yes | 74 (54.81) | 46 (59.74) | ||
| No | 61 (45.19) | 31 (40.26) | ||
| History of drinking | 0.536 | 0.463 | ||
| Yes | 79 (58.52) | 49 (63.64) | ||
| No | 56 (41.48) | 28 (36.36) | ||
| History of smoking | 0.316 | 0.573 | ||
| Yes | 86 (63.70) | 52 (67.53) | ||
| No | 49 (36.30) | 25 (32.47) | ||
| History of DM | 0.775 | 0.378 | ||
| Yes | 74 (54.81) | 47 (61.04) | ||
| No | 61 (45.19) | 30 (38.96) | ||
| Hyperlipemia | 0.020 | 0.886 | ||
| Yes | 82 (60.74) | 46 (59.74) | ||
| No | 53 (39.26) | 31 (40.26) | ||
| Chol (mmol/L) | 1.575 | 0.117 | ||
| 4.78±1.03 | 4.55±1.01 | |||
| LDL (mmol/L) | 1.183 | 0.238 | ||
| 3.02±0.94 | 3.18±0.96 | |||
| HDL (mmol/L) | 1.857 | 0.064 | ||
| 1.18±0.35 | 1.09±0.32 |
miR-497 remarkably upregulated in serum of ATCI patients
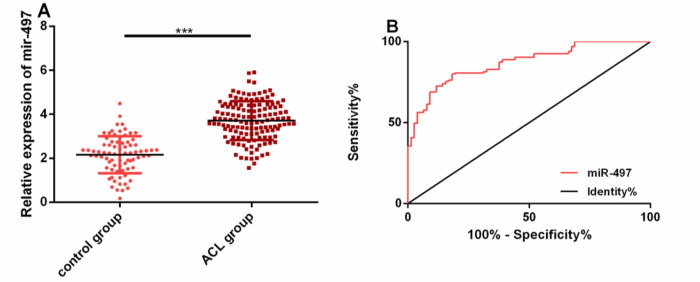
For knowing whether miR-497 was imbalanced in ATCI patients, its expression in the patients’ serum was detected. The expression in the ATCI group was remarkably higher than that in the control group (P<0.05). The ROC curve of miR-497 for identifying ATCI was also plotted. Its area under the curve (AUC), sensitivity, specificity and optimal cut-off value were 0.868 (95%CI: 0.821–0.915), 80.00%, 81.82% and 2.15, respectively (Fig. 1).
Fig. 1:
miR-497 remarkably upregulated in serum of ATCI patients.
A: miR-497 expression in the serum of patients in both groups.
B: The ROC curve of miR-497 for identifying ATCI.
Note: *** indicates P<0.001 when there is a comparison between two groups
Correlation of miR-497 with pathological parameters
According to the observation of the correlation of miR-497 with ATCI patients’ pathological parameters, this miR was correlated with histories of hypertension, smoking and DM (P<0.001). According to the plotted ROC curves, the AUCs of miR-497 for these pathological parameters were 0.803, 0.817 and 0.819, respectively. (Table 3, and 4 and Fig. 2).
Table 3:
Correlation of miR-497 with pathological parameters (mean±SD)
| Parameters | n | miR-497 | t | P |
|---|---|---|---|---|
| Gender | 1.448 | 0.149 | ||
| Male | 125 | 2.31±0.75 | ||
| Female | 87 | 2.16±0.73 | ||
| Age (yr) | 1.456 | 0.147 | ||
| ≥53 | 126 | 2.29±0.72 | ||
| <53 | 86 | 2.14±0.76 | ||
| BMI (kg/m2) | 1.473 | 0.142 | ||
| ≥23 | 101 | 2.19±0.73 | ||
| <23 | 111 | 2.34±0.75 | ||
| History of hypertension | 5.351 | <0.001 | ||
| Yes | 120 | 2.76±0.79 | ||
| No | 92 | 2.19±0.74 | ||
| History of drinking | 1.866 | 0.063 | ||
| Yes | 128 | 2.36±0.79 | ||
| No | 84 | 2.16±0.72 | ||
| History of smoking | 4.289 | <0.001 | ||
| Yes | 138 | 2.73±0.81 | ||
| No | 74 | 2.24±0.76 | ||
| History of DM | 5.873 | <0.001 | ||
| Yes | 121 | 2.88±0.79 | ||
| No | 91 | 2.25±0.75 | ||
| Hyperlipemia | 1.515 | 0.131 | ||
| Yes | 128 | 2.34±0.74 | ||
| No | 84 | 2.18±0.77 | ||
| Chol (mmol/L) | 0.634 | 0.526 | ||
| High expression | 131 | 2.21±0.81 | ||
| Low expression | 81 | 2.14±0.73 | ||
| LDL (mmol/L) | 0.854 | 0.393 | ||
| High expression | 129 | 2.36±0.76 | ||
| Low expression | 83 | 2.27±0.73 | ||
| HDL (mmol/L) | 1.225 | 0.221 | ||
| High expression | 138 | 2.23±0.82 | ||
| Low expression | 74 | 2.09±0.74 |
Table 4:
Diagnostic values of serum miR-497 for clinicopathological parameters
| Pathological parameters | AUC | 95%CI | S.E | Cut-off value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| History of hypertension | 0.804 | 0.744–0.863 | 0.030 | >2.476 | 75.83 | 79.35 |
| History of smoking | 0.816 | 0.756–0.878 | 0.031 | >2.224 | 71.74 | 78.38 |
| History of DM | 0.819 | 0.762–0.876 | 0.028 | >2.908 | 59.50 | 80.01 |
Fig. 2:
Correlation of miR-497 with pathological parameters.
The correlation of miR-497 with histories of hypertension (A), smoking (B) and DM (C). The diagnostic values of miR-497 for histories of hypertension (D), smoking (E) and DM (F).
Note: *** indicates P<0.001 when there is a comparison between two groups
miR-497 expression remarkably upregulated in ATCI patients with recurrence and poor prognoses
The predictive values of miR-497 for the recurrence and prognosis of ATCI patients were further explored. miR-497 expression was higher in the serum of the patients with recurrence and poor prognoses (P<0.001). According to the plotted ROC curves for predicting recurrence, the four ROC parameters of this miR were 0.924 (95%CI: 0.881–0.966), 86.87%, 88.89% and 2.694, respectively. According to the curves for predicting poor prognoses, the parameters were 0.937 (95%CI: 0.898–0.975), 89.53%, 87.76% and 2.911, respectively (Fig. 3, Table 5).
Fig. 3:
miR-497 expression in and its predictive values for ATCI patients with recurrence and poor prognoses.
A: miR-497 remarkably upregulated in ATCI patients with recurrence.
B: miR-497 remarkably upregulated in ATCI patients with poor prognoses. C: The ROC curve of miR-497 for predicting recurrence.
D: The ROC curve of miR-497 for predicting poor prognoses.
Note: *** indicates P<0.001 when there is a comparison between two groups
Table 5:
ROC parameters of miR-497 for predicting recurrence and poor prognoses
| Objectives | AUC | 95%CI | Standard error | Cut-off value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| Predicting recurrence | 0.924 | 0.881–0.966 | 0.022 | 2.694 | 86.87 | 88.89 |
| Predicting poor prognoses | 0.937 | 0.898–0.975 | 0.019 | 2.911 | 89.53 | 87.76 |
Correlation of miR-497 with prognostic survival
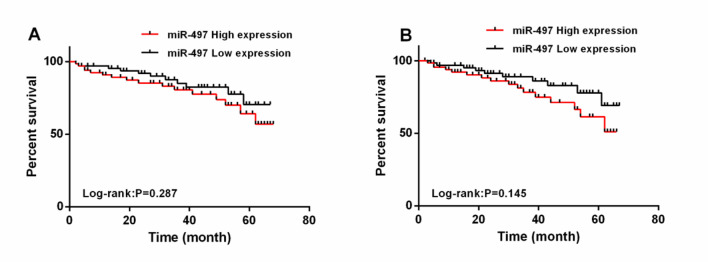
All ATCI patients were followed up for 3 and 5 years. Their 3-year and 5-year overall survival rates (OSRs) were 73.33% (99/135) and 51.85% (70/135), respectively. With the median expression of serum miR-497 (3.643) as the threshold, the OSRs of patients with low expression were remarkably higher than those of patients with high expression (P=0.287, P=0.145). According to the multivariate Cox regression, history of hypertension (P=0.041), history of smoking (P=0.048), history of DM (P=0.036) and miR-497 (P=0.001) were independent prognostic factors affecting the 3-year OSR of ATCI patients (Table 6 and 7 and Fig. 4).
Table 6:
Assignment for COX regression analysis
| Factors | Variables | Assignment |
|---|---|---|
| Gender | X1 | Male = 1, female = 2 |
| Age | X2 | ≥53 = 1, <53 = 2 |
| BMI | X3 | ≥23 = 1, <23 = 2 |
| History of hypertension | X4 | Yes = 1, no =2 |
| History of drinking | X5 | Yes = 1, no =2 |
| History of smoking | X6 | Yes = 1, no =2 |
| History of DM | X7 | Yes = 1, no =2 |
| Hyperlipemia | X8 | Yes = 1, no =2 |
| Chol | X9 | High expression = 1, low expression = 2 |
| LDL | X10 | High expression = 1, low expression = 2 |
| HDL | X11 | High expression = 1, low expression = 2 |
| miR-497 | X12 | ≥3.643 = 1, <3.643 = 2 |
Table 7:
Univariate and multivariate Cox regression analyses on influencing factors of 3-year OSR
| Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95CI%) | P | HR (95CI%) | P | |
| Gender | 1.053 (0.548–2.033) | 0.137 | ||
| Age | 1.105 (0.893–1.538) | 0.112 | ||
| BMI | 1.346 (0.378–2.196) | 0.198 | ||
| History of hypertension | 0.694 (0.347–1.384) | 0.037 | 0.533 (0.266–1.066) | 0.041 |
| History of drinking | 1.528 (1.078–3.973) | 0.107 | ||
| History of smoking | 0.619 (0.309–1.238) | 0.039 | 0.604 (0.302–1.208) | 0.048 |
| History of DM | 0.579 (1.289–1.158) | 0.023 | 0.597 (0.298–1.194) | 0.036 |
| Hyperlipemia | 1.793 (1.047–2.182) | 0.162 | ||
| Chol | 0.881 (0.783–1.893) | 0.127 | ||
| LDL | 1.973 (1.062–1.839) | 0.182 | ||
| HDL | 1.172 (0.625–1.983) | 0.263 | ||
| miR-497 | 0.473 (0.237–0.946) | 0.003 | 0.267 (0.133–0.534) | 0.004 |
Fig. 4:
Correlation of miR-497 with 3- and 5-year OS.
A: High miR-497 levels were associated with a lower 3-year OSR in ATCI patients.
B: High miR-497 levels were associated with a lower 5-year OSR in ATCI patients
Discussion
As the pathogenic basis of many cardiocerebrovascular diseases in clinical practice and a major incentive of ACI, atherosclerosis (AS) causes lipid accumulation in arterial vascular endothelium due to abnormal lipid metabolism in the body. As a result, the lipids form plaques, block arteries and produce lesions (15–17). As reported by clinical research, ATCI patients can suffer repeated attacks of ATCI, which aggravates their conditions and even endangers their life in serious cases (18). Therefore, improving the prognosis of patients is particularly important to study influencing factors of ATCI prognosis.
miRNAs are expressed in many malignant tumors, which include ATCI. miR-497 has a great effect on various tumors and ischemic diseases. According to Chen X and other researchers, it is highly expressed in the serum of patients with ischemic stroke, and plays a pivotal role in the pathogenesis of nervous system diseases (19). According to Yin KJ et al., its functional incapacitation leads to neuronal damage after focal cerebral ischemia, so knocking down it can reduce ischemic cerebral infarction and improve neurological functions (20). In our study, miR-497 had remarkably high expression in the serum of ATCI patients, which indicates that it may be involved in the pathological process of the disease. In the report of Wang YY et al., this miR is highly expressed in the serum of AS patients (21), which is similar to our research results. After further analyzing the diagnostic value of miR-497 for ATCI patients, we found that its AUC for distinguishing the patients was 0.868, which suggests that this miR may be a potential target for diagnosing and treating the disease. According to the analysis of its correlation with the patients’ pathological parameters, this miR was correlated with histories of hypertension, smoking and DM. As reported by Luo L and other researchers, history of hypertension, history of smoking and history of DM have relatively high diagnostic values for ATCI (22), which is similar to our findings. According to the ROC curves, miR-497 also had relatively high diagnostic values.
As reported by a previous study, both untimely treatment and various factors after treatment cause reinfarction in ATCI patients, thus increasing disability and mortality rates (23). In our study, the recurrence rate of 135 patients was 26.67%, similar to 25.12% from Yu LH and others (24). The 3- and 5-year OSRs of ATCI patients were 73.33% and 51.85%, respectively; the AUCs of miR-497 for predicting the recurrence and prognosis of ATCI were 0.924 and 0.937, respectively. These findings reveal that miR-497 has multiple predictive performance for ATCI, and that it can be used as a potential marker for predicting the recurrence and prognosis of the disease. In the research of Cui J et al., miR-497 upregulation is helpful to prompt the pathological condition of advanced AS, so this miR can be a potential therapeutic target for AS (25), which is similar to our results. According to the analysis of survival and prognostic factors, high miR-497 levels (>3.643) were correlated with lower 3- and 5-year OSRs and poor prognoses. According to results of Cox regression analyses, miR-497 and histories of hypertension, smoking and DM were independent prognostic factors for the poor prognosis of ATCI patients; the patients with high miR-497 (>3.643) and these histories had an increased risk of poor prognoses.
Our research has confirmed that miR-497 as a serological index has a relatively high value for the diagnosis and prognosis of ATCI patients. However, there are still some deficiencies. Firstly, we can supplement the analysis on the predictive value of this miR for efficacy in the patients. Secondly, we can analyze the risk factors that affect the curative effect on or the recurrence of ATCI. Additionally, we can focus on the molecular mechanism of this miR in ATCI or its influences on cell biological functions, so as to carry out basic research for exploring potential treatments for the disease. Therefore, this research will be gradually improved based on the above aspects.
Conclusion
miR-497 expression rises in ATCI patients, so this miR is expected to become a serum diagnostic marker for ATCI.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
No funding was received in this study.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Zhang JY, Liu B, Wang YN, et al. (2014). Effect of rosuvastatin on OX40L and PPAR-gamma expression in human umbilical vein endothelial cells and atherosclerotic cerebral infarction patients. J Mol Neurosci, 52(2): 261–268. [DOI] [PubMed] [Google Scholar]
- 2.Wufuer Y, Shan X, Sailike M, et al. (2017). GPVIFcPEG improves cerebral infarct volume and cerebral thrombosis in mouse model with cerebral thrombosis. Mol Med Rep, 16(5): 7561–7568. [DOI] [PubMed] [Google Scholar]
- 3.Poncyljusz W, Falkowski A, Kojder I, et al. (2007). Treatment of acute ischemic brain infarction with the assistance of local intraarterial thrombolysis with recombinant tissue-type plasminogen activator. Acta Radiol, 48(7): 774–780. [DOI] [PubMed] [Google Scholar]
- 4.Chu H, Zhang S, Fu J, et al. (2017). [TIE’s flying acupuncture for acute cerebral infarction hemiplegia: a randomized controlled trial]. Zhongguo Zhen Jiu, 37(11): 1153–1156. [DOI] [PubMed] [Google Scholar]
- 5.Sun DJ, Zhuang AX, Zeng QH, et al. (2014). A study of microemboli monitoring of atherosclerotic thrombotic cerebral infarction and artery stenosis. Genet Mol Res, 13(3): 6734–6745. [DOI] [PubMed] [Google Scholar]
- 6.Leclercq M, Diallo AB, Blanchette M. (2013). Computational prediction of the localization of microRNAs within their pre-miRNA. Nucleic Acids Res, 41(15): 7200–7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ha J, Kim H, Yoon Y, et al. (2015). A method of extracting disease-related microRNAs through the propagation algorithm using the environmental factor based global miRNA network. Biomed Mater Eng, 26 Suppl 1: S1763–1772. [DOI] [PubMed] [Google Scholar]
- 8.Fleming NH, Zhong J, da Silva IP, et al. (2015). Serum-based miRNAs in the prediction and detection of recurrence in melanoma patients. Cancer, 121(1): 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahasi K, Iinuma H, Wada K, et al. (2018). Usefulness of exosome-encapsulated microRNA-451a as a minimally invasive biomarker for prediction of recurrence and prognosis in pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci, 25(2): 155–161. [DOI] [PubMed] [Google Scholar]
- 10.Zhang R, Qin Y, Zhu G, et al. (2016). Low serum miR-320b expression as a novel indicator of carotid atherosclerosis. J Clin Neurosci, 33: 252–258. [DOI] [PubMed] [Google Scholar]
- 11.Zhang YZ, Wang J, Xu F. (2017). Circulating miR-29b and miR-424 as Prognostic Markers in Patients with Acute Cerebral Infarction. Clin Lab, 63(10): 1667–1674. [DOI] [PubMed] [Google Scholar]
- 12.Xu GS, Li ZW, Huang ZP, et al. (2019). MiR-497-5p inhibits cell proliferation and metastasis in hepatocellular carcinoma by targeting insulin-like growth factor 1. Mol Genet Genomic Med, 7(10): e00860. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Wang J, Lin M, Ren H, et al. (2019). Expression and Clinical Significance of Serum miR-497 in Patients with Acute Cerebral Infarction. Clin Lab, 65(4):10.7754/Clin.Lab.2018.181001. [DOI] [PubMed] [Google Scholar]
- 14.Xue MZ, Li YJ, Gao XG, et al. (2011). [Atherosclerotic stenosis of intracranial and extracranial cerebral arteries in patients with cerebral infarction and the correlative factors]. Zhonghua Yi Xue Za Zhi, 91(11): 762–765. [PubMed] [Google Scholar]
- 15.QiaoZhen X, AiGuo M, Tong W, et al. (2019). Correlation between of small dense low-density lipoprotein cholesterol with acute cerebral infarction and carotid atherosclerotic plaque stability. J Clin Lab Anal, 33(6): e22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobiyama K, Saigusa R, Ley K. (2019). Vaccination against atherosclerosis. Curr Opin Immunol, 59: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Libby P, Buring JE, Badimon L, et al. (2019). Atherosclerosis. Nat Rev Dis Primers, 5(1): 56. [DOI] [PubMed] [Google Scholar]
- 18.Zhao L, Zhai Z, Hou W. (2016). Analysis of Carotid color ultrasonography and high sensitive C-reactive protein in patients with atherosclerotic cerebral infarction. Pak J Med Sci, 32(4): 931–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Lin S, Gu L, et al. (2019). Inhibition of miR-497 improves functional outcome after ischemic stroke by enhancing neuronal autophagy in young and aged rats. Neurochem Int, 127: 64–72. [DOI] [PubMed] [Google Scholar]
- 20.Yin KJ, Deng Z, Huang H, et al. (2010). miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis, 38(1): 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang YY, Li H, Wang XH, et al. (2016). Probucol inhibits MMP-9 expression through regulating miR-497 in HUVECs and apoE knockout mice. Thromb Res, 140: 51–58. [DOI] [PubMed] [Google Scholar]
- 22.Luo L, Zhu M, Zhou J. (2018). Association between CTSS gene polymorphism and the risk of acute atherosclerotic cerebral infarction in Chinese population: a case-control study. Biosci Rep, 38(6): BSR20180586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higashi Y. (2009). Edaravone for the treatment of acute cerebral infarction: role of endothelium-derived nitric oxide and oxidative stress. Expert Opin Pharmacother, 10(2): 323–331. [DOI] [PubMed] [Google Scholar]
- 24.Yu LH, Wang DX, Li YH, et al. (2015). [Recurrence of Cerebral Infarction Associated Aspirin Resistance or Chinese Medical Constitutions: a Correlation Study]. Zhongguo Zhong Xi Yi Jie He Za Zhi, 35(10): 1205–1209. [PubMed] [Google Scholar]
- 25.Cui J, Ren Z, Zou W, et al. (2017). miR-497 accelerates oxidized low-density lipoprotein-induced lipid accumulation in macrophages by repressing the expression of apelin. Cell Biol Int, 41(9): 1012–1019. [DOI] [PubMed] [Google Scholar]