Abstract
Purpose:
To determine whether MRI-detected suspicious (BIRADS 4 & 5) breast lesions can be downgraded using second-look ultrasound (SLU) and thus reduce unnecessarily performed breast biopsies.
Materials Methods:
A retrospective single-center review of consecutive patients, who underwent breast MRI studies during a 12-month time period was performed. 94 patients with 103 lesions undergoing SLU of incidentally detected MRI BI-RADS 4&5 lesions which were not identified on previous ultrasound were included in the study. The SLU detection rate and SLU features of the lesions were assessed. Histology (91/103) or two year follow up (n=12) were defined as the reference standard for lesion diagnosis.
Results:
57 (55.3%) of the 103 lesions were identified on SLU. 17 of the identified lesions were malignant (29.8%). Lesions detected on ultrasound presented on MRI as masses in 66.7% (38/57) and non-mass in 33.3% (19/57). Our findings showed that it is possible to distinguish between malignant and benign lesions with SLU. The results were significant (p<0.05) for the following morphological features: shape, orientation, margins, architectural distortion, hyperechoic rim/ edema. All lesions classified as SLU BI-RADS 2 in our study were benign and thus, 30% of all unnecessary biopsies could potentially have been avoided. Including SLU BI-RADS 3 lesions, this rate increased 60%, while yielding one (of 17, 5.8%) false negative result. All three BI-RADS 5 lesions detected by SLU presented as malignant on ultrasound.
Conclusion:
SLU can potentially downgrade incidental MRI BIRADS 4 lesions. This may reduce the number of unnecessarily performed biopsies by 30–60%, thus simplifying patient management.
Keywords: Second-look ultrasound, Breast Cancer, Magnetic Resonance Imaging, Image- guided biopsy, BI-RADS
Introduction
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) of the breast is highly sensitive for detection of breast cancer (1, [1]. Suspicious imaging findings are not specific for a malignant diagnosis, leading to unnecessary breast biopsies of incidentally detected lesions that were not visible on prior conventional imaging [2]. Whereas in probably benign lesions a short-term follow-up to document stability per BI-RADS 3 guidelines can be performed, lesions that are suspicious or highly suggestive of malignancy need to be histopathologically verified [3]. Although MRI-guided breast biopsy is an accurate tool, its availability and costs limit its application [4–6] and other means for image-guided breast biopsy or follow-up in probably benign lesions are desirable.
Second-look Ultrasound (SLU) is a clinically feasible method for the assessment of MRI-detected lesions [7–9]. Though SLU performance varies and depends on both patient and lesion features [7,9,10], a systematic review reported that a large fraction of lesions can be correctly identified with SLU [9]. While lesion detection rates by SLU have been quite comprehensively addressed in the literature, it remains unclear whether SLU may also provide clinically decisive additional diagnostic information to distinguish benign from malignant lesions. Therefore, the aim of this study was to evaluate, whether MRI-detected suspicious breast lesions appearing benign on SLU could be downgraded to potentially avoid unnecessary breast biopsies.
Methods
Patients
This retrospective single-center study was approved by the institutional review board. The need for written informed written consent for the retrospective review of routine medical data records was waived by the IRB.
For this study, all consecutive 1692 MRI studies in 1522 patients who were diagnosed at our institution, a tertiary care university hospital were reviewed. The MRI examinations were either performed and read in our hospital or the patients were referred for assessment and second opinion. The main indications for the MRI examinations were 1) problem solving [8,11] (clarification of discrepant and unclear conventional and clinical findings such as asymmetric densities and architectural distortions without ultrasound correlates), 2) high-risk screening of women with either known BRCA-1 or BRCA-2 mutation or fulfilling the familial risk criteria [8,11], 3) follow-up examinations after breast conserving therapy or 4) staging of breast cancer. Of those, all patients who underwent SLU due to MRI-detected, contrast-enhancing BI-RADS 4 or 5 lesions not previously documented by conventional imaging, were included in our study. Excluded were patients without at least two years of follow-up or invasive tissue sampling. If no lesion was detected on SLU, MRI-guided biopsy was performed subsequently in these patients. Finally, 94 patients (mean age: 52 years, range: 26– 78 years) with 103 lesions were included. Patients of this database have been reported on in another paper in another scientific context [10].
The images were retrospectively reviewed by a fellowship-trained, board-certified radiologist with breast imaging experience of more than five years.
MRI protocol and interpretation
Due to our institution being an assessment center, different 1.5T and 3T MRI devices and sequences were used. However, all MRI studies adhered to international guidelines (EUSOBI guidelines)[12]. A T2w and/or STIR sequence and a T1-weighted contrast sequence with a temporal resolution of around one minute per measurement were always part of the protocol, approximately 80% of all examinations further included a DWI sequence.
MRI examinations were interpreted according to BI-RADS in conjunction with the clinical history and any available prior breast imaging by one out of five dedicated breast radiologists with more than 5 years of experience. All lesions detected with MRI were documented in accordance with the MRI BI-RADS lexicon [13].
Ultrasound Technique and analysis
All SLUs were conducted within 6 weeks after MRI using a Siemens Acuson S3000 device. A 18L6HD linear transducer was used for B-mode ultrasound. Five dedicated breast radiologists, all of them having more than five years of experience in breast imaging, performed the SLUs.
SLU is defined as a targeted ultrasound examination of lesions detected by MRI and not previously documented on ultrasound. SLU targeted to the region of the MRI-detected enhancing lesion was conducted. Lesions corresponding in depth, position, size and similar shape of the MRI lesion were assumed to be correctly identified and used as a target for US-guided breast biopsy.
The ultrasound features of the lesions were described in accordance with the US BI-RADS lexicon [13] and compared to the lesion type (benign vs. malignant). The following ultrasound features were analyzed: shape, orientation, margin, echo pattern, posterior features, calcifications, architectural distortion, vascularity and edema/ hyperechoic rim.
Histopathology
In lesions detected on SLU, US-guided biopsies were conducted either using a 14G core needle biopsy or 9G console-based vacuum assisted biopsy (ATEC). If the MRI-lesion was not found on SLU MRI-guided biopsy was performed using a console-based 9G vacuum-assisted biopsy device (ATEC). In case of a benign histopathological diagnosis at image-guided needle biopsy, the final diagnosis was benign. In case of a high-risk lesion with uncertain potential for malignancy, the final diagnosis was established with open surgery.
Histology was obtained for 91 lesions in total; 49 by ultrasound-guided biopsy, 35 by MRI-guided biopsy, 6 by surgery and 1 by stereotactic biopsy.
Follow-up protocol
All lesions that were benign on histopathology and deemed to be concordant were subject to follow-up. MRI follow-up was performed every 6 months for 2 years. When a lesion was no longer visible, significantly smaller or had obvious benign features, no further follow-up was performed.
Data analysis
Data was collected by using the PACS-system of our institution. The collected data included: patient age, breast size, morphological features on MRI and US according to the BI-RADS lexicon, the method of tissue sampling, the result of the histological and immunohistochemical analysis and the last instance the patient underwent follow-up, i.e. date, method and result of follow-up.
All statistical analyses were performed using SPSS 20 (IBM, USA). Fisher’s exact test and Chi-square-test were used, P-values <0.05 were considered significant. The ROC curve was also calculated by plotting BI-RADS categories and the standard of reference (i.e. lesion type).
Results
One-hundred-and-three lesions were included in our study. 94 (91.3%) lesions were documented as MRI BI-RADS 4 and 9 (8.7%) as MRI BI-RADS 5.
Of these 57 (55.3%) lesions were identified by SLU. Forty of those (70.2%) were benign, 17(29.8%) were malignant. SLU identified 54/94 (57.4%) with BI-RADS 4, 3/9 with BI-RADS 5 (33%).
Seventeen malignant lesions were detected by SLU. Eleven (64.7%) were invasive ductal carcinomas, two were DCIS (11.8%) and four belonged to other types of carcinomas. Forty benign lesions were identified on SLU. Eleven (27.5%) were papillomas, 7 (17.5%) were fibroadenomas, 7 (17.5%) were fibrocystic changes and 9 (22.5%) belonged to other types of benign lesions. 6 (15%) lesions were managed through follow-up. Detection rates and histological results are listed in Tab. 1
Table 1:
SLU lesion detection rates stratified by histological results and MRI appearance
| Lesion type (Number) | Identified (%) | Not identified (%) |
|---|---|---|
| All lesions (103) | 57 (55.3%) | 46 (44.7%) |
| Malignant lesions (31) | 17 (54.8%) | 14 (45.2%) |
| Inv. duct. Carcinoma | 11 (64.7%) | 8 (57.1%) |
| DCIS | 2 (11.8%) | 3 (21.4%) |
| Others | 4 (23.5%) | 3 (21.4%) |
| Benign Lesions (72) | 40 (55.6%) | 32 (44.4%) |
| Papilloma | 11 (27.5%) | 5 (15.6%) |
| Fibroadenoma | 7 (17.5%) | 2 (6.25%) |
| Fibrocystic changes | 7 (17.5%) | 15 (46.9%) |
| Others | 9 (12.5%) | 4 (12.5%) |
| Follow-up only | 6 (15%) | 6 (18.8%) |
| Mass lesions (58) | 38 (65.5%) | 20 (34.5%) |
| Non Mass lesions (41) | 17 (41.5%) | 24 (58.5%) |
| Foci (4) | 2 (50%) | 2 (50%) |
Second-look ultrasound detection rate
The SLU detection rate in this study was 55.3% (57/103). Forty-six lesions were not detected on SLU, including 14 (30.4%) malignant lesions and 32 (69.6%) benign lesions. The ultrasound detection rate for malignant lesions was comparable to that of benign lesions (54.8% vs. 55.6%). Mass lesions had a higher SLU detection rate than non-mass lesions (65.5% vs. 41.5%). The mean size of SLU-detected mass and non-mass lesions was bigger than that of not-detected mass and non-mass lesions (Mass: 13.5mm vs. 9.2mm; non-mass: 31.9mm vs. 21mm). The portion of malignant lesions among SLU-detected and not detected lesions was comparable (29.8% and 30.4%).
Ultrasound features associated with malignancy
Five ultrasound features significantly differed between benign and malignant lesions: shape (p= 0.035), orientation (p= 0.023), margin (p< 0.001), architectural distortion (p= 0.003) and edema/hyperechoic rim (p< 0.001). (see Table 2 for distribution of significant features and Table 3 for distribution of not significant features)
Table 2:
Distribution of ultrasound features in benign and malignant breast lesions (significant)
| Ultrasound Feature | Number of malignant lesions (%) | Number of benign lesions (%) | P-Value |
|---|---|---|---|
| Shape | P= 0.035 | ||
| Oval | 1 (6.2%) | 15 (93.8%) | |
| Round | 3 (30%) | 7 (70%) | |
| Irregular | 13 (41.9%) | 18 (58.1%) | |
| Orientation | P= 0.023 | ||
| Parallel | 4 (14.8%) | 23 (85.2%) | |
| Not parallel | 13 (43.3%) | 17 (56.7%) | |
| Margin | P <0.001 | ||
| Circumscribed | 0 | 21 (100%) | |
| Indistinct | 12 (41.4%) | 17 (58.6%) | |
| Angular | 3 (100%) | 0 | |
| Spiculated | 2 (100%) | 0 | |
| Architectural distortions | P= 0.003 | ||
| None | 7 (17.1%) | 34 (82.9%) | |
| Present | 10 (62.5%) | 6 (37.5%) | |
| Hyperechoic rim /Edema | P <0.001 | ||
| None | 4 (9,3%) | 39 (90.7%) | |
| Present | 13 (92.9%) | 1 (7.1%) | |
Table 3:
Distribution of ultrasound features in benign and malignant lesions (not significant), note: there was calcification within the malignant lesion; in one benign lesion there was calcification within the lesion, in the other was outside the lesion.
| Ultrasound feature | Number of malignant lesions (%) | Number of benign lesions (%) | P-value |
|---|---|---|---|
| Echo pattern | P= 0.503 | ||
| Hypoechoic | 7 (25.9%) | 20 (74.1%) | |
| Isoechoic | 3 (42.9%) | 4 (57.1%) | |
| Heterogeneous | 7 (61.1%) | 11 (61.1%) | |
| Others | 0 | 5 (100%) | |
| Posterior features | P= 0.078 | ||
| None | 12 (25%) | 36 (75%) | |
| Enhancement | 0 | 1 (100%) | |
| Shadowing | 4 (66.7%) | 2 (33.3%) | |
| Combined | 1 (50%) | 1 (50%) | |
| Calcifications | P=0.662 | ||
| None | 16 (29.6%) | 38 (70.4%) | |
| Present | 1 (33.3%) | 2 (66.7%) | |
| Vascularity | P= 0.295 | ||
| Absent | 3 (15.8%) | 16 (84.2%) | |
| Internal | 3 (27.3%) | 8 (72.7%) | |
| Vessels in rim | 1 (50%) | 1 (50%) | |
| No data | 10 (40%) | 15 (60%) | |
Downgrading lesions with SLU
Twelve (12/57= 21.1%) MRI BI-RADS 4 lesions were assigned a BI-RADS 2 category on SLU. These 12 lesions were benign in subsequent pathological examination or follow-up.
Thirteen MRI BI-RADS 4 lesions were classified as BI-RADS 3 on SLU. One of these lesions (1/13, 7.7%) was an invasive ductal carcinoma. The remaining lesions (92.3%) were benign. All MRI MR BI-RADS 5 lesions were rated BI-RADS 4 on SLU.
ROC-Analysis
The area under the curve (AUC) was calculated for the US BI-RADs ratings of the SLU-detected MR BI-RADS 4 and 5 lesions. The AUC was 80.3%, showing a good diagnostic accuracy for SLU to diagnose breast cancer (see Image 1).Setting the threshold at SLU BI-RADS >2 showed 100% sensitivity and 30% specificity, meaning that no malignant lesion would have been overlooked and 30% of unnecessarily performed biopsies could have been avoided. Setting the threshold at BI-RADS >3 resulted in a sensitivity of 94.1% and a specificity of 60%. This means that 60% of unnecessarily conducted biopsies could have been avoided, but 5.8% (1/17, 1 invasive ductal carcinoma) of all malignant lesions would have been missed.
Figure 1:
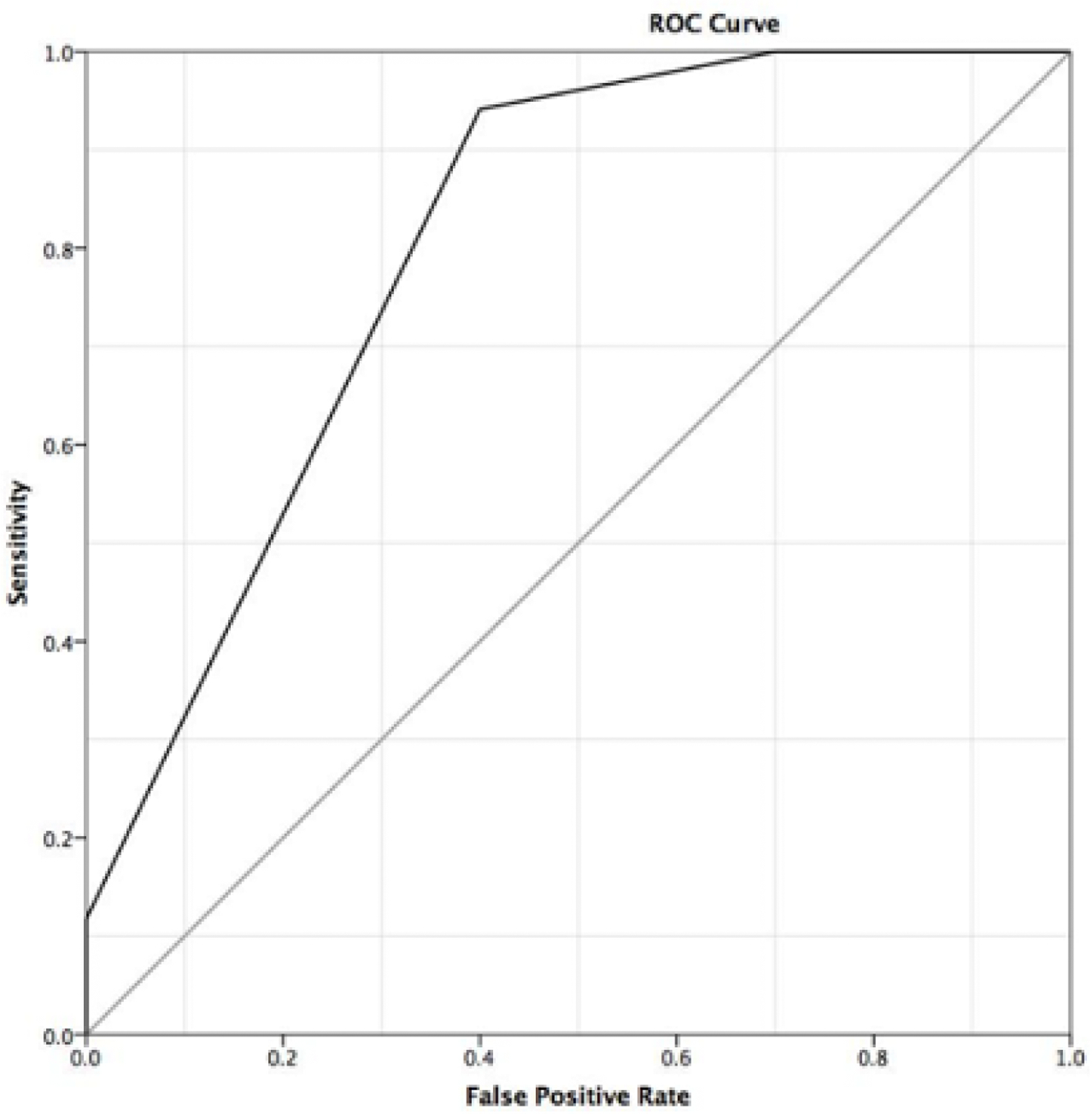
Receiver-Operating- Characteristics (ROC) curve for diagnosis of malignancy in SLU detected breast lesions by US-BIRADS category assignment revealing an area under the curve of 80.3% and two operating points with rule-out criteria at BI-RADS >2 (no FN results) and BI-RADS >3 (1 FN result, DCIS).
Discussion
Our study shows that SLU can downgrade suspicious (BI-RADS 4) MRI-detected breast lesions and therefore potentially avoid unnecessary biopsies. Twelve MR BI-RADS 4 lesions were downgraded to a BI-RADS 2 category assignment, all of them confirmed benign in pathological examination or follow-up. No MRI BI-RADS 5 lesion was rated BI-RADS 2 on SLU. We thereby demonstrated that SLU-BIRADS 2 can downgrade MRI-detected breast lesions that would either require biopsy or short-term follow-up.
With SLU, benign and malignant lesions can be accurately distinguished. The amount of unnecessary invasive tissue sampling could have been substantially reduced by 30% in this study; our findings demonstrate the possibility to lower the amount of unnecessarily conducted image-guided biopsies, thereby reducing costs and simplifying the management of MRI-detected breast lesions.
This number could have potentially been increased to 60%, if all SLU BI-RADS 3 lesions would not have been biopsied. In this case, one malignant lesion would have been missed. However, a SLU BI-RADS 3 finding warrants short-term follow-up which could have been performed safely using US in 6 months, thereby revealing potential progression and leading to a lesion upgrade. Naturally, the small sample size of our study precludes definite recommendations for patient care, but the encouraging findings corroborate the general possibility of using SLU, not only to detect, but also to downgrade MRI-detected breast lesions.
SLU has previously been used to reduce biopsies; in a single-center study by Abe et al., 36 MRI BI-RADS 3 lesions were not biopsied as they were benign-appearing in SLU. All of these lesions were benign in follow-up [14]. In another study SLU was used to evaluate MRI-detected lesions; among BI-RADS 4 lesions all malignancies presented with suspicious ultrasound findings on SLU (17/23 were malignant, 73.9%) [2].
A fraction of malignant lesions in the aforementioned studies (Abe: 11/33= 33% and Lee: 5/18= 27.7%) did not show any typical malignant findings, which included non-parallel orientation, echogenic halo, angular or spiculated margins and posterior shadowing [2,14]. In this study one malignant lesion (5.8%) did not show any of those features on SLU. Yet, it was rated BI-RADS 4 on SLU due to its irregular shape, which yields 40% risk of malignancy according to our data (see above) and being hypoechoic, a feature associated with malignancy [15]. The lesion was biopsied and turned out to be a DCIS. Both mentioned studies did not report the SLU BI-RADS classification of those lesions. In this study all malignant lesions, except one (BI-RADS 3), were classified as either BI-RADS 4 (14/17, 82.4%) or BI-RADS 5 (2/17, 11.8%) on SLU.
We showed that- in addition to the typical features margins and orientation- shape, architectural distortions and the presence of a hyperechoic rim/ edema on SLU were significantly associated with malignancy.
Irregular shape, non-parallel orientation, angular and spiculated margins are known malignant features [2,14–17]. The role of hyperechoic rim/ edema is not as well researched; we could only find one study on this topic [18]. Architectural distortion was an important malignant feature in this study as the malignancy rate for such lesions was 62.5%. However, only one study from 1992 was found and it showed a similar malignancy rate [19]. Vascularity was not a significant feature in this study, because it was not assessed for many lesions. Increased vascularity was demonstrated to be suggestive for malignancy in earlier studies [14,20]; a more recent study showed that the vascular anatomy and not increased vascularity per se was crucial for differentiating malignant from benign lesions [21]. Echogenicity was also not significant in our study (p=0.078). Its role as a malignant feature is not clear as there are studies reporting hypoechogenicity to be indicative for malignancies [14,15] and others reporting the opposite [2,16].
The SLU detection rate in this study was 55.3%, which is consistent with other studies [2,14,22]. In contrast to a majority of other studies, our SLU detection rates were similar in benign and malignant lesions (55.6% and 54.8%) [9]. However, three prior reports noted a similar of even higher prevalence of breast cancer in lesions without SLU correlates as compared to those detected by SLU [23–25]. A potential reason for this perceived discrepancy is a potential selection bias in retrospective previous studies. Our experience that benign US features may downgrade MRI-suspicious lesions that is reported in this paper is likely already applied by some centers even without published empirical evidence. Mass lesions demonstrated a higher detection rate than non-mass lesions (65.5% and 41.5%) [9,26]. 14 malignant lesions could not be identified by SLU. Therefore, this study is consistent with the findings of previous studies, that the absence of a suspicious MRI-detected lesion on SLU necessitates MR-guided biopsy [2,14,22,27].
Our study has several limitations. The design of our study was retrospective; there was no defined protocol for the conduction of the SLU. At our institution, SLU is usually performed in MRI-detected lesions that were previously either missed or not examined by ultrasound. The rate of incidental BI-RADS 4/5 findings has been reported with 10% in a study by Mahoney and colleagues [28]. The only exemption are very small foci and non-mass lesions in bigger breasts (cup size D or higher), that are regularly scheduled for immediate MRI-guided biopsy. Therefore, our rate of SLU examinations is well within what is expected from the literature, largely excluding any database review/patient selection bias though the retrospective review could potentially have missed single SLU cases not specifically documented as such.
In addition, a Duplex-ultrasound was not performed in a substantial number of lesions. The number of lesions was small, especially in sub-group analysis and therefore no further conclusions can be drawn on e.g. whether a a formal combination of US BI-RADS criteria may exclude malignancy.
In conclusion, this study indicates, that SLU can accurately downgrade MRI-detected breast lesions if visible upon SLU. These findings have the potential to change clinical practice as unnecessary image-guided biopsies could be avoided. Patients with BI-RADS 4 lesions visible upon SLU and classified as SLU BI-RADS 2 (or 3), may undergo short-term follow-up instead of invasive diagnostic procedures. This approach has the potential to simplify and speed up patient management in assessment centers and could also reduce costs. Further, best prospective research is needed to independently confirm our results and to define the potential of SLU to avoid unnecessary biopsies in the investigated setting.
Figure 2:
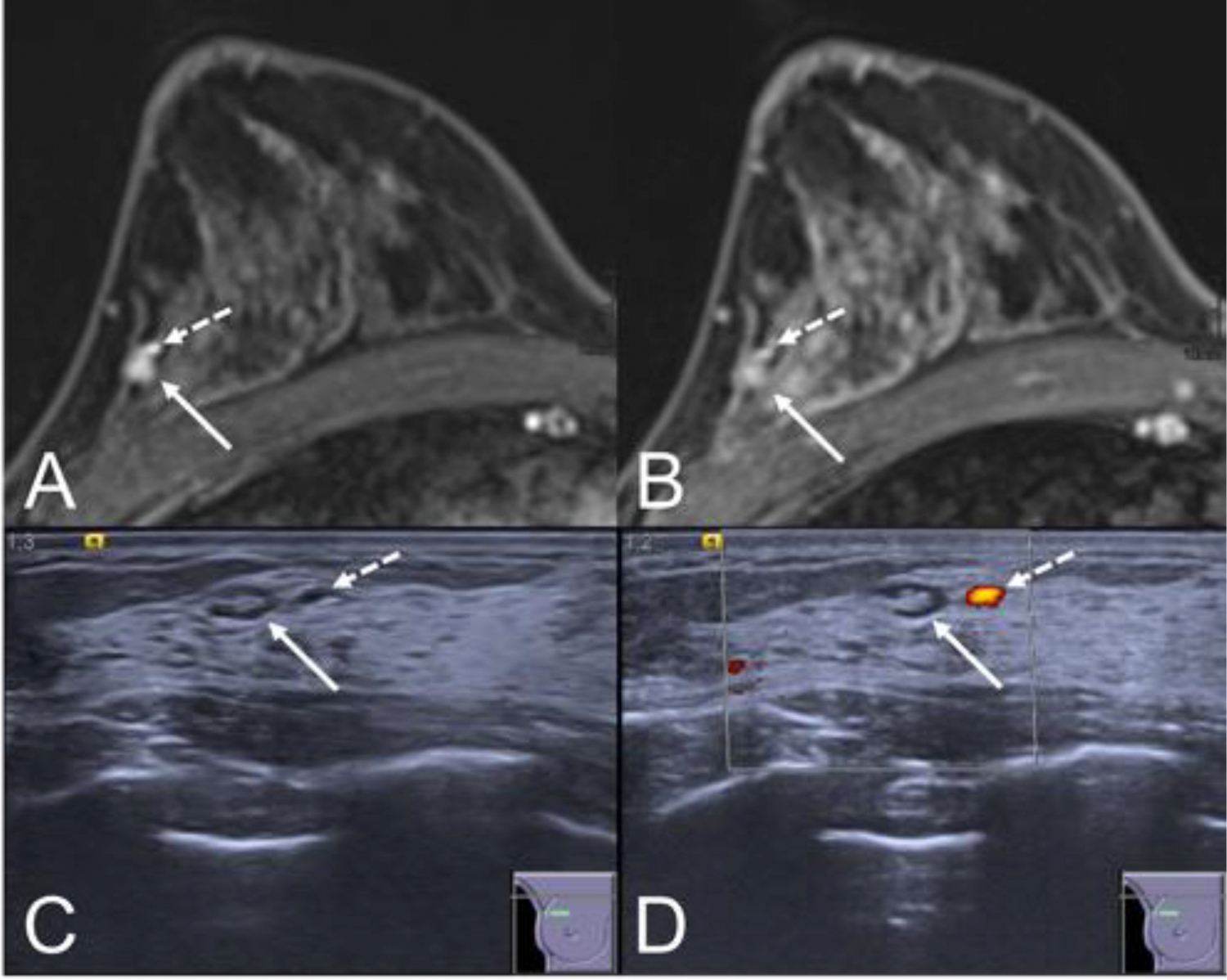
Example for a lesion that could be downgraded by second-look ultrasound. MRI (A: early, B: late contrast enhanced fat suppressed T1w) shows an irregular shaped mass lesion (white arrow) with rather circumscribed margins and a signal decrease in time, corresponding to a washout curve type. The lesion was assigned a BI-RADS 4 category and underwent second-look ultrasound. Ultrasound (C: B-mode, D: Power Doppler) shows the lesion (white arrow) showing the imaging criteria of a benign lymph node. Note the adjacent vessel (dashed arrow) that is both visible on MRI and US and further hints at the diagnosis of a lymph node. The SLU examination was assigned a BI-RADS 2 rating and proved stable on follow-up over more than two years.
Table 4:
ROC-Analysis for BI-RADS ≤ 2 and ≤ 3
| BI-RADS | Sensitivity | 95% CI | Specificity | 95% CI | +LR | −LR |
|---|---|---|---|---|---|---|
| ≤ 2 | 100 | 80.5 −100 | 30 | 16.6– 46.5 | 1.43 | 0 |
| ≤ 3 | 94.12 | 80.5 −100 | 60 | 43.3– 75.1 | 2.35 | 0.098 |
Highlights:
A benign appearance on second-look ultrasound can downgrade MRI BI-RADS 4 lesions.
Second-look ultrasound BI-RADS 2 lesions did not yield false-negative results.
This study demonstrates the potential to avoid 30% of all unnecessary biopsies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
None
References
- [1].Kim TH, Contralateral Enhancing Lesions on Magnetic Resonance Imaging, (2012) 903–913. [DOI] [PubMed]
- [2].Lee SH, Kim SM, Jang M, Yun BL, Kang E, Kim SW, Park SY, Ahn HS, Chang JH, Yoo Y, Song TK, Moon WK, Role of second-look ultrasound examinations for MR-detected lesions in patients with breast cancer., Ultraschall in Der Medizin 36 (2015) 140–148. 10.1055/s-0034-1399143. [DOI] [PubMed] [Google Scholar]
- [3].Bick U, Trimboli RM, Athanasiou A, Balleyguier C, Baltzer PAT, Bernathova M, Borbély K, Brkljacic B, Carbonaro LA, Clauser P, Cassano E, Colin C, Esen G, Evans A, Fallenberg EM, Fuchsjaeger MH, Gilbert FJ, Helbich TH, Heywang-Köbrunner SH, Herranz M, Kinkel K, Kilburn-Toppin F, Kuhl CK, Lesaru M, Lobbes MBI, Mann RM, Martincich L, Panizza P, Pediconi F, Pijnappel RM, Pinker K, Schiaffino S, Sella T, Thomassin-Naggara I, Tardivon A, Ongeval CV, Wallis MG, Zackrisson S, Forrai G, Herrero JC, Sardanelli F, European Society of Breast Imaging (EUSOBI), with language review by Europa Donna–The European Breast Cancer Coalition, Image-guided breast biopsy and localisation: recommendations for information to women and referring physicians by the European Society of Breast Imaging, Insights Imaging. 11 (2020) 12. 10.1186/s13244-019-0803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Clauser P, Mann R, Athanasiou A, Prosch H, Pinker K, Dietzel M, Helbich TH, Fuchsjäger M, Camps-herrero J, Sardanelli F, Forrai G, Baltzer PAT, A survey by the European Society of Breast Imaging on the utilisation of breast MRI in clinical practice, (2018) 1909–1918. [DOI] [PMC free article] [PubMed]
- [5].Evans A, Trimboli RM, Athanasiou A, Balleyguier C, Baltzer PA, Bick U, Herrero JC, Clauser P, Colin C, Cornford E, Fallenberg EM, Fuchsjaeger MH, Gilbert FJ, Helbich TH, Kinkel K, Heywang-köbrunner SH, Breast ultrasound : recommendations for information to women and referring physicians by the European Society of Breast Imaging, (2018) 449–461. [DOI] [PMC free article] [PubMed]
- [6].Woitek R, Spick C, Schernthaner M, Rudas M, Kapetas P, Bernathova M, Furtner J, Pinker K, A simple classification system ( the Tree flowchart ) for breast MRI can reduce the number of unnecessary biopsies in MRI-only lesions, (2017) 3799–3809. 10.1007/s00330-017-4755-6. [DOI] [PMC free article] [PubMed]
- [7].Meissnitzer M, Dershaw DD, Lee CH, Morris EA, Targeted ultrasound of the breast in women with abnormal MRI findings for whom biopsy has been recommended, AJR Am J Roentgenol. 193 (2009) 1025–1029. 10.2214/AJR.09.2480. [DOI] [PubMed] [Google Scholar]
- [8].Mann RM, Balleyguier C, Baltzer PA, Bick U, Colin C, Cornford E, Evans A, Fallenberg E, Forrai G, Fuchsjäger MH, Gilbert FJ, Helbich TH, Heywang-Köbrunner SH, Camps-Herrero J, Kuhl CK, Martincich L, Pediconi F, Panizza P, Pina LJ, Pijnappel RM, Pinker-Domenig K, Skaane P, Sardanelli F, European Society of Breast Imaging (EUSOBI), with language review by Europa Donna–The European Breast Cancer Coalition, Breast MRI: EUSOBI recommendations for women’s information, Eur Radiol. 25 (2015) 3669–3678. 10.1007/s00330-015-3807-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Spick C, Baltzer PAT, Diagnostic Utility of Second- Look US for Breast Lesions Identified at MR Imaging : Sytematic Review and Meta-Analysis, Radiology. 273 (2014) 401–409. 10.1148/radiol.14140474. [DOI] [PubMed] [Google Scholar]
- [10].Bumberger A, Clauser P, Kolta M, Kapetas P, Bernathova M, Helbich TH, Pinker K, Baltzer PA, Can we predict lesion detection rates in second-look ultrasound of MRI-detected breast lesions? A systematic analysis, Eur J Radiol. 113 (2019) 96–100. 10.1016/j.ejrad.2019.02.008. [DOI] [PubMed] [Google Scholar]
- [11].Sardanelli F, Boetes C, Borisch B, Decker T, Federico M, Gilbert FJ, Helbich T, Heywang-Köbrunner SH, Kaiser WA, Kerin MJ, Mansel RE, Marotti L, Martincich L, Mauriac L, Meijers-Heijboer H, Orecchia R, Panizza P, Ponti A, Purushotham AD, Regitnig P, Del Turco MR, Thibault F, Wilson R, Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group, Eur. J. Cancer. 46 (2010) 1296–1316. 10.1016/j.ejca.2010.02.015. [DOI] [PubMed] [Google Scholar]
- [12].Mann RM, Kuhl CK, Kinkel K, Boetes C, Breast MRI: guidelines from the European Society of Breast Imaging., European Radiology. 18 (2008) 1307–1318. 10.1007/s00330-008-0863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].A.C. of R. 2013 D’Orsi CJ, SickleD’Orsi CJ, Sickles EA, Mendelson EB, Morris EA et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology; 2013s EA, Mendelson EB, Morris EA et al. ACR BI-RADS® Atlas, Breast Imaging Rep, ACR BI-RADS Atlas Fifth Edition, (n.d.) 6. [Google Scholar]
- [14].Abe H, Schmidt RA, Shah RN, Shimauchi A, Kulkarni K, Sennett CA, Newstead GM, MR-directed (“second-look”) ultrasound examination for breast lesions detected initially on MRI: MR and sonographic findings, American Journal of Roentgenology. 194 (2010) 370–377. 10.2214/AJR.09.2707. [DOI] [PubMed] [Google Scholar]
- [15].Stavros A, Rapp L, Dennis MA, Parker SH, Sisney GA, Nodules : Use of Sonography to Distinguish Lesions ‘, (1995). [DOI] [PubMed]
- [16].Hsu H-H, Chang T-H, Chou Y-C, Peng Y-J, Ko K-H, Chang W-C, Lin Y-P, Hsu G-C, Yu J-C, Breast Nonmass Enhancement Detected with MRI: Uility and Lesion Characterization with Second-Look Ultrasonography, The Breast Journal. 21 (2015) 579–587. 10.1111/tbj.12491. [DOI] [PubMed] [Google Scholar]
- [17].Ferre R, Pare M, Mesurolle B, Ultrasound features of retroareolar breast carcinoma, Diagnostic and Interventional Imaging. 99 (2018) 343–344. 10.1016/j.diii.2018.03.005. [DOI] [PubMed] [Google Scholar]
- [18].Skaane P, Ultrasonography as adjunct to mammography in the evaluation of breast tumors., Acta Radiologica. Supplementum. 420 (1999) 1–47. [PubMed] [Google Scholar]
- [19].Nishimura S, Matsusue S, Hospital T, ARCHITECTURAL DISTORTION OF SUBCUTANEOUS FASCIAL LAYER IN BREAST TUMORS: ULTRASONOGRAPHIC EVALUATION, 18 (1992) 815–820. [DOI] [PubMed] [Google Scholar]
- [20].Lee S, Choi HY, Baek SY, Lim SM, Role of Color and Power Doppler Imaging in Differentiating between Malignant and Benign Solid Breast Masses, (2002) 459–464. 10.1002/jcu.10100. [DOI] [PubMed]
- [21].Svensson WE, Pandian AJ, Hashimoto H, The Use of Breast Ultrasound Color Doppler Vascular Pattern Morphology Improves Diagnostic Sensitivity with Minimal Change in Specificity, (2010) 466–474. [DOI] [PubMed]
- [22].Meissnitzer M, Dershaw DD, Lee CH, Morris EA, Targeted ultrasound of the breast in women with abnormal MRI findings for whom biopsy has been recommended, American Journal of Roentgenology. 193 (2009) 1025–1029. 10.2214/AJR.09.2480. [DOI] [PubMed] [Google Scholar]
- [23].Carbognin G, Girardi V, Calciolari C, Brandalise A, Bonetti F, Russo A, Pozzi Mucelli R, Utility of second-look ultrasound in the management of incidental enhancing lesions detected by breast MR imaging, Radiol Med. 115 (2010) 1234–1245. 10.1007/s11547-010-0561-9. [DOI] [PubMed] [Google Scholar]
- [24].Laguna AD, Arranz SJ, Checa VQ, Roca SA, Jimenez DE, Oliver-Goldaracena J, Sonographic Findings of Additional Malignant Lesions in Breast Carcinoma Seen by Second Look Ultrasound, J Clin Imaging Sci. 1 (2011). 10.4103/2156-7514.82338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Luciani ML, Pediconi F, Telesca M, Vasselli F, Casali V, Miglio E, Passariello R, Catalano C, Incidental enhancing lesions found on preoperative breast MRI: management and role of second-look ultrasound, Radiol Med. 116 (2011) 886–904. 10.1007/s11547-011-0630-8. [DOI] [PubMed] [Google Scholar]
- [26].Leung JWT, Utility of second-look ultrasound in the evaluation of MRI-detected breast lesions, Seminars in Roentgenology. 46 (2011) 260–274. 10.1053/j.ro.2011.08.002. [DOI] [PubMed] [Google Scholar]
- [27].Wiratkapun C, Duke D, Nordmann AS, Lertsithichai P, Narra V, Barton PT, Hildebolt CF, Bae KT, Indeterminate or Suspicious Breast Lesions Detected Initially with MR Imaging. Value of MRI-directed Breast Ultrasound, Academic Radiology. 15 (2008) 618–625. 10.1016/j.acra.2007.10.016. [DOI] [PubMed] [Google Scholar]
- [28].Mahoney MC, Gatsonis C, Hanna L, DeMartini WB, Lehman C, Positive predictive value of BI-RADS MR imaging, Radiology. 264 (2012) 51–58. 10.1148/radiol.12110619. [DOI] [PMC free article] [PubMed] [Google Scholar]


