Abstract
A total of 300 weanling pigs (Line 400 × 200, DNA, Columbus, NE, initially 4.83 kg) were used in a 46-d trial to evaluate the effects of different nutritional strategies to replace pharmacological levels of Zn, provided by zinc oxide (ZnO), in nursery diets on growth performance and fecal dry matter (DM). Six treatments with 10 replicate pens per treatment and 5 pigs per pen were used. Diets consisted of: (1) positive control (ZnO providing 3,000 mg/kg added Zn from d 0 to 7 and 2,000 mg/kg added Zn from d 8 to 25 and 21% crude protein, CP); (2) negative control (NC; no added ZnO); (3) NC plus 1.2% Na diformate; (4) NC with 4% coarse ground wheat bran; (5) NC but formulated to 18% CP; and (6) the combination of NC with 18% CP, 1.2% Na diformate, and 4% coarse ground wheat bran. The diets formulated to 18% CP contained 1.2% standardized ileal digestible (SID) Lys from d 0 to 25, whereas the 21% CP diets contained 1.4% SID Lys from d 0 to 7 and 1.35% SID Lys from d 7 to 25. From d 25 to 46, all pigs were fed a common diet. From d 0 to 7, no differences in any variables were observed between treatments. From d 7 to 25, pigs fed the diet with added ZnO had greater (P < 0.01) average daily gain (ADG) and average daily feed intake (ADFI) than all other treatments. Pigs fed the diet formulated to 18% CP had decreased (P < 0.01) ADG when compared with pigs fed the other diets. From d 25 to 46, no previous treatment effects on ADG or gain to feed ratio (G:F) were observed. Overall (d 0 to 46), pigs fed the diet with added ZnO from d 0 to 25 had greater (P < 0.01) ADG, ADFI, and final body weight than pigs fed added Na Diformate, or 4% coarse ground wheat bran, or with the 18% CP diet, or with pigs fed the combination of the additives intermediate. There was no evidence for differences in overall G:F. Pigs fed the NC diet had the lowest fecal DM and highest fecal scores (P < 0.05), indicating the greatest incidence of loose stools. Pigs fed added ZnO had greater fecal DM than pigs fed the NC, 4% added wheat bran, or 18% CP diets, or with pigs fed the combination of additives intermediate (P < 0.01). These results suggest that adding pharmacological levels of Zn from ZnO improves nursery pig performance and increases DM content of feces when compared with pigs fed diets with either Na diformate, 4% course wheat bran, or 18% CP alone. However, a combination of all three alternatives appeared to be additive and partially restored growth performance similar to adding pharmacological levels of Zn.
Keywords: crude protein, growth performance, Na diformate, nursery pigs, wheat bran, zinc oxide
INTRODUCTION
The addition of pharmacological levels of Zn (2,000–3,000 mg/kg Zn from ZnO) to nursery pig diets is a common practice in the U.S. swine industry. Feeding pharmacological levels of Zn to nursery pigs began when Poulsen (1995) observed that high concentrations of added dietary Zn decreased the incidence of nonspecific postweaning diarrhea. Later, other studies showed that pharmacological concentrations of inorganic Zn as Zn oxide improved growth performance of nursery pigs (Carlson et al., 1999; Hill et al., 2001).
It is well established that feeding pharmacological levels of Zn consistently reduces postweaning diarrhea because Zn ions alter microbiota to prevent attachment (Starke et al., 2014) and translocation (Huang et al., 1999) of pathogenic bacteria, such as Escherichia coli in the gastrointestinal tract (GIT). Hampson (1986) suggested that physiological changes in the GIT may enhance nutrient absorption by altering intestinal morphology. The use of ZnO has been questioned in some parts of the world because of environmental concerns as feeding pharmacological concentrations of Zn result in increased Zn excretion due to low retention rates and bioavailability of ZnO (Case and Carlson, 2002). High Zn accumulation in the soil has been implicated in reduced plant growth (Chaney, 1993). It is believed that the benefits generated by using pharmacological levels of Zn in swine farming do not outweigh the harm caused by long-term environmental contamination. For these reasons, the European Commission has decided to phase out the use of pharmacological levels of Zn in pig diets starting 1 June 2022 (European Medicines Agency, 2016). In addition, the use of pharmacological levels of Zn in the diet could favor increased populations of antimicrobial resistant bacteria (Sargeant et al., 2010; Slifierz et al., 2015). However, restrictions on using pharmacological levels of Zn could make nursery pigs more susceptible to common infectious agents, which could lead to economic losses and decrease animal welfare.
Some reports support that low-crude protein (CP) diets or added coarse wheat bran may help to maintain gut health by lowering protein fermentation and promoting the proliferation of commensal microbiota (Nyachoti et al., 2006; Heo et al., 2010). Other studies suggest the use of acidifiers as an option to improve weanling pig’s health and growth performance (Pettigrew, 2006; Stein, 2006). This is a result of their potential to reduce the pH of the GIT, which improves nutrient digestion and protects the GIT from pathogenic invasion and proliferation.
Although replacing pharmacological levels of Zn has been widely studied in production settings, the results are still inconsistent, and few studies evaluated the impact of different products in combination in nursery diets. Therefore, the objective of this study was to evaluate diet acidification, added coarse wheat bran, low CP, and their combination as alternatives to replace pharmacological levels of Zn on growth performance and fecal dry matter (DM) during the nursery phase.
MATERIALS AND METHODS
The Kansas State University Institutional Animal Care and Use Committee approved the protocol used in this experiment (Approval number 4035).
Animals, Housing, and Procedures
The study was conducted at the Kansas State Segregated Early Weaning Facility in Manhattan, KS. A total of 300 pigs (Line 400 × 200, DNA, Columbus, NE; initially 4.83 kg and 18.5 d of age) were used in a 46-d growth trial. At weaning, pigs were individually weighed and assigned to pens to achieve balanced weight across treatments. Pens of pigs were randomly allotted to 1 of 6 treatments in a completely randomized design, with 10 replicate pens per treatment and 5 pigs per pen. Gender was balanced within each block. Pigs were housed in two identical buildings with all treatments equally represented in each building. Pens had tri-bar floors and allowed approximately 0.25 m2/pig. Each pen contained a four-hole, dry self-feeder and a cup waterer to provide ad libitum access to feed and water.
Diets were corn and soybean meal-based and were fed in three phases: Phase 1 from d 0 to 7 in pellet form; Phase 2 from d 8 to 25 in meal form; and Phase 3 from d 26 to 46 in meal form (Tables 1 and 2). Phase 1 diets were pelleted under the following parameters: 62°C average conditioning temperature, 72°C average hot pellet temperature, 4.76 × 31.75 mm die (L/D = 6.0), and 23°C ambient temperature. Diets were formulated to meet or exceed NRC (2012) nutrient requirement estimates.
Table 1.
Ingredient composition and nutrient profile of Phase 1 diets fed from d 0 to 7 postweaning (as-fed basis)
| Treatment | ||||||
|---|---|---|---|---|---|---|
| Item | Positive control | Negative control (NC) | NC + Na diformate | NC + wheat bran | NC + 18% CP | NC + Na diformate + wheat bran + 18% CP |
| Ingredient, % | ||||||
| Corn | 44.40 | 44.80 | 43.70 | 41.20 | 52.25 | 47.55 |
| Soybean meal (46.5% CP) | 18.10 | 18.10 | 18.15 | 17.75 | 10.55 | 10.35 |
| Fish meal | 4.50 | 4.50 | 4.50 | 4.50 | 4.50 | 4.50 |
| Dried whey powder | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| Enzymatically treated soybean meal1 | 3.75 | 3.75 | 3.75 | 3.75 | 3.75 | 3.75 |
| Wheat bran (coarse) | — | — | — | 4.00 | — | 4.00 |
| Choice white grease | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Calcium carbonate | 0.25 | 0.25 | 0.25 | 0.30 | 0.25 | 0.30 |
| Monocalcium phosphate | 0.30 | 0.30 | 0.30 | 0.20 | 0.40 | 0.30 |
| Sodium chloride | 0.30 | 0.30 | 0.15 | 0.30 | 0.32 | 0.30 |
| L-lysine-HCl | 0.42 | 0.42 | 0.42 | 0.42 | 0.41 | 0.41 |
| DL-methionine | 0.22 | 0.22 | 0.22 | 0.21 | 0.17 | 0.16 |
| L-threonine | 0.21 | 0.21 | 0.21 | 0.20 | 0.19 | 0.18 |
| L-tryptophan | 0.06 | 0.06 | 0.06 | 0.05 | 0.07 | 0.07 |
| L-valine | 0.11 | 0.11 | 0.11 | 0.10 | 0.12 | 0.12 |
| Vitamin premix2 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Trace mineral premix3 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Phytase4 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| Zinc oxide | 0.40 | — | — | — | — | — |
| Na diformate5 | — | — | 1.20 | — | — | 1.20 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| SID amino acids, % | ||||||
| Lysine | 1.40 | 1.40 | 1.40 | 1.40 | 1.20 | 1.20 |
| Isoleucine: lysine | 56 | 56 | 56 | 56 | 55 | 55 |
| Leucine: lysine | 109 | 110 | 109 | 108 | 113 | 111 |
| Methionine: lysine | 38 | 38 | 38 | 37 | 37 | 36 |
| Methionine and cystine: lysine | 58 | 58 | 58 | 58 | 58 | 58 |
| Threonine: lysine | 65 | 65 | 65 | 65 | 65 | 65 |
| Tryptophan: lysine | 20.3 | 20.3 | 20.3 | 20.1 | 21.0 | 21.3 |
| Valine: lysine | 68 | 68 | 68 | 68 | 70 | 70 |
| Histidine: lysine | 32 | 32 | 32 | 32 | 32 | 32 |
| Total lysine, % | 1.54 | 1.54 | 1.54 | 1.54 | 1.32 | 1.32 |
| ME, kcal/kg | 3,402 | 3,417 | 3,378 | 3,378 | 3,421 | 3,344 |
| NE, kcal/kg | 2,565 | 2,574 | 2,545 | 2,536 | 2,613 | 2,551 |
| SID lysine: NE, g/Mcal | 5.43 | 5.41 | 5.47 | 5.49 | 4.57 | 4.68 |
| Crude protein, % | 21.0 | 21.0 | 20.9 | 21.1 | 18.0 | 18.0 |
| Ca, % | 0.65 | 0.65 | 0.65 | 0.65 | 0.64 | 0.64 |
| P, % | 0.64 | 0.64 | 0.64 | 0.65 | 0.63 | 0.64 |
| Available P, % | 0.55 | 0.55 | 0.55 | 0.54 | 0.57 | 0.55 |
| STTD P, % | 0.56 | 0.56 | 0.56 | 0.56 | 0.56 | 0.56 |
| STTD Ca, % | 0.53 | 0.53 | 0.53 | 0.53 | 0.53 | 0.53 |
| Ca:P | 1.00 | 1.00 | 1.01 | 1.00 | 1.01 | 1.01 |
| STTD Ca:STTD P | 0.95 | 0.95 | 0.95 | 0.95 | 0.93 | 0.94 |
1HP 300, Hamlet Protein, Findlay, OH.
2Provided per kg of premix: 3,527,399 IU vitamin A; 881,850 IU vitamin D; 17,637 IU vitamin E; 1,764 mg vitamin K; 15.4 mg vitamin B12; 33,069 mg niacin; 11,023 mg pantothenic acid; 3,307 mg riboflavin.
3Provided per kg of premix: 73 g Zn from Zn sulfate; 73 g Fe from iron sulfate; 22 g Mn from manganese oxide; 11 g Cu from copper sulfate; 0.2 g I from calcium iodate; 0.2 g Se from sodium selenite.
4Rhonozyme 2700 (DSM Nutritional Products, Inc., Parsippany, NJ). Provided 181.8 phytase units (FYT) per kg of diet, with a release of 0.10% available P.
5FORMI NDF (ADDCON Group GmbH, Bitterfeld-Wolfen, Germany), provided 1.2% Na diformate.
Table 2.
Ingredient composition and nutrient profile of Phase 2 diets fed from d 7 to 25 postweaning (as-fed basis)
| Treatment | ||||||
|---|---|---|---|---|---|---|
| Item | Positive control | Negative control (NC) | NC + Na diformate | NC + wheat bran | NC + 18% CP | NC + Na diformate + wheat bran + 18% CP |
| Ingredient, % | ||||||
| Corn | 56.75 | 57.00 | 55.95 | 53.45 | 64.10 | 59.45 |
| Soybean meal, (46.5% CP) | 29.05 | 29.05 | 29.10 | 28.70 | 21.65 | 21.40 |
| Milk, whey powder | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Wheat bran (coarse) | — | — | — | 4.00 | — | 4.00 |
| Calcium carbonate | 0.92 | 0.92 | 0.92 | 0.95 | 0.92 | 0.97 |
| Monocalcium phosphate | 0.90 | 0.90 | 0.90 | 0.80 | 1.02 | 0.90 |
| Sodium chloride | 0.55 | 0.55 | 0.35 | 0.55 | 0.57 | 0.35 |
| L-lysine-HCl | 0.50 | 0.50 | 0.50 | 0.50 | 0.54 | 0.54 |
| DL-methionine | 0.21 | 0.21 | 0.21 | 0.20 | 0.23 | 0.22 |
| L-threonine | 0.24 | 0.24 | 0.24 | 0.23 | 0.24 | 0.24 |
| L-tryptophan | 0.04 | 0.04 | 0.04 | 0.03 | 0.07 | 0.07 |
| L-valine | 0.10 | 0.10 | 0.10 | 0.10 | 0.16 | 0.16 |
| Vitamin premix1 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Trace mineral premix2 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Phytase3 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| Zinc oxide | 0.26 | --- | --- | --- | --- | --- |
| Na diformate4 | --- | --- | 1.20 | --- | --- | 1.20 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| SID amino acids, % | ||||||
| Lysine | 1.35 | 1.35 | 1.35 | 1.35 | 1.20 | 1.20 |
| Isoleucine: lysine | 55 | 55 | 55 | 55 | 52 | 52 |
| Leucine: lysine | 112 | 112 | 111 | 111 | 111 | 109 |
| Methionine: lysine | 36 | 36 | 36 | 35 | 39 | 38 |
| Methionine and cystine: lysine | 57 | 57 | 57 | 57 | 60 | 60 |
| Threonine: lysine | 65 | 65 | 65 | 65 | 65 | 65 |
| Tryptophan: lysine | 19.1 | 19.1 | 19.1 | 19.0 | 21.2 | 21.0 |
| Valine: lysine | 67 | 67 | 67 | 67 | 70 | 70 |
| Histidine: lysine | 35 | 35 | 35 | 35 | 33 | 33 |
| Total lysine, % | 1.48 | 1.48 | 1.48 | 1.49 | 1.31 | 1.32 |
| ME, kcal/kg | 3,269 | 3,278 | 3,242 | 3,236 | 3,282 | 3,209 |
| NE, kcal/kg | 2,426 | 2,433 | 2,406 | 2,395 | 2,475 | 2,411 |
| SID lysine: NE, g/Mcal | 5.55 | 5.54 | 5.60 | 5.62 | 4.84 | 4.97 |
| Crude protein, % | 20.6 | 20.6 | 20.5 | 20.7 | 17.8 | 17.9 |
| Ca, % | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 |
| P, % | 0.62 | 0.62 | 0.61 | 0.62 | 0.61 | 0.61 |
| Available P, % | 0.47 | 0.47 | 0.47 | 0.46 | 0.49 | 0.47 |
| STTD P, % | 0.51 | 0.51 | 0.51 | 0.51 | 0.51 | 0.51 |
| STTD Ca, % | 0.57 | 0.57 | 0.57 | 0.57 | 0.57 | 0.57 |
| Ca:P | 1.22 | 1.22 | 1.22 | 1.20 | 1.23 | 1.23 |
| STTD Ca: STTD P | 1.13 | 1.13 | 1.13 | 1.12 | 1.11 | 1.13 |
1Provided per kg of premix: 3,527,399 IU vitamin A; 881,850 IU vitamin D; 17,637 IU vitamin E; 1,764 mg vitamin K; 15.4 mg vitamin B12; 33,069 mg niacin; 11,023 mg pantothenic acid; 3,307 mg riboflavin.
2Provided per kg of premix: 73 g Zn from Zn sulfate; 73 g Fe from iron sulfate; 22 g Mn from manganese oxide; 11 g Cu from copper sulfate; 0.2 g I from calcium iodate; 0.2 g Se from sodium selenite.
3Rhonozyme 2700 (DSM Nutritional Products, Inc., Parsippany, NJ). Provided 181.8 phytase units (FYT) per kg of diet, with a release of 0.10% available P.
4FORMI NDF (ADDCON Group GmbH, Bitterfeld-Wolfen, Germany). Provided 1.2% of Na diformate.
Treatments consisted of six different diets: (1) positive control with 3,000 mg/kg Zn from Zn oxide (ZnO) from d 0 to 7 and 2,000 mg/kg Zn from d 7 to 25; (2) negative control without added ZnO (NC); (3) NC with 1.2% Na diformate; (4) NC containing 4% coarse ground wheat bran (946 μm particle size); (5) NC formulated to low CP (18% CP) with high levels of feed grade amino acids; and (6) NC with the combination of Na diformate, 4% coarse ground wheat bran, and 18% CP. The diets formulated to 18% CP contained 1.2% standardized ileal digestible (SID) Lys from d 0 to 25, whereas the 21% CP diets contained 1.4% SID Lys from d 0 to 7 and 1.35% SID Lys from d 7 to 25. The lysine concentration was reduced in the 18% CP diet to provide a total lys:CP ratio of no more than 7.3:1 to ensure adequate nitrogen for the synthesis of nonessential amino acids. All other nutrients were formulated to meet or exceed the pigs’ estimated requirements.
Experimental diets were fed for 25 d (Phase 1 and Phase 2). From d 26 to 46, all pigs were fed the same diet (Phase 3; Table 3). Pigs were weighed, and feed disappearance measured on d 7, 14, 25, 37, and 46 to determine body weight (BW), average daily gain (ADG), average daily feed intake (ADFI), and gain to feed ratio (G:F). All experimental diets were manufactured at the Kansas State University, O.H. Kruse Feed Technology Innovation Center in Manhattan, KS. Diet samples were collected during manufacturing for chemical analysis.
Table 3.
Ingredient composition and nutrient profile of Phase 3 diet fed from d 25 to 46 postweaning (as-fed basis)
| Item | % |
|---|---|
| Ingredient | |
| Corn | 65.60 |
| Soybean meal, (46.5% CP) | 30.20 |
| Calcium carbonate | 1.00 |
| Monocalcium phosphate | 0.95 |
| Sodium chloride | 0.58 |
| L-lysine-HCl | 0.55 |
| DL-methionine | 0.23 |
| L-threonine | 0.25 |
| L-tryptophan | 0.07 |
| L-valine | 0.14 |
| Vitamin premix1 | 0.25 |
| Trace mineral premix2 | 0.15 |
| Phytase3 | 0.07 |
| Total | 100 |
| SID amino acids, % | |
| Lysine | 1.35 |
| Isoleucine: lysine | 54 |
| Leucine: lysine | 112 |
| Methionine: lysine | 37 |
| Methionine and cystine: lysine | 58 |
| Threonine: lysine | 64 |
| Tryptophan: lysine | 20.9 |
| Valine: lysine | 68 |
| Histidine: lysine | 35 |
| Total lysine, % | 1.49 |
| ME, kcal/kg | 3,271 |
| NE, kcal/kg | 2,422 |
| SID lysine: NE, g/Mcal | 5.56 |
| Crude protein, % | 20.8 |
| Ca, % | 0.73 |
| P, % | 0.59 |
| Available P, % | 0.42 |
| STTD P, % | 0.46 |
| STTD Ca, % | 0.55 |
| Ca:P | 1.24 |
| STTD Ca:STTD P | 1.17 |
1Provided per kg of premix: 3,527,399 IU vitamin A; 881,850 IU vitamin D; 17,637 IU vitamin E; 1,764 mg vitamin K; 15.4 mg vitamin B12; 33,069 mg niacin; 11,023 mg pantothenic acid; 3,307 mg riboflavin.
2Provided per kg of premix: 73 g Zn from Zn sulfate; 73 g Fe from iron sulfate; 22 g Mn from manganese oxide; 11 g Cu from copper sulfate; 0.2 g I from calcium iodate; 0.2 g Se from sodium selenite.
3Rhonozyme 2700 (DSM Nutritional Products, Inc., Parsippany, NJ). Provided 181.8 phytase units (FYT) per kg of diet, with a release of 0.10% available P.
All pens were scored daily by the same person to determine fecal consistency. Fecal consistency was based on a numerical scale from 1 to 5, in which 1 represented a hard, dry fecal pellet; 2 represented firmly formed feces; 3 represented soft, moist feces that retained its shape; 4 represented soft, unformed feces that assumed the shape of its container; and 5 represented a watery liquid that could be poured. Fecal samples were collected on d 7, 14, 25, and 37. Samples were collected from 3 randomly selected pigs per pen and pooled for a total of 10 samples per treatment. To determine percentage DM, samples were dried in an oven at 55ºC for 16 h.
Statistical analysis was performed using GLIMMIX procedure of SAS version 9.4 (SAS Institute Inc., Cary, NC). Nursery growth performance and fecal DM data were analyzed as a completely randomized design with pen as the experimental unit using a linear mixed model. Fixed effects in the model included dietary treatment, and barn was included in the model as a random effect. Fecal DM was analyzed as repeated measures over time. Fecal score was analyzed as a split plot in time using a cumulative logit ordinal regression model. Dietary treatment, day of evaluation, and the associated interaction were included in the model as fixed effects. Pen was included within the statistical model as a random intercept, and data were analyzed as a repeated measures over time. Fecal score data are reported as frequency distribution of fecal scores within each fecal score category for each treatment or each day of evaluation. Results were considered significant at P ≤ 0.05 and considered a trend at P ≤ 0.10.
RESULTS
In Phase 1 (d 0 to 7), no effect was observed in ADG, ADFI, and BW between the six treatments (Table 4).
Table 4.
Growth performance of nursery pigs fed diets with different alternatives to replace pharmacological levels of Zn1
| Positive control | Negative control | Na diformate | Coarse wheat bran | 18% CP | Na diformate + wheat bran + 18% CP | SEM | P-value | |
|---|---|---|---|---|---|---|---|---|
| BW, kg | ||||||||
| Weaning | 4.8 | 4.8 | 4.8 | 4.8 | 4.8 | 4.8 | 0.02 | 0.49 |
| d 7 | 5.2 | 5.3 | 4.8 | 5.2 | 5.0 | 5.3 | 0.11 | 0.12 |
| d 25 | 11.7a | 10.0b,c | 10.1b | 10.0b,c | 9.2c | 10.1b | 0.29 | <0.01 |
| d 46 | 24.9a | 22.9b | 22.5bc | 22.8b,c | 20.9c | 23.1a,b,c | 0.70 | <0.01 |
| Phase 1 (d 0 to 7) | ||||||||
| ADG, g | 55 | 63 | 0 | 48 | 35 | 64.3 | 14.8 | 0.21 |
| ADFI, g | 85 | 110 | 103 | 97 | 95 | 109 | 11.4 | 0.29 |
| G/F, g/kg | 557 | 547 | 30.3 | 455 | 347 | 509 | 10.1 | 0.12 |
| Phase 2 (d 7 to 25) | ||||||||
| ADG, g | 358a | 262b | 275b | 269b | 221c | 267b | 14.2 | <0.01 |
| ADFI, g | 457a | 358b,c | 360b,c | 357b,c | 340b | 375c | 16.4 | <0.01 |
| G/F, g/kg | 758a | 705a,b | 734a,b | 714a,b | 626c | 683b | 26.4 | <0.01 |
| Phase 3 (d 25 to 46)2 | ||||||||
| ADG, g | 629 | 608 | 597 | 605 | 561 | 620 | 25.4 | 0.14 |
| ADFI, g | 928a | 886a,b | 864b | 874a,b | 796c | 893a,b | 31.5 | <0.01 |
| G/F, g/kg | 679 | 686 | 694 | 693 | 697 | 697 | 18.1 | 0.90 |
| Overall (d 0 to 46) | ||||||||
| ADG, g | 437a | 389b | 379b,c | 383b,c | 341c | 397a,b | 15.8 | <0.01 |
| ADFI, g | 626a | 566b | 526b,c | 553b,c | 505c | 578a,b | 21.0 | <0.01 |
| G/F, g/kg | 700 | 687 | 695 | 692 | 672 | 688 | 13.3 | 0.40 |
a,b,cDifferent superscripts within a row indicate evidence for difference (P < 0.05) in average daily gain (ADG), average daily feed intake (ADFI), gain-to-feed ratio (G:F), body weight (BW). CP = crude protein.
1A total of 300 weaned pigs (Line 241 × 600, DNA Genetics, Columbus, NE) with an initial BW of 4.83 kg and 18.5 d of age were used. Pigs were allotted to pens in a completely randomized design with 5 pigs per pen and 10 replicates per treatment.
2Common period in which all pigs fed the same diet without feed additives from d 25 to d 46.
In Phase 2 (day 7 to 25), pigs fed the diet containing pharmacological Zn had greater (P < 0.01) ADG and ADFI than pigs fed any other treatment. Pigs fed the combination diet had greater ADG and ADFI than those fed the 18% CP diet and the same ADG and ADFI of those fed the negative control, 1.2% Na diformate, and 4% coarse ground wheat bran intermediate. Pigs fed 18% CP had the poorest (P < 0.05) G:F compared with pigs fed other diets. Pigs fed the combination diet had decreased (P < 0.05) G:F than those fed the positive control, with other treatments intermediate.
During Phase 3 (d 25 to 46), no difference (P > 0.10) was observed for ADG and G:F. Pigs previously fed added Zn had greater (P < 0.01) ADFI than those previously fed the 18% CP diet followed by those previously fed 1.2% Na diformate, with the others intermediate.
Overall (d 0 to 46), pigs fed diets with pharmacological Zn had greater (P < 0.01) ADG, ADFI, and final BW than those fed the negative control, 1.2% Na diformate, 4% coarse ground wheat bran, or 18% CP diets. However, there was no difference in ADG, ADFI, and final BW between pigs fed added Zn or the combination treatment. No difference was observed for overall G:F.
For fecal DM, no differences were observed between treatments on d 7 after weaning (Table 5). On d 14, pigs fed negative control had lower (P < 0.01) fecal DM content than all other treatments. Pigs fed diets with Na diformate, coarse wheat bran, or 18% CP had lower (P < 0.05) fecal DM than the positive control with ZnO; however, there were no differences between pigs fed added Zn and those fed the combination diet. On d 25, pigs fed negative control diet had lower (P < 0.01) fecal DM content than all other treatments. No differences were observed between pigs fed the added Zn diet, 1.2% Na diformate, 18% CP, and the combination diet. The 4% coarse ground wheat bran treatment was intermediate. On d 37, the response followed the same pattern as observed on d 25; however, pigs fed negative control and 4% coarse ground wheat bran had lower fecal DM content compared with those previously fed added Zn. For the overall mean of the four collection periods, pigs fed the negative control diet had lower (P < 0.010) fecal DM than all other treatments. Pigs fed diets containing Na diformate, coarse wheat bran, or 18% CP diet had lower (P < 0.010) fecal DM content than those fed added Zn. No differences were observed between pigs fed added Zn and those fed the combination diet.
Table 5.
Effect of different alternatives to replace pharmacological Zn in nursery diets on fecal dry matter, %1,2,3
| Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|
| Day of collection | Positive control | Negative control | Na diformate | Coarse wheat bran | 18% CP | Na diformate + wheat bran + 18% CP | SEM | P-value |
| d 7 | 22.01 | 19.03 | 21.35 | 21.46 | 20.26 | 24.37 | 2.668 | 0.15 |
| d 14 | 25.50a | 19.11c | 22.17b | 22.80b | 23.14b | 24.12a,b | 0.791 | <0.01 |
| d 25 | 24.73a | 20.91c | 23.11a,b | 21.87b | 22.97a,b | 23.39a,b | 0.709 | <0.01 |
| d 372 | 25.83a | 21.91c | 23.94a,b | 22.73b,c | 24.79a | 24.97a | 0.698 | <0.01 |
| Mean | 24.52a | 20.24c | 22.64b | 22.22b | 22.79b | 24.21a,b | 0.771 | <0.01 |
a,b,cDifferent superscripts within a row indicate evidence for difference (P < 0.05) in fecal DM.
1A total of 300 weaned pigs (Line 241 × 600, DNA Genetics, Columbus, NE) with an initial BW of 4.83 kg and 18.5 d of age were used. Pigs were allotted to pens in a completely randomized design with 5 pigs per pen and 10 replicates per treatment.
2Common period in which all pigs fed the same diet without feed additives from d 25 to d 46.
3Three pigs per pen were randomly selected and sampled. The fecal samples were collected from the same pigs and dried.
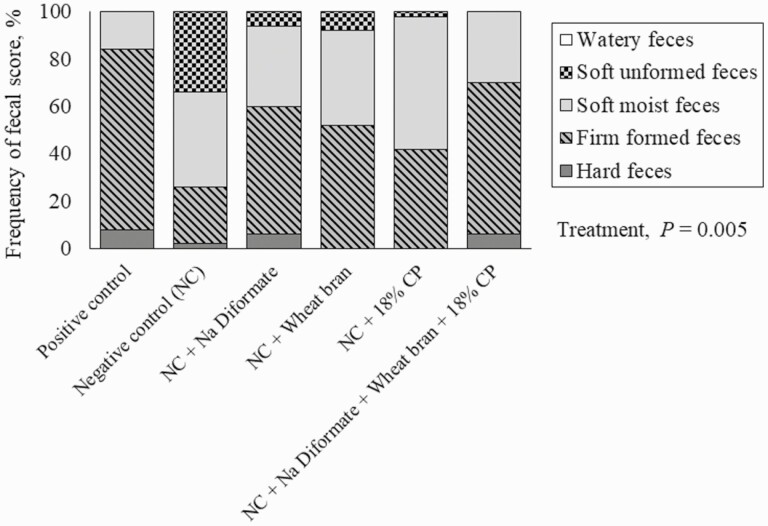
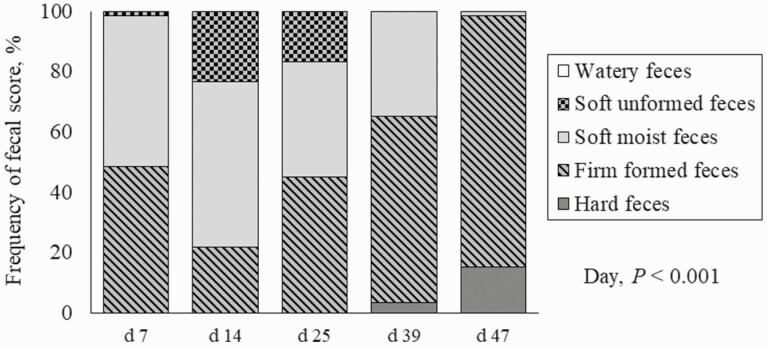
For fecal score, there was no evidence of a dietary treatment × day interaction (P = 0.543). However, there was evidence of main effects for both treatment and day. Pigs fed the negative control had softer feces (P = 0.005) than all other treatments (Figure 1). From d 7 to 25, pigs had higher frequency distribution (P < 0.001) of soft unformed and soft moist feces compared with d 37 and 46 (Figure 2).
Figure 1.
Frequency distribution of fecal score in nursery pigs per treatment (d 0 to 42).
Figure 2.
Frequency distribution of fecal score in nursery pigs per day.
DISCUSSION
Regulatory agencies have been pressured to eliminate the use of pharmacological levels of Zn in weanling pig diets (European Medicines Agency, 2016). The environmental concerns have led to a ban on pharmacological levels of added Zn in starter diets in Europe. Because of the possibility for a ban on the use of pharmacological additions of Zn in the United States, the purpose of this study was to evaluate nutrition alternatives to pharmacological levels of Zn in nursery pig diets.
The present data support previous studies that demonstrated that pharmacological doses of Zn, provided by Zn oxide, increase growth performance in weanling pigs (Carlson et al., 1999; Hill et al., 2001). It has been suggested that Zn promotes growth of weanling pigs by controlling pathogenic bacterial scours (Huang et al., 1999; Katouli et al., 1999). Additionally, studies indicated that Zn promotes growth in weaned pigs regardless of diarrhea incidence or effect on the intestinal microbial count (Li et al., 2001). In the current study, pigs fed added Zn had improved growth performance and a reduction in the incidence of diarrhea, which corroborates those previous findings and more recent observations as well (Ren et al., 2020, Wei et al., 2020).
Acidifiers have been commonly used in diets for weanling pigs. Kil et al. (2011) proposed that acidifiers might improve growth performance due to a reduction in pH in the stomach and lower GIT, a modulation of microbial populations, and improvements in nutrient digestion. Efficient protein digestion requires the maintenance of a low gastric pH (Kil et al., 2011), and previous studies have shown that formic acid supplementation improves protein digestibility in weaned pigs (Roth and Kirchgessner, 1998). We hypothesized that the use of an acidifier may improve protein digestibility which might have offset the low dietary lysine level used.
Organic acids have also received much attention as alternatives to replace antibiotic growth promoters (Øverland et al., 2000). Formic acid has shown to be effective against pathogenic bacteria (Gedek et al., 1992; Kirchgessner et al., 1992) and usually Na or Ca formate and K diformate are used. Some studies have observed that formic acids are effective in promoting growth of weanling pigs (Paulicks et al., 1996; Luise et al., 2017). However, the magnitude and consistency of the responses of the acidifiers are variable depending on the nature of the acids, inclusion rate, and other dietary factors (Jacela et al., 2009). Grecco et al. (2018) suggested that the benefits of using an acidifier usually are less noticeable when pigs are not exposed to a disease challenge status. Therefore, we speculate that the lack of improvement in growth performance with added Na diformate alone may be due to the relatively good health status of pigs in the current study.
The reason for the addition of 4% coarse ground wheat bran as an alternative to pharmacological levels of Zn was based on supposed improvements in gut health when feeding coarse wheat bran (Mikkelsen et al., 2004; Canibe et al., 2005). The inclusion of wheat bran in the diet has been observed to stimulate lactic and butyric acid production in the small and large intestines (Bikker et al., 2006, Carneiro et al., 2007) and reduce coliform bacteria counts in the small intestine (Bikker et al., 2006). In the absence of pharmacological Zn for early weaned pigs, the use of wheat bran increased intestinal fermentation (Molist et al., 2010). Coarse ground wheat bran also has been shown to reduce E. coli attachment in the small intestine, presumably by increasing passage rate (Molist et al., 2010). However, in the present study, the inclusion of 4% coarse ground wheat bran failed to improve performance relative to pigs fed the added Zn diet. The use of different cereals or fiber in nursery diets is still equivocal (Hermes et al., 2010). The level and type of fiber in diet may affect diet palatability (Solà-Oriol et al., 2009), feed intake (Mateos et al., 2006), and gut maturation (Montagne et al., 2003).
A low-CP amino-acid-fortified diet might alleviate postweaning diarrhea (Nyachoti et al., 2006). Heo et al. (2010) observed that pigs fed low protein diets had decreased nitrogen entering the large intestine which resulted in less nitrogen fermentation and higher fecal DM scores. That was the reason a low CP diet was considered as a potential alternative to pharmacological levels of added Zn in the present study. Le Bellego and Noblet (2002) observed that reducing CP in postweaning diets to 16.9% with correct amino acid supplementation may reduce diarrhea, without affecting weight gain and protein deposition. However, in the present study, the low CP (18%) diet used resulted in reduced nursery performance. This is likely because in addition to decreasing CP, SID lysine was also decreased to maintain a maximum lysine:CP ratio. Studies have observed that up to a 4-percentage unit reduction in CP, with the addition of essential amino acids, can be achieved without negatively affecting growth performance (Le Bellego and Noblet, 2002). It might be speculated that in the present study, the amount of L-lysine HCl and other feed grade amino acids were already high in the 21% CP diet and already near the maximum lysine:CP ratio of 7.3:1. Therefore, a reduction in CP of 3 percentage units, maintaining the 7.3:1 lysine:CP ratio, was enough to reduce growth performance.
Interestingly, the combination of Zn alternatives (Na diformate, wheat bran, and 18% CP) resulted in similar overall growth performance, fecal DM content, and fecal score as those fed added Zn. Although some alternatives for pharmacological Zn in nursery diets have been widely evaluated, few studies have used the strategy of combined alternatives and their impact on growth performance and fecal characteristics. From our results, we speculate that the combination of the different components of each alternative may have promoted absorption of nutrients and improved fecal DM and consequently growth performance.
Gut health of pigs can be clinically assessed by evaluating fecal consistency and appearance (Pedersen et al., 2011). It has been used by many researchers to quantify the severity of diarrhea (Adewole et al., 2016). Diarrhea has been defined as an increased frequency of defecation accompanied by feces that contains an increased amount of water and decreased DM (Radostits et al., 2000). In the present study, diets without pharmacological Zn (NC and alternatives) had high fecal scores and low fecal DM. These results agree with previous studies that demonstrate the importance of pharmacological Zn in diets for weaned pigs to reduce the incidence of diarrhea (Ou et al., 2007; Wang et al., 2009). Similar to overall growth performance, the combination of reduced CP, diet acidification, and use of coarse wheat bran allowed fecal DM to be similar to pigs fed pharmacological Zn.
In the field of Zn replacements, several feed alternatives, combined or not, may be potentially used in pig diets. The main challenges are the inconsistency and the wide range of responses obtained. The efficiency of each alternative depends on factors such as the stage of growth, diet complexity, ingredient type and inclusion level, and health status of pigs (Liu et al., 2018).
In conclusion, the present study suggests that diets without pharmacological levels of Zn reduce nursery performance, and lowering dietary CP exacerbates the reduction in growth, which is a result of the reduction in dietary SID lysine content and intake. The use of a combination of the three alternatives (1.2% Na diformate, 4% coarse ground wheat bran, and 18% CP) partially improved the appearance of feces and resulted in similar fecal DM content and overall growth performance compared with pigs fed diets containing pharmacological levels of added Zn. Therefore, studies are required to identify and further refine combinations of alternatives and strategies that sustain growth performance and fecal DM when high levels of ZnO are removed from nursery pig diets.
ACKNOWLEDGMENTS
We wish to thank ADDCON Group GMBH for providing the Na diformate used in this study and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Brazil, for the financial support to the first author. Contribution no. 21-211-J of the Kansas Agricultural Experiment Station, Manhattan, KS.
Conflict of interest statement. The authors declare no conflict of interest.
LITERATURE CITED
- Adewole, D. I., Kim I. H., and Nyachoti C. M.. . 2016. Gut health of pigs: challenge models and response criteria with a critical analysis of the effectiveness of selected feed additives—a review. Asian Austral. J. Anim. 29(7):909–924. doi: 10.5713/ajas.15.0795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikker, P., Dirkzwager A., Fledderus J., Trevisi P., le Huërou-Luron I., Lallès J. P., and Awati A.. . 2006. The effect of dietary protein and fermentable carbohydrates levels on growth performance and intestinal characteristics in newly weaned piglets. J. Anim. Sci. 84:3337–3345. doi: 10.2527/jas.2006-076 [DOI] [PubMed] [Google Scholar]
- Canibe, N., Højberg O., Højsgaard S., and Jensen B. B.. . 2005. Feed physical form and formic acid addition to the feed affect the gastrointestinal ecology and growth performance of growing pigs. J. Anim. Sci. 83:1287–1302. doi: 10.2527/2005.8361287x [DOI] [PubMed] [Google Scholar]
- Carlson, M. S., Hill G. M., and Link J. E.. . 1999. Early- and traditionally weaned nursery pigs benefit from phase-feeding pharmacological concentrations of zinc oxide: effect on metallothionein and mineral concentrations. J. Anim. Sci. 77(5):1199–1207. doi: 10.2527/1999.7751199x [DOI] [PubMed] [Google Scholar]
- Carneiro, M., Lordelo M., Cunha L. F., and Freire J.. . 2007. Microbial activity in the gut of piglets: II. Effect of fibre source and enzyme supplementation. Livest. Sci. 108(1–3):262–265. doi: 10.1016/j.livsci.2007.01.069 [DOI] [Google Scholar]
- Case, C. L., and Carlson M. S.. . 2002. Effect of feeding organic and inorganic sources of additional zinc on growth performance and zinc balance in nursery pigs. J. Anim. Sci. 80:1917–1924. doi: 10.2527/2002.8071917x [DOI] [PubMed] [Google Scholar]
- Chaney, R. L. 1993. Zinc phytotoxicity. In: Robson, A.D., editor. Zinc in soils and plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; p. 135−150. [Google Scholar]
- European Medicines Agency. 2016. European Medicines Agency—news and events—Committee for Medicinal Products for Veterinary Use (CVMP Meeting 6–8 December 2016). Available from https://www.ema.europa.eu/en/news/committee-medicinal-products-veterinary-use-cvmp-meeting-6-8-december-2016.
- Gedek, B., Kirchgessner M., Eidelburger U., Wiehler S., Bott A., and Roth F. X.. . 1992. Zum Einfluß von Ameisensa¨ure auf die Keimzahlen der Mikroflora und deren Zusammensetzung in verschiedenen Segmenten des Gastrointestinaltraktes. 5 Mitteilung zur nutritiven Wirksamkeit von organischen Sa¨uren in der Ferkelaufzucht. J. Anim. Physiol. Anim. Nutr. 67:206–214. [Google Scholar]
- Grecco, H. A. T., Amorim A. B., Saleh M. A. D., L. P. Tse M., Telles F. G., Miassi G. M., Pimenta G. M., and Berto D. A.. . 2018. Evaluation of growth performance and gastro-intestinal parameters on the response of weaned piglets to dietary organic acids. An. Acad. Bras. Cienc. 90:401–414. doi: 10.1590/0001-3765201820160057 [DOI] [PubMed] [Google Scholar]
- Hampson, D. J. 1986. Alterations in piglet small intestinal structure at weaning. Res. Vet. Sci. 40:32–40. [PubMed] [Google Scholar]
- Heo, J. M., Kim J. C., Hansen C. F., Mullan B. P., Hampson D. J., Maribo H., and Pluske J. R.. . 2010. Effects of dietary protein level and zinc oxide supplementation on the incidence of post-weaning diarrhoea in weaner pigs challenged with an enterotoxigenic strain of Escherichia coli. Livest. Sci. 133(1–3):210–213. doi: 10.1016/j.livsci.2010.06.066 [DOI] [Google Scholar]
- Hermes, R. G., Molist F., Ywazaki M., de Segura A. G., Gasa J., Torrallardona D., and Pérez J. F.. . 2010. Effects of type of cereal and fibre level on growth and parameters of the gastrointestinal tract in young pigs. Livest. Sci. 133(1–3):225–228. doi: 10.1016/j.livsci.2010.06.071 [DOI] [Google Scholar]
- Hill, G. M., Mahan D. C., Carter S. D., Cromwell G. L., Ewan R. C., Harrold R. L., Lewis A. J., Miller P. S., Shurson G. C., and Veum T. L.. . 2001. Effect of pharmacological concentrations of zinc oxide with or without the inclusion of an antibacterial agent on nursery pig performance. J. Anim. Sci. 79:934–941. doi: 10.2527/2001.794934x [DOI] [PubMed] [Google Scholar]
- Huang, S. X., McFall M., Cegielski A. C., and Kirkwood R. N.. . 1999. Effect of dietary zinc supplementation on Escherichia coli septicemia in weaned pigs. J. Swine Health Prod. 7:109–111. [Google Scholar]
- Jacela, J. Y., DeRouchey J. M., Tokach M. D., Goodband R. D., Nelssen J. L., Renter D. G., and Dritz S. S.. . 2009. Feed additives for swine: fact sheets—acidifiers and antibiotics. J. Swine Health Prod. 17(5):270–275. [Google Scholar]
- Katouli, M., Melin L., Jensen-Waern M., Wallgren P., and Möllby R.. . 1999. The effect of zinc oxide supplementation on the stability of the intestinal flora with special reference to composition of coliforms in weaned pigs. J. Appl. Microbiol. 87:564–573. doi: 10.1046/j.1365-2672.1999.00853.x. [DOI] [PubMed] [Google Scholar]
- Kil, D. Y., Kwon W. B., and Kim B. G.. . 2011. Dietary acidifiers in weanling pig diets: a review. Rev. Colom. Cienc. Pecua. 24(3):231–247. [Google Scholar]
- Kirchgessner, M., Gedek B., Wiehler S., Eidelburger U., and Roth F. X.. . 1992. Zum Einfluß von Ameisensa¨ure, Calcium formiat und Natrium-hydrogencarbonat auf die Keimzahlen der Mikroflora und deren Zusammensetzung in verschiedenen Segmenten des Gastrointestinaltraktes. J. Anim. Physiol. Anim. Nutr. 67:73–81. [Google Scholar]
- Le Bellego, L., and Noblet J.. . 2002. Performance and utilization of dietary energy and amino acids in piglets fed low protein diets. Livest. Prod. Sci. 76(1–2):45–58. doi: 10.1016/S0301-6226(02)00008-8 [DOI] [Google Scholar]
- Li, B. T., Van Kessel A. G., Caine W. R., Huang S. X., and Kirkwood R. N.. . 2001. Small intestinal morphology and bacterial populations in ileal digesta and feces of newly weaned pigs receiving a high dietary level of zinc oxide. Can. J. Anim. Sci. 81:511–516. doi: 10.4141/A01-043 [DOI] [Google Scholar]
- Liu, Y., Espinosa C. D., Abelilla J. J., Casas G. A., Lagos L. V., Lee S. A., Kwon W. B., Mathai J. K., Navarro D. M. D. L., Jaworski N. W., . et al. 2018. Non-antibiotic feed additives in diets for pigs: a review. Anim. Nutr. 4:113–125. doi: 10.1016/j.aninu.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luise, D., Motta V., Salvarani C., Chiappelli M., Fusco L., Bertocchi M., Mazzoni M., Maiorano G., Costa L. N., Milgen J. V., . et al. 2017. Long-term administration of formic acid to weaners: influence on intestinal microbiota, immunity parameters and growth performance. Anim. Feed Sci. Technol. 232:160–168. doi: 10.1016/j.anifeedsci.2017.06.015 [DOI] [Google Scholar]
- Mateos, G. G., Martin F., Latorre M. A., Vicente B., and Lazaro R.. . 2006. Inclusion of oat hulls in diets for young pigs based on cooked maize or cooked rice. Anim. Sci. 82(1):57–63. doi: 10.1079/ASC20053 [DOI] [Google Scholar]
- Mikkelsen, L. L., Naughton P. J., Hedemann M. S., and Jensen B. B.. . 2004. Effects of physical properties of feed on microbial ecology and survival of Salmonella enterica serovar Typhimurium in the pig gastrointestinal tract. Appl. Environ. Microbiol. 70:3485–3492. doi: 10.1128/AEM.70.6.3485-3492.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molist, F., de Segura A. G., Pérez J. F., Bhandari S. K., Krause D. O., and Nyachoti C. M.. . 2010. Effect of wheat bran on the health and performance of weaned pigs challenged with Escherichia coli K88+. Livest. Sci. 133(1–3):214–217. doi: 10.1016/j.livsci.2010.06.067 [DOI] [Google Scholar]
- Montagne, L., Pluske J. R., and Hampson D. J.. . 2003. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol. 108(1–4):95–117. doi: 10.1016/S0377-8401(03)00163-9 [DOI] [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th ed. Washington (DC): National Academies Press. [Google Scholar]
- Nyachoti, C. M., Omogbenigun F. O., Rademacher M., and Blank G.. . 2006. Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets. J. Anim. Sci. 84:125–134. doi: 10.2527/2006.841125x [DOI] [PubMed] [Google Scholar]
- Ou, D. Y., Li D. F., Ca Y. H., Li X. L., Yin J. D., Qiao S. Y., and Wu G. Y.. . 2007. Dietary supplementation with zinc oxide decreases expression of the stem cell factor in the small intestine of weanling pigs. J. Nutr. Biochem. 18:820–826. doi: 10.1016/j.jnutbio.2006.12.022 [DOI] [PubMed] [Google Scholar]
- Overland, M., Granli T., Kjos N. P., Fjetland O., Steien S. H., and Stokstad M.. . 2000. Effect of dietary formates on growth performance, carcass traits, sensory quality, intestinal microflora, and stomach alterations in growing-finishing pigs. J. Anim. Sci. 78:1875–1884. doi: 10.2527/2000.7871875x. [DOI] [PubMed] [Google Scholar]
- Paulicks, B. R., Roth F. X., and Kirchgessner M.. . 1996. Dose effects of potassium diformate (Formi™ LHS) on the performance of growing piglets. Agribiol. Res. 49:318–326. [Google Scholar]
- Pedersen, K. S., Stege H., and Nielsen J. P.. . 2011. Evaluation of a microwave method for dry matter determination in faecal samples from weaned pigs with or without clinical diarrhoea. Prev. Vet. Med. 100:163–170. doi: 10.1016/j.prevetmed.2011.04.014 [DOI] [PubMed] [Google Scholar]
- Pettigrew, J. E. 2006. Reduced use of antibiotic growth promoters in diets fed to weanling pigs: dietary tools. Anim. Biotechnol. 17(1):207–215. doi: 10.1080/10495390600956946 [DOI] [PubMed] [Google Scholar]
- Poulsen, H. D. 1995. Zinc oxide for weanling piglets. Acta Agric. Scand. A Anim. 45(3):159–167. doi: 10.1080/09064709509415847 [DOI] [Google Scholar]
- Radostits, O. M., Gay C. C., Blood D. C., and Hinchcliff K. W.. . 2000. Diseases of the alimentary tract−1. Veterinary medicine. 9th ed. London: WB Saunders; p. 173. [Google Scholar]
- Ren, P., Chen J., Wedekind K., Hancock D., and Vázquez-Añón M.. . 2020. Interactive effects of zinc and copper sources and phytase on growth performance, mineral digestibility, bone mineral concentrations, oxidative status, and gut morphology in nursery pigs. Transl. Anim. Sci. 4:txaa083. doi: 10.1093/tas/txaa083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, F. X., and Kirchgessner M.. . 1998. Organic acids as feed additives for young pigs: nutritional and gastrointestinal effects. J. Anim. Feed Sci. 7(1):25−33. doi: 10.22358/jafs/69953/1998 [DOI] [Google Scholar]
- Sargeant, H. R., McDowall K. J., Miller H. M., and Shaw M. A.. . 2010. Dietary zinc oxide affects the expression of genes associated with inflammation: transcriptome analysis in piglets challenged with ETEC K88. Vet. Immunol. Immunopathol. 137:120–129. doi: 10.1016/j.vetimm.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Slifierz, M. J., Friendship R., and Weese J. S.. . 2015. Zinc oxide therapy increases prevalence and persistence of methicillin-resistant Staphylococcus aureus in pigs: a randomized controlled trial. Zoonoses Public Health 62(4):301–308. 10.1111/zph.12150 [DOI] [PubMed] [Google Scholar]
- Solà-Oriol, D., Roura E., and Torrallardona D.. . 2009. Feed preference in pigs: effect of cereal sources at different inclusion rates. J. Anim. Sci. 87:562–570. doi: 10.2527/jas.2008-0949 [DOI] [PubMed] [Google Scholar]
- Starke, I. C., Pieper R., Neumann K., Zentek J., and Vahjen W.. . 2014. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS Microbiol. Ecol. 87:416–427. doi: 10.1111/1574-6941.12233 [DOI] [PubMed] [Google Scholar]
- Stein, H. H. 2006. Reduced use of antibiotic growth promoters in diets fed to weanling pigs: dietary tools. Anim. Biotechnol. 17(2):217–231. doi: 10.1080/10495390600956946 [DOI] [PubMed] [Google Scholar]
- Wang, J. J., Chen L. X., Li P., Li X. L., Zhou H. J., Wang F. L., Li D. F., Yin Y. L., and Wu G. Y.. . 2009. Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J. Nutr. 138:1025–1032. doi: 10.1093/jn/138.6.1025 [DOI] [PubMed] [Google Scholar]
- Wei, X., Tsai T., Knapp J., Bottoms K., Deng F., Story R., Maxwell C., and Zhao J.. . 2020. ZnO modulates swine gut microbiota and improves growth performance of nursery pigs when combined with peptide cocktail. Microorganisms 8:146. doi: 10.3390/microorganisms8020146 [DOI] [PMC free article] [PubMed] [Google Scholar]