Abstract
Objective
Research has linked early adverse childhood experiences (ACEs) with asthma development; however, existing studies have generally relied on parent report of exposure and outcome. We aimed to examine the association of early life ACEs with empirically determined trajectories of childhood asthma risk, using independent register information on both exposures and outcome.
Methods
Based on nationwide registries, we established a study cohort of 466 556 children born in Denmark (1997–2004). We obtained information on ACEs during the first 2 years of life (bereavement, parental chronic somatic and/or mental illness) and childhood asthma diagnosis or medication use from birth through age 10 years from the Danish National Patient and Prescription Registries, respectively. We identified asthma phenotypes using group-based trajectory modelling. We then used multinomial logistic regression to examine the association between early ACEs and asthma phenotypes.
Results
We identified four asthma phenotypes: non-asthmatic, early-onset transient, early-onset persistent and late-onset asthma. Girls with early-onset transient asthma (OR 1.13, 95% CI 1.04 to 1.24), early-onset persistent asthma (1.27, 95% CI 1.08 to 1.48) or late-onset asthma (OR 1.28, 95% CI 1.11 to 1.48) vs no asthma were more likely to have early life ACE exposure compared with girls without ACE exposure. Results were similar for boys who also had experienced early life ACEs with ORs of 1.16 (95% CI 1.08 to 1.25), 1.34 (95% CI 1.20 to 1.51) and 1.11 (95% CI 0.98 to 1.25), respectively.
Conclusion
In a nationwide-population study, we identified three childhood onset asthma phenotypes and found that ACEs early in life were associated with increased odds for each of these asthma phenotypes among both girls and boys.
Keywords: asthma epidemiology, asthma, paediatric asthma
Key messages.
What is the key question?
What are the age-dependent asthma phenotypes in children, and are adverse childhood experiences early in life associated with increased risk of these asthma phenotypes?
What is the bottom line?
Using group-based trajectory modelling, we identified four asthma phenotypes (non-asthmatic, early-onset transient, early-onset persistent and late-onset asthma), and found that adverse childhood experiences early in life were associated with increased odds of all three childhood asthma onset phenotypes.
Why read on?
This is the first nationwide population-based study to characterise early childhood asthma trajectories. This study, furthermore, adds knowledge to the field of adverse childhood experiences and childhood asthma, using independent register data of both exposures and outcomes.
Introduction
Asthma is the most common chronic disease in childhood and is associated with reduced quality of life and substantial direct and indirect societal costs.1–3 Despite much research on the aetiology and risk factors, the development and progression of asthma is not well understood.4 Asthma is an umbrella term for multiple distinct pathophysiological mechanisms that vary in their clinical expression of disease. Given this heterogeneity, inconsistencies between previous studies investigating the incidence and aetiology of asthma are to be expected.5
Findings reported in the current literature indicate that adverse childhood experiences (ACEs) influence mental and physical health later in life.6–8 Experience of severe ACEs in childhood (eg, abuse, bereavement or neglect) has been linked with elevated rates of morbidity and mortality.9–14 Although the biological mechanisms linking ACEs with health outcomes later in life are undoubtedly multifaceted, recent research suggests dysregulation of the immune system may be one key underlying biological pathway.15 16 A few studies have examined the influence of ACEs on later development of asthma.13 Their findings indicate strong associations, including significant dose–response relationships, but the majority of the studies suffer from methodological limitations, such as parental report of ACEs and asthma outcomes in their children, small sample sizes, cross-sectional designs and low response rates.13 14 We addressed a number of these limitations by leveraging a nationwide cohort to examine whether the experience of ACEs during early life (0–2 years of age) is associated with asthma among children followed up to the age of 10 years. We used a data-driven approach to examine trajectories of asthma phenotypes using Danish nationwide data in relation to ACEs. We hypothesised that exposure to ACEs would increase the risk of asthma. Prior research also suggests that both dose and type of ACE affect the association with asthma risk.13 Previous studies have also identified sex-differential effects, suggesting that girls may be more vulnerable to maternal stress postnatal, whereas boys may be more vulnerable to maternal stress during pregnancy.17 18 Given these previous findings, we also investigated dose dependency of ACE exposure relative to development of asthma and sex-specific associations, with the hypothesis that girls would be more vulnerable to ACEs than boys.
Material and methods
Study subjects: study design
We used nationwide register data of live-born singletons in Denmark, born from 1 January 1997 to 31 December 2004, and their parents and siblings (based on records of the mother), obtained from the Danish National Medical Birth Registry.19 A total of 466 556 children were included in this register based cohort study after exclusion of children with missing or extreme gestational ages (<154 or >315 days), children that could not be linked to their fathers, or children that emigrated or died before their 10th birthday (figure 1). Baseline characteristics of the non-included population are available in online supplemental table S1. We excluded children with missing data on gestational age or extreme gestational age because of the high risk of misclassifications as well as errors, obviously a result of typing errors in these administrative data, within these specific records of pregnancies. Baseline characteristics of this specific non-included population are available in online supplemental table S2. The large number of missing in this specific population supports a lower data quality. The children were followed from birth to ten years of age.
Figure 1.
Flow chart illustrating the identification of the study population.
thoraxjnl-2020-214528supp001.pdf (232.4KB, pdf)
thoraxjnl-2020-214528supp002.pdf (233.3KB, pdf)
We linked the cohort to multiple nationwide administrative registers by the unique social security number assigned to all citizens in Denmark at birth.20 Data from the Danish Civil Registration System, which contains information about sex, date of birth, identity of the parents, and emigration,21 was linked with:
The Danish National Patient Register, which includes information on all hospital admissions (non-psychiatric) since 1977, and since 1995 also psychiatric inpatients, emergency department and outpatient contacts.22 For each patient contact since 1994, physician-assigned diagnoses are recorded according to the International Classification of Diseases, 10th revision (ICD-10).
The Danish National Prescription Registry,23 which includes information on all claimed prescriptions since 1995 from Danish pharmacies classified according to the Anatomical Therapeutic Chemical (ATC) classifications system.24
The Danish National Medical Birth Registry, which includes information pertaining to obstetrical outcomes and health of the new born.19
The Population Education Register,25 which includes educational level based on the International Standard Classification of Education (ISCED-2011).26
The Danish Register of Causes of Death, which records all deaths of citizens in Denmark according to cause of death based on ICD-10 codes.27
ACEs early in life
We defined three categories of ACEs occurring in the first 2 years of life: (1) bereavement, that is, the death of a parent or sibling,28 identified through the Danish Register of Causes of Death, (2) parental chronic somatic illnesses29 based on the Charlson Comorbidity Index30 but excluding diagnoses of asthma (ICD-10 codes: J45 and J46), using data from the National Patient Register and (3) parental mental illnesses29 defined as any psychiatric diagnosis (ICD-10: F00–F99) as well as suicide attempts (ICD-10: X60-84 and Y87.0), obtained from the Danish National Patient Register. Multiple objective factors were chosen, as ‘stress’ is undoubtedly multifaceted. However, it has been speculated whether positive or negative events impact health later in life to a similar degree.5 Therefore, childhood events were operationalised as events that could be assumed to be negative with reasonable confidence.
Childhood asthma
Childhood asthma (age 0–10 years) was defined based on a diagnosis of asthma (ICD-10: J45) or status asthmaticus (ICD-10: J46), obtained from the Danish National Patient Register, or claims of at least two prescriptions of any antiasthmatic medication within 1 year31 obtained from the Danish National Prescription Register. The ATC codes for inhaled asthma drugs were: inhaled b2-agonists (R03AC02-04, R03AC12 and R03AC13), inhaled glucocorticoids (R03BA01, R03BA02 and R03BA05), fixed-dose combination of inhaled b2-agonists and glucocorticoids (R03AK06 and R03AK07) and leukotriene receptor antagonists (R03DC03).23 24 The registers contain administrative data, and therefore the number of data points varies between individuals. We dichotomised the outcome into asthma/no-asthma yearly for each of the ten years, and each child, therefore, had 10 data points collected during the study period.
Statistical approach
We used Group-based Trajectory Modelling (GBTM)32 to identify clusters of children following similar progression for asthma development between birth and 10 years of age using the SAS PROC TRAJ macro.33 The response variable used in estimation of the trajectories was yearly asthma status (yes/no). When fitting trajectory models, we iteratively changed the number of groups (3–6 group models) and shape of the trajectories and evaluated the Bayesian information criterion (BIC)34 across models to select the model with the best fit (negative BIC closest to 0). We also ensured that distinct groups could be identified, as indicated by a p<0.05. Based on previous findings,17 35 we a priori stratified the sample by sex of the child before fitting trajectories. We also examined the posterior probability of trajectory assignment to evaluate the quality of the classification further. We identified potential confounders based on prior literature and the use of Directed Acyclic Graphs (see online supplemental figure S1). The confounders included were: maternal age at delivery (<25/25–34/≥35 years), parental highest educational level at delivery (vocational education or below (ISCED level 0–3) or higher education (ISCED level 4–8)), maternal smoking during pregnancy (no/yes), maternal place of living at delivery (urban/rural), parity (nulliparous/multiparous), calendar year of birth (1997–1999/2000–2002/2003–2004) and season of birth (spring/summer/fall/winter).
thoraxjnl-2020-214528supp003.pdf (404KB, pdf)
We used multinomial logistic regression to examine sex-specific associations between ACEs and asthma phenotypes (childhood onset phenotypes vs never/infrequent asthma). We considered ACE scores in three ways: (1) any ACE vs none, (2) cumulative score ranging from 0 to 3 in which each category of ACE with at least one event was summed (ie, 0=no ACE in any category and 3=at least one ACE in each of the bereavement, somatic illness and mental illness categories) and (3) separate ACE-specific models (any bereavement vs none, any somatic illness vs none, any mental illness vs none).
In all multinomial models, we treated the non-asthmatic (NA) trajectory as the reference group. Statistical tests for interaction further justified our hypotheses by revealing p<0.0001 of sex.
To account for missing covariate data, we performed multiple imputations by chained equations using the fully conditional method using SAS PROC MIANALYZE. All statistical analyses were performed using SAS V.14.1 (SAS Institute).
Results
A total of 3.8% and 3.9% of the girls and boys, respectively, were exposed to at least one ACE during the first 2 years of their life. ACE-exposed children were slightly more likely to have either a younger (<25 years) or older (≥35 years) mother compared with non-exposed children (table 1). Furthermore, exposed children were more likely to have lower educated parents, be first-born, and their mothers were more likely to smoke during pregnancy. No differences were observed for maternal place of living at delivery, or season of birth.
Table 1.
Baseline characteristics of girls and boys by exposed and non-exposed to ACEs early in life
| Characteristic | Girls | Boys | ||||||
| Exposed (N=8673) | Non-exposed (N=218 483) | Total (N=227 156) | P value* | Exposed (N=9257) | Non-exposed (N=230 143) | Total (N=239 400) | P value* | |
| Maternal age at delivery (years) | ||||||||
| <25 | 1624 (18.7) | 30 994 (14.2) | 32 618 (14.4) | 1771 (19.1) | 32 820 (14.3) | 34 591 (14.4) | ||
| 25–34 | 5575 (64.3) | 154 868 (70.9) | 160 443 (70.6) | 5831 (63.0) | 162 856 (70.8) | 168 687 (70.5) | ||
| ≥35 | 1474 (17.0) | 32 621 (14.9) | 34 095 (15.0) | <0.0001 | 1655 (17.9) | 34 467 (15.0) | 36 122 (15.1) | <0.0001 |
| Parents highest educational level at conception | ||||||||
| Vocational education or below | 5797 (66.8) | 126 051 (57.7) | 131 848 (58.0) | 6150 (66.4) | 132 562 (57.6) | 138 712 (57.9) | ||
| Higher education | 2834 (32.7) | 91 925 (42.1) | 94 759 (41.7) | 3060 (33.1) | 97 016 (42.2) | 100 076 (41.8) | ||
| Missing | 42 (0.5) | 507 (0.2) | 549 (0.2) | <0.0001 | 47 (0.5) | 565 (0.2) | 612 (0.3) | <0.0001 |
| Maternal smoking during pregnancy | ||||||||
| No | 4991 (57.5) | 142 825 (65.4) | 147 816 (65.1) | 5482 (59.2) | 150 299 (65.3) | 155 781 (65.1) | ||
| Yes | 2315 (26.7) | 37 719 (17.3) | 40 034 (17.6) | 2356 (25.5) | 39 582 (17.2) | 41 938 (17.5) | ||
| Missing | 1367 (15.8) | 37 939 (17.4) | 39 306 (17.3) | <0.0001 | 1419 (15.3) | 40 262 (17.5) | 41 681 (17.4) | <0.0001 |
| Maternal place of living at delivery | ||||||||
| Rural | 7678 (88.5) | 194 375 (89.0) | 202 053 (88.9) | 8201 (88.6) | 204 485 (88.9) | 212 686 (88.8) | ||
| Urban | 981 (11.3) | 23 895 (10.9) | 24 876 (11.0) | 1047 (11.3) | 25 406 (11.0) | 26 453 (11.0) | ||
| Missing | 14 (0.2) | 213 (0.1) | 227 (0.1) | 0.2647 | 9 (0.1) | 252 (0.1) | 261 (0.1) | 0.4169 |
| Parity | ||||||||
| First | 3475 (40.1) | 93 633 (42.9) | 97 108 (42.7) | 3776 (40.8) | 98 459 (42.8) | 102 235 (42.7) | ||
| ≥2 | 5154 (59.4) | 124 116 (56.8) | 129 270 (56.9) | 5445 (58.8) | 130 850 (56.9) | 136 295 (56.9) | ||
| Missing | 44 (0.5) | 734 (0.3) | 778 (0.3) | <0.0001 | 36 (0.4) | 834 (0.4) | 870 (0.4) | 0.0002 |
| Calendar year of birth | ||||||||
| 1997–1999 | 2772 (32.0) | 81 939 (37.5) | 84 711 (37.3) | 2922 (31.6) | 86 465 (37.6) | 89 387 (37.3) | ||
| 2000–2002 | 3556 (41.0) | 82 300 (37.7) | 85 856 (37.8) | 3861 (41.7) | 86 580 (37.6) | 90 441 (37.8) | ||
| 2003–2004 | 2345 (27.0) | 54 244 (24.8) | 56 589 (24.9) | <0.0001 | 2474 (26.7) | 57 098 (24.8) | 59 572 (24.9) | <0.0001 |
| Season of birth | ||||||||
| Winter | 2138 (24.7) | 56 040 (25.6) | 58 178 (25.6) | 2350 (25.4) | 58 511 (25.4) | 60 861 (25.4) | ||
| Spring | 2301 (26.5) | 58 129 (26.6) | 60 430 (26.6) | 2375 (25.7) | 61 390 (26.7) | 63 765 (26.6) | ||
| Summer | 2116 (24.4) | 53 727 (24.6) | 55 843 (24.6) | 2315 (25.0) | 56 780 (24.7) | 59 095 (24.7) | ||
| Fall | 2118 (24.4) | 50 587 (23.2) | 52 705 (23.2) | 0.0272 | 2217 (23.9) | 53 562 (23.3) | 55 779 (23.3) | 0.1186 |
| Offspring asthma trajectories | ||||||||
| No asthma | 7778 (89.7) | 199 857 (91.5) | 207 635 (91.4) | 7788 (84.1) | 199 479 (86.7) | 207 267 (86.6) | ||
| Early-onset transient asthma | 532 (6.1) | 11 208 (5.1) | 11 740 (5.2) | 871 (9.4) | 17 937 (7.8) | 18 808 (7.9) | ||
| Early-onset persistent asthma | 165 (1.9) | 3346 (1.5) | 3511 (1.5) | 320 (3.5) | 6140 (2.7) | 6460 (2.7) | ||
| Late-onset asthma | 198 (2.3) | 4072 (1.9) | 4270.0 (1.9) | <0.0001 | 278 (3.0) | 6587 (2.9) | 6865 (2.9) | <0.0001 |
*The p values are computed using a χ2 distribution.
ACE, adverse childhood experiences.
While we identified that a five-group model had a slightly better fit as determined by BIC values compared with a four-group model, the difference was minor, thus we chose to use the four-group model (figure 2) to be consistent with earlier studies and improve comparability.5 36 Patterns were similar in girls and boys, although with slightly different proportions of children assigned to each trajectory. The four groups can be characterised as: (1) NA (girls=91%, boys=87%), 2) early-onset transient asthma (EOTA) (girls=5%, boys=8%), early-onset persistent asthma (EOPA) (girls=2%, boys=3%) and late-onset asthma (LOA) (girls=2%, boys=3%). The average posterior probability of group membership was 0.98 for girls and 0.97 for boys, indicating excellent classification of trajectory. Children assigned to the EOTA trajectory are characterised by an early-life asthma diagnosis or treatment around the age of 2 years, resolving by approximately 6 years of age. Odds of asthma among children assigned to the EOPA trajectory increases until approximately 4 years of age and persists throughout the remainder of the study period. In children assigned to the LOA trajectory, odds of asthma increases between four and 5 years of age and continues to increase in a consistent manner until approximately 9 years of age.
Figure 2.
Phenotypes of asthma identified by group-based trajectories models using register data from a nationwide cohort of children born in Denmark between 1997 and 2004, by sex (girls, n=227 156, boys, n=239 400). Shaded area portray CIs of the trajectories.
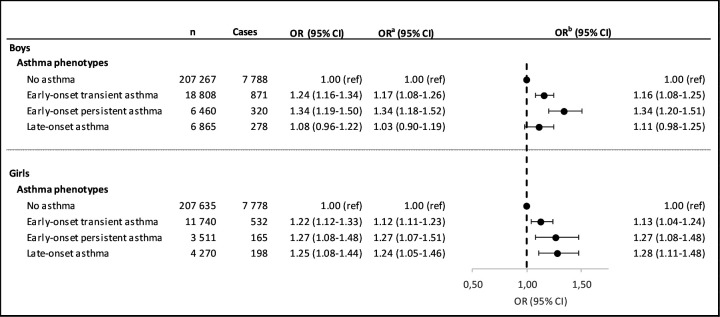
Adjusted analyses showed that any vs no ACE exposure increased the odds of EOTA for both girls (OR 1.13, 95% CI 1.04 to 1.24) and boys (OR 1.16, 95% CI 1.08 to 1.25) and EOPA for both girls (OR 1.27, 95% CI 1.08 to 1.48) and boys (OR 1.34, 95% CI 1.20 to 1.51). Girls with any ACE also had higher odds of being assigned to the LOA group (OR 1.28, 95% CI 1.11 to 1.48). The direction of this association was similar in boys (OR 1.11, 95% CI 0.98 to 1.25) (figure 3).
Figure 3.
Results from sex-stratified multinomial logistic regression analyses between ACEs in early life (0/≥1) and asthma phenotypes. The population includes children born in Denmark between 1997 and 2004. aAdjusted for maternal age at delivery (years), parental highest educational level at conception, maternal smoking during pregnancy, maternal place of living at delivery, parity, calendar year of birth and season of birth. bIncludes adjustment and imputation by chained equations using a fully conditional method. ACEs, adverse childhood experiences.
Subgroup analyses
Analysis of the early life ACE cumulative score in relation to asthma phenotypes did not indicate a dose–response association (table 2).
Table 2.
Results from sex-separated multinomial logistic regression analyses between cumulative ACEs in early life (0 vs 1, and 0 vs >1) and asthma phenotypes
| Asthma phenotypes | Girls, n=227 156 | Boys, n=239 400 | ||||
| n | Cases | OR* (95% CI) | n | Cases | OR* (95% CI) | |
| Children who had experienced 1 vs 0 ACE | ||||||
| No asthma | 207 635 | 7413 | 1 | 207 267 | 7430 | 1 |
| Early-onset transient asthma | 11 740 | 508 | 1.12 (1.01 to 1.24) | 18 808 | 832 | 1.17 (1.08 to 1.27) |
| Early-onset persistent asthma | 3511 | 155 | 1.25 (1.05 to 1.50) | 6460 | 306 | 1.34 (1.18 to 1.52) |
| Late-onset asthma | 4270 | 190 | 1.24 (1.05 to 1.47) | 6865 | 267 | 1.04 (0.90 to 1.20) |
| Children who had experienced >1 vs 0 ACE | ||||||
| No asthma | 207 635 | 365 | 1 | 207 267 | 358 | 1 |
| Early-onset transient asthma | 11 740 | 24 | 1.08 (0.69 to 1.69) | 18 808 | 39 | 1.15 (0.81 to 1.64) |
| Early-onset persistent asthma | 3511 | 10 | 1.77 (0.91 to 3.44) | 6460 | 14 | 1.40 (0.80 to 2.44) |
| Late-onset asthma | 4270 | 8 | 1.21 (0.57 to 2.56) | 6865 | 11 | 0.96 (0.49 to 1.86) |
The response variable is the asthma phenotypes, and no asthma is the reference. The population studied comprised of children born in Denmark between 1997 and 2004.
*Adjusted for maternal age at delivery(years), parents highest educational level at conception, maternal smoking during pregnancy, maternal place of living at delivery, parity & calendar year of birth and season of birth.
ACE, adverse childhood experiences.
Subgroup analysis of each ACE category in relation to asthma phenotypes (table 3) support the results of any ACE exposure.
Table 3.
Results from sex-separated multinomial logistic regression analyses between specific early-life ACEs (bereavement/parental chronic diseases/parental mental illness or suicide attempt), each examined in a separate model and asthma phenotypes
| Asthma phenotypes | n | Cases | OR* (95% CI) | n | Cases | OR* (95% CI) |
| Children exposed to bereavement (yes vs no) | ||||||
| No asthma | 207 635 | 417 | 1 | 207 267 | 456 | 1 |
| Early-onset transient asthma | 11 740 | 25 | 1.121 (1.01 to 1.23) | 18 808 | 40 | 1.17 (1.08 to 1.26) |
| Early-onset persistent asthma | 3511 | 7 | 1.27 (1.07 to 1.51) | 6460 | 15 | 1.34 (1.18 to 1.52) |
| Late-onset asthma | 4270 | 9 | 1.24 (1.05 to 1.46) | 6865 | 14 | 1.03 (0.90 to 1.20) |
| Children exposed to parental chronic (yes vs no) diseases | ||||||
| No asthma | 207 635 | 3391 | 1 | 207 267 | 3995 | 1 |
| Early-onset transient asthma | 11 740 | 269 | 1.14 (0.99 to 1.31) | 18 808 | 442 | 1.22 (1.09 to 1.36) |
| Early-onset persistent asthma | 3511 | 88 | 1.37 (1.09 to 1.73) | 6460 | 176 | 1.38 (1.17 to 1.64) |
| Late-onset asthma | 4270 | 108 | 1.32 (1.06 to 1.65) | 6865 | 147 | 1.04 (0.86 to 1.26) |
| Children exposed to parental mental illness or suicide attempt (yes vs no) | ||||||
| No asthma | 207 635 | 3601 | 1 | 207 267 | 3564 | 1 |
| Early-onset transient asthma | 11 740 | 255 | 1.09 (0.95 to 1.25) | 18 808 | 412 | 1.14 (1.02 to 1.27) |
| Early-onset persistent asthma | 3511 | 76 | 1.1920 (0.93 to 1.534) | 6460 | 138 | 1.32 (1.09 to 1.57) |
| Late-onset asthma | 4270 | 86 | 1.15 (0.901 to 1.46) | 6865 | 123 | 1.02 (0.83 to 1.25) |
The response variable is the asthma phenotypes, no asthma is the reference. The population studied comprised of children born in Denmark between 1997 and 2004.
*Adjusted for maternal age atdelivery (years), parents' highest educational level at conception, maternal smoking during pregnancy, maternal place of livingat delivery, parity & calendar year of birth and season of birth.
ACEs, adverse childhood experiences.
Discussion
In this nationwide cohort study of 466 556 children followed from birth to the age of 10 years, we identified four trajectories reflecting no/infrequent asthma and three asthma phenotypes among children. Notably, the phenotypes were similar for girls and boys, despite sex differences in the overall asthma prevalence. ACEs early in life were associated with an increased risk of asthma, which did not differ by the number or specific types of ACE. In general, no sex differences were observed in the associations between ACEs and asthma.
We used GBTM to distinguish empirically between trajectories of asthma, which is advantageous as it does not require the definition of asthma phenotypes by a predefined age of onset. To our knowledge, this is the first study based on a nationwide population to identify asthma phenotypes by sex. The average of the posterior probability of group membership was high for both girls and boys (close to 1), indicating that the trajectories were well defined and that misclassification of outcome was minimal. One published study identified similar trajectories in a population-based Canadian cohort with combined sex,5 and similar approaches have been used to identify wheezing phenotypes, which demonstrated comparable trajectories to ours.36
The three asthma phenotypes identified by GBTM were similar for girls and boys, though with slightly different prevalence of asthma between sexes. Specifically, we found that more boys developed asthma before the age of 10 years compared with girls. This is in line with existing knowledge; for example, in the USA, the percentage of current asthma in boys <18 years is 9.2% vs 7.2% in girls.37 Our results showed more pronounced associations among girls for LOA consistent with earlier studies.17 18
Our data did not confirm a dose–response relationship as suggested by previous studies.38 This might be due to a lack of power in our subgroup analyses, as only few children experienced more than one ACE. Our study used independent measures of exposures, outcomes and covariates. Furthermore, we included only events that most children would be assumed to perceive as adverse. While this provides a design that diminishes reporting bias, we were unable to capture the subjective experience of ACEs.
Each specific category of ACE was associated with roughly a similar increase in risk of childhood asthma. A systematic review provides evidence that the impact of ACE on the development of asthma may be age-dependent.13 For example, a recent publication reported that postnatal maternal depression was associated with higher odds of asthma at the age of 6–8 years (an age span included in our study), but not after 9 years of age.39
Methodological considerations
This study is based on a nationwide population, which minimises selection bias. Only 8% of the overall population was excluded. We did not see any large difference between the included and the excluded population with regard to the exposure (included girls 3.8% and boys 3.9%; excluded girls 3.9% and boys 4%), nor the outcome (included girls 8.6% and boys 13.5%; excluded girls 9.8% and boys 14.8%) (please see online supplemental table S1 for more details). We adjusted for a wide range of baseline characteristics but did not include potential confounding factors such as air pollution levels, or other environmental exposures (eg, parental occupational exposure implying a risk of exposing the children via a carrier home-effect) that might influence the associations.13 38 However, adjustment for various possible confounders, including maternal place of living at the birth of the child (urban/rural) as well as the highest educational level of the parents, did not substantially alter the associations. However, we cannot rule out residual confounding. In addition, a limitation of the register-based studies is that we lacked information on some important confounders such as body mass index and environmental factors that could be related to both ACEs and asthma risk. Furthermore, some misclassification of both exposure and outcome cannot be ruled out.
ACEs early in life were obtained through registry data of death and morbidity, reflecting illness severe enough for the parents to seek professional help. This definition of ACEs most likely increases the specificity of ACEs, though it might also decrease in sensitivity, which is a limitation for register-based data. Ascertainment of asthma was based on a diagnosis of asthma or at least two claimed prescriptions of anti-asthmatic drugs. While healthcare services in Denmark are free of charge, prescription medicine is not, and any interpersonal differences in healthcare seeking behaviour, for example, due to economic restraint, might lead to misclassification of asthma phenotypes and likely result in estimates that are more conservative.
We measured ACEs between birth and 2 years of age. It is, therefore, possible that children assigned to the early-onset asthma trajectory experienced ACEs after asthma onset (reverse causation); however, it is unlikely that the included ACEs would occur because the child developed asthma. The mechanisms by which early life adverse events impact on asthma still needs further investigation.
Conclusion
In this large nationwide cohort study based on register data, three childhood asthma onset phenotypes were identified, and each of these was more common in children experiencing adverse experiences early in life. Our results do not suggest marked differences in relation to child sex or differences with regard to the type or dose of ACEs.
Footnotes
Contributors: KP, WC, CSS, NWA, CS, HAK, XL, KSH, RJW and VS: Conception and design of the analysis, interpretation of data and drafting the work. All authors contributed to the manuscript drafts revising for important intellectual content and have read and approved the manuscript.
Funding: KP received a 1-year PhD scholarship by Aarhus University (560.000 Danish kroner) and travel grants from Oticon (grant no. 18–0681 & 18–2812 & 18–0681) and Knud Højgaards Fund (Grant no. 18-02-2016 & 19-03-0406). VS received a grant for the study from The Danish Working Environment Research Fund (grant no. 17–2015–09/20150067134). Characterisation of asthma phenotypes/trajectories was supported through a US National Institutes of Environmental Health Centre grant (P30ES023515). During the preparation of this manuscript, WC was supported by the US National Institutes of Health (T32HD049311). XL was supported by the Danish Council for Independent Research (DFF-5053-00156B) and is supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 891079.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data cannot be made freely available as they are subject to regulations at Statistic Denmark and EU General Data Protection Regulation, however, data can be made available on request for authorised research units.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the Danish Data Protection Agency. Informed consent is not required for a register-based study based on encrypted data according to the legislation in Denmark.
References
- 1. Asher I, Pearce N. Global burden of asthma among children. int j tuberc lung dis 2014;18:1269–78. 10.5588/ijtld.14.0170 [DOI] [PubMed] [Google Scholar]
- 2. Global Initiative for Asthma . (*NEW) 2017 GINA report: global strategy for asthma management and prevention, 2017. Available: http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/ [Accessed 5 Feb 2018].
- 3. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: Executive summary of the GINA dissemination Committee report. Allergy 2004;59:469–78. 10.1111/j.1398-9995.2004.00526.x [DOI] [PubMed] [Google Scholar]
- 4. Campbell DE, Mehr S, Sam M. Fifty years of allergy: 1965-2015. J Paediatr Child Health 2015;51:91–3. 10.1111/jpc.12806 [DOI] [PubMed] [Google Scholar]
- 5. Sbihi H, Koehoorn M, Tamburic L, et al. Asthma trajectories in a population-based birth cohort. impacts of air pollution and Greenness. Am J Respir Crit Care Med 2017;195:607–13. 10.1164/rccm.201601-0164OC [DOI] [PubMed] [Google Scholar]
- 6. Burke NJ, Hellman JL, Scott BG, et al. The impact of adverse childhood experiences on an urban pediatric population. Child Abuse Negl 2011;35:408–13. 10.1016/j.chiabu.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun 2013;27:8–12. 10.1016/j.bbi.2012.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steptoe A, Marteau T, Fonagy P, et al. ACEs: evidence, gaps, evaluation and future priorities. Social Policy and Society 2019;18:415–24. 10.1017/S1474746419000149 [DOI] [Google Scholar]
- 9. Danese A, Caspi A, Williams B, et al. Biological embedding of stress through inflammation processes in childhood. Mol Psychiatry 2011;16:244–6. 10.1038/mp.2010.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Danese A, Moffitt TE, Pariante CM, et al. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry 2008;65:409–15. 10.1001/archpsyc.65.4.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull 2011;137:959–97. 10.1037/a0024768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pace TWW, Mletzko TC, Alagbe O, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry 2006;163:1630–3. 10.1176/ajp.2006.163.9.1630 [DOI] [PubMed] [Google Scholar]
- 13. Exley D, Norman A, Hyland M. Adverse childhood experience and asthma onset: a systematic review. Eur Respir Rev 2015;24:299–305. 10.1183/16000617.00004114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wing R, Gjelsvik A, Nocera M, et al. Association between adverse childhood experiences in the home and pediatric asthma. Ann Allergy Asthma Immunol 2015;114:379–84. 10.1016/j.anai.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 15. Beijers R, Buitelaar JK, de Weerth C. Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes: beyond the HPA axis. Eur Child Adolesc Psychiatry 2014;23:943–56. 10.1007/s00787-014-0566-3 [DOI] [PubMed] [Google Scholar]
- 16. Wright RJ. Stress-Related programming of autonomic imbalance: role in allergy and asthma. Chem Immunol Allergy 2012;98:32–47. 10.1159/000336496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee A, Mathilda Chiu Y-H, Rosa MJ, et al. Prenatal and postnatal stress and asthma in children: Temporal- and sex-specific associations. J Allergy Clin Immunol 2016;138:740–7. 10.1016/j.jaci.2016.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosa MJ, Just AC, Tamayo Y Ortiz M, et al. Prenatal and postnatal stress and wheeze in Mexican children: sex-specific differences. Ann Allergy Asthma Immunol 2016;116:306–12. 10.1016/j.anai.2015.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knudsen LB, Olsen J. The Danish medical birth registry. Dan Med Bull 1998;45:320–3. [PubMed] [Google Scholar]
- 20. Thygesen LC, Daasnes C, Thaulow I, et al. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health 2011;39:12–16. 10.1177/1403494811399956 [DOI] [PubMed] [Google Scholar]
- 21. Pedersen CB. The Danish civil registration system. Scand J Public Health 2011;39:22–5. 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 22. Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health 2011;39:30–3. 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 23. Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health 2011;39:38–41. 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 24. WHO Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC classification and DDD assignment 2013. Oslo: WHO Collaborating Centre for Drug Statistics Methodology, 2012. https://www.whocc.no/atc_ddd_index/ [Google Scholar]
- 25. UNESCO UNES and CO, UNESCO Institute for Statistics . International standard classification of education: ISCED 2011. Monteral: UNESCO Institute for Statistics, 2012. http://www.uis.unesco.org/Education/Documents/isced-2011-en.pdf [Google Scholar]
- 26. Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health 2011;39:91–4. 10.1177/1403494810394715 [DOI] [PubMed] [Google Scholar]
- 27. Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health 2011;39:26–9. 10.1177/1403494811399958 [DOI] [PubMed] [Google Scholar]
- 28. Li J, Olsen J, Vestergaard M, et al. Prenatal stress exposure related to maternal bereavement and risk of childhood overweight. PLoS One 2010;5:e11896. 10.1371/journal.pone.0011896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bergink V, Larsen JT, Hillegers MHJ, et al. Childhood adverse life events and parental psychopathology as risk factors for bipolar disorder. Transl Psychiatry 2016;6:e929. 10.1038/tp.2016.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 31. Liu X, Madsen KP, Sejbaek CS, et al. Risk of childhood asthma following prenatal exposure to negative life events and job stressors: a nationwide register-based study in Denmark. Scand J Work Environ Health 2019;45:174–82. 10.5271/sjweh.3785 [DOI] [PubMed] [Google Scholar]
- 32. Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res 2007;35:542–71. 10.1177/0049124106292364 [DOI] [Google Scholar]
- 33. Jones BL, Nagin DS, ROEDER K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res 2001;29:374–93. 10.1177/0049124101029003005 [DOI] [Google Scholar]
- 34. Nagin DS, Odgers CL, Modeling G-BT. Group-Based trajectory modeling (nearly) two decades later. J Quant Criminol 2010;26:445–53. 10.1007/s10940-010-9113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brunst KJ, Rosa MJ, Jara C, et al. Impact of maternal lifetime interpersonal trauma on children's asthma: mediation through maternal active asthma during pregnancy. Psychosom Med 2017;79:91–100. 10.1097/PSY.0000000000000354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen Q, Just AC, Miller RL, et al. Using latent class growth analysis to identify childhood wheeze phenotypes in an urban birth cohort. Annals of Allergy, Asthma & Immunology 2012;108:311–5. 10.1016/j.anai.2012.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. CDC . Most recent national asthma data, 2019. Available: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm [Accessed 08 Nov 2019].
- 38. Rosa MJ, Lee AG, Wright RJ. Evidence establishing a link between prenatal and early-life stress and asthma development. Curr Opin Allergy Clin Immunol 2018;18:148–58. 10.1097/ACI.0000000000000421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kozyrskyj AL, Letourneau NL, Kang LJ, et al. Associations between postpartum depressive symptoms and childhood asthma diminish with child age. Clin Exp Allergy 2017;47:324–30. 10.1111/cea.12837 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2020-214528supp001.pdf (232.4KB, pdf)
thoraxjnl-2020-214528supp002.pdf (233.3KB, pdf)
thoraxjnl-2020-214528supp003.pdf (404KB, pdf)
Data Availability Statement
Data cannot be made freely available as they are subject to regulations at Statistic Denmark and EU General Data Protection Regulation, however, data can be made available on request for authorised research units.