Abstract
Intestinal resident macrophages are at the front line of host defence at the mucosal barrier within the gastrointestinal tract and have long been known to play a crucial role in the response to food antigens and bacteria that are able to penetrate the mucosal barrier. However, recent advances in single-cell RNA sequencing technology have revealed that resident macrophages throughout the gut are functionally specialised to carry out specific roles in the niche they occupy, leading to an unprecedented understanding of the heterogeneity and potential biological functions of these cells. This review aims to integrate these novel findings with long-standing knowledge, to provide an updated overview on our understanding of macrophage function in the gastrointestinal tract and to speculate on the role of specialised subsets in the context of homoeostasis and disease.
Keywords: neural-immune interactions, macrophages, immunology, neuropathy
Key messages.
The intestinal macrophage pool is a heterogeneous mix of diversified and functionally specialised macrophage subsets.
Specialised intestinal macrophage subsets appear to receive cues from surrounding cells, a concept otherwise known as macrophage niche.
Neuron-associated macrophages are crucial for the survival and homoeostasis of enteric neurons that control gastrointestinal motility and intestinal secretion.
Blood vessel-associated macrophages ensure blood vessel integrity and regulate blood vessel permeability.
Specialised macrophage subsets are located in association with intestinal crypts and Peyer’s patches.
Introduction
The first studies on macrophages revealed the potent ability of these cells to scavenge and phagocytose, and they were rapidly identified as cells crucial to host defence and tissue homoeostasis. As macrophage populations were progressively discovered in most tissues, these cells became known as ‘housekeepers’, cells whose purpose is to clear apoptotic cells and defend the host from pathogen invasion. In the intestine, the most characterised macrophage population resides in the lamina propria, interspersed within the villi, in prime position to engulf and clear pathogens and dietary antigens that breach the intestinal barrier. The constant exposure to the content of the lumen and the low-grade inflammation generated by exposure to commensal microbes, promotes a rapid turnover of lamina propria macrophages, which require continuous replenishment by monocytes circulating in the bloodstream. While in other organs macrophages were shown to derive mainly, if not exclusively, from self-maintaining macrophages that colonise the embryonic tissue, similar studies in the intestine revealed that macrophages seeded embryonically had been replaced by incoming monocytes at around 3 weeks of age, and that the macrophage pool within the intestine relies solely on replenishment by circulating monocytes.1 For many years, this dogma held true, and intestinal macrophages were frequently cited as being the ‘ontological exception’, as the only tissue-resident macrophages deriving exclusively from circulating monocytes. Using fate-mapping techniques, we and others demonstrated that the intestine also harbours self-maintaining macrophages that reside in specific anatomical locations within the intestinal wall. These macrophages interact closely with surrounding cells and carry out specific functions, suggesting that they receive cues from the surrounding environment, a concept otherwise known as the macrophage ‘niche’. Here, we will discuss the recent novel insights in the role of these specialised macrophage populations and speculate how these subsets may be implicated in gastrointestinal disease.
Origin of intestinal resident macrophages
Since their first description by Nobel Prize laureate Elie Metchnikoff in 1878, subsequent research uncovered that tissue resident macrophages throughout different organs have similar morphological and functional characteristics, suggesting that they share a common progenitor. This led to the introduction of the mononuclear phagocyte system (MPS) in the 1970s, which postulated that all tissue resident macrophages derive from monocytes circulating in the bloodstream migrating into peripheral tissue where they undergo further differentiation.2 3 However, the advent of fate-mapping technologies and parabiosis experiments have largely refined this hypothesis. It is now clear that most tissue resident macrophage populations originate largely, if not exclusively, from precursors seeded embryonically and are able to self-maintain within the tissue.4–6 Resident macrophages can only be replaced by circulating haematopoietic monocytes under specific conditions, such as depletion or disappearance over time. Thus, macrophage pools in different tissues constitute a heterogeneous population with varying contributions of macrophages of embryonic and haematopoietic origin (figure 1). Macrophages within the central nervous system represent one extreme of this ontological spectrum, as they are almost exclusively derived from embryonic progenitors that give rise to an adult macrophage pool that self-maintains throughout adulthood without contribution of circulating monocytes, with exception of choroid plexus macrophages.7 In other organs, such as skin, heart, pancreas and the liver, embryonic precursors and circulating monocytes both give rise to the resident macrophage pool, although the contribution of monocytes tends to increase over time.8–12
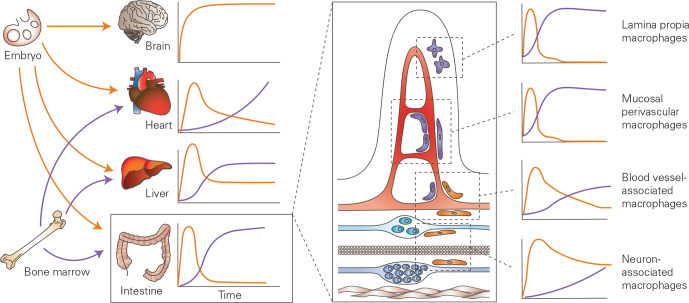
Figure 1.
Ontogeny of tissue resident macrophages. Most organs contain a heterogeneous mix of bone marrow derived macrophages and macrophages deriving from embryonic progenitors; however, in the brain, the resident macrophage population (microglia) derives solely from the proliferation of progenitors seeded in the embryo. Of note, the two first embryonic waves are represented together in this figure, for simplicity. Within the intestine, the large majority of macrophages derive from the bone marrow, however, a small population of macrophages derives from embryonic progenitors and is able to self-maintain in adulthood. These self-maintaining macrophage populations colonise specific niches in close proximity to the vasculature in the submucosa and enteric neurons. Other macrophage populations, located in the villi and surrounding the mucosal vascular network, derive entirely from precursors that originate in the bone marrow. Adapted from Ginhoux and Guilliams, 2016.13
Embryonic precursors of macrophages are seeded before birth and derive from either yolk-sac erythro-myeloid progenitors (EMPs) or fetal-liver precursors. In mice, embryonic hematopoiesis occurs in three sequential waves and begins at E7.0 (7th day of embryonic life) with the primitive wave in the yolk sac, which gives rise to erythroblasts, megakaryocytes and macrophages.13–16 Of note, microglia are the only macrophage population known to derive directly from primitive myeloid progenitors that arise in the yolk sac before E8.0.17 18 Once the blood circulation is established at E8.5, a second wave of EMPs migrates from the yolk sac to the fetal liver, where EMP-derived fetal monocytes will give rise to tissue-resident macrophages.19 From E10.5, the third and final wave starts, giving rise to fetal hematopoietic stem cells for lifelong haematopoiesis, which eventually seed the bone marrow at E17.5.20 However, the exact contribution of each wave of embryonic precursors to the different mature macrophage pools in the adult is subject to intense debate; as yet, not all transcription factors involved have been identified. Within the intestinal lamina propria, initial studies revealed that, although the intestine is seeded by embryonic precursors from E8.5 onwards, these cells do not persist to adulthood and they are replaced by incoming bone marrow-derived monocytes around the time of weaning.1 Recently, however, using murine fate-mapping models, we and others were able to show that in the gut, as in other organs, embryonic progenitors give rise to a subset of macrophages that are able to self-maintain and persist into adulthood.21 22 These long-lived, self-maintaining macrophages occupy well-defined niches within the gut wall, notably the submucosal and myenteric plexus, intestinal crypts and the vasculature (figure 1).
In humans, there is also mounting evidence that points towards a similar mixed ontogeny for tissue resident macrophages. Studies in sex-mismatched organ transplantation enable the assessment of longevity/self-maintenance of donor-derived tissue-resident macrophages and their replenishment by circulating monocytes of the recipient. In some organs, such as the derma, donor-derived tissue resident macrophages are rapidly replaced by circulating monocytes of the host, whereas in other organs, such as the lung and heart, a population of donor-derived tissue resident macrophages persists through local proliferation and is not replenished.23–25 A similar transplantation study in the intestine by Bujko and colleagues elegantly showed that macrophage subsets are replaced at differing rates by circulating cells, suggesting that a similar ontological heterogeneity exists also within the human intestine.26 These studies suggest that in human tissues, mature resident macrophage pools can be replenished by circulating cells, but that there is likely also a population of self-maintaining resident macrophages. Further evidence for self-maintaining resident macrophages derives from several reports of patients with congenital monocytopenia in which the number of tissue-resident macrophages in various organs was not affected.27 28 These findings support the hypothesis that also in humans, resident macrophages are able to develop and maintain in the absence of monocytes, likely through self-maintenance. In line with murine findings, this study revealed that these long-lived macrophage subsets tend to occupy specific niches and may be functionally distinct from their incoming counterparts.
The macrophage niche
Niche requirements
Although macrophages have been studied for over a century, advances in RNA sequencing and single-cell RNA sequencing have only recently revealed highly specialised macrophage populations, insight that has led to the introduction of the concept of macrophage niche. In recent years, studies have uncovered populations such as immunoregulatory neuron-associated macrophages in the lung, collagen-degrading Lyve1+ macrophages associated with blood vessels, lipid-associated macrophages regulating adipocyte size and metabolic homoeostasis in adipose tissue, and many more.29–31 These macrophage subsets occupy distinct anatomical locations and perform unique functions, suggesting that the surrounding cells participate in the instruction of macrophages to imprint functions specific to the niche (figure 2). Furthermore, when circulating cells of hematopoietic origin differentiate into tissue-resident macrophages, they acquire a similar phenotype, functional specialisation and, often, ability to self-maintain as their embryonic resident counterparts.32 The concept that macrophages adapt functionally to the niche in which they reside implies the existence of a combination of trophic factors, signalling molecules and physical scaffolding present within a specific tissue that is able to imprint this precise function and phenotype on the macrophage.33 In turn, it is likely that the adopted phenotype of the macrophage is of benefit, or may even be critically important, to the imprinting niche, as illustrated by the examples above.
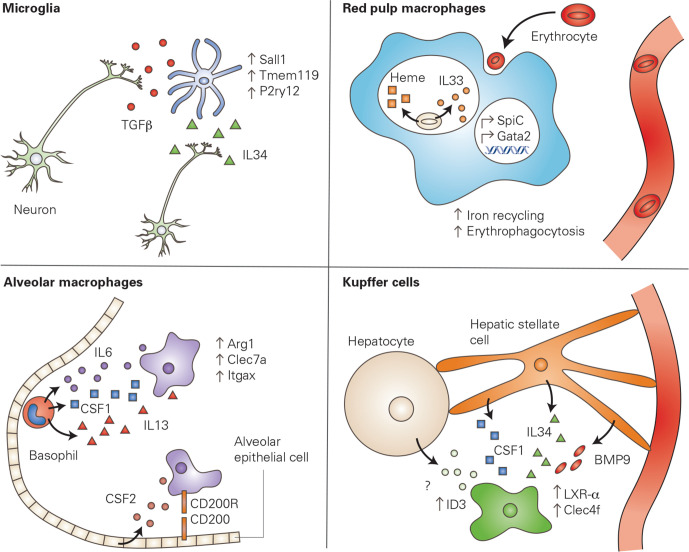
Figure 2.
Local signals imprint macrophage phenotype. The local cellular environment produces signals which are able to activate specific transcriptional profiles within tissue resident macrophages, conferring a niche-specific phenotype or function to the macrophage. In the brain, neurons produce IL34, a growth factor required for the survival and proliferation of macrophages. In addition, neurons produce TGFβ, which has been shown to induce the expression of microglia-specific genes. In the spleen, red pulp macrophages clear aged erythrocytes via phagocytosis (erythrophagocytosis). The phagocytosed erythrocytes release mediators that upregulate transcription factors including SpiC and GATA2, leading to an increase of iron recycling and further erythrophagocytosis. Alveolar macrophages in the lung interact with alveolar epithelial cells via CD200-CD200R interaction. Epithelial cells are also a critical source of CSF2, a growth factor necessary for alveolar macrophage survival. Furthermore, basophils produce a variety of signalling molecules which are critical for the transcriptional signature of alveolar macrophages. Finally, Kupffer cells in the liver require multiple signalling molecules produced by hepatic stellate cells to upregulate Kupffer cell-specific genes, including Clec4f. Furthermore, hepatocytes seem to be necessary for the upregulation of Id3, another Kupffer cell-specific gene, however, the mechanism through which this signalling occurs is unknown. Kupffer cell panel adapted from Bonnardel et al 55 CSF1, colony stimulating factor 1; IL34, interleukin 34; TGFβ, transforming growth factor beta.
Macrophages depend on a continuous supply of trophic factors such as colony stimulating factor 1 (CSF1 or M-CSF), CSF 2 (CSF2 or granulocyte macrophage-CSF) and interleukin (IL)-34 for their development and maintenance, and express the corresponding receptors Csf1r or Csf2r.34–39 Csf1r is required for the development, proliferation, survival and recruitment of tissue resident macrophages in most organs, whereas Csf2r deficiency mainly affects lung macrophages.40–43 Intestinal macrophages express Csf1r and depend on CSF1 to differing levels, as Csf1op/op mice present almost complete loss of macrophages in the muscularis externa, and only a reduction of macrophages in the lamina propria.44–46 Within the intestine, neurons and interstitial cells of Cajal (ICC) produce CSF1 to maintain the macrophage population within the muscularis externa.45 47 However, while Csf1r-expressing macrophages are present within the lamina propria and are ablated on Csf1r blockade, CSF1 production has so far only been reported by unidentified cells located at the base of intestinal crypts, most likely Paneth cells, and by epithelial cells located at the base of gastric pyloric glands.46 48–50
Additional signalling molecules may be involved in the niche-specific instruction of the macrophage, as shown for transforming growth factor beta (TGFb) in the case of microglia and colonic macrophages, TGFb and bone morphogenetic protein 7 (BMP7) for mucosal Langerhans cells and TGFb, BMP9 and desmosterol for liver Kupffer cells.51–55 Further programming of macrophages may also occur through phagocytosis of specific metabolites. This process occurs in red pulp macrophages in the spleen, which upon phagocytosis of aged erythrocytes sense heme and upregulate transcription factor Spi-C, which controls iron-recycling in macrophages (figure 2).56 Thus, imprinting of specific functions within the niche can occur due to cell-to-cell interaction via secreted molecules, or engulfment of metabolites that can activate transcriptional programming.
Niche accessibility and niche availability
Colonisation of a specific niche is determined largely by niche accessibility and niche availability, which regulate the contribution of circulating cells to the niche.57 Niche accessibility is determined by the presence of a barrier and whether it impedes the passage of circulating cells to the niche. Microglia, for example, being isolated from the blood stream by the blood–brain barrier, are uniquely of embryonic origin, whereas spleen, peritoneal and liver macrophages, which are more readily accessible from the blood stream, receive a progressively higher input by circulating cells with age (figure 3).58 Niche availability, on the other hand, is determined by whether there are empty niches that can be colonised, due to neonatal tissue growth, for example, or loss of resident cells due to inflammation (figure 3). Finally, engraftment by circulating cells is subject to competition from the presence of local resident self-maintaining macrophages, which are able to proliferate, differentiate in response to local signals, and occupy the niche. In most tissues, in the absence of inflammation, maintenance of the local tissue-resident macrophage pool occurs through self-maintenance. However, inflammation can lead to loss of resident macrophages and render the niche accessible to blood monocyte engraftment. It can induce a rapid and substantial recruitment of monocytes, triggered by the release of chemokines. These monocytes must first differentiate into macrophages, before they compete with resident proliferating cells for the available niche. The role of inflammation in facilitating engraftment of monocytes may be the reason why macrophages in the lamina propria of the intestine are so readily replaced by circulating cells, whereas their counterparts deeper within the gut wall are not readily replaced, as will be discussed in further detail in the following paragraph. The lamina propria is continuously exposed to the contents of the intestinal lumen and the microbiota, which has been proposed to constitute a source of permanent ‘physiological’ or ‘controlled’ inflammation.59 Similarly, in organs where monocyte engraftment increases progressively with age, it is possible that low-grade inflammation associated with ageing (‘inflamm-ageing’) or metabolic stress, in addition to a potential loss of self-renewal capacity of resident cells, may drive infiltration of incoming monocytes.60 61
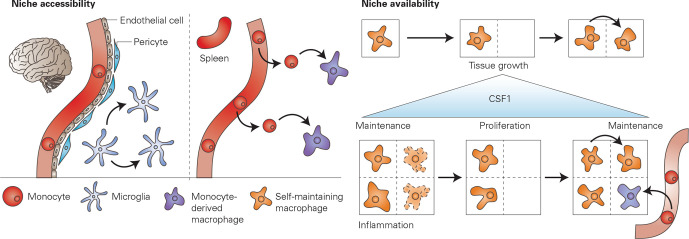
Figure 3.
Accessibility and availability of the macrophage niche. The colonisation of a macrophage niche by self-maintenance of resident cells or incoming monocytes that differentiate into macrophages depends on niche accessibility and niche availability. Niche accessibility: in the brain, the blood–brain barrier made up of endothelial cells, pericytes and astrocytes (not shown) impedes the egression of monocytes, therefore, maintenance of tissue resident macrophages (microglia) occurs solely through local proliferation. In organs such as the spleen, where no vascular barrier is present, monocytes can easily leave the blood stream and differentiate to macrophages within the tissue. Niche availability: a macrophage niche may become available due to tissue growth, leading to the proliferation of local macrophages that then occupy the open niche. If a macrophage niche becomes available due to inflammation and loss of macrophages (represented here using a dotted line), there may be engraftment of monocytes that differentiate to macrophages. It is plausible that the regulation of macrophage numbers may be determined by the levels of available CSF1, which has been shown to promote macrophage maintenance and proliferation. CSF1, colony stimulating factor 1.
The fact that resident macrophages proliferate when a niche becomes available implies that macrophages possess the ability to sense loss of their counterparts. Furthermore, tissue-resident macrophages tend to display a regular pattern and distribution within the tissue, suggesting that they can detect, and are potentially repelled by, neighbouring cells.62 63 This phenomenon is somewhat reminiscent to quorum-sensing, the system that bacteria use to regulate population density. The mechanisms underlying macrophage quorum sensing are unknown, however, availability of trophic factors may be involved.57 Indeed, low levels of CSF1 are necessary for macrophage survival while high levels are able to induce macrophage proliferation.64–66 Macrophages, as the predominant cell type that express Csf1r, consume available CSF1, thereby keeping CSF1 levels low and thus inhibiting macrophage proliferation. On loss of resident macrophages, unconsumed CSF1 leads to a rise in CSF1 levels, thereby unlocking macrophage proliferation (figure 3). Furthermore, CSF1 may also regulate monocyte replenishment, as increased CSF1 leads to monocytopenia in the circulation, and recruitment of monocytes to the tissue through induction of C-C motif chemokine receptor 2 (CCR2) ligands.64 66 67
Functional heterogeneity of niche-specific macrophages in health and disease
Macrophages can be found throughout the gastrointestinal tract, where they occupy distinct niches and carry out a variety of functions. The most abundant macrophage population is represented by lamina propria macrophages that reside within the villi. Lamina propria macrophages are crucial for host defence and intestinal homoeostasis and require constant replenishment by circulating cells. In addition, we recently demonstrated that deeper within the gut wall, there are several populations of macrophages that are able to self-maintain, and derive partially from precursors seeded embryonically.21 22 To demonstrate this, we made use of a murine fate-mapping model, which enables irreversible labelling of Cx3cr1+ cells present at the time of tamoxifen administration. Using this approach, we were able to demonstrate that a subset of intestinal macrophages, labelled at 4–6 weeks of age, retained labelling even 35 weeks after tamoxifen administration.21 Furthermore, by administering tamoxifen to pregnant dams, we were able to demonstrate that a population of macrophages seeded within the embryo persists until adulthood. These findings clearly demonstrate that a subset of intestinal macrophages is able to self-maintain within the tissue, and does not require replenishment from circulating monocytes. Interestingly, when studying the anatomical location of self-maintaining macrophages in the intestine, we observed that these cells occupy specific areas within the gut wall, distant from the lumen, and in close proximity to the enteric neurons and blood vessels found in the submucosa and muscularis externa, and Paneth cells. Using single-cell RNA sequencing, we showed that these subpopulations are transcriptionally distinct, suggesting that they receive imprinting from the surrounding cells. It is thus clear that, in addition to short-lived lamina propria macrophages, specialised macrophage subsets subject to reduced turnover and self-maintaining, occupy specific niches within the gut (table 1). In the following paragraphs, we will discuss the functional specialisations of these different subsets and speculate on how they may be implicated in gastrointestinal disease.
Table 1.
Overview of macrophage populations present within the intestine, with details regarding niche, location, markers and function where known.
| Niche | Location | Marker | Function | Notes | Key reference |
| Blood vessel | Small Intestine | Adamdec1* | Vascular integrity maintenance |
|
De Schepper et al, 21 Cell 2018 |
| Neuron | Small Intestine | Fcrls* | Required for the survival of enteric neurons |
|
De Schepper et al,21 Cell 2018 |
| Unknown | Small Intestine, Colon | Tim4 CD4 | Unknown |
|
Shaw et el,22 JEM 2018 |
| Blood vessel/ Crypt |
Small Intestine, Colon | CD169 | Monocyte recruitment in colitis | Asano et al,123 Nature Communications 2015 | |
| Crypt | Small Intestine | Unknown | Unknown |
|
De Schepper et al,21 Cell 2018 |
| Peyer’s Patch | Small Intestine | Tim4 CD4 | Tim4+: Phagocytosis Tim4-: Microbial defence |
|
Bonnardel et al,140 Cell Reports 2015 |
| Blood vessel | Small Intestine | Unknown | Regulation of bacterial translocation to blood |
|
Honda et al,62 Nature Communications 2020 |
| Epithelium | Colon | Cd11c Cd121b | Unknown |
|
Kang et al,82 Mucosal Immunology 2020 |
Markers included are either surface markers, or proposed gene markers derived from single-cell RNA sequencing experiments (labelled with *). Further comments on longevity and microbiota-dependence, in addition to the key reference, are also included.
*denotes candidate gene markers identified via single-cell RNA sequencing.
Lamina propria macrophages: the classical view
The most abundant and amply characterised macrophage population within the GI tract resides within the intestinal mucosa and villi. Lamina propria macrophages are positioned in the first line of defence to respond to bacteria and food antigens that breach the intestinal barrier and are thus crucial for maintaining the delicate balance between pathogen defence and oral tolerance (figure 4). Lamina propria macrophages are highly phagocytic; however, upon ingestion of food antigens or harmless commensal bacteria, they do not release pro-inflammatory mediators or nitric oxide, thereby preventing the influx of other immune cells (that might conceivably induce collateral damage) and maintaining tissue homoeostasis.68–70 In addition, lamina propria macrophages participate in oral tolerance via the presentation of trapped antigens to CD103+ dendritic cells, which then migrate to mesenteric lymph nodes to induce antigen-specific regulatory T cells.71–73 Conversely, the expansion within the intestine of FoxP3+ regulatory T cells, which are involved in oral tolerance to dietary antigens, relies on IL-10. IL-10 is produced by lamina propria macrophages in the presence of microbiota. However, macrophage-derived IL-10 is dispensable for oral tolerance and regulatory T cell maintenance, whereas IL-10 produced by the regulatory T cells themselves is critical for oral tolerance.74–76 These findings, in addition to contradicting reports regarding the importance of Cx3cr1-expressing macrophages in the establishment of oral tolerance, suggest that our understanding of how macrophages may participate in oral tolerance is incomplete and warrants further investigation. Cx3cr1high macrophages may participate in antigen presentation also by luminal sampling, as these cells extend dendritic projections through the epithelium to capture antigens within the lumen.77 78 Projection of dendrites is enhanced by lactic acid and pyruvic acid, two common microbial metabolites, via GPR31 in Cx3cr1high macrophages.79 Finally, lamina propria macrophages help to preserve epithelial integrity by phagocytosing apoptotic cells in the intestinal epithelium, and this is illustrated by Csf1 deficient mice, which display impaired epithelial differentiation and renewal.80 81 Interestingly, a recent single-cell RNA sequencing study uncovered a novel population of CD121b+ macrophages located close to the tips of the villi, thereby in close proximity to apoptotic cells of the epithelium and intestinal microbes.82 In line with this finding, this population was greatly reduced in germ-free mice, further supporting a role of the intestinal microbiota in shaping the phenotype of intestinal macrophage populations.
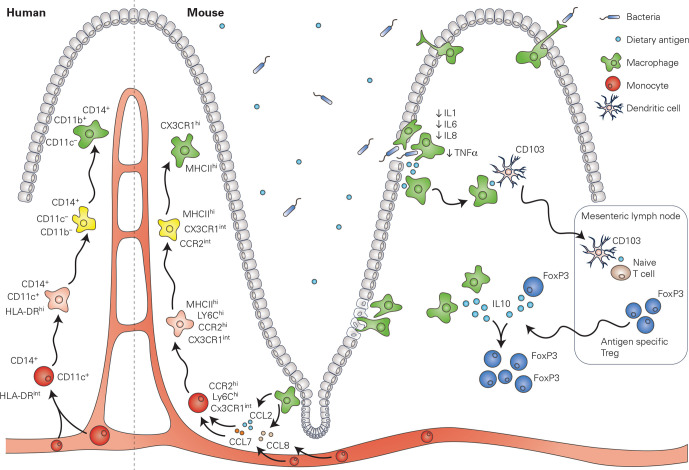
Figure 4.
Lamina propria macrophages in mouse and in human: the classical view. The pool of macrophages located within the lamina propria requires a high level of replenishment by circulating monocytes, which upon egression from the blood stream rapidly undergo a series of well-defined steps (known as the monocyte to macrophage ‘waterfall’) to differentiate into macrophages. This process has been extensively studied within mouse, however, studies suggest that a similar process is present also within the human lamina propria. Fully matured lamina propria macrophages -play a major role in host defence and are responsible for the phagocytosis of bacteria and dietary antigens which breach the mucosal barrier. On ingestion, lamina propria macrophages downregulate the expression of pro-inflammatory cytokines and mediators, thereby promoting oral tolerance. Lamina propria macrophages have also been implicated in the development of oral tolerance, as they have been shown to present trapped antigens to dendritic cells, which then migrate to the mesenteric lymph nodes to induce antigen-specific regulatory T cells. Antigen-specific regulatory T cells then migrate to the intestine, where their expansion is mediated by IL-10 produced by lamina propria macrophages and the regulatory T cells themselves. IL-10, interleukin 10.
As will be discussed in the following paragraphs, there is compelling evidence for the existence of specialised niches within the lamina propria, such as crypt-associated macrophages. However, it is a subject of debate whether the macrophage population that is located within the villi (and is dedicated to host defence and phagocytosis), truly represents a differentiated niche. While these cells present a certain degree of functional specialisation, the pool of macrophages in the lamina propria requires a high level of replenishment by bone marrow-derived monocytes. Parabiosis and bone-marrow chimaera experiments have demonstrated that circulating donor cells will readily engraft within the gut wall and contribute to the lamina propria macrophage pool.1 4 Once within the tissue, newly recruited monocytes rapidly undergo differentiation to adopt a mature macrophage phenotype through a series of well-defined intermediate stages, a process otherwise known as the monocyte to macrophage waterfall (figure 4).1 83 In this process, which depends on the transcription factor Nr4a1, newly recruited cells rapidly acquire major histocompatibility complex (MHC) type II (MHCII) expression, downregulate Ly6C and extravasation genes, and finally, upregulate Cx3cr1 to acquire a mature macrophage phenotype.51 62 68 83 The acquisition of the mature Cx3cr1high MHCIIhigh Ly6C- phenotype occurs in 5–6 days and is paralleled by significant changes in gene expression, most notably genes involved in phagocytosis, genes of the complement pathway and IL-10.51 Newly recruited macrophages, however, still differ transcriptionally from their resident counterparts 12 days after engraftment, and likely continue to undergo further differentiation with time. Interestingly, the dynamics of differentiation of engrafted cells appears to diverge between the small and the large intestine, suggesting that the environmental cues, such as the microbiome, imprinting incoming macrophage cells may be region-specific.84 A monocyte-to-macrophage ‘waterfall’ has also been described in the human intestinal mucosa, in which CD14hi CCR2+ CD11Chi monocytes transition through intermediate populations, giving rise to mature CD14lo CCR2- CD11Clo macrophages (figure 4).26 68 85 In contrast to murine intestinal macrophage maturation, however, human monocytes downregulate CX3CR1 as they differentiate into macrophages.85
The high turnover and dependence of lamina propria macrophages on circulating monocytes argues against the lamina propria constituting a niche. However, it is possible that lamina propria macrophages merely require such a high rate of replenishment and replacement due to the permanent ‘physiological’ inflammation generated by exposure to commensal microbiota and dietary antigens. This hypothesis is supported by the fact that lamina propria macrophages themselves upregulate monocyte chemoattractants such as CCL7, CCL8 and CCL12 as they differentiate from monocytes.51 59 This continuous inflammatory stress may drive premature macrophage death, in addition to providing the inflammatory stimuli for the recruitment of incoming cells. In line with this hypothesis, replenishment of lamina propria macrophages by circulating monocytes occurs at a much slower rate in germ-free or antibiotic-treated mice.1 22 86 There is little evidence, however, to support the notion that there are self-maintaining macrophages within the lamina propria pool. In mice lacking the CCR2, which controls Ly6Chi monocyte egress from the bone marrow, and mice in which monocytes are depleted, lamina propria macrophages are significantly reduced.68 87 88 Furthermore, on thermal injury, local Cx3cr1+ macrophages surrounding the injury site did not proliferate and the injury site was replenished by CCR2+ monocytes, which then differentiated into Cx3cr1+ macrophages.62 Replenishment of the injury site by monocytes also occurred in antibiotic-treated mice, although at a slower rate, further supporting the notion that inflammatory stimuli generated by the presence of the microbiota favours monocyte engraftment, and that the lamina propria may not harbour resident cells capable of self-maintenance. However, experiments employing depletion of lamina propria macrophages, followed by adoptive transfer of labelled monocytes, demonstrated that monocyte-derived macrophages in the lamina propria can proliferate within the niche.89 Although these experiments were carried out in an arguably artificial setting, they do demonstrate that monocyte-derived macrophages are able to proliferate and colonise a niche, if the niche is empty. It is plausible that self-maintaining macrophages alone may not be sufficient to guarantee host defence at the mucosal barrier, and that only a continuous supply of newly differentiated macrophages is able to protect the lamina propria from bacterial invasion.
Neuron-associated macrophages
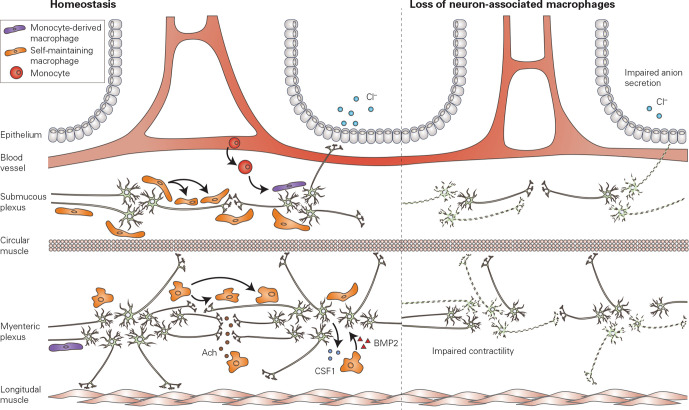
The gastrointestinal tract receives extrinsic input by sympathetic and parasympathetic fibres, which together with intrinsic enteric neurons coordinate intestinal functions including smooth muscle contractility, intestinal secretion and blood flow.90 Throughout the intestine, enteric neurons are organised into plexuses, the main ones being the myenteric and submucosal plexuses, which are located respectively between the circular and the longitudinal muscle in the muscularis externa, and luminal side relative to the muscularis externa. Using a fate-mapping model, we identified populations of self-maintaining neuron-associated macrophages critical for neuronal survival in both the myenteric and the submucosal plexus (figure 5).21
Figure 5.
Neuron-associated macrophages. Neuron-associated macrophages are located in close proximity to enteric neurons within the submucous plexus and the myenteric plexus, located deep within the gut wall. Neuron-associated macrophages are largely self-maintaining, and receive little input from circulating cells. Neurons in the myenteric plexus maintain the macrophage population via production of CSF-1, which is necessary for macrophage survival. Furthermore, acetylcholine released from enteric neurons can modulate the phenotype of macrophages in the context of intestinal inflammation. Conversely, macrophages within the muscularis externa produce BMP2, which is critical for peristalsis. Selective depletion of neuron-associated macrophages leads to a loss of enteric neurons in both the myenteric and submucosal plexuses, leading to impaired intestinal contractility and impaired anion secretion. Ach, acetylcholine; aBMP2, bone morphogenetic protein 2; CSF-1, colony stimulating factor 1.
Submucosal neuron-associated macrophages
The submucosal plexus, also known as Meissner’s plexus, is situated in the submucosal region between the circular muscle and mucosa. In close association to the neurons of the submucous plexus, there is a population of macrophages that is primarily self-maintaining, as over 90% of cells located in this niche is derived from macrophages that were present, and labelled, at birth. Submucosal neuron-associated macrophages occupy a niche that is relatively distant from the intestinal lumen, food antigens and bacteria, and are thus characterised by a distinct transcriptome and turnover compared with lamina propria macrophages. Single-cell RNA sequencing of all self-maintaining macrophages revealed a subpopulation of macrophages that upregulate neuron-associated genes, and can be localised to the submucosal plexus via in situ hybridisation.21 These neuron-associated macrophages upregulate genes which have been reported to be enriched in microglia, including Fcrls, Mef2a, Hexb and Gpr34, suggesting that they receive predominant imprinting from neuronal signals, as occurs for microglia in the brain. In line with this concept, a similar imprinting of a ‘microglia-like’ signature has recently been reported in neuron-associated macrophages in peripheral nerves located throughout the body.91 Wang et al studied macrophages associated to dorsal root ganglia, vagal nerve, subcutaneous fascial nerves and sciatic nerves and found a core ‘microglia-like’ expression signature, characterised by genes such as Tmem119, P2ry12, Siglech, Trem2 and Olfml3.91 However, a recent study profiling sciatic nerve associated macrophages found these cells to be rather more similar to border-associated macrophages, that represent a macrophage population in the brain distinct from microglia.92 Furthermore, microglia express the unique transcription factor Sall1, that is not expressed in other tissue-resident macrophages, suggesting that the distinct embryonic origin of microglia is able to confer a specific transcriptional profile that cannot be fully recapitulated by tissue resident macrophages located in proximity to nerve fibers.93 94 It is thus clear that ontogeny plays a part in defining transcriptional identity, in addition to further imprinting by local, niche-specific signals.
Neuron-associated macrophages within the submucous plexus are crucial for neuronal survival, as their selective depletion leads to apoptosis of submucosal neurons mediated by Caspase-3, and impaired neuron-evoked intestinal anion secretion (figure 5).21 Intestinal anion secretion is crucial for increase in luminal fluid and propulsion of intestinal content, and dysfunction of submucous neurons may underlie delayed transit as in slow transit constipation. Indeed, loss of submucous neurons has been described in patients with slow transit constipation.95 96 Given the relatively recent discovery of neuron-associated macrophages, no data are available on these cells in the context of slow transit constipation. However, these recent advances warrant further exploration of their potential role in gastro-intestinal disorders with delayed transit.
Muscularis neuron-associated macrophages
A similar neuron-associated macrophage population is located in the muscularis externa, in close association to the enteric neurons of the myenteric plexus. Using a fate-mapping model which labels Cx3cr1+ macrophages at 3 weeks of age, we showed that over 80% of macrophages in the myenteric plexus retained labelling after 35 weeks, suggesting that this niche receives little input from circulating monocytes, and consists largely of self-maintaining cells of embryonic origin.21 Indeed, monocytes could only be detected at very low levels within the muscularis externa at steady state conditions.97 98 However, on selective depletion of self-renewing macrophages, the myenteric plexus was repopulated by bone marrow-derived macrophages.21 It is most likely that these incoming cells gain the neuron-associated phenotype imprinted by signals from the neuronal niche. Indeed, bulk RNA sequencing of long-lived, self-maintaining macrophages and newly recruited macrophages revealed few differentially expressed genes, suggesting that incoming cells are efficiently imprinted by the niche and are able to adopt a neuron-associated phenotype.21 It is as yet unclear which molecular cues produced by myenteric neurons are responsible for the imprinting of neuron-associated macrophage phenotype; however, it has been shown that neurons are a source of CSF-1, which is critical for macrophage survival.99 Furthermore, in the context of inflammation, we showed that vagus nerve stimulation reduced intestinal inflammation by activating cholinergic enteric neurons in close proximity to macrophages expressing the α7 nicotinic acetylcholine receptor.100 101 These findings were recapitulated in patients undergoing abdominal surgery, where activation of cholinergic enteric neurons using the 5-HT4 agonist prucalopride reduced the inflammatory response and improved clinical recovery.100 These studies suggest that neuronal signalling is able to polarise macrophage phenotype and inflammatory response, a finding that is further supported by recent work in the context of infection-induced inflammation, where macrophages in the myenteric plexus were shown to respond to β2-adrenergic signalling via an Arginase 1-polyamine neuroprotective pathway, thereby limiting loss of enteric neurons.102 Taken together, these findings clearly demonstrate that neurons are able to imprint a specific phenotype to the macrophages present within the niche, and that, in response to inflammation or infection, neuronal signalling is able to instruct macrophages so as to preserve the neuronal network.
Neuron-associated macrophages in the myenteric plexus are crucial for the survival of myenteric neurons, as their depletion leads to Caspase-3-mediated apoptosis and loss of over 50% of neurons in the myenteric plexus, with consequent impaired peristalsis and prolonged intestinal transit (figure 5).21 Furthermore, macrophages associated to neurons in the myenteric plexus have been shown to produce BMP2, which is critical for the regulation of peristalsis.99 Finally, in Csf1op/op mice where macrophages are completely absent, the myenteric plexus is disorganised and neuronal density is increased, suggesting that macrophages may also participate in shaping connectivity in the enteric nervous system.45 Taken together, these findings demonstrate that neuron-associated macrophages are crucial for the maintenance of the neuronal population in the myenteric plexus and may play a critical role in disorders of the gastrointestinal tract characterised by altered or delayed gastrointestinal transit accompanied by neurodegeneration, as occurs in diabetes or ageing. The increasing prevalence of these chronic conditions in the western society underscores the urgent need for therapeutic options, and neuron-associated macrophages may represent a novel and exciting target.
In diabetes, neuropathy occurs in up to 50% of patients, and can affect the gastrointestinal tract, leading to vomiting, constipation, diarrhoea, and most notably, gastroparesis.103–106 Delayed gastric emptying is attributed to loss of gastric nitrergic neurons and ICCs both in patients and in animal models of diabetes.107–111 However, neurodegeneration in the context of diabetes is not limited to the stomach, as the number in cholinergic, nitrergic and total neurons is also reduced in the colonic myenteric plexus of animal model of diabetes.112 Similarly, in patients with diabetes, apoptosis and loss of colonic myenteric neurons with consequent impaired contractile and inhibitory responses have been observed.113 While the role of macrophages in diabetic neuropathy of the colon has not been explored, macrophages are known to play a crucial role in diabetic gastroparesis. In animal models, loss of CD206+ macrophages in the gastric muscularis externa has been linked to the loss of neuronal nitric oxide synthase-expressing neurons, increased oxidative stress and pro-inflammatory cytokine levels and the development of delayed gastric emptying.114 115 In line with these findings, CD206+ macrophages are reduced in the gastric antrum of patients with diabetic gastroparesis, a finding that correlates with loss of ICC.107 In mice with diabetic gastroparesis, macrophages were found to express higher levels of inflammatory genes, including IL-6 and iNOS, and lower levels of anti-inflammatory genes such as HO-1, Arg1 and Fizz1.116 In the complete absence of muscularis macrophages, as in Csf1op/op mice, mice do not develop gastroparesis following induction of diabetes and replenishment of macrophages after diabetes induction led to development of delayed gastric emptying and ICC damage.116 117 These data suggest that macrophage polarisation is central to the development of diabetic gastroparesis. On the one hand, loss of CD206+ macrophages is instrumental to ICC damage and long-standing diabetes in the absence of gastroparesis is associated with high levels of CD206 and HO-1. On the other hand, mice lacking macrophages are protected from gastroparesis.118 It is plausible that loss of a neuroprotective population of macrophages may drive neuropathy in the stomach, although it is unclear whether the CD206+ macrophage population described above corresponds to a neuron-associated population. Whether neuron-associated macrophages are lost or adopt an altered phenotype remains to be studied; however, findings support the hypothesis that in the context of diabetes, the gastric muscularis externa niche is altered and unable to imprint a neurosupportive phenotype to macrophages. The effect of diabetes on the macrophage niche and the signalling molecules involved are, to this date unknown, and warrant further study as they represent an intriguing and promising target for therapeutic intervention in the context of diabetic gastroparesis.
Blood vessel-associated macrophages
The vasculature represents an additional example of highly specialised niche, and vasculature-associated macrophages have been identified in the heart, adipose tissue and the dermis.119 120 Macrophages associated to the blood vessels are exposed to particulate material flowing in the blood stream and may thus act as immune sentinels at the interface between the organ and the rest of the body. Also within the intestine, several specialised populations of blood-vessel associated macrophages have been described. While these populations share common features, it is unclear whether these populations represent a single population or whether distinct subpopulations are present within the intestine. In the following paragraph, we will summarise recent findings on blood vessel-associated macrophage populations in the intestine.
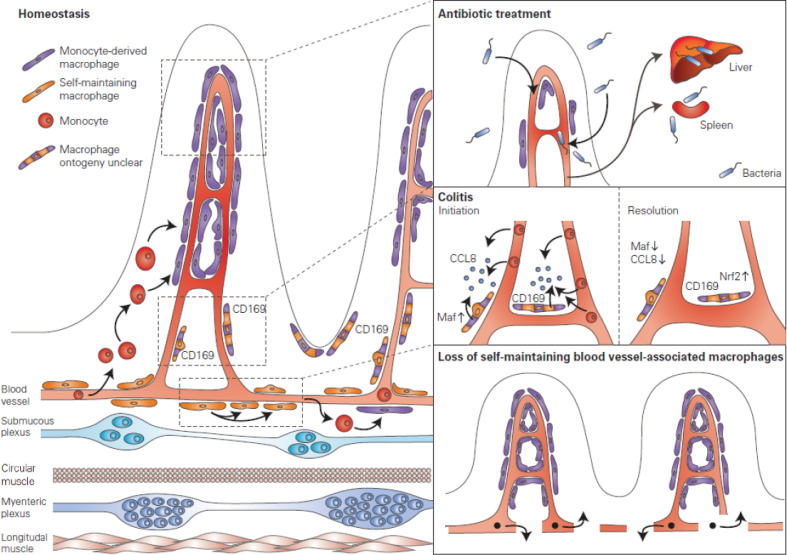
We recently described a specialised population of self-maintaining macrophages in the lamina propria that is closely associated to the vascular plexus, where large veins and arteries give rise to the capillary bed of the mucosa (figure 6).21 These macrophages present a distinct transcriptome, as revealed by single-cell RNA sequencing, and upregulate genes involved in angiogenesis, such as Ecm, Tnfaip2, Anpep, Hif1a, Mmp2 and Mmp14, in addition to candidate marker gene Adamdec1. Depletion of self-maintaining blood vessel-associated macrophages led to loss of VE-cadherin+ blood vessels and increased vascular leakage (figure 6).21 These findings suggest that blood-vessel associated macrophages are essential for vascular integrity and may be critical in preventing dissemination of luminal microbes to the blood stream. In light of these findings, it is plausible that blood vessel-associated macrophages contribute to the recently described gut-vascular barrier, a barrier similar to the blood–brain barrier, that is, crucial to prevent dissemination of intestinal microbes to the liver and the bloodstream.121 122
Figure 6.
Blood vessel-associated macrophages. Various populations of blood vessel associated macrophages have been identified within the intestine. Mucosal perivascular macrophages in the villi derive from monocytes and form an interdigitated network surrounding the blood vessel. Antibiotic treatment reduces the population of mucosal perivascular macrophages, thus impairing the perivascular network and leading to bacterial translocation to the liver and the spleen. Furthermore, a population of CD169+ macrophages which appears to be associated to the vasculature and lymphatics, distant from the epithelial border, was identified. While the ontogeny of this population remains to be defined, these macrophages were shown to be critical in the initiation phase of colitis, where upregulation of the transcription factor Maf leads to upregulation of CCL8 and recruitment of monocytes. Conversely, in the later stages of colitis, these macrophages downregulate Maf and CCL8, leading to an upregulation of Nrf2-dependent genes that favour the resolution of inflammation. Finally, we identified a population of self-maintaining macrophages that are associated to the vascular plexus located at the base of the villi. These macrophages were shown to regulate blood vessel integrity, as their selective depletion led to loss of blood vessels and increased vascular leakage.
Intriguingly, a recent study identified macrophages intimately associated to the blood vessels within the villi.62 Using advanced in vivo imaging technology, Honda et al showed that these mucosal perivascular macrophages form an interdigitated network surrounding the microcirculation, and that in the absence of microbiota these cells retracted, leaving large portions of the circulation uncovered. Furthermore, the consequence of this retraction was shown to be increased dissemination of intestinal pathogens to systemic sites, suggesting that these macrophages act to support and protect the gut vascular barrier within the villi (figure 6). Perivascular mucosal macrophages derive from circulating monocytes and their differentiation was shown to depend on the transcription factor Nr4a1 and the presence of the microbiota. It is plausible that these cells are functionally specialised due to the close proximity to blood vessels, in a similar manner to the self-maintaining blood vessel-associated population of the submucosa; however, they require a high turnover due to their anatomical location and the constant exposure to luminal contents. In line with this hypothesis, these cells displayed delayed turnover in antibiotic-treated mice.
In addition to the self-maintaining blood vessel-associated macrophages and the mucosal perivascular macrophages described above, another specialised, CD169+ macrophage population in close association with the vasculature and lymphatics of the intestine has been described. CD169, or sialoadhesin, is expressed by macrophage populations localised at the boundary between tissues and circulating fluids throughout a variety of organs, in particular spleen, the subcapsular and medullary sinus of the lymph node, and in the small intestine along the length of the central lacteal and lymphatic vessel at the base of the villi.123 124 Notably, CD169+ macrophages were initially described to be associated to intestinal crypts, however, increasing evidence points towards an intimate association of these cells to vessels, and therefore, this population has been included in this paragraph.82 124 It may be, however, that CD169+ macrophages also associate to intestinal crypts, and that the latter may represent a distinct population.
CD169+ macrophages capture particulate materials including apoptotic cells, viruses and immune complexes, and are implicated in the development of tolerance to circulating antigens.124 125 It is thus likely that the exposure of CD169+ macrophages to the circulating fluid drives the imprinting of a specific function. However, it is unclear whether within the intestine this population represents a distinct macrophage population to the self-maintaining population described above, as these cells have not been transcriptionally defined. Furthermore, while circulating monocytes are able to engraft in the pool of CD169+ macrophages, it is unclear whether embryonic progenitors give rise to this population, and whether these cells are able to self-maintain.123 Further research is certainly warranted on the ontogeny and transcriptome of intestinal CD169+ macrophages; however, compelling evidence has already been provided to suggest a functional specialisation of these cells and that they play a crucial role in murine models of colitis.123
Intestinal CD169+ macrophages have been shown to be critical in promoting infiltration of monocytes via CCL8 expression in experimental models of colitis, a mechanism dependent on the CD169+ macrophage transcription factor Maf (figure 6).123 125 In addition to regulating CCL8 expression, Maf also appears to repress cytoprotective genes downstream of the antioxidative transcription factor Nrf2, suggesting it acts as a molecular switch governing the phenotype of CD169+ macrophages.125 In mice, selective depletion of CD169+ macrophages, or anti-CCL8 treatment, ameliorated disease progression, suggesting that CCL8-mediated monocyte recruitment is central in initiating mucosal inflammation.123 Following the initial proinflammatory response, CD169+ macrophages subsequently downregulate Maf expression, leading to a progressive upregulation of Nrf2-dependent genes and downregulation of CCL8 during resolution of inflammation. Thus, Maf expression appears to govern the functional and phenotypical response of CD169+ macrophages to environmental cues, further illustrating the plasticity of macrophages in adapting to the niche and surrounding environmental cues. Future studies should elucidate the signals that regulate Maf expression in CD169+ macrophages, and how this signalling pathway could be targeted for therapeutic intervention during colitis. Furthermore, it is unclear whether a comparable population is present in human, and whether a similar mechanism is present in inflammatory bowel diseases (IBD).
It is crucial to increase our understanding of the role of blood vessel-associated macrophages in maintaining intestinal blood vessel integrity, as this subpopulation may be implicated in diseases characterised by gut vascular barrier dysfunction and bacterial translocation. High levels of endotoxaemia can be detected in patients with IBD and liver cirrhosis, suggesting that in these diseases, the intestinal vascular barrier is impaired.126–128 In mice it has been demonstrated that the establishment of the mucosal perivascular macrophage network relies on the presence of the microbiota, and upon antibiotic treatment mice showed reduced vascular adhesion by perivascular macrophages and increased systemic bacterial dissemination, leading to a reduction in survival and impaired vascular healing in the context of colitis.62 Intriguingly, in colitis, antibiotic-treated mice showed impaired differentiation of monocytes to macrophages compared with untreated controls. As stasis of immature monocytes has widely been described in patients with IBD, these new findings shed light on the role of the microbiota in promoting the differentiation of monocytes to mucosal perivascular macrophages, in addition to providing a mechanism through which impaired macrophage differentiation could promote endotoxaemia in colitis.68 129–131 Furthermore, the finding that the microbiota is crucial for vascular protection by mucosal perivascular macrophages to prevent systemic dissemination of intestinal pathogens, becomes highly relevant when considering that paediatric patients are more susceptible to Staphylococcus aureus colonisation following a course of antibiotics.62 132 Uncovering the mechanisms through which the microbiota contributes to regulating macrophage phenotype and function will be critical to exploit these novel findings and therapeutically target blood-vessel associated macrophages in the context of IBD.
Also in liver disease, gut microbiota and bacterial translocation play an important role in the pathogenesis of non-alcoholic or metabolic-associated fatty liver disease (NAFLD) and steatohepatitis. In NAFLD patients and a high-fat diet (HFD) mouse models, gut vascular barrier dysfunction has been observed leading to increased bacterial translocation to systemic sites.122 133 Whether HFD drives dysfunction of blood vessel-associated macrophages is as yet unknown, however, it has been shown to promote an accumulation of pro-inflammatory macrophages in the colon, suggesting that local inflammation may affect local macrophage phenotype imprinting.134 Further research should focus on the effect of HFD on specific macrophage populations within the intestine, as therapeutic interventions preventing bacterial dissemination may be key to reducing systemic low-grade inflammation and metabolic endotoxaemia.
Additional macrophage niches within the intestine
Crypt-associated macrophages
The intestinal crypt is a highly specialised environment, harbouring intestinal stem cells for epithelial regeneration, Paneth cells for antimicrobial defence and mucus-producing goblet cells. This unique niche is also supported by macrophages, and recent studies have shed light on a population of CSF1R-dependent macrophages intimately associated with the crypt epithelium.46 Depletion of these macrophages led to a reduction of Lgr5+ intestinal stem cells, and aberrant differentiation of Paneth cells and goblet cell density. Furthermore, single-cell RNA sequencing revealed a population of self-maintaining macrophages expressing typical Paneth cell markers, such as Itln1 and Defa, suggesting physical interaction between these macrophages and Paneth cells.21 Paneth cells produce antimicrobial compounds, such as α-defensins and lysozyme, in addition to Wnt3, which is critical for Lgr5+ intestinal stem cell maintenance.135 The antimicrobial products secreted by Paneth cells prevent bacterial translocation across the gut epithelium, and the reduction of lysozyme release observed on loss of crypt-associated macrophages may promote intestinal dysbiosis and intestinal expansion of Escherichia coli.136 Intriguingly, as Paneth cells have been reported to produce CSF-1, it is possible that Paneth cells maintain the local macrophage subset in a similar manner as neurons in the myenteric plexus.48 Future studies should focus on unravelling the communication between macrophages, intestinal stem cells and Paneth cells, to identify the molecular mechanisms provided by macrophages that are crucial for stem and Paneth cell homoeostasis, in addition to defining whether crypt-associated macrophages represent a transcriptionally distinct subset of macrophages. In an experimental model of colitis, it was shown that macrophages are essential for the regeneration of the damaged epithelium. Indeed, during the regenerative response, there was an upregulation of macrophage activation genes such as chemokines, chemokine ligands and interferon response genes in the crypt niche.137 Macrophages were shown to extend processes to directly contact colonic epithelial stem cells near the crypt base, and in the absence of macrophages, epithelial proliferation was impaired.137 Similarly, in a murine model of epithelial damage, Trem2+ macrophages were shown to promote wound healing via IL-4 and IL-13.138
The crypt-associated macrophage niche has not yet been well-defined; however, considering the critical role of macrophages in promoting wound healing and epithelial regeneration, these cells may be important in the context of colonic dysplasia and carcinoma. Indeed, dysregulated wound healing and epithelial regeneration are known to promote colorectal cancer.139
Peyer’s patch-associated macrophages
A distinct population of macrophages can be found located in Peyer’s patches. It has been shown that Peyer’s patch-associated macrophages derive from CCR2+ monocytes and fail to express typical macrophage markers such as CD64, F4/80 and CD206.140 Instead, Tim4+ and Tim4- CD4+ macrophage subsets have been defined within the Peyer’s patch niche. Peyer’s patch-associated macrophages have been shown to efficiently phagocytose particles and apoptotic cells, and present an antimicrobial and antiviral gene signature.140 In line with these findings, single-cell RNA sequencing revealed a population of macrophages upregulating B cell genes, including CD79a, CD79b and Ms4a1, further supporting the hypothesis that Peyer’s patch-associated macrophages play an important role in the clearance of apoptotic cells.21
Concluding remarks
While intestinal macrophages were long considered the housekeepers of the intestinal mucosa, it has become clear that these cells carry out a plethora of additional functions vital to the correct functioning and homoeostasis of the gastrointestinal tract. The proof that also within the intestine, macrophage subsets are able to self-maintain and do not only derive from circulating monocytes, sets the stage for differentiated and specialised macrophage subsets. Furthermore, the concept that surrounding cells are able to imprint specific functions on the resident macrophage (the macrophages niche) further highlights the functional plasticity of macrophages, and opens the possibility of the existence of additional macrophage niches within the intestine that have yet to be described. We have only scratched the surface of the true diversity and importance of intestinal macrophages, and the coming years will see a major overhaul in our understanding of the role of these cells in both intestinal homoeostasis and disease.
Acknowledgments
We would like to thank Michael Camilleri for reading and correcting the manuscript and providing insightful and constructive feedback.
Footnotes
Contributors: MFV and GB wrote and edited the manuscript.
Funding: MFV is funded by FWO PhD fellowship 11C2219N. GB is funded by ERC Advanced grant number 833816-NEUMACS.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Bain CC, Bravo-Blas A, Scott CL, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 2014;15:929–37. 10.1038/ni.2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Furth R. Origin and kinetics of mononuclear phagocytes. Ann N Y Acad Sci 1976;278:161–75. 10.1111/j.1749-6632.1976.tb47027.x [DOI] [PubMed] [Google Scholar]
- 3. van Furth R, Cohn ZA, Hirsch JG, et al. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ 1972;46:845–52. [PMC free article] [PubMed] [Google Scholar]
- 4. Yona S, Kim K-W, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013;38:79–91. 10.1016/j.immuni.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013;38:792–804. 10.1016/j.immuni.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 2014;14:392–404. 10.1038/nri3671 [DOI] [PubMed] [Google Scholar]
- 7. Goldmann T, Wieghofer P, Jordão MJC, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol 2016;17:797–805. 10.1038/ni.3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoeffel G, Wang Y, Greter M, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med 2012;209:1167–81. 10.1084/jem.20120340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 2014;40:91–104. 10.1016/j.immuni.2013.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 2012;9:286–94. 10.1038/nrgastro.2012.32 [DOI] [PubMed] [Google Scholar]
- 11. Calderon B, Carrero JA, Ferris ST, et al. The pancreas anatomy conditions the origin and properties of resident macrophages. J Exp Med 2015;212:1497–512. 10.1084/jem.20150496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scott CL, Zheng F, De Baetselier P, et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun 2016;7:1–10. 10.1038/ncomms10321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ginhoux F, Guilliams M. Tissue-Resident macrophage ontogeny and homeostasis. Immunity 2016;44:439–49. 10.1016/j.immuni.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 14. Mass E, Ballesteros I, Farlik M, et al. Specification of tissue-resident macrophages during organogenesis. Science 2016;353:aaf4238. 10.1126/science.aaf4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tober J, Koniski A, McGrath KE, et al. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood 2007;109:1433–41. 10.1182/blood-2006-06-031898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palis J, Robertson S, Kennedy M, et al. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 1999;126:5073–84. [DOI] [PubMed] [Google Scholar]
- 17. Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010;330:841–5. 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoeffel G, Ginhoux F. Ontogeny of tissue-resident macrophages. Front Immunol 2015;6:486. 10.3389/fimmu.2015.00486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palis J, Yoder MC. Yolk-Sac hematopoiesis: the first blood cells of mouse and man. Exp Hematol 2001;29:927–36. 10.1016/S0301-472X(01)00669-5 [DOI] [PubMed] [Google Scholar]
- 20. Hoeffel G, Chen J, Lavin Y, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 2015;42:665–78. 10.1016/j.immuni.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Schepper S, Verheijden S, Aguilera-Lizarraga J, et al. Self-Maintaining gut macrophages are essential for intestinal homeostasis. Cell 2018;175:400–15. 10.1016/j.cell.2018.07.048 [DOI] [PubMed] [Google Scholar]
- 22. Shaw TN, Houston SA, Wemyss K, et al. Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. J Exp Med 2018;215:1507–18. 10.1084/jem.20180019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McGovern N, Schlitzer A, Gunawan M, et al. Human dermal CD14⁺ cells are a transient population of monocyte-derived macrophages. Immunity 2014;41:465–77. 10.1016/j.immuni.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bajpai G, Schneider C, Wong N, et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med 2018;24:1234–45. 10.1038/s41591-018-0059-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nayak DK, Zhou F, Xu M, et al. Long-Term persistence of donor alveolar macrophages in human lung transplant recipients that influences donor-specific immune responses. Am J Transplant 2016;16:2300–11. 10.1111/ajt.13819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bujko A, Atlasy N, Landsverk OJB, et al. Transcriptional and functional profiling defines human small intestinal macrophage subsets. J Exp Med 2018;215:441–58. 10.1084/jem.20170057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Emile JF, Geissmann F, Martin OC, et al. Langerhans cell deficiency in reticular dysgenesis. Blood 2000;96:58–62. [PubMed] [Google Scholar]
- 28. Vinh DC, Patel SY, Uzel G, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood 2010;115:1519–29. 10.1182/blood-2009-03-208629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ural BB, Yeung ST, Damani-Yokota P, et al. Identification of a nerve-associated, lung-resident interstitial macrophage subset with distinct localization and immunoregulatory properties. Sci Immunol 2020;5:eaax8756. 10.1126/sciimmunol.aax8756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lim HY, Lim SY, Tan CK, et al. Hyaluronan receptor LYVE-1-Expressing macrophages maintain arterial tone through hyaluronan-mediated regulation of smooth muscle cell collagen. Immunity 2018;49:326–41. 10.1016/j.immuni.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 31. Jaitin DA, Adlung L, Thaiss CA, et al. Lipid-Associated macrophages control metabolic homeostasis in a Trem2-Dependent manner. Cell 2019;178:686–98. 10.1016/j.cell.2019.05.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van de Laar L, Saelens W, De Prijck S, et al. Yolk sac macrophages, fetal liver, and adult monocytes can colonize an empty niche and develop into functional tissue-resident macrophages. Immunity 2016;44:755–68. 10.1016/j.immuni.2016.02.017 [DOI] [PubMed] [Google Scholar]
- 33. Guilliams M, Thierry GR, Bonnardel J, et al. Establishment and maintenance of the macrophage niche. Immunity 2020;52:434–51. 10.1016/j.immuni.2020.02.015 [DOI] [PubMed] [Google Scholar]
- 34. Huffman JA, Hull WM, Dranoff G, et al. Pulmonary epithelial cell expression of GM-CSF corrects the alveolar proteinosis in GM-CSF-deficient mice. J Clin Invest 1996;97:649–55. 10.1172/JCI118461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lavin Y, Mortha A, Rahman A, et al. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol 2015;15:731–44. 10.1038/nri3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Y, Szretter KJ, Vermi W, et al. Il-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol 2012;13:753–60. 10.1038/ni.2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW, et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A 1990;87:4828–32. 10.1073/pnas.87.12.4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shibata Y, Berclaz PY, Chroneos ZC, et al. Gm-Csf regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 2001;15:557–67. 10.1016/S1074-7613(01)00218-7 [DOI] [PubMed] [Google Scholar]
- 39. Yoshida M, Ikegami M, Reed JA, et al. Gm-Csf regulates protein and lipid catabolism by alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 2001;280:L379–86. 10.1152/ajplung.2001.280.3.L379 [DOI] [PubMed] [Google Scholar]
- 40. Pridans C, Raper A, Davis GM, et al. Pleiotropic Impacts of Macrophage and Microglial Deficiency on Development in Rats with Targeted Mutation of the Csf1r Locus. J Immunol 2018;201:2683–99. 10.4049/jimmunol.1701783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dai X-M, Ryan GR, Hapel AJ, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 2002;99:111–20. 10.1182/blood.V99.1.111 [DOI] [PubMed] [Google Scholar]
- 42. Guilliams M, De Kleer I, Henri S, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 2013;210:1977–92. 10.1084/jem.20131199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nishinakamura R, Wiler R, Dirksen U, et al. The pulmonary alveolar proteinosis in granulocyte macrophage colony-stimulating factor/interleukins 3/5 beta C receptor-deficient mice is reversed by bone marrow transplantation. J Exp Med 1996;183:2657–62. 10.1084/jem.183.6.2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mikkelsen HB, Thuneberg L. Op/op mice defective in production of functional colony-stimulating factor-1 lack macrophages in muscularis externa of the small intestine. Cell Tissue Res 1999;295:485–93. 10.1007/s004410051254 [DOI] [PubMed] [Google Scholar]
- 45. Muller PA, Koscsó B, Rajani GM, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 2014;158:300–13. 10.1016/j.cell.2014.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sehgal A, Donaldson DS, Pridans C, et al. The role of CSF1R-dependent macrophages in control of the intestinal stem-cell niche. Nat Commun 2018;9:1–17. 10.1038/s41467-018-03638-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Avetisyan M, Rood JE, Huerta Lopez S, et al. Muscularis macrophage development in the absence of an enteric nervous system. Proc Natl Acad Sci U S A 2018;115:4696–701. 10.1073/pnas.1802490115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ryan GR, Dai XM, Dominguez MG, et al. Rescue of the colony-stimulating factor 1 (CSF-1)-nullizygous mouse (Csf1(op)/Csf1(op)) phenotype with a CSF-1 transgene and identification of sites of local CSF-1 synthesis. Blood 2001;98:74–84. 10.1182/blood.V98.1.74 [DOI] [PubMed] [Google Scholar]
- 49. Abboud SL, Bunegin M, Ghosh-Choudhury N, et al. Analysis of the mouse CSF-1 gene promoter in a transgenic mouse model. J Histochem Cytochem 2003;51:941–9. 10.1177/002215540305100709 [DOI] [PubMed] [Google Scholar]
- 50. Huynh D, Dai X-M, Nandi S, et al. Colony stimulating factor-1 dependence of Paneth cell development in the mouse small intestine. Gastroenterology 2009;137:136–44. 10.1053/j.gastro.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schridde A, Bain CC, Mayer JU, et al. Tissue-Specific differentiation of colonic macrophages requires TGFβ receptor-mediated signaling. Mucosal Immunol 2017;10:1387–99. 10.1038/mi.2016.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yasmin N, Bauer T, Modak M, et al. Identification of bone morphogenetic protein 7 (BMP7) as an instructive factor for human epidermal Langerhans cell differentiation. J Exp Med 2013;210:2597–610. 10.1084/jem.20130275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Capucha T, Koren N, Nassar M, et al. Sequential BMP7/TGF-β1 signaling and microbiota instruct mucosal Langerhans cell differentiation. J Exp Med 2018;215:481–500. 10.1084/jem.20171508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sakai M, Troutman TD, Seidman JS, et al. Liver-Derived signals sequentially reprogram myeloid enhancers to initiate and maintain Kupffer cell identity. Immunity 2019;51:655–70. 10.1016/j.immuni.2019.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bonnardel J, T'Jonck W, Gaublomme D, et al. Stellate cells, hepatocytes, and endothelial cells imprint the Kupffer cell identity on monocytes colonizing the liver macrophage niche. Immunity 2019;51:638–54. 10.1016/j.immuni.2019.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haldar M, Kohyama M, So AY-L, et al. Heme-Mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell 2014;156:1223–34. 10.1016/j.cell.2014.01.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Guilliams M, Scott CL. Does niche competition determine the origin of tissue-resident macrophages? Nat Rev Immunol 2017;17:451–60. 10.1038/nri.2017.42 [DOI] [PubMed] [Google Scholar]
- 58. Sawai CM, Babovic S, Upadhaya S, et al. Hematopoietic stem cells are the major source of multilineage hematopoiesis in adult animals. Immunity 2016;45:597–609. 10.1016/j.immuni.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 2009;136:2003–14. 10.1053/j.gastro.2009.01.075 [DOI] [PubMed] [Google Scholar]
- 60. Berry MR, Mathews RJ, Ferdinand JR, et al. Renal sodium gradient orchestrates a dynamic antibacterial defense zone. Cell 2017;170:860–74. 10.1016/j.cell.2017.07.022 [DOI] [PubMed] [Google Scholar]
- 61. Lambrecht B, Guilliams M. Monocytes find a new place to dwell in the niche of heartbreak hotel. J Exp Med 2014;211:2136. 10.1084/jem.21111insight1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Honda M, Surewaard BGJ, Watanabe M, et al. Perivascular localization of macrophages in the intestinal mucosa is regulated by NR4A1 and the microbiome. Nat Commun 2020;11:1329. 10.1038/s41467-020-15068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mikkelsen HB, Garbarsch C, Tranum-Jensen J, et al. Macrophages in the small intestinal muscularis externa of embryos, newborn and adult germ-free mice. J Mol Histol 2004;35:377–87. 10.1023/B:HIJO.0000039840.86420.b7 [DOI] [PubMed] [Google Scholar]
- 64. Tagliani E, Shi C, Nancy P, et al. Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1. J Exp Med 2011;208:1901–16. 10.1084/jem.20110866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tushinski RJ, Oliver IT, Guilbert LJ, et al. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell 1982;28:71–81. 10.1016/0092-8674(82)90376-2 [DOI] [PubMed] [Google Scholar]
- 66. Jenkins SJ, Ruckerl D, Thomas GD, et al. Il-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J Exp Med 2013;210:2477–91. 10.1084/jem.20121999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ulich TR, del Castillo J, Watson LR, et al. In vivo hematologic effects of recombinant human macrophage colony-stimulating factor. Blood 1990;75:846–50. 10.1182/blood.V75.4.846.846 [DOI] [PubMed] [Google Scholar]
- 68. Bain CC, Scott CL, Uronen-Hansson H, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 2013;6:498–510. 10.1038/mi.2012.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Smythies LE, Sellers M, Clements RH, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest 2005;115:66–75. 10.1172/JCI200519229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Roberts PJ, Riley GP, Morgan K, et al. The physiological expression of inducible nitric oxide synthase (iNOS) in the human colon. J Clin Pathol 2001;54:293–7. 10.1136/jcp.54.4.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mazzini E, Massimiliano L, Penna G, et al. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1⁺ macrophages to CD103⁺ dendritic cells. Immunity 2014;40:248–61. 10.1016/j.immuni.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 72. Worbs T, Bode U, Yan S, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med 2006;203:519–27. 10.1084/jem.20052016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schulz O, Jaensson E, Persson EK, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med 2009;206:3101–14. 10.1084/jem.20091925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hadis U, Wahl B, Schulz O, et al. Intestinal tolerance requires gut homing and expansion of Foxp3+ regulatory T cells in the lamina propria. Immunity 2011;34:237–46. 10.1016/j.immuni.2011.01.016 [DOI] [PubMed] [Google Scholar]
- 75. Kim M, Galan C, Hill AA, et al. Critical Role for the Microbiota in CX3CR1+ Intestinal Mononuclear Phagocyte Regulation of Intestinal T Cell Responses. Immunity 2018;49:151–63. 10.1016/j.immuni.2018.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cassani B, Villablanca EJ, Quintana FJ, et al. Gut-tropic T cells that express integrin α4β7 and CCR9 are required for induction of oral immune tolerance in mice. Gastroenterology 2011;141:2109–18. 10.1053/j.gastro.2011.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Niess JH, Brand S, Gu X, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 2005;307:254–8. 10.1126/science.1102901 [DOI] [PubMed] [Google Scholar]
- 78. Chieppa M, Rescigno M, Huang AYC, et al. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med 2006;203:2841–52. 10.1084/jem.20061884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Morita N, Umemoto E, Fujita S, et al. GPR31-dependent dendrite protrusion of intestinal CX3CR1+ cells by bacterial metabolites. Nature 2019;566:110–4. 10.1038/s41586-019-0884-1 [DOI] [PubMed] [Google Scholar]
- 80. Huynh D, Akçora D, Malaterre J, et al. Csf-1 receptor-dependent colon development, homeostasis and inflammatory stress response. PLoS One 2013;8:e56951. 10.1371/journal.pone.0056951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sauter KA, Pridans C, Sehgal A, et al. Pleiotropic effects of extended blockade of CSF1R signaling in adult mice. J Leukoc Biol 2014;96:265–74. 10.1189/jlb.2A0114-006R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kang B, Alvarado LJ, Kim T, et al. Commensal microbiota drive the functional diversification of colon macrophages. Mucosal Immunol 2020;13:216–29. 10.1038/s41385-019-0228-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tamoutounour S, Henri S, Lelouard H, et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol 2012;42:3150–66. 10.1002/eji.201242847 [DOI] [PubMed] [Google Scholar]
- 84. Gross-Vered M, Trzebanski S, Shemer A, et al. Defining murine monocyte differentiation into colonic and ileal macrophages. Elife 2020;9:e49998. 10.7554/eLife.49998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bernardo D, Marin AC, Fernández-Tomé S, et al. Human intestinal pro-inflammatory CD11chighCCR2+CX3CR1+ macrophages, but not their tolerogenic CD11c−CCR2−CX3CR1− counterparts, are expanded in inflammatory bowel disease. Mucosal Immunol 2018;11:1114–26. 10.1038/s41385-018-0030-7 [DOI] [PubMed] [Google Scholar]
- 86. Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol 2010;184:2026–37. 10.4049/jimmunol.0901936 [DOI] [PubMed] [Google Scholar]
- 87. Panea C, Farkas AM, Goto Y, et al. Intestinal monocyte-derived macrophages control Commensal-Specific Th17 responses. Cell Rep 2015;12:1314–24. 10.1016/j.celrep.2015.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kinnebrew MA, Buffie CG, Diehl GE, et al. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity 2012;36:276–87. 10.1016/j.immuni.2011.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Varol C, Vallon-Eberhard A, Elinav E, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity 2009;31:502–12. 10.1016/j.immuni.2009.06.025 [DOI] [PubMed] [Google Scholar]
- 90. Steadman RH, Braunfeld M, Park H. Liver and Gastrointestinal Physiology. In: Pharmacology and physiology for anesthesia: foundations and clinical application. Elsevier Inc, 2013: 475–86. [Google Scholar]
- 91. Wang PL, Yim AKY, Kim K-W, et al. Peripheral nerve resident macrophages share tissue-specific programming and features of activated microglia. Nat Commun 2020;11:2552. 10.1038/s41467-020-16355-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ydens E, Amann L, Asselbergh B, et al. Profiling peripheral nerve macrophages reveals two macrophage subsets with distinct localization, transcriptome and response to injury. Nat Neurosci 2020;23:676–89. 10.1038/s41593-020-0618-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Buttgereit A, Lelios I, Yu X, et al. Sall1 is a transcriptional regulator defining microglia identity and function. Nat Immunol 2016;17:1397–406. 10.1038/ni.3585 [DOI] [PubMed] [Google Scholar]
- 94. Utz SG, See P, Mildenberger W, et al. Early fate defines microglia and non-parenchymal brain macrophage development. Cell 2020;181:557–73. 10.1016/j.cell.2020.03.021 [DOI] [PubMed] [Google Scholar]
- 95. Bassotti G, Villanacci V, Nascimbeni R, et al. Colonic neuropathological aspects in patients with intractable constipation due to obstructed defecation. Mod Pathol 2007;20:367–74. 10.1038/modpathol.3800748 [DOI] [PubMed] [Google Scholar]
- 96. Wedel T, Roblick UJ, Ott V, et al. Oligoneuronal hypoganglionosis in patients with idiopathic slow-transit constipation. Dis Colon Rectum 2002;45:54–62. 10.1007/s10350-004-6114-3 [DOI] [PubMed] [Google Scholar]
- 97. Hori M, Nobe H, Horiguchi K, et al. MCP-1 targeting inhibits muscularis macrophage recruitment and intestinal smooth muscle dysfunction in colonic inflammation. Am J Physiol Cell Physiol 2008;294:C391–401. 10.1152/ajpcell.00056.2007 [DOI] [PubMed] [Google Scholar]
- 98. Pohl J-M, Gutweiler S, Thiebes S, et al. Irf4-dependent CD103+CD11b+ dendritic cells and the intestinal microbiome regulate monocyte and macrophage activation and intestinal peristalsis in postoperative ileus. Gut 2017;66:2110–20. 10.1136/gutjnl-2017-313856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gabanyi I, Muller PA, Feighery L, et al. Neuro-Immune interactions drive tissue programming in intestinal macrophages. Cell 2016;164:378–91. 10.1016/j.cell.2015.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Stakenborg N, Labeeuw E, Gomez-Pinilla PJ, et al. Preoperative administration of the 5-HT4 receptor agonist prucalopride reduces intestinal inflammation and shortens postoperative ileus via cholinergic enteric neurons. Gut 2019;68:1406–16. 10.1136/gutjnl-2018-317263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Matteoli G, Gomez-Pinilla PJ, Nemethova A, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 2014;63:938–48. 10.1136/gutjnl-2013-304676 [DOI] [PubMed] [Google Scholar]
- 102. Matheis F, Muller PA, Graves CL, et al. Adrenergic signaling in muscularis macrophages limits infection-induced neuronal loss. Cell 2020;180:64–78. 10.1016/j.cell.2019.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. KASSANDER P. Asymptomatic gastric retention in diabetics (gastroparesis diabeticorum). Ann Intern Med 1958;48:797–812. 10.7326/0003-4819-48-4-797 [DOI] [PubMed] [Google Scholar]
- 104. Bagyánszki M, Bódi N. Diabetes-related alterations in the enteric nervous system and its microenvironment. World J Diabetes 2012;3:80–93. 10.4239/wjd.v3.i5.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zandecki M, Vanden Berghe P, Depoortere I, et al. Characterization of myenteric neuropathy in the jejunum of spontaneously diabetic BB-rats. Neurogastroenterol Motil 2008;20:818–28. 10.1111/j.1365-2982.2008.01091.x [DOI] [PubMed] [Google Scholar]
- 106. Hicks CW, Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diab Rep 2019;19:86. 10.1007/s11892-019-1212-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Grover M, Bernard CE, Pasricha PJ, et al. Diabetic and idiopathic gastroparesis is associated with loss of CD206-positive macrophages in the gastric antrum. Neurogastroenterol Motil 2017;29:e13018. 10.1111/nmo.13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Harberson J, Thomas RM, Harbison SP, et al. Gastric neuromuscular pathology in gastroparesis: analysis of full-thickness antral biopsies. Dig Dis Sci 2010;55:359–70. 10.1007/s10620-009-1071-2 [DOI] [PubMed] [Google Scholar]
- 109. Wrzos HF, Cruz A, Polavarapu R, et al. Nitric oxide synthase (NOS) expression in the myenteric plexus of streptozotocin-diabetic rats. Dig Dis Sci 1997;42:2106–10. 10.1023/A:1018830820537 [DOI] [PubMed] [Google Scholar]
- 110. Takahashi T, Nakamura K, Itoh H, et al. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology 1997;113:1535–44. 10.1053/gast.1997.v113.pm9352855 [DOI] [PubMed] [Google Scholar]
- 111. Grover M, Farrugia G, Stanghellini V. Gastroparesis: a turning point in understanding and treatment. Gut 2019;68:2238–50. 10.1136/gutjnl-2019-318712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Du F, Wang L, Qian W, et al. Loss of enteric neurons accompanied by decreased expression of GDNF and PI3K/Akt pathway in diabetic rats. Neurogastroenterol Motil 2009;21:1229–e114. 10.1111/j.1365-2982.2009.01379.x [DOI] [PubMed] [Google Scholar]
- 113. Chandrasekharan B, Anitha M, Blatt R, et al. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil 2011;23:131–e26. 10.1111/j.1365-2982.2010.01611.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Choi KM, Kashyap PC, Dutta N, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology 2010;138:2399–409. 10.1053/j.gastro.2010.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Choi KM, Gibbons SJ, Nguyen TV, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology 2008;135:2055–64. 10.1053/j.gastro.2008.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cipriani G, Gibbons SJ, Miller KE, et al. Change in populations of macrophages promotes development of delayed gastric emptying in mice. Gastroenterology 2018;154:2122–36. 10.1053/j.gastro.2018.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cipriani G, Gibbons SJ, Verhulst P-J, et al. Diabetic Csf1op/op mice lacking macrophages are protected against the development of delayed gastric emptying. Cell Mol Gastroenterol Hepatol 2016;2:40–7. 10.1016/j.jcmgh.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bernard CE, Gibbons SJ, Mann IS, et al. Association of low numbers of CD206-positive cells with loss of ICC in the gastric body of patients with diabetic gastroparesis. Neurogastroenterol Motil 2014;26:1275–84. 10.1111/nmo.12389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Chakarov S, Lim HY, Tan L, et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 2019;363:aau0964. 10.1126/science.aau0964 [DOI] [PubMed] [Google Scholar]
- 120. Silva HM, Báfica A, Rodrigues-Luiz GF, et al. Vasculature-associated fat macrophages readily adapt to inflammatory and metabolic challenges. J Exp Med 2019;216:786–806. 10.1084/jem.20181049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Spadoni I, Zagato E, Bertocchi A, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 2015;350:830–4. 10.1126/science.aad0135 [DOI] [PubMed] [Google Scholar]
- 122. Mouries J, Brescia P, Silvestri A, et al. Microbiota-Driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol 2019;71:1216–28. 10.1016/j.jhep.2019.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Asano K, Takahashi N, Ushiki M, et al. Intestinal CD169(+) macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nat Commun 2015;6:1–7. 10.1038/ncomms8802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Asano K, Kikuchi K, Tanaka M. Cd169 macrophages regulate immune responses toward particulate materials in the circulating fluid. J Biochem 2018;164:77–85. 10.1093/jb/mvy050 [DOI] [PubMed] [Google Scholar]
- 125. Kikuchi K, Iida M, Ikeda N, et al. Macrophages switch their phenotype by regulating Maf expression during different phases of inflammation. J Immunol 2018;201:635–51. 10.4049/jimmunol.1800040 [DOI] [PubMed] [Google Scholar]
- 126. Pastor Rojo O, López San Román A, Albéniz Arbizu E, et al. Serum lipopolysaccharide-binding protein in endotoxemic patients with inflammatory bowel disease. Inflamm Bowel Dis 2007;13:269–77. 10.1002/ibd.20019 [DOI] [PubMed] [Google Scholar]
- 127. Gardiner KR, Halliday MI, Barclay GR, et al. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut 1995;36:897–901. 10.1136/gut.36.6.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Han D-W. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol 2002;8:961–5. 10.3748/wjg.v8.i6.961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kamada N, Hisamatsu T, Okamoto S, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest 2008;118:2269–80. 10.1172/JCI34610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ogino T, Nishimura J, Barman S, et al. Increased Th17-inducing activity of CD14+ CD163 low myeloid cells in intestinal lamina propria of patients with Crohn's disease. Gastroenterology 2013;145:1380–91. 10.1053/j.gastro.2013.08.049 [DOI] [PubMed] [Google Scholar]
- 131. Jones G-R, Bain CC, Fenton TM, et al. Dynamics of colon monocyte and macrophage activation during colitis. Front Immunol 2018;9:2764. 10.3389/fimmu.2018.02764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Grishin A, Papillon S, Bell B, et al. The role of the intestinal microbiota in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg 2013;22:69–75. 10.1053/j.sempedsurg.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Thaiss CA, Levy M, Grosheva I, et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018;359:1376–83. 10.1126/science.aar3318 [DOI] [PubMed] [Google Scholar]
- 134. Kawano Y, Nakae J, Watanabe N, et al. Colonic pro-inflammatory macrophages cause insulin resistance in an intestinal Ccl2/Ccr2-Dependent manner. Cell Metab 2016;24:295–310. 10.1016/j.cmet.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 135. Sato T, van Es JH, Snippert HJ, et al. Paneth cells constitute the niche for LGR5 stem cells in intestinal crypts. Nature 2011;469:415–8. 10.1038/nature09637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Riba A, Olier M, Lacroix-Lamandé S, et al. Paneth cell defects induce microbiota dysbiosis in mice and promote visceral hypersensitivity. Gastroenterology 2017;153:1594–606. 10.1053/j.gastro.2017.08.044 [DOI] [PubMed] [Google Scholar]
- 137. Pull SL, Doherty JM, Mills JC, et al. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A 2005;102:99–104. 10.1073/pnas.0405979102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Seno H, Miyoshi H, Brown SL, et al. Efficient colonic mucosal wound repair requires TREM2 signaling. Proc Natl Acad Sci U S A 2009;106:256–61. 10.1073/pnas.0803343106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Low D, Mino-Kenudson M, Mizoguchi E. Recent advancement in understanding colitis-associated tumorigenesis. Inflamm Bowel Dis 2014;20:2115–23. 10.1097/MIB.0000000000000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bonnardel J, Da Silva C, Henri S, et al. Innate and adaptive immune functions of Peyer's patch monocyte-derived cells. Cell Rep 2015;11:770–84. 10.1016/j.celrep.2015.03.067 [DOI] [PubMed] [Google Scholar]