Abstract
Purpose
Functional electrical stimulation-assisted cycle ergometry (FESCE) enables in-bed leg exercise independently of patients’ volition. We hypothesised that early use of FESCE-based progressive mobility programme improves physical function in survivors of critical care after 6 months.
Methods
We enrolled mechanically ventilated adults estimated to need >7 days of intensive care unit (ICU) stay into an assessor-blinded single centre randomised controlled trial to receive either FESCE-based protocolised or standard rehabilitation that continued up to day 28 or ICU discharge.
Results
We randomised in 1:1 ratio 150 patients (age 61±15 years, Acute Physiology and Chronic Health Evaluation II 21±7) at a median of 21 (IQR 19–43) hours after admission to ICU. Mean rehabilitation duration of rehabilitation delivered to intervention versus control group was 82 (IQR 66–97) versus 53 (IQR 50–57) min per treatment day, p<0.001. At 6 months 42 (56%) and 46 (61%) patients in interventional and control groups, respectively, were alive and available to follow-up (81.5% of prespecified sample size). Their Physical Component Summary of SF-36 (primary outcome) was not different at 6 months (50 (IQR 21–69) vs 49 (IQR 26–77); p=0.26). At ICU discharge, there were no differences in the ICU length of stay, functional performance, rectus femoris cross-sectional diameter or muscle power despite the daily nitrogen balance was being 0.6 (95% CI 0.2 to 1.0; p=0.004) gN/m2 less negative in the intervention group.
Conclusion
Early delivery of FESCE-based protocolised rehabilitation to ICU patients does not improve physical functioning at 6 months in survivors.
Trial registration number
Keywords: critical care, complementary medicine
Key messages.
What is the key question?
Functional-electrical stimulation cycle ergometry allows delivery of exercise to patients who are sedated and unconscious and can enhance progressive mobility programme, but its effects on patients-centred outcomes are unknown.
What is the bottom line?
Application of very early intensive cycling-based progressive mobility programmes to intensive care unit (ICU)-long stayers did not improve muscle mass and power in ICU or physical function at 6 months.
Why read on?
This is the first large randomised controlled trial on the use of early cycling-based protocolised rehabilitation in the critically ill.
Introduction
Preserving independent functioning and acceptable quality of life is as important as survival for most patients in intensive care. Unfortunately, functional disability, a natural consequence of weakness, is a frequent and long‐lasting complication in survivors of critical illness.1 2 Minimising sedation and a culture of early mobility has potential to reduce long-term sequelae of critical illness.3–5 Protocolised physical therapy has been shown to reduce the duration of mechanical ventilation and intensive care unit (ICU) length of stay,6 but these benefits are not consistently translated into improved long-term functional outcomes.7–10 The delivery of protocolised physical therapy requires the concomitant presence of a cooperative patient and a trained physiotherapist, often a precious resource in the ICU. In turn, implementation of early mobility strategies may fail in randomised controlled trials and in clinical practice. Only six randomised controlled trials out of 43 published to date in the field reported data of protocol implementation.6 Moreover, during acute critical illness no active exercise can be delivered.11 12 Yet, immobility‐associated muscle loss is evident as early as within 18–48 hours of onset of acute critical illness13 14 and during the first week patients lose 10%–20% of rectus femoris muscle cross-sectional diameter15 and up to 40% of muscle strength.16
Neuromuscular electrical stimulation (NMES) may mimic active exercise in patients, who lack voluntary muscle activity.17–25 During NMES, cutaneous electrodes placed over specific muscle groups electrically trigger muscle contractions. Passive cycling and NMES can be delivered simultaneously and synchronised to produce a coordinated pattern of movements (see online supplemental video 1) and increase whole-body energy expenditure.26 The technique is called functional electrical stimulation-assisted cycle ergometry (FESCE). FESCE is beneficial to patients with stroke and spinal cord injuries (reviewed in Doucet et al 27) as it prevents the loss of muscle mass28 and improved anabolic resistance and insulin sensitivity in quadriplegic patients.29 30 In a pilot study, FESCE seems to be safe and feasible in the critically ill.31
thoraxjnl-2020-215755supp001.mov (12.4MB, mov)
In the light of this we aimed to test early FESCE-based protocolised rehabilitation in a randomised controlled trial powered to test treatment effects on patient-centred outcomes. We hypothesised that protocolised progressive mobility programme, which includes FESCE and starts within 72 hours after ICU admission, would improve functional outcomes of ICU survivors at 6 months when compared with the standard of care.
Methods
This was a single centre, prospective, randomised controlled parallel group trial with a blinded outcome assessor, which had been registered prior to enrolling the first patient at www.clinicaltrials.gov and the full protocol has been published.32 We used a deferred consent procedure, where patients without capacity were enrolled based on assent gained from legal representatives and asked to provide consent as soon as they regained capacity.
Participants
Participants were recruited in two multidisciplinary ICUs of 11 and 10 level three beds, respectively, at tertiary FNKV University Hospital in Prague, Czech Republic. We included adult (≥18 years) patients who received mechanical ventilation for less than 72 hours but were predicted to need ICU for a week or more. We excluded patients bedridden before ICU admission, with missing or injured lower limbs, irreversible paralysis or those with pacemakers (see online supplemental appendix 1 for full list of eligibility criteria).
thoraxjnl-2020-215755supp002.pdf (7.4MB, pdf)
Standard care group
Both groups received usual best medical and nursing care in the ICU, which included daily sedation holds when applicable, respiratory physiotherapy and management as usual in the routine practice. Both groups received standard physiotherapy delivered two times a day 6 days in a week in a routine way by physiotherapists not involved in the study and adhering to the published safety criteria.33 Most importantly, a fraction of inspired oxygen less than 0.6 with a percutaneous oxygen saturation more than 90% and a respiratory rate less than 30 breaths/min and normal and stable intracranial pressure (ICP) were required for in-bed and out-of-bed mobilisation. In the control group the therapy was initiated on request of the treating physician and was documented, but not protocolised. It included passive and active range of motion, application of stretch reflex to upper and lower extremities and activation of global motor response according to Vojta reflex locomotion, positioning in bed, sitting, mobility activities progressing from activity in-bed to out-of-bed activities such as up to chair or ambulation, multi-component intervention (eg, combination with respiratory physiotherapy) and education.
Intervention group
The intervention began the calendar day after randomisation and consisted of a progressive mobility programme tailored to patients’ condition and supplemented by the use of FESCE (online supplemental table 1). The goal was to deliver a total of 90 min of active exercise a day until ICU discharge or day 28 whichever occurred earlier. Early in the course of the disease the intervention included FESCE (RT300 System, Restorative Therapies 2005-2016. LB100108 V.37).31 See online supplemental appendix 1—online supplemental table 1 for details. In brief, after warm-up phase (5 min of passive cycling), patients received therapy consisting of functional electrical stimulation or active cycling with duration adjusted per protocol and patient’s tolerance) followed by relaxation phase (5 min of passive cycling). FES impulses had pulse width 250 μs, pulse frequency 40 Hz and the lowest output per channel (in a range 0–60 mA) that allowed locomotive movement of lower extremities. Once the patient was more alert and able to participate, they were encouraged to engage in therapy. To increase the intervention workload, both resistance (3–10 Nm) and cycling cadence were increased incrementally. Face-to-face individual therapy was delivered two times a day by a certified physical therapist (MSc) specially trained in FESCE application in ICU.
Measures to ensure protocol implementation
Study physiotherapists (NH, KR) were appointed as 1.8 full working time equivalent specifically for this study and delivered the intervention 7 days/week. Throughout the study, 20 randomly selected exercise sessions were monitored by a hidden observer to ensure reliability and consistency of protocol implementation data reported by physiotherapists. Rehabilitation after discharge from ICU was not altered nor monitored in either group. Data on safety outcomes (ICP elevation, dialysis interruptions) were collected from clinical information system Metavision V.5, iMDsoft, Israel. A multi-step approach was used to minimise number of patients lost to follow-up (see online supplemental appendix 1 for more details).
Outcomes
The primary outcome of this trial was the Physical Component Summary (PCS) score of the SF-36 quality of life questionnaire measured in ICU survivors at 6 months and calculated as per RAND methodology, V.1.34 Because there was no study in similar population reporting on PCS, we calculated the power of the study based on an important determinant of PCS, which is physical function. Based on the study by Kayambu et al,35 where physical function score was 60.0±29.4 points in the control group, 108 patients are required in order to have 80% chance to detect a difference (at p<0.05) a change by 15.8 points or more, which is within the limits determined as clinically important for patients with COPD, asthma and myocardial infarction.36 To compensate for 28% mortality, we aimed to randomise 150 patients. More details on power analysis are in online supplemental appendix 1.
Secondary outcomes were Four-item Physical Fitness in Intensive Care Test (PFIT-s),37 rectus muscle cross-sectional diameter on B-mode ultrasound, mean daily nitrogen balance, muscle power as per the Medical Research Council score, number of ventilator-free days and ICU length of stay, all measured at discharge from ICU or day 28, whichever occurred earlier. Prespecified secondary safety outcomes were the number of episodes of elevated ICP and dialysis interruptions. Detailed description of secondary outcome assessment is in online supplemental appendix 1.
Randomisation
Eligible patients were randomly assigned (1:1) to receive either standard care or the intervention using offsite independent randomisation protocol embedded in the electronic case report form. Randomisation was stratified according to the presence or absence of sepsis and whether a specific consent was given to be involved in a nested metabolic substudy that included serial muscle biopsies.32 38 There were permuted blocks of four in each stratum. Both the study team and clinical personnel were aware of subject treatment allocation. The outcome assessors (JG, BB) were not involved in patient care and remained blinded to treatment allocations.
Statistical methods
The primary outcome and all secondary outcomes were reported as medians (IQR) in an intention-to-treat population and compared between the intervention and standard of care groups, with all tests two-sided using the level of significance set at p<0.05. Normality of data distribution was tested by Shapiro-Wilks’ test and data are reported as means±SD or median (IQR), as appropriate. We used log-rank test for time-to-event analyses, t-test or Wilcoxon test for continuous variables (depending on normality of distribution), and χ2 for frequency of event comparisons. No imputation of missing data was used. All calculations were performed in R, V.4.0.3 (updated on 10 October 2020) and ggplot2 package was used to create figures.
Results
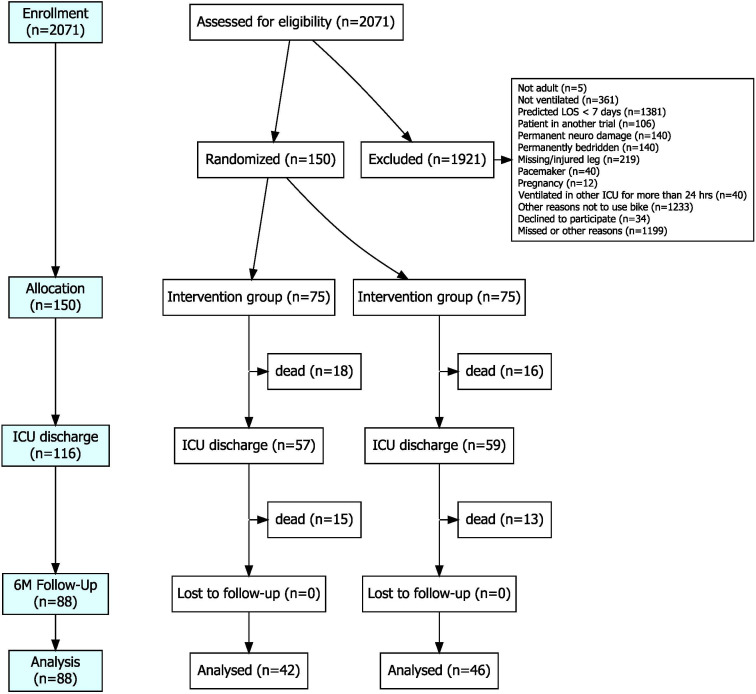
Between October 2016 and November 2019 (see online supplemental figure 3), 2071 patients were screened in order to enrol the prespecified number of 150 (7.2%) participants into the trial. Participant flow is shown in figure 1 and baseline characteristics of randomised patients in table 1.
Figure 1.
Flowchart of patients enrolled into the trial. Each patient could have one or more reasons not to be included and therefore the sum of reasons exceed the number of patients excluded. Other reasons included missed patients due to logistical reasons or patients who were deemed unlikely to survive; all patients who had been enrolled based on legal representative assent and regained capacity, gave written informed consent by the end of the follow-up period. ICU, intensive care unit; LOS, length of stay
Table 1.
Study subject characteristics
| Baseline characteristics | Intervention (n=75) | Control (n=75) | P value | |
| Demographic | Sex male/female (% male) | 53/22 (71%) | 57/18 (76%) | 0.46 |
| Age (years) | 59.9±15.1 | 62.3±15.4 | 0.34 | |
| Body mass index (kg/m2) | 29.3±6.3 | 30.7±8.3 | 0.24 | |
| Pre-admission health and function | Charlson Comorbidity Score | 2.8±2.3 | 3.4±2.4 | 0.15 |
| Physical activity (RAPA score) | 1 (IQR 1–3) | 2 (IQR 1–5) | 0.17 | |
| Level of independence (IAPA score) | 8 (IQR 7–8) | 8 (IQR 7–8) | 0.52 | |
| Current disease severity | Sepsis on admission (n, %) | 19 (25.3%) | 18 (24.0%) | 0.85 |
| APACHE II | 22.1±5.2 | 22.2±7.7 | 0.91 | |
| SOFA score at enrolment | 8.8±2.6 | 8.8±3.2 | 0.89 | |
| Primary reason for admission | Respiratory failure (COPD, pneumonia) | 20 (27%) | 17 (23%) | 0.7 |
| Isolated TBI | 16 (21%) | 10 (13%) | 0.28 | |
| Multiple trauma with TBI | 12 (16%) | 9 (12%) | 0.64 | |
| Multiple trauma without TBI | 2 (3%) | 5 (7%) | 0.44 | |
| Septic shock (non-respiratory) | 8 (11%) | 10 (13%) | 0.8 | |
| Out-of-hospital cardiac arrest | 5 (7%) | 6 (8%) | 1 | |
| Haemorrhagic stroke (operated) | 2 (3%) | 6 (8%) | 0.28 | |
| Congestive heart failure | 2 (3%) | 4 (5%) | 0.68 | |
| Haemorrhagic shock, non-traumatic | 1 (1%) | 3 (4%) | 0.62 | |
| Meningitis, encephalitis | 2 (3%) | 2 (3%) | 1 | |
| Other diagnoses | 5 (7%) | 3 (4%) | 0.72 | |
| Time from admission to enrolment (hours)* | 31.5±19.0 | 30.8±17.4 | 0.80 | |
CCS31; IAPA ranges 0–8 with higher number meaning higher functional independence32; RAPA score ranges from 1 ‘I almost never do any physical activities’ to 5 ‘I do 30 min or more per day of moderate physical activity 5 or more days per week’33.
*Intervention began next calendar day after enrolment.
APACHE, Acute Physiology and Chronic Health Evaluation; CCS, Charlson Comorbidity Score; IAPA, Instrumental Activities Of Daily Living Scale; RAPA, Rapid Assessment of Physical Activity; SOFA, Sequential Organ Failure Assessment; TBI, traumatic brain injury.
Protocol implementation
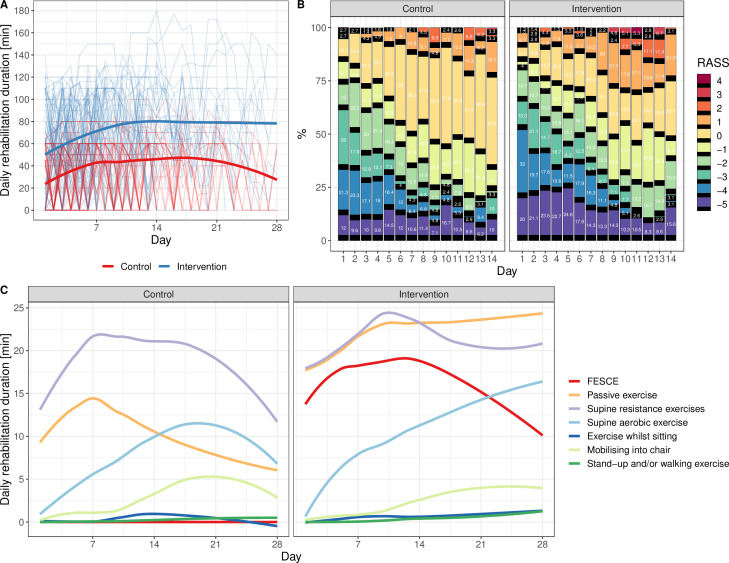
Patients in intervention and control arms stayed for a median of 12 (IQR 7–21) and 12 (IQR 6–19) days in ICU (p=0.76 log-rank test). Six and eleven patients randomised to intervention and control group, respectively, received no rehabilitation. At least one physiotherapy session was delivered in 817 out of 932 (88%) versus 615 out of 895 (69%) ICU days (p<0.001, χ2 test) and the first rehabilitation occurred 63 (IQR 45–84) versus 68 (48–95) hours after ICU admission (p=0.14 Wilcoxon) in the intervention versus control groups, respectively. During the days where rehabilitation was delivered, the median daily duration of it was 82.2 (IQR 65.6–96.6) versus 53.3 (IQR 50.1–57.1) min in the intervention and control group, respectively (median difference 29 min, p<0.001, Wilcoxon test). This included in the intervention group 33 (IQR 22–39) min per treatment day of FESCE (figure 2). Further details on rehabilitation in both groups can be found in online supplemental appendix 1 (online supplemental tables 2A, 2B and 3).
Figure 2.
Protocol implementation indices. (A) Average duration of rehabilitation in intervention (blue line) and control (red line) groups in all days of all patients (ie, including days without rehabilitation). Thin lines are individual patients (one outlier received up to 180 min of rehabilitation a day due to protocol violation). (B) Sedation level heatmap. (C) Average types of exercise delivered daily. FESCE, functional electrical stimulation-assisted cycle ergometry; RASS, Richmond Agitation-Sedation Scale, where 0 (alert and calm) or −1 (drowsy) were target levels of sedation management.
Outcomes
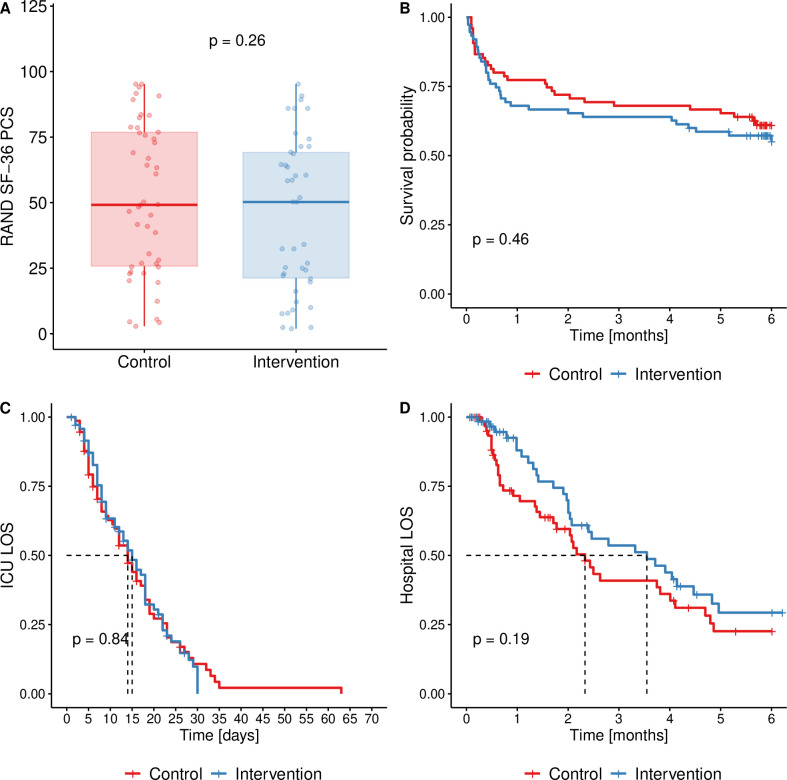
Forty-two (56%) and forty-six (61%) patients were alive and all available to follow-up at 6 months in intervention and control groups, respectively (p=0.51, χ2 test). This represents 81.5% (88/108) of prespecified sample size. Median physical component score of SF-36 in survivors (primary outcome) was 50 (IQR 21–69) in the intervention group and 49 (IQR 26–77) in controls (p=0.261, Wilcoxon test, see also online supplemental figures 4–6 and Table S5 in online supplemental data file). Patients’ in the intervention group had by 0.6 (95% CI 0.2 to 1.0) g/m2 of body surface area less negative mean daily nitrogen balance (p=0.004, t-test) as compared with control group, in the small subgroup with ICP monitoring in place (n=4 vs 3) more ICP elevations in the interventional (23 elevations/15 ICP days vs 0/15; p=0.018, Wilcoxon test), none of which occur during or immediately after FESCE exercise (see online supplemental appendix 1). There were no significant differences in any of seven other prespecified secondary outcomes (see figure 3 and table 2).
Figure 3.
(A) Physical component summary of SF-36 score (primary outcome); (B) Kaplan-Meier curve of survival in the study; (C) Kaplan-Meier curve of patients in the ICU (censored for non-survivors); (D) Kaplan-Meier curve of patients at hospital (censored for non-survivors). P values are from Wilcoxon in (A) and log-rank test in (B), (C) and (D). ICU, intensive care unit; LOS, length of stay; PCS, Physical Component Summary.
Table 2.
Secondary outcomes
| Secondary outcomes | Intervention | Standard of care | P value |
| PFIT-s at ICU discharge | 9.4 (8.0 to 10.8) n=37 |
9.6 (8.3 to 10.9) n=42 |
0.77* |
| Rectus muscle diameter at ICU discharge (mean difference from baseline (cm)) | −11 (−17 to −6) % n=57 | −13 (−19 to −7) % n=54 | 0.64 |
| MRC score at ICU discharge | 42.4 (39.2 to 45.6) |
39.4 (36.5 to 42.4) |
0.13 |
| Nitrogen balance (gN/m2/day) | −2.7 (−3.1 to −2.4) n=852 days of 75 patients |
−3.4 (−3.7 to −3.0) n (days)=759 days of 75 patients |
0.004 |
| Ventilator-free days at D28 | 9.3 (6.5 to 12.0) n=75 |
11.0 (8.2 to 13.8) n=75 |
0.33 |
| Number of untoward dialysis interruptions/days of rehabilitation during dialysis | 0/17 | 0/41 | N/A |
| Numbers of ICP elevations/days with ICP measured | 1.5 (0.2 to 2.9) (n=4 patients, 15 ICP days) |
0 (n=3 patients, 15 ICP days) | 0.018* |
Unless stated otherwise, data presented as means (95% CIs) and p values are from t-test.
PFIT-s ranging from 0 to 12 points with lower scores meaning higher degree of disability, see also online supplemental figure 1 and online supplemental table 4 in online supplemental appendix 1.
MRC score ranging from 0 to 60 points with higher scores meaning increasing muscle power.
Bold values indicate statistical significance.
*Wilcoxon test.
ICP, intracranial pressure; ICU, intensive care unit; MRC, Medical Research Council; PFIT-s, Four-item Physical Fitness in Intensive Care Test.
Ancillary analyses
Of note, although not a prespecified outcome, in the intervention group there was worse mental component summary score of SF-36 at 6 months 54.8 (IQR 37.1–69.6) versus 70.2 (IQR 51.5–81.3), p=0.009, Wilcoxon test (see online supplemental figures 5 and 7 in online supplemental appendix 1). Despite neither number of ICU days on pharmacological treatment for delirium (36% vs 37%, p=0.86, χ2 test) nor doses of sedatives (see online supplemental figure 8 in online supplemental appendix 1) were different, patients in the intervention group spent more time in the ICU either agitated or deeply sedated as seen on the heatmap in online supplemental figure 2B and online supplemental table 10 in online supplemental appendix 1.
Discussion
The main finding of this study is that in mechanically ventilated patients with anticipated long ICU length of stay, progressive mobility programme started very early and containing FESCE did not improve physical disability 6 months after surviving critical illness. The intervention led to 0.6 gN/m2/day improvement in nitrogen balance, which during a median of 11 days equals to sparing of approximately 380 g of lean body mass. This did not translate into measurable preservation into leg muscle mass, muscle power, physical fitness at ICU discharge or shortening of mechanical ventilation or ICU stay.
There are only limited number of other randomised controlled trials looking at long term effects on functional outcomes of a rehabilitation intervention delivered in ICU. Randomised controlled trials investigating in-bed cycling only39 40 and most studies on progressive mobility programmes7–10 41 42 demonstrated no difference in physical health after 6 months. The lack of effect in these trials could have been caused by problems with protocol implementation6 as in the only study reporting on duration of rehabilitation that was delivered,7 it was only 24% of prescribed duration (22 min vs 90 min per protocol). Largest trial so far by Morris et al 9 randomised 300 ICU patients very similar to ours to receive up to three sessions of resistance exercise delivered 7 days/week or a standard rehabilitation. There was no effect on the duration of hospital stay (primary outcome) and physical function was identical at hospital discharge; interestingly, patients in the intervention group improved faster after discharge and reached significantly better physical function scores after 6 months.9 Kayambu et al 35 also demonstrated better physical function at 6 months in ICU patients with sepsis exposed to protocoled rehabilitation, but this study is criticised due to small sample size and 40% loss of follow-up. Therefore, when designing our trial, we put emphasis on achieving protocol implementation and minimising loss of follow-up. Indeed, rigorously monitored delivery of exercise and successful protocol implementation is the main strength of this trial. Intervention group received exercise on 88% ICU days (as compared with 66% in the control group, see also online supplemental figure 9) with median duration per treatment day of 82 min with clear and significant separation of the rehabilitation duration from the control group. Despite successful implementation, we failed to demonstrate short-term or long-term effects, with the exception of the slight improvement of nitrogen economy. Preservation of lean body mass could be clinically meaningful, but in our study, it occurred unaccompanied by any signal of improvement of muscle function and its significance is therefore questionable. Indeed, the difference could have also occurred by chance due to multiple testing.
The lack of effect of the intervention could have been caused by multiple factors. First, median rehabilitation duration in our control group of 53 min per treatment day was far longer than expected and rare among rehabilitation trials.43 Our patients were discharged from ICU in better functional status (higher PFIT-s scores) then in other trials,44 45 which could mean that our discharge policy is conservative or reflect the fact that the rehabilitation in the control group was effective and FESCE-based intervention added no further benefit. On the same note, if rehabilitation delivered to the control group was close to the tolerable maximum, the intervention could have overstretched physiological reserves of some patients and offset potential benefits. In a study on healthy volunteers26 we have found that unloaded FESCE as used in our study can lead to aerobic lactate production and increase whole-body energy to 138%±29% and leg blood flow to 160%±30% of baseline, analogously to 25 W aerobic exercise. In contrast, physical therapy in the critically ill is known to cause very little increase in energy expenditure only analogous to 6 W exercise.46 Second, as shown in figure 2, in the intervention group there were more patients who were either agitated or unresponsive, possibly due to unequal distribution of patients with traumatic brain injury at baseline (37% vs 25%, in the intervention vs control groups, respectively p=0.11). Therefore, the increment in the duration of rehabilitation in the interventional group mostly consisted of passive elements of therapy (for details see online supplemental appendix 1) while out of bed mobilisation therapy duration was very similar to control group.
With regards of safety of the intervention, during 1000 FESCE sessions delivered to ICU patients, we have not observed any immediate impairment of cardiorespiratory function nor dialysis malfunction. We aimed to specifically look at safety of FESCE in patients with neurological injuries and allowed the intervention in patients with ICP monitoring in place, provided that ICP was normal and stable and the patient had not been receiving any second-tier therapy. The subgroup of enrolled patients with ICP monitoring in place was small (n=7) and we have not observed any immediate effect of FESCE or control rehabilitation on ICP. In line, none of the sessions had to be interrupted due to ICP elevation. Nonetheless, delayed ICP elevations only occurred in the intervention group and after 6 months mental health as well as emotional and social functions were worse in interventional compared with control group. The use of sedatives and antipsychotics was not different between groups offering no explanation for these phenomena. It should be stressed that mental function after 6 months was measured as a part of SF-36 score, but on its own it was not a prespecified secondary outcome and the difference may have occurred by chance. Nonetheless, we cannot rule out that the use of FESCE itself was responsible for the impairment of central nervous system function, as progressive mobility programme alone was safe in neuro patients47 or led to improvement of mental functions in unselected ICU patients.39 In the most recent multicentre RCT of Berney et al 34 randomised 162 patients with sepsis or systemic inflammation to receive 60 min/day of FESCE in addition to usual rehabilitation or usual rehabilitation alone (median of 15 min of active exercise per day). FESCE was delivered for a median of 53 min per day for a median of 5 days in the intervention group, there was no difference in muscle strength at hospital discharge and no major adverse events. Patients with neurological injuries at baseline had been excluded from Berney et al’s study. Although underpowered, this trial also did not demonstrate any influence of the intervention on the incidence of cognitive impairment at 6 months, in keeping with our results.
Indeed, although our study adds important knowledge to the field, its limitations are to be recognised, too. Due to higher-than-expected mortality (in fact, 41% of enrolled patients were not alive after 6 months) the study only achieved 81.5% of the prespecified sample size evaluated for primary outcome (88 out of 108) and it is therefore underpowered. In addition, our sample size was based on surrogate physical function in the control group of 16 patients in the study of Kayambu.35 Based on data in our study (PCS=51.7±28.8 in the control group), 133 patients would be needed to demonstrate 15 points difference in PCS at α=0.8 and p<0.05. The generalisability of our results is limited by single-centre design and relatively very intensive exercise in the control group. It is possible and likely that in different clinical environment with less intense rehabilitation in the control group, results would be different. In addition, we have not controlled nor monitored patient recovery pathway between ICU discharge and collection of the primary outcome.
Future outcome-based trials should certainly put emphasis on delivering progressive mobility element in the interventional group, enrol more homogeneous and specific patients’ populations.37 So far, the safety of FESCE-based is uncertain in patients with neurological injuries and needs investigation. There is also a burning need for studies focused on understanding physiology of FES-triggered contraction of healthy muscle versus muscle altered by underlying critical illness.3 In the meantime, protocolised physical therapy delivered by appropriately trained personnel remains the only evidence-based intervention to shorten duration of ICU stay and possibly improve long-term outcomes.
In conclusion, early FESCE-based protocolised physiotherapy delivered to mechanically ventilated patients does not change PCS score 6 months after discharge, nor duration of mechanical ventilation or any parameters of skeletal muscle mass, power and function at ICU discharge, apart from borderline improvement of nitrogen balance. These results must be interpreted in the context of very high dose and early start of rehabilitation in the control group, and relatively good physical functional status achieved by patients in the control group compared with other studies of long-stay ICU patients.
Acknowledgments
We thank the team of physiotherapists and research nurses, namely Marie Hejnová, Irena Kozáková, Jana Kukulová, Šárka Gregorová, Šárka Vosalová and Kateřina Ťopková. The study was funded exclusively from Agentura pro zdravotnický výzkum (grant agency of Czech Ministry of Health) AZV number 16-28663A.
Footnotes
Twitter: @FrantaDuska
Contributors: PW and FD are the authors of the main idea and overlooked the conduct of the study. PW, KJ and MF were responsible for consenting and recruiting patients and performing clinical procedures. PW is data analyst and biostatistician. NH and KR are the study physiotherapists. JG and BB were blinded outcome assessors. All authors have access to record-level data and have seen and approved the final version of the manuscript.
Funding: Grant Agency for Research in Healthcare AZV 16-28663A, email info@azvcr.cz ISDS: f7eike4, Phone: +420 271 019 257 For updated contact details, see http://www.azvcr.cz/kontakt.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: FD, KAR FNKV, Charles University, Fac Med 3, Srobarova 50, 10034 Prague, Czech Republic. Phone: +420267162451, Email: frantisek.duska@lf3.cuni.cz.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. We will sent de-identified patient-level data upon reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The trial design is in accordance with Declaration of Helsinki and the protocol, care report form and informed consent formularies were reviewed and approved by FNKV University Hospital Research Ethics Board ('Ethical Committee') on 24 June 2015 (decision number EK-VP-27-0-2015). All patients or their legal representatives gave their prospective written informed consent to participate in the study.
References
- 1. Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011;364:1293–304. 10.1056/NEJMoa1011802 [DOI] [PubMed] [Google Scholar]
- 2. Herridge MS, Moss M, Hough CL, et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med 2016;42:725–38. 10.1007/s00134-016-4321-8 [DOI] [PubMed] [Google Scholar]
- 3. Herridge MS, Mobile HMS. Mobile, awake and critically ill. CMAJ 2008;178:725–6. 10.1503/cmaj.080178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Needham DM. Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA 2008;300:1685–90. 10.1001/jama.300.14.1685 [DOI] [PubMed] [Google Scholar]
- 5. Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med 2014;370:1626–35. 10.1056/NEJMra1209390 [DOI] [PubMed] [Google Scholar]
- 6. Waldauf P, Jiroutková K, Krajčová A, et al. Effects of rehabilitation interventions on clinical outcomes in critically ill patients: systematic review and meta-analysis of randomized controlled trials. Crit Care Med 2020;48:1055–65. 10.1097/CCM.0000000000004382 [DOI] [PubMed] [Google Scholar]
- 7. Wright SE, Thomas K, Watson G, et al. Intensive versus standard physical rehabilitation therapy in the critically ill (EPICC): a multicentre, parallel-group, randomised controlled trial. Thorax 2018;73:213–21. 10.1136/thoraxjnl-2016-209858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hodgson CL, Bailey M, Bellomo R, et al. A binational multicenter pilot feasibility randomized controlled trial of early goal-directed mobilization in the ICU. Crit Care Med 2016;44:1145–52. 10.1097/CCM.0000000000001643 [DOI] [PubMed] [Google Scholar]
- 9. Morris PE, Berry MJ, Files DC, et al. Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: a randomized clinical trial. JAMA 2016;315:2694. 10.1001/jama.2016.7201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moss M, Nordon-Craft A, Malone D, et al. A randomized trial of an intensive physical therapy program for patients with acute respiratory failure. Am J Respir Crit Care Med 2016;193:1101–10. 10.1164/rccm.201505-1039OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bailey P, Thomsen GE, Spuhler VJ, et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med 2007;35:139–45. 10.1097/01.CCM.0000251130.69568.87 [DOI] [PubMed] [Google Scholar]
- 12. Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874–82. 10.1016/S0140-6736(09)60658-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levine S, Nguyen T, Taylor N, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 2008;358:1327–35. 10.1056/NEJMoa070447 [DOI] [PubMed] [Google Scholar]
- 14. Hermans G, De Jonghe B, Bruyninckx F, et al. Clinical review: critical illness polyneuropathy and myopathy. Crit Care 2008;12:238. 10.1186/cc7100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013;310:1591–600. 10.1001/jama.2013.278481 [DOI] [PubMed] [Google Scholar]
- 16. Topp R, Ditmyer M, King K, et al. The effect of bed rest and potential of prehabilitation on patients in the intensive care unit. AACN Clin Issues 2002;13:263–76. 10.1097/00044067-200205000-00011 [DOI] [PubMed] [Google Scholar]
- 17. Zanotti E, Felicetti G, Maini M, et al. Peripheral muscle strength training in bed-bound patients with COPD receiving mechanical ventilation: effect of electrical stimulation. Chest 2003;124:292–6. 10.1378/chest.124.1.292 [DOI] [PubMed] [Google Scholar]
- 18. Gerovasili V, Stefanidis K, Vitzilaios K, et al. Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study. Crit Care 2009;13:R161. 10.1186/cc8123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Routsi C, Gerovasili V, Vasileiadis I, et al. Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial. Crit Care 2010;14:R74. 10.1186/cc8987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abu-Khaber HA, Abouelela AMZ, Abdelkarim EM. Effect of electrical muscle stimulation on prevention of ICU acquired muscle weakness and facilitating weaning from mechanical ventilation. Alexandria J Med 2013;49:309–15. 10.1016/j.ajme.2013.03.011 [DOI] [Google Scholar]
- 21. Kho ME, Truong AD, Zanni JM, et al. Neuromuscular electrical stimulation in mechanically ventilated patients: a randomized, sham-controlled pilot trial with blinded outcome assessment. J Crit Care 2015;30:32–9. 10.1016/j.jcrc.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goll M, Wollersheim T, Haas K, et al. Randomised controlled trial using daily electrical muscle stimulation (EMS) in critically ill patients to prevent intensive care unit (ICU) acquired weakness (ICUAW). Intensive Care Med Exp 2015;3:1–2. 10.1186/2197-425X-3-S1-A809 [DOI] [Google Scholar]
- 23. Fischer A, Spiegl M, Altmann K, et al. Muscle mass, strength and functional outcomes in critically ill patients after cardiothoracic surgery: does neuromuscular electrical stimulation help? the Catastim 2 randomized controlled trial. Crit Care 2016;20:1–13. 10.1186/s13054-016-1199-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fontes Cerqueira TC, Cerqueira Neto MLde, Cacau LdeAP, et al. Ambulation capacity and functional outcome in patients undergoing neuromuscular electrical stimulation after cardiac valve surgery: a randomised clinical trial. Medicine 2018;97:e13012. 10.1097/MD.0000000000013012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koçan Kurtoğlu D, Tastekin N, Birtane M, et al. Effectiveness of neuromuscular electrical stimulation on auxiliary respiratory muscles in patients with chronic obstructive pulmonary disease treated in the intensive care unit. Turk J Phys Med Rehab 2015;61:12–17. 10.5152/tftrd.2015.04378 [DOI] [Google Scholar]
- 26. Gojda J, Waldauf P, Hrušková N, et al. Lactate production without hypoxia in skeletal muscle during electrical cycling: crossover study of femoral venous-arterial differences in healthy volunteers. PLoS One 2019;14:e0200228. 10.1371/journal.pone.0200228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Doucet BM, Lam A, Griffin L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J Biol Med 2012;85:201–15. [PMC free article] [PubMed] [Google Scholar]
- 28. Bauman WA, Spungen AM, Adkins RH, et al. Metabolic and endocrine changes in persons aging with spinal cord injury. Assist Technol 1999;11:88–96. 10.1080/10400435.1999.10131993 [DOI] [PubMed] [Google Scholar]
- 29. Kjaer M, Pollack SF, Mohr T, et al. Regulation of glucose turnover and hormonal responses during electrical cycling in tetraplegic humans. Am J Physiol 1996;271:R191–9. 10.1152/ajpregu.1996.271.1.R191 [DOI] [PubMed] [Google Scholar]
- 30. Gorgey AS, Dolbow DR, Dolbow JD, et al. The effects of electrical stimulation on body composition and metabolic profile after spinal cord injury--Part II. J Spinal Cord Med 2015;38:23–37. 10.1179/2045772314Y.0000000244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parry SM, Berney S, Warrillow S, et al. Functional electrical stimulation with cycling in the critically ill: a pilot case-matched control study. J Crit Care 2014;29:695.e1–695.e7. 10.1016/j.jcrc.2014.03.017 [DOI] [PubMed] [Google Scholar]
- 32. Waldauf P, Gojda J, Urban T, et al. Functional electrical stimulation-assisted cycle ergometry in the critically ill: protocol for a randomized controlled trial. Trials 2019;20:724. 10.1186/s13063-019-3745-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sommers J, Engelbert RHH, Dettling-Ihnenfeldt D, et al. Physiotherapy in the intensive care unit: an evidence-based, expert driven, practical statement and rehabilitation recommendations. Clin Rehabil 2015;29:1051–63. 10.1177/0269215514567156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berney S, Hopkins RO, Rose JW, et al. Functional electrical stimulation in-bed cycle ergometry in mechanically ventilated patients: a multicentre randomised controlled trial. Thorax 2021;76:656–63. 10.1136/thoraxjnl-2020-215093 [DOI] [PubMed] [Google Scholar]
- 35. Kayambu G, Boots R, Paratz J. Early physical rehabilitation in intensive care patients with sepsis syndromes: a pilot randomised controlled trial. Intensive Care Med 2015;41:865–74. 10.1007/s00134-015-3763-8 [DOI] [PubMed] [Google Scholar]
- 36. Wyrwich KW, Tierney WM, Babu AN, et al. A comparison of clinically important differences in health-related quality of life for patients with chronic lung disease, asthma, or heart disease. Health Serv Res 2005;40:577–92. 10.1111/j.1475-6773.2005.0l374.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Denehy L, de Morton NA, Skinner EH, et al. A physical function test for use in the intensive care unit: validity, responsiveness, and predictive utility of the physical function ICU test (scored). Phys Ther 2013;93:1636–45. 10.2522/ptj.20120310 [DOI] [PubMed] [Google Scholar]
- 38. Ziak J, Krajcova A, Jiroutkova K, et al. Assessing the function of mitochondria in cytosolic context in human skeletal muscle: adopting high-resolution respirometry to homogenate of needle biopsy tissue samples. Mitochondrion 2015;21:106–12. 10.1016/j.mito.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 39. Fossat G, Baudin F, Courtes L, et al. Effect of in-bed leg cycling and electrical stimulation of the quadriceps on global muscle strength in critically ill adults: a randomized clinical trial. JAMA 2018;320:368–78. 10.1001/jama.2018.9592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eggmann S, Verra ML, Luder G. Physiological effects and safety of an early, combined endurance and resistance training in mechanically ventilated, critically ill patients. PLoS One 2018;101:e344–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Amundadottir OR, Jónasdóttir RJ, Sigvaldason K. Effects of intensive upright mobilisation on outcomes of mechanically ventilated patients in the intensive care unit: a randomised controlled trial with 12-months follow-up. Eur J Physiother 2019;0:1–11. [Google Scholar]
- 42. Denehy L, Skinner EH, Edbrooke L, et al. Exercise rehabilitation for patients with critical illness: a randomized controlled trial with 12 months of follow-up. Crit Care 2013;17:R156. 10.1186/cc12835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dall' Acqua AM, Sachetti A, Santos LJ, et al. Use of neuromuscular electrical stimulation to preserve the thickness of abdominal and chest muscles of critically ill patients: a randomized clinical trial. J Rehabil Med 2017;49:40–8. 10.2340/16501977-2168 [DOI] [PubMed] [Google Scholar]
- 44. Nordon-Craft A, Schenkman M, Edbrooke L, et al. The physical function intensive care test: implementation in survivors of critical illness. Phys Ther 2014;94:1499–507. 10.2522/ptj.20130451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parry SM, Denehy L, Beach LJ, et al. Functional outcomes in ICU – what should we be using? – an observational study. Crit Care 2015;19:127. 10.1186/s13054-015-0829-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hickmann CE, Roeseler J, Castanares-Zapatero D, et al. Energy expenditure in the critically ill performing early physical therapy. Intensive Care Med 2014;40:548–55. 10.1007/s00134-014-3218-7 [DOI] [PubMed] [Google Scholar]
- 47. Bahouth MN, Power MC, Zink EK, et al. Safety and feasibility of a neuroscience critical care program to mobilize patients with primary intracerebral hemorrhage. Arch Phys Med Rehabil 2018;99:1220–5. 10.1016/j.apmr.2018.01.034 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2020-215755supp001.mov (12.4MB, mov)
thoraxjnl-2020-215755supp002.pdf (7.4MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. We will sent de-identified patient-level data upon reasonable request to the corresponding author.