Abstract
BACKGROUND
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the ongoing pandemic of coronavirus disease 2019 (COVID-19), and has caused more than 80 million infections and 1.7 million deaths worldwide. Although it is primarily a respiratory virus, SARS-CoV-2 also has extra-pulmonary effects. Pancreatic injury and cases of acute pancreatitis (AP) have been recognized and attributed to SARS-CoV-2, but the mechanisms of pancreatic injury are still a subject of debate. There is also controversy on whether SARS-CoV-2 can cause AP or if it is an epiphenomenon.
AIM
To review and to explore the relationship between SARS-CoV-2 infection and AP, and to provide an overview of the existing literature on possible mechanisms of SARS-CoV-2-induced pancreatic lesion.
METHODS
A systematic review was conducted in accordance with PRISMA guidelines for papers on SARS-CoV-2 infection and AP. A narrative review on possible mechanisms of SARS-CoV-2-induced pancreatic lesion was also performed.
RESULTS
A literature review revealed a growing body of evidence on SARS-CoV-2-induced pancreatic lesions including the mechanisms of direct virus-mediated injury, systemic inflammatory response and circulating pro-inflammatory interleukins, virus-induced lipotoxicity, and drug-induced injury. A systematic review of the literature revealed 22 cases of AP in COVID-19 patients. However, limitations of the reported cases make it difficult to establish a causal relationship between SARS-CoV-2 infection and AP. All of the studies agreed on special monitoring and surveillance of this subset of patients due to the still unknown clinical progression, therapeutic implications, and prognosis.
CONCLUSION
AP should be considered in COVID-19 patients, especially in those exhibiting abdominal pain and systematic, and complete reporting of these cases should be general practice. However, there is still insufficient evidence showing that COVID-19 can cause AP or negatively impact prognosis. Additional studies are needed to clarify the relationship between these two entities and their theragnostic significance.
Keywords: Pancreatitis, Pancreas, COVID-19, SARS-CoV-2
Core Tip: Recently, an association between acute pancreatitis (AP) and coronavirus disease 2019 (COVID-19) has been proposed, but the mechanisms of pancreatic injury of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are not fully understood. Although viral-induced AP is an established diagnosis, there is still insufficient evidence clearly showing COVID-19 as AP etiology. We conducted an in-depth analysis on the mechanisms of pancreatic injury by SARS-CoV-2 and reviewed published cases of AP in COVID-19 patients.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) emergence in December 2019 brought unprecedented challenges to global health care. Until December 28, 2020, more than 80 million cases had been confirmed globally and were responsible for more than 1.7 million deaths[1].
The most common clinical manifestations of COVID-19 are respiratory, particularly fever and cough[2], but as cases have increased of widespread severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) across the globe, other symptoms and clinical scenarios have emerged. Gastrointestinal (GI) and hepatic involvement, among others, have been recognized and are mediated by the expression of angiotensin-converting enzyme 2 (ACE2) on the GI tract, the main receptor of SARS-CoV-2[3,4]. A recent systematic review and meta-analysis by Mao et al[5] showed that the estimated prevalence of digestive symptoms is 15%. Nausea, vomiting, diarrhea, and loss of appetite are the most frequent symptoms. Nineteen percent of patients present with liver injury, which may be more prevalent in fatal cases[5]. Furthermore, approximately 10% of COVID-19-positive patients may present with only GI symptoms. This clinical presentation may be associated with the delayed diagnosis of COVID-19 and a tendency of the disease to progress to more severe forms[5].
The expression of ACE2 in pancreatic cells (both exocrine glands and islets) renders the pancreas a potential target for SARS-CoV-2, but only recently has it received attention for its role in the COVID-19 clinical picture. Several case reports of pancreatic injury and acute pancreatitis (AP) caused by the novel coronavirus have been reported. About 1%-2% of non-severe and 17% of severe cases of COVID-19 exhibit pancreatic injury, which may have developed before the patient’s admission[6]. However, there is still uncertainty about the physiopathological mechanisms involved and the precise etiology of pancreatic injury in the reported cases.
We conducted a literature review to clarify the relationship between SARS-CoV-2 infection and AP.
MATERIALS AND METHODS
A systematic review was conducted according to PRISMA guidelines[7]. We searched the PubMed and EMBASE databases on November 1, 2020 for published articles using the medical subject headings keywords “COVID-19” and “pancreatitis.” Considering the urgency of the topic, a grey literature search using the same keywords was done on Google Scholar to increase the sensitivity of the search. Articles were included if they reported AP cases in COVID-19 patients. Review studies and articles dealing with pediatric patients and COVID-19 patients without the diagnosis of AP, even if pancreatic lesion was suspected, were excluded from our systematic review. References of eligible manuscripts were screened for additional articles. The literature search was restricted to articles published in English, Spanish, and Portuguese.
The titles and abstracts of studies retrieved by the search strategy and those from additional sources, namely by cross-referencing, were screened independently by all authors to identify studies that potentially met the inclusion criteria. Then full texts of these potentially eligible studies were retrieved and independently assessed for eligibility. Any disagreements between them over the eligibility of particular studies was resolved through discussion with the three authors, and a consensus was met in all included papers. When not available, the full texts were requested from the authors, and only full text articles were included in this review. Records were managed with Zotero (version 5.0) to exclude duplicates.
Data were extracted from each of the papers undergoing full text review, and included authors, study design, population number, gender of included patients, age, co-morbidities, symptoms on admission, AP etiology, severity and both local and systemic complications reported, method for COVID-19 diagnosis, intensive care unit admission, and need for mechanical ventilation. Descriptive statistics were used to analyze the data. Given the heterogeneity of the study population and design, a meta-analysis was not possible to perform. The results have been reported according to PRISMA and AMSTAR guidelines.
Secondary literature on the physiopathology of pancreatic involvement by SARS-CoV-2 was conducted and the review of paper titles, abstracts, and filtering for those requiring full text evaluation was made by the lead author. A cross-referencing search was done for additional articles.
RESULTS
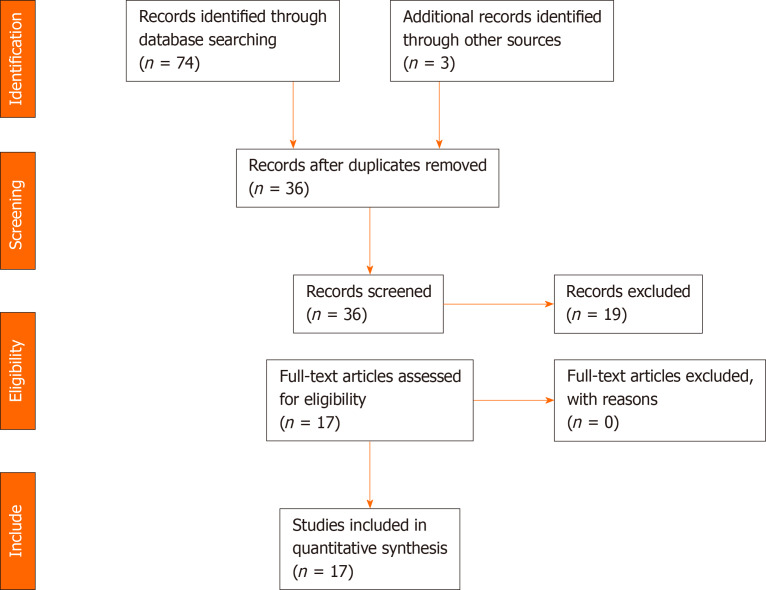
For the systematic review, the initial search yielded 74 papers. After duplicate removal, abstract screening, cross-referencing search, and individual paper analysis, 17 were included in the study. The PRISMA flow diagram in Figure 1 depicts the reasons for paper exclusion.
Figure 1.
PRISMA flow diagram.
Data were collected and the results are summarized in Table 1. A total of 22 cases of AP in COVID-19 patients was reported in 17 articles (11 case reports, 2 case series and 4 letters to Editor). Some of the included cases do not include important medical data, including patient previous medical history, a complete characterization and classification of the AP episode or of the COVID-19 infection. While all cases of AP were diagnosed based on the Atlanta guidelines, in many, the severity of the AP episode was not adequately recorded or there was insufficient available data to support the given severity score (mild, moderately severe, and severe). Even so, when clearly stated, we used the indicated severity score in this review. In very few reports, COVID-19 severity was assessed and different criteria were used, so we decided to remove this item from our review.
Table 1.
Case reports/series of acute pancreatitis and coronavirus disease 2019 infection
| Ref. | Study design | Population, No. | Gender | Age (yr) | Co-morbidities |
Symptoms on admission
|
AP etiology | AP severity | Local complications | Systemic complications | COVID-19 diagnosis | ICU | Mechanical ventilation | |
|
Resp
|
GI
|
|||||||||||||
| Kumar et al[30], 2020 | Case report | 1 | F | 67 | HT, abdominal surgery | X | √ | Unknown | (Moderately) severe1 | Pancreatic necrosis | Respiratory dysfunction | NP swabRT-PCR2 | √ | X |
| Ibrahim et al[31], 2020 | Case report | 23 | M | 33 | NA | √ | √ | Unknown | Severe | None | Respiratory and renal failureIleus | NP swabRT-PCR2 | √ | √ |
| Elhence et al[24], 2020 | Case series | 5 | F | 31 | NA | √ | √ | Biliary | Severe | Infected WON | Respiratory failure | NA4 | NA | X |
| M | 40 | Chronic alcoholism | √ | √ | Alcohol | Severe | Infected WON | Respiratory and renal failures | RT-PCR5 | NA | X | |||
| M | 42 | NA | √ | √ | Biliary | Severe | Infected WON | Respiratory failure | NA6 | NA | NA | |||
| NA | NA | NA | √ | √ | Unknown | Mild | X | NA | NA2 | NA | NA | |||
| NA | NA | NA | √ | √ | Unknown | Mild | X | NA | NA2 | NA | NA | |||
| Cheung et al[32], 2020 | Case report | 1 | M | 38 | None | X7 | √ | Unknown | Mild8 | X | X | NP swab; RT-PCR2 | X | NA |
| Brikman et al[33], 2020 | Case report | 1 | M | 61 | None | √ | √ | Unknown | NA | X | NA | NP swab; RT-PCR9 | NA | X |
| Liaquat et al[34], 2020 | Case report | 1 | M | 53 | None | √ | √ | Type 1 AIP due to elevated IgG4 levels | Severe | Acute infected necrotic collection7 | NA | NP swab; RT-PCR10 | NA | X |
| Bokhari and Mahmood[35], 2020 | Case report | 1 | M | 32 | None | √ | √ | Unknown | NA | Acute peripancreatic fluid collection | NA | RT-PCR11 | X | NA |
| Gonzalo-Voltas et al[36], 2020 | Case report | 1 | F | 76 | GERD, dislipidemia | √ | √ | Unknown | Mild | X | None | RT-PCR2 | X | NA |
| Gadiparthi et al[28], 2020 | Letter to editor | 1 | M | 40 | Obesity (grade II) | X | √ | Metabolic (hypertriglyceridemia) | Moderately severe | Acute fluid collections | Respiratory | NP swab; RT-PCR2 | √ | X |
| Karimzadeh et al[37], 2020 | Case report | 1 | F | 65 | HT, asthma | X | √ | Unknown | NA | X | Respiratory failure | RT-PCR2 | √ | X |
| Pinte and Baicus[38], 2020 | Letter to editor | 1 | M | 47 | None | √ | X | Unknown | NA | X | NA | NA | NA | NA |
| Schepis et al[21], 2020 | Case report | 1 | F | 67 | NA | X | √ | Unknown | Moderately severe | Pseudocyst | NA | NP swab and pseudocyst fluid RT-PCR2 | NA | NA |
| Miao et al[39], 2020 | Letter to editor | 1 | F | 26 | None | X | √ | Unknown | NA | X | NA | RT-PCR2 | NA | NA |
| Aloysius et al[40], 2020 | Case report | 1 | F | 36 | Obesity (grade II), chronic anxiety | √ | √ | Unknown | Severe | X | Respiratory | NP swab; RT-PCR12 | √ | X |
| Hadi et al[41], 2020 | Case series | 313 | F | 47 | None | √ | X | Unknown | Severe | X | Respiratory and renal failure | NP swab and tracheal aspirates RT-PCR2 | √ | √ |
| F | 68 | HT, hypothyroidism, osteoporosis | √ | √ | Unknown | Severe | NA | Respiratory and renal failure | NP swab and tracheal aspirates RT-PCR2 | √ | √ | |||
| Anand et al[42], 2020 | Letter to editor | 1 | F | 59 | Thrombophilia, cholecystectomy | √ | X14 | Unknown | NA | X | NA | RT-PCR15 | X | NA |
| Meireles et al[43], 2020 | Case report | 1 | F | 36 | CKD, HT | √ | X | Unknown | NA | X | NA | NA16 | X | NA |
Respiratory disfunction not completely stratified.
Cases are signaled with when coronavirus disease 2019 (COVID-19) diagnosis was established on the admission for acute pancreatitis (AP).
Only one patient had diagnoses of AP.
COVID-19 diagnosis made 62 d after AP onset.
COVID-19 diagnosis made 34 d after AP onset.
COVID-19 diagnosis made 91 d after AP onset.
When presented to ED with AP (1 wk previously he was diagnosed with COVID-19, but no medical history is given).
The patient was readmitted 1 wk after the initial episode. In both, he had a mild course with favorable evolution under conservative management.
COVID-19 diagnosis established 14 d earlier to AP episode.
On the 2nd readmission due to autoimmune pancreatitis.
COVID-19 diagnosis established 1 wk before AP admission.
COVID-19 diagnosis after AP admission.
One patient without evidence of AP.
Gastrointestinal symptoms present on readmission, but not on initial admission with COVID-19 complicated by streptococcal pneumonia.
AP diagnosis established after COVID-19 diagnosis.
AP diagnosis established 11 d after initial COVID-19 disease (on the 7th day of admission).
AIP: Autoimmune pancreatitis; AP: Acute pancreatitis; CKD: Chronic kidney disease; COVID-19: Coronavirus disease 2019; F: Female; GERD: Gastroesophageal reflux disease; GI: Gastrointestinal; HT: Arterial hypertension; ICU: Intensive care units; M: Male; NA: Not available; NP: Nasopharyngeal; Resp: Respiratory; RT-PCR: Real-time polymerase chain reaction.
In the narrative review, expert opinions, review articles, and experimental studies, including in vitro experiments were included. Several plausible explanations on pancreatic injury by SARS-CoV-2 are noted and can be grouped into direct virus-mediated injury, systemic inflammatory response and circulating pro-inflammatory interleukins and cytokines, virus-induced lipotoxicity, and drug-induced injury. These mechanisms are further explored in the Discussion.
DISCUSSION
Pancreatic lesions, usually defined by serum amylase and/or lipase elevations, and cases of AP have been reported in COVID-19 patients. Autopsy studies in patients previously infected by SARS-CoV-2 identified areas of focal pancreatitis and pancreatic and/or peripancreatic necrosis and calcifications, but only two-thirds of these patients had exhibited symptoms suggestive of AP[8]. The diagnosis of AP, based on the modified Atlanta criteria, requires two of the following three features: Abdominal pain consistent with AP (acute onset of a persistent, severe, epigastric pain often radiating to the back), serum lipase activity (or amylase activity) at least three times greater than the upper limit of normal, and characteristic findings of AP on contrast-enhanced computed tomography (CECT) and less commonly magnetic resonance imaging or transabdominal ultrasonography[9]. This classification also divides AP into interstitial edematous pancreatitis and necrotizing pancreatitis and identifies local and systemic complications, which have a clear impact on disease progression, morbidity, and mortality[9]. All cases included in our study fulfilled the above criteria for the diagnosis of AP, but only a few included AP classification and local and systemic complications in the case report.
Mechanisms of pancreatic injury by SARS-CoV-2
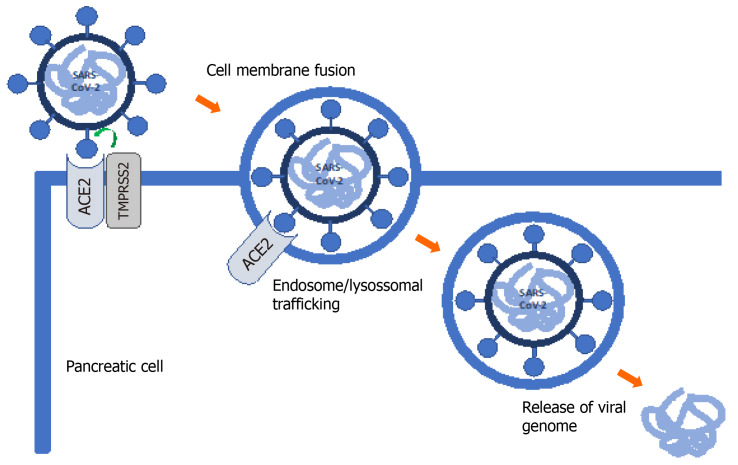
SARS-CoV-2 infection requires entry of the virus into the host cell. Metallopeptidase ACE2 has been identified as the cell receptor. Transmembrane serine protease 2 (TMPRSS2) facilitates viral entry at the plasma membrane surface. As such, co-expression of both ACE2 and TMPRSS2 is critical for successful SARS-CoV-2 infection (Figure 2)[10].
Figure 2.
Severe acute respiratory syndrome coronavirus 2 mechanism of cell entry. Host cell entry is caused by the binding of the spike S glycoprotein found on the viral cell surface to angiotensin-converting enzyme 2 (ACE2), a protease on the host cell surface. This entry process is assisted by priming of the S protein by the host cell transmembrane serine protease 2. After S protein binding, the virus is internalized, uncoated and the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) genome is released into the cytoplasm. The viral RNA is then replicated and translated. Following reproduction of all viral components, coronavirus is assembled and released via vesicular exocytosis.
ACE2 is normally expressed in the pancreas. Liu et al[6] explored its expression and distribution, finding higher levels of ACE2 in the pancreas than in the lung and ACE2 expression in both exocrine glands and islets. Most studies have focused on ACE2 expression and there are few reports on TMPRSS2 expression in the pancreas. In one of these studies, Coate et al[11] found that ACE2 is mainly expressed in islet and exocrine tissue capillaries and some ductal cells, while TMPRSS2 is mainly expressed in ductal cells. However, ACE2 and TMPRSS2 are rarely co-expressed in pancreatic ducts. Pancreatic beta cells do not co-express ACE2 and TMPRSS2 and several authors have questioned the direct cytotoxic effects of SARS-CoV-2 on beta cells. It is still unknown whether SARS-CoV-2 directly and/or indirectly affects beta cell function[11]. However, COVID-19-associated glucose metabolism changes and diabetes appear to be multifactorial, resulting from systemic inflammation and metabolic changes in other organs, including the liver, muscle and adipose tissues, and are not exclusively the result of pancreatic damage. Further studies evaluating SARS-CoV-2 entry into beta cells and not only receptor expression are needed[11].
Severe cases of AP and COVID-19 are characterized by a cytokine storm, which ultimately leads to multiorgan failure and increased mortality. A recent meta-analysis by Hegyi et al[12] found similar patterns of cytokine expression in both COVID-19 and AP. In this scenario, pancreatic damage may result in interstitial leakage of pancreatic lipase and consequently fat tissue lipolysis increasing unsaturated fatty acid levels, which in turn causes mitochondrial injury and excessive production and release of proinflammatory mediators–a cytokine storm. Levels of interleukin (IL)-6, IL-8, and IL-10 were increased in severe cases of both AP and COVID-19 compared to non-severe cases[12]. Consequently, some authors have hypothesized a beneficial role of extracorporeal cytokine absorption in severe cases[13]. At this point, there are insufficient data to differentiate between severe AP caused by COVID-19 from severe AP with COVID-19.
After the cytokine storm associated with severe COVID-19 cases, there is a migration of inflammatory cells to the inflammation/infection site, promoting a pro-inflammatory feedback loop. Tissue factor is upregulated on platelets, white blood and endothelial cells, leading to activation of both extrinsic and intrinsic coagulation pathways and thrombin generation. This microthrombotic event described in lung vasculature[14] can also take place in the pancreatic vasculature, causing hypoperfusion and ischemia[15] with the subsequent induction of an inflammatory response and AP.
Current evidence and open research questions
After reviewing the clinical cases, certain issues are worth analyzing: Abdominal pain, a cardinal symptom in most cases of AP, may be due to SARS-CoV-2 injury to the GI tract, and it may not be possible to differentiate it from the AP pain; SARS-CoV-2 pancreatic injury causing serum lipase and/or amylase elevation has been recognized, mainly in severe cases, but per se it is not diagnostic of AP; serum lipase elevation is not specific of pancreatic pathology, and can be seen in other GI pathology, including gastroparesis, gastritis, enteritis and colitis[16], which are also recognized to be part of the COVID-19 clinical picture; and although infrequent, there are cases of severe AP due to respiratory dysfunction in COVID-19 patients without abnormalities on CECT scan (most severe cases exhibit local peripancreatic complications).
A multiple-hit theory in AP has been recognized and multiple etiological factors can contribute to AP development. However, a complete work-up determining AP etiology is of utmost importance, to avoid deeming cases as idiopathic or establishing an incorrect diagnosis. Viral-attributed AP is rare, but well described, more frequently affecting immunocompromised patients[17,18]. The underlying pathophysiology varies with the type of virus involved. Although still controversial, the main receptors of SARS-CoV-2 are expressed in the pancreas and pancreatic injury has been recognized in COVID-19 patients, especially in severe cases. The thrombogenic state of COVID-19 can also contribute to pancreatic hypoperfusion and ischemia, another established etiology of AP. However, in some cases, it is difficult to exclude other causes of AP, including certain medications. (1) Tocilizumab, which has been proposed for the treatment of COVID-19, has been associated with the development of AP from 2 wk of treatment onset, and hypertriglyceridemia, an established etiology of AP[19]. (2) Propofol infusion in critically ill patients increases serum triglyceride levels, secondary to the lipid emulsion vehicle, which can contribute to hypertriglyceridemia and pancreatic injury in COVID-19 patients[19]. (3) Lopinavir/ritonavir, which are associated with lipid metabolism abnormalities in COVID-19 patients[20], have not been implied as causative agents of AP, but clinicians should be aware of possible treatment side-effects. And (4) Doxycycline, lisinopril, estrogens and steroids are associated with AP development, and constituted the chronic medications of some of the patients included in the case reports.
Even SARS-CoV-2 detection in pseudocyst fluid, as reported by Schepis and colleagues[21], is not unequivocal evidence of AP caused by the novel coronavirus, and several hypotheses arise, including retrograde contamination from the GI tract and/or SARS-CoV-2 infection through inflammatory cells, as a Trojan horse. In a retrospective multicenter study on unusual manifestations of COVID-19, Miró et al[22] did not find an increased frequency of AP in these patients.
Furthermore, idiopathic AP (IAP) is an exclusion diagnosis, which indicates that no etiology has been determined after a complete diagnostic evaluation, including a detailed history, laboratory serum tests, and adequate imaging. Numerous studies have suggested that microlithiasis and sludge may be the cause of a large subset of previously diagnosed IAP and endoscopic ultrasound imaging and magnetic resonance cholangiopancreatography can detect biliary etiology in one-third of patients diagnosed with IAP[23]. The etiologic evaluation of the reported cases is highly heterogeneous and doubts may arise in their classification as IAP, viral-attributed AP cases or even SARS-CoV-2 induced AP. The controversy broadens when determining the role of SARS-CoV-2 in cases of AP with a defined etiology.
It is also important to consider the temporal relationship between COVID-19 diagnosis and AP. In some cases, COVID-19 was diagnosed several days after AP admission, which leads us to question the impact of COVID-19 in these cases.
In a case series, published by Elhence et al[24], three cases of severe AP with respiratory failure (systemic complication of AP) tested positive for COVID-19 several days after the diagnosis of AP (34 to 91 d from admission). These patients did not develop severe respiratory complications due to the novel coronavirus. A marked inflammatory response early in the course of AP can lead to organ failure and the development of a compensatory anti-inflammatory response syndrome, a state of immune exhaustion[9], preventing a strong inflammatory response to SARS-CoV-2. It has also been postulated that the immune response in AP, determined by individual genetic factors, can also modulate the inflammatory response to SARS-CoV-2 and that COVID-19 may be associated with a milder course in most of these cases, especially in younger patients.
In relation to AP treatment in COVID-19 patients, currently no guidelines are available and no specific recommendations can be made. General supportive measures and fluid resuscitation guided by the patient’s hemodynamic status are the mainstay of AP treatment. However, careful monitoring is advised as many COVID-19 patients may pose specific and unpredictable challenges. Drugs known to cause pancreatic lesion should be considered for suspension, based on their indication, the patient´s clinical status, and their risk-benefit relationship.
Several sequelae of AP including new onset diabetes and exocrine insufficiency are more frequent in severe cases of AP, alcohol-induced pancreatitis, and when pancreatic and/or peripancreatic necrosis develops[25,26]. These sequelae are also recognized as indirect signs of pancreatic lesion in COVID-19 patients, which have a higher incidence than previously considered and can develop even in milder cases. Wang and colleagues[27], in a retrospective analysis, indicated that 6 of 9 patients with COVID-19 pneumonia and pancreatic injury developed blood glucose abnormalities and Gadiparthi et al[28] reported an AP case with new-onset type 2 diabetes. Exocrine insufficiency was not mentioned in the included cases, and to the best of our knowledge, no other pancreas-related sequelae are currently reported in the literature. Further studies and follow-up of patients with presumed COVID-19-induced AP are needed to evaluate the incidence and prognosis of these sequelae.
The paucity of published literature associated with short follow-up periods and some inconsistent findings, have rendered prognostic evaluation in this subset of patients difficult to determine. At this moment, no considerations in the prognosis of AP in COVID-19 patients can be drawn.
This review had some limitations. The case reports included in our review have a risk of bias, as certain clinical information was not included and none of the case reports were written according to CARE guidelines[29]. The etiologic work-up of some of these patients was also incomplete when considering current guidelines, and the attribution of SARS-CoV-2 as the causative agent of AP in some causes may be abusive.
CONCLUSION
Despite the trend in recent literature of trying to establish or refute the role of SARS-CoV-2 in AP cases, currently, there is no sufficient evidence showing that COVID-19 can cause AP or negatively impact prognosis. Adherence to AP guidelines, namely diagnosis and etiological work-up, and careful monitoring of patients are of utmost importance to ensure the most adequate orientation and avoid convenience diagnosis. Prediction of disease course, assessment of disease severity in the background of COVID-19 and overall outcome when these two entities coexist are some of the most pertinent open research questions. Further studies are needed to clarify SARS-CoV-2 pancreatic injury and to directly exploit a causal relation between SARS-CoV-2 and AP. Furthermore, patients with suspected SARS-CoV-2-induced AP should be followed in a timely manner to assess patient’s recovery and/or associated complications including new-onset diabetes, pancreatic exocrine insufficiency, pancreatitis-induced local complications, and/or chronic pancreatitis.
ARTICLE HIGHLIGHTS
Research background
There is increasing literature connecting acute pancreatitis (AP) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, but whether SARS-CoV-2 can cause AP or is an epiphenomenon remains a subject of debate.
Research motivation
To explore current literature and provide a concise overview of the current evidence as well as possible mechanisms of pancreatic injury in coronavirus disease 2019 (COVID-19) patients.
Research objectives
To provide an overview of current evidence on AP in COVID-19 patients and to promote and enhance future studies on this special subset of patients.
Research methods
Systematic and narrative review of the literature.
Research results
Available studies on AP in COVID-19 patients present important limitations and mechanisms of pancreatic injury are debatable and not completely understood.
Research conclusions
Currently there is insufficient evidence showing that SARS-CoV-2 infection can cause AP and the therapeutic and prognostic significance of AP in COVID-19 patients is largely unknown.
Research perspectives
This is a very important issue, requiring ongoing research.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Sociedade Portuguesa de Cirurgia; Asociación Española de Cirujanos; Sociedade Portuguesa de Cirurgia Minimamente Invasiva; Sociedade Portuguesa da Hérnia e Parede Abdominal.
Peer-review started: January 10, 2021
First decision: February 14, 2021
Article in press: June 1, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Portugal
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Badovinac D, Fujino Y, Zhan Q, Zhao CF S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
Contributor Information
Tiago Correia de Sá, Department of General Surgery, Centro Hospitalar do Tâmega e Sousa, Penafiel 4564-007, Portugal. tiago.rc.sa@gmail.com.
Carlos Soares, Department of General Surgery, Centro Hospitalar do Tâmega e Sousa, Penafiel 4564-007, Portugal.
Mónica Rocha, Department of General Surgery, Centro Hospitalar do Tâmega e Sousa, Penafiel 4564-007, Portugal.
References
- 1.Worldometer W. Coronavirus Update (live)-Worldometer. [cited 25 December 2020]. Available from: https://www.worldometers.info/coronavirus/?
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020; 158: 1831-1833. :e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors AA, Osterhaus AD, van der Voort PH, Mulder DJ, van Goor H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020;251:228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin Gastroenterol Hepatol 2020; 18: 2128-2130. :e2. doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome : Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, Cui X, Xiao J, Zhan J, Meng T, Zhou W, Liu J, Xu H. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69:1010–1018. [Google Scholar]
- 11.Coate KC, Cha J, Shrestha S, Wang W, Gonçalves LM, Almaça J, Kapp ME, Fasolino M, Morgan A, Dai C, Saunders DC, Bottino R, Aramandla R, Jenkins R, Stein R, Kaestner KH, Vahedi G Consortium H. Brissova M, Powers AC. SARS-CoV-2 Cell Entry Factors ACE2 and TMPRSS2 are Expressed in the Pancreas but are Not Enriched in Islet Endocrine Cells. bioRxiv. 2020 doi: 10.1016/j.cmet.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hegyi P, Szakács Z, Sahin-Tóth M. Lipotoxicity and Cytokine Storm in Severe Acute Pancreatitis and COVID-19. Gastroenterology. 2020;159:824–827. doi: 10.1053/j.gastro.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Convertino I, Tuccori M, Ferraro S, Valdiserra G, Cappello E, Focosi D, Blandizzi C. Exploring pharmacological approaches for managing cytokine storm associated with pneumonia and acute respiratory distress syndrome in COVID-19 patients. Crit Care. 2020;24:331. doi: 10.1186/s13054-020-03020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price LC, McCabe C, Garfield B, Wort SJ. Thrombosis and COVID-19 pneumonia: the clot thickens! Eur Respir J. 2020;56 doi: 10.1183/13993003.01608-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hackert T, Hartwig W, Fritz S, Schneider L, Strobel O, Werner J. Ischemic acute pancreatitis: clinical features of 11 patients and review of the literature. Am J Surg. 2009;197:450–454. doi: 10.1016/j.amjsurg.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Kahil K, El Halabi M, Bou Daher H, Rustom LBO, Marrache M, Ichkhanian Y, El Sayed M, Sharara AI. Significant elevations in serum lipase in the emergency department: When it is not pancreatitis! Am J Emerg Med. 2020;38:1033–1034. doi: 10.1016/j.ajem.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Rawla P, Bandaru SS, Vellipuram AR. Review of Infectious Etiology of Acute Pancreatitis. Gastroenterology Res. 2017;10:153–158. doi: 10.14740/gr858w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simons-Linares CR, Imam Z, Chahal P. Viral-Attributed Acute Pancreatitis: A Systematic Review. Dig Dis Sci. 2020 doi: 10.1007/s10620-020-06531-9. [DOI] [PubMed] [Google Scholar]
- 19.Morrison AR, Johnson JM, Ramesh M, Bradley P, Jennings J, Smith ZR. Acute hypertriglyceridemia in patients with COVID-19 receiving tocilizumab. J Med Virol. 2020;92:1791–1792. doi: 10.1002/jmv.25907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubel AR, Chong PL, Abdullah MS, Asli R, Momin RN, Mani BI, Chong VH. Lipemic serum in patients with Coronavirus Disease 2019 (COVID-19) undergoing treatment. J Med Virol. 2020;92:1810–1811. doi: 10.1002/jmv.25942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schepis T, Larghi A, Papa A, Miele L, Panzuto F, De Biase L, Annibale B, Cattani P, Rapaccini GL. SARS-CoV2 RNA detection in a pancreatic pseudocyst sample. Pancreatology. 2020;20:1011–1012. doi: 10.1016/j.pan.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miró Ò, Llorens P, Jiménez S, Piñera P, Burillo-Putze G, Martín A, Martín-Sánchez FJ, González Del Castillo J Spanish Investigators in Emergency Situations TeAm (SIESTA) network. Frequency of five unusual presentations in patients with COVID-19: results of the UMC-19-S1. Epidemiol Infect. 2020;148:e189. doi: 10.1017/S0950268820001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan J, Ouyang Y, Yu C, Yang X, Xia L, Lu N. Comparison of EUS with MRCP in idiopathic acute pancreatitis: a systematic review and meta-analysis. Gastrointest Endosc 2018; 87: 1180-1188. :e9. doi: 10.1016/j.gie.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Elhence A, Mahapatra SJ, Vajpai T, Garg PK. Acute pancreatitis and nosocomial COVID-19: Cause specific host responses may determine lung injury. Pancreatology. 2020;20:1258–1261. doi: 10.1016/j.pan.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhi M, Zhu X, Lugea A, Waldron RT, Pandol SJ, Li L. Incidence of New Onset Diabetes Mellitus Secondary to Acute Pancreatitis: A Systematic Review and Meta-Analysis. Front Physiol. 2019;10:637. doi: 10.3389/fphys.2019.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jawaid S, Forsmark CE. Exocrine Pancreatic Insufficiency Following Acute Pancreatitis: True Association or EPIphenomenon? Dig Dis Sci. 2019;64:1731–1733. doi: 10.1007/s10620-019-05653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang F, Wang H, Fan J, Zhang Y, Zhao Q. Pancreatic Injury Patterns in Patients With Coronavirus Disease 19 Pneumonia. Gastroenterology. 2020;159:367–370. doi: 10.1053/j.gastro.2020.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gadiparthi C, Bassi M, Yegneswaran B, Ho S, Pitchumoni CS. Hyperglycemia, Hypertriglyceridemia, and Acute Pancreatitis in COVID-19 Infection: Clinical Implications. Pancreas. 2020;49:e62–e63. doi: 10.1097/MPA.0000000000001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, Kiene H, Helfand M, Altman DG, Sox H, Werthmann PG, Moher D, Rison RA, Shamseer L, Koch CA, Sun GH, Hanaway P, Sudak NL, Kaszkin-Bettag M, Carpenter JE, Gagnier JJ. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 2017;89:218–235. doi: 10.1016/j.jclinepi.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 30.Kumaran NK, Karmakar BK, Taylor OM. Coronavirus disease-19 (COVID-19) associated with acute necrotising pancreatitis (ANP) BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-237903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibrahim YS, Karuppasamy G, Parambil JV, Alsoub H, Al-Shokri SD. Case Report: Paralytic Ileus: A Potential Extrapulmonary Manifestation of Severe COVID-19. Am J Trop Med Hyg. 2020;103:1600–1603. doi: 10.4269/ajtmh.20-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung S, Delgado Fuentes A, Fetterman AD. Recurrent Acute Pancreatitis in a Patient with COVID-19 Infection. Am J Case Rep. 2020;21:e927076. doi: 10.12659/AJCR.927076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brikman S, Denysova V, Menzal H, Dori G. Acute pancreatitis in a 61-year-old man with COVID-19. CMAJ. 2020;192:E858–E859. doi: 10.1503/cmaj.201029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liaquat H, Shupp B, Kapoor S, Matin A. High-Dose Prednisone for Treatment of Autoimmune Pancreatitis in a Patient with Coronavirus Disease 2019 (COVID-19) due to Infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Am J Case Rep. 2020;21:e926475. doi: 10.12659/AJCR.926475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bokhari SMMA, Mahmood F. Case Report: Novel Coronavirus-A Potential Cause of Acute Pancreatitis? Am J Trop Med Hyg. 2020;103:1154–1155. doi: 10.4269/ajtmh.20-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalo-Voltas A, Uxia Fernández-Pérez-Torres C, Baena-Díez JM. Acute pancreatitis in a patient with COVID-19 infection. Med Clin (Engl Ed) 2020; 155: 183-184. doi: 10.1016/j.medcle.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karimzadeh S, Manzuri A, Ebrahimi M, Huy NT. COVID-19 presenting as acute pancreatitis: Lessons from a patient in Iran. Pancreatology. 2020;20:1024–1025. doi: 10.1016/j.pan.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinte L, Baicus C. Pancreatic involvement in SARS-CoV-2: case report and living review. J Gastrointestin Liver Dis. 2020;29:275–276. doi: 10.15403/jgld-2618. [DOI] [PubMed] [Google Scholar]
- 39.Miao Y, Lidove O, Mauhin W. First case of acute pancreatitis related to SARS-CoV-2 infection. Br J Surg. 2020;107:e270. doi: 10.1002/bjs.11741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aloysius MM, Thatti A, Gupta A, Sharma N, Bansal P, Goyal H. COVID-19 presenting as acute pancreatitis. Pancreatology. 2020;20:1026–1027. doi: 10.1016/j.pan.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hadi A, Werge M, Kristiansen KT, Pedersen UG, Karstensen JG, Novovic S, Gluud LL. Coronavirus Disease-19 (COVID-19) associated with severe acute pancreatitis: Case report on three family members. Pancreatology. 2020;20:665–667. doi: 10.1016/j.pan.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anand ER, Major C, Pickering O, Nelson M. Acute pancreatitis in a COVID-19 patient. Br J Surg. 2020;107:e182. doi: 10.1002/bjs.11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meireles PA, Bessa F, Gaspar P, Parreira I, Silva VD, Mota C, Alvoeiro L. Acalculous Acute Pancreatitis in a COVID-19 Patient. Eur J Case Rep Intern Med. 2020;7:001710. doi: 10.12890/2020_001710. [DOI] [PMC free article] [PubMed] [Google Scholar]