Abstract
BACKGROUND
Evidence for exercise as an efficacious strategy to improve aerobic capacity of breast cancer survivors (BCS) has come largely from intervention studies conducted in laboratory settings. There is an increasing need to translate to community-type settings, but the efficacy of those interventions using gold standard evaluation is not well-established.
AIM
To investigate whether similar improvement in aerobic capacity (maximal oxygen consumption [VO2]) measured with gold standard testing can be achieved through a community-based setting in BCS.
METHODS
A peak cardiopulmonary exercise test (VO2peak), 6-min walk test (6MWT), and timed up and go test (TUG) were assessed pre- and post-16 wk of progressive intensity aerobic and strength training exercise at a community center.
RESULTS
The sample consisted of 31 early BCS (< 1 year since treatment completion) and 15 controls (CTLs). Both groups significantly improved VO2peak (+1.2 mL/kg/min; P = 0.030), 6MWT (+35 meters; P < 0.001), and TUG (-0.44 s; P < 0.01) following training. Both groups improved peak cycling power during the cardiopulmonary exercise test with BCS improving by +10 watts more than the CTLs (P = 0.020). Average exercise attendance was 71% (34 of 48 possible days), but compliant days averaged only 60% of total days for aerobic, and < 40% for strength in both groups.
CONCLUSION
Community-based exercise programs can be an effective strategy to improve aerobic capacity and physical function for early-stage BCS but potentially not to the same extent observed in laboratory-based randomized controlled trials. Further research is needed to explore barriers and facilitators of exercise engagement in community-based centers to maximize training benefits for adults with cancer.
Keywords: Aerobic capacity, Breast cancer, Community-based, Exercise, Physical function
Core Tip: Breast cancer survivors can improve aerobic capacity and physical function through participation in community-based exercise programs. However, these improvements may not be as substantial as those observed in laboratory-based randomized controlled trials. While community-based programs may provide cancer survivors better flexibility and access than laboratory settings, to maximize training benefits, continued work developing and testing exercise intervention prescriptions and associated outcomes is necessary in addition to exploring barriers and facilitators of exercise engagement for adults with cancer.
INTRODUCTION
Cardiorespiratory fitness describes the ability to use oxygen to produce energy for physical work and can be quantified as aerobic capacity (maximal oxygen consumption [VO2]; mL O2/kg/min) using the gold standard maximal cardiopulmonary exercise testing (CPET). Breast cancer survivors (BCS) have substantially impaired aerobic capacities[1,2], with a decline in VO2 similar to that experienced with a 10 year increase in age, which can be attributed to both cancer treatment-related toxicities and increased sedentary lifestyle habits[3,4]. This impairment places BCS at substantial risk of death from cardiovascular (CV) disease and loss of functional independence[5]. Fortunately, exercise is a known promoter of CV health in the general population[6] and is an effective strategy for improving aerobic capacity in BCS during and post-treatment[7-9].
Multiple well-designed and controlled exercise intervention trials have demonstrated the benefits of aerobic and strength training exercise on aerobic capacity and other physical function outcomes, resulting in the development of exercise prescription guidelines for adults with cancer[10]. Guidelines were recently updated in 2019 and recommend at least 30 min of moderate intensity aerobic exercise at least 3 d/wk, and at least 2 d/wk of strength training for people with cancer[11]. Participation in exercise interventions reflecting these guidelines have demonstrated increases in aerobic capacity by 2.3-2.9 mL/kg/min (approximately 10%-15%)[7,9,12]. However, these changes in aerobic capacity have been observed primarily from interventions in highly controlled laboratory randomized controlled trials (RCTs), and the efficacy of this “trial-proven” exercise prescription delivered through real-world settings such as community-based centers has not been well-studied[13]. These settings likely differ from laboratory settings because they may be more relaxed environments with the opportunity for increased socialization between participants, and may provide additional flexibility in terms of training modes and session attendance (ATT) than what laboratory-controlled trials may provide or allow, regardless that the intention to improve fitness and quality of life may be the same. Of the limited work available on evaluating outcomes in community-based settings, cardiorespiratory fitness appears to be maintained or improved but has not been evaluated using direct, gold standard exercise testing methods (i.e. maximal CPET)[14-17]. Instead, alternative indicators of functional capacity such as the 6-min walk test (6MWT), and the timed up and go (TUG) test have been used, which may offer improved utility and feasibility in clinical/community-based centers. As exercise is increasingly recommended to survivors, and as interventions transition from highly controlled laboratory based settings to more practical venues like community-based centers, it is important to precisely evaluate whether similar cardiorespiratory fitness benefits can still be achieved to properly support and accommodate a large volume of cancer survivors in need of improving or even maintaining their overall health, physical function, and quality of life through participation in regular exercise[13,18].
Exercise programming for cancer survivors is a well-recognized challenge in exercise oncology[19-21] because survivors frequently require tailored exercise accommodations based on cancer-related treatments and associated side effects. Oncology-trained staff in community-based settings may be uniquely positioned to modify workouts, but these necessary adjustments have the potential to significantly impact exercise engagement[20,22]. Therefore, collecting and reporting exercise efficacy, ATT, and compliance in community-based intervention studies will improve understanding of how patients engage in these settings and how exercise engagement may relate to physical fitness and health outcomes[23-25].
The primary aim of this study was to evaluate the impact of a community-based exercise program on change in aerobic capacity in women with breast cancer measured using the gold standard CPET. A secondary aim was to evaluate if changes in aerobic capacity in the community-based setting differ between women with and without cancer. A third aim evaluated how exercise engagement (ATT and compliance) relates to changes in aerobic capacity. For the primary aim, we hypothesized that aerobic capacity would significantly improve following community-based training. For the second and third aims, we hypothesized that women in both groups would benefit similarly from the community-based protocol and that the observed changes in aerobic capacity would be directly related to training ATT and compliance.
MATERIALS AND METHODS
This study used a parallel-group, pre-post design. Two groups (one BCS, one non-cancer control [CTL]) of women were engaged in an identical supervised exercise evaluation and intervention at Get REAL and Heel (GRH), an established, community-based exercise facility for cancer survivors in Chapel Hill, North Carolina that has offered exercise training reflective of recommended guidelines to cancer survivors for the past 10 years. This study leveraged an associated exercise oncology lab with gold standard testing capacity to evaluate the existing community-based program as it has currently operated. For data collection, two consecutive visits to the Exercise Oncology Research Laboratory of the UNC Department of Exercise and Sport Science occurred prior to (pre-intervention) and immediately following (post-intervention) the 16-wk exercise program. Outcomes evaluated included peak aerobic capacity (VO2peak), the 6MWT, and the TUG test. Patient demographics and cancer-specific clinical data were extracted from the UNC Health Care electronic medical record by the research team. The study (NCT03760536) was approved by the Protocol Review Committee of the UNC Lineberger Comprehensive Cancer Center and the UNC Institutional Review Board. All participants provided written informed consent.
Study participants
Women ≥ 21-years-old diagnosed with early-stage (Stage 0-III) breast cancer and within 1 year of completing primary therapy (surgery, chemotherapy, radiation) were eligible for the BCS arm. Women in the CTL group were age-matched with no history of cancer. Both groups self-reported no more than 2 d of physical activity per week and not meeting recommended physical activity guidelines. Both groups self-reported no cardiovascular, metabolic, or orthopedic limitations, and were cleared by a cardiologist (both groups) and oncologists (BCS only) prior to participating. Participants in the BCS group were recruited from the Medical Oncology clinic at the North Carolina Cancer Hospital, and by word of mouth from local oncologists and cancer centers. Participants in the CTL group were residents from nearby communities and recruited via electronic and paper fliers and word of mouth. The recruitment period was April 2017-2019 for BCS and February to August 2019 for CTL.
Exercise intervention
Both BCS and CTL groups participated in a 16-wk supervised, small-group (approximately 4-8 participants per session) exercise intervention including combination aerobic and strength exercise training 3 d a week for approximately 1-h total per d (48 total days of training opportunity) at the GRH facility. A variety of equipment for training was used and adapted to individual participant fitness and mobility needs, allowing trainers to maximize patient safety and exercise engagement while helping participants strive towards reaching guideline-prescribed levels of activity. Participants could choose treadmills, stationary bikes or ellipticals for aerobic work and body weight, resistance bands, dumbbells, or machine weights for strength training, depending on their abilities and preferences.
The specific exercise prescription for the supervised intervention at the GRH facility is presented in Table 1. In the first 2 wk, all study participants were asked to engage in low intensity aerobic exercise for 10-15 min plus approximately 30 min of light-to-moderate strength training each day at the facility. Participants were encouraged to challenge themselves to safely engage in exercise intensity that elicited the prescribed exertion level (Borg’s Rating of Perceived Exertion[26], RPE) throughout the intervention. RPE was used as a surrogate measure of intensity in this study in place of heart rate monitoring since it has historically been used at GRH because heart rate transmitter signals tended to overlap between participants in the limited training space available. Intensity could be increased under the direction of trained exercise staff by adding grade (treadmill), resistance (cycling), or speed (mph or rpm) for aerobic training, and by progressing from body weight to resistance bands to free weights or machines for resistance training. The exercise training program has been implemented and designed to create a dose-response to help participants safely attain weekly exercise goals reflective of national guidelines[11].
Table 1.
Exercise progression in the Get REAL & HEEL Exercise Program
|
Aerobic exercise
|
Week 1-2
|
Week 3-7
|
Week 8-16
|
| Duration (min) | 10-15 | 10-30 | 30 |
| Intensity | Low | Moderate | |
| RPE | 8-11 | 12-14 | |
| Resistance exercise | Week 1-2 | Week 3-7 | Week 8-16 |
| Duration (min) | ~30 | ||
| Intensity | Low to moderate | High | |
| RPE | 7-13 | 14-15 | |
| Sets x Reps / exercise | 1 x 15 | 2 x 10 - 15 | 2 x 10 |
Data collection
Aerobic capacity: Participants completed a familiarization session for the maximal CPET on the first day of data collection using a Corival (Lode B.V., Groningen, The Netherlands) electronically-braked cycle ergometer. Participants were fitted with a Polar heart rate monitor (Polar Electro Inc., Lake Success, NY, United States) and a mask for indirect calorimetry using the ParvoMedics metabolic system. No gas was collected or analyzed on the first day but participants completed a 5-min unloaded and 20 watts loaded cycling warm-up followed by an incremental 15 watts/min with a protocol up to 75% heart rate reserve for familiarization. On day 2 of data collection, participants completed the same CPET protocol but continued until they reached volitional exhaustion or were stopped when oxygen consumption plateaued despite an increase in wattage. Testing was also terminated if cadence was < 50 rpm. Gas exchange data were exported in 5 s average bins, and VO2peak was recorded as the average of the three highest recordings within the final minute of the maximal test. Time to test termination (TTE) was recorded as the ramp-only portion (excluding the 5-min warm up portion), and peak power was the highest wattage recorded before test termination and were used as additional descriptive data for better interpretation of testing performance. This protocol was repeated at the end of the 16-wk exercise intervention.
Intervention ATT and compliance: Training records were maintained by GRH staff to track participant ATT and compliance over 16 wk. ATT was calculated as the number of days participants came to the facility out of 48 total days of opportunity. Compliance was also included in our study to: help distinguish ATT from fulfillment of the workout prescription; and to quantify completed exercise load and progression, which improves the ability to evaluate effects of the intervention on VO2peak with more granularity than ATT alone. An aerobic compliant (aCOMP) day was defined as the participant achieving ≥ 80% of the prescribed duration of aerobic exercise within the prescribed RPE range (Borg 6-20 scale[26]). A strength compliant (sCOMP) day was defined as the participant achieving ≥ 80% of prescribed strength volume (sets x repetitions) within the prescribed RPE range. The 80% duration/volume cutoff in conjunction with RPE as an indicator of intensity was used to help quantify specific training load, not just ATT, and was determined by an experienced exercise oncology research team member as training criteria reasonable to induce changes in aerobic capacity.
Additional outcomes: 6MWT and TUG. On the first day of data collection and prior to CPET familiarization, participants completed one 6MWT and two TUG tests to assess functional capacity. The fastest TUG time was recorded for analysis. This protocol was repeated at the end of the 16-wk intervention. These outcomes were included for additional context about participant performance and may be useful for reference/implementation in future work as a more clinically feasible measure of cardiorespiratory fitness and overall functionality alternate to gold standard VO2peak outcomes, since most community-based program usually do not have the capacity to conduct maximal CPETs.
Power calculations
Published systematic reviews and meta analyses support exercise therapy to increase aerobic capacity in breast cancer survivors by approximately 2.3-2.9 mL O2/kg/min[7,9,12] in laboratory RCTs. For our analysis, power was calculated based on a primary outcome of mean difference in delta VO2peak of BCS following training. Given 80% power and an alpha of 0.05, and assuming a mean difference following training of 2.5 mL O2/kg/min and standard deviation of 3 (d = 0.83), 11 BCS participants would be required. Oversampling was performed to account for potential dropouts and missing data.
Statistical analyses
Statistical analyses were performed using Jamovi open source software (The Jamovi project, version 1.2.5). Baseline descriptive statistics were computed to summarize participant demographics, breast cancer diagnosis/treatment characteristics, and ATT and compliance with the exercise intervention. The α-level was set a priori for all statistical procedures at < 0.05.
Aerobic capacity following training: The primary aim of this study was to evaluate the impact of a community-based exercise program on change in aerobic capacity (deltaVO2peak) in women with breast cancer measured using the gold standard CPET.
Aerobic capacity between groups following training: A linear mixed model was used to evaluate the impact of the exercise training program on change in aerobic capacity (deltaVO2peak) between women with and without cancer. The model used fixed effects of time (pre- vs post-testing) and condition (BCS vs CTL), and a random effect of subject with adjustment for age. If time-by-condition interactions were not significant, the final models estimated the main effects of condition and time on each outcome. Cohen’s d effect size was calculated per group as the mean difference from pre- to post-testing, divided by the standard deviation (SD) of the mean difference. For a priori interpretation of effect sizes, Cohen’s “rules of thumb” were used: small = 0.20, medium = 0.50, and large = 0.80[27].
Intervention ATT and compliance: Independent samples t-tests were used to compare exercise ATT and compliance between groups after the intervention. Univariate linear regression models were used to evaluate associations of deltaVO2peak with days of exercise ATT, days of aCOMP, and days of sCOMP per group. For an exploratory analysis, the two groups were also pooled and reevaluated.
TTE, peak power, 6MWT, and TUG: Separate linear mixed models were used to evaluate the effects of time (pre- vs post-testing) and condition (BCS vs CTL) on time to test termination, peak power, 6MW, and TUG. If time-by-condition interactions were not significant, the final models estimated the main effects of condition and time on each outcome. These analyses were completed to provide supplemental information about the performance changes from participating in a real-world community-based program; programs that provided BCS with flexible opportunities to engage in regular supervised exercise training.
RESULTS
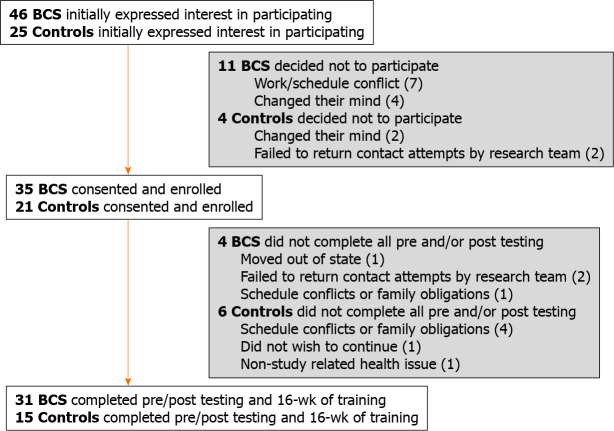
Thirty-five women with breast cancer were enrolled, of which 31 completed all study assessments. Twenty-one non-cancer CTLs were enrolled, of which 15 completed all study assessments (Figure 1). Those who did not complete post-testing were excluded from the analyses due to missing data but were otherwise very similar to women with complete data. At baseline (Table 2), BCS participants were taller (P = 0.035) and had worse TUG performance (P < 0.01) than CTL participants.
Figure 1.
Recruitment and retention.
Table 2.
Baseline characteristics – mean (SD)
|
|
Breast cancer (n = 31)
|
Control (n = 15)
|
Total (n = 46)
|
| Demographics | |||
| Age (yr) | 54 (12) | 55 (8) | 54 (11) |
| Height (cm) | 167 (7) | 162 (7)1 | 165 (8) |
| Weight (kg) | 77 (12) | 75 (14) | 76 (13) |
| BMI categories (kg/m2) | |||
| Normal (18.5 to < 25) | 29% | 13% | 24% |
| Overweight (25 to < 30) | 48% | 60% | 52% |
| Obese (30 to < 35) | 10% | 7% | 9% |
| Obese II (≥ 35) | 13% | 20% | 15% |
| Body fat (%) | 41 (6) | 40 (4) | 41 (5) |
| Lean mass (kg) | 42 (6) | 42 (7) | 42 (6) |
| Postmenopausal (%) | 65% | 67% | 65% |
| Race (Caucasian, %) | 87% | 100% | 91% |
| Clinical variables | |||
| VO2peak (mL/kg/min) | 20.9 (5.3) | 22.4 (2.8) | 21.4 (4.6) |
| Time toexertion (mm:ss) | 9:41 (1:42) | 10:21 (1:20) | 9:54 (1:36) |
| Peak power (Watt) | 120 (26) | 130 (20) | 123 (24) |
| Six minute walk (m) | 538 (72) | 557 (53) | 544 (67) |
| Timed up & go (sec) | 4.8 (1.2) | 3.9 (0.7)1 | 4.5 (1.2) |
| Breast cancer details | |||
| Tumor stage | |||
| 0 | 3% | -- | -- |
| I | 27% | -- | -- |
| II | 47% | -- | -- |
| III | 23% | -- | -- |
| HR status | |||
| ER positive | 81% | -- | -- |
| HER2 status | |||
| Positive (all received trastuzumab) | 26% | -- | -- |
| Surgery | |||
| Lumpectomy | 71% | -- | -- |
| Mastectomy | 29% | -- | -- |
| Cardiotoxic therapies | |||
| Anthracycline | 23% | -- | -- |
| Trastuzumab | 26% | -- | -- |
| Anthra + Tras | 3% | -- | -- |
| Endocrine therapy | |||
| Aromatase inhibitor | 45% | -- | -- |
| Tamoxifen | 19% | -- | -- |
| Days since end of primary treatment | 101 (91) | -- | -- |
P value < 0.05, significantly different between groups.
Aerobic capacity between settings
Women with breast cancer improved VO2peak following community-based exercise training by 1.2 (3.3) mL O2/kg/min.
Aerobic capacity between participants
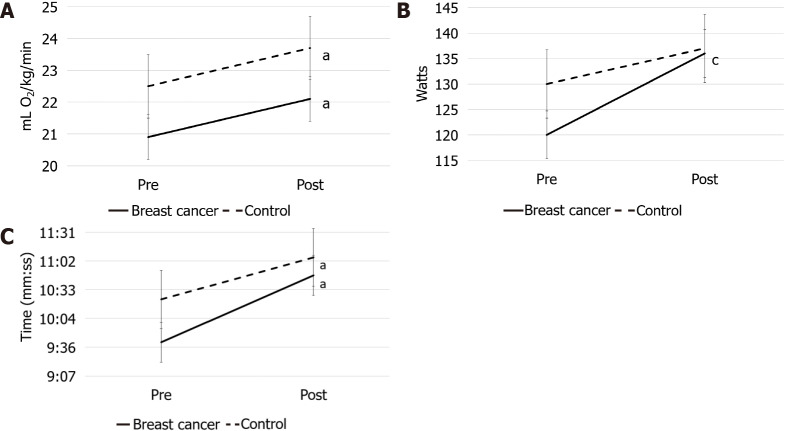
There was no significant time x group interaction for VO2peak but women in both the BCS and CTL groups had significantly improved aerobic capacity by approximately 1.2 mL O2/kg/min (95%confidence interval [CI]: 0.15-2.27, Cohen’s d = 0.36; P = 0.03) from pre- to post-testing (Figure 2A).
Figure 2.
Maximal testing results (standard error bars). A: Aerobic capacity; B: Peak Power; C: Time to Exhaustion in breast cancer survivors and controls before and after 16-wk of training. aP < 0.05 for time; cP < 0.05 for group time interaction.
Intervention ATT and compliance
Both groups attended the same mean number of training sessions (approximately 34 d of 48 planned; P = 0.420) for an average ATT of 71% (Table 3). Differences in days of aerobic compliance between groups did not reach statistical significance but demonstrated a medium to large effect size (Cohen’s d = 0.63; P = 0.06) with CTL exhibiting more days of aerobic compliance than BCS - 32 (9) vs 26 (10) d. Control participants also completed a greater number of compliant strength training days compared to BCS - 18 (3) vs 14 (5) d (Cohen’s d = 1.0; P = 0.011). The total number of compliant strength training days was < 40% total days in both groups. There were no significant associations found in univariate analyses between deltaVO2peak and ATT or compliance for either group, independently or in the pooled sample (data not shown).
Table 3.
Attendance and compliance (out of 48 total days of training opportunity)
|
|
Breast cancer mean (SD) (n = 31)
|
Control mean (SD) (n = 15)
|
Cohen’s d
|
P
value
|
| Intervention Attendance (days) | 35 (9) | 33 (9) | 0.22 | 0.420 |
| Aerobic Compliance (days) | 26 (10) | 32 (9) | -0.63 | 0.060 |
| Strength Compliance (days) | 14 (5) | 18 (3) | -1.00 | 0.011 |
TTE, peak power, 6MWT, and TUG
There was a significant time x group interaction for peak power with both groups demonstrating increased power at post-testing similar to other studies[23], but BCS increased by approximately 10 more watts than the CTLs (95%CI: 1.8-17.5; P = 0.02) (Figure 2B). A significant main effect of time was observed for TTE and 6MWT. Both groups completed almost a minute more of exercise during the peak test (95%CI: 0.60-1.29; P < 0.001) (Figure 2C) and walked approximately 35 meters more in 6 min (95%CI: 21.5-49.1; P < 0.001), which is considered clinically meaningful in clinical populations[28,29], at post-testing. Significant main effects of time and group were observed for TUG with both groups improving time by approximately 0.4 s (95%CI: -0.72-(-0.17); P < 0.01) with an approximately 0.8 s difference between groups at baseline sustained after exercise training (95%CI: 0.21-1.5; P = 0.012).
DISCUSSION
Participation in community-based exercise training reflective of recommended guidelines increased aerobic capacity in women with breast cancer in our study, but not to the extent observed in laboratory RCT settings. The 1.2 mL O2/kg/min (+6%) improvement following community-based training is less than the 2.3-2.9 mL/kg/min (approximately 10%-15%) improvement reported in recent meta-analyses; however, the latter interventions were completed in laboratory RCT settings[7,9,12]. Changes in VO2peak following training in non-RCT settings have been only minimally evaluated using gold standard methodology[13] in contrast to alternatives such as submaximal VO2peak testing, 6MWT, and/or TUG[14,16,17,30] but have nonetheless supported beneficial changes. However, while the transition of interventions from laboratory settings to community settings is increasing[13,31,32], it is important to evaluate outcomes with gold standard testing to ensure survivors are receiving effective intervention in this newer setting. While the aerobic capacity improvement in our study may be small, substantial decline in aerobic capacity is a known manifestation of cancer survivorship[1,3,33]. Therefore, maintenance or even slight improvement is encouraging especially in conjunction with the observed improvements in peak cycling power and cycling endurance. Furthermore, while functional capacity (6MWT and TUG) of BCS in our study is not equivalent to non-cancer population norms[34], it reflects improvement commonly observed for women with breast cancer[35], which is of clinical relevance[28,29] and underscores the beneficial impact of exercise for demands of daily life.
Both women with and without breast cancer in our study demonstrated similar improvements in aerobic capacity (+1.2 mL/kg/min; P = 0.03) following the community-based exercise program. Interestingly, VO2peak of both groups reflected that of published norms for women with breast cancer who are post-treatment (21.5 mL O2/kg/min)[2]. For CTL participants, this is substantially lower than conventional standards[36]. Recently, our laboratory has consistently observed impaired VO2peak in both BCS and middle-aged, sedentary women without cancer[37,38]. We speculate that this may reflect a regional fitness characteristic but warrants further investigation. In terms of training response for non-cancer populations, interventions similar to our study have generally elicited up to a 15% improvement in VO2peak[39,40]. Therefore, the approximate 6% improvement observed in our study is also less than what is considered clinically significant in non-cancer populations[41,42]. The relatively poor compliance observed in our study indicates that prescribed training progression was not followed and may provide a partial explanation for the relatively modest improvements observed in VO2peak. Exercise dosing and progression are important components related to the FITT principle[20,21,43] and are important factors for impacting patient physical and functional outcomes[8,11,44]. Furthermore, while ATT in our study (71%, attending about 2 of 3 training days per week) is similar to other exercise oncology interventions (approximately 70%-75%)[19,20,45], it reflects less than that recommended by national guidelines (≥ 3 d/wk, ≥ 30 min/d)[11]. It is reasonable to consider that improved exercise ATT closer to or matching the established, recommended guidelines in addition to improved compliance (which considers progressing intensity) would lead to more optimal physiological changes. However, despite modest intervention engagement, participants in our study still demonstrated positive/beneficial training adaptations which reiterates the need to investigate the fundamental question of what is the optimal training prescription for this population[9,23,43,46].
The ability to provide a community-based program that may improve physical and functional health and that survivors enjoy is paramount, especially when a survivor needs to prioritize aspects of work, life, and family around exercise[20,46]. Documenting exactly why patients missed sessions was not specifically recorded in this study but were anecdotally related to job, family, or general life obligations. Survivors also frequently expressed enjoyment with study participation and the GRH program, and many women regretted not having started their exercise journey earlier in treatment process. With respect to our generally positive findings, community-based programs such as GRH appear to have a place in cancer survivorship. Continued focus and conversations with survivors to better understand challenges around exercise compliance may help identify targets to improve engagement, and should remain a priority.
Difficulties with exercise engagement and compliance reflect the well-recognized challenges of exercise programming for clinical populations[19-21,47,48]. Based on the observations of our exercise staff and research team, participant compliance was primarily hindered by not achieving prescribed intensity, as measured by self-reported RPE. This method has the inherent weakness of subjectivity; however, it was the most feasible option in our relatively small community-based setting where, historically, heart rate monitor transmitter signals have overlapped between participants, displayed incorrectly, and caused unnecessary worry/confusion to an otherwise upbeat and positive environment. In our study, it was observed that while participants would and could complete both prescribed duration (aerobic) and volume (sets x reps, strength), there was reluctance to increase intensity (grade/speed/weight, etc.) especially in the second half of training when intensity targets increased. Strength training intensity targets were achieved less frequently than aerobic for both groups, leading to fewer strength compliant days than aerobic (Table 3), which has been observed previously in this population[20,49,50]. GRH training staff encouraged and guided participants how to safely reach more difficult intensities and no adverse events were documented during training; therefore, lack of training support, injury, or unnecessary discomfort were unlikely contributors to sub-optimal compliance, yet successfully increasing intensity remained a challenge. While participants were reminded how to contextualize and use the RPE scale, it is possible there was misunderstanding or concern from participants that reporting a higher RPE somehow suggested they were less capable of completing the prescribed exercise.
The primary limitation of our study is that it was a small, self-selected sample of mostly White women willing who were able to engage in exercise training for 4 consecutive months. This is not representative of the majority of women with breast cancer, especially younger women who may have acute family and job demands. The greatest strength of this study was the well-established, long-standing community-based exercise oncology program with veteran training staff, but we recognize that this experienced environment is difficult to replicate[13,51-53] especially in partnership with facilities capable of gold standard testing.
Future research would benefit from the inclusion of more diverse participants and from continued efforts to explore barriers and facilitators of exercise engagement. Calculation of both ATT and compliance in community-based settings similar to the methods presented in our study would improve the ability to quantify exercise load, progression, and dose responses more precisely. Heart rate monitoring in addition to self-reported RPE may also help better communicate and indicate training intensity, especially as technology for self-monitoring progresses. Directly collecting more information from participants about missed sessions and live feedback during training sessions would help provide better understanding of participant barriers, concerns, and potential limitations to engagement. These improvements would help contribute to the development of more specific future exercise prescriptions that are both suitable and effective in community-based settings. Furthermore, a third group of BCS who did not exercise for 16-wk, or who completed prescriptions with differing levels of exercise volume (days/reps/time) and/or undulating intensity progression (high vs low intensity) would help clarify specific findings from the current study and enhance future designs.
CONCLUSION
A community-based exercise program such as GRH can improve aerobic capacity and overall physical function in women with breast cancer, potentially not to the extent of an exercise oncology laboratory based RCT setting but similar to that of women without a cancer diagnosis participating in a similar program. Survivors may not meet recommended physical activity guidelines immediately, but proper training accommodations may help facilitate and encourage the integration of exercise as a daily routine. This is an important first step to life-long exercise commitment and provides the foundation to achieve recommended guidelines. Community-based venues offering exercise oncology programs have the potential to augment long term cancer care[13], will be essential for accommodating the growing number and needs of survivors[18], and should be continually evaluated as interventions are implemented in new settings.
ARTICLE HIGHLIGHTS
Research background
Exercise is an efficacious strategy to improve aerobic capacity of breast cancer survivors (BCS) but has not been consistently evaluated with gold standard testing in community-based settings.
Research motivation
As a growing number of BCS are in need of community-based exercise access, providing effective interventions is paramount to their long term health and functionality.
Research objectives
The objective was to use gold standard testing to determine whether breast cancer survivors exhibit similar improvement in aerobic capacity (maximal oxygen consumption [VO2]) following community-based exercise compared to interventions in laboratory settings.
Research methods
A peak cardiopulmonary exercise test (VO2peak), 6-min walk test (6MWT), and timed up and go test (TUG) were assessed pre- and post-16 wk of progressive intensity aerobic and strength training exercise at a community center.
Research results
Both BCS (n = 31) and CTL (n = 15) groups significantly improved physical and functional capacity following training (VO2peak +1.2 mL/kg/min, P = 0.030; 6MWT +35 meters, P < 0.001; TUG -0.44 s; P < 0.01). Peak cycling power improved in both groups with BCS exhibiting 10 watts more improvement than CTL (P = 0.020). Average exercise ATT of 71%, 34/48 possible days) is in accordance with previous work, and the modest compliance (60% aerobic, < 40% resistance) emphasizes the challenges of exercise engagement in clinical populations.
Research conclusions
A community-based exercise program can effectively improve aerobic capacity and physical function for early stage breast cancer survivors but potentially not to the same extent observed in laboratory-based randomized controlled trials. Further research is needed to explore barriers and facilitators of exercise engagement in community-based centers to maximize training benefits for adults with cancer.
Research perspectives
Providing BCS with accessible and effective exercise interventions is a critical component in survivorship and should be continually evaluated with gold standard outcomes, especially because it is not yet a standard intervention of oncology practice.
Footnotes
Institutional review board statement: The study approved by the Protocol Review Committee of the UNC Lineberger Comprehensive Cancer Center and the UNC Institutional Review Board.
Clinical trial registration statement: This registration policy applies to registry trials. This study is registered at Get REAL and HEEL Research Program - Full Text View - ClinicalTrials.gov.
Informed consent statement: All participants provided written informed consent.
Conflict-of-interest statement: The authors do not have any conflict of interest disclaimers.
Manuscript source: Invited manuscript
Peer-review started: February 10, 2021
First decision: March 17, 2021
Article in press: May 27, 2021
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu J S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Yuan YY
Contributor Information
Jordan T Lee, Department of Exercise and Sport Science, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, United States.
Chad W Wagoner, Department of Exercise and Sport Science, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, United States.
Stephanie A Sullivan, Department of Exercise and Sport Science, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, United States.
Dean J Amatuli, Department of Exercise and Sport Science, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, United States.
Kirsten A Nyrop, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, United States.
Erik D Hanson, Department of Exercise and Sport Science, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, United States.
Lee Stoner, Department of Exercise and Sport Science, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, United States.
Brian C Jensen, Department of Medicine, Division of Cardiology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, United States.
Hyman B Muss, Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC 27599, United States.
Claudio L Battaglini, Department of Exercise and Sport Science, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, United States. claudio@email.unc.edu.
Data sharing statement
No data sharing.
References
- 1.Lakoski SG, Barlow CE, Koelwyn GJ, Hornsby WE, Hernandez J, Defina LF, Radford NB, Thomas SM, Herndon JE 2nd, Peppercorn J, Douglas PS, Jones LW. The influence of adjuvant therapy on cardiorespiratory fitness in early-stage breast cancer seven years after diagnosis: the Cooper Center Longitudinal Study. Breast Cancer Res Treat. 2013;138:909–916. doi: 10.1007/s10549-013-2478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peel AB, Thomas SM, Dittus K, Jones LW, Lakoski SG. Cardiorespiratory fitness in breast cancer patients: a call for normative values. J Am Heart Assoc. 2014;3:e000432. doi: 10.1161/JAHA.113.000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, Hornsby WE, Coan AD, Herndon JE 2nd, Douglas PS, Haykowsky M. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30:2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–1441. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 5.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaminsky LA, Arena R, Beckie TM, Brubaker PH, Church TS, Forman DE, Franklin BA, Gulati M, Lavie CJ, Myers J, Patel MJ, Piña IL, Weintraub WS, Williams MA American Heart Association Advocacy Coordinating Committee. Council on Clinical Cardiology, and Council on Nutrition, Physical Activity and Metabolism. The importance of cardiorespiratory fitness in the United States: the need for a national registry: a policy statement from the American Heart Association. Circulation. 2013;127:652–662. doi: 10.1161/CIR.0b013e31827ee100. [DOI] [PubMed] [Google Scholar]
- 7.Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, Moskowitz CS, Matsoukas K, Iyengar NM, Dang CT, Jones LW. Efficacy of Exercise Therapy on Cardiorespiratory Fitness in Patients With Cancer: A Systematic Review and Meta-Analysis. J Clin Oncol. 2018;36:2297–2305. doi: 10.1200/JCO.2017.77.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkham AA, Bland KA, Sayyari S, Campbell KL, Davis MK. Clinically Relevant Physical Benefits of Exercise Interventions in Breast Cancer Survivors. Curr Oncol Rep. 2016;18:12. doi: 10.1007/s11912-015-0496-3. [DOI] [PubMed] [Google Scholar]
- 9.Battaglini CL, Mills RC, Phillips BL, Lee JT, Story CE, Nascimento MG, Hackney AC. Twenty-five years of research on the effects of exercise training in breast cancer survivors: A systematic review of the literature. World J Clin Oncol. 2014;5:177–190. doi: 10.5306/wjco.v5.i2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen VE, Schwartz AL American College of Sports Medicine. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 11.Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, Zucker DS, Matthews CE, Ligibel JA, Gerber LH, Morris GS, Patel AV, Hue TF, Perna FM, Schmitz KH. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51:2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones LW, Liang Y, Pituskin EN, Battaglini CL, Scott JM, Hornsby WE, Haykowsky M. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16:112–120. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Covington KR, Hidde MC, Pergolotti M, Leach HJ. Community-based exercise programs for cancer survivors: a scoping review of practice-based evidence. Support Care Cancer. 2019;27:4435–4450. doi: 10.1007/s00520-019-05022-6. [DOI] [PubMed] [Google Scholar]
- 14.Schneider CM, Hsieh CC, Sprod LK, Carter SD, Hayward R. Effects of supervised exercise training on cardiopulmonary function and fatigue in breast cancer survivors during and after treatment. Cancer. 2007;110:918–925. doi: 10.1002/cncr.22862. [DOI] [PubMed] [Google Scholar]
- 15.Irwin ML, Cartmel B, Harrigan M, Li F, Sanft T, Shockro L, O'Connor K, Campbell N, Tolaney SM, Mayer EL, Yung R, Freedman RA, Partridge AH, Ligibel JA. Effect of the LIVESTRONG at the YMCA exercise program on physical activity, fitness, quality of life, and fatigue in cancer survivors. Cancer. 2017;123:1249–1258. doi: 10.1002/cncr.30456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leach HJ, Danyluk JM, Nishimura KC, Culos-Reed SN. Benefits of 24 vs 12 weeks of exercise and wellness programming for women undergoing treatment for breast cancer. Support Care Cancer. 2016;24:4597–4606. doi: 10.1007/s00520-016-3302-3. [DOI] [PubMed] [Google Scholar]
- 17.Marker RJ, Cox-Martin E, Jankowski CM, Purcell WT, Peters JC. Evaluation of the effects of a clinically implemented exercise program on physical fitness, fatigue, and depression in cancer survivors. Support Care Cancer. 2018;26:1861–1869. doi: 10.1007/s00520-017-4019-7. [DOI] [PubMed] [Google Scholar]
- 18.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the "Silver Tsunami": Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25:1029–1036. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, Ladha AB, Proulx C, Vallance JK, Lane K, Yasui Y, McKenzie DC. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 20.Kirkham AA, Bonsignore A, Bland KA, McKenzie DC, Gelmon KA, VAN Patten CL, Campbell KL. Exercise Prescription and Adherence for Breast Cancer: One Size Does Not FITT All. Med Sci Sports Exerc. 2018;50:177–186. doi: 10.1249/MSS.0000000000001446. [DOI] [PubMed] [Google Scholar]
- 21.Neil-Sztramko SE, Winters-Stone KM, Bland KA, Campbell KL. Updated systematic review of exercise studies in breast cancer survivors: attention to the principles of exercise training. Br J Sports Med. 2019;53:504–512. doi: 10.1136/bjsports-2017-098389. [DOI] [PubMed] [Google Scholar]
- 22.Kirkham AA, Campbell KL, McKenzie DC. Comparison of aerobic exercise intensity prescription methods in breast cancer. Med Sci Sports Exerc. 2013;45:1443–1450. doi: 10.1249/MSS.0b013e3182895195. [DOI] [PubMed] [Google Scholar]
- 23.Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst Rev. 2018;1:CD011292. doi: 10.1002/14651858.CD011292.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nyrop KA, Deal AM, Choi SK, Wagoner CW, Lee JT, Wood WA, Anders C, Carey LA, Dees EC, Jolly TA, Reeder-Hayes KE, Muss HB. Measuring and understanding adherence in a home-based exercise intervention during chemotherapy for early breast cancer. Breast Cancer Res Treat. 2018;168:43–55. doi: 10.1007/s10549-017-4565-1. [DOI] [PubMed] [Google Scholar]
- 25.Wagoner CW, Choi SK, Deal AM, Lee JT, Wood WA, Muss HB, Nyrop KA. Establishing physical activity in breast cancer: self-report vs activity tracker. Breast Cancer Res Treat. 2019;176:395–400. doi: 10.1007/s10549-019-05263-3. [DOI] [PubMed] [Google Scholar]
- 26.Borg GA. Perceived exertion: a note on "history" and methods. Med Sci Sports. 1973;5:90–93. [PubMed] [Google Scholar]
- 27.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Academic Press; 1977. [Google Scholar]
- 28.Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017;23:377–381. doi: 10.1111/jep.12629. [DOI] [PubMed] [Google Scholar]
- 29.Shoemaker MJ, Curtis AB, Vangsnes E, Dickinson MG. Clinically meaningful change estimates for the six-minute walk test and daily activity in individuals with chronic heart failure. Cardiopulm Phys Ther J. 2013;24:21–29. [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider CM, Repka CP, Brown JM, Lalonde TL, Dallow KT, Barlow CE, Hayward R. Demonstration of the need for cardiovascular and pulmonary normative data for cancer survivors. Int J Sports Med. 2014;35:1134–1137. doi: 10.1055/s-0034-1375691. [DOI] [PubMed] [Google Scholar]
- 31.De Jesus S, Fitzgeorge L, Unsworth K, Massel D, Suskin N, Prapavessis H, Sanatani M. Feasibility of an exercise intervention for fatigued breast cancer patients at a community-based cardiac rehabilitation program. Cancer Manag Res. 2017;9:29–39. doi: 10.2147/CMAR.S117703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noble M, Russell C, Kraemer L, Sharratt M. UW WELL-FIT: the impact of supervised exercise programs on physical capacity and quality of life in individuals receiving treatment for cancer. Support Care Cancer. 2012;20:865–873. doi: 10.1007/s00520-011-1175-z. [DOI] [PubMed] [Google Scholar]
- 33.Jones LW, Haykowsky M, Pituskin EN, Jendzjowsky NG, Tomczak CR, Haennel RG, Mackey JR. Cardiovascular reserve and risk profile of postmenopausal women after chemoendocrine therapy for hormone receptor--positive operable breast cancer. Oncologist. 2007;12:1156–1164. doi: 10.1634/theoncologist.12-10-1156. [DOI] [PubMed] [Google Scholar]
- 34.Rikli RE, Jones CJ. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist. 2013;53:255–267. doi: 10.1093/geront/gns071. [DOI] [PubMed] [Google Scholar]
- 35.Swartz MC, Lewis ZH, Lyons EJ, Jennings K, Middleton A, Deer RR, Arnold D, Dresser K, Ottenbacher KJ, Goodwin JS. Effect of Home- and Community-Based Physical Activity Interventions on Physical Function Among Cancer Survivors: A Systematic Review and Meta-Analysis. Arch Phys Med Rehabil. 2017;98:1652–1665. doi: 10.1016/j.apmr.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fitzgerald MD, Tanaka H, Tran ZV, Seals DR. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: a meta-analysis. J Appl Physiol (1985) 1997;83:160–165. doi: 10.1152/jappl.1997.83.1.160. [DOI] [PubMed] [Google Scholar]
- 37.Wagoner CW, Hanson ED, Ryan ED, Brooks R, Wood WA, Jensen BC, Lee JT, Coffman EM, Battaglini CL. Two weeks of lower body resistance training enhances cycling tolerability to improve precision of maximal cardiopulmonary exercise testing in sedentary middle-aged females. Appl Physiol Nutr Metab. 2019;44:1159–1164. doi: 10.1139/apnm-2018-0623. [DOI] [PubMed] [Google Scholar]
- 38.Evans ES, Hackney AC, Pebole MM, McMurray RG, Muss HB, Deal AM, Battaglini CL. Adrenal Hormone and Metabolic Biomarker Responses to 30 min of Intermittent Cycling Exercise in Breast Cancer Survivors. Int J Sports Med. 2016;37:921–929. doi: 10.1055/s-0042-110654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warburton DE, Nicol CW, Bredin SS. Prescribing exercise as preventive therapy. CMAJ. 2006;174:961–974. doi: 10.1503/cmaj.1040750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 42.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 43.Campbell KL, Neil SE, Winters-Stone KM. Review of exercise studies in breast cancer survivors: attention to principles of exercise training. Br J Sports Med. 2012;46:909–916. doi: 10.1136/bjsports-2010-082719. [DOI] [PubMed] [Google Scholar]
- 44.Scott JM, Thomas SM, Peppercorn JM, Herndon JE 2nd, Douglas PS, Khouri MG, Dang CT, Yu AF, Catalina D, Ciolino C, Capaci C, Michalski MG, Eves ND, Jones LW. Effects of Exercise Therapy Dosing Schedule on Impaired Cardiorespiratory Fitness in Patients With Primary Breast Cancer: A Randomized Controlled Trial. Circulation. 2020;141:560–570. doi: 10.1161/CIRCULATIONAHA.119.043483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santa Mina D, Au D, Brunet J, Jones J, Tomlinson G, Taback N, Field D, Berlingeri A, Bradley H, Howell D. Effects of the community-based Wellspring Cancer Exercise Program on functional and psychosocial outcomes in cancer survivors. Curr Oncol. 2017;24:284–294. doi: 10.3747/co.23.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sweegers MG, Altenburg TM, Chinapaw MJ, Kalter J, Verdonck-de Leeuw IM, Courneya KS, Newton RU, Aaronson NK, Jacobsen PB, Brug J, Buffart LM. Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? Br J Sports Med. 2018;52:505–513. doi: 10.1136/bjsports-2017-097891. [DOI] [PubMed] [Google Scholar]
- 47.Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Friedenreich CM, Yasui Y, Reid RD, Cook D, Jespersen D, Proulx C, Dolan LB, Forbes CC, Wooding E, Trinh L, Segal RJ. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst. 2013;105:1821–1832. doi: 10.1093/jnci/djt297. [DOI] [PubMed] [Google Scholar]
- 48.van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, de Maaker-Berkhof M, Boven E, Schrama J, Geenen MM, Meerum Terwogt JM, van Bochove A, Lustig V, van den Heiligenberg SM, Smorenburg CH, Hellendoorn-van Vreeswijk JA, Sonke GS, Aaronson NK. Effect of Low-Intensity Physical Activity and Moderate- to High-Intensity Physical Exercise During Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J Clin Oncol. 2015;33:1918–1927. doi: 10.1200/JCO.2014.59.1081. [DOI] [PubMed] [Google Scholar]
- 49.Santos WDND, Vieira A, de Lira CAB, Mota JF, Gentil P, de Freitas Junior R, Battaglini CL, Bottaro M, Vieira CA. Once a Week Resistance Training Improves Muscular Strength in Breast Cancer Survivors: A Randomized Controlled Trial. Integr Cancer Ther. 2019;18:1534735419879748. doi: 10.1177/1534735419879748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ottenbacher A, Yu M, Moser RP, Phillips SM, Alfano C, Perna FM. Population Estimates of Meeting Strength Training and Aerobic Guidelines, by Gender and Cancer Survivorship Status: Findings From the Health Information National Trends Survey (HINTS) J Phys Act Health. 2015;12:675–679. doi: 10.1123/jpah.2014-0003. [DOI] [PubMed] [Google Scholar]
- 51.Foley MP, Barnes VA, Hasson SM. Effects of a community-based multimodal exercise program on physical function and quality of life in cancer survivors: a pilot study. Physiother Theory Pract. 2015;31:303–312. doi: 10.3109/09593985.2015.1004390. [DOI] [PubMed] [Google Scholar]
- 52.Rajotte EJ, Yi JC, Baker KS, Gregerson L, Leiserowitz A, Syrjala KL. Community-based exercise program effectiveness and safety for cancer survivors. J Cancer Surviv. 2012;6:219–228. doi: 10.1007/s11764-011-0213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leach HJ, Danyluk JM, Nishimura KC, Culos-Reed SN. Evaluation of a Community-Based Exercise Program for Breast Cancer Patients Undergoing Treatment. Cancer Nurs. 2015;38:417–425. doi: 10.1097/NCC.0000000000000217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data sharing.