Abstract
Reclaiming municipal wastewater for agricultural, environmental, and industrial purposes is increasing in the United States to combat dwindling freshwater supplies. However, there is a lack of data regarding the microbial quality of reclaimed water. In particular, no previous studies have evaluated the occurrence of vancomycin-resistant enterococci (VRE) in reclaimed water used at spray irrigation sites in the United States. To address this knowledge gap, we investigated the occurrence, concentration, and antimicrobial resistance patterns of VRE and vancomycin-susceptible enterococci at three U.S. spray irrigation sites that use reclaimed water. We collected 48 reclaimed water samples from one Mid-Atlantic and two Midwest spray irrigation sites, as well as their respective wastewater treatment plants, in 2009 and 2010. Samples were analyzed for total enterococci and VRE using standard membrane filtration. Isolates were purified and then confirmed using biochemical tests and PCR. Antimicrobial susceptibility testing was conducted using the Sensititre® microbroth dilution system. Data were analyzed by two-sample proportion tests and one-way analysis of variance. We detected total enterococci and VRE in 71% (34/48) and 4% (2/48) of reclaimed water samples, respectively. E. faecalis was the most common species identified. At the Mid-Atlantic spray irrigation site, UV radiation decreased total enterococci to undetectable levels; however, subsequent storage in an open-air pond at this site resulted in increased concentrations of enterococci. E. faecalis isolates recovered from the Mid-Atlantic spray irrigation site expressed intrinsic resistance to quinupristin/dalfopristin; however, non-E. faecalis isolates expressed resistance to quinupristin/dalfopristin (52% of isolates), vancomycin (4%), tetracycline (13%), penicillin (4%) and ciprofloxacin (17%). Our findings show that VRE are present in low numbers in reclaimed water at point-of-use at the sampled spray irrigation sites; however, resistance to other antimicrobial classes is more prevalent, particularly among non-E. faecalis isolates.
Keywords: antibiotic-resistant bacteria, enterococci, vancomycin-resistant enterococci, wastewater, reclaimed water, spray irrigation
1. Introduction1
As the world population increases and global water use escalates, freshwater resources continue to dwindle. To alleviate pressures on freshwater resources, countries—including the United States—are reclaiming treated municipal wastewater for potable and nonpotable reuse (EPA, 2012). This reclaimed water has been defined as “municipal wastewater that has been treated to meet specific water quality criteria with the intent of being used for a range of purposes”(EPA, 2012). In the United States, reclaimed water is used in landscape irrigation, food crop irrigation, snowmaking, groundwater recharge, power production, and indirect and direct potable reuse (EPA, 2012). With increasing reclaimed water use, the potential public health impacts due to microbial contamination of reclaimed water need to be explored and addressed.
Previous studies have shown that a number of bacterial pathogens can survive wastewater treatment including methicillin-resistant Staphylococcus aureus, Escherichia coli, Salmonella, and enterococci (Levantesi et al., 2010; Nagulapally et al., 2009; Rosenberg Goldstein et al., 2012; Rosenberg Goldstein et al., 2014). Vancomycin-resistant enterococci (VRE), in particular, have recently been isolated from wastewater effluent (Garcia et al., 2007; Nagulapally et al., 2009; Rosenberg Goldstein et al., 2014) and could persist in distribution systems that supply reclaimed water to spray irrigation sites.
VRE are gram-positive, opportunistic human pathogens that are resistant to vancomycin (a drug of last resort) and can cause urinary tract infections, wound infections, bacteremia and endocarditis (CDC, 2009). Between 2006 and 2007, Enterococcus spp. was the third most commonly reported pathogen causing healthcare-acquired infections in the United States (Hidron et al., 2008). Twelve percent and 4% of pathogens recovered from healthcare-acquired infections were Enterococcus spp. and VRE, respectively (Hidron et al., 2008). And by 2010, Enterococcus spp. became the second leading cause of healthcare-acquired infections (Sievert et al., 2013). Enterococci, in general, are tolerant to an array of environmental stressors, including extreme temperatures (5–65°C), variable pH levels (4.5–10), and high NaCl concentrations (Fisher and Phillips, 2009). Due to the higher tolerance of enterococci to chlorination, these microorganisms can withstand wastewater treatment processes—including tertiary treatments involving chlorination—and persist in the environment (Castillo-Rojas et al., 2013; Varela et al., 2013).
Hospital effluent discharged to municipal wastewater treatment plants has been identified as an important initial source of environmental contamination of VRE (Varela et al., 2013). Antibiotic-resistant enterococci has been recovered from treated municipal wastewater effluent in the United States, China, and Portugal (Ferreira da Silva et al., 2006; Garcia et al., 2007; Huang et al., 2012; Martins da Costa et al., 2006), and VRE, specifically, has been isolated from treated wastewater effluent in the United States and the United Kingdom (Beier et al., 2008; Caplin et al., 2008; Rosenberg Goldstein et al., 2014).
However, to our knowledge, there are no published studies analyzing reclaimed water recovered from U.S. spray irrigation sites (at point-of-use) for the presence of VRE and total enterococci. In this study, we evaluated the occurrence, concentration, and antimicrobial susceptibilities of VRE and total enterococci recovered from reclaimed water used at three U.S. spray irrigation sites. We also evaluated the impact of storing reclaimed water in open-air ponds on levels of VRE and total enterococci.
2. Materials and Methods
2.1. Study Sites
We sampled three spray irrigation sites that use reclaimed water: one Mid-Atlantic site and two Midwest sites. All sites were chosen based on the willingness of the site operator to participate.
The Mid-Atlantic spray irrigation site (Mid-Atlantic SI1) receives wastewater effluent from a tertiary wastewater treatment plant (WWTP) in an urban area that has been described previously as Mid-Atlantic WWTP1 (Rosenberg Goldstein et al., 2012). Briefly, the raw wastewater influent (681,390 m3/day) at this plant is comprised of domestic and hospital wastewater and the plant employs the following treatment steps: screens, primary clarifier, primary aeration tank, secondary aeration tank, secondary clarifier, multimedia filter, chlorination, dechlorination and discharge. The chlorination dose at this plant was 2–3 mg/L, followed by dechlorination with sodium bisulfite such that the chlorine residual in effluent is < 0.1 mg/L. This treated effluent is then piped to Mid-Atlantic SI1. Once it arrives at Mid-Atlantic SI1 the effluent passes through a double-walled aluminum screen and is then treated with 254 nanometer wavelength ultraviolet (UV) radiation bulbs that produce a minimum of 30,000 microwatt seconds per square centimeter. After UV treatment, the water is pumped into an open-air storage pond at a rate of 230,000 gallons per day with a peak capacity of 4 million gallons. The reclaimed water is then pumped from the storage pond to spray irrigation heads for use in landscaping (Figure 1).
Figure 1.
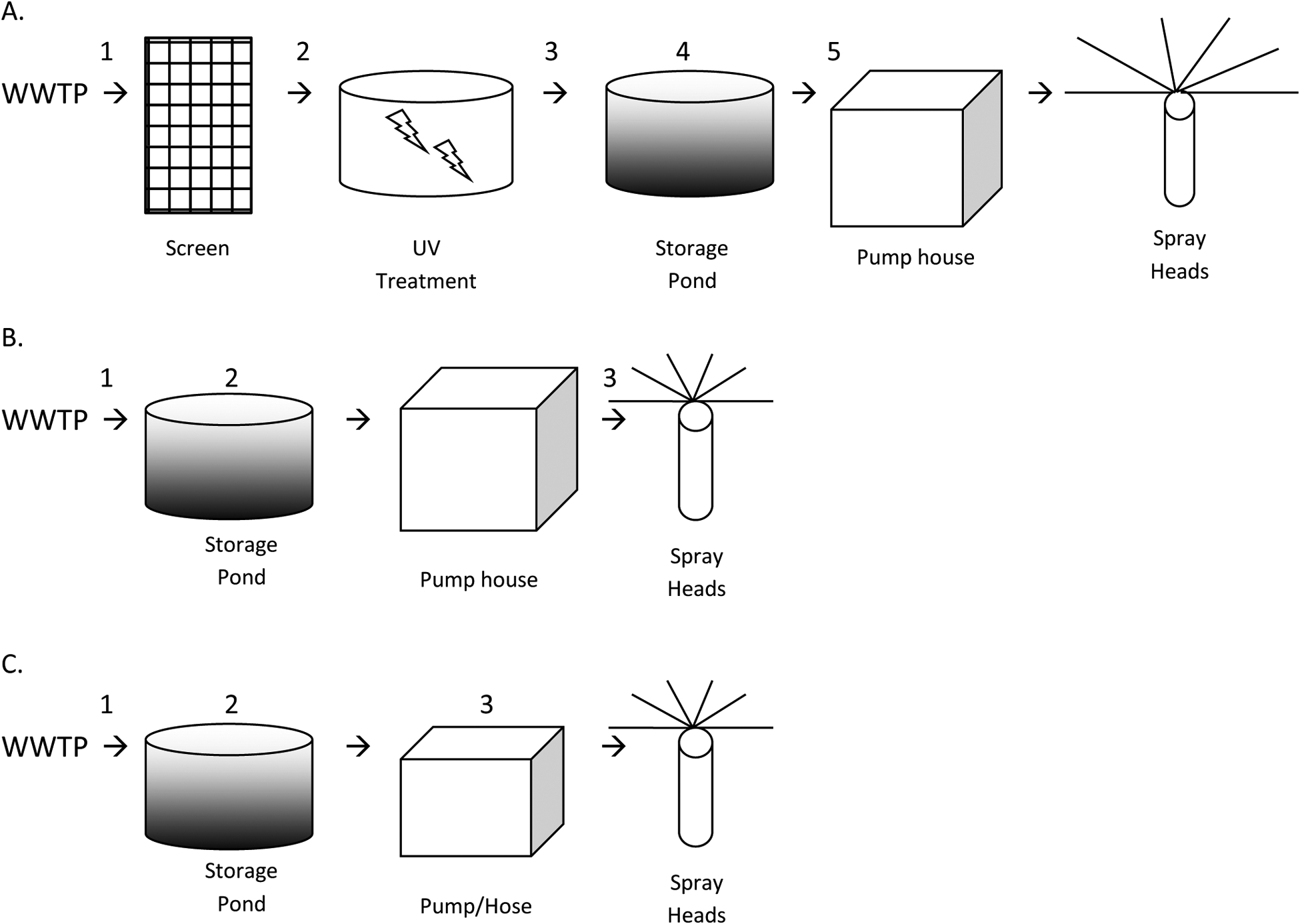
Treatment and/or storage processes at the spray irrigation sites: A) Mid-Atlantic SI1, B) Midwest SI1, and C) Midwest SI2. Numbers depict sampling locations at each site: A) 1=Effluent, 2=Before UV, 3=After UV, 4=Pond, 5=Inlet to Pumphouse; B) and C) 1=Effluent, 2=Storage Pond, 3=Pump/Hose.
Midwest spray irrigation site 1 (Midwest SI1) receives wastewater effluent from a tertiary WWTP in a rural area that has been described previously as Midwest WWTP1 (Rosenberg Goldstein et al., 2012). Briefly, the raw wastewater influent (1,363 m3/day) at this plant is comprised of domestic wastewater and agriculturally influenced stormwater, and the plant employs the following treatment steps: screens, activated sludge lagoons, clarifiers, seasonal chlorination (and dechlorination), and discharge. Seasonal chlorination occurs at this plant in June, July, and August, and during these times the chlorination dose is 4 mg/L with a contact time to assure a chlorine residual of 0 mg/L in effluent. The effluent is then piped to Midwest SI1 where it undergoes no additional treatment, is stored in an open-air storage pond and is then pumped to spray irrigation heads for use in landscaping (Figure 1).
Midwest spray irrigation site 2 (Midwest SI2) receives wastewater effluent from a tertiary WWTP in a rural area that has been described previously as Midwest WWTP2 (Rosenberg Goldstein et al., 2012). Briefly, the raw wastewater influent (1,439 m3/day) at this plant is comprised of domestic wastewater, wastewater from a food production facility, and agriculturally influenced stormwater, and the plant employs the following treatment steps: screens, sequencing batch reactor, lagoon cell A, lagoon cell B, lagoon cell C, lagoon cell D, lagoon cell E, and discharge. The unchlorinated effluent from this plant is piped to Midwest SI2 where it undergoes no additional treatment, is stored in an open-air storage pond and is then pumped to spray irrigation heads for use in landscaping and crop irrigation (Figure 1).
2.2. Sample collection
A total of 48 reclaimed water samples were included in this study (Table 1). All samples were collected between August 2009 and October 2010, and the timing of sample collection was determined by the site operators. Figure 1 indicates the specific locations where the samples were collected. All samples were collected in 1-L sterile polyethylene Nalgene® Wide Mouth Environmental Sample Bottles and transported to the laboratory at 4°C.
Table 1.
Average concentrations of total enterococci and vancomycin-resistant enterococci (VRE) and percentage of VRE out of total recovered isolates by spray irrigation site and treatment or storage step across all sample collection dates.
| Sampling Location (# of samples) |
Total Enterococci (CFU/100mL) |
VRE (CFU/100mL) |
Percentage of VRE | |
|---|---|---|---|---|
| Mid-Atlantic SI1 | ||||
| Mid-Atlantic WWTP1 Effluent (n=2) | 0.41 | 0.0005 | 0.1% | |
| Before UV (n=8) | 35.78 | 0.039 | 0.1% | |
| After UV (n=8) | 0 | 0 | 0% | |
| Pond (n=8) | 0.39 | 0 | 0% | |
| Inlet to Pumphouse (n=8) | 173.63 | 0.13 | 0.07% | |
| Midwest SI1 | ||||
| Midwest WWTP1 Effluent (n=3) | 12.08 | 5.14 | 42.6% | |
| Pond (n=3) | 120.5 | 0.67 | 0.6% | |
| Midwest SI2 | ||||
| Midwest WWTP2 Effluent (n=4) | 56.08 | 0 | 0 | |
| Hose (n=4) | 30.88 | 0 | 0 |
2.3. Isolation
Standard membrane filtration was used to isolate total enterococci and VRE from the reclaimed water samples (EPA, 2002). Ten-fold dilutions of each sample were filtered through 0.45 μm, 47 mm mixed cellulose ester filters (Millipore, Billerica, MA). Filters were then plated in duplicate on membrane-Enterococcus Indoxyl-β-D-Glucoside (mEI) agar (EMD Millipore, Billerica, MA) to isolate total enterococci, and mEI agar modified with 16 μg/mL of vancomycin to isolate VRE. Plates were incubated at 41°C for 24 hr. Colonies with blue halos were considered presumptive total enterococci and VRE. These colonies were purified on Brain Heart Infusion (BHI) agar (Becton, Dickinson and Company, Franklin Lakes, NJ) and archived in Brucella broth (Becton, Dickinson and Company) with 15% glycerol at −80°C. E. faecalis ATCC 29212 was used as a positive control and phosphate buffered saline was used as a negative control throughout the isolation process.
2.4. Identification
Total enterococci and VRE were confirmed and identified using the Gram stain, the catalase test, detection of pyrrolidonyl peptidase (pyr) activity (Remel, Lenexa, KS), and a multiplex PCR assay developed by Micallef et al. (2013). Genomic DNA was extracted by heat lysis as described previously (Micallef et al., 2013). Briefly, the PCR reaction targeted the D-alanine:D-alanine ligase (ddl) genes of E. faecalis and E. faecium, the vancomycin resistance-encoding vanC1 and vanC2/3 genes of E. gallinarum and E. casseliflavus, respectively, and an internal control targeting a 350 base pair portion of the 16S rRNA gene. PCR amplification consisted of an initial denaturing step of 95°C for 3 min, followed by 35 cycles of denaturing at 94°C for 30 s, annealing at 54°C for 30 s, and extension at 72°C for 30 s, with a final extension at 72°C for 5 min. Positive controls used for PCR amplification were E. faecalis ATCC 51299, E. faecium ATCC 51559, E. casseliflavus ATCC 25788, and E. gallinarum ATCC 49573. Molecular grade water was used as a negative control for PCR amplification.
2.5. Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed on all PCR-confirmed Enterococcus isolates (n = 41) using the Sensititre® microbroth dilution system (Trek Diagnostic Systems Inc., Cleveland, OH) following the manufacturer’s recommendations. Cultures incubated overnight were transferred to sterile, demineralized water (Trek Diagnostic Systems) to achieve a 0.5 McFarland standard. Then, 50 μL of each suspension was transferred to sterile cation-adjusted Mueller Hinton broth (Trek Diagnostic Systems), and 50 μL of the broth solution was then dispensed into minimal inhibitory concentration (MIC) plates (Trek Diagnostic Systems) that included the following antibiotics: erythromycin, quinupristin/dalfopristin, vancomycin, tetracycline, gentamicin, linezolid, streptomycin, penicillin, and ciprofloxacin. E. faecalis ATCC 29212 and Staphylococcus aureus ATCC 29213 strains were used for quality control. All plates were read manually. MICs were recorded as the lowest concentration of an antimicrobial that completely inhibited bacterial growth. Resistance break points published by the CLSI were used (CLSI, 2012). Multidrug resistance was defined as resistance to two or more classes of antibiotics.
2.6. Statistical analyses
Descriptive statistics included the percentage of reclaimed water samples that were positive for total enterococci and VRE, as well as the antimicrobial susceptibility patterns of all tested isolates. A two-sample proportion test was performed to compare the percentages of enterococci-positive samples between the Mid-Atlantic spray irrigation sites and the Midwest spray irrigation sites. One-way analysis of variance (ANOVA) was performed to compare average log concentrations of total enterococci by treatment step for each spray irrigation site and its corresponding wastewater treatment plant. For the Mid-Atlantic spray irrigation site, ANOVA was followed by linear contrasts as a post-hoc test to compare average log concentrations of total enterococci between specific treatment/storage steps. In all cases, p-values ≤ 0.05 were defined as statistically significant. All statistical analyses were performed using Stata/IC 10 (StataCorp LP, College Station, TX).
3. Results
3.1. Occurrence of total enterococci and VRE
Enterococcus spp. were detected at all spray irrigation sites (Table 1) and in the majority of reclaimed water samples. Specifically, 71% (34/48) of reclaimed water samples were positive for enterococci: 56% (18/32) of samples from Mid-Atlantic SI1; 100% (2/2) of samples from Mid-Atlantic WWTP1; 100% (3/3) of samples from Midwest SI1; 100% (3/3) of samples from Midwest WWTP1; 100% (5/5) of samples from Midwest SI2; and 100% (3/3) of samples from Midwest WWTP2. The percentage of enterococci-positive samples collected from the Midwest region (100%; 14/14) was greater than that of the Mid-Atlantic region (59%; 20/34) (p=0.002).
VRE were detected at 2 out of 3 of the spray irrigation sites. Specifically, 4% (2/48) of reclaimed water samples were positive for VRE: 3% (1/32) of samples from Mid-Atlantic SI1; 0% (0/2) of samples from Mid-Atlantic WWTP1; 0% (0/3) of samples from Midwest SI1; and 33% (1/3) of samples from Midwest WWTP1. No samples recovered from Midwest SI2 or its associated WWTP were positive for VRE.
Average concentrations of total enterococci and VRE, as well as the percentage of VRE out of total recovered isolates, are shown in Table 1. At the Mid-Atlantic SI1 and Midwest SI1, average concentrations of enterococci were lower in the WWTP effluent samples collected directly from their respective WWTPs compared to the first reclaimed water samples tested at the sites (“Before UV” sample at Mid-Atlantic SI1, and “Pond” sample at Midwest SI1) (Table 1). In contrast, at the Midwest SI2 site, average concentrations of enterococci were higher in the WWTP effluent samples collected directly from the WWTP compared to the first reclaimed water samples tested at the spray irrigation site (“Hose” samples at Midwest SI2) (Table 1). However, these differences were not statistically significant based on one-way ANOVA and post-hoc analyses. At Mid-Atlantic SI1, the concentration of total enterococci in reclaimed water decreased to undetectable levels after on-site UV treatment, but then increased after the water was pumped to and stored in the open-air pond (Table 1; Figure 2). Levels of total enterococci in samples collected from the “Inlet to Pumphouse” at Mid-Atlantic SI1 were statistically significantly higher than levels of total enterococci detected in “Before UV” (p = 0.02), “After UV” (p = 0.005), and “Pond” (p = 0.005) samples (Table 1; Figure 2).
Figure 2.
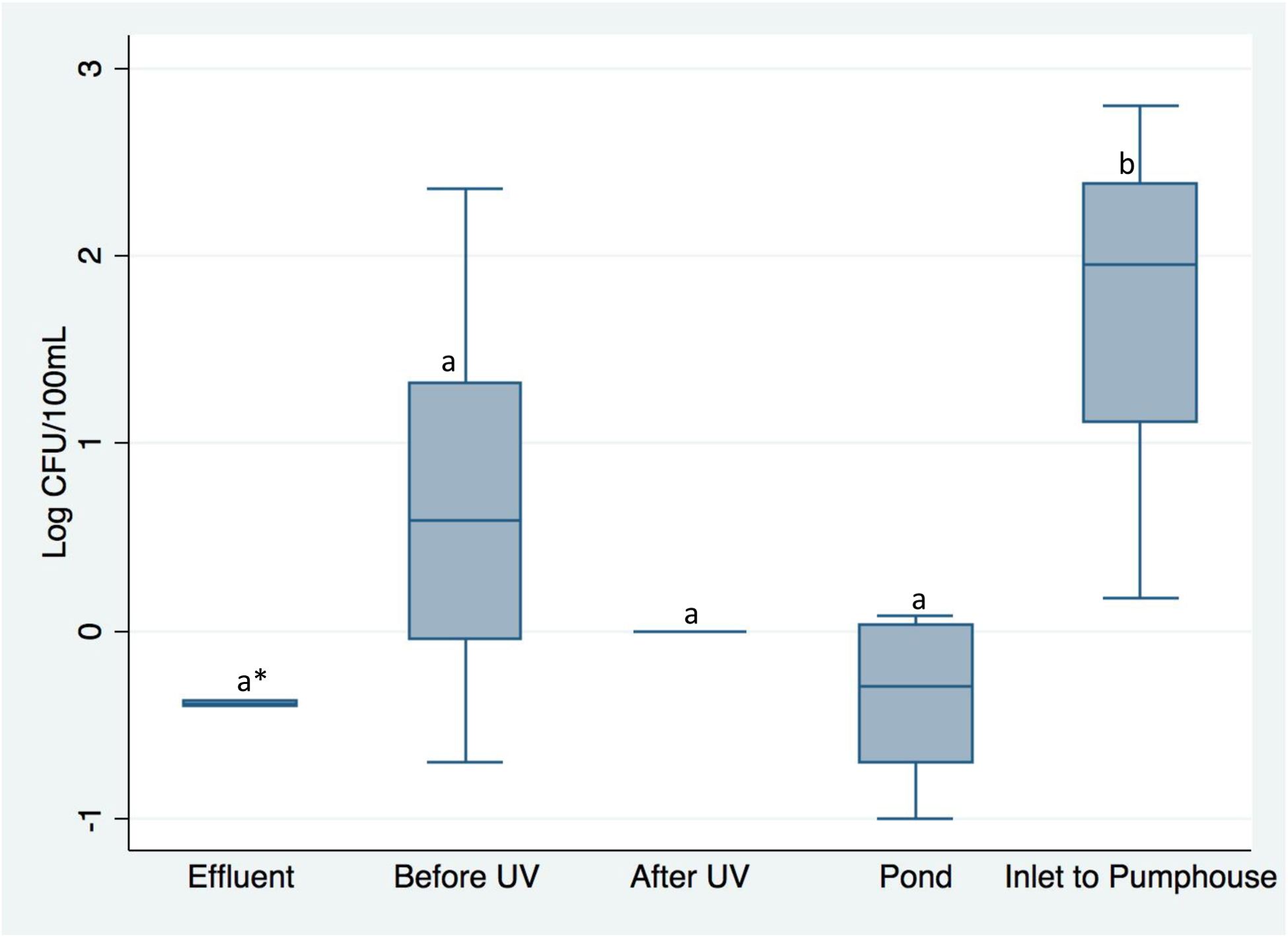
Average log concentrations of total enterococci (Log CFU/100mL) as reclaimed water flows through treatment and storage locations at Mid-Atlantic Spray Irrigation Site 1. Whiskers are drawn from the 75th percentile to the upper adjacent value and from the 25th percentile to the lower adjacent value, the mid-line is the median, letters indicate statistical significance based on post-hoc analyses (p ≤ 0.05), and * indicates that the difference between the effluent and inlet to pumphouse samples was marginally significant (p ≤ 0.06).
In total, 41 enterococci isolates were recovered from all sampled sites. The majority of these isolates were identified as E. faecalis (44%), followed by E. faecium (27%), E. casseliflavus (12%), E. gallinarum (5%) and other (12%) (Table 2).
Table 2.
Number and percentage of total enterococci isolates by species and spray irrigation site
| Number of Isolates (%) | ||||
|---|---|---|---|---|
| Enterococcus | Mid-Atlantic | Midwest | Midwest | |
| species | SI1 (n=36) | SI1 (n=4) | SI2 (n=1) | Total (n=41) |
| E. faecalis | 13 (36) | 4 (100) | 1 (100) | 18 (44) |
| E. faecium | 11 (31) | 0 (0) | 0 (0) | 11 (27) |
| E. casseliflavus | 5 (14) | 0 (0) | 0 (0) | 5 (12) |
| E. gallinarum | 2 (6) | 0 (0) | 0 (0) | 2 (5) |
| Other | 5 (14) | 0 (0) | 0 (0) | 5 (12) |
3.2. Antibiotic resistance patterns
Few isolates were recovered from Midwest SI1 and Midwest SI2; therefore, antibiotic resistance patterns are presented only for isolates recovered from Mid-Atlantic SI1 (n=36). E. faecalis recovered from Mid-Atlantic S11 (n=13) were intrinsically resistant to quinupristin/dalfopristin as expected, but did not express resistance to erythromycin, vancomycin, tetracycline, gentamicin, linezolid, streptomycin, penicillin or ciprofloxacin. In contrast, non-E. faecalis isolates recovered from Mid-Atlantic SI1 (n=23) expressed resistance to several antibiotics: 52% (12/23) of isolates were resistant to quinupristin/dalfopristin; 4% (1/23) were resistant to vancomycin; 13% (3/23) were resistant to tetracycline; 4% (1/23) were resistant to penicillin; and 17% (4/23) were resistant to ciprofloxacin. Figure 3 depicts the antibiotic resistance patterns of non-E. faecalis recovered from each sampling location of Mid-Atlantic SI1.
Figure 3:
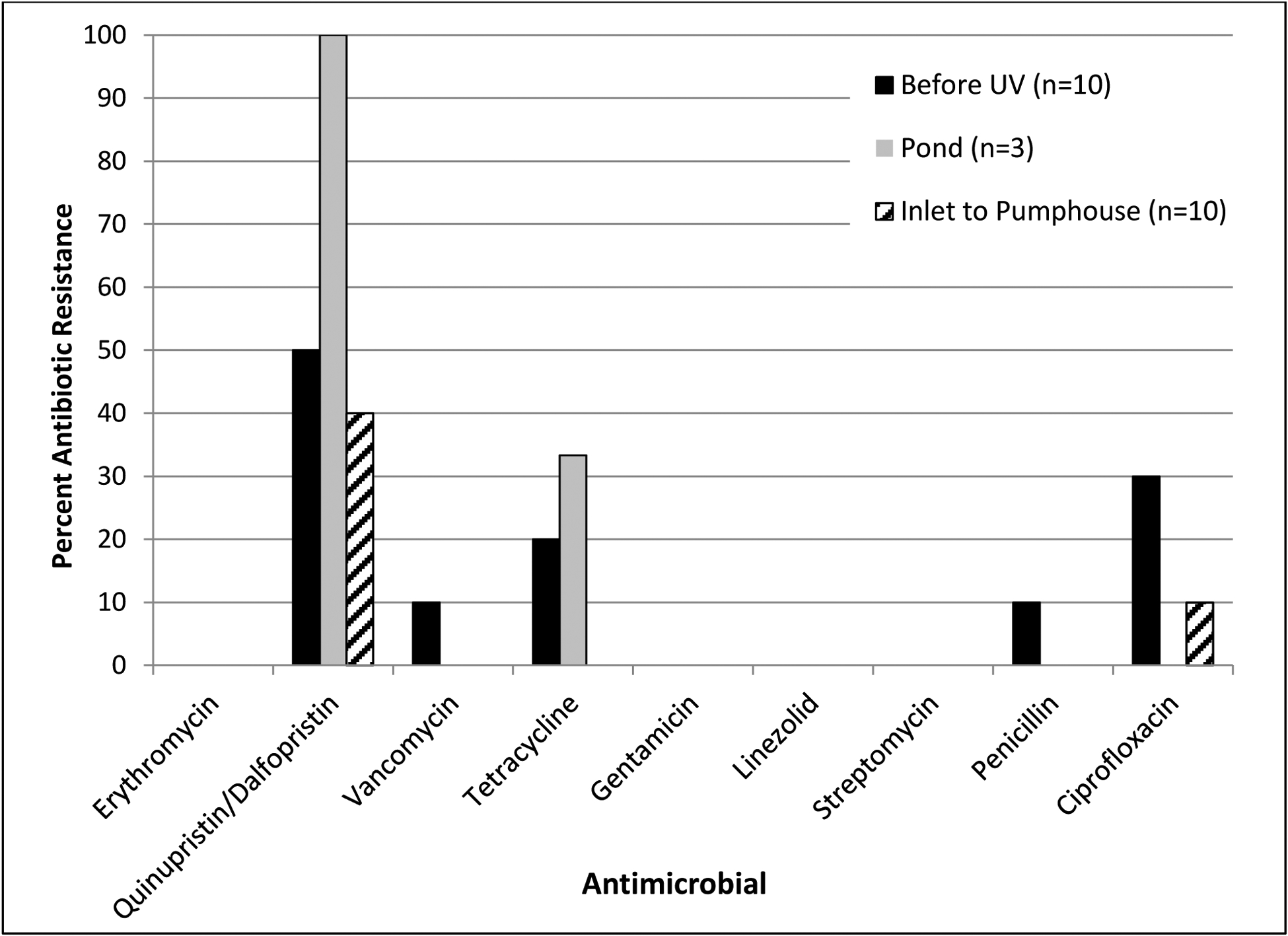
Antimicrobial resistance patterns among non-E. faecalis isolates (E. faecium, E. casseliflavus, E. gallinarum, other) recovered from different sampling locations at Mid-Atlantic SI1.
Three out of 23 (13%) non-E. faecalis isolates from Mid-Atlantic SI1 expressed multidrug resistance. One E. faecium isolate expressed resistance to quinupristin/dalfopristin, tetracycline and penicillin; one Enterococcus spp. isolate that could not be identified to the species level expressed resistance to vancomycin, tetracycline and ciprofloxacin; and one other Enterococcus spp. that could not be identified to the species level expressed resistance to quinupristin/dalfopristin and tetracycline.
4. Discussion
4.1. Occurrence of Enterococcus spp.
To our knowledge, this is the first report of the occurrence of enterococci, VRE and other antibiotic-resistant enterococci in reclaimed water recovered from point-of-use at spray irrigation sites in the United States. Previous studies primarily focused on the detection of VRE and Enterococcus spp. at wastewater treatment plants and in treated effluent (Beier et al., 2008; Caplin et al., 2008; Ferreira da Silva et al., 2006; Garcia et al., 2007; Huang et al., 2012; Martins da Costa et al., 2006; Rosenberg Goldstein et al., 2014). Other studies have identified the presence of enteric bacteria and fecal indicators (in general) in reclaimed water at point-of-use (Abreu-Acosta and Vera, 2011; Bahri et al., 2001; Ryu et al., 2005). Building upon these previous studies, we detected antibiotic-resistant and -susceptible enterococci in the effluent delivered from wastewater treatment plants to spray irrigation sites, reclaimed water stored at these sites, and reclaimed water at point-of-use.
At the Mid-Atlantic spray irrigation site, where the treated wastewater effluent was further disinfected through on-site UV radiation treatment, total enterococci were significantly reduced to an undetectable level after UV treatment. This finding is consistent with previous studies that have identified UV radiation as a successful disinfectant for enterococci (Conner-Kerr et al., 1998; Luczkiewicz et al., 2011; Nagulapally et al., 2009; Ryu et al., 2005). Of particular note, Nagulapally, et al. (2009) determined that VRE were eliminated to undetectable levels in WWTP effluent after UV disinfection. However, at the Mid-Atlantic spray irrigation site, the benefits of UV-disinfection were negated once the treated water was piped to an open-air pond, where concentrations of total enterococci subsequently increased, possibly due to contamination associated with precipitation/run-off events and wildlife. These data suggest that future guidelines regarding the safe use of reclaimed water at spray irrigation sites need to include water storage recommendations in addition to any on-site treatment requirements.
4.2. Enterococcus Species Diversity
Out of the enterococci isolates that could be identified to the species level, 71% were identified as either E. faecium or E. faecalis. These findings are in line with our previous study of the wastewater treatment plants that supplied the reclaimed water, where nearly all of the characterized VRE isolates were E. faecium or E. faecalis (Rosenberg Goldstein et al., 2014). These data are also similar to the recent findings of Ben Said et al. (2015) that demonstrated the predominance of E. faecium and E. faecalis in both wastewater and surface water samples recovered from Tunisia (Ben Said et al., 2015). These two species are the predominant enterococci species found in the human gastrointestinal tract (Silva et al., 2011); therefore, it is not surprising to detect them in significant numbers in municipal wastewater and reclaimed water (Fisher and Phillips, 2009; Murray, 1990). E. faecalis is also the most common species isolated from human clinical enterococcal infections (Fisher and Phillips, 2009; Murray, 1990; Silva et al., 2011) and generally expresses more virulence traits compared to E. faecium (Banerjee and Anupurba, 2015). Thus, the common occurrence of E. faecalis in reclaimed water samples tested in this study may represent a potential public health concern among spray irrigation workers who are heavily exposed to this water source, especially if these individuals are immuno-compromised. However, the fact that the infectious dose of enterococci remains unknown hampers our ability to estimate the potential risk of enterococcal infections among spray irrigators or others exposed to reclaimed water.
4.3. Antibiotic Resistance Patterns
While E. faecalis was the most predominant enterococcal species isolated in this study, these isolates expressed only intrinsic resistance to quinupristin/dalfopristin, as expected. In contrast, the non-E. faecalis isolates recovered from the Mid-Atlantic spray irrigation site expressed resistance to several antibiotics, including quinupristin/dalfopristin (52%), vancomycin (4%), tetracycline (13%), penicillin (4%) and ciprofloxacin (17%) (Figure 3). These resistance rates were considerably lower than those associated with vancomycin-resistant E. faecium isolates that were recovered directly from the wastewater treatment plant that supplied reclaimed water to this site (Rosenberg Goldstein et al., 2014), suggesting that the tertiary wastewater treatment process that was employed at Mid-Atlantic WWTP1 resulted in reduced percentages of antibiotic-resistant enterococci exiting the plant. However, our finding that 52% of non-E. faecalis isolates (including E. faecium) recovered from reclaimed water at point-of-use were resistant to quinupristin/dalfopristin is concerning. Currently, quinupristin/dalfopristin is one of only two chemotherapeutic agents approved by the U.S. Food and Drug Administration for the treatment of vancomycin-resistant E. faecium infections (Arias et al., 2012); therefore, the release of quinupristin/dalfopristin-resistant E. faecium strains into the environment through reclaimed water could potentially have implications for the successful treatment of VRE infections. Nevertheless, additional studies are needed to better understand the linkages between rates of resistant bacteria detected in environmental media (such as reclaimed water) and resistant bacterial infections occurring in clinical settings.
4.5. Limitations
As with any field study, there were limitations to our work. Our study was based on culture-dependent methods; therefore, if VRE were present in viable but non-culturable states within our reclaimed water samples they would not have been detected by our protocols, resulting in possible underestimations of the prevalence and concentrations of VRE in the tested reclaimed water (Lleo et al., 2001). In addition, because we aimed to generate data on concentrations of enterococci and VRE in reclaimed water samples, we refrained from using enrichment steps in our protocols, which resulted in relatively low numbers of recovered isolates. Finally, because only three spray irrigation sites located in two U.S. regions could be included in the study, our findings are not representative of all spray irrigation sites in the U.S. that are utilizing reclaimed water as an alternative freshwater source.
5. Conclusions
To our knowledge, our study is the first to demonstrate the occurrence, concentration, and antimicrobial susceptibility of enterococci and VRE in reclaimed water at point-of-use at U.S. spray irrigation sites. In summary, total enterococci were recovered from the majority of reclaimed water samples and the prevalence of VRE within these samples was low. However, resistance to antibiotics other than vancomycin among enterococci recovered from reclaimed water was prevalent. In addition, we showed that UV radiation of reclaimed water at a spray irrigation site was effective in reducing levels of enterococci to undetectable levels; yet, subsequent storage of the reclaimed water in an open-air pond resulted in concentrations of enterococci that were higher than those before UV treatment. Future regulations regarding the use of reclaimed water in spray irrigation activities should include recommendations concerning additional onsite treatment of the water as well as appropriate storage conditions that would limit increases in bacterial growth.
Acknowledgements:
We thank the operators of the wastewater treatment plants and spray irrigation sites for their participation and assistance. This work was supported by the R03 Small Grants Program, Grant #1-R03-OH009598-01, from the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention (CDC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC.
Footnotes
Abbreviations: Analysis of variance (ANOVA); Clinical and Laboratory Standards Institute (CLSI); minimal inhibitory concentration (MIC); vancomycin-resistant enterococci (VRE); wastewater treatment plant (WWTP)
References
- Abreu-Acosta N, Vera L. Occurrence and removal of parasites, enteric bacteria and faecal contamination indicators in wastewater natural reclamation systems in Tenerife-Canary Islands, Spain. Ecol Eng 2011; 37: 496–503. [Google Scholar]
- Arias CA, Mendes RE, Stilwell MG, Jones RN, Murray BE. Unmet needs and prospects for oritavancin in the management of vancomycin-resistant enterococcal infections. Clin Infect Dis 2012; 54 Suppl 3: S233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahri A, Basset C, Oueslati F, Brissaud F. Reuse of reclaimed wastewater for golf course irrigation in Tunisia. Water Sci Technol 2001; 43: 117–24. [PubMed] [Google Scholar]
- Banerjee T, Anupurba S. Prevalence of Virulence Factors and Drug Resistance in Clinical Isolates of Enterococci: A Study from North India. J Pathog 2015; 2015: 692612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier RC, Duke SE, Ziprin RL, Harvey RB, Hume ME, Poole TL, et al. Antibiotic and disinfectant susceptibility profiles of vancomycin-resistant Enterococcus faecium (VRE) isolated from community wastewater in Texas. Bull Environ Contam Toxicol 2008; 80: 188–94. [DOI] [PubMed] [Google Scholar]
- Ben Said L, Klibi N, Lozano C, Dziri R, Ben Slama K, Boudabous A, et al. Diversity of enterococcal species and characterization of high-level aminoglycoside resistant enterococci of samples of wastewater and surface water in Tunisia. Sci Total Environ 2015; 530–531: 11–7. [DOI] [PubMed] [Google Scholar]
- Caplin JL, Hanlon GW, Taylor HD. Presence of vancomycin and ampicillin-resistant Enterococcus faecium of epidemic clonal complex-17 in wastewaters from the south coast of England. Environ Microbiol 2008; 10: 885–92. [DOI] [PubMed] [Google Scholar]
- Castillo-Rojas G, Mazari-Hiriart M, Ponce de Leon S, Amieva-Fernandez RI, Agis-Juarez RA, Huebner J, et al. Comparison of Enterococcus faecium and Enterococcus faecalis Strains isolated from water and clinical samples: antimicrobial susceptibility and genetic relationships. PLoS One 2013; 8: e59491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Diseases and organisms in healthcare settings, 2009.
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. 32, 2012. [Google Scholar]
- Conner-Kerr TA, Sullivan PK, Gaillard J, Franklin ME, Jones RM. The effects of ultraviolet radiation on antibiotic-resistant bacteria in vitro. Ostomy Wound Manage 1998; 44: 50–6. [PubMed] [Google Scholar]
- EPA. Method 1600: Enterococci in Water by Membrane Filtration Using Membrane-Enterococcus Indoxyl-B-D-Glucoside Agar (mEI), 2002.
- EPA. 2012 Guidelines for Water Reuse, Washington, D.C., 2012. [Google Scholar]
- Ferreira da Silva M, Tiago I, Verissimo A, Boaventura RA, Nunes OC, Manaia CM. Antibiotic resistance of enterococci and related bacteria in an urban wastewater treatment plant. FEMS Microbiol Ecol 2006; 55: 322–9. [DOI] [PubMed] [Google Scholar]
- Fisher K, Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiology 2009; 155: 1749–57. [DOI] [PubMed] [Google Scholar]
- Garcia S, Wade B, Bauer C, Craig C, Nakaoka K, Lorowitz W. The effect of wastewater treatment on antibiotic resistance in Escherichia coli and Enterococcus sp. Water Environ Res 2007; 79: 2387–95. [DOI] [PubMed] [Google Scholar]
- Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 2008; 29: 996–1011. [DOI] [PubMed] [Google Scholar]
- Huang JJ, Hu HY, Lu SQ, Li Y, Tang F, Lu Y, et al. Monitoring and evaluation of antibiotic-resistant bacteria at a municipal wastewater treatment plant in China. Environ Int 2012; 42: 31–6. [DOI] [PubMed] [Google Scholar]
- Levantesi C, La Mantia R, Masciopinto C, Bockelmann U, Ayuso-Gabella MN, Salgot M, et al. Quantification of pathogenic microorganisms and microbial indicators in three wastewater reclamation and managed aquifer recharge facilities in Europe. Sci Total Environ 2010; 408: 4923–30. [DOI] [PubMed] [Google Scholar]
- Lleo MM, Bonato B, Tafi MC, Signoretto C, Boaretti M, Canepari P. Resuscitation rate in different enterococcal species in the viable but non-culturable state. J Appl Microbiol 2001; 91: 1095–102. [DOI] [PubMed] [Google Scholar]
- Luczkiewicz A, Jankowska K, Bray R, Kulbat E, Quant B, Sokolowska A, et al. Antimicrobial resistance of fecal indicators in disinfected wastewater. Water Sci Technol 2011; 64: 2352–61. [DOI] [PubMed] [Google Scholar]
- Martins da Costa P, Vaz-Pires P, Bernardo F. Antimicrobial resistance in Enterococcus spp. isolated in inflow, effluent and sludge from municipal sewage water treatment plants. Water Res 2006; 40: 1735–40. [DOI] [PubMed] [Google Scholar]
- Micallef SA, Goldstein RE, George A, Ewing L, Tall BD, Boyer MS, et al. Diversity, distribution and antibiotic resistance of Enterococcus spp. recovered from tomatoes, leaves, water and soil on U.S. Mid-Atlantic farms. Food Microbiol 2013; 36: 465–74. [DOI] [PubMed] [Google Scholar]
- Murray BE. The life and times of the Enterococcus. Clin Microbiol Rev 1990; 3: 46–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagulapally SR, Ahmad A, Henry A, Marchin GL, Zurek L, Bhandari A. Occurrence of ciprofloxacin-, trimethoprim-sulfamethoxazole-, and vancomycin-resistant bacteria in a municipal wastewater treatment plant. Water Environ Res 2009; 81: 82–90. [DOI] [PubMed] [Google Scholar]
- Rosenberg Goldstein RE, Micallef SA, Gibbs SG, Davis JA, He X, George A, et al. Methicillin-resistant Staphylococcus aureus (MRSA) detected at four U.S. wastewater treatment plants. Environ Health Perspect 2012; 120: 1551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg Goldstein RE, Micallef SA, Gibbs SG, George A, Claye E, Sapkota A, et al. Detection of vancomycin-resistant enterococci (VRE) at four U.S. wastewater treatment plants that provide effluent for reuse. Sci Total Environ 2014; 466–467: 404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Alum A, Abbaszadegan M. Microbial characterization and population changes in nonpotable reclaimed water distribution systems. Environ Sci Technol 2005; 39: 8600–5. [DOI] [PubMed] [Google Scholar]
- Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34: 1–14. [DOI] [PubMed] [Google Scholar]
- Silva N, Igrejas G, Goncalves A, Poeta P. Commensal gut bacteria: distribution of Enterococcus species and prevalence of Escherichia coli phylogenetic groups in animals and humans in Portugal. Ann Microbiol 2011; 62: 449–459. [Google Scholar]
- Varela AR, Ferro G, Vredenburg J, Yanik M, Vieira L, Rizzo L, et al. Vancomycin resistant enterococci: from the hospital effluent to the urban wastewater treatment plant. Sci Total Environ 2013; 450–451: 155–61. [DOI] [PubMed] [Google Scholar]


