Abstract
The gray mouse lemur (Microcebus murinus, GML) is a nocturnal, arboreal, prosimian primate that is native to Madagascar. Captive breeding colonies of GMLs have been established primarily for noninvasive studies on questions related to circadian rhythms and metabolism. GMLs are increasingly considered to be a strong translational model for neurocognitive aging due to overlapping histopathologic features shared with aged humans. However, little information is available describing the clinical presentations, naturally occurring diseases, and histopathology of aged GMLs. In our colony, a 9 y-old, male, GML was euthanized after sudden onset of weakness, lethargy, and tibial fracture. Evaluation of this animal revealed widespread fibrous osteodystrophy (FOD) of the mandible, maxilla, cranium, appendicular, and vertebral bones. FOD and systemic metastatic mineralization were attributed to underlying chronic renal disease. Findings in this GML prompted periodic colony-wide serum biochemical screenings for azotemia and electrolyte abnormalities. Subsequently, 3 additional GMLs (2 females and 1 male) were euthanized due to varying clinical and serum biochemical presentations. Common to all 4 animals were FOD, chronic renal disease, uterine adenocarcinoma (females only), cataracts, and osteoarthritis. This case study highlights the concurrent clinical and histopathologic abnormalities that are relevant to use of GMLs in the expanding field of aging research.
Abbreviations: FOD, fibrous osteodystrophy; GML, gray mouse lemur
Within the past 5 y, recognition of the translational utility of the gray mouse lemur (Microcebus murinus, GML) has greatly expanded, in part due to the sequencing of its genome.27 GMLs have been proposed as an animal model in the context of aging research,14,35 most notably within the fields of Alzheimer disease and dementia33,39 and circadian rhythms.15,20 GMLs are nocturnal, arboreal, prosimian primates (family Cheirogaleidae) that are endemic to Madagascar. They are among the smallest primates, with a body weight of 49 to 80 g in the wild37 (60 to 110 g in captivity) and have a life expectancy of approximately 8 to 10 y in captivity.14 A small number of captive breeding colonies have been established throughout Europe and the United States, many of which have arisen from a closed captive breeding colony at the Muséum National d'Histoire Naturelle (MNHN) in Brunoy, France.
Despite an ever-growing interest in the GML as a model organism, clinical and pathologic case reports focusing on naturally occurring disease are rare for this species.1,4,10,16,17,20,28,31,34,38 Reports of spontaneous disease often focus on neoplasia28,31,34 or on ocular abnormalities, which are accessible without invasive interventions.1,4,12 Apart from age-related neurodegenerative disease and cognitive impairment,5,23,25,26,32,36 little is known about the natural disease predilection and histologic aging phenotypes of GMLs.
In June 2017, a 9 y-old male GML was euthanized after the sudden onset of weakness, lethargy, and tibial fracture. Necropsy and histopathology revealed chronic renal disease, widespread fibrous osteodystrophy (FOD), and systemic metastatic mineralization. These findings prompted colony-wide serum biochemical screenings for potential underlying renal disease and subsequent metabolic bone disease within the population.
Herein, we report the clinical, gross, and histologic multisystemic pathology of 4 aged GMLs. This is the first documentation of FOD secondary to chronic renal disease in GMLs in a captive research colony. In addition, we corroborate previous reports31,34 of uterine adenocarcinoma in aged female GMLs. Together, these findings aid in providing appropriate clinical care to GMLs as their use in the field of aging research continues to expand.
Materials and Methods
Animals and Husbandry.
GMLs were maintained for noninvasive phenotyping and genetic research as approved by the Stanford University Administrative Panel on Laboratory Animal Care (APLAC #27439) and in accordance with the Guide for the Care and Use of Laboratory Animals.21 All animals were transferred to Stanford University from the University of Texas at Austin in 2015. The colony consisted of GMLs and their offspring originating from the closed captive breeding colony at the MNHN in Brunoy, France. At the time of the initial case report in June 2017, the colony consisted of 7 adult animals with an average age of 9 y (range: 6.7 to 10.7 y). GMLs were individually or group housed indoors in an AAALAC-accredited facility using modified marmoset caging with multiple polyvinyl chloride perches and nest boxes (Britz and Company, Wheatland, WY). Temperature was maintained at 23.3 to 24.4 °C and light cycles were altered from 14:10 h (March to August) to 10:14 h (September to February) light:dark every 6 mo to stimulate photoperiod-dependent breeding behavior and metabolic changes. All GMLs were fed crushed Teklad Global 20% Protein Primate Diet (Envigo, Indianapolis, IN, #2050), which made up approximately 20% of the total diet by weight. The remainder of the diet consisted of fresh fruits and vegetables. Twice a week, one to 2 insect larvae, such as mealworms, were provided as enrichment items.
Complete Blood Counts and Serum Biochemistry.
Annual colony-wide physical examinations included complete blood counts (CBC) and serum biochemistry panels (including glucose, AST, ALT, alkaline phosphatase, total bilirubin, cholesterol, blood urea nitrogen (BUN), creatinine, calcium, phosphorus, total protein, albumin, globulin, and electrolyte panel). Blood (250 μL) was collected from the medial saphenous vein for analysis. After the identification of chronic renal disease in the colony (animal GML1), additional renal screening panels were performed on the 6 remaining animals to evaluate serum BUN, creatinine, calcium, phosphorus, and total protein levels. All values were compared with previously obtained values for individual animals to track changes over time, as well as with reference ranges for the 25% to 75% quartiles for male and female GMLs during the appropriate long-day and short-day intervals.29
Radiographs.
GML1 was anesthetized with 4% isoflurane delivered by induction chamber and maintained with 1% to 2% isoflurane delivered by face mask for pelvic limb radiographs. Postmortem radiographs of the right pelvic and right thoracic limbs were also obtained from GML1. Radiographs were obtained with the VetVision DC Dental X-Ray Digital System (Midmark Corporation, Lincolnshire, IL). Radiographs were only performed on GML1 due to his clinical presentation – pelvic limb lameness and mid-diaphyseal swelling. None of the other GMLs had clinical musculoskeletal indications for radiographs.
Necropsy and Histopathology.
All GMLs were euthanized by barbiturate overdose under isoflurane anesthesia. Terminal cardiac blood was immediately collected via cardiocentesis for serum biochemistry and CBC. Due to limited blood volumes, serum biochemistry parameters were prioritized. Terminal body weights were obtained, and a thorough external examination and gross necropsy were performed. Postmortem body condition (that is, thin) was based on the subjective assessment of subcutaneous and intracavitary adipose stores.
Tissues were immersion-fixed in 10% neutral buffered formalin for 72 h. After fixation, bones were decalcified in Cal-Ex II Fixative/Decalcifier (Fisher Scientific, Upland, CA). The following formalin-fixed tissues were processed routinely, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin (H and E): heart, aorta (ascending and descending segments), lungs, trachea, thyroid gland, parathyroid gland, kidneys, urinary bladder, male reproductive tract (testicle, epididymis, seminal vesicle, prostate, and penile urethra), female reproductive tract (uterus, cervix, vagina, and ovaries), salivary glands, tongue, epiglottis, esophagus, stomach, small and large intestine, liver (with gallbladder), adrenal gland, spleen, lymph nodes, bone (right scapulohumeral joint, right radius and ulna, stifle joints, right tibia, skull, mandible, maxilla, nasal turbinates, vertebral column), spinal cord, eyes, and bone marrow.
Select tissues were stained with Von Kossa (to identify mineralization), Masson's trichrome (to identify collagen), Congo Red (to identify amyloid), and Gram stain (to identify bacteria).
Ancillary Diagnostics.
Based on the gross and histologic findings, ancillary diagnostics were performed. Additional diagnostics for GML2 included microbiologic culture (aerobic and anaerobic) and follow-up PCR speciation of lung and uterine tissue. Microbiologic cultures were evaluated inhouse using the Omnilog Gen III (Biolog, Hayward, CA). Formalin-fixed paraffin-embedded tissues scrolls (3, 25-µm-thick) of lesioned lung and uterine tissue from GML2 were submitted to Charles River Laboratories for speciation of Klebsiella sp. via TaqMan PCR (Charles River Research Animal Diagnostic Services, Wilmington, MA).
Results
GML1 Clinical Presentation.
GML1 was a 9 y-old male cohoused with a male cagemate. Medical history included conservative management for conspecific fight wounds approximately 1 y prior to presentation. GML1 presented with sudden onset weakness and lethargy. On physical examination, he was quiet, moderately dehydrated, exhibited bilateral pelvic limb weakness (right limb weaker than left), and a palpable swelling over the middiaphysis of the right tibia. Pelvic limb radiographs revealed a closed, craniolaterally-displaced, oblique fracture of the middiaphysis of the right tibia (Figure 1 A). Additional radiographic findings included “moth-eaten” lucencies of all bones and degenerative joint disease of the tibiotarsal and tarso-metatarsal joints. The lemur's right pelvic limb was splinted, and he received carprofen (5 mg/kg SC once daily for 3 d), 0.9% saline (2 mL SC), and was housed individually. He initially improved, exhibiting normal activity levels with ambulation and climbing behaviors. However, 6 d later, GML1 presented obtunded, laterally recumbent, and tachypneic. Repeat pelvic limb radiographs showed no evidence of callus formation at the fracture site (not shown). Based on poor prognosis, GML1 was euthanized.
Figure 1.
Appendicular fractures in gray mouse lemur 1 (GML1). (A) Closed, oblique, middiaphyseal fracture of the right tibia (asterisk) and diffuse “moth eaten” lucenies of the right tibia and tarsus. (B) Postmortem medial deviation of the right tibia (arrow) relative to the stifle and tarsus. (C) Postmortem lateral deviation of the right middiaphyseal radius and ulna (arrow). (D) Postmortem radiographs revealed comminuted fractures of the radius and ulna (asterisk) in addition to “moth eaten” lucencies of the distal humerus, radius, and ulna.
Terminal serum biochemistry (Table 1) revealed severe azotemia (BUN 221 mg/dL, creatinine 1.68 mg/dL), and hyperphosphatemia (15.5 mg/dL). Calcium levels could not be measured due to low sample volume.
Table 1.
Serum biochemistry values for male gray mouse lemurs (GMLs). Abbreviations: SD = short-day season (10:14-h light:dark); LD = long-day season (14:10-h light:dark); H = higher than reference value; L = lower than reference value.
|
GML1 |
GML4 |
||||||
| 07/30/2015 (LD) | 01/23/2017 (SD) | 06/01/2017 (LD) | 01/23/2017 (SD) | 08/01/2017 (LD) | 08/29/2018 (LD) | ||
| Parameter | Units | Baseline screen | Baseline screen | Necropsy | Baseline screen | Renal screen | Necropsy |
| Glucose | mg/dL | 92 | 91 | — | 109 (H) | — | 101 |
| ALT | U/L | — | 149 (L) | 205 | 168 | — | 294 (H) |
| ALP | IU/L | — | 175 (H) | — | 95 (H) | — | 45 (L) |
| T. Bilirubin | mg/dL | — | 0.4 | 0.2 (L) | 0.5 (H) | — | 0.2 (L) |
| BUN | mg/dL | 21 | 18 | 221 (H) | 26 (H) | 25 (H) | 33 (H) |
| Creatinine | mg/dL | 1.4 (H) | 0.1 | 1.68 (H) | 0 (L) | 0.39 (H) | 0.28 |
| Calcium | mg/dL | 13 (H) | 14.1 (H) | 10.6 (H) | 8.5 (L) | 8.4 (L) | |
| Phosphorus | mg/dL | — | 5.2 | 15.5 (H) | 6.2 (H) | 6.3 | 5.0 |
| T. Protein | g/dL | — | 9.8 | — | 8.6 (L) | 7.3 (L) | 6.7 (L) |
| Albumin | g/dL | — | 6.7 (H) | — | 6.1 | — | 4.0 (L) |
| Globulin | g/dL | — | 3.1 | — | 2.5 | — | 2.7 (L) |
| Sodium | mmol/L | 148 | — | 141 | — | — | 149 |
| Potassium | mmol/L | 6.8 | — | — | — | — | 4.4 (L) |
GML1 Necropsy.
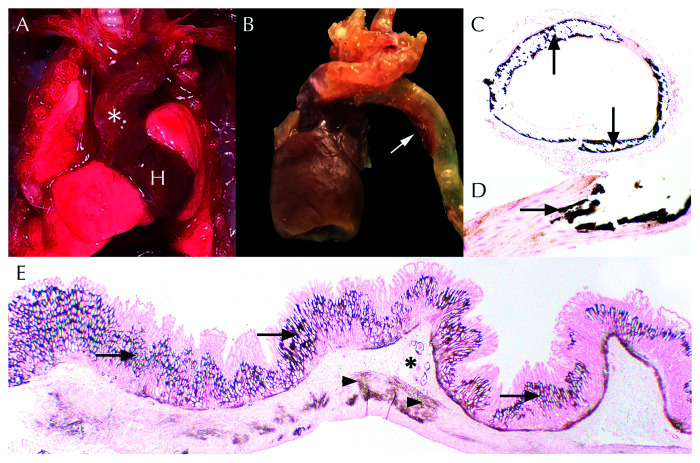
GML1 presented to necropsy in thin body condition, weighing 55.0 g. Corresponding to the radiographically observed fracture, medial deviation of the right tibia relative to the stifle and tarsus was present (Figure 1 B). Distal to the right elbow was a 0.4-cm-diameter, smoothly contoured nodule that circumferentially surrounded the radius and ulna (Figure 1 C). Postmortem radiographs revealed closed oblique fractures of the radius and ulna with significant callus formation (Figure 1 D). The calvarial, axial, and appendicular bones were thin and extremely pliable upon manipulation. Vessels exiting the left ventricle were slightly tortuous, increased in diameter (approximately 0.2- to 0.3-cm diameter), mottled tan to red, and firm (Figures 2 A and 2 B). Affected vessels comprised the ascending and descending aorta to the level of the diaphragm, the aortic arch, and the proximal aspect of the brachiocephalic trunk. Bilaterally, the kidneys were diffusely pale tan and pitted along the cortical surface.
Figure 2.
Systemic metastatic mineralization in gray mouse lemur 1 (GML1). (A) In-vivo thickening and tortuosity (white asterisk) of the aorta and brachiocephalic trunk as it exits the heart (H). (B) Ex-vivo dilation and opacity (white arrow) of the aortic arch and descending aorta. (C) Transverse sections of the aorta exhibited circumferential deposition of dark brown mineral (black arrows). Von Kossa, 4×. (D) Higher magnification of aorta with replacement of tunica media smooth muscle by dark brown mineral (black arrow). Von Kossa, 40× . (E) Widespread gastric mineralization (dark brown) seen throughout the lamina propria (arrows), submucosal vessels (asterisk), and smooth muscle (arrowheads). Von Kossa, 1.25×.
GML1 Histopathology.
Histologically, all examined bones exhibited features of FOD (Figures 3 A and 3 B). Affected bones included the mandible, maxilla, calvarium, appendicular bones, and vertebral bones. Bone cortices were expanded and replaced by highly cellular, trichrome-positive fibrous connective tissue that surrounded poorly mineralized islands of osteoblast-lined trichrome-positive immature woven bone. Intratrabecular resorption cavities and Howship lacunae were associated with high numbers of osteoclasts.
Figure 3.
Fibrous osteodystrophy (FOD) in gray mouse lemurs (GMLs) (A) Transverse section of the sacroiliac joint (S = sacrum; I = ilium) of GML1 exhibits diffuse expansion and replacement of bone by highly cellular fibrous connective tissue. Sc = spinal cord. H and E, 1.25×. (B) Cortical and medullary bone is replaced by fibrosis (arrows) that surrounds poorly mineralized islands of woven bone (asterisk). High numbers of osteoclasts (arrowheads) are scattered throughout. H and E, 20 ×. (C) Transverse section of the oral cavity in GML2 revealed widespread replacement of the maxillary (asterisk) and mandibular (arrowhead) alveolar bone by fibrous connective tissue and immature woven bone. (D) Spicules of woven bone (asterisk) were trichrome-positive. Trichrome, 20×. MxM = maxillary molar; MdM = mandibular molar; Bc = buccal cavity. H and E, 1.25×.
As noted clinically, a complete oblique nonunion fracture was present in the right tibia. Variably sized islands of necrotic bone (sequestra) were present at the fracture site. Similarly, complete, closed, nonunion fractures capped by multinodular fibrocartilaginous calluses were present in the midradial diaphysis and midbody of the scapula.
Nearly all examined vessels (that is aorta, pulmonary artery, coronary arteries, renal, splenic, lingual, gastric, mesenteric, scleral) exhibited vascular mineralization. Vessels displayed segmental to circumferential mural thickening of the vessel walls by wispy to amorphous, basophilic, Von-Kossa-positive mineralization (Figures 2 C and 2 D). Mineral deposits were present within the tunica intima and/or media of large caliber arteries. Widespread basement membrane mineralization was present in the renal tubules, glomeruli, gastric mucosa (Figure 2 E), pulmonary alveoli, and trachea. Basement membrane mineralization was also identified in the parathyroid gland in conjunction with glandular ectasia, vascular dilation, and multifocal epithelial degeneration and necrosis.
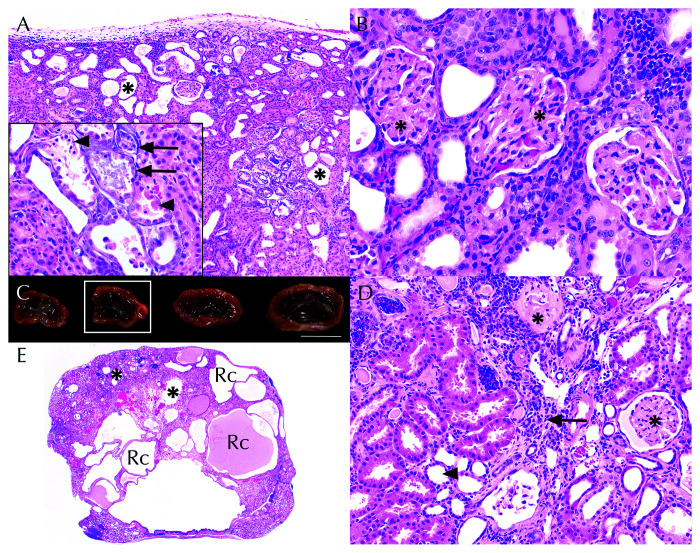
Within the kidneys, features of chronic renal disease and acute tubular necrosis (due to basement membrane mineralization) were identified (Figures 4 A and 4 B). Approximately 40% of renal glomeruli exhibited globally increased mesangial matrix deposition and increased cellularity. Glomerular capillary profiles were obscured and admixed with karyorrhectic debris and rare fibrin thrombi. Glomerular tufts were occasionally adherent to Bowman capsule. Parietal cells were occasionally piled up (hyperplasia) or were thin and attenuated. Frequently, glomerular basement membranes, Bowman capsule, or parietal epithelial cells were obscured by mineralization. Renal tubular basement membranes (particularly within the medulla) were frequently mineralized (Figure 4 A, inset). Overlying renal tubular epithelial cells were necrotic and sloughed within the tubular lumen or enlarged and basophilic (regeneration). Other tubules were dilated and contained proteinaceous fluid or luminal mineralized concretions. Low numbers of neutrophils, histiocytes, lymphocytes, and plasma cells were scattered throughout the interstitium where they were occasionally admixed with fibrosis. Additional histologic findings unique to GML1 are shown in (Figure 5).
Figure 4.
Chronic renal disease in gray mouse lemurs (GMLs) (A) In GML1, renal tubular dilation (asterisks) was compounded by widespread basement membrane mineralization (inset, arrows) and acute tubular necrosis (inset, arrowheads). H and E, 10×; inset, 40×. (B) Increased glomerular mesangial matrix (asterisks) obscured capillary loop profiles. H and E, 40× (C) Undulating cortical surfaces, size variability, and renal pelvis dilation in gray mouse lemur 2 (GML2). Scale bar = 1 cm. (D). Residual cortical tissue comprised interstitial fibrosis and lymphoplasmacytic interstitial nephritis (arrow), tubular dilation (arrowhead), and variable glomerular mesangial matrix thickening (asterisks). H and E, 20×. (E). Renal histology of kidney from Figure 4 C (white box) demonstrating marked cortical thinning, renal cysts (Rc), and tubular dilation (asterisks). H and E, 1.25×.
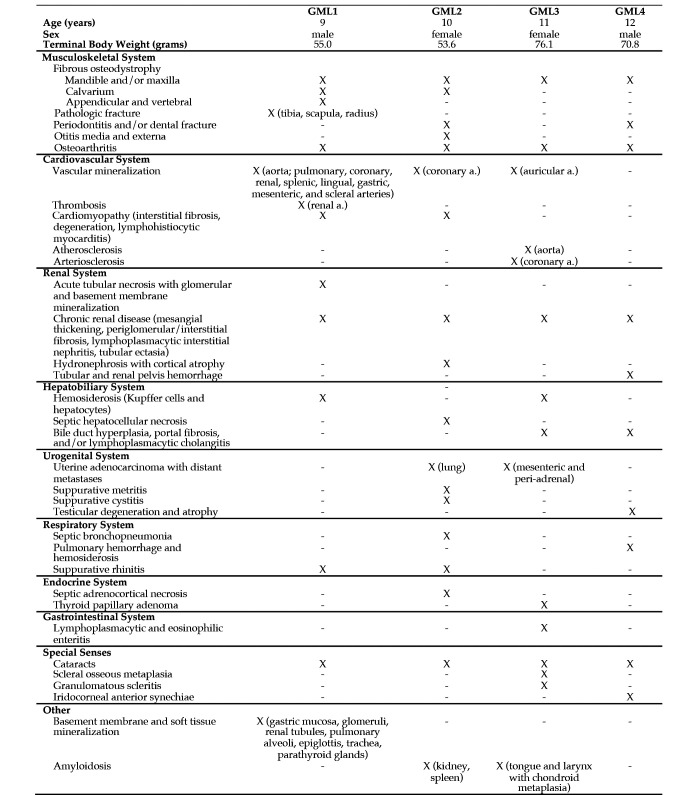
Figure 5.
Histologic lesions within gray mouse lemurs (GMLs). X = feature present; - = feature absent.
Colony-Wide Serum Biochemical Screenings.
Based on the serum biochemical, gross, and histologic findings identified in GML1, periodic colony-wide screens for subclinical renal disease (semiannually for animals over 10 y old, annually for younger animals) were implemented (Table 1 and Table 2) beginning in August of 2017. As none of the GMLs (including GML1) had shown indications of renal disease on an annual serum biochemistry panel performed in January 2017, the additional screening panels were performed to detect subclinical renal disease in the colony. Of the 6 remaining colony animals, 2 additional GMLs (GML2 and GML4) had serum biochemical evidence of azotemia based on periodic renal screenings (Table 2). GML2 had significant azotemia, hyperphosphatemia, and hypercalcemia and thus received 0.9% saline (2 mL SC) 3 times per week. Approximately one month later, GML2 declined acutely and was euthanized. GML4 exhibited mild azotemia, hypocalcemia, and normophosphatemia. Despite continued clinical monitoring, GML4 was euthanized approximately one year later due to progressive neurologic signs (circling and repetitive head movements). GML3 was not azotemic but was euthanized after being found acutely nonresponsive in her enclosure. Histologic evidence of chronic renal disease was identified; therefore, this animal was included in this case series.
Table 2.
Serum biochemistry values for female gray mouse lemurs (GMLs). Abbreviations: SD = short-day season (10:14-h light:dark); LD = long-day season (14:10-h light:dark); H = higher than reference value; L = lower than reference value.
|
GML2 |
GML3 |
|||||||
| 01/23/2017 (SD) | 08/02/2017 (LD) | 09/01/2017 (LD) | 09/06/2017 (SD) | 01/23/2017 (SD) | 08/01/2017 (LD) | 07/23/2018 (LD) | ||
| Parameter | Units | Baseline screen | Renal screen | Renal screen | Necropsy | Baseline screen | Renal screen | Necropsy |
| Glucose | mg/dL | 102 | — | — | 126 (H) | 77 | — | — |
| ALT | U/L | 74 (L) | — | — | 339 (H) | 582 (H) | — | 218 |
| ALP | IU/L | 48 | — | — | 48 | 115 (H) | — | 43 (L) |
| T. Bilirubin | mg/dL | 0.4 | — | — | 0.3 | 0.4 | — | — |
| BUN | mg/dL | 72 (H) | 192 (H) | 173 (H) | 200 (H) | 22 | 25 | 42 (H) |
| Creatinine | mg/dL | 0.47 (H) | 1.45 (H) | 1.01 (H) | 1.37 (H) | 0.08 (L) | 0.24 (L) | 0.15 (L) |
| Calcium | mg/dL | 10.8 (H) | 10.8 (H) | 10.7 (H) | 9.0 (L) | 10.2 | 9.1 (L) | 7.4 (L) |
| Phosphorus | mg/dL | 7.8 (H) | 11.3 (H) | 4.1 (L) | 8.9 (H) | 7.0 | 7.7 | 6.9 |
| T. Protein | g/dL | 10.2 | 7.9 | 7.3 (L) | 5.8 (L) | 10.4 | 9.5 (H) | 6.6 (L) |
| Albumin | g/dL | 6.4 | — | — | 3.3 (L) | 7.0 (H) | — | 4.1 (L) |
| Globulin | g/dL | 3.8 | — | — | 2.5 | 3.4 | — | 2.5 (L) |
| Sodium | mmol/L | — | — | — | 147 | — | — | 151 (H) |
| Potassium | mmol/L | — | — | — | 6.8 | — | — | 3.8 (L) |
GML2, GML3, and GML4.
Several histopathologic features in GML2, GML3, and GML4 had also been identified in GML1. FOD was found in GML2, GML3, and GML4 with varying degrees of severity and anatomic distributions (Figure 3 C and Figure 5). All GMLs had FOD in the mandibular and/or maxillary bone; these anatomic locations are typically impacted in the earliest stages of FOD development.11 None of these GMLs showed evidence of secondary pathologic fracture. However, GML2 and GML4 both had periodontitis, and GML4 also had fracture of the molars.
Chronic renal disease, as evidenced by histopathologic lesions, was present in GML2, GML3, and GML4 (Figure 4 C, 4 D, and 4 E). Like GML1, the other 3 GMLs also had glomerular mesangial thickening, periglomerular and interstitial fibrosis, lymphoplasmacytic interstitial nephritis, and tubular ectasia. Hydronephrosis, cortical atrophy, and interstitial amyloidosis were prominent findings in GML2. GLM4 had hemorrhage in the tubules and renal pelvis. The acute tubular necrosis and mineralization found in GML1 were not seen in the other 3 GMLs.
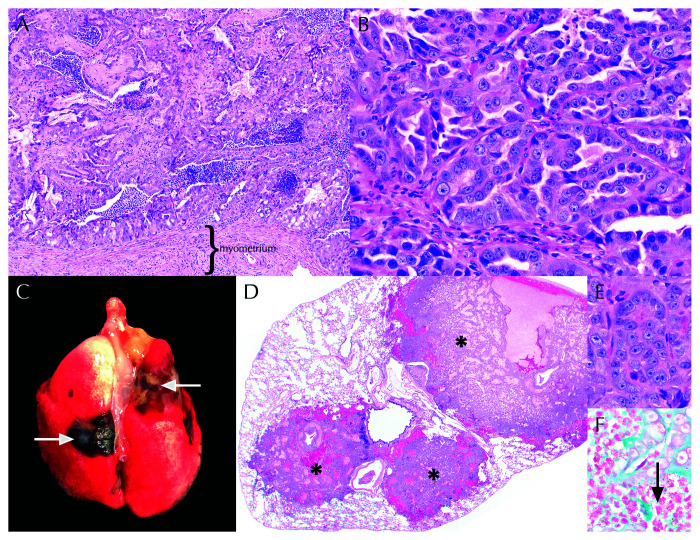
Both female lemurs (GML2 and GML3) had uterine adenocarcinoma with metastases to the lungs (GML2, Figure 6) or mesenteric/peri-adrenal lymph nodes (GML3). In both female GMLs, the uterine adenocarcinoma was composed of tubules, glands, and islands of neoplastic cells that filled the uterine lumen and invaded the submucosa and myometrium (GML2 only). In GML2, a concurrent metritis (Klebsiella sp., moderate numbers of Enterococcus faecalis, and large numbers of aerobic gram-positive rods), suppurative bronchopneumonia (Klebsiella oxytoca), and generalized septicemia were noted. A summary of histologic findings that were unique to GML2, GML3, and GML4 can be found in Figure 5.
Figure 6.
Metastatic uterine adenocarcinoma in female gray mouse lemurs (GMLs) (A) Uterine adenocarcinoma effaced the endometrium and filled the uterine lumen of GML2. H and E, 10×. (B) Neoplastic cells in GML2 and GML3 comprised polygonal epithelial cells arranged in tubular formations. H and E, 40×. (C) Tan to black pulmonary nodules (arrows) were visible on the pleural surface and extended within the parenchyma of GML2. D. Nodules in Figure 6 C corresponded to multifocal metastatic uterine adenocarcinoma (asterisks). H and E, 1.25×. (E) Metastatic neoplastic cells were cytologically indistinguishable from the primary uterine tumors of GML2 and GML3. (Figure 6 B). H and E, 40 ×. (F) Suppurative bronchopneumonia with gram-negative rods (arrow) was also present within the lung tissue of GML2. Gram stain, 100×.
Discussion
As the use of GMLs in behavioral and biomedical research increases,13,26 a generalized understanding of species-specific disease predispositions is critical for 1) providing adequate husbandry, 2) appropriately screening for age- or sex-related clinical abnormalities and, 3) early implementation of clinical interventions. For models of aging, awareness of naturally occurring diseases and comparing their characteristics to those of humans and other models is necessary for appropriate model selection. The GMLs in our cohort either met or exceeded the average lifespan of captive housed GMLs (8 to 10 y).14 Despite significant variation in clinical presentation, all GMLs exhibited widespread multisystemic pathology. Disease processes common to all mouse lemurs included FOD, chronic renal disease, uterine adenocarcinoma (females only), cataracts, and osteoarthritis. Routine colony-wide clinical evaluation and health-screening is critical to detection of these morbidities.
Metabolic bone disease has been reported in colony-housed marmosets,18,30,42 rhesus macaques,41 and a Golden Lion Tamarin,9 but not in Microcebus sp. or other prosimian primates. FOD is a metabolic bone disease characterized by widespread bone resorption and replacement with fibrous connective tissue and immature woven bone.11 Bone lesions manifest due to primary hyperparathyroidism (that is, parathyroid gland hyperplasia/neoplasia), secondary hyperparathyroidism (that is, stemming from underlying chronic renal disease or dietary imbalances of calcium, phosphorus, and vitamin D), or paraneoplastic syndromes (that is, elevated parathyroid hormone-related protein). New World nonhuman primates are exceptionally predisposed to develop FOD due to their unique calcium and vitamin D requirements.8,22 However, less is known about species-specific dietary requirements for prosimian primates40 and how these relate to the overlapping manifestations of chronic renal disease and altered serum parathyroid hormone (PTH) levels. The GMLs in this report were fed a commercial diet that is designed to support the gestation, lactation, and growth of most nonhuman primates. Relevant composition of vitamins and minerals include the following: 1.0% calcium; 0.8% phosphorus; 0.5% nonphytate phosphorus; 0.2% magnesium; 8.0 IU/g vitamin D3. Thus, while diet cannot be definitively excluded as a contributory cause of FOD, the seemingly balanced nutritional profile, lack of concurrent gastrointestinal disease, and presence of significant renal disease render nutritional pathogenesis less likely.
In marmosets, spontaneous development of gastrointestinal disease and metabolic bone disease is well-documented.3,22,30 Recently, PTH has been identified as an effective marker for discriminating between marmosets with and without spontaneous metabolic bone disease.3 However, in our cases, serum PTH levels were not evaluated at the time of euthanasia due to lack of clinical disease suspicion (in GML1) and lack of species-specific assay availability. Thus, aberrations in serum PTH levels could not be confirmed. In addition, histologic evaluation of the parathyroid gland did not show hyperplasia, which would have suggested elevated serum PTH levels. Thus, we are currently evaluating species-specific PTH assays that may aid in enhanced detection of FOD in captive-housed mouse lemur colonies.
In all GMLs, serum biochemistry or histologic lesions suggested chronic renal disease as a potential underlying pathogenesis for FOD development. Chronic renal disease has been documented in other aged prosimians, including Eulemur sp., Varecia sp., Hapalemur sp., and Loris tardigradis.40 Moreover, chronic renal disease has been described as the most frequent postmortem pathologic finding of GMLs.31 A histopathologic survey conducted at the MNHN mouse lemur colony found that approximately 90% of GMLs surveyed over a 10-y period had histologic evidence of chronic renal disease, with or without clinical signs.31 However, that study did not evaluate bone pathology; thus, the presence of concurrent FOD in this population is unknown. In addition, our colony diet is expected to be appropriate for GMLs, yet the dietary protein content may nonetheless have potentially exacerbated or contributed to the development of renal disease.
The gross and histologic presentation of renal disease varied between the cases in this report. Although GML1 had diffuse renal lesions, GML1 lacked the marked hydronephrosis and marked chronic corticomedullary atrophy noted in GML2. Despite this, widespread FOD predominated in GML1, yet was only seen in the mandibular/maxillary alveolar bone and cranium of GML2. Similarly, metastatic mineralization of the vasculature (that is aorta, pulmonary artery, coronary arteries, renal, splenic, lingual, gastric, mesenteric, and scleral vessels) and basement membranes (that is, renal tubules, glomeruli, gastric mucosa, pulmonary alveoli, trachea, parathyroid gland) were widespread throughout GML1, but only noted in the aorta and a peri-auricular artery of GML3, and in a coronary artery of GML2. Thus, perhaps the presence of underlying renal disease, compounded with superimposed renal basement membrane mineralization, resulted in significant disease exacerbation in GML1, as compared with GML2. GML3 and GML4 had moderate renal disease, mild BUN elevation, and a correspondingly lower burden of FOD (limited to alveolar bone of the mandible or maxilla).
Aside from pathologic fracture (GML1), the GMLs in our cohort had relatively nonspecific clinical signs. Periodic colony-wide serum biochemical testing to monitor for azotemia, hyperphosphatemia, and serum calcium alterations may help to identify animals with chronic renal disease and potentially trigger intervention in the onset and progression of FOD development. However, published serum biochemical values29 for GMLs must be interpreted in terms of both the season (that is, long-day compared with short-day) and institutional variation. In our case study, our values were compared with previously published values;29 however, we recognize the need for internal validation based on our own colony-housed animals.
In addition to renal disease and FOD, uterine adenocarcinoma with distant metastases was identified in both female mouse lemurs (GML2 and GML3). In 2009, a comprehensive literature review and colony-wide retrospective analysis of captive prosimian neoplasia was conducted.34 This study identified GMLs as the most frequently affected prosimian species (28 of 117 [13%]), with uterine adenocarcinomas (papillary and tubulopapillary) accounting for 2 of 28 neoplasms. A similar histopathologic analysis conducted within the MNHN colony identified uterine papillary adenomas/adenocarcinomas in 27 of 99 (27%) of examined females.31 Of those, 2 had distant metastases to the kidney (n = 1) or lungs (n = 1). Thus, our identification of uterine adenocarcinoma with distant metastasis (lung and lymph nodes) adds to the growing body of literature regarding the prevalence of this neoplasm in female GMLs and may have implications for successful breeding colonies.
Klebsiella oxytoca pneumonia, metritis, and septicemia were significant comorbidities in GML2. In New World and Old World primates, Klebsiella sp, pneumonia and/or septicemia are typically attributed to Klebsiella pneumoniae isolates.7,8,24 However, K. oxytoca has been identified as an opportunistic bacterial pathogen in laboratory animal species, particularly those that are immunosuppressed.2,6 Infection with K. oxytoca can result in suppurative metritis, otitis media, and pneumonia in rodents.6 Given the multitude of concurrent disease conditions in GML2 (that is FOD, chronic renal disease, metastatic uterine adenocarcinoma), immunosuppression was considered a key factor in the induction and development of K. oxytoca septicemia. Because of this organism's predilection for the urogenital tract, infection was postulated to have begun in the uterus and spread via hematogenous dissemination.
Lastly, the underlying cause of circling in GML4 was never fully elucidated. However, the combination of acute pulmonary and renal hemorrhage, coupled with thrombocytopenia, suggest that the neurologic signs may have resulted from intracerebral hemorrhage. The renal disease in GML4 was moderate in degree and unlikely to have caused neurologic signs.
In summary, this is the first report of FOD in GMLs. Based on serum biochemistry and histologic evaluation, underlying chronic renal disease was considered the most likely pathogenesis for developing metabolic bone disease. Because the animals showed relatively nonspecific clinical signs, semiannual to annual colony-wide serum biochemical panels are recommended to screen for early evidence of azotemia and electrolyte abnormalities, which can affect research use as well as animal health. In addition, uterine adenocarcinoma should be considered a differential diagnosis in aged female GMLs. Due to the small numbers of animals in our colony, we cannot speculate on the age at which these conditions are likely to arise. The findings in this case series add to the body of literature describing spontaneous diseases of captive GMLs and help provide clinicopathologic context for their clinical care and use in aging research.
Acknowledgments
The authors would like to thank Elias Godoy for his assistance with tissue harvesting and the Stanford Animal Histology Services for help with the preparation of histologic specimens. This work was supported by the Howard Hughes Medical Institute and the Vera Moulton Wall Center of Stanford University.
References
- 1.Alleaume C, Mrini ME, Laloy E, Marchal J, Aujard F, Chahory S. 2017. Scleral and corneal xanthomatous inflammation in a gray mouse lemur (Microcebus murinus). Vet Ophthalmol 20:177–180. 10.1111/vop.12374. [DOI] [PubMed] [Google Scholar]
- 2.Barthold SW, Griffey SM, Percy DH. 2016. Mouse, p 1–118. Chapter 1. In: Barthold SW, Griffey SM, Percy DH, editors. Pathology of laboratory rodents and rabbits, 4th ed. Ames (IA): John Wiley and Sons. [Google Scholar]
- 3.Baxter VK, Shaw GC, Sotuyo NP, Carlson CS, Olson EJ, Zink MC, Mankowski JL, Adams RJ, Hutchinson EK, Metcalf Pate KA. 2013. Serum albumin and body weight as biomarkers for the antemortem identification of bone and gastrointestinal disease in the common marmoset. PLoS One 8:1–10. 10.1371/journal.pone.0082747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beltran WA, Vanore M, Ollivet F, Nemoz-Bertholet F, Aujard F, Clerc B, Chahory S. 2007. Ocular findings in two colonies of gray mouse lemurs (Microcebus murinus). Vet Ophthalmol 10:43–49. 10.1111/j.1463-5224.2007.00491.x. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand A, Pasquier A, Petiet A, Wiggins C, Kraska A, Joseph-Mathurin N, Aujard F, Mestre-Francés N, Dhenain M. 2013. Micro-MRI study of cerebral aging: ex vivo detection of hippocampal subfield reorganization, microhemorrhages and amyloid plaques in mouse lemur primates. PLoS One 8:1–8. 10.1371/journal.pone.0056593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleich A, Kirsch P, Sahly H, Fahey J, Smoczek A, Hedrich HJ, Sundberg JP. 2008. Klebsiella oxytoca: opportunistic infections in laboratory rodents. Lab Anim 42:369–375. 10.1258/la.2007.06026e. [DOI] [PubMed] [Google Scholar]
- 7.Burke RL, Whitehouse CA, Taylor JK, Selby EB. 2009. Epidemiology of invasive Klebsiella pneumoniae with hypermucoviscosity phenotype in a research colony of nonhuman primates. Comp Med 59:589–597. [PMC free article] [PubMed] [Google Scholar]
- 8.Calle PP, Ott Joslin J. 2015. New World and Old World Monkeys, p 301–335. Chapter 37. In: Miller RE, Fowler ME, editors. Fowler's zoo and wild animal medicine, vol 8. St Louis (MO): WB Saunders. 10.1016/B978-1-4557-7397-8.00037-2 [DOI] [Google Scholar]
- 9.Choi E, Childs-Sanford SE, Abou-Madi N, King EE, Caserto BG, Priest H, Behling-Kelly E, Miller AD. 2016. Hepatic osteodystrophy in a golden lion tamarin (Leontopithecus rosalia). J Zoo Wildl Med 47:907–911. 10.1638/2015-0201.1. [DOI] [PubMed] [Google Scholar]
- 10.Cichon N, Lampe K, Bremmer F, Becker T, Mätz-Rensing K. 2017. Unique case of granulomatous arteritis in a grey mouse lemur (Microcebus murinus)—first case description. Primate Biol 4:71–75. 10.5194/pb-4-71-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craig LE, Dittmer KE, Thompson KG. 2016. Bones and joints, pp. 16–163.e161. Chapter 2. In: Maxie MG.editor. Jubb, Kennedy & Palmer's pathology of domestic animals, vol 1, 6th ed. St Louis (MO): WB Saunders. [Google Scholar]
- 12.Dubicanac M, Joly M, Strüve J, Nolte I, Mestre-Francés N, Verdier JM, Zimmermann E. 2018. Intraocular pressure in the smallest primate aging model: the gray mouse lemur. Vet Ophthalmol 21:319–327. 10.1111/vop.12434. [DOI] [PubMed] [Google Scholar]
- 13.Ezran C, Karanewsky CJ, Pendleton JL, Sholtz A, Krasnow MR, Willick J, Razafindrakoto A, Zohdy S, Albertelli MA, Krasnow MA. 2017. The mouse lemur, a genetic model organism for primate biology, behavior, and health. Genetics 206:651–664. 10.1534/genetics.116.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer KE, Austad SN. 2011. The development of small primate models for aging research. ILAR J 52:78–88. 10.1093/ilar.52.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerber L. 2020. The art of growing old: environmental manipulation, physiological rhythms, and the advent of Microcebus murinus as a primate model of aging. Hist Philos Life Sci 42:26. 10.1007/s40656-020-00321-2. [DOI] [PubMed] [Google Scholar]
- 16.Hämäläinen A. 2012. A case of adult cannibalism in the gray mouse lemur, Microcebus murinus. Am J Primatol 74:783–787. 10.1002/ajp.22034. [DOI] [PubMed] [Google Scholar]
- 17.Hämäläinen A, Raharivololona B, Ravoniarimbinina P, Kraus C. 2015. Host sex and age influence endoparasite burdens in the gray mouse lemur. Front Zool 12:1–14. 10.1186/s12983-015-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatt JM, Sainsbury AW. 1998. Unusual case of metabolic bone disease in a common marmoset (Callithrix jacchus). Vet Rec 143:78–80. 10.1136/vr.143.3.78. [DOI] [PubMed] [Google Scholar]
- 19.Hozer C, Pifferi F, Aujard F, Perret M. 2019. The biological clock in gray mouse lemur: adaptive, evolutionary and aging considerations in an emerging non-human primate model. Front Physiol 10:1–23. 10.3389/fphys.2019.01033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hülskötter K, Schmidtke D, Dubicanac M, Siesenop U, Zimmermann E, Gerhauser I, Baumgärtner W, Herder V. 2017. Spontaneous listeriosis in grey mouse lemurs (Microcebus murinus), but not in Goodman's mouse lemurs (Microcebus lehilahytsara) of the same colony. Vet Microbiol 208:94–96. 10.1016/j.vetmic.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 22.Jarcho MR, Power ML, Layne-Colon DG, Tardif SD. 2013. Digestive efficiency mediated by serum calcium predicts bone mineral density in the common marmoset (Callithrix jacchus). Am J Primatol 75:153–160. 10.1002/ajp.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joly M, Ammersdörfer S, Schmidtke D, Zimmermann E. 2014. Touchscreen-based cognitive tasks reveal age-related impairment in a primate aging model, the grey mouse lemur (Microcebus murinus). PLoS One 9:1–12. 10.1371/journal.pone.0109393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasuya K, Takayama K, Bito M, Shimokubo N, Kawashima R, Shibahara T. 2017. Septicemic invasive Klebsiella pneumoniae infection in a cynomolgus monkey (Macaca fascicularis) with severe diffused suppurative meningoencephalitis. J Vet Med Sci 79:1167–1171. 10.1292/jvms.17-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraska A, Dorieux O, Picq JL, Petit F, Bourrin E, Chenu E, Volk A, Perret M, Hantraye P, Mestre-Frances N, Aujard F, Dhenain M. 2011. Age-associated cerebral atrophy in mouse lemur primates. Neurobiol Aging 32:894–906. 10.1016/j.neurobiolaging.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Languille S, Blanc S, Blin O, Canale CI, Dal-Pan A, Devau G, Dhenain M, Dorieux O, Epelbaum J, Gomez D, Hardy I, Henry PY, Irving EA, Marchal J, Mestre-Francés N, Perret M, Picq JL, Pifferi F, Rahman A, Schenker E, Terrien J, Théry M, Verdier JM, Aujard F. 2012. The grey mouse lemur: a non-human primate model for ageing studies. Ageing Res Rev 11:150–162. 10.1016/j.arr.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Larsen PA, Harris RA, Liu Y, Murali SC, Campbell CR, Brown AD, Sullivan BA, Shelton J, Brown SJ, Raveendran M, Dudchenko O, Machol I, Durand NC, Shamim MS, Aiden EL, Muzny DM, Gibbs RA, Yoder AD, Rogers J, Worley KC. 2017. Hybrid de novo genome assembly and centromere characterization of the gray mouse lemur (Microcebus murinus). BMC Biol 15:1–17. 10.1186/s12915-017-0439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liptovszky M, Perge E, Molnár V, Sós E. 2011. Osteoblastic osteosarcoma in a Grey Mouse Lemur (Microcebus murinus)—short communication. Acta Vet Hung 59:433–437. 10.1556/avet.2011.030. [DOI] [PubMed] [Google Scholar]
- 29.Marchal J, Dorieux O, Haro L, Aujard F, Perret M. 2012. Characterization of blood biochemical markers during aging in the Grey Mouse Lemur (Microcebus murinus): impact of gender and season. BMC Vet Res 8:1–9. 10.1186/1746-6148-8-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson EJ, Shaw GC, Hutchinson EK, Schultz-Darken N, Bolton ID, Parker JB, Morrison JM, Baxter VK, Pate KA, Mankowski JL, Carlson CS. 2015. Bone disease in the common marmoset: radiographic and histological findings. Vet Pathol 52:883–893. 10.1177/0300985815589354. [DOI] [PubMed] [Google Scholar]
- 31.Perret M. 1982. Stress-effects in Microcebus murinus. Folia Primatol (Basel) 39:63–114. 10.1159/000156069. [DOI] [PubMed] [Google Scholar]
- 32.Picq JL, Villain N, Gary C, Pifferi F, Dhenain M. 2015. Jumping stand apparatus reveals rapidly specific age-related cognitive impairments in mouse lemur primates. PLoS One 10:1–11. 10.1371/journal.pone.0146238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pifferi F, Epelbaum J, Aujard F. 2019. Strengths and weaknesses of the gray mouse lemur (Microcebus murinus) as a model for the behavioral and psychological symptoms and neuropsychiatric symptoms of dementia. Front Pharmacol 10:1–11. 10.3389/fphar.2019.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remick AK, Van Wettere AJ, Williams CV. 2009. Neoplasia in prosimians: case series from a captive prosimian population and literature review. Vet Pathol 46:746–772. 10.1354/vp.08-VP-0154-R-FL. [DOI] [PubMed] [Google Scholar]
- 35.Roberts L. 2019. Small, furry and powerful: are mouse lemurs the next big thing in genetics? Nature 570:151–154. 10.1038/d41586-019-01789-0. [DOI] [PubMed] [Google Scholar]
- 36.Roy M, Cardoso C, Dorieux O, Malgorn C, Epelbaum S, Petit F, Kraska A, Brouillet E, Delatour B, Perret M, Aujard F, Dhenain M. 2015. Age-associated evolution of plasmatic amyloid in mouse lemur primates: relationship with intracellular amyloid deposition. Neurobiol Aging 36:149–156. 10.1016/j.neurobiolaging.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmid J, Kappeler PM. 1994. Sympatric mouse lemurs (Microcebus spp.) in Western Madagascar. Folia Primatol (Basel) 63:162–170. 10.1159/000156812. [DOI] [PubMed] [Google Scholar]
- 38.Schmidtke D, Lempp C, Dubicanac M, Radespiel U, Zimmermann E, Baumgärtner W, Kästner S, Meier M, Balkema-Buschmann A, Harris RA, Raveendran M, Muzny DM, Worley KC, Rogers J. 2018. Spontaneous spongiform brainstem degeneration in a young mouse lemur (Microcebus murinus) with conspicuous behavioral, motor, growth, and ocular pathologies. Comp Med 68:489–495. 10.30802/AALAS-CM-18-000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidtke D, Zimmermann E, Trouche SG, Fontès P, Verdier JM, Mestre-Francés N. 2020. Linking cognition to age and amyloid-β burden in the brain of a nonhuman primate (Microcebus murinus). Neurobiol Aging 94:207–216. 10.1016/j.neurobiolaging.2020.03.025. [DOI] [PubMed] [Google Scholar]
- 40.Williams CV. 2015. Prosimians, p 291–301. Chapter 36. In: Miller RE, Fowler ME, editors. Fowler's Zoo and Wild Animal Medicine, vol 8. St Louis (MO): WB Saunders. [Google Scholar]
- 41.Wolfensohn SE. 2003. Case report of a possible familial predisposition to metabolic bone disease in juvenile rhesus macaques. Lab Anim 37:139–144. 10.1258/00236770360563787. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi A, Kohno Y, Yamazaki T, Takahashi N, Shinki T, Horiuchi N, Suda T, Koizumi H, Tanioka Y, Yoshiki S. 1986. Bone in the marmoset: a resemblance to vitamin D-dependent rickets, type II. Calcif Tissue Int 39:22–27. 10.1007/BF02555736. [DOI] [PubMed] [Google Scholar]