Abstract
Sheep are a commonly used and validated model for cardiovascular research and, more specifically, for heart valve research. Implanting a heart valve on the arrested heart in sheep is complex and is often complicated by difficulties in restarting the heart, causing significant on-table mortality. Therefore, optimal cardioprotective management during heart valve implantation in sheep is essential. However, little is known about successful cardioprotective management techniques in sheep. This article reports our experience in the cardioprotective management of 20 female sheep that underwent surgical aortic valve replacement with a stented tissue-engineered heart valve prosthesis. During this series of experiments, we modified our cardioprotection protocol to improve survival. We emphasize the importance of total body hypothermia and external cooling of the heart. Furthermore, we recommend repeated cardioplegia administration at 20 min intervals during surgery, with the final dosage of cardioplegia given immediately before the de-clamping of the aorta. To reduce the number of defibrillator shocks during a state of ventricular fibrillation (VF), we have learned to restart the heart by reclamping the aorta, administering cardioplegia until cardiac arrest, and de-clamping the aorta thereafter. Despite these encouraging results, more research is needed to finalize a protocol for this procedure.
Abbreviations: CPB, cardiopulmonary bypass; SR, sinus rhythm; VF,ventricular fibrillation
Sheep are a commonly used and well-validated model for cardiovascular research, particularly for heart valve research, as blood pressure, heart rate, cardiac output, and intracardiac pressures are similar between sheep and humans. Sheep are particularly useful for heart valve research because observable changes in implanted heart valve bioprostheses that would take several years to develop in humans are apparent after only a few months in sheep.3,11 This feature allows the ovine model to provide relevant and important information about heart valve prostheses in a relatively short time span. The first preclinical step in developing novel heart valves is to test the valve in the pulmonary position in sheep. This surgical technique is relatively easy, as the procedure can be performed on a beating heart in a low-pressure circulation. However, aortic valve surgery is the most frequently performed valvular surgical intervention in human patients.12 Thus, an important next step is to prove the clinical applicability of a new valve by testing the valve in-vivo in the aortic position in an animal model. In contrast to pulmonary valve replacement, aortic valve replacement must be performed on an arrested heart, which makes the surgical procedure significantly more complex. The sheep is a difficult model for aortic valve replacements due to its narrow annulus, short distance between the annulus and coronary ostia, a short ascending aorta, and difficulty in de-airing of the heart prior to suturing the aortotomy.19 Consequently, high on-table mortality rates, ranging from 9% to 33%, have been reported.1,18,21,24 Furthermore, the incidence of mortality during the first 30 d after surgery, directly related to the surgical procedure, is often high, ranging from 17% to 50%.1,2,16,18,21 Therefore, optimizing cardioprotective strategies during surgery would improve postoperative survival. However, little is known about protective strategies in sheep. In the current series of experiments, we implanted stented, tissue engineered, aortic heart valve prostheses in 20 adult domestic sheep and developed cardioprotective techniques to increase survival rates. In this observational study, we share our experience and insights regarding cardioprotective management to potentially improve the outcome of future surgeries that require an arrested heart in sheep.
Materials and Methods
Animals.
We performed surgery on 20 female Swifter sheep (mean age 1.97 ± 0.23year, mean weight 69.4 kg ± 6.6 kg) with a follow-up period of between 1 and 12 mo. Approval for the animal studies was obtained by the Amsterdam University Medical Centers Animal Care Ethics Committee (AVD1180020197705) and is consistent with the current Dutch law on animal experimentation (WOD). The sheep were acquired from a local vendor and were bred for educational purposes. After receipt, the sheep were quarantined for at least 14 d prior to surgery. During and after the quarantine period, animal caretakers assessed the general health of all sheep at least twice a day during feeding and general caretaking, with particular attention paid to sheep that had recently undergone surgery. Husbandry personnel also checked the sheep twice a week for healthy rumination. A detailed animal welfare assessment was conducted once a week, during which all sheep were thoroughly checked for any clinical symptoms or signs of discomfort. Welfare assessments were individually recorded for every sheep. All animals were socially housed in groups of 2 to 10 animals, on wheat straw bedding (Nijssen Fourages, Nieuw-Vennep, the Netherlands). Animals were fed sheep feed pellets (250 gr per day; Kasper Faunafood, Woerden, the Netherlands) and had ad libitum access to meadow hay (Nijssen Fourages, Nieuw-Vennep, the Netherlands) and tap water. The sheep were housed indoors in an air-conditioned room with a temperature ranging between 15 to 21 °C and with 12h:12h light:dark cycle.
Anesthesia.
A buprenorphine patch (5 mcg/h patch; BuTrans, Mundipharma, Cambridge, UK) was taped to the shaved, ventral side of the proximal part of the tail one day prior to the aortic valve surgery to provide preemptive analgesia. On the day of surgery, ketamine hydrochloride (10 mg/kg IM, Narketan 100 mg/mL, Vétoquinol, Paris, France) and midazolam (0.4 mg/kg IM, Midazolam Actavis 5 mg/mL, Actavis, NJ, US) were administered as preanesthetic medication. A single dose of amoxicillin/clavulanic acid (20 mg/kg IV, Amoxicillin/clavulanic acid 500 mg/50 mg, Sandoz, Holzkirchen, Germany) was administered before the incision to provide prophylactic antibiotic therapy. Propofol was used to induce (2 to 4 mg/kg IV; Propofol 20 mg/mL, Fresenius Kabi, Bad Homburg, Germany) and maintain anesthesia (20 mg/kg/h IV) during surgery. Sufentanil (5 mcg/kg/h IV, Sufentanil-Hameln 50 mcg/mL, Hameln, Gloucester, UK) was administered for pain relief during surgery. A single dose of amiodarone hydrochloride (300 mg IV, Cordarone 50 mg/mL, Sanofi, Paris, France) was added to the saline infusion bag before starting cardiopulmonary bypass (CPB).
Aortic valve replacement.
All sheep were monitored during surgery by ECG, arterial blood pressure measurement, and capnography. After anesthesia, sheep were placed in the right lateral position, and the left thorax and neck were prepared for surgery. The sheep were placed on CPB after heparinization (15,000 to 20,000 IU IV, heparin 5000 IU/mL, LEO, Ballerup, Denmark), with CPB flow rates between 3.5 to 4 L/min. An activated clotting time of greater than 400 s was maintained during CPB and arterial blood gas analyses were performed. For arterial access, a 16 Fr arterial cannula (Edwards Lifesciences, Irvine, US) was inserted in the left carotid artery. A 24 Fr venous cannula (Edwards Lifesciences, Irvine, US) was inserted in the left jugular vein. The Seldinger technique was used for both canulation sites.23 A left-sided anterolateral thoracotomy was performed in the third or fourth intercostal space. The pericardium was opened and the pulmonary artery was dissected free from the aorta. A 13 Fr cannula (Medtronic, Minneapolis, US) was placed in the left ventricle via the left atrial appendage for left ventricular venting. The animal was cooled down to 32 °C. The ascending aorta was clamped just proximal to the junction of the brachiocephalic trunk. A needle was inserted into the aorta proximal to the cross clamp, and one liter of cardioplegic solution (the commercially available formulation of St Thomas’ Hospital Cardioplegic Solution number 1, supplemented with mannitol (80 mL; Mannitol 10%, Baxter, Utrecht, the Netherlands)) was administered into the ascending aorta (Table 1) at a flow-rate of 250 mL/min. Cold saline was poured directly in the pericardial space. A subtotal transverse aortotomy was made distal to the sinotubular junction, after which the cusps of the native aortic valve were excised. The heart valve prosthesis was implanted using nonpledgeted interrupted sutures (2-0 Ti-Cron, Covidien, Dublin, Ireland). The aorta was closed in a continuous fashion (5-0 Prolene C1, Ethicon, Somerville, US) and carefully de-aired. The animal was rewarmed. The aorta clamp was removed. If needed, the heart was defibrillated (20J) to induce to a stable sinus rhythm. The left venting cannula was removed, and the incision in the left auricular appendage was closed with a running suture (5-0 Prolene C1, Ethicon, Somerville, US). After weaning from extracorporeal circulation, epicardial echocardiography was performed, using a TEE probe on a Philips iE33 echocardiography machine to assess valve function. Protamine hydrochloride (10 mL IV, protaminehydrochloride 10 mg/mL, Mylan, Canonsburg, US) was administered intravenously before removal of the cannulas. A chest tube was placed in the left thoracic cavity, and the wound was closed in layers. The chest tube was removed shortly before extubation.
Table 1.
Composition of the cardioplegia
| St Thomas cardioplegia | Number 1, per liter |
| Sodium chloride | 5377 mg |
| Potassium chloride | 1109 mg |
| Magnesium sulfate | 297 mg |
| Magnesium chloride | 3063 mg |
| Monopotassium phosphate | 164 mg |
| Calcium chloride dehydrate | 177 mg |
| Procaine hydrochloride | 274 mg |
| Additive to St Thomas cardioplegia, per liter | |
| Mannitol | 10%, 80 mL |
Results
Surgery and recovery.
All sheep but one could be weaned from cardiopulmonary bypass. The mean cardiopulmonary bypass (CPB) time was 163 ± 58 min, and mean aortic cross clamp time was 83 ± 29 min. One sheep (number 13) died during surgery due to refractory VF. Surgery on this sheep was difficult due to a lesion of the aortic arch, which required extensive reconstruction, resulting in a prolonged clamp time (173 min). Four sheep died on days one and two after surgery. Sheep number 5 died due to severe aortic regurgitation because an aortic valve leaflet remained in open position. This was caused by one loose fiber of the electrospun valve leaflet scaffold that was caught in a suture, either during aortic closure or during one additional hemostatic stitch that was placed after the aorta was closed. Sheep number 10 died due to high lactate levels during CPB support. Sheep number 12 died due to hemorrhage. Sheep number 20 died due to prolonged CPB time (340 min). The remaining 15 sheep were sacrificed according to schedule or are still alive at the time of writing (the follow-up period of the sheep is ongoing). Three sheep developed complications during the postoperative period. Sheep 18 had postoperative pleural effusion, and sheep 19 developed cardiac tamponade; both were treated with a thorax drain. The third sheep (number 3) required inotropic support with dobutamine (10 mcg/kg/min IV, Dobutamine 12.5 mg/mL, Centrafarm, Etten-Leur, Netherlands) during the first night. All 20 sheep are included in this analysis (Table 2).
Table 2.
Cardioprotective management.
| Sheep | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| CPB time (min) | 144 | 140 | 234 | 130 | 202 | 127 | 258 | 114 | 110 | 114 | 97 | 165 | X | 199 | 144 | 127 | 150 | 172 | 140 | 340 |
| Aorta clamp time (min) | 70 | 69 | 89 | 67 | 82 | 75 | 102 | 71 | 71 | 77 | 65 | 76 | 173 | 108 | 77 | 90 | 90 | 110 | 81 | 105 |
| Number of applied shocks | 0 | 20 | 25 | 4 | 25 | 1 | 35 | 3 | 2 | 0 | 0 | 29 | 100 | 10 | 15 | 0 | 0 | 5 | 0 | 5 |
| Dosage of cardioplegia (mL) | 850 | 1500 | 900 | 1550 | 1650 | 1000 | 2500 | 1350 | 1350 | 1600 | 1400 | 2100 | 2700 | 2000 | 1600 | 1500 | 1400 | 2500 | 1600 | 2350 |
| Repetitive selective cardioplegia (each 20 min) | No | No | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Administration of a final dose of cardioplegia before de-clamping | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | Yes | Yes | Yes | Yes | Yes |
| Reclamping the aorta | No | No | No | No | No | No | Yes | No | No | No | No | Yes | Yes | Yes | Yes | No | No | Yes | No | Yes |
| Spontaneous sinus rhythm after reclamping aorta | NA | NA | NA | NA | NA | NA | Yes | NA | NA | NA | NA | Yes | No | Yes | Yes | NA | NA | Yes | NA | Yes |
| Survival of the surgery | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 30-day survival | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No |
CPB = cardiopulmonary bypass, NA = not applicable
The cardioprotection protocol.
When starting our experimental series, we administered only a single dose of cardioplegic solution into the ascending aorta after clamping. We did not initially administer multiple doses of cardioplegia during surgery. We cooled the heart by pouring cold saline (4 °C) into the pericardium, both before and during valve implantation. After the first 2 surgeries, both sheep recovered fairly easily after de-clamping the aorta.
Recovery of the third and fourth sheep after surgery was more difficult. These animals showed persistent VF during recovery and respectively required 20 or 25 shocks with the defibrillator (20Joule) before a stable sinus rhythm could be established. These difficult recoveries led us to modify our cardioprotective protocol. Our first change was to further cool the saline and cardioplegic solution to a temperature between 0 to 4 °C by storing packages in an ice-filled container. Our second change was to routinely administer multiple doses of cardioplegic solution given 20 min apart, rather than using a single dose. The repeated doses of cardioplegia were administered directly into the ostia of both coronary arteries via a perfusion cannula (Medtronic, Minneapolis US).
Our third change is relevant to sheep that develop persistent VF that does not respond to defibrillation attempts. During the eighth experiment, the sheep experienced persistent VF during recovery; the fibrillating heart was not responsive to 35 defibrillation attempts. We clamped the aorta for a second time and administered another dose of cardioplegia until cardiac arrest was achieved. We then de-clamped the aorta; a spontaneous sinus rhythm began immediately thereafter. A comparable case of persistent VF occurred during surgery on the 13th sheep, in which 29 shocks did not induce a stable sinus rhythm. In this sheep we also clamped the aorta for the second time and administered cardioplegia until cardiac arrest. We de-clamped the aorta immediately after cardiac arrest, and a sinus rhythm occurred spontaneously. This method, in which the aorta was reclamped during a state of persistent VF, was our third adaptation to the protocol, and also led to spontaneous sinus rhythm after declamping in sheep fifteen and sixteen.
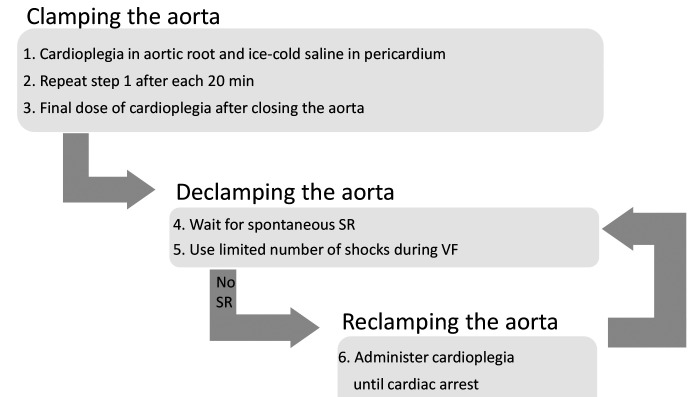
Finally, we observed that a final dose of cardioplegia administered just before de-clamping seemed to lead to an easier conversion to spontaneous sinus rhythm. Our fourth adaptation to the protocol was to routinely apply a last dose of cardioplegia right after closing the aorta to assure complete cardiac arrest before de-clamping. We implemented this fourth adaptation during surgery of sheep 16 to 20. This final protocol resulted in uncomplicated procedures with recovery to a spontaneous sinus rhythm after declamping in 3 of these sheep. The other 2 sheep in this group developed VF and were unsuccessfully defibrillated 5 times, after which we reclamped the aorta and administered cardioplegia until cardiac arrest. Both of these 2 sheep recovered with a spontaneous stable sinus rhythm after removing the clamp for the second time, allowing us to limit the number of shocks administered, thus reducing the potential for myocardial damage. Figure 1 shows the schematic view of our final protocol.
Figure 1.
Schematic overview of the cardioprotection protocol, SR, sinus rhythm; VF, ventricular fibrillation
Discussion
Surgery on the sheep is difficult, as perioperative arrhythmias are often refractory and may result in sudden death.5 Light manipulation of the heart or a short ischemic period can easily cause immediate and intractable VF in sheep.22 When operating on the arrested heart, VF often occurs after declamping. Therefore, myocardial tissue must be protected throughout the process of valve implantation.
Since the 1950s, total body hypothermia has been used to reduce myocardial oxygen consumption and preserve cardiac function during cardiac surgery.4 In the present study, we reduced the blood temperature of the sheep to 32 °C to provide tissue protection. In other similar studies, the internal temperature was lowered even further, to 28 °C6,10 or 25 °C.18 We found that administering ice-cold cardioplegic solution and routinely applying ice-cold saline directly into the pericardium and left ventricle were cardioprotective. Cooling down the cardioplegic solution to 4 °C or using saline slush has also been described in literature.1,8,10,17-19 The cardioplegia solution arrests the heart and prevents repolarization of the membranes of cardiomyocytes due to its high extracellular potassium level, resulting in a decreased cellular energy requirement from the myocardium. Using cold cardioplegic solution to arrest the heart is an excellent method of myocardial preservation, resulting in improved postoperative myocardial performance.9 Moreover, multiple applications of cardioplegia has been described by many authors and seems to be more cardioprotective than administering a single dose.1,6,17,19,21 We observed that inducing complete cardiac arrest throughout surgery by administering multiple doses of cardioplegia and by giving a final dose immediately before declamping the aorta protects the myocardium and promotes spontaneous recovery to a stable sinus rhythm.
VF cannot always be avoided in sheep. VF increases myocardial oxygen demands7,14 while the blood flow to the myocardium is reduced, even during CPB support.20 This combination of factors causes a severe and progressive state of energy imbalance leading to intramyocardial acidosis, accumulation of intramyocardial Na+ and depletion of adenosine triphosphate in the cardiomyocytes.7,14,15 These conditions are not favorable for successful defibrillation, and prolongation of the VF period can impair successful recovery.14,15 Moreover, open chest defibrillation during cardiac surgery can lead to serious complications, such as myocardial necrosis. Higher cumulative energy levels due to repeated defibrillation are likewise related to a higher risk of damage.13 Therefore, defibrillating the ovine heart should be performed as sparingly as possible. Delaying electrical shocks during VF until more favorable myocardial metabolic conditions are restored can improve outcomes.14 In our experience, the metabolic conditions in the myocardium during VF can be improved by reclamping the aorta and arresting the heart. This method of reclamping the aorta has been previously applied in a single experiment.16 In our surgeries, the technique of reclamping, administering cardioplegia, and de-clamping was highly effective during VF, as 6 of 7 sheep spontaneously converted to sinus rhythm without the need for more defibrillation.
In these surgeries, we carefully de-aired the heart before closing the aorta and removing the left venting cannula, but we did not use echocardiography to look for residual air in the heart. Coronary air embolisms can occlude the coronary arteries and lead to VF, so echocardiographic assessment of air in the heart may be warranted to reduce the risk of VF.
Our mean aortic cross clamp time was rather long (mean CPB time was 163 min and mean aortic cross clamp time was 83 min) because we implanted a 19 mm stented heart valve prosthesis with high struts. The size of the prosthesis, compared with the native aortic annulus and the notable height of the struts, tremendously complicated the procedure, particularly the closure of the aorta. Before starting this experimental series, we implanted one 17 mm stented heart valve prosthesis in an acute trial in a sheep of comparable size. In that experiment, the 17 mm valve prosthesis was implanted without any difficulties in a short period (CPB time was 126 min and aortic cross clamp time was 52 min). However, to avoid a size mismatch between the valve prosthesis and the native aortic annulus that may occur with 17 mm prostheses, we decided to implant 19 mm prostheses in this experimental series.
Our insights into cardioprotection for sheep are purely based on observations, which is a major limitation of this study. Because we continuously modified our cardioprotection protocol, we could not compare different treatments in a randomized fashion. Furthermore, we do not have a control group for comparison. Further research should be conducted to experimentally determine the optimal cardioprotection protocol for the ovine heart.
In these surgeries, we observed benefits of effective cardioprotection during surgery on the arrested heart during aorta valve implantation in sheep. We emphasize the importance of total body hypothermia and frequent administration of ice-cold saline on the heart throughout surgery. Furthermore, we recommend the administration of cardioplegia repeatedly during surgery at 20 min intervals, with the final dose of cardioplegia administered just before de-clamping the aorta. This procedure appears to facilitate the recovery of a stable sinus rhythm. To reduce the number of shocks needed to resolve a state of VF and the associated risk of damage to the myocardium, we experienced successful outcomes with reclamping the aorta, administering cardioplegia until cardiac arrest, and de-clamping the aorta thereafter.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Cardiovasculair Onderzoek Nederland [grant number CVON2012-01].
References
- 1.Amaral JJ, Pomerantzeff PM, Casagrande IS, Cestari IA, Gutierrez PS, Stolf NG. 2010. Analysis of hemodynamic performance of the bovine pericardium valved conduit, implanted in the aortic position in ovines. Rev Bras Cir Cardiovasc 25:543–551. 10.1590/S0102-76382010000400019. [Article in English, Portuguese]. [DOI] [PubMed] [Google Scholar]
- 2.Baraki H, Tudorache I, Braun M, Höffler K, Görler A, Lichtenberg A, Bara C, Calistru A, Brandes G, Hewicker-Trautwein M, Hilfiker A, Haverich A, Cebotari S. 2009. Orthotopic replacement of the aortic valve with decellularized allograft in a sheep model. Biomaterials 30:6240–6246. 10.1016/j.biomaterials.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 3.Barnhart GR, Jones M, Ishihara T, Rose DM, Chavez AM, Ferrans VJ. 1982. Degeneration and calcification of bioprosthetic cardiac valves. Bioprosthetic tricuspid valve implantation in sheep. Am J Pathol 106:136–139. [PMC free article] [PubMed] [Google Scholar]
- 4.Bigelow WG, Lindsay WK, Greenwood WF. 1950. Hypothermia; its possible role in cardiac surgery: an investigation of factors governing survival in dogs at low body temperatures. Ann Surg 132:849–866. 10.1097/00000658-195011000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dardenne A, Fernandez C, Wagner A, Milewski K, Ordanes DR, Mount PA, Cheng Y, Yi GH, Conditt GB, Tellez A, Kaluza GL, Granada JF, Feeney WP. 2013. Benefits of standardizing the treatment of arrhythmias in the sheep (Ovis aries) model of chronic heart failure after myocardial infarction. J Am Assoc Lab Anim Sci 52:290–294. [PMC free article] [PubMed] [Google Scholar]
- 6.Doss M, Risteski P, Wood JP, Wimmer-Greinecker G, Moritz A. 2008. In-vivo evaluation of the BioPhysio valve prosthesis in the aortic position. J Heart Valve Dis 17:105–109. [PubMed] [Google Scholar]
- 7.El-Menyar AA. 2005. The resuscitation outcome: revisit the story of the stony heart. Chest 128:2835–2846. 10.1378/chest.128.4.2835. [DOI] [PubMed] [Google Scholar]
- 8.Goetz WA, Tan TE, Lim KH, Salgues Sle H, Grousson N, Xiong F, Chua YL, Yeo JH. 2008. Truly stentless molded autologous pericardial aortic valve prosthesis with single point attached commissures in a sheep model. Eur J Cardiothorac Surg 33:548–553. 10.1016/j.ejcts.2007.12.044 [DOI] [PubMed] [Google Scholar]
- 9.Harlan BE, Starr A, Harwin FM. 2012. Manual of cardiac surgery: volume 1. New York (NY): Springer–Verlag. [Google Scholar]
- 10.Harvey L, Bianco R, Lahti M, Carney J, Zhang L, Robinson N. 2015. Carpentier-Edwards aortic pericardial bioprosthetic valve as a valid control in preclinical in vivo ovine studies. Eur J Pharmacol 759:192–199. 10.1016/j.ejphar.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 11.Hill AJ, Iaizzo PA. 2009. Comparative cardiac anatomy. p 104–107. In: Iaizzo PA, editor. Handbook of cardiac anatomy, physiology, and devices. New York (NY): Springer Science. 10.1007/978-1-60327-372-5 [DOI] [Google Scholar]
- 12.Iung B, Vahanian A. 2011. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol 8:162–172. 10.1038/nrcardio.2010.202. [DOI] [PubMed] [Google Scholar]
- 13.Kerber RE, Carter J, Klein S, Grayzel J, Kennedy J. 1980. Open chest defibrillation during cardiac surgery: energy and current requirement. Am J Cardiol 46:393–396. 10.1016/0002-9149(80)90006-5. [DOI] [PubMed] [Google Scholar]
- 14.Kolarova J, Ayoub IM, Yi Z, Gazmuri RJ. 2003. Optimal timing for electrical defibrillation after prolonged untreated ventricular fibrillation. Crit Care Med 31:2022–2028. 10.1097/01.CCM.0000070446.84095.F4. [DOI] [PubMed] [Google Scholar]
- 15.Liakopoulos OJ, Allen BS, Buckberg GD, Hristov N, Tan Z, Villablanca JP, Trummer G. 2010. Resuscitation after prolonged cardiac arrest: role of cardiopulmonary bypass and systemic hyperkalemia. Ann Thorac Surg 89:1972–1979. 10.1016/j.athoracsur.2010.02.052. [DOI] [PubMed] [Google Scholar]
- 16.Meuris B, Ozaki S, Neethling W, De Vleeschauwer S, Verbeken E, Rhodes D, Verbrugghe P, Strange G. 2016. Trileaflet aortic valve reconstruction with a decellularized pericardial patch in a sheep model. J Thorac Cardiovasc Surg 152:1167–1174. 10.1016/j.jtcvs.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang DW, Salerno CT, Pederson TS, Bolman RM, 3rd, Bianco RW. 1998. Long-term evaluation of orthotopically implanted stentless bioprosthetic aortic valves in juvenile sheep. J Invest Surg 11:175–183. 10.3109/08941939809098032 [DOI] [PubMed] [Google Scholar]
- 18.Rakow N, Nelson DA, Falkner P, Wahlberg P, Grangaard R, Capps M, Billstrom T, Shecterle LM, St Cyr JA. 2007. Orthotopic total aortic root replacement model in adult sheep. J Inves Surg 20:55–59. 10.1080/08941930601126322 [DOI] [PubMed] [Google Scholar]
- 19.Salerno CT, Pederson TS, Ouyang DW, Bolman RM, 3rd, Bianco RW. 1998. Chronic evaluation of orthotopically implanted bileaflet mechanical aortic valves in adult domestic sheep. J Inves Surg 11:341–347. 10.3109/08941939809032210 [DOI] [PubMed] [Google Scholar]
- 20.Salerno TA, Chiong MA. 1983. Should ventricular fibrillation be induced prior to the infusion of cardioplegic solution? Ann Thorac Surg 35:367–371. 10.1016/S0003-4975(10)61586-X. [DOI] [PubMed] [Google Scholar]
- 21.Santos PC, Gerola LR, Casagrande I, Buffolo E, Cheung DT. 2007. Stentless valves treated by the L-hydro process in the aortic position in sheep. Asian Cardiovasc Thorac Ann 15:413–417. 10.1177/021849230701500511. [DOI] [PubMed] [Google Scholar]
- 22.Shofti R, Zaretzki A, Cohen E, Engel A, Bar-El Y. 2004. The sheep as a model for coronary artery bypass surgery. Lab Anim 38:149–157. 10.1258/002367704322968821. [DOI] [PubMed] [Google Scholar]
- 23.Seldinger SI. 1953. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol 39:368–376. 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- 24.Theodoridis K, Tudorache I, Cebotari S, Calistru A, Meyer T, Sarikouch S, Bara C, Haverich A, Hilfiker A. 2017. Six-year-old sheep as a clinically relevant large animal model for aortic valve replacement using tissue-engineered grafts based on decellularized allogenic matrix. Tissue Eng Part C Methods 23:953–963. 10.1089/ten.tec.2017.0163. [DOI] [PubMed] [Google Scholar]