Abstract
Background:
Glycation, inflammation, and oxidative stress are the cardinal motivators of diabetes vascular complications. Here, we studied the effect of eucalyptol (EUC) on the formation of atheromatous lesions, glycation, oxidative stress, and inflammatory markers as well as insulin resistance, lipid profile, and activity of glyoxalase-1 (GLO-I) in the atherosclerotic rat model.
Methods:
Diabetic-atherosclerosis induced in rats with a combination of streptozotocin and atherogenic diet. Two groups of rats, normal and diabetic-atherosclerotic, were treated intragastrically with EUC (200 mg/kg) once daily for 3 months. Fasting blood sugar (FBS), insulin, insulin resistance index, lipid profile, the activity of GLO-I, low-density lipoprotein (LDL) glycation and oxidation markers, inflammatory markers, creatinine in the serum, and proteinuria in the urine of all rats were determined.
Results:
EUC inhibited the formation of any atheromatous lesions in atherosclerotic rats. Further, EUC displayed the lowering effect on glycemia, insulin resistance, LDL glycation, and oxidation products, and tumor necrosis factor (TNF)-α as well as it exhibited the improving effect on lipid profile, the activity of GLO-I, and renal function in the diabetic rat (P < 0.001).
Conclusions:
EUC prevented the formation of the atheromatous lesions and improved renal function in the atherosclerotic rat model due to a reduction of glycation, oxidative stress, and inflammatory mediators.
Keywords: Atherosclerosis, eucalyptol, inflammation, oxidative stress
Introduction
Diabetic vascular complications are the reason for high percentage of morbidity and mortality associated with diabetes.[1] Hyperglycemia, insulin resistance, glycation, inflammation, and oxidative stress are the main motivators of it.[2,3] Early, intermediate, and end glycation products by induction of oxidative stress and inflammation contribute to diabetes vascular complications. Furthermore, especially glycated albumin and pentosidine (PEN) have a role as predictors for diabetic vascular complications.[4,5] Cytotoxic reactive carbonyl species as methylglyoxal (MGO) contribute to carbonyl stress and the formation of advanced glycation end products that is momentous for the activation of a series of inflammatory responses leading to diabetic vascular complications.[6] Furthermore, the activity of the glyoxalase system as the main enzymatic system for the detoxification of MGO reduces in diabetic patients.[7] Oxidized LDL and glycated albumin (g-Alb) consociate with severeness of atherosclerosis.[3,8] Over and above, Ox-LDL as the main contributor of macrovascular complications is the result of glycation and free radicals that accelerates diabetes.
Eucalyptol (EUC) or 1,8 cineole, a monoterpene naturally found in essential oils of various plant species, has been widely reported for the bioactivities. Moveover, it possesses diverse pharmacological activities including antimicrobial, anticancer, anti-inflammatory, antioxidation.[9] The effect of EUC has been studied only in an atherosclerotic model of Zebrafish, which indicates its reductive effect on lipid profiles in the atherosclerotic model and in vitro human LDL oxidation.[8] Up to now, the effect of EUC on glycation, oxidative stress, and inflammatory markers, as well as the activity of glyoxalase-1 (GLO-I) in the diabetic-atherosclerotic model, has not been studied. Therefore, in this study, for the first time, the effect of EUC on the formation of atheromatous lesions, glycation, oxidative stress, and inflammatory markers as well as insulin resistance, lipid profile, and activity of GLO-I in atherosclerotic rat model have been investigated.
Methods
Materials
EUC, dihydroxyacetone, sodium carbonate, sodium mono and dihydrogen phosphate, iodoacetamide, glucose, sodium azide, acetonitrile, ethanol, trichloroacetic acid, 2,4-dinitrophenylhydrazine, potassium iodide, cyclohexane, heptafluorobutyric acid, CuSo4, thiobarbituric acid, Chol, cholic acid, 2-methylpropanol, CaCl2, NaCl, and EDTA from Merck Chemical Co. Streptozotocin (STZ), methylglyoxal (MGO), pentosidine (PEN), nitroblue tetrazolium (NBT), oxalic acid, 5-hydroxymethylfurfuraldehyde, and Triton X-100, were purchased from Sigma Chemical Co. The 0.45 μm syringe filters were obtained from Millipore.
In vivo studies
Animal model of diabetic-atherosclerosis
In this study, with the approval of the ethics committee at Ardabil University of Medical Sciences with the code of Ethics IR.ARUMS.REC.1397.176 and by the standard principles of working with laboratory animals, 40 male Wistar rats housed under controlled conditions. After two weeks, they were divided into two main groups: normal and diabetic-atherosclerotic rats. Diabetic-atherosclerosis induced in rat with a combination of intraperitoneal (i.p) injection of STZ (45 mg/kg) and atherogenic diet (chow diet containing 1% cholesterol and 0.5% cholic acid) that explained in our previous study.[2] Then, each of the two main (diabetic and normal) groups were divided into two subgroups, containing 10 rats in each. These groups were named as follows: subgroup 1: normal and atherosclerotic groups that received 0.9% as vehicle and subgroup 2: normal and atherosclerotic groups that received EUC (200 mg/kg). Further, EUC and vehicle were administered intragastrically once daily for 12 weeks. The treatment was dissolved in Tween 20 as a stock solution and diluted with normal saline before intragastric administration to rats. The dose of EUC was selected based on the best activities of antioxidant and anti-inflammatory in the cited dose[10] and our pilot study.
The experimental protocol was approved by the Animal Ethical Committee in accordance with the guidelines for the care and use of laboratory animals prepared by Ardabil University of medical sciences.
The blood samples of each animal were taken from heart at the end of the study and were allowed to clot at room temperature. Serum was separated by centrifugation at 5000 rpm for 15 min and was stored at −70°C for measurements.
Determination of biochemical parameters
Fasting blood sugar (FBS), triglyceride (TG), total cholesterol (TC), LDL, and high-density lipoprotein (HDL), as well as serum creatinine (Cr) and protein excretion in urine (PU), were measured by photometric methods. Besides, the atherogenic index was computed with LDL/HDL ratio. The sera insulin levels were assayed with the enzyme-linked immunosorbent assay (ELISA) rat kit (Mercodia, Uppsala, Sweden) and homeostasis model assessment of insulin resistance (HOMA-IR) as an index of insulin resistance and beta-cell function[11] was calculated as explained before.[12]
Determination of glycated products
Glycated LDL was determined by the reaction of extracting LDL with oxalic acid and thiobarbituric acid to yield the hydroxymethylfurfuraldehyde chromogen.[13,14] MGO was assayed by a reverse-phase high-performance liquid chromatography (HPLC) that have been cited in our previous studies.[2]
Determination of oxidative stress markers
Early and end LDL oxidation products was measured based on photometric methods that fully has been expressed in our recent paper.[2] Briefly, conjugated dienes (CD) in LDL lipids, as early products of LDL oxidation, were measured spectrometrically at 234 nm.[15] Fluorescence intensity of end oxidation product of LDL (oxidation products of apo-B100), was recorded at the emission maximum of 430 nm upon excitation at 360 nm.[16] Advanced oxidation protein product (AOPP) was measured based on the spectrophotometric method using chloramine-T as a standard.[17] Levels of malondialdehyde (MDA) and glutathione (GSH) were determined with a commercial assay kit (Cayman Chemical Co., Ann Arbor, MI).
Determination of inflammatory markers
Tumor necrosis factor-α (TNF-α) was assayed using ELISA kits (Immunotech, France). The assay was carried out according to the instructions provided by the manufacturer. All samples were measured in duplicate. The plate was read on an ELISA reader and analyzed using SoftMax (Molecular Devices Corporation, Menlo Park, CA) software. The detection limit of the rat TNF-α ELISA kit was determined to be 10 pg/ml.
Pathological study
The whole aortas were collected and were fixed with 10% buffered formalin. Formalin-fixed paraffin-embedded (FFPE) tissue sections were stained with hematoxylin and eosin (H&E).
Statistical analysis
All data were expressed as mean ± standard deviation (SD). Different variables in all four groups were compared with multiple analysis of variance (MANOVA-TUKEY) test by using Statistical Package for the Social Sciences (SPSS) version 16 statistical. Besides, significance was defined as P < 0.05.
Results
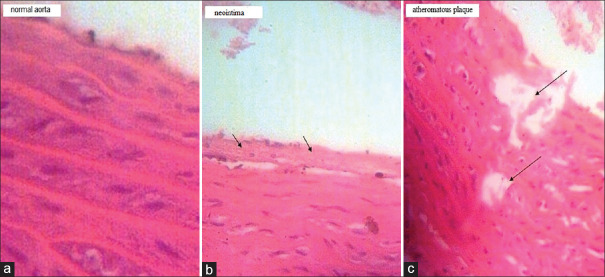
The effect of EUC administration on the formation of aortic lesions in normal and atherosclerotic rats is shown in Figure 1a-c. The atheromatous plaques and neointima were generated in the diabetic-atherosclerotic rats but the treatment inhibited the generation of any aortic lesions in the diabetic-atherosclerotic rats.
Figure 1.
Effect of eucalyptol (EUC) on atheromatous plaque formation in normal (N) and diabetic-atherosclerotic (D) rats (stained by H and E & original magnification ×200). (a) Normal aorta in N, N (EUC) and D (EUC) groups. (b) Formation of neointima in the untreated D group. (c) Atherosclerosis plaque formation in the untreated D group
Table 1 represented the levels of fasting blood sugar (FBS), insulin, HOMA-IR, lipid profile and kidney function parameters in all rat groups. The levels of FBS, HOMA-IR TG, TC, atherogenic index, Cr, and PU increased in the atherosclerotic rat group. Furthermore, EUC compensated for the cited changes (P < 0.001). Therefore, the treatment had an advantageous effect on glucose and lipid metabolism as well as insulin function in diabetic rats.
Table 1.
The effect of eucalyptol on fasting blood sugar, insulin, HOMA-IR, lipid profile and kidney function parameters in the normal (N) and diabetic-atherosclerotic rats (D)
| Parameters | Groups | |||
|---|---|---|---|---|
| N | N (Eucalyptol) | D | D (Eucalyptol) | |
| Fasting blood sugar (mmol/L) | 4.47±0.27 | 4.34±0.31 | 15.81±0.61 | 8.03±0.46 |
| Insulin (µU/mL) | 17.92±1.16 | 18.07±0.95 | 7.86±0.64N | 11.31±0.57N,D |
| HOMA-IR | 3.56±0.18 | 3.48±0.17 | 5.52±0.28N | 4.03±0.24N,D |
| Triglyceride (mmol/l) | 0.90±0.03 | 0.88±0.02 | 2.83±0.08N | 1.02±0.03N,D |
| Total cholesterol (mmol/l) | 2.25±0.07 | 2.13±0.09 | 6.63±0.16N | 4.39±0.13N,D |
| HDL (mmol/l) | 1.32±0.05 | 128±0.05 | 0.44±0.01N | 0.81±0.02N,D |
| LDL (mmol/l) | 0.45±0.02 | 0.37±0.01 | 4.39±0.12N | 2.98±0.06N,D |
| HDL/LDL | 0.34±0.01 | 0.29±0.01 | 9.97±0.21N | 3.67±0.11N,D |
| Creatinine (µmol/l) | 65.50±4.24 | 58.33±1.86 | 98.50±5.74N | 76.45±3.687N,D |
| Proteinuria (mg/24 h) | 16.27±0.57 | 13.25±0.40 | 303.50±16.54N | 187.68±911N,D |
NIndicates significance of data comparing normal group (N) with other groups (P<0.001). DIndicates significance of data comparing diabetic- atherosclerotic group (D) with other groups (P<0.001). HOMA-IR: Homeostasis model assessment of insulin resistance; LDL: Low-density lipoprotein; HDL: High-density lipoprotein
Comparison of the effect of the treatment on glycation (g-LDL, MGO, and PEN), oxidative stress (early and end oxidation products of LDL, AOPP, MDA, and GSH), and inflammatory (TNF-α) markers, as well as the activity of GLO-I in the named groups, are exhibited in Table 2. Induction of diabetic-atherosclerosis in the rats elevated glycation, oxidative stress, and inflammatory markers and decreased the level of GSH and the activity of GLO-I but the levels of glycation, oxidative stress, and inflammatory markers expect GSH and the activity of GLO-I decreased in the treated atherosclerotic rats than the untreated ones (P < 0.001).
Table 2.
The effect of eucalyptol on glycation, oxidative stress and inflammatory markers as well as activity of glyoxalase-I in the normal (N) and diabetic-atherosclerotic rats (D)
| Parameters | Groups | |||
|---|---|---|---|---|
| N | N (Eucalyptol) | D | D (Eucalyptol) | |
| Glycated LDL (µmol/l) | 60.25±2.34 | 58.43±2.65 | 173.98±8.09N | 128.16±6.90N,D |
| Methylglyoxal (µmol/l) | 17.45±1.36 | 9.64±0.83N | 155.31±8.61N | 69.42±3.75N,D |
| Early LDL oxidation products (µmol/l) | 20.43±1.34 | 18.65±0.97 | 87.65±5.03N | 47.45±3.66N,D |
| End LDL oxidation products (µmol/l) | 254.43±17.98 | 247.65±15.09 | 487.16±23.09N | 403.73±19.12N,D |
| Advanced oxidation protein products (µmol/l) | 30.71±4.08 | 26.32±3.95 | 89.65±6.01N | 48.74±375N,D |
| Malondialdehyde (µmol/l) | 9.84±0.62 | 9.96±0.73 | 101.57±7.36N | 80.82±5.54N,D |
| Glutathione (µmol/l) | 188.15±9.03 | 192.67±10.67 | 99.43±5.05N | 139.81±7.33N,D |
| Tumor necrosis factor-α (pg/ml) | 131.54±8.12 | 100.06±6.23N | 321.76±18.06N | 245.22±14.81N,D |
| Glyoxalase-I (U/ml) | 39.95±4.64 | 47.68±2.92N | 20.85±3.05N | 35.16±3.37N,D |
NIndicates significance of data comparing normal group (N) with other groups (P<0.001). DIndicates significance of data comparing diabetic-atherosclerotic group (D) with other groups (P<0.001). LDL: Low-density lipoprotein
Discussion
In this study, EUC prevented the formation of an atheromatous lesion due to the reduction of various glycation, oxidative stress, and inflammatory markers as risk factors for vascular complications in the atherosclerotic rats. Moreover, the treatment showed an advantageous effect on glucose and lipid metabolism, glyoxalase system activity, and kidney function.
According to the pathologic investigation, both of the neointima and the atheromatous plaque were formed in the aorta of the untreated diabetic-atherosclerotic rats [Figure 1b and c]. However, any lesions were observed in the aorta of other groups [Figure 1a]. Presumably, EUC decreased the accumulation of macrophage-derived foam cells in the aortic intima of the diabetic-atherosclerotic rats and inhibited the thickening of intima.
Hyperglycemia and insulin resistance are the main participants in the pathogenesis of vascular disorders of diabetes.[18] EUC diminished the levels of blood glucose and insulin resistance in the diabetic-atherosclerotic rats [Table 1] owing to the beneficial effect on insulin action with antioxidant antiglycation properties (P < 0.001).
The rising levels of TG, TC, and LDL along with a reduction of HDL as dyslipidemia are viewed in atherosclerotic rats. The treatment represented an advantageous effect on the lipid profile [Table 1]. Previously, the lowering effect of EUC in atherosclerotic model of zebrafish was reported.[19]
MGO, a highly reactive dicarbonyl compound, is the main precursor in the formation of advanced glycation end products. It is detoxified with glyoxalase-I (GLO-1) as the key enzyme in the antiglycation defense.[7] New evidence shows that elevating levels of MGO along with reduced activity of GLO-I are linked to diabetic vascular complications.[2,12,20] The treatment represented antiglycating property with the induction of the GLO-I activity as well as the reduction of MGO in the diabetic-atherosclerotic rats [Table 2] rather than the untreated ones (P < 0.001). Based on our literature review, the effect of EUC on the activity of the GLO system activity and MGO level has not been exhibited.
Oxidative stress and inflammation motivate vascular complications.[21] The treatment diminished of AOPP and MDA levels as markers of protein and lipid oxidation along with the elevation of the GSH level. Furthermore, it decreased TNF-α [Table 2] as an inflammatory marker (P < 0.001). Previously, the antioxidant and anti-inflammatory effects of EUC with a reduction of MDA and elevation of GSH together within a decline on TNF-α level in acute pancreatitis model of Swiss mice[22], as well as the anti-inflammatory effect of it in vascular endothelium dysfunction model of mice[23], and atherosclerosis model of zebrafish was reported.[19]
LDL glycation and oxidation are the consequence of glycemia, dyslipidemia, oxidative stress, and glycation.[24,25] Furthermore, LDL glycation and oxidation contribute to atherosclerosis and nephropathy.[26] Our results represent the concomitant inhibitory effect of the treatment on LDL glycation as well as early and end LDL oxidation products [Table 2] in the diabetic-atherosclerotic group (P < 0.001). Previously, the reductive effect of EUC on the in vitro formation of the early oxidation product of LDL was presented.[19] As shown in the results, EUC not only improved lipid profile in atherosclerotic rats but also interfered with the LDL glycation and oxidation, thus it indicates a potent antiatherogenic activity, which is confirmed by other results.
Glycemia causes more damage to the tissues like kidneys that are an insulin-independent organ and the flow of glucose into the cells is via the circulating glucose level.[27] Moreover, hyperglycemia due to the raising of glycated products increases the gene ration of TNF α may directly interfere with insulin signaling and renal function.[5] Atherogenic diet and dyslipidemia motivate nephropathy.[12,28] The levels o f Cr in serum and PU [Table 1] in the atherosclerotic ra t confirms it (0.001). While, EUC ameliorated renal disorder in the atherosclerotic one owing to a beneficial effect on lipid profile as well as antioxidant, antiglycation, and anti-inflammatory properties. According to our research, compounds that have antioxidant, anti-inflammatory, and antiglycation properties have a potent renoprotective effect.[2,3,29]
Conclusions
EUC prevented the formation of the atheromatous lesions and improved renal function in the atherosclerotic rat model due to a reduction of glycation, oxidative stress, and inflammatory mediators as well as the beneficial effect on glucose and lipid metabolism and the activity of glyoxalase system.
Financial support and sponsorship
Ardabil University of Medical Sciences.
Conflicts of interest
There is no conflicts interest.
Acknowledgments
The authors are thankful from Ardabil University of Medical Sciences for financial support
References
- 1.Forbes JM CM. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–88. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 2.Mahdavifard S, Nakhjavani M. Effect of glutamine on oxidative stress, inflammatory, and glycation markers, and the activity of glyoxalase system in diabetic rats with atherosclerosis. J Mazandaran Univ Med Sci. 2019;28:33–42. [Google Scholar]
- 3.Mahdavifard S, Nakhjavani M. Effect of cysteine on transforming growth factor β1 as the main cause of renal disorder in a rat model of diabetic nephropathy. J Mazandaran Univ Med Sci. 2019;29:95–101. [Google Scholar]
- 4.Raghav A, Ahmad J, Noor S, Alam K, Mishra BK. Glycated albumin and the risk of chronic kidney disease in subjects with type 2 diabetes: A study in North Indian population. Diabetes Metab Syndr. 2018;12:381–5. doi: 10.1016/j.dsx.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Song F, Schmidt AM. Glycation and insulin resistance novel mechanisms and unique targets? Arterioscler Thromb Vasc Biol. 2012;32:1760–5. doi: 10.1161/ATVBAHA.111.241877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shayesteh R, Kamalinejad M, Adiban H, Kardan A, Keyhanfar F, Eskandari MR. Cytoprotective effects of pumpkin (Cucurbita Moschata) fruit extract against oxidative stress and carbonyl stress. Drug Res (Stuttg) 2017;67:576–82. doi: 10.1055/s-0043-110484. [DOI] [PubMed] [Google Scholar]
- 7.Maessen DE, Stehouwer CD, Schalkwijk CG. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin Sci (Lond) 2015;128:839–61. doi: 10.1042/CS20140683. [DOI] [PubMed] [Google Scholar]
- 8.Nichols TC, Merricks EP, Bellinger DA, Raymer RA, Yu J, Lam D3, et al. Oxidized LDL and fructosamine associated with severity of coronary artery atherosclerosis in insulin resistant pigs fed a high fat/high NaCl Diet. PLoS One. 2015;10:e0132302. doi: 10.1371/journal.pone.0132302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linghu KG, Wu GP, Fu LY, Yang H, Li HZ, Chen Y, et al. 1,8-cineole ameliorates lps-induced vascular endothelium dysfunction in mice via PPAR-gamma dependent regulation of NF-kappaB. Front Pharmacol. 2019;10:178. doi: 10.3389/fphar.2019.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lima PR, de Melo MT, Carvalho KM, de Oliveira ØB, Arruda BR, de Castro Brito GA, et al. 1,8-cineole (eucalyptol) ameliorates cerulein-induced acute pancreatitis via modulation of cytokines, oxidative stress and NF-κB activity in mice. Life Sci. 2013;10:24–6. doi: 10.1016/j.lfs.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR HJ, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologi. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Mahdavifard S, Bathaie SZ, Nakhjavani M, Taghikhani M. The synergistic effect of antiglycating agents (MB-92) on inhibition of protein glycation, misfolding and diabetic complications in diabetic-atherosclerotic rat. Eur J Med Chem. 2016;121:892–902. doi: 10.1016/j.ejmech.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer EJ, Otokozawa S, Ai M. Limitations of direct methods and the reference method for measuring HDL and LDL cholesterol. Clin Chem. 2011;57:1081–3. doi: 10.1373/clinchem.2010.159483. author reply 3. [DOI] [PubMed] [Google Scholar]
- 14.Younis N, Charlton-Menys V, Sharma R, Soran H, Durrington PN. Glycation of LDL in non-diabetic people: Small dense LDL is preferentially glycated both in vivo and in vitro. Atherosclerosis. 2009;202:162–8. doi: 10.1016/j.atherosclerosis.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Ahotupa M, Vasankari TJ. Baseline diene conjugation in LDL lipids: An indicator of circulating oxidized LDL. Free Radic Biol Med. 1999;27:1141–50. doi: 10.1016/s0891-5849(99)00201-4. [DOI] [PubMed] [Google Scholar]
- 16.Mahdavifard S, Bathaie S, Nakhjavani M, Heidarzadeh H. L-cysteine is a potent inhibitor of protein glycation on both albumin and LDL, and prevents the diabetic complications in diabetic–atherosclerotic rat. Food Res Int. 2014;62:909–16. [Google Scholar]
- 17.Witko-Sarsat V, Nguyen-Khoa T, Jungers P, Drüeke T, Descamps-Latscha B. Advanced oxidation protein products as a novel molecular basis of oxidative stress in uraemia. Nephrol Dial Transplant. 1999;14(Suppl 1):76–8. doi: 10.1093/ndt/14.suppl_1.76. [DOI] [PubMed] [Google Scholar]
- 18.Huang D, Refaat M, Mohammedi K, Jayyousi A, Al Suwaidi J, Abi Khalil C. Macrovascular complications in patients with diabetes and prediabetes. BioMed Res Int. 2017;2017:7839101. doi: 10.1155/2017/7839101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho KH. 1,8-cineole protected human lipoproteins from modification by oxidation and glycation and exhibited serum lipid-lowering and anti-inflammatory activity in zebrafish. BMB Rep. 2012;45:565–70. doi: 10.5483/bmbrep.2012.45.10.044. [DOI] [PubMed] [Google Scholar]
- 20.Tezuka Y, Nakaya I, Nakayama K, Nakayama M, Yahata M, Soma J. Methylglyoxal as a prognostic factor in patients with chronic kidney disease. Nephrology (Carlton) 2019;24:943–50. doi: 10.1111/nep.13526. [DOI] [PubMed] [Google Scholar]
- 21.Al-Trad B, Alkhateeb H, Alsmadi W, Al-Zoubi M. Eugenol ameliorates insulin resistance, oxidative stress and inflammation in high fat-diet/streptozotocin-induced diabetic rat. Life Sci. 2019;216:183–8. doi: 10.1016/j.lfs.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 22.Lima PR, de Melo TS, Carvalho KM, de Oliveira IB, Arruda BR, de Castro Brito GA, et al. 1,8-cineole (eucalyptol) ameliorates cerulein-induced acute pancreatitis via modulation of cytokines, oxidative stress and NF-kappaB activity in mice. Life Sci. 2013;92:1195–201. doi: 10.1016/j.lfs.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Menezes RR, Godin A, Rodrigues FF, Coura GME, Melo ISF, Brito AMS, et al. Thiamine and riboflavin inhibit production of cytokines and increase the anti-inflammatory activity of a corticosteroid in a chronic model of inflammation induced by complete Freund's adjuvant. Pharmacol Rep. 2017;69:1036–43. doi: 10.1016/j.pharep.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2311–6. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 25.Cai R, Chen S, Jiang S. [Chlorogenic acid inhibits non-enzymatic glycation and oxidation of low density lipoprotein] Zhejiang Da Xue Xue Bao Yi Xue Ban. 2018;47:27–34. doi: 10.3785/j.issn.1008-9292.2018.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawanami D, Matoba K, Utsunomiya K. Dyslipidemia in diabetic nephropathy. Replace Ther. 2016;2:16. [Google Scholar]
- 27.Gnudi L, Thomas SM, Viberti G. Mechanical forces in diabetic kidney disease: A trigger for impaired glucose metabolism. J Am Soc Nephrol. 2007;18:2226–32. doi: 10.1681/ASN.2006121362. [DOI] [PubMed] [Google Scholar]
- 28.Nozako M, Koyama T, Nagano C, Sato M, Matsumoto S, Mitani K, et al. An atherogenic paigen-diet aggravates nephropathy in type 2 diabetic OLETF rats. PLoS One. 2015;10:e0143979. doi: 10.1371/journal.pone.0143979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmadvand H, Mahdavifard S. Protective effect of thioctic acid on renal ischemia-reperfusion injury in rat. Int J Prev Med. 2019;10:176. doi: 10.4103/ijpvm.IJPVM_396_17. [DOI] [PMC free article] [PubMed] [Google Scholar]