Abstract
Background:
Multiple sclerosis (MS) is an inflammatory disease while there are controversies regarding the role of vitamin D supplements in controlling relapse and disability improvement during treatment.
Objective:
The goal of this systematic review and meta-analysis was to evaluate the effect of vitamin D supplements on MS-related relapse and the Expanded Disability Status Scale (EDSS).
Methods:
We searched databases to include randomized clinical trials (RCTs) which were published up to October 2018. We included RCTs, being single-blinded or double-blinded or open-label trials in which one of the main outcomes was EDSS and/or relapse after vitamin D supplementation. All statistical analyses were performed using RevMan 5.3. Odds ratios (OR) and 95% confidence intervals (CI) were calculated for relapse between treatment arms. The mean difference was calculated for EDSS comparisons.
Results:
Nine articles were included for analysis. Of these nine studies, five compared vitamin D supplement groups with placebo (group 1 studies), and four compared high- and low-dose vitamin D groups. A total of 561 patients were analyzed. Being treated with vitamin D instead of placebo showed no effect on relapse rate (OR = 0.66, 95% CI = 0.28–1.54) as well as EDSS (mean difference = 0.06, 95%CI [-0.31, 0.42]). The results of studies comparing high- vs. low-dose vitamin D interventions showed no significant effect on relapse rate (OR = 1.08, 95%CI [0.29–4.08] as well as final EDSS (mean difference = 0.17, 95% CI = -0.73, 1.07).
Conclusions:
Our findings show that vitamin D supplements (high or low dose) have no significant effect on relapse rate and disability during treatment in MS patients.
Keywords: Disability, multiple sclerosis, relapse, systematic review, vitamin D
Introduction
Multiple sclerosis (MS) is an autoimmune chronic demyelinating disease of the central nervous system thought to have an increasing incidence worldwide.[1] It is the one of the most common cause of disability due to neurological disease in young adults.[2]
Environmental factors along with genetics play an important role in disease incidence.[3] Duration and intensity of sunlight exposure, the latitude of birth, and serum vitamin D level are considered to be correlated with the incidence of MS.[4,5,6] Vitamin D has a significant effect on cytokine profiles, neurological disease development, and regulates inflammation in immune cells[7] as well as regulation of 900 genes.[8,9] It also regulates Th1 and Th2 cell proliferation.[10]
In a nested case-control study using stored serum samples from the U.S. military, the authors concluded that a 50 nmol/L increase in serum vitamin D level was associated with a 50% decreased risk of developing MS.[11] While the literature shows a decreased level of vitamin D during MS relapse as compared to other times in MS cases,[12,13,14] the role of vitamin D supplements on MS-related relapses is controversial.[15,16,17,18] A systematic review and meta-analysis looking at the results of five randomized clinical trials (RCTs), demonstrated that vitamin D supplementation did not affect controlling MS relapses.[19]
In this meta-analysis, we evaluate the effect of vitamin D supplements on MS-related relapse and Expanded Disability Status Scale (EDSS)
Methods
The protocol of this systematic review has been published.[20]
Literature search
We searched PubMed, Scopus, EMBASE, CINAHL, Web of Science, Ovid, ProQuest, American College of Physicians Journal Club database, Health Technology Assessment Database (The Cochrane Collaboration), and National Health System (NHS), Economic Evaluation Database (The Cochrane Collaboration) and gray literature including the reference of included studies, conference abstracts which were published up to October 2018.
Inclusion and exclusion criteria
We included RCTs, being single-blinded or double-blinded or open-label trials in which one of the main outcomes was EDSS and/or relapse after vitamin D supplementation. Only articles that had been published in the English language were included. Studies comparing high- and low-dose vitamin D therapies were also considered for analysis.
Cohort studies, case-control studies, and any other types of studies were excluded.
Data extraction
Two independent researchers independently assessed the articles. Data on the number of participants in each group, relapse in each treatment arm of the study, final EDSS, study duration, first author, publication year, and sample size were extracted from the included studies. In the case of disagreement, two researchers solved it by consultation with a third reviewer.
Statistical analysis
All statistical analyses were performed using RevMan 5.3 (The Cochrane Community, London, United Kingdom). Odds ratios (OR) and 95% confidence intervals (CI) were calculated for relapse between treatment arms.
We used the inverse variance with a random-effects model.
The mean difference was calculated for EDSS comparisons.
In one study (Stein et al.), the authors reported the median and interquartile range (IQR) for the final EDSS which we transformed to mean and SD.
Inconsistency (I2) was calculated to determine heterogeneity.
Risk of bias assessment
We evaluated the risk of potential biases using Cochrane Collaboration's tool for assessing the risk of bias [Figures 1 and 2].[21]
Figure 1.
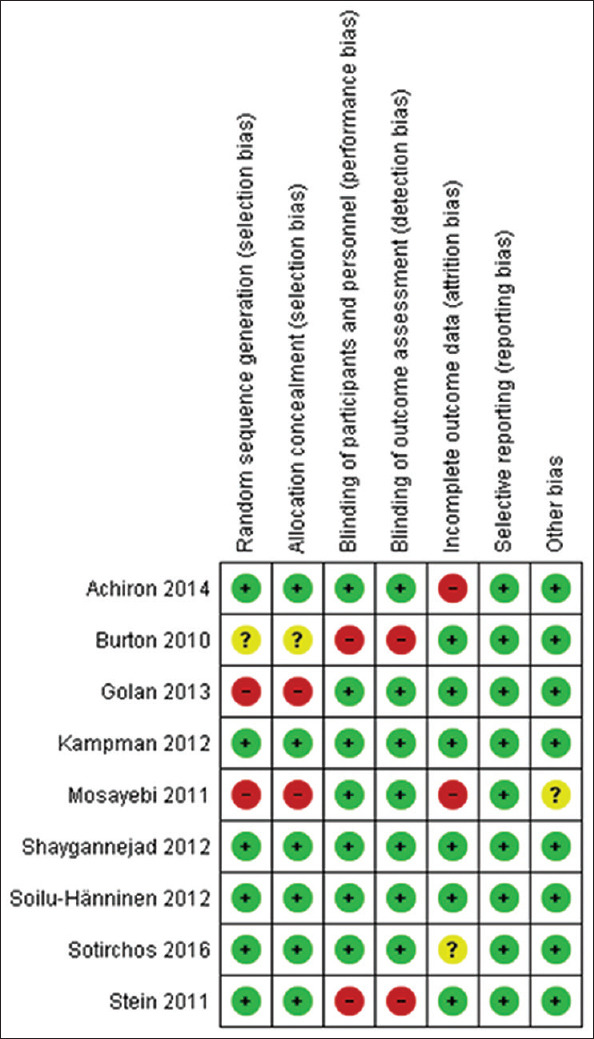
Methodologic quality assessment graph
Figure 2.
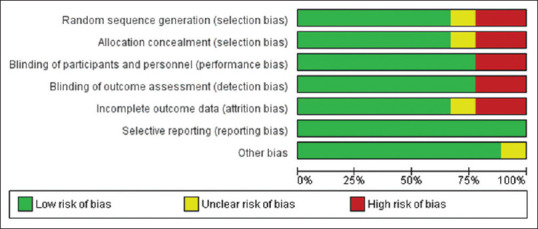
Risk-of-bias assessment for each study included in the meta-analysis
Funnel plots created in Review Manager 5.1 were used to assess publication bias.
A P value of less than 0.05 was considered statistically significant.
Results
The literature search found 102 articles that were screened. When discounting observational studies, reviews, case reports, and non-randomized trials, 21 studies remained. Finally, nine articles were included for analysis [Figure 3].
Figure 3.
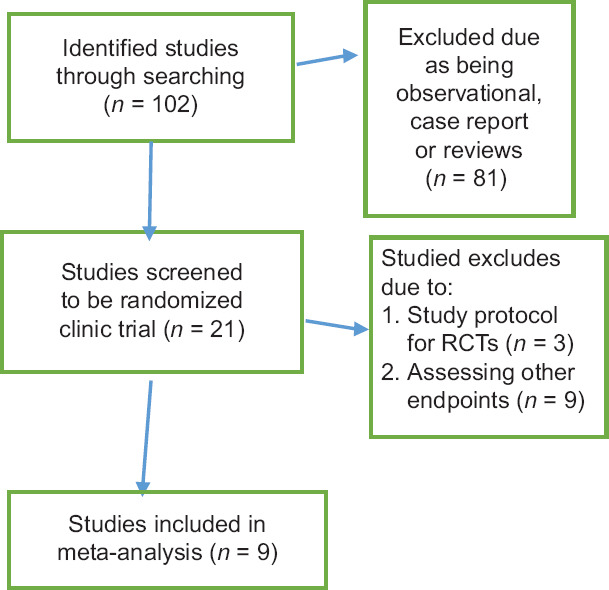
Flow diagram summarizing the selection of eligible studies
Of these nine studies, five compared vitamin D supplement groups with placebo (group 1 studies), and four compared high- and low-dose vitamin D groups (group 2 studies). Study duration varied among included studies and the included studies were from different countries [Table 1].
Table 1.
Characteristics of included studies
| Characteristics | Results |
|---|---|
| First author and date[22] | Achiron, 2014 |
| Title | Effect of Alfacalcidol on multiple sclerosis-related fatigues: A randomized, double-blind placebo-controlled study |
| Total number of participants | 158 |
| Country | Israel |
| Intervention | Alfacalcidol (1 mcg/d) |
| Control | Placebo |
| Duration | Six consecutive months |
| First author and date[25] | Shaygannejad, 2012 |
| Title | Effects of Adjunct Low-Dose Vitamin D on Relapsing-Remitting Multiple Sclerosis Progression: Preliminary Findings of a Randomized Placebo-Controlled Trial |
| Total number of participants | 50 |
| Country | Iran |
| Intervention | 0.25 μg adjunct calcitriol per day and increased to 0.5 μg/day after 2 weeks and continued for 12 months |
| Control | placebo |
| Duration | 1 year |
| First author and date[18] | Soilu-Hanninen, 2014 |
| Title | A randomized, double-blind, placebo-controlled trial with vitamin D3 as an add on treatment to interferon b-1b in patients with multiple sclerosis |
| Total number of participants | 66 |
| Country | Finland |
| Intervention | 20 mg of cholecalciferol, corresponding to 20 000 IU or 0.5 mg of vitamin D3, once weekly for 1 year |
| Control | Placebo |
| Duration | 1 year |
| First author and date[16] | Kampman, 2012 |
| Title | Effect of vitamin D3 supplementation on relapses, disease progression, and measures of function in persons with multiple sclerosis: exploratory outcomes from a double-blind randomized controlled trial |
| Total number of participants | 68 |
| Country | Norway |
| Intervention | 20,000 IU vitamin D3 (cholecalciferol) once a week |
| Control | Placebo |
| Duration | 2 years |
| First author and date[24] | Mosayebi, 2011 |
| Title | Therapeutic Effect of Vitamin D3 in Multiple Sclerosis Patients |
| Total number of participants | 62 |
| Country | Iran |
| Intervention | 300,000 IU/month vitamin D3 IM |
| Control | Placebo |
| Duration | 6 months |
| First author and date[23] | Sotirchos, 2015 |
| Title | Safety and immunologic effects of high- vs low-dose cholecalciferol in multiple |
| Total number of participants | 40 |
| Country | USA |
| Intervention | 10,000 IU of cholecalciferol+400 IU cholecalciferol and 1,000 mg calcium. |
| Control | 4,000 IU of cholecalciferol+400 IU cholecalciferol and 1,000 mg calcium |
| Duration | 6 months |
| First author and date[26] | Golan 2013 |
| Title | Vitamin D supplementation for patients with multiple sclerosis treated with interferon-beta: a randomized controlled trial assessing the effect on flu-like symptoms and immunomodulatory properties |
| Total number of participants | 45 |
| Country | Israel |
| Intervention | 75,000 IU of vitamin D3 solution every 3 weeks in addition to 800 IU of vitamin D3 by daily tablets (total of 4370 IU/d) |
| Control | One bottle of placebo solution every 3 weeks besides 800 IU of vitamin D3 by daily tablets (total of 800 IU/d) |
| Duration | 1 year |
| First author and date[27] | Stein, 2011 |
| Title | A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis |
| Total number of participants | 23 |
| Country | Australia |
| Intervention | 1,000 IU vitamin D2 daily plus a high-dose vitamin D2 supplement |
| Control | (1,000 IU) vitamin D2 daily |
| Duration | 6 months |
| First author and date[15] | Burton, 2010 |
| Title | A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis |
| Total number of participants | 49 |
| Country | Canada |
| Intervention | 40,000 IU/day over 28 weeks followed by 10,000 IU/day (12 weeks), and further down titrated to 0 IU/day |
| Control | =<4,000 IU/day of vitamin D |
| Duration | 1 year |
One study in group 1 and one in group 2 had no results regarding final EDSS (Achiron et al. and Sotirchos[22,23] (the corresponding authors were contacted with no response).
A total of 561 patients were analyzed. Information regarding relapse and final EDSS in group 1 studies are shown in Table 2 and information regarding group 2 studies are shown in Table 3.
Table 2.
Number of patients, the number who experienced relapses, and final EDSS in group one studies (vitamin d vs placebo)
| First author | Vitamin D group | Placebo group | ||||
|---|---|---|---|---|---|---|
| Total number | No of relapses | Final EDSS (mean±SD) | Total number | No of relapses | Final EDSS (mean±SD) | |
| Achiron | 80, | 8 | - | 78, | 25 | - |
| Shaygannejad | 25, | 8 | 1.6±0.7 | 25, | 9 | 1.94±1.4 |
| Soilu-Hanninen | 34 | 9 | 1.8±1.2 | 32 | 9 | 1.6±1.3 |
| Kampman | 35 | 6 | 2.77±0.39 | 33 | 4 | 2.42±0.4 |
| Mosayebi | 26 | - | 2.31±1.3 | 33 | - | 2.67±1.25 |
Table 3.
Number of patients, the number who experienced relapses, and final EDSS in group one studies (high vs low dose vitamin D)
| First author | High dose group | Low dose group | ||||
|---|---|---|---|---|---|---|
| Total number | No of relapses | Final EDSS (mean±SD) | Total number | No of relapses | Final EDSS (mean±sd) | |
| Sotirchos | 19 | 1 | - | 21 | 1 | - |
| Golan | 24 | 8 | 3.3±2.4 | 21 | 6 | 3.6±2.3 |
| Stein | 11 | 4 | 3±1.48 | 12 | 0 | 2±0.74 |
| Burton | 25 | 4 | 1.15±1.39 | 24 | 9 | 1.45±1.78 |
Relapse in group 1 studies (vitamin D vs. placebo groups)
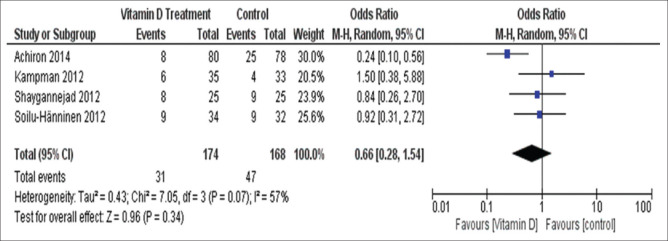
Being treated with vitamin D instead of placebo showed no effect on relapse rate during treatment in four studies (one study [Mosayebi et al.[24]] had no relapse rate, the corresponding authors were contacted with no response) included for this analysis (OR = 0.66, 95% CI = 0.28–1.54) with no significant heterogeneity (I2 = 57%, Chi2 = 7.05, P = 0.07) [Figure 4].
Figure 4.
Number of patients who experienced relapses in group 1 studies
EDSS in group 1 studies (vitamin D vs. placebo groups)
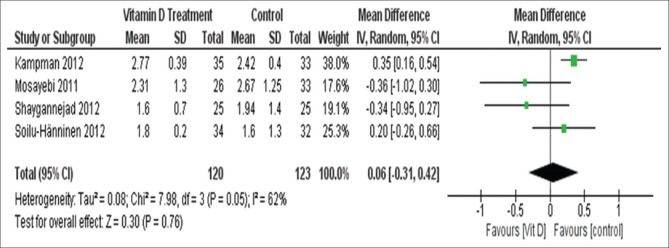
Four studies included in this analysis (one study, Achiron et al. had no final EDSS) and the results suggested no effect of vitamin D supplementation on final EDSS (mean difference = 0.06, 95%CI (-0.31, 0.42).
There was no significant heterogeneity (I2 = 62%, Chi2 = 7.98, P = 0.05) [Figure 5].
Figure 5.
Final EDSS in patients of group 1 studies
Relapse in group 2 studies (High- vs. Low-vitamin D groups)
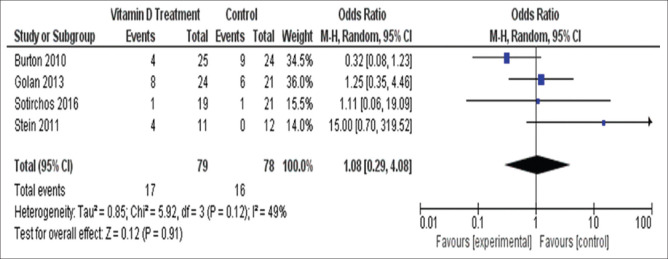
The four studies using high- vs. low-vitamin D interventions showed no significant effect on relapse rate (OR = 1.08, 95%CI (0.29–4.08) with no significant heterogeneity (I2 = 49%, Chi2 = 5.92, P = 0.12) [Figure 6].
Figure 6.
Number of patients who experienced relapses in group 2 studies
EDSS in group 2 studies (High- vs. Low-vitamin D groups)
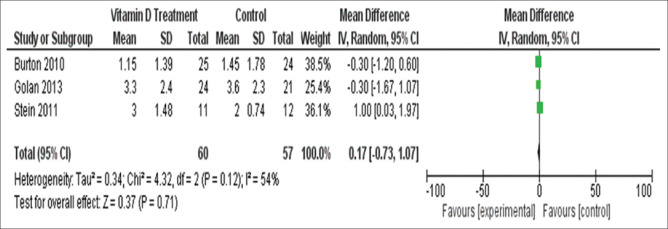
Three out of four studies provided data regarding final EDSS which showed no significant effect (mean difference = 0.17, 95%CI = -0.73, 1.07). The results indicated no significant heterogeneity (I2 = 54%, Chi2 = 4.32, P = 0.12) [Figure 7].
Figure 7.
Final EDSS in patients of group 2 studies
Discussion
This meta-analysis showed that neither vitamin D supplements in comparison with placebo nor high- vs. low-dose vitamin D intervention had a significant association with relapse rate during treatment and final EDSS.
In a previous systematic review conducted by James et al.,[19] results of five clinical trials were pooled and analyzed. Their results showed that there was no significant association between vitamin D (either low, high, or calcitriol) administration and relapse rate in MS cases.[19] They reported no significant heterogeneity in their subgroup analysis which is as per our findings.
In a recent meta-analysis, Zheng et al. assessed the effects of vitamin D supplements on EDSS and annual relapse rate in MS cases.[28] Including six RCTs, they reported no significant effect on EDSS (mean difference = -0.01, 95%C = 0.34, 0.33); however, they found significant effect on annual relapse rate (mean difference = 0.05, 95%CI = 0.01–0.1).[28]
This finding could be due to applying the mean difference of annual relapse rate instead of solely looking at relapse numbers.
In one of the included studies in this meta-analysis (Achiron et al.),[22] the relapse rate during the study period differed significantly between an intervention (Alfacalcidol 1 mcg/d) and placebo-controlled groups while the others showed no significant differences.
This meta-analysis included studies used different doses of vitamin D supplements in study arms.
There is no consensus regarding the optimal dose of vitamin D supplements for preventing relapse in MS although, Pierrot-Deseilligny et al. investigated a plateau effect of vitamin D on the rate of relapse.[29] They suggested that every 10 nmol increase in serum vitamin D level will result in a relapse rate reduction of 13.7%.[29]
Our results also suggest that high-dose vitamin D supplements in comparison with low-dose vitamin D supplements were not associated with better outcomes (reduced relapse rates and a significant decrease of EDSS). Four included studies in this part applied different doses of high- vs. low-dose supplements. We also should consider that duration of administration differed between studies. A delayed vitamin D onset of action is thought to be near 2 months, could explain this diversity. By considering 415 German patients, Embry et al. reported a 2-month lag time between vitamin D administration and magnetic resonance imaging (MRI) lesions.[30]
In one of the RCTs of this meta-analysis, 38% of relapses in the vitamin D intervention group occurred during 1 month of administration.[18] Achiron et al. claimed that the reduction of relapses in the vitamin D intervention group compared with the placebo group was significant after 4 months of treatment.[22] Shaygannejad et al. found that there is no effect on the relapse rate in the 1st year of vitamin D therapy in comparison with placebo in MS.[25] In Kampman et al. study, the median time to relapse was 39 and 29 weeks in the vitamin D supplement and placebo groups, respectively (P = 0.4).[16]
It may be helpful for future systematic reviews to consider the evaluation of relapses in vitamin D intervention groups after 2 months of administration.
Different factors could affect the effectiveness of vitamin D supplementation on the relapse rate. One factor could be the baseline vitamin D level as sun exposure and nutrition differ among participants of different studies. The results of Kampman et al. show relapse in six cases of the intervention vs four of the control group while Golan et al. and Stein et al. reported higher relapse rates in the high-dose vs low-dose group.[16,26,27]
In the studies included in this meta-analysis, the duration of treatment, and a dose of vitamin D supplements differed along with different formulations. For instance, Stein et al. administered vitamin D2 which is thought to have less effectiveness than vitamin D3.[27] It has been also demonstrated that the duration of action and potency of vitamin D2 is less than vitamin D3.[31]
Previous observational studies showed that sunlight exposure, vitamin D supplements, and serum vitamin D levels were associated with a lower risk of MS as well as relapse rate[3,4] but these studies were observational.
Multicentric, large, randomized trials may solve this problem.
Conclusions
Our findings show that vitamin D supplements (high or low dose) have no significant effect on relapse rate and disability during treatment in MS patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ghajarzadeh M, Jalilian R, Eskandari G, Sahraian MA, Azimi A and Mohammadifar M. Fatigue in multiple sclerosis: Relationship with disease duration, physical disability, disease pattern, age and sex. Acta Neurol Belg. 2013;113:411–4. doi: 10.1007/s13760-013-0198-2. [DOI] [PubMed] [Google Scholar]
- 2.Ghajarzadeh M, Owji M, Sauraian MA, Naser Moghadasi A, Azimi A. Emotional Intelligence (EI) of Patients with Multiple Sclerosis (MS) Iran J Public Health. 2014;43:1550–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Eskandari G, Ghajarzadeh M, Yekaninejad MS, Sahraian MA, Gorji R, Rajaei F, et al. Comparison of serum vitamin D level in multiple sclerosis patients, their siblings, and healthy controls. Iran J Neurol. 2015;14:81–5. [PMC free article] [PubMed] [Google Scholar]
- 4.van der Mei IA, Ponsonby A-L, Blizzard L, Dwyer T. Regional variation in multiple sclerosis prevalence in Australia and its association with ambient ultraviolet radiation. Neuroepidemiology. 2001;20:168–74. doi: 10.1159/000054783. [DOI] [PubMed] [Google Scholar]
- 5.Bäärnhielm M, Hedström A, Kockum I, Sundqvist E, Gustafsson SA, Hillert J, et al. Sunlight is associated with decreased multiple sclerosis risk: No interaction with human leukocyte antigen-DRB1* 15. Eur J Neurol. 2012;19:955–62. doi: 10.1111/j.1468-1331.2011.03650.x. [DOI] [PubMed] [Google Scholar]
- 6.Bivona G, Gambino CM, Iacolino G, Ciaccio M. Vitamin D and the nervous system. Neurol Res. 2019;41:827–35. doi: 10.1080/01616412.2019.1622872. [DOI] [PubMed] [Google Scholar]
- 7.Smolders J, Damoiseaux J, Menheere P, Hupperts R. Vitamin D as an immune modulator in multiple sclerosis, a review. J Neuroimmunol. 2008;194:7–17. doi: 10.1016/j.jneuroim.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Bivona G, Agnello L, Bellia C, Iacolino G, Scazzone C, Lo Sasso B, et al. Non-skeletal activities of vitamin D: From physiology to brain pathology. Medicina (Kaunas) 2019;55:341. doi: 10.3390/medicina55070341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bivona G, Lo Sasso B, Iacolino G, Gambino CM, Scazzone C, Agnello L, et al. Standardized measurement of circulating vitamin D [25(OH) D] and its putative role as a serum biomarker in Alzheimer's disease and Parkinson's disease. Clin Chim Acta. 2019;497:82–7. doi: 10.1016/j.cca.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Bivona G, Agnello L, Ciaccio M. The immunological implication of the new vitamin D metabolism. Cent-Eur J Immunol. 2018;43:331–4. doi: 10.5114/ceji.2018.80053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 12.Mowry EM, Krupp LB, Milazzo M, Chabas D, Strober JB, Belman AL, et al. Vitamin D status is associated with relapse rate in pediatric-onset multiple sclerosis. Ann Neurol. 2010;67:618–24. doi: 10.1002/ana.21972. [DOI] [PubMed] [Google Scholar]
- 13.Smolders J, Menheere P, Kessels A, Damoiseaux J, Hupperts R. Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mul Scler. 2008;14:1220–4. doi: 10.1177/1352458508094399. [DOI] [PubMed] [Google Scholar]
- 14.Runia TF, Hop WC, de Rijke YB, Buljevac D, Hintzen RQ. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology. 2012;79:261–6. doi: 10.1212/WNL.0b013e31825fdec7. [DOI] [PubMed] [Google Scholar]
- 15.Burton J, Kimball S, Vieth R, Bar-Or A, Dosch HM, Cheung R, et al. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology. 2010;74:1852–9. doi: 10.1212/WNL.0b013e3181e1cec2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kampman MT, Steffensen LH, Mellgren SI, Jørgensen L. Effect of vitamin D3 supplementation on relapses, disease progression, and measures of function in persons with multiple sclerosis: Exploratory outcomes from a double-blind randomised controlled trial. Mult Scler. 2012;18:1144–51. doi: 10.1177/1352458511434607. [DOI] [PubMed] [Google Scholar]
- 17.Laursen JH, Søndergaard HB, Sørensen PS, Sellebjerg F, Oturai AB. Vitamin D supplementation reduces relapse rate in relapsing-remitting multiple sclerosis patients treated with natalizumab. Multi Scler Relat Disord. 2016;10:169–73. doi: 10.1016/j.msard.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Soilu-Hänninen M, Åivo J, Lindström B-M, Elovaara I, Sumelahti ML, Färkkilä M, et al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon β-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012;83:565–71. doi: 10.1136/jnnp-2011-301876. [DOI] [PubMed] [Google Scholar]
- 19.James E, Dobson R, Kuhle J, Baker D, Giovannoni G and Ramagopalan SV. The effect of vitamin D-related interventions on multiple sclerosis relapses: A meta-analysis. Mult Scler. 2013;19:1571–9. doi: 10.1177/1352458513489756. [DOI] [PubMed] [Google Scholar]
- 20.Ghajarzadeh M, Keshtkar AA, Azimi A, Sahraian MA, Mohammadifar M, Ramagopalan SV. The effect of vitamin D supplements on clinical and para-clinical outcomes in patients with multiple sclerosis: Protocol for a systematic review. JMIR Res Protoc. 2019;8:e12045. doi: 10.2196/12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Achiron A, Givon U, Magalashvili D, Dolev M, Liraz Zaltzman S, Kalron A, et al. Effect of alfacalcidol on multiple sclerosis-related fatigue: A randomized, double-blind placebo-controlled study. Mult Scler. 2015;21:767–75. doi: 10.1177/1352458514554053. [DOI] [PubMed] [Google Scholar]
- 23.Sotirchos ES, Bhargava P, Eckstein C, Van Haren K, Baynes M, Ntranos A, et al. Safety and immunologic effects of high- vs low-dose cholecalciferol in multiple sclerosis. Neurology. 2016;86:382–90. doi: 10.1212/WNL.0000000000002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosayebi G, Ghazavi A, Ghasami K, Jand Y, Kokhaei P. Therapeutic effect of vitamin D3 in multiple sclerosis patients. Immunol Invest. 2011;40:627–39. doi: 10.3109/08820139.2011.573041. [DOI] [PubMed] [Google Scholar]
- 25.Shaygannejad V, Janghorbani M, Ashtari F, Dehghan H. Effects of adjunct low-dose vitamin d on relapsing-remitting multiple sclerosis progression: Preliminary findings of a randomized placebo-controlled trial. Mult Scler Int. 2012;2012:452541. doi: 10.1155/2012/452541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golan D, Halhal B, Glass-Marmor L, Staun-Ram E, Rozenberg O, Lavi I, et al. Vitamin D supplementation for patients with multiple sclerosis treated with interferon-beta: A Randomized controlled trial assessing the effect on flu-like symptoms and immunomodulatory properties. BMC Neurol. 2013;13:60. doi: 10.1186/1471-2377-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein MS, Liu Y, Gray OM, Baker JE, Kolbe SC, Ditchfield MR, et al. A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology. 2011;77:1611–8. doi: 10.1212/WNL.0b013e3182343274. [DOI] [PubMed] [Google Scholar]
- 28.Zheng C, He L, Liu L, Zhu J, Jin T. The efficacy of vitamin D in multiple sclerosis: A meta-analysis. Mult Scler Relat Disord. 2018;23:56–61. doi: 10.1016/j.msard.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Pierrot-Deseilligny C, Rivaud-Péchoux S, Clerson P, de Paz R, Souberbielle J-C. Relationship between 25-OH-D serum level and relapse rate in multiple sclerosis patients before and after vitamin D supplementation. Ther Adv Neurol Disord. 2012;5:187–98. doi: 10.1177/1756285612447090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Embry AF, Snowdon LR, Vieth R. Vitamin D and seasonal fluctuations of gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol. 2000;48:271–2. [PubMed] [Google Scholar]
- 31.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–91. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]