ABSTRACT
A prophylactic vaccine that confers durable protection against human immunodeficiency virus (HIV) would provide a valuable tool to prevent new HIV/AIDS cases. As herpesviruses establish lifelong infections that remain largely subclinical, the use of persistent herpesvirus vectors to deliver HIV antigens may facilitate the induction of long-term anti-HIV immunity. We previously developed recombinant (r) forms of the gamma-herpesvirus rhesus monkey rhadinovirus (rRRV) expressing a replication-incompetent, near-full-length simian immunodeficiency virus (SIVnfl) genome. We recently showed that 8/16 rhesus macaques (RMs) vaccinated with a rDNA/rRRV-SIVnfl regimen were significantly protected against intrarectal (i.r.) challenge with SIVmac239. Here we investigated the longevity of this vaccine-mediated protection. Despite receiving no additional booster immunizations, the protected rDNA/rRRV-SIVnfl vaccinees maintained detectable cellular and humoral anti-SIV immune responses for more than 1.5 years after the rRRV boost. To assess if these responses were still protective, the rDNA/rRRV-SIVnfl vaccinees were subjected to a second round of marginal-dose i.r. SIVmac239 challenges, with eight SIV-naive RMs serving as concurrent controls. After three SIV exposures, 8/8 control animals became infected, compared to 3/8 vaccinees. This difference in SIV acquisition was statistically significant (P = 0.0035). The three vaccinated monkeys that became infected exhibited significantly lower viral loads than those in unvaccinated controls. Collectively, these data illustrate the ability of rDNA/rRRV-SIVnfl vaccination to provide long-term immunity against stringent mucosal challenges with SIVmac239. Future work is needed to identify the critical components of this vaccine-mediated protection and the extent to which it can tolerate sequence mismatches in the challenge virus.
IMPORTANCE We report on the long-term follow-up of a group of rhesus macaques (RMs) that received an AIDS vaccine regimen and were subsequently protected against rectal acquisition of simian immunodeficiency virus (SIV) infection. The vaccination regimen employed included a live recombinant herpesvirus vector that establishes persistent infection in RMs. Consistent with the recurrent SIV antigen expression afforded by this herpesvirus vector, vaccinees maintained detectable SIV-specific immune responses for more than 1.5 years after the last vaccination. Importantly, these vaccinated RMs were significantly protected against a second round of rectal SIV exposures performed 1 year after the first SIV challenge phase. These results are relevant for HIV vaccine development because they show the potential of herpesvirus-based vectors to maintain functional antiretroviral immunity without the need for repeated boosting.
KEYWORDS: herpesviruses, human immunodeficiency virus, nonhuman primate models, simian immunodeficiency virus, vaccines
INTRODUCTION
The development of a prophylactic human immunodeficiency virus (HIV) vaccine faces significant challenges. Not only must vaccine-induced immune responses protect against diverse HIV isolates, but antiviral responses should also persist at protective levels without the need for regular boosting. A vaccine that elicits long-lasting anti-HIV immunity would be particularly useful in regions with a high incidence of HIV/AIDS where regular mass immunization campaigns would be too costly or impractical to implement. To further complicate matters, anti-Envelope (Env) antibodies (Abs) induced by nonpersistent vectors tend to decay rapidly following vaccination, and strategies based on repeated boosting with HIV-1 gp120 subunit vaccines have had limited success in extending the half-life of anti-Env Ab responses in humans (1–5).
In devising vaccine regimens against HIV, it may be instructive to consider some of the properties of live-attenuated simian immunodeficiency virus (SIV) vaccines, as they remain the most effective active immunization strategy against homologous SIV challenge in nonhuman primates (6–8). Notably, a single inoculation of live-attenuated SIV results in persistent and high levels of Env-specific Ab responses, as well as broadly targeted effector-differentiated CD8+ T cells, in rhesus macaques (RMs) (8–11). Furthermore, the ability of live-attenuated SIV vaccines to persist in vivo is critical for their protective efficacy. Indeed, protection against SIV challenge is inversely associated with the degree of attenuation of the vaccine strain, and it takes several months after inoculation for vaccine efficacy to mature to fully protective levels (9, 12). These observations are in line with the notion that persistent, low-level antigen expression facilitates the generation of protective immunity against immunodeficiency virus infection (13, 14).
Because safety concerns have precluded the development of live-attenuated HIV vaccines, there is considerable interest in creating vaccine strategies that safely extend antigen availability in vivo, with the hope of recapitulating the protective effects of live-attenuated SIV strains in monkeys. One approach that seems well suited for this purpose is the use of live recombinant herpesviruses to deliver HIV antigens. Because herpesviruses establish latent lifelong infections that remain largely subclinical in their hosts, a herpesvirus-based HIV vaccine could promote chronic low-level expression of HIV antigens in vivo. Another argument for using herpesviruses as vehicles for delivering HIV antigens is their capacity to accommodate large amounts of inserted DNA in a stable fashion. We have taken advantage of these properties and generated live recombinant (r) forms of the gamma2-herpesvirus rhesus monkey rhadinovirus (rRRV) expressing a 9,343-bp, near-full-length SIVmac239 (SIVnfl) genome (15). SIVnfl is replication incompetent due to deletions of critical elements for viral replication. SIVnfl can assemble noninfectious SIV particles and express all nine SIV gene products. Similar to RMs inoculated with live-attenuated SIV strains, rRRV-SIVnfl-vaccinated monkeys develop effector-differentiated T-cell responses against the entire SIV proteome and durable anti-Env Abs. However, the overall magnitude of SIV-specific immune responses induced by first-generation rRRV-SIVnfl vectors still fell short of what is seen after live-attenuated SIV vaccination (15).
We recently evaluated the efficacy of SIVnfl vaccination against challenge with the pathogenic, neutralization-resistant SIVmac239 clone in RMs. In order to increase vaccine immunogenicity, we vaccinated animals with rRRV vectors expressing SIVnfl and SIV env in combination with SIVnfl-expressing rDNA plasmids. These rDNA plasmids were administered by intramuscular electroporation as a primer or booster for the rRRV vaccine. In one study, an rRRV/rDNA vaccine regimen induced robust SIV-specific immune responses and conferred significant protection against intravenous (i.v.) acquisition of SIVmac239 (16). In a separate study, priming with rDNA and then boosting with rRRV were also highly immunogenic and afforded significant protection against intrarectal (i.r.) challenges with SIVmac239 (17). Of note, it is unclear whether the rDNA vaccinations contributed to the efficacy of the rRRV/rDNA and rDNA/rRRV vaccine regimens. Since 8 of the 16 rDNA/rRRV vaccinees from the latter experiment remained uninfected after the i.r. SIVmac239 challenge series, we set out to investigate the longevity of this vaccine-mediated protection. To that end, we kept those protected animals alive for 1 year after the end of the first SIV challenge phase, during which time we continued to monitor their vaccine-elicited T-cell and anti-Env Ab responses. At the end of this 1-year period, we subjected these animals to a second round of i.r. SIVmac239 challenges, with eight concurrent unvaccinated RMs serving as controls. The present study reports the results of our immunogenicity measurements and the outcome of the second i.r. SIVmac239 challenge series.
RESULTS
Experimental design.
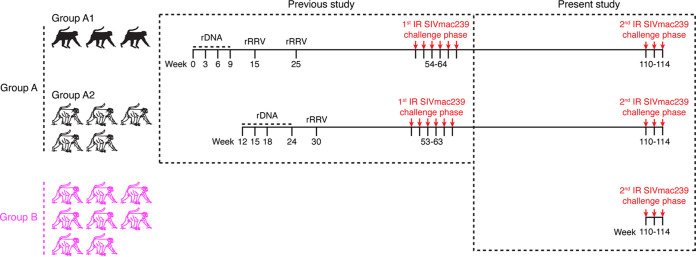
We recently showed that a rDNA/rRRV vaccine regimen encoding SIVnfl conferred significant protection against rectal acquisition of SIVmac239 in RMs (17). After 6 repeated i.r. exposures to a marginal-dose of SIVmac239, 8/16 rDNA/rRRV-vaccinated RMs remained uninfected, compared to 0/8 control animals. The 8 protected rDNA/rRRV vaccinees in question were kept alive after the conclusion of this first round of SIV challenges and are the subject of the present study. Those animals are collectively referred to as group A here (Fig. 1).
FIG 1.
Experimental design. The eight RMs in group A were used in a recent SIV vaccine trial conducted by our group (17). Those animals were part of a larger cohort of 16 RMs that were primed with rDNA plasmids (delivered by intramuscular electroporation) and then boosted with a mixture of five rRRV vectors expressing SIVnfl or SIV env. After six i.r. SIVmac239 challenges performed at weeks 53 to 64, eight of the 16 rDNA/rRRV-vaccinated RMs remained aviremic, whereas all (8/8) contemporaneous controls became infected. This difference in SIVmac239 acquisition between controls and vaccinees was statistically significant. To assess the durability of this vaccine-mediated protection, the eight rDNA/rRRV vaccinees that remained uninfected (referred to as group A here) were kept alive for another year without further boosters and then subjected to a second round of i.r. SIVmac239 challenges beginning at week 110. Eight additional unvaccinated RMs (group B) served as the concurrent controls for the second challenge phase, which utilized the same dose (200 TCID50) of the same stock of SIVmac239 used in the first challenge round. As part of our previous efforts to optimize the performance of the rDNA/rRRV vaccine regimen, three of the group A animals received two rRRV boosters (group A1), while the remaining five animals (group A2) were given one booster.
As part of previous attempts by our group to improve vaccine performance, we tested two modifications to the rDNA/rRRV vaccine regimen. The first one was an additional rRRV booster, which was delivered to three animals in group A (referred to as group A1) (Fig. 1). The remaining five RMs in group A were given only one booster with rRRV at week 30 and are referred to as group A2 (Fig. 1). As we described in our recent paper (17), the second rRRV booster had little or no effect on vaccine-induced SIV-specific immune responses, likely as a result of preexisting anti-vector immunity. The second modification consisted of i.v. infusions of the cytotoxic T-lymphocyte antigen-4 (CTLA-4)-blocking monoclonal antibody (MAb) Ipilimumab (Ipi) after each rDNA priming immunization (17). The original goal was to amplify vaccine-induced SIV-specific immune responses by transiently blocking CTLA-4 in vivo. One RM in group A1 (r13053) and two in group A2 (r14070 and r15018) were treated with Ipi (Fig. 1). The addition of Ipi to the rDNA/rRRV vaccine regimen resulted in modest and mostly transient effects on vaccine immunogenicity (17).
The first round of i.r. SIVmac239 challenges (spanning study weeks 53 to 64) unfolded as follows: 5/8 rDNA/rRRV vaccinees and 3/8 rDNA+Ipi/rRRV vaccinees remained uninfected after six i.r. SIVmac239 challenges. Compared to the control group, these differences were statistically significant for the rDNA/rRRV group (P = 0.028) but not for the rDNA+Ipi/rRRV group (P = 0.199). Since there was no significant difference in the rate of SIV acquisition between the rDNA+Ipi/rRRV and rDNA/rRRV groups, we explored whether the rate of infection in vaccinees from both groups combined (n = 16) differed from that in the control group. This analysis showed that vaccine efficacy remained statistically significant even when RMs from the two groups were pooled (P = 0.031). This finding is consistent with the minor differences in vaccine-induced SIV-specific T-cell responses between the rDNA/rRRV and rDNA+Ipi/rRRV regimens reported in our recent paper (17). Based on these similarities, we combined the protected rDNA/rRRV (n = 5) and rDNA+Ipi/rRRV (n = 3) vaccinees into one group (i.e., group A) for the follow-up study and analyses described here.
Dynamics of rDNA/rRRV-induced SIV-specific T-cell responses over a 2-year period.
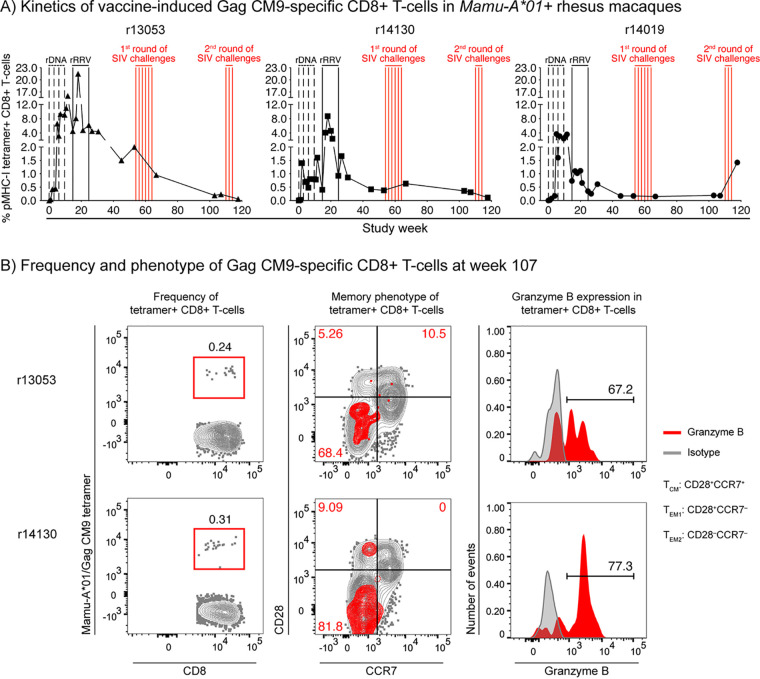
We used peptide/major histocompatibility complex class I (pMHC-I) tetramer staining to monitor vaccine-induced CD8+ T-cell responses in peripheral blood mononuclear cells (PBMC) from the three Mamu-A*01+ vaccinees in group A1. At study week 53, just before the first round of SIV challenges, vaccine-elicited Gag CM9-specific CD8+ T cells constituted 0.17%, 0.38%, and 2.0% of peripheral CD8+ T cells in r14019, r14130, and r13053, respectively (Fig. 2A). One year later, prior to the second SIV challenge phase (week 107), these responses had remained virtually unchanged in r14019 (0.18%) and r14130 (0.31%) but decreased to 0.24% in r13053 (Fig. 2A and B). Similar to their memory profile measured at week 53 (17), the majority of vaccine-elicited Gag CM9-specific CD8+ T cells in monkeys r13053 and r14130 were skewed toward an effector memory (TEM) phenotype, with the fully differentiated TEM2 signature (CD28− CCR7−) dominating the pMHC-I tetramer-positive population (Fig. 2B). Consistent with this effector-differentiated phenotype, >60% of pMHC-I tetramer-positive CD8+ T cells in monkeys r13053 and r14130 expressed the cytotoxic marker granzyme B ex vivo (Fig. 2B). We could not evaluate the functional profile of Gag CM9-specific CD8+ T cells in r14019 because of the low number of events in the pMHC-I tetramer gate.
FIG 2.
Kinetics and memory phenotype of vaccine-induced Gag CM9-specific CD8+ T cells in Mamu-A*01+ RMs. (A) The kinetics of vaccine-induced Gag CM9-specific CD8+ T-cell responses were monitored in PBMC from the three Mamu-A*01+ animals in group A1 using peptide/MHC-I tetramer staining. The time scale in the x axis matches that in Fig. 1. The times of each vaccination (vertical black lines) and i.r. SIVmac239 exposures (vertical red lines) are shown in the graph. Background levels of tetramer staining on the day of the first rDNA priming immunization averaged 0.02%. (B) Frequency, memory phenotype, and granzyme B expression levels of vaccine-induced Gag CM9-specific CD8+ T cells at week 110 in monkeys r13053 and r14130. The memory profile of tetramer-positive CD8+ T cells is overlaid onto the bulk CD3+CD8+ T-cell population to provide context for the distribution of central memory (TCM; CD28+ CCR7+) and effector memory (TEM) subsets, which include the transitional memory (TEM1; CD28+ CCR7−) and fully differentiated (TEM2; CD28− CCR7−) signatures. The frequencies shown in red in the memory phenotype contour plots indicate the frequencies of each memory subset within the tetramer gate. Because the number of events in the pMHC-I tetramer gate from monkey r14019 was too low, we could not evaluate the functional profile of Gag CM9-specific CD8+ T cells in this animal.
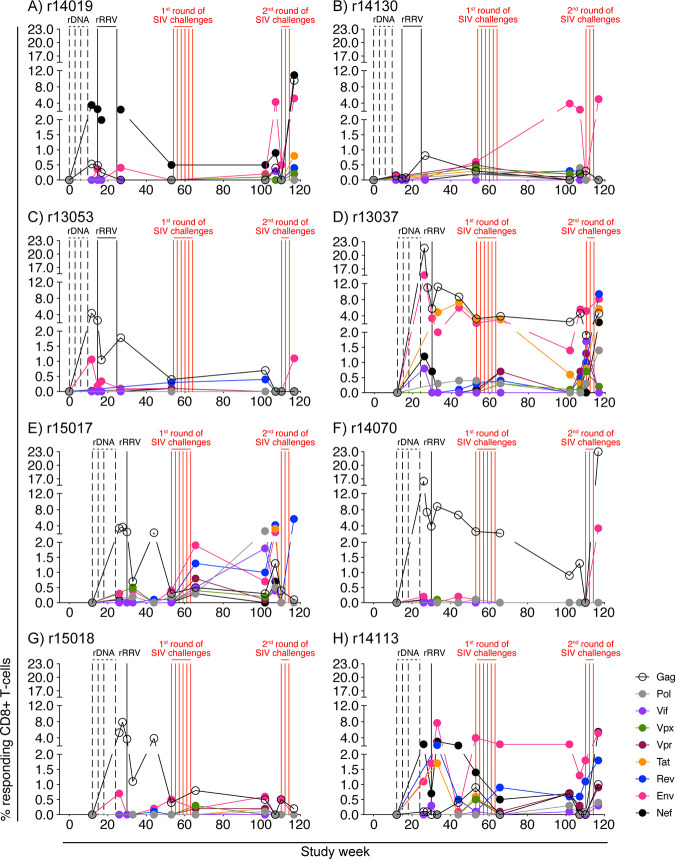
We also employed intracellular cytokine staining (ICS) to characterize the breadth and magnitude of vaccine-induced SIV-specific T-cell responses in the group A vaccinees. The stimuli for these assays consisted of pools of peptides (15-mers overlapping by 11 amino acids) spanning the entire SIVmac239 proteome. Consistent with the ability of SIVnfl to express all nine SIV gene products, vaccine-induced CD8+ T cells against the entire SIV proteome were detected, but not in every monkey (Fig. 3). Gag and Env were the main targets of CD8+ T cells, but the overall breadth and magnitude of CD8+ T-cell responses also varied considerably among animals. On one side of the spectrum was monkey r13037, whose Gag- and Env-specific CD8+ T cells peaked at 16% to 22% of peripheral CD8+ T cells at week 26 and then remained in the 1.4%-to-5.6% range until the second round of SIV challenges at week 110 (Fig. 3D). At that time, the sum of all SIV protein-specific CD8+ T-cell responses in r13037 accounted for 13% of its peripheral CD8+ T-cell compartment (see Fig. 5A). In contrast, monkey r14130 displayed low T-cell reactivity against SIV antigens during most of the vaccine phase, except for a transient increase in Env-specific CD8+ T cells in the weeks preceding the second SIV challenge round (Fig. 3B). At week 110, the total frequency of SIV-specific CD8+ T cells in r14130 was 0.4% (see Fig. 5A).
FIG 3.
Kinetics of vaccine-induced CD8+ T-cell responses against all nine SIV proteins. ICS was used to quantify vaccine-induced CD8+ T-cell responses against Gag, Pol, Vif, Vpx, Vpr, Tat, Rev, Env, and Nef in PBMC at multiple time points for each group A animal. The time scale in the x axis matches that in Fig. 1. The times of each vaccination (vertical black lines) and i.r. SIVmac239 exposures (vertical red lines) are shown in the graph. The percentages of responding CD8+ T cells shown in the y axes were calculated by adding the background-subtracted frequencies of positive responses producing any combination of IFN-γ, TNF-α, and CD107a. Protein-specific CD8+ T-cell responses are color coded according to the legend. Panels A to C show the frequencies of protein-specific CD8+ T cells in each of the three group A1 vaccinees (r14019, r14130, and r13053). Panels D to H show the frequencies of protein-specific CD8+ T cells in each of the five group A2 vaccinees (r13037, r15017, r14070, r15018, and r14113).
FIG 5.
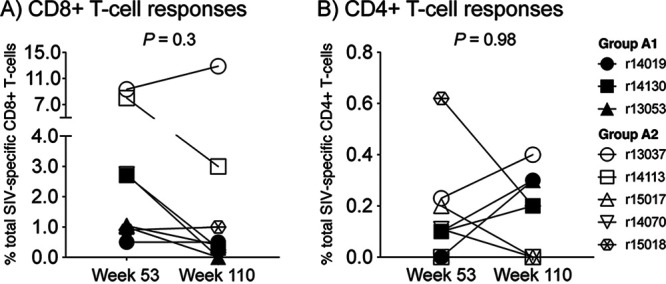
Vaccine-induced SIV-specific T-cell responses exhibit similar frequencies before the first and second SIV challenge rounds. To assess the durability of anti-SIV cellular immunity induced by the rDNA/rRRV vaccine regimen, the sum of all SIV protein-specific CD8+ (A) and CD4+ (B) T cells measured in each group A monkey were compared between two time points: week 53 (i.e., the beginning of the first SIV challenge phase) and week 110 (i.e., beginning of the second SIV challenge phase). P values were calculated using the Wilcoxon matched-pairs signed-rank test.
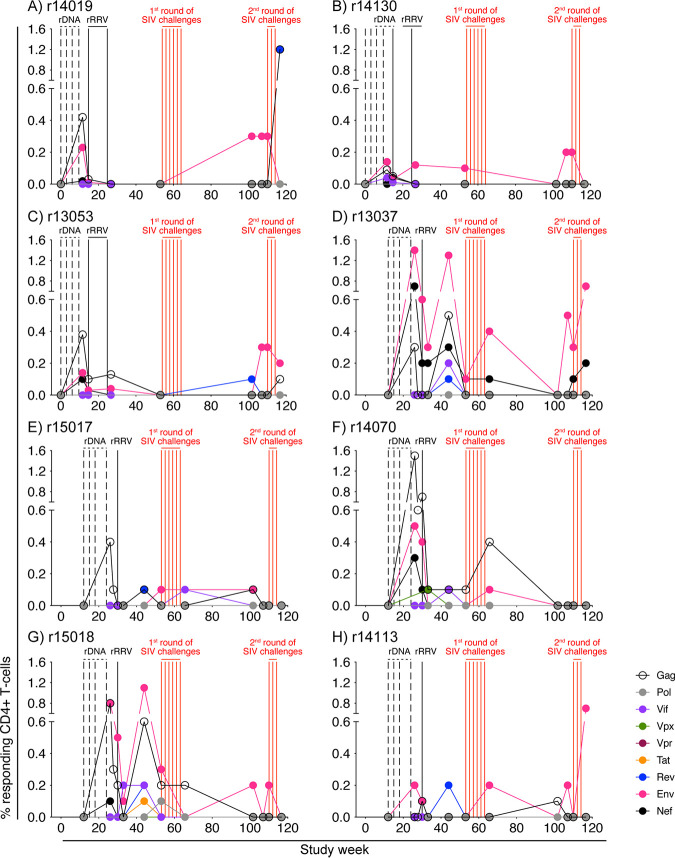
The rDNA/rRRV vaccine regimen also elicited SIV-specific CD4+ T-cell responses, but at much lower frequencies than those seen for CD8+ T-cell responses (Fig. 4). Although broadly targeted CD4+ T cells were detected in several animals prior to the first SIV challenge phase, the repertoire of these responses became more restricted as time passed, leaving Env-specific CD4+ T cells as the dominant specificity prior to the second SIV challenge round (Fig. 4).
FIG 4.
Kinetics of vaccine-induced CD4+ T-cell responses against all nine SIV proteins. ICS was used to quantify vaccine-induced CD4+ T-cell responses against Gag, Pol, Vif, Vpx, Vpr, Tat, Rev, Env, and Nef in PBMC at multiple time points for each group A animal. The time scale in the x axis matches that in Fig. 1. The times of each vaccination (vertical black lines) and i.r. SIVmac239 exposures (vertical red lines) are shown in the graph. The percentages of responding CD4+ T cells shown in the y axes were calculated by adding the background-subtracted frequencies of positive responses producing any combination of IFN-γ, TNF-α, and CD107a. Protein-specific CD4+ T-cell responses are color coded according to the legend. Panels A to C show the frequencies of protein-specific CD4+ T cells in each of the three group A1 vaccinees (r14019, r14130, and r13053). Panels D to H show the frequencies of protein-specific CD4+ T cells in each of the five group A2 vaccinees (r13037, r15017, r14070, r15018, and r14113).
To assess whether vaccine-induced T-cell responses declined over time, we determined the sum of all SIV protein-specific T cells measured in each animal before each SIV challenge phase (Fig. 5). On average, vaccine-elicited SIV-specific CD8+ T cells constituted 2.3% and 3.3% of peripheral CD8+ T cells at weeks 53 and 110, respectively (Fig. 5A). This difference was not statistically significant (P = 0.3). Likewise, there was no significant difference between the total frequencies of SIV-specific CD4+ T-cell responses measured at weeks 53 (0.17%) and 110 (0.18%) (Fig. 5B) (P = 0.98). Taken together, these data show that SIV-specific T-cell responses induced by the rDNA/rRRV vaccine regimen persisted for at least 80 weeks without additional booster immunizations. Additionally, the observation that vaccine-elicited Gag-specific CD8+ T cells measured at week 107 exhibited a TEM2 phenotype and expressed granzyme B suggests that these responses remained functional over time.
Dynamics of rDNA/rRRV-induced anti-Env antibody responses over a 2-year period.
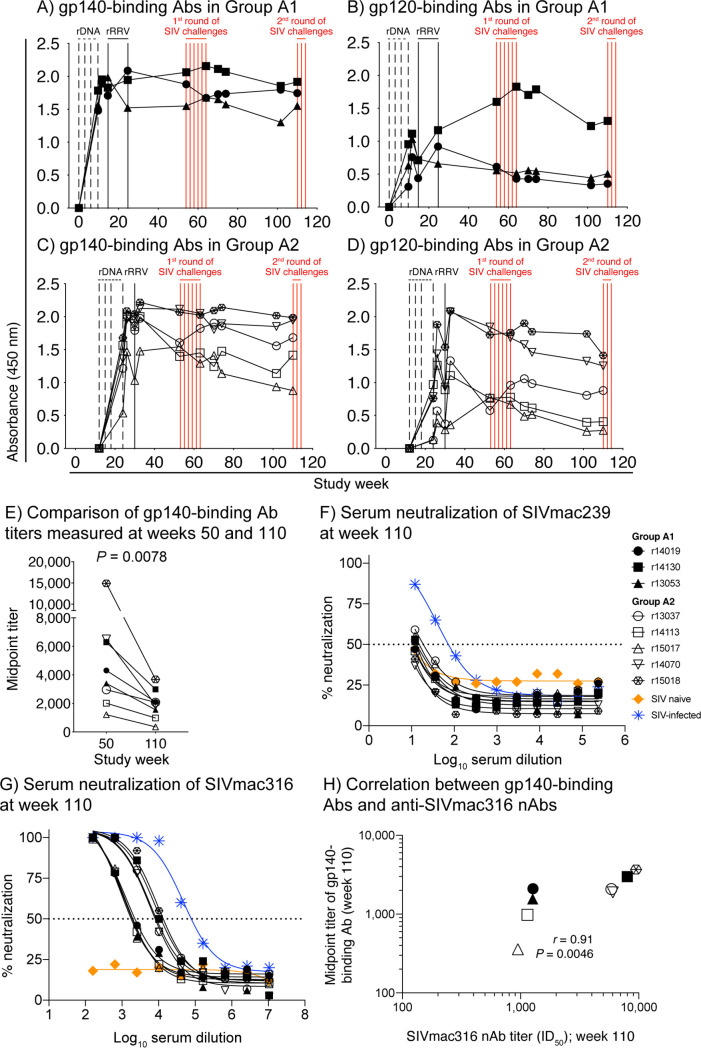
To characterize the longevity of Env-binding Abs induced by the rDNA/rRRV vaccine regimen, we performed longitudinal enzyme-linked immunosorbent assays (ELISAs) using a fixed dilution (1:1,000) of plasma collected at multiple time points throughout the vaccine phase. This analysis showed that the rDNA/rRRV vaccine regimen induced durable gp140- and gp120-binding Abs in all RMs in groups A1 and A2 (Fig. 6A to D). In fact, after peaking following the rDNA prime or rRRV boost, vaccine-induced anti-Env Abs remained detectable throughout the first (weeks 53 to 64) and second (week 110) rounds of SIV challenges (Fig. 6A to D). This pattern deviates from the ones observed in previous clinical trials of HIV-1 gp120 subunit vaccines, where the kinetics of anti-Env Abs resembled a sawtooth wave, increasing after each immunization but then decaying rapidly in the ensuing months (1, 2, 18, 19). The longevity of anti-Env Ab responses generated by the rDNA/rRRV vaccine regimen is consistent with the ability of rRRV vectors to establish persistent infection in RMs and promote chronic low-level Env expression in vivo.
FIG 6.
Vaccine-induced anti-Env antibody responses in group A remain detectable and functional for more than 1.5 years after the last vaccination. (A to D) Env-binding antibodies (Abs) were measured by ELISA using plate-bound SIVmac239 gp140 (A and C) and gp120 (B and D) at multiple time points throughout the vaccine phase. Straight 1:1,000 plasma dilutions were used for this analysis. The time scale in the x axes matches that in Fig. 1. The times of each vaccination (vertical black lines) and i.r. SIVmac239 exposures (vertical red lines) are shown in the graph. (E) Comparison of vaccine-induced gp140-binding Ab titers measured at weeks 50 and 110. Serial dilutions of sera collected prior to the first (week 50) and second (week 110) SIV challenge phases were used to determine the midpoint titers of gp140-binding Abs at these time points. The P value was calculated using the Wilcoxon matched-pairs signed-rank test. (F and G) Serially diluted sera from vaccinated animals collected at week 110 were used to determine the lowest reciprocal dilution that results in 50% reduction of SIVmac239 (F) or SIVmac316 (G) infectivity in TZM-bl assays (ID50). Internal controls included serum from an SIV-naive RM (negative control; orange line) and an RM that had been infected with SIVmac239 for 15 weeks (positive control; blue line). (H) The ID50 titers of anti-SIVmac316 nAbs measured at week 110 correlated positively with the midpoint titer of gp140-binding Abs measured at the same time point. The P value and correlation coefficient (r) were calculated using Spearman’s rank correlation test.
The long-lasting anti-Env Abs elicited by the rDNA/rRRV vaccine regimen prompted us to investigate whether these responses decayed over time. To do that, we set up gp140 ELISAs using serially diluted sera collected at weeks 50 and 110 to determine the midpoint titers of gp140-binding Abs at these two time points. This analysis revealed that the titers of gp140-binding Abs at week 50 (mean, 5,202) were significantly higher than those at week 110 (mean, 1,955; P = 0.008) (Fig. 6E). This difference remained significant even when an apparent outlier animal (r15018) was excluded from the comparison (P = 0.0156).
Next, we assessed the ability of rDNA/rRRV-induced anti-Env Abs to neutralize SIV in vitro. Since both the rDNA and rRRV vectors expressed the Env proteins of SIVmac239 (tier 3) and SIVmac316 (tier 1) (17), we screened sera from the group A vaccinees for neutralizing Abs (nAbs) against these two SIV clones. Consistent with our previous analysis of rDNA/rRRV-induced anti-SIV nAbs at week 53 (17), there was little neutralizing activity against the difficult-to-neutralize SIVmac239 in week 110 serum samples (Fig. 6F). In four of the animals (r14130, r13053, r13037, and r15017), we detected reductions in SIVmac239 infectivity that marginally exceeded 50% only at the lowest dilution tested (1:12) (Fig. 6F). However, since complete confidence intervals could not be calculated for the neutralization curves of those animals, we chose not to report their interpolated 50% inhibitory dilution (ID50) titers. In contrast, nAbs against the more readily neutralizable SIVmac316 were detectable in all animals, with ID50 titers ranging from 949 to 9,477 (Fig. 6G). In keeping with previous reports by our group (17, 20), there was a strong positive correlation between the ID50 titers of anti-SIVmac316 nAbs and the midpoint titers of gp140-binding Abs (Fig. 6H). Collectively, these data show that the rDNA/rRRV vaccine regimen elicited long-lasting anti-Env Ab responses. Despite some contraction over time, vaccine-elicited anti-Env Abs in the group A animals remained functional at study week 110, more than 1.5 years after the rRRV booster.
Outcome of the second round of i.r. SIVmac239 challenges.
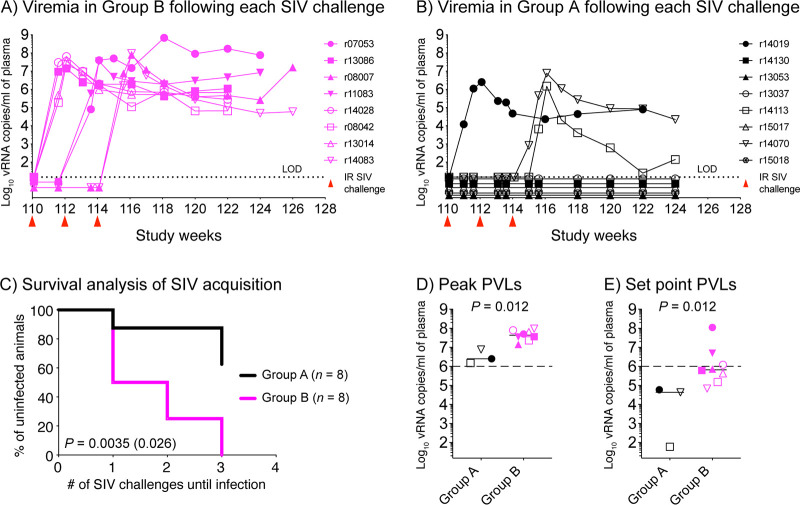
To assess the antiviral efficacy of the persistent vaccine-induced SIV-specific immune responses in group A, those animals were subjected to a second round of i.r. SIVmac239 challenges beginning at week 110. A group of 8 unvaccinated control RMs (group B) served as controls. Virus exposures occurred every 2 weeks and contained the same marginal dose (200 50% tissue culture infective doses [TCID50]) of the SIVmac239 stock utilized previously. Plasma viral loads (PVLs) measured on days 6 to 10 after each SIV exposure were used to determine whether RMs were rechallenged. Monkeys with a positive PVL on days 6 to 10 were no longer challenged, whereas animals that remained aviremic were re-exposed to SIV on day 14. The SIV challenges were halted once all RMs in group B became infected.
Following the first i.r. SIVmac239 challenge, half (4/8) of the animals in group B, but only one vaccinee in group A (r14019), became productively infected (Fig. 7A and B). Two additional control monkeys, but no other vaccinee, became viremic after the second SIV exposure (Fig. 7A and B). It was only after the third SIV exposure that two additional group A vaccinees (r14070 and r14113) acquired SIV infection (Fig. 7B). However, since the third SIV exposure also infected the last two control monkeys in group B (Fig. 7A), the SIV challenges were halted after this point. Overall, the rate of SIV acquisition in group A was significantly delayed compared to that in group B (P = 0.0035, log-rank test) (Fig. 7C). The ratio of uninfected to infected animals at the end of the SIV challenge phase was also significantly higher in group A (5/3) than in group B (0/8) (P = 0.026; Fisher’s exact test) (Fig. 7C). Although three group A vaccinees acquired SIV infection, they experienced significantly lower peak and set point PVLs than the group B animals (Fig. 7D and E), consistent with vaccine-induced SIV-specific immune responses mediating partial control of viral replication. Thus, while the protection afforded by the rDNA/rRRV regimen was incomplete, significant vaccine efficacy could still be demonstrated >1.5 years after the last immunization.
FIG 7.
Outcome of SIV challenge. Beginning at study week 110, all vaccinees in group A were subjected to a second round of i.r. SIVmac239 challenges utilizing the same dose (200 TCID50) of the same stock of SIVmac239 used in the first challenge phase. The animals were exposed to SIV every other week. A monkey was rechallenged only if it remained aviremic after each virus exposure. The challenge phase was halted after the third SIV exposure, since all control RMs in group B had become infected by that point. (A and B) Plasma viral loads (PVLs) of RMs in group B (A) and group A (B) after each of the three i.r. SIVmac239 exposures performed as part of the second SIV challenge phase. Once infected, PVLs in each animal were followed for 10 to 12 weeks. The time scale in the x axis matches that in Fig. 1. The time of each i.r. SIVmac239 exposure is indicated by red arrowheads below the x axis of each panel. LOD, limit of detection (15 vRNA copies/ml of plasma). (C) Kaplan-Meier survival analysis of SIV acquisition in group A (vaccinees; n = 8) versus group B (controls; n = 8). The P value outside the parentheses was determined using the log-rank test. The P value in parentheses was determined by Fisher’s exact test by comparing the numbers of infected and uninfected animals in group A versus group B at the end of the second SIV challenge phase. (D and E) Comparison of peak (D) and set point (E) PVLs between group A (vaccinees) and group B (controls). Peak PVLs were determined as the highest PVL measurement within the first 4 weeks postinfection. Set point PVLs were calculated as the geometric means of all PVLs measured at week 8 postinfection and at subsequent time points. Lines correspond to medians, and P values were calculated using the Mann-Whitney U test. The dashed lines in panels D and E are for reference only and indicate a PVL of 106 vRNA copies/ml of plasma.
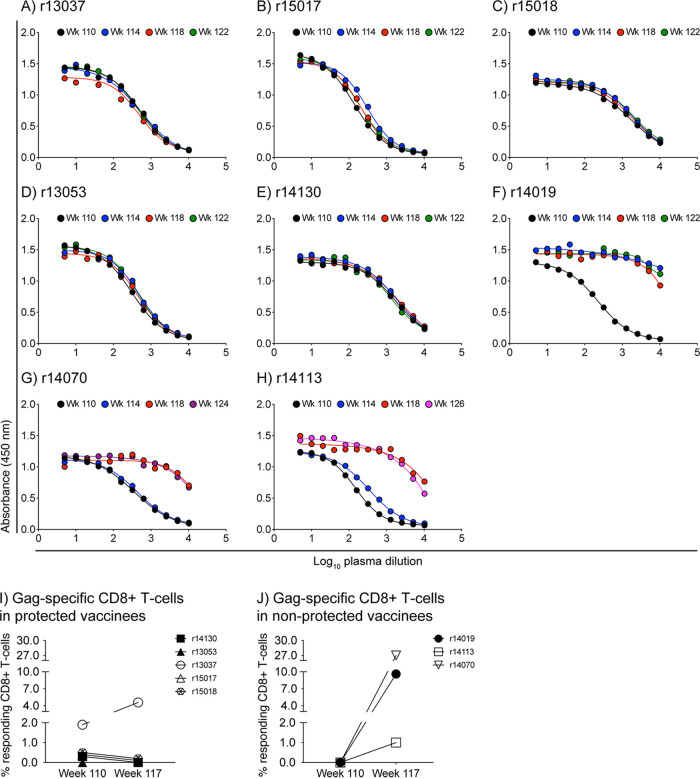
Importantly, the five protected vaccinees (r14130, r13053, r13037, r15017, and r15018) remained aviremic for at least 10 weeks after the third SIV exposure (Fig. 7B). Vaccine efficacy in those animals was also evidenced by the lack of anamnestic expansion of vaccine-induced gp120-binding Abs in the weeks that followed the second SIV challenge phase (Fig. 8A to E). Furthermore, monkeys r14130, r13053, r15017, and r15018 exhibited similar frequencies of Gag-specific CD8+ T cells at week 110 (day of first SIV challenge) and week 117 (3 weeks after the third SIV exposure) (Fig. 8I). Although monkey r13037 displayed a marginal increase in its Gag-specific CD8+ T-cell response at week 117 (Fig. 8I), similar fluctuations in T-cell responses were observed in this animal prior to the second SIV challenge phase (Fig. 3D). In contrast, postchallenge levels of gp120-binding Abs and Gag-specific CD8+ T cells were markedly increased in the three nonprotected vaccinees (r14019, r14113, and r14070) (Fig. 8F to J), consistent with productive SIV infection.
FIG 8.
Protected vaccinees show no evidence of anamnestic expansion of vaccine-induced SIV-specific immune responses after the second SIV challenge phase. (A to H) ELISA was used to determine the profile of gp120-binding Abs in serially diluted plasma from the group A animals. The samples used for this analysis were collected at week 110 (day of first SIV exposure), week 114 (day of third SIV exposure), week 118, and weeks 122 to 126. Data for the five protected vaccinees are shown in panels A (r13037), B (r15017), C (r15018), D (r13053), and E (r14130). Data for the three vaccinees that acquired SIV infection are shown in panels F (r14019), G (r14070), and H (r14113). (I and J) ICS was used to quantify Gag-specific CD8+ T-cell responses in PBMC from the group A animals at week 117, 3 weeks after the third SIV exposure. Those responses were then compared to those measured at week 110 in protected (I) and nonprotected (J) vaccinees. The percentages of responding CD8+ T cells shown on the y axes were calculated by adding the background-subtracted frequencies of positive responses producing any combination of IFN-γ, TNF-α, and CD107a.
Lastly, we searched for immune correlates that could distinguish the group A vaccinees that acquired SIV infection from those that did not. Neither the total frequency of vaccine-induced SIV-specific T-cell responses nor the titers of gp140-binding Abs at week 110 correlated with the outcome of the second SIV challenge phase. Anti-SIVmac316 nAb titers also had no predictive value. Curiously, four of the five group A vaccinees that resisted SIV infection exhibited borderline serological neutralizing activity against SIVmac239 at week 110, the time of the first challenge (Fig. 6F). Monkey r15018 was the exception, as its serum reduced SIVmac239 infectivity by less than 50% at the lowest dilution tested, and yet this animal remained aviremic following the second SIV challenge phase. Week 110 sera from the three vaccinees that acquired SIV infection also reduced SIVmac239 by less than 50% at the lowest dilution tested (Fig. 6F). However, these differences were not statistically significant (P = 0.14). Thus, conventional measures of anti-SIV immune responses did not fully explain the outcome of the second SIV challenge phase.
DISCUSSION
We recently showed that a rDNA/rRRV vaccine regimen encoding SIVnfl conferred significant protection against rectal acquisition of SIVmac239 in RMs (17). The present study not only validates this finding but also expands upon it in important ways. We show here that the protected vaccinees from our previous study maintained detectable SIV-specific immune responses for >1.5 years after the rRRV boost. Critically, the efficacy the rDNA/rRRV vaccine regimen was also long-lived, as 5/8 group A vaccinees resisted a second round of i.r. SIVmac239 exposures performed 1 year after the first SIVmac239 challenge phase. It is worth noting that protection against acquisition of SIVmac239 in RMs is a high bar for evaluating preclinical AIDS vaccine concepts, considering the high replicative kinetics of SIVmac239 and the unusual resistance of its Env protein to Ab-mediated neutralization (21).
Viewed against the backdrop of previous clinical trials of HIV vaccines, the present study stands out by the persistence of rDNA/rRRV-induced SIV-specific immune responses. In the AIDSVAX trials, for example, which tested the efficacy of six doses of an alum-adjuvanted HIV-1 gp120 subunit vaccine given at 6-month intervals, the kinetics of vaccine-induced Env-specific Ab levels exhibited a sawtooth behavior, increasing after each immunization but then decaying rapidly in the ensuing months (18). McCormack et al. reported a similar pattern of anti-Env Ab responses in a phase I trial of different adjuvant formulations of an HIV-1 gp120 subunit vaccine given at weeks 0, 4, and 28 (19). The ALVAC-HIV/gp120 vaccine utilized in the RV144 trial also induced short-lived Env-specific Ab responses, with half-lives ranging between 12 and 24 weeks (22). While recent studies in RMs indicate that the durability of anti-Env Abs elicited by Env protein vaccination can be enhanced by new adjuvants (23), more work is needed to determine the safety of these compounds and their impact on vaccine efficacy. Although vaccine-induced gp140-binding Abs in group A decreased over time, these responses remained detectable and functional in all animals for >1.5 years after the last vaccination.
RRV is a gamma2-herpesvirus that is closely related to the human Kaposi sarcoma-associated herpesvirus (24). Since one of the hallmarks of herpesviruses is their ability to establish persistent infections in their hosts, recurrent rRRV-driven expression of SIV antigens was likely the primary factor underlying the durability of vaccine-elicited SIV-specific immune responses in group A. A recent study by Hansen et al. (25) also illustrates the potential of herpesvirus-vectored vaccines to generate lasting antiviral immune responses. Using recombinant versions of the 68-1 strain of the beta-herpesvirus rhesus cytomegalovirus (RhCMV), the authors showed that RhCMV 68-1-induced SIV-specific T-cell responses remained detectable and efficacious for ∼3 years after the last vaccination (25). Since the RhCMV 68-1 vector platform has attracted considerable interest from the scientific community, a comparison between its performance and that of rRRV seems appropriate. While both vectors induce TEM-biased responses, rRRV does not share the ability of RhCMV 68-1 to elicit broadly targeting CD8+ T-cell responses restricted by MHC-II and MHC-E molecules (26, 27). Rather, rRRV induces CD8+ T cells that recognize immunodominant SIV epitopes restricted by classical MHC-I molecules (16, 17, 20, 28–32). Furthermore, whereas the latest generation of Env-expressing rRRV vectors can elicit anti-Env Abs in RMs (16, 17, 33), the same is not true for RhCMV 68-1 constructs. In fact, several studies have shown that anti-Env Abs remain below detection limits in RhCMV 68-1/Env-vaccinated RMs (25, 34, 35). Additionally, although ∼50% of RhCMV 68-1-vaccinated RMs stringently control and eventually clear SIVmac239 infection, this vaccine modality does not block SIV acquisition (35, 36). In contrast, we show here and elsewhere that vaccination with SIVnfl utilizing rRRV as a primer or as a booster for rDNA immunizations confers significant, albeit partial, protection against i.v. and i.r. challenges with SIVmac239 (16, 17). Of note, recombinant versions of the alpha-herpesviruses herpes simplex virus 1 and simian varicella virus (the monkey homolog of varicella-zoster virus) expressing SIV antigens have also been described and tested in RMs (37–40). Collectively, these observations support continued study of recombinant herpesviruses as vectors for HIV vaccines.
Since the group A vaccinees had been rectally exposed to SIVmac239 prior to the present study, and given our inability to identify a clear correlate of protection for the rDNA/rRRV vaccine regimen, we point out the following potential weaknesses to the present study. The first one is the possibility that host factors other than adaptive immune responses contributed to the group A vaccinees’ resistance to SIV infection. Certain combinations of TRIM5 alleles, for example, are associated with intrinsic resistance to mucosal challenge with SIVsmE660 in RMs (41, 42). However, because the SIVmac239 capsid protein is refractory to TRIM5α-mediated restriction, expression of SIVsmE660-restrictive TRIM5 alleles has not been linked to delayed SIVmac239 acquisition in rectally challenged RMs (42). A homozygous 32-bp deletion in the CCR5 chemokine receptor gene (i.e., the CCR5Δ32 allele) that abrogates cell surface expression of CCR5 is also known to confer potent resistance to R5-tropic HIV infection in humans (43). However, an RM equivalent of the CCR5Δ32 allele has not yet been identified (44). Although the aforementioned studies suggest that polymorphisms in the TRIM5 and CCR5 genes did not affect the outcome of the present study, we cannot rule out the possibility that unknown factors modulated the susceptibility of the group A and group B animals to rectal SIVmac239 infection.
The second caveat is the possibility that the first round of i.r. SIVmac239 challenges resulted in occult SIV infections in some of the group A vaccinees, thereby increasing their resistance to subsequent virus exposures. Although we cannot formally exclude this possibility, it seems unlikely considering that none of group A animals exhibited PVL “blips” during or after the first round of i.r. SIVmac239 challenges. Of note, transient viremia is often observed in RMs experiencing abortive or subclinical infections following mucosal SIV challenges (45–47). Furthermore, such atypical infection outcomes would be expected to trigger anamnestic SIV-specific immune responses in vaccinated RMs, but that did not seem to occur. In fact, there was no significant difference in the magnitude of vaccine-induced SIV-specific T-cell responses measured in PBMC before and after the first round of i.r. SIVmac239 challenges (17). Although the frequencies of SIV protein-specific T-cell responses fluctuated in some of the group A vaccinees in the weeks preceding the second SIVmac239 challenge phase, those changes coincided with a period of frequent sampling and could have resulted from sporadic reactivations of the rRRV vectors. Consistent with this notion, herpesvirus-specific CD8+ T-cell responses in PBMC from humans persistently infected with CMV and Epstein-Barr virus display similar oscillations over time, especially during times of virus reactivation (48–52). Thus, notwithstanding possible contributing factors as described above, it seems clear that persistent, vaccine-induced SIV-specific immune responses were principally or exclusively responsible for the protective effects against SIVmac239 infection reported here.
A few questions remain unanswered by the current study. First, is there an immune signature that predicts vaccine efficacy? As mentioned above, we have so far been unable to identify immune correlates of protection in SIVnfl vaccinees. Second, considering the partial efficacy of SIVnfl vaccination, can the rDNA/rRRV and rRRV/rDNA regimens be optimized to match the apparent sterilizing immunity afforded by live-attenuated SIV strains against homologous SIVmac239 challenge? Third, would an optimized SIVnfl vaccine regimen confer protection against heterologous SIV challenges? Fourth, do the rDNA vaccinations contribute significantly to the protection? Addressing these questions could provide new insights into how to elicit protective immunity against HIV.
MATERIALS AND METHODS
Research animals.
The Indian-origin RMs (Macaca mulatta) utilized in this study were housed at the Wisconsin National Primate Research Center (WNPRC). All animals were cared for in accordance with the guidelines of the Weatherall report and the principles described in the National Research Council’s Guide for the Care and Use of Laboratory Animals (53) under a protocol approved by the University of Wisconsin Graduate School Animal Care and Use Committee. Vaccinations were performed under anesthesia (ketamine administered at 5 to 12 mg/kg depending on the animal), and all efforts were made to minimize suffering. Euthanasia was performed at the end of the study in accordance with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. The MHC-I genotype, sex, and age of each monkey at the beginning of the second SIV challenge phase are shown in Table 1.
TABLE 1.
Animal characteristics
| Exptl group | Animal ID | Relevant MHC-I allele | Age (yrs)a | Sex |
|---|---|---|---|---|
| A1 | r14019 | Mamu-A*01 | 5.2 | Male |
| A1 | r14130 | Mamu-A*01 | 4.5 | Female |
| A1 | r13053 | Mamu-A*01 | 5.9 | Female |
| A2 | r13037 | 6.0 | Female | |
| A2 | r14113 | Mamu-A*01 | 4.6 | Male |
| A2 | r15017 | 4.0 | Male | |
| A2 | r14070 | 4.8 | Male | |
| A2 | r15018 | 4.0 | Male | |
| B | r07053 | Mamu-A*01 | 11.5 | Male |
| B | r13086 | Mamu-A*01 | 5.6 | Male |
| B | r08007 | Mamu-A*02 | 11.1 | Male |
| B | r11083 | Mamu-A*02 | 7.6 | Female |
| B | r14028 | Mamu-B*08 | 5.1 | Female |
| B | r08042 | Mamu-B*08 | 10.1 | Male |
| B | r13014 | Mamu-B*17 | 6.2 | Male |
| B | r14083 | Mamu-B*17 | 4.8 | Male |
Age at the time of the second i.r. SIVmac239 challenge phase.
Vaccinations.
A detailed description of vaccine vectors and immunization protocols used in this study can be found elsewhere (17).
SIVmac239 challenges.
The RMs in Groups A and B were subjected to the same i.r. SIVmac239 challenge regimen described in our previous study (17). Briefly, the animals were exposed to 200 TCID50 (4.8 × 105 viral RNA [vRNA] copies) of an in vivo-titrated SIVmac239 stock every 2 weeks.
SIV RNA viral load measurements.
PVLs were measured using 0.5 ml of EDTA-anticoagulated RM plasma based on a modification of a previously published method (54). Total RNA was extracted from plasma samples using Qiagen DSP virus/pathogen midikits, on a QIASymphonyXP laboratory automation instrument platform. Six replicate two-step reverse transcription-PCRs (RT-PCRs) were performed per sample using a random primed reverse transcription reaction, followed by 45 cycles of PCR using the following primers and probe: forward primer, SGAG21 [5′-GTCTGCGTCAT(dP)TGGTGCATTC-3′]; reverse primer, SGAG22 [5′-CACTAG(dK)TGTCTCTGCACTAT(dP)TGTTTTG-3′]; probe, PSGAG23 [5′-FAM-CTTC(dP)TCAGT(dK)TGTTTCACTTTCTCTTCTGCG-BHQ1-3′]. The threshold of detection on an input volume of 0.5 ml of plasma was 15 vRNA copies/ml.
ELISA measurements.
The kinetics of vaccine-induced gp140- and gp120-binding Ab responses were measured by ELISA using plasma samples collected at several time points during the vaccine phase, as part of the analysis presented in Fig. 6. These plasma samples were used at a fixed dilution of 1:1,000. The midpoint titers of vaccine-induced gp140-binding Abs were also determined by ELISA, except that serially diluted serum was used for this analysis. The plasma samples used in the analysis presented in Fig. 8 were serially diluted in the same way as the aforementioned sera, except that the plasma samples were centrifuged through 0.22-μm filters prior to the ELISA to remove debris. The purified SIVmac239 gp140 and gp120 proteins used in ELISAs were obtained from Immune Technology Corp. A detailed description of the ELISAs can be found elsewhere (17).
SIV neutralization assays.
Sera from the group A animals were screened for neutralizing activity against SIVmac239 and SIVmac316 by the luciferase-based TZM-bl assay, as described previously (55). Stocks of replication-competent SIVmac239 and SIVmac316 were produced by transfecting HEK293T cells (ATCC) with full-length DNA using jetPRIME technology (Polyplus-transfection). Supernatant was harvested after 72 h and stored at −80°C until use. Neutralization was tested by incubating SIVmac239 or SIVmac316 and monkey sera for 1 h at 37°C before transferring them onto TZM-bl cells (AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH). Neutralization was measured in duplicate wells within each experiment. The ID50 titer was defined by the log(inhibitor) versus response (three parameters) equation in Prism (GraphPad Software).
Quantification of pMHC-I tetramer-positive CD8+ T cells.
The Mamu-A*01/Gag CM9 (amino acids 181 to 189) tetramer used in this study was obtained from MBL International and was labeled with allophycocyanin (APC). A detailed description of the pMHC-I tetramer staining assays employed here can be found elsewhere (56). MAbs against the following molecules were used in the pMHC-I tetramer staining assays: CD3 (clone SP34-2; labeled with peridinin chlorophyll protein [PerCP-Cyanine5.5; BD Biosciences), CD8α (clone RPA-T8; BV785; BioLegend), CD14 (clone M5E2; BV510; BioLegend), CD16 (clone 3G8; BV510; BioLegend), CD20 (clone 2H7; BV510; BioLegend), CD28 (clone 28.2; phycoerythrin [PE]-Cyanine7; BioLegend), CCR7 (clone 150503; FITC; BioLegend), and granzyme B (clone GB12; PE; Life Technologies). An amine-reactive dye (ARD; LIVE/DEAD fixable aqua dead cell stain; Life Technologies) was included in the surface staining MAb cocktail. FlowJo v9.9 and FlowJo v10.7 (FlowJo, LLC) were used to analyze the data according to a workflow described previously (17). All tetramer frequencies mentioned in this paper correspond to percentages of live CD14− CD16− CD20− CD3+ CD8+ tetramer-positive lymphocytes.
ICS assay.
ICS assays were performed in freshly isolated PBMC as described previously (17). The MAbs against CD3, CD8α, CD14, CD16, and CD20 described above were used in the ICS assays. The ARD aqua reagent was also included, although cells were stained with it prior to the surface staining step. MAbs against the following molecules were also used in the ICS assays: CD28 (clone L293; unconjugated; BD Biosciences), CD49d (clone 9F10; unconjugated; BD Pharmingen), CD107a (clone H4A3; PE; BioLegend, Inc.), CD4 (clone OKT4; BV605; BioLegend), gamma interferon (IFN-γ) (clone 4S.B3; BV421; BioLegend), tumor necrosis factor alpha (TNF-α) (clone Mab11; APC; BD Biosciences), and CD69 (clone FN50; PE-Cy7; BioLegend). Details about sample acquisition and analysis can be found elsewhere (17). Two criteria were used to determine whether a response was positive. First, the frequency of gated events had to be ≥2-fold higher than their corresponding values in background-subtracted negative-control tests. Second, the gates for each response had to contain ≥10 events. These calculations were performed with Microsoft Excel, and results are presented as the percentages of responding CD4+ or CD8+ T cells, that is, live CD14− CD16− CD20− CD3+ lymphocytes of either subset producing any combination of IFN-γ, TNF-α, or CD107a.
Statistics.
We used Kaplan-Meier survival analysis to determine whether the rate of SIVmac239 acquisition differed between vaccinees and control animals. The P value for this comparison was calculated using the log-rank (Mantel-Cox) test. Differences between two immune parameters were assessed by the Wilcoxon matched-pairs signed rank test. Differences in PVLs between groups A and B were determined by the Mann-Whitney U test. The correlation between titers of gp140-binding Abs and anti-SIVmac316 nAbs in Fig. 6 was determined by the Spearman rank correlation method. A significance threshold of 0.05 was used for each statistical test. All P values reported in this paper are two tailed.
ACKNOWLEDGMENTS
We are grateful to a number of individuals that contributed to the execution of the present research. These individuals include all members of the Watkins laboratory at the University of Miami; all members of Immunology Services and Scientific Protocol Units at the WNPRC; and Rebecca Shoemaker, Kelli Oswald, and Randy Fast at the AIDS and Cancer Virus Program at the Frederick National Laboratory.
This work was funded by Public Health Service grant K01 OD023032 (M.A.M.) from the Office of the Director, National Institutes of Health (NIH), and by R37 AI052056 (D.I.W.) and R37 AI063928 (R.C.D.) from the National Institute of Allergy and Infectious Diseases at NIH. Partial support came from federal funds from the Office of Research Infrastructure Programs (P51 OD011106) and the National Cancer Institute, NIH, under contract no. HHSN261200800001E and 75N91019D00024 (J.D.L.). We also acknowledge the Miami Center for AIDS Research (P30 AI073961) for their support.
M.A.M. has a consulting financial interest in Emmune, Inc., a company that is developing HIV immunotherapies based on the immunoadhesin eCD4-Ig. This potential conflict of interest is being managed by Scripps Research.
Contributor Information
Mauricio A. Martins, Email: mmartins@scripps.edu.
Frank Kirchhoff, Ulm University Medical Center.
REFERENCES
- 1.Lewis GK, DeVico AL, Gallo RC. 2014. Antibody persistence and T-cell balance: two key factors confronting HIV vaccine development. Proc Natl Acad Sci U S A 111:15614–15621. 10.1073/pnas.1413550111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klasse PJ, Sanders RW, Cerutti A, Moore JP. 2012. How can HIV-type-1-Env immunogenicity be improved to facilitate antibody-based vaccine development. AIDS Res Hum Retroviruses 28:1–15. 10.1089/aid.2011.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klasse PJ, Ozorowski G, Sanders RW, Moore JP. 2020. Env exceptionalism: why are HIV-1 Env glycoproteins atypical immunogens. Cell Host Microbe 27:507–518. 10.1016/j.chom.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pitisuttithum P, Nitayaphan S, Chariyalertsak S, Kaewkungwal J, Dawson P, Dhitavat J, Phonrat B, Akapirat S, Karasavvas N, Wieczorek L, Polonis V, Eller MA, Pegu P, Kim D, Schuetz A, Jongrakthaitae S, Zhou Y, Sinangil F, Phogat S, Diazgranados CA, Tartaglia J, Heger E, Smith K, Michael NL, Excler J-L, Robb ML, Kim JH, O'Connell RJ, Vasan S, Pitisuthitham A, Sabmee Y, Sirisopana N, Eamsila C, Savaraj P, Labwech W, Teerachia S, Chotirosniramit N, Supindham T, Pruenglampoo B, Sugandhavesa P, Kosashunhanan N, Kaewthip O, Sroysuwan P, Jarujareet P, Ratto-Kim S, Molnar S, Schoen J, Churikanont N, Getchalarat S, Sangnoi N, for the RV306 Study Group , et al. 2020. Late boosting of the RV144 regimen with AIDSVAX B/E and ALVAC-HIV in HIV-uninfected Thai volunteers: a double-blind, randomised controlled trial. Lancet HIV 7:e238–e248. 10.1016/S2352-3018(19)30406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rerks-Ngarm S, Pitisuttithum P, Excler JL, Nitayaphan S, Kaewkungwal J, Premsri N, Kunasol P, Karasavvas N, Schuetz A, Ngauy V, Sinangil F, Dawson P, deCamp AC, Phogat S, Garunathan S, Tartaglia J, DiazGranados C, Ratto-Kim S, Pegu P, Eller M, Karnasuta C, Montefiori DC, Sawant S, Vandergrift N, Wills S, Tomaras GD, Robb ML, Michael NL, Kim JH, Vasan S, O’Connell RJ, for the RV305 Study Team . 2017. Randomized, double-blind evaluation of late boost strategies for HIV-uninfected vaccine recipients in the RV144 HIV Vaccine Efficacy Trial. J Infect Dis 215:1255–1263. 10.1093/infdis/jix099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniel MD, Kirchhoff F, Czajak SC, Sehgal PK, Desrosiers RC. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938–1941. 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 7.Manrique J, Piatak M, Lauer W, Johnson W, Mansfield K, Lifson J, Desrosiers R. 2013. Influence of mismatch of Env sequences on vaccine protection by live attenuated simian immunodeficiency virus. J Virol 87:7246–7254. 10.1128/JVI.00798-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukazawa Y, Park H, Cameron MJ, Lefebvre F, Lum R, Coombes N, Mahyari E, Hagen SI, Bae JY, Reyes MD, Swanson T, Legasse AW, Sylwester A, Hansen SG, Smith AT, Stafova P, Shoemaker R, Li Y, Oswald K, Axthelm MK, McDermott A, Ferrari G, Montefiori DC, Edlefsen PT, Piatak MJ, Lifson JD, Sekaly RP, Picker LJ. 2012. Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat Med 18:1673–1681. 10.1038/nm.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyand MS, Manson KH, Garcia-Moll M, Montefiori D, Desrosiers RC. 1996. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol 70:3724–3733. 10.1128/JVI.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauduin MC, Yu Y, Barabasz A, Carville A, Piatak M, Lifson JD, Desrosiers RC, Johnson RP. 2006. Induction of a virus-specific effector-memory CD4+ T cell response by attenuated SIV infection. J Exp Med 203:2661–2672. 10.1084/jem.20060134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adnan S, Reeves RK, Gillis J, Wong FE, Yu Y, Camp JV, Li Q, Connole M, Li Y, Piatak M, Lifson JD, Li W, Keele BF, Kozlowski PA, Desrosiers RC, Haase AT, Johnson RP. 2016. Persistent low-level replication of SIVΔnef drives maturation of antibody and CD8 T cell responses to induce protective immunity against vaginal SIV infection. PLoS Pathog 12:e1006104. 10.1371/journal.ppat.1006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connor RI, Montefiori DC, Binley JM, Moore JP, Bonhoeffer S, Gettie A, Fenamore EA, Sheridan KE, Ho DD, Dailey PJ, Marx PA. 1998. Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J Virol 72:7501–7509. 10.1128/JVI.72.9.7501-7509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koff WC, Johnson PR, Watkins DI, Burton DR, Lifson JD, Hasenkrug KJ, McDermott AB, Schultz A, Zamb TJ, Boyle R, Desrosiers RC. 2006. HIV vaccine design: insights from live attenuated SIV vaccines. Nat Immunol 7:19–23. 10.1038/ni1296. [DOI] [PubMed] [Google Scholar]
- 14.Picker LJ, Hansen SG, Lifson JD. 2012. New paradigms for HIV/AIDS vaccine development. Annu Rev Med 63:95–111. 10.1146/annurev-med-042010-085643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin YC, Bischof GF, Lauer WA, Gonzalez-Nieto L, Rakasz EG, Hendricks GM, Watkins DI, Martins MA, Desrosiers RC. 2018. A recombinant herpesviral vector containing a near-full-length SIVmac239 genome produces SIV particles and elicits immune responses to all nine SIV gene products. PLoS Pathog 14:e1007143. 10.1371/journal.ppat.1007143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martins MA, Bischof GF, Shin YC, Lauer WA, Gonzalez-Nieto L, Watkins DI, Rakasz EG, Lifson JD, Desrosiers RC. 2019. Vaccine protection against SIVmac239 acquisition. Proc Natl Acad Sci U S A 116:1739–1744. 10.1073/pnas.1814584116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Nieto L, Castro IM, Bischof GF, Shin YC, Ricciardi MJ, Bailey VK, Dang CM, Pedreño-Lopez N, Magnani DM, Ejima K, Allison DB, Gil HM, Evans DT, Rakasz EG, Lifson JD, Desrosiers RC, Martins MA. 2019. Vaccine protection against rectal acquisition of SIVmac239 in rhesus macaques. PLoS Pathog 15:e1008015. 10.1371/journal.ppat.1008015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert PB, Peterson ML, Follmann D, Hudgens MG, Francis DP, Gurwith M, Heyward WL, Jobes DV, Popovic V, Self SG, Sinangil F, Burke D, Berman PW. 2005. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis 191:666–677. 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 19.McCormack S, Tilzey A, Carmichael A, Gotch F, Kepple J, Newberry A, Jones G, Lister S, Beddows S, Cheingsong R, Rees A, Babiker A, Banatvala J, Bruck C, Darbyshire J, Tyrrell D, Van Hoecke C, Weber J. 2000. A phase I trial in HIV negative healthy volunteers evaluating the effect of potent adjuvants on immunogenicity of a recombinant gp120W61D derived from dual tropic R5X4 HIV-1ACH320. Vaccine 18:1166–1177. 10.1016/S0264-410X(99)00388-6. [DOI] [PubMed] [Google Scholar]
- 20.Martins MA, Gonzalez-Nieto L, Ricciardi MJ, Bailey VK, Dang CM, Bischof GF, Pedreño-Lopez N, Pauthner MG, Burton DR, Parks CL, Earl P, Moss B, Rakasz EG, Lifson JD, Desrosiers RC, Watkins DI. 2020. Rectal acquisition of simian immunodeficiency virus (SIV) SIVmac239 infection despite vaccine-induced immune responses against the entire SIV proteome. J Virol 94:e00979-20. 10.1128/JVI.00979-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martins MA, Watkins DI. 2018. What is the predictive value of animal models for vaccine efficacy in humans? Rigorous simian immunodeficiency virus vaccine trials can be instructive. Cold Spring Harb Perspect Biol 10:a029504. 10.1101/cshperspect.a029504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yates NL, Liao H-X, Fong Y, deCamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang Z-Y, Seaton KE, Berman PW, Alpert MD, Evans DT, O'Connell RJ, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Pinter A, Zolla-Pazner S, Gilbert PB, Nabel GJ, Michael NL, Kim JH, Montefiori DC, Haynes BF, Tomaras GD. 2014. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 6:228ra39. 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasturi SP, Rasheed MAU, Havenar-Daughton C, Pham M, Legere T, Sher ZJ, Kovalenkov Y, Gumber S, Huang JY, Gottardo R, Fulp W, Sato A, Sawant S, Stanfield-Oakley S, Yates N, LaBranche C, Alam SM, Tomaras G, Ferrari G, Montefiori D, Wrammert J, Villinger F, Tomai M, Vasilakos J, Fox CB, Reed SG, Haynes BF, Crotty S, Ahmed R, Pulendran B. 2020. 3M-052, a synthetic TLR-7/8 agonist, induces durable HIV-1 envelope-specific plasma cells and humoral immunity in nonhuman primates. Sci Immunol 5:eabb1025. 10.1126/sciimmunol.abb1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desrosiers RC, Sasseville VG, Czajak SC, Zhang X, Mansfield KG, Kaur A, Johnson RP, Lackner AA, Jung JU. 1997. A herpesvirus of rhesus monkeys related to the human Kaposi’s sarcoma-associated herpesvirus. J Virol 71:9764–9769. 10.1128/JVI.71.12.9764-9769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen SG, Marshall EE, Malouli D, Ventura AB, Hughes CM, Ainslie E, Ford JC, Morrow D, Gilbride RM, Bae JY, Legasse AW, Oswald K, Shoemaker R, Berkemeier B, Bosche WJ, Hull M, Womack J, Shao J, Edlefsen PT, Reed JS, Burwitz BJ, Sacha JB, Axthelm MK, Früh K, Lifson JD, Picker LJ. 2019. A live-attenuated RhCMV/SIV vaccine shows long-term efficacy against heterologous SIV challenge. Sci Transl Med 11:eaaw2607. 10.1126/scitranslmed.aaw2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, Gilbride RM, Lewis MS, Gilliam AN, Ventura AB, Malouli D, Xu G, Richards R, Whizin N, Reed JS, Hammond KB, Fischer M, Turner JM, Legasse AW, Axthelm MK, Edlefsen PT, Nelson JA, Lifson JD, Fruh K, Picker LJ. 2013. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science 340:1237874–1237874. 10.1126/science.1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen SG, Wu HL, Burwitz BJ, Hughes CM, Hammond KB, Ventura AB, Reed JS, Gilbride RM, Ainslie E, Morrow DW, Ford JC, Selseth AN, Pathak R, Malouli D, Legasse AW, Axthelm MK, Nelson JA, Gillespie GM, Walters LC, Brackenridge S, Sharpe HR, Lopez CA, Fruh K, Korber BT, McMichael AJ, Gnanakaran S, Sacha JB, Picker LJ. 2016. Broadly targeted CD8(+) T cell responses restricted by major histocompatibility complex E. Science 351:714–720. 10.1126/science.aac9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bilello JP, Manrique JM, Shin YC, Lauer W, Li W, Lifson JD, Mansfield KG, Johnson RP, Desrosiers RC. 2011. Vaccine protection against simian immunodeficiency virus in monkeys using recombinant gamma-2 herpesvirus. J Virol 85:12708–12720. 10.1128/JVI.00865-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martins MA, Tully DC, Shin YC, Gonzalez-Nieto L, Weisgrau KL, Bean DJ, Gadgil R, Gutman MJ, Domingues A, Maxwell HS, Magnani DM, Ricciardi M, Pedreño-Lopez N, Bailey V, Cruz MA, Lima NS, Bonaldo MC, Altman JD, Rakasz E, Capuano S, Reimann KA, Piatak M, Lifson JD, Desrosiers RC, Allen TM, Watkins DI. 2017. Rare control of SIVmac239 infection in a vaccinated rhesus macaque. AIDS Res Hum Retroviruses 33:843–858. 10.1089/AID.2017.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martins MA, Shin YC, Gonzalez-Nieto L, Domingues A, Gutman MJ, Maxwell HS, Castro I, Magnani DM, Ricciardi M, Pedreño-Lopez N, Bailey V, Betancourt D, Altman JD, Pauthner M, Burton DR, von Bredow B, Evans DT, Yuan M, Parks CL, Ejima K, Allison DB, Rakasz E, Barber GN, Capuano S, Lifson JD, Desrosiers RC, Watkins DI. 2017. Vaccine-induced immune responses against both Gag and Env improve control of simian immunodeficiency virus replication in rectally challenged rhesus macaques. PLoS Pathog 13:e1006529. 10.1371/journal.ppat.1006529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martins MA, Tully DC, Pedreño-Lopez N, von Bredow B, Pauthner MG, Shin YC, Yuan M, Lima NS, Bean DJ, Gonzalez-Nieto L, Domingues A, Gutman MJ, Maxwell HS, Magnani DM, Ricciardi MJ, Bailey VK, Altman JD, Burton DR, Ejima K, Allison DB, Evans DT, Rakasz EG, Parks CL, Bonaldo MC, Capuano S, Lifson JD, Desrosiers RC, Allen TM, Watkins DI. 2018. Mamu-B*17+ rhesus macaques vaccinated with env, vif, and nef manifest early control of SIVmac239 replication. J Virol 92:e00690-18. 10.1128/JVI.00690-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martins MA, Gonzalez-Nieto L, Shin YC, Domingues A, Gutman MJ, Maxwell HS, Magnani DM, Ricciardi MJ, Pedreño-Lopez N, Bailey VK, Altman JD, Parks CL, Allison DB, Ejima K, Rakasz EG, Capuano S, Desrosiers RC, Lifson JD, Watkins DI. 2018. The frequency of vaccine-induced T-cell responses does not predict the rate of acquisition after repeated intrarectal SIVmac239 challenges in Mamu-B*08 rhesus macaques. J Virol 93:e01626-18. 10.1128/JVI.01626-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin YC, Bischof GF, Lauer WA, Desrosiers RC. 2015. Importance of codon usage for the temporal regulation of viral gene expression. Proc Natl Acad Sci U S A 112:14030–14035. 10.1073/pnas.1515387112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak MJ, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med 15:293–299. 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak MJ, Lifson JD, Picker LJ. 2011. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473:523–527. 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen SG, Piatak MJ, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, Gilliam AN, Xu G, Whizin N, Burwitz BJ, Planer SL, Turner JM, Legasse AW, Axthelm MK, Nelson JA, Fruh K, Sacha JB, Estes JD, Keele BF, Edlefsen PT, Lifson JD, Picker LJ. 2013. Immune clearance of highly pathogenic SIV infection. Nature 502:100–104. 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy CG, Lucas WT, Means RE, Czajak S, Hale CL, Lifson JD, Kaur A, Johnson RP, Knipe DM, Desrosiers RC. 2000. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J Virol 74:7745–7754. 10.1128/JVI.74.17.7745-7754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaur A, Sanford HB, Garry D, Lang S, Klumpp SA, Watanabe D, Bronson RT, Lifson JD, Rosati M, Pavlakis GN, Felber BK, Knipe DM, Desrosiers RC. 2007. Ability of herpes simplex virus vectors to boost immune responses to DNA vectors and to protect against challenge by simian immunodeficiency virus. Virology 357:199–214. 10.1016/j.virol.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Traina-Dorge V, Pahar B, Marx P, Kissinger P, Montefiori D, Ou Y, Gray WL. 2010. Recombinant varicella vaccines induce neutralizing antibodies and cellular immune responses to SIV and reduce viral loads in immunized rhesus macaques. Vaccine 28:6483–6490. 10.1016/j.vaccine.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pahar B, Gray WL, Phelps K, Didier ES, deHaro E, Marx PA, Traina-Dorge VL. 2012. Increased cellular immune responses and CD4+ T-cell proliferation correlate with reduced plasma viral load in SIV challenged recombinant simian varicella virus - simian immunodeficiency virus (rSVV-SIV) vaccinated rhesus macaques. Virol J 9:160. 10.1186/1743-422X-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Letvin NL, Rao SS, Montefiori DC, Seaman MS, Sun Y, Lim SY, Yeh WW, Asmal M, Gelman RS, Shen L, Whitney JB, Seoighe C, Lacerda M, Keating S, Norris PJ, Hudgens MG, Gilbert PB, Buzby AP, Mach LV, Zhang J, Balachandran H, Shaw GM, Schmidt SD, Todd JP, Dodson A, Mascola JR, Nabel GJ. 2011. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci Transl Med 3:81ra36. 10.1126/scitranslmed.3002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds MR, Sacha JB, Weiler AM, Borchardt GJ, Glidden CE, Sheppard NC, Norante FA, Castrovinci PA, Harris JJ, Robertson HT, Friedrich TC, McDermott AB, Wilson NA, Allison DB, Koff WC, Johnson WE, Watkins DI. 2011. The TRIM5α genotype of rhesus macaques affects acquisition of simian immunodeficiency virus SIVsmE660 infection after repeated limiting-dose intrarectal challenge. J Virol 85:9637–9640. 10.1128/JVI.05074-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367–377. 10.1016/S0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 44.Weiler A, May GE, Qi Y, Wilson N, Watkins DI. 2006. Polymorphisms in eight host genes associated with control of HIV replication do not mediate elite control of viral replication in SIV-infected Indian rhesus macaques. Immunogenetics 58:1003–1009. 10.1007/s00251-006-0166-6. [DOI] [PubMed] [Google Scholar]
- 45.Ma ZM, Abel K, Rourke T, Wang Y, Miller CJ. 2004. A period of transient viremia and occult infection precedes persistent viremia and antiviral immune responses during multiple low-dose intravaginal simian immunodeficiency virus inoculations. J Virol 78:14048–14052. 10.1128/JVI.78.24.14048-14052.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McChesney MB, Collins JR, Lu D, Lu X, Torten J, Ashley RL, Cloyd MW, Miller CJ. 1998. Occult systemic infection and persistent simian immunodeficiency virus (SIV)-specific CD4(+)-T-cell proliferative responses in rhesus macaques that were transiently viremic after intravaginal inoculation of SIV. J Virol 72:10029–10035. 10.1128/JVI.72.12.10029-10035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller CJ, Marthas M, Torten J, Alexander NJ, Moore JP, Doncel GF, Hendrickx AG. 1994. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J Virol 68:6391–6400. 10.1128/JVI.68.10.6391-6400.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bharadwaj M, Burrows SR, Burrows JM, Moss DJ, Catalina M, Khanna R. 2001. Longitudinal dynamics of antigen-specific CD8+ cytotoxic T lymphocytes following primary Epstein-Barr virus infection. Blood 98:2588–2589. 10.1182/blood.V98.8.2588. [DOI] [PubMed] [Google Scholar]
- 49.Catalina MD, Sullivan JL, Bak KR, Luzuriaga K. 2001. Differential evolution and stability of epitope-specific CD8(+) T cell responses in EBV infection. J Immunol 167:4450–4457. 10.4049/jimmunol.167.8.4450. [DOI] [PubMed] [Google Scholar]
- 50.Hislop AD, Annels NE, Gudgeon NH, Leese AM, Rickinson AB. 2002. Epitope-specific evolution of human CD8(+) T cell responses from primary to persistent phases of Epstein-Barr virus infection. J Exp Med 195:893–905. 10.1084/jem.20011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rentenaar RJ, Gamadia LE, van DerHoek N, van Diepen FN, Boom R, Weel JF, Wertheim-van Dillen PM, van Lier RA, ten Berge IJ. 2000. Development of virus-specific CD4(+) T cells during primary cytomegalovirus infection. J Clin Invest 105:541–548. 10.1172/JCI8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sester M, Sester U, Gärtner BC, Girndt M, Meyerhans A, Köhler H. 2002. Dominance of virus-specific CD8 T cells in human primary cytomegalovirus infection. J Am Soc Nephrol 13:2577–2584. 10.1097/01.ASN.0000030141.41726.52. [DOI] [PubMed] [Google Scholar]
- 53.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
- 54.Li H, Wang S, Kong R, Ding W, Lee FH, Parker Z, Kim E, Learn GH, Hahn P, Policicchio B, Brocca-Cofano E, Deleage C, Hao X, Chuang GY, Gorman J, Gardner M, Lewis MG, Hatziioannou T, Santra S, Apetrei C, Pandrea I, Alam SM, Liao HX, Shen X, Tomaras GD, Farzan M, Chertova E, Keele BF, Estes JD, Lifson JD, Doms RW, Montefiori DC, Haynes BF, Sodroski JG, Kwong PD, Hahn BH, Shaw GM. 2016. Envelope residue 375 substitutions in simian-human immunodeficiency viruses enhance CD4 binding and replication in rhesus macaques. Proc Natl Acad Sci U S A 113:E3413–E3422. 10.1073/pnas.1606636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montefiori DC. 2009. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol 485:395–405. 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez-Nieto L, Domingues A, Ricciardi M, Gutman MJ, Maxwell HS, Pedreño-Lopez N, Bailey V, Magnani DM, Martins MA. 2016. Analysis of simian immunodeficiency virus-specific CD8+ T cells in rhesus macaques by peptide-MHC-I tetramer staining. J Vis Exp 2016:54881. 10.3791/54881. [DOI] [PMC free article] [PubMed] [Google Scholar]