Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder that affects millions worldwide. Due to population ageing, the incidence of AD is increasing. AD patients develop cognitive decline and dementia, features for which is known, requiring permanent care. This poses a major socio-economic burden on healthcare systems as AD patients’ relatives and healthcare workers are forced to cope with rising numbers of affected people. Despite recent advances, AD pathological mechanisms are not fully understood. Nevertheless, it is clear that the amyloid beta (Aβ) peptide, which forms amyloid plaques in AD patients’ brains, plays a key role. Type 2 diabetes, the most common form of diabetes, affects hundreds of million people globally. Islet amyloid polypeptide (IAPP) is a hormone co-produced and secreted with insulin in pancreatic β-cells, with a key role in diabetes, as it helps regulate glucose levels and control adiposity and satiation. Similarly to Aβ, IAPP is very amyloidogenic, generating intracellular amyloid deposits that cause β-cell dysfunction and death. It is now clear that IAPP can also have a pathological role in AD, decreasing cognitive function. IAPP harms the blood-brain barrier, directly interacts and co-deposits with Aβ, promoting diabetes-associated dementia. IAPP can cause a metabolic dysfunction in the brain, leading to other diabetes-related forms of AD. Thus, here we discuss IAPP association with diabetes, Aβ and dementia, in the context of what we designate a “diabetes brain phenotype” AD hypothesis. Such approach helps to set a conceptual framework for future IAPP-based drugs against AD.
Keywords: aggregation, Alzheimer, amylin, amyloid, diabetes, islet amyloid polypeptide
Dementia and Amyloid Beta Peptide
Dementia affects around 50 million people worldwide, being a social and economic burden for the patients, their families and health systems around the world. AD, a very common form of dementia, is behind the increased demand for research on this key topic. It is linked to amyloid beta peptide (Aβ) amyloidogenesis and/or aggregation. Aβ is predominantly found as Aβ40 or Aβ42, with either 40 or 42 amino acids, respectively. Increases of total Aβ and/or of the Aβ42/Aβ40 ratio correlate significantly with AD and cognitive decline (Martins et al., 2008; Kuperstein et al., 2010). Importantly, extracellular Aβ42 amyloid plaques and intracellular Tau deposits are tell-tale signs of AD, being explored as diagnostic tools, alongside other changes in the brain, with neuronal loss being invariably seen and related with cognitive decline (Martins et al., 2008; Kuperstein et al., 2010).
Diabetes and Islet Amyloid Polypeptide
Diabetes, particularly type 2, is characterized by insulin resistance or insufficient insulin production, leading to hyperglycaemia, and aggregation and deposition of islet amyloid polypeptide (IAPP), or amylin. This neuroendocrine hormone is produced and secreted in concert with insulin, inhibiting both insulin and glucagon secretion, and controlling adiposity and satiation. IAPP is highly amyloidogenic, leading to intracellular aggregates and, ultimately, to extracellular amyloid structures which cause β-cell death and are present in about 90% of diabetic patients, being thus a key disease marker (Westermark et al., 2011).
Key Role of Islet Amyloid Polypeptide, also Known as Amylin
This hormone is first synthesized as 89 residue, preproIAPP, afterwards losing its signal peptide in the endoplasmic reticulum, forming proIAPP, which matures in the late Golgi complex into IAPP. IAPP is then stored alongside insulin in secretory vesicles to be released in response to glucose stimulus (Westermark et al., 2011). When demand for insulin rises, IAPP synthesis also increases, overloading the β-cell processing machinery and leading to the accumulation of unprocessed IAPP forms. These intermediates are considered highly amyloidogenic, promoting IAPP oligomerization and amyloid deposition (Raimundo et al., 2020).
Islet Amyloid Polypeptide & Alzheimer’s Disease
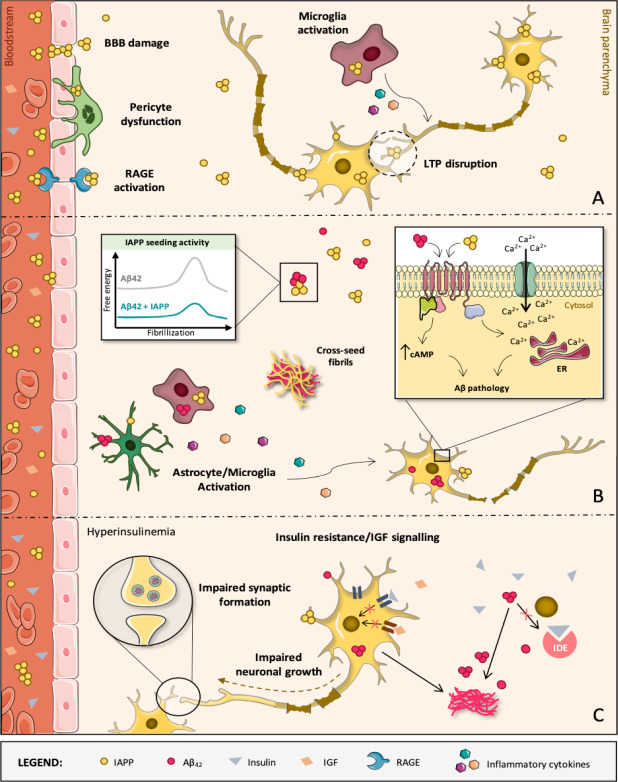
It is current consensus that this “diabetes-associated peptide” can contribute to AD. The indubitable relevance of aggregated Aβ-42 and phosphorylated Tau on AD pathophysiology led AD research to be very focused on these proteins and in their aggregation, making other contributing factors for AD development to be somewhat disregarded (Tiwari et al., 2019; Raimundo et al., 2020). One such relevant factor, partially overlooked before, is the contribution of diabetes and IAPP towards developing AD. There is evidence that besides the known effect in the pancreas, IAPP has a significant impact at the cognitive level that are relevant for AD, via several mechanisms (Figure 1).
Figure 1.
Diabetes-induced molecular, cellular and structural alterations in the AD brain.
(A) IAPP oligomers are mostly formed within secretory vesicles of pancreatic β-cells, being then co-released with insulin into the blood stream in response to specific stimuli. IAPP oligomers are membrane-permeable and may compromise the BBB and diffuse into brain parenchyma. In brain microvascular pericytes of AD patients with T2D, IAPP forms intracellular toxic inclusions leading to nuclei fragmentation, autophagy impairment and loss of cell viability and function. IAPP oligomers may also engage receptor for advanced glycation end products, promoting inflammation and exacerbating cerebrovascular damage, namely to the BBB. It also facilitates toxic accumulation of IAPP in the brain, where IAPP can directly interact with neurons as well as microglia and astrocytes, activating them and contributing to AD pathology development. At high concentrations, IAPP can act upon neuronal receptors (e.g., AMY3) to modulate signaling cascades that are associated with long-term potentiation (LTP) disruption and, consequently, synaptic failure. (B) Despite IAPP being able to affect brain functions aside from Aβ42 pathology, IAPP and Aβ42 are clearly able to interact with each other, with IAPP accelerating Aβ42 aggregation and deposition. In fact, diabetic AD patients have cross-seeded fibrils and oligomers accumulated in the brain and constituted by these two peptides. Both individual and co-aggregates of IAPP and Aβ42 are prone to activate glial cells which, in response, produce and release inflammatory mediators (e.g., cytokines), creating a pathological environment detrimental for neurons. Direct activation of AMY3 neuronal receptors via IAPP and Aβ42 leads to increased cytosolic cAMP levels and downstream activation of molecular pathways (e.g., PKA, MAPK, AKT and cFos) involved in neuroinflammation, Aβ pathology and cell death. As a result, a Ca2+ perturbed influx may occur and disturb the ER homeostasis, contributing to neuronal apoptosis. (C) AD is also a metabolic disease in some instances. IDE degrades not only excess insulin in the brain but also other substrates, such as Aβ. If IDE is occupied with insulin, it is no longer free to degrade Aβ. This impairs Aβ clearance, causing senile plaques to be formed. When the brain loses capacity to deal with glucose, insulin and IGF, classical AD molecular biomarkers (Aβ aggregates) appear and negatively impact crucial neuronal functions. These events progressively impair brain homeostasis and promote massive neurodegeneration, leading to AD and dementia. AD: Alzheimer’s disease; AMY3: amylin-3 receptors; Aβ: amyloid beta peptide; BBB: blood-brain barrier; ER: endoplasmic reticulum; IAPP: islet amyloid polypeptide; IDE: insulin-degrading enzyme; IGF: insulin-like growth factor; RAGE: receptor for advanced glycation end products; T2D: type 2 diabetes.
It was shown that circulating oligomerized IAPP is found in AD patients’ plasma and may accumulate in extra-pancreatic tissues, such as the brain (Schultz et al., 2019). IAPP aggregation is linked with hyperamylinemia, as increased IAPP production leads to the appearance of misfolded and aggregated species, by a seeding-nucleation model (Mukherjee et al., 2017), which then originate deleterious effects on the brain and peripheral organs. IAPP is able to affect brain functions independently of Aβ42 (Srodulski et al., 2014). IAPP deposits are found in AD patients’ brains (Jackson et al., 2013), that are not necessarily co-localized with Aβ42 and even if there is no clear sign of diabetes (Lutz & Meyer, 2015). Moreover, elevated levels of IAPP can directly cause brain microvascular injuries (Ly et al., 2017). IAPP and Aβ42 can also interact, with IAPP acting as a seed for Aβ42 deposition, originating cross-seeded oligomers (Oskarsson et al., 2015). This is corroborated by the fact that Aβ42 self-assembly can be prevented by an aggregation blocker based on IAPP (Yan et al., 2007) and that pramlintide, an IAPP analogue, protects against AD related neurodegeneration and dementia in general (Patrick et al., 2019). Thus, the regions responsible for Aβ42 -IAPP cross interaction are probably high-affinity binding sites involved in self-aggregation. Pramlintide, as an IAPP analogue, likely prevents the cross interactions, or promotes off-pathways, not conducive to fibril formation (Raimundo et al., 2020). IAPP may also aggravate Aβ42 effects via ROS generation and the failure of insulin-degrading enzyme to degrade insulin, IAPP and Aβ42 (Lim et al., 2010). Aβ40, the major component of AD cerebrovascular plaques, also interacts and cross-seeds with IAPP (Kandimalla et al., 2017; Raimundo et al., 2020). All mechanisms, both Aβ-dependent and -independent, aid onset and progression of AD.
Alzheimer’s Disease & Diabetes
AD is also related with insulin resistance, as glucose levels in the brain are unbalanced, giving rise to the terminology type 3 diabetes, as a form of AD (Kandimalla et al., 2017). Although this creates a novel perspective of AD, as a metabolic disease, it is somewhat misleading and limitative. AD is above all a brain disease and it can certainly be triggered solely by Aβ related pathways, independently of any role of diabetes and/or IAPP. Still, some forms of AD, under the general brain disease umbrella, correspond to a “diabetes brain phenotype” (Raimundo et al., 2020). This is a larger concept than type 3 diabetes, as it includes all forms of AD where IAPP and related players interfere, including in the absence of typical diabetes but where the brain is affected. For instance, a brain that lost the ability to respond to glucose, insulin and/or insulin-like growth factor (IGF), can easily suffer neuronal loss (Rivera et al., 2005). In addition, decreasing the activity of insulin/IGF signalling cascades appears to prevent AD-like neurodegeneration in other organisms, probably by favouring more compact amyloid fibrils that are less bioactive and more innocuous (El-Ami et al., 2014). Although insulin metabolism clearly has a role on at least some form(s) of AD, the mechanisms behind it are not well understood. Insulin resistance leads to higher activation of kinases, leading to Tau phosphorylation and, later, cell death (Arnold et al., 2018). Insulin resistance directly increases Aβ42 and its precursor protein levels, thus contributing to AD via Aβ-related mechanisms (Kandimalla et al., 2017; Raimundo et al., 2020). In sum, alterations in insulin/IGF metabolism and signalling increase AD biomarkers and deprive the brain of physiological actions, such as neuronal growth, synapses formation/differentiation, and the overall synaptic plasticity, required for the cognitive function and lacking in dementia (Kandimalla et al., 2017).
Diabetes Brain Phenotype Hypothesis
Given the molecular evidence, one cannot ignore the link between diabetes and AD. At the epidemiologic level, it is also clear that diabetic patients have higher incidence of dementia and AD (Ott et al., 1999). There are two possible explanations: on one hand, IAPP may damage the brain, whether by self-assembly or in concomitant action with Aβ-42 (it is even possible to consider it the second amyloid in AD); on the other hand, IAPP dyshomeostasis affects the whole body, including the brain, promoting AD as a result (Ott et al., 1999; Kandimalla et al., 2017; Srodulski et al., 2014; Mukherjee et al., 2017; Raimundo et al., 2020). More research is needed to clarify the extent of these deleterious effects. We propose the concept of a “brain diabetes phenotype” as a working hypothesis, in which AD may be caused by a dysregulation of glucose metabolism in the brain, a lack of function of insulin and IGF signalling, as well as by IAPP directly, with or without Aβ-mediated mechanisms. AD is above all a brain disease, and of course there are diabetes independent mechanisms that certainly occur. However, the contribution of diabetes related mechanisms and of IAPP directly (with and without Aβ) must also be thoroughly researched, under that working hypothesis.
Overall, this review aims at sparking the interest of investigators and highlighting the importance of glucose metabolism, insulin/IGF resistance and IAPP in AD. As AD is a multi-factorial disease, such perspective shift may pave the way for much-needed effective therapies for AD patients.
Acknowledgments:
We acknowledge the funding agencies as indicated.
Footnotes
C-Editors: Zhao M, Qiu Y; T-Editor: Jia Y
Conflicts of interest: None.
Financial support: This work was supported by iNOVA4Health – UID/Multi/04462/2019, a program financially supported by Fundação para a Ciência e Tecnologia / Ministério da Educação e Ciência, through national funds and co-funded by FEDER under the PT2020 Partnership Agreement, Funding from INTERFACE Programme, through the Innovation, Technology and Circular Economy Fund (FITEC), FCT via PTDC/BIA-MOL/31104/2017 and UID/Multi/04462/2019-SubProj iNOVA4Health C44 (to RM), PD/BD/135504/2018 (to AFR), Sociedade Portuguesa de Diabetologia for the Nuno Castelo-Branco Prize – 2016 (to RM), and ICM acknowledges FCT-MCTES Program “Concurso de Estímulo ao Emprego Científico” (CEECIND/01670/2017).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by iNOVA4Health – UID/Multi/04462/2019, a program financially supported by Fundação para a Ciência e Tecnologia/ Ministério da Educação e Ciência, through national funds and co-funded by FEDER under the PT2020 Partnership Agreement, Funding from INTERFACE Programme, through the Innovation, Technology and Circular Economy Fund (FITEC), FCT via PTDC/BIA-MOL/31104/2017 and UID/Multi/04462/2019-SubProj iNOVA4Health C44 (to RM), PD/BD/135504/2018 (to AFR), Sociedade Portuguesa de Diabetologia for the Nuno Castelo-Branco Prize – 2016 (to RM), and ICM acknowledges FCT-MCTES Program “Concurso de Estímulo ao Emprego Científico” (CEECIND/01670/2017).
References
- 1.Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS, Craft S, Gandy S, Buettner C, Stoeckel LE, Holtzman DM, Nathan DM. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14:168–181. doi: 10.1038/nrneurol.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Ami T, Moll L, Carvalhal Marques F, Volovik Y, Reuveni H, Cohen E. A novel inhibitor of the insulin/IGF signaling pathway protects from age-onset, neurodegeneration-linked proteotoxicity. Aging Cell. 2014;13:165–174. doi: 10.1111/acel.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson K, Barisone GA, Diaz E, Jin LW, DeCarli C, Despa F. Amylin deposition in the brain: A second amyloid in Alzheimer disease? Ann Neurol. 2013;74:517–526. doi: 10.1002/ana.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kandimalla R, Thirumala V, Reddy PH. Is Alzheimer’s disease a type 3 diabetes. A critical appraisal. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1078–1089. doi: 10.1016/j.bbadis.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuperstein I, Broersen K, Benilova I, Rozenski J, Jonckheere W, Debulpaep M, Vandersteen A, Segers-Nolten I, Van Der Werf K, Subramaniam V, Braeken D, Callewaert G, Bartic C, D’Hooge R, Martins IC, Rousseau F, Schymkowitz J, De Strooper B. Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 2010;29:3408–3420. doi: 10.1038/emboj.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim YA, Rhein V, Baysang G, Meier F, Poljak A, Raftery MJ, Guilhaus M, Ittner LM, Eckert A, Götz J. Abeta and human amylin share a common toxicity pathway via mitochondrial dysfunction. Proteomics. 2010;10:1621–1633. doi: 10.1002/pmic.200900651. [DOI] [PubMed] [Google Scholar]
- 7.Lutz TA, Meyer U. Amylin at the interface between metabolic and neurodegenerative disorders. Front Neurosci. 2015;9:216. doi: 10.3389/fnins.2015.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ly H, Verma N, Wu F, Liu M, Saatman KE, Nelson PT, Slevin JT, Goldstein LB, Biessels GJ, Despa F. Brain microvascular injury and white matter disease provoked by diabetes-associated hyperamylinemia. Ann Neurol. 2017;82:208–222. doi: 10.1002/ana.24992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martins IC, Kuperstein I, Wilkinson H, Maes E, Vanbrabant M, Jonckheere W, Van Gelder P, Hartmann D, D’Hooge R, De Strooper B, Schymkowitz J, Rousseau F. Lipids revert inert Aβ amyloid fibrils to neurotoxic protofibrils that affect learning in mice. EMBO J. 2008;27:224–233. doi: 10.1038/sj.emboj.7601953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukherjee A, Morales-Scheihing D, Salvadores N, Moreno-Gonzalez I, Gonzalez C, Taylor-Presse K, Mendez N, Shahnawaz M, Gaber AO, Sabek OM, Fraga DW, Soto C. Induction of IAPP amyloid deposition and associated diabetic abnormalities by a prion-like mechanism. J Exp Med. 2017;214:2591–2610. doi: 10.1084/jem.20161134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oskarsson ME, Paulsson JF, Schultz SW, Ingelsson M, Westermark P, Westermark GT. In vivo seeding and cross-seeding of localized amyloidosis: A molecular link between type 2 diabetes and Alzheimer disease. Am J Pathol. 2015;185:834–846. doi: 10.1016/j.ajpath.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 13.Patrick S S, Corrigan R, Grizzanti J, Mey M, Blair J J, Pallas M, Camins A, Lee HG, Casadesus G. Neuroprotective effects of the amylin analog, pramlintide, on Alzheimer’s disease are associated with oxidative stress regulation mechanisms. J Alzheimers Dis. 2019;69:157–168. doi: 10.3233/JAD-180421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raimundo AF, Ferreira S, Martins IC, Menezes R. Islet amyloid polypeptide: a partner in crime with Aβ in the pathology of Alzheimer’s disease. Fron Mol Neurosci. 2020;13:35. doi: 10.3389/fnmol.2020.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 16.Schultz N, Janelidze S, Byman E, Minthon L, Nägga K, Hansson O, Wennström M. Levels of islet amyloid polypeptide in cerebrospinal fluid and plasma from patients with Alzheimer’s disease. PLoS One. 2019;14:e0218561. doi: 10.1371/journal.pone.0218561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srodulski S, Sharma S, Bachstetter AB, Brelsfoard JM, Pascual C, Xie XS, Saatman KE, Van Eldik LJ, Despa F. Neuroinflammation and neurologic deficits in diabetes linked to brain accumulation of amylin. Mol Neurodegener. 2014;9:30. doi: 10.1186/1750-1326-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int J Nanomedicine. 2019;14:5541–5554. doi: 10.2147/IJN.S200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westermark P, Andersson A, Westermark GT. Islet amyloid, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 20.Yan LM, Velkova A, Tatarek-Nossol M, Andreetto E, Kapurniotu A. IAPP mimic blocks Aβ cytotoxic self-assembly: Cross-suppression of amyloid toxicity of Aβ and IAPP suggests a molecular link between Alzheimer’s disease and type II diabetes. Angew Chem Int Ed Engl. 2007;46:1246–1252. doi: 10.1002/anie.200604056. [DOI] [PubMed] [Google Scholar]