Abstract
Transcranial magnetic stimulation, a type of noninvasive brain stimulation, has become an ancillary therapy for motor function rehabilitation. Most previous studies have focused on the effects of repetitive transcranial magnetic stimulation (rTMS) on motor function in stroke patients. There have been relatively few studies on the effects of different modalities of rTMS on lower extremity motor function and corticospinal excitability in patients with stroke. The MEDLINE, Embase, Cochrane Library, ISI Science Citation Index, Physiotherapy Evidence Database, China National Knowledge Infrastructure Library, and ClinicalTrials.gov databases were searched. Parallel or crossover randomized controlled trials that addressed the effectiveness of rTMS in patients with stroke, published from inception to November 28, 2019, were included. Standard pairwise meta-analysis was conducted using R version 3.6.1 with the “meta” package. Bayesian network analysis using the Markov chain Monte Carlo algorithm was conducted to investigate the effectiveness of different rTMS protocol interventions. Network meta-analysis results of 18 randomized controlled trials regarding lower extremity motor function recovery revealed that low-frequency rTMS had better efficacy in promoting lower extremity motor function recovery than sham stimulation. Network meta-analysis results of five randomized controlled trials demonstrated that high-frequency rTMS led to higher amplitudes of motor evoked potentials than low-frequency rTMS or sham stimulation. These findings suggest that rTMS can improve motor function in patients with stroke, and that low-frequency rTMS mainly affects motor function, whereas high-frequency rTMS increases the amplitudes of motor evoked potentials. More high-quality randomized controlled trials are needed to validate this conclusion. The work was registered in PROSPERO (registration No. CRD42020147055) on April 28, 2020.
Keywords: cortical excitability, lower extremity, motor function, network meta-analysis, noninvasive brain stimulation, stroke, systematic review, transcranial magnetic stimulation
Introduction
Stroke is the dominant cause of disability among adults worldwide (Lavados et al., 2007; Zhou et al., 2019), often causing motor impairment. Motor impairment is related to a higher risk of falling because of gait impairments (Kim et al., 2014a), as well as limitations in activities of daily life and poor quality of life (Robinson et al., 2011). Clinical therapists therefore normally focus on improving walking ability and lower extremity motor function in patients with stroke (Winstein et al., 2016; Hankey, 2017). As a result of abnormally increased transcallosal inhibition from the contralateral to ipsilateral hemisphere, an imbalance in interhemispheric inhibition often occurs after stroke (Calautti et al., 2001; Zappasodi et al., 2019). This imbalance is associated with the degree of motor impairment and the limitation of sensorimotor recovery (Murase et al., 2004; Peters et al., 2018).
Based on the concept of disrupting interhemispheric balance after stroke, transcranial magnetic stimulation (TMS), a type of noninvasive brain stimulation, has become an essential ancillary therapy for motor function rehabilitation, modulating cortical excitability and inducing neural plasticity (Nowak et al., 2009). TMS can either increase or decrease excitability of the stimulated cerebral cortex site, and of remote regions via functional anatomical connections (Kobayashi and Pascual-Leone, 2003; Gu et al., 2013; Yang et al., 2020). Theoretically, repetitive TMS (rTMS) can induce different changes in cortical excitability that vary with the stimulated frequency. High-frequency rTMS (HF-rTMS) increases brain activity, whereas low-frequency rTMS (LF-rTMS) induces the opposite effect (Benussi et al., 2019). Lately, novel forms of rTMS therapies have emerged, such as deep TMS (dTMS), which uses a different coil type (Hesed coil) that can purportedly stimulate deeper cortical and subcortical regions (Chieffo et al., 2013), and theta-burst stimulation (TBS), including continuous TBS and intermittent TBS (iTBS) (Harrington and Hammond-Tooke, 2015; Strzalkowski et al., 2019).
Previous meta-analyses have examined the efficacy of rTMS compared with sham therapy, and have demonstrated that rTMS can improve motor function, balance, and walking speed compared with the sham group (Li et al., 2018; Ghayour-Najafabadi et al., 2019; Tung et al., 2019). However, these meta-analyses did not compare the effectiveness of different modalities of rTMS (e.g., HF-rTMS, LF-rTMS, and iTBS) on lower extremity motor function. Furthermore, pairwise meta-analyses provide limited insights into overall treatment hierarchies because treatment effects are only estimated from relevant treatment comparisons. In contrast to standard pairwise meta-analyses, network meta-analysis (NMA) allows the comparison of different modalities of rTMS, although it does not allow direct comparisons of head-to-head trials (Mills et al., 2013). Thus, NMA provides a more complete insight into the efficacy of rTMS interventions, and should be considered as the highest level of evidence in treatment guidelines (Leucht et al., 2016). The objective of this systematic review and NMA was to compare the efficacy of different rTMS interventions for lower extremity motor function in patients with stroke, and to obtain clinical significance levels of these interventions from the perspective of motor function improvement. Furthermore, this review aimed to explore the effectiveness of rTMS on cortical excitability.
Data and Methods
We established the protocol of this systematic review, registered in PROSPERO (registration No. CRD42020147055) on April 28, 2020, in line with guidance from the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) statement (Additional file 1) (Moher et al., 2015; Shamseer et al., 2015). This review was also in accordance with the PRISMA statement (Liberati et al., 2009) and its extension for NMA (Hutton et al., 2015), and with the Cochrane Collaboration recommendations (Higgins and Green, 2011).
PRISMA 2009 Checklist.
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 1-2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 2-4 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 4 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | 4 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 4-5 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 5 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 5 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 6 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 6 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 6 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 6 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 7 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 8-10 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 7 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 11 |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 11 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 11-12 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 12 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 13-16 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 13-16 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 12-13 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | 16 |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 16-20 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 20 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 20-21 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 21 |
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097
Eligibility criteria
The inclusion criteria were stroke patients with lower extremity dysfunction who were diagnosed according to the stroke diagnostic criteria formulated by The Fourth National Cerebrovascular Disease Conference in 1995 (Chinese Society of Neuroscience and Chinese Neurosurgical Society, 1996), and parallel or crossover randomized controlled trials (RCTs) that explored the effectiveness of rTMS on lower extremity recovery in stroke patients. Quasi-randomized trials or studies including adolescents (under 18 years of age) with stroke, bilateral stroke patients, or subtentorial stroke patients were excluded. Outcome measures included lower extremity motor function and cortical excitability. The primary outcome was motor recovery of the lower extremity, measured by the Fugl-Meyer assessment (FMA). Secondary outcomes included balance function, speed, motor evoked potential (MEP), and the Barthel Index (BI). Balance function was measured using the Berg Balance Scale (BBS) along with the Timed Up and Go Test (TUG). Post-treatment values of motor function and similar measurements were pooled.
Data sources and searches
The following online electronic databases were searched for eligible studies from inception to November 28, 2019: MEDLINE, Embase, Cochrane Library, ISI Science Citation Index, Physiotherapy Evidence Database (PEDro), and China National Knowledge Infrastructure Library (CNKI). Keywords included transcranial magnetic stimulation or TMS, stroke, cerebrovascular accident, and the combination of these words. We also searched for studies in progress, unpublished research, and research reported in the gray literature and ClinicalTrials.gov. The full search strategy is illustrated in Additional file 2 (93.3KB, pdf) . We re-ran the searches just before the final analysis, and further studies were retrieved for inclusion.
Data collection and analysis
Study selection
Two reviewers (YJX and YC) independently screened the titles and abstracts of the included studies using the search strategy, to identify studies that potentially met the predefined inclusion criteria. The full text versions of these potential studies were then retrieved and evaluated independently by two reviewers (YJX and YC). Any disagreements were settled by discussion or by reaching a consensus with a third reviewer.
Data extraction and management
Based on predefined criteria, relevant information from the eligible studies was extracted independently by two reviewers (YJX and YC) using electronic data collection forms. Discrepancies were resolved by consensus with a third reviewer (QFG). The main components of the identified studies (sample size, population characteristics, and study design), rTMS modalities, and outcome measures were extracted. We contacted the authors for any unpublished data that was necessary for the data analysis.
Risk of bias assessment
The PEDro scale was applied to appraise the methodological quality of the included studies (de Morton, 2009). The final score on the PEDro scale is the number of positive answers to 11 questions. An excellent-quality study was defined by a score of 9 to 10, a good-quality study by a score of 6 to 8, a normal-quality study by a score of 4 to 5, and a poor-quality study by a score of less than 4 (Maher et al., 2003). We excluded poor-quality studies (scores lower than 4). Two independent reviewers conducted the quality assessment, and any divergences between the reviewers were resolved by discussion or agreement with a third reviewer.
Quality of evidence
The global quality of our results was evaluated using an approach to extend the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) system (Atkins et al., 2004) to NMAs. In this way, we were able to integrate quality ratings for direct comparisons to evaluate the certainty of the evidence (confidence in evidence/quality of evidence) from an NMA (Brignardello-Petersen et al., 2018). According to the GRADE rating standards, which contain five categories—limitations in study design, inconsistency, indirectness, imprecision, and publication bias (Atkins et al., 2004)—the quality of evidence can be graded as high, moderate, low, or very low (Puhan et al., 2014). The evidence profiles were generated using GRADEpro GDT (https://gdt.gradepro.org/app/) (Brignardello-Petersen et al., 2019).
Measures of treatment effect
When diverse measures were used to appraise identical outcomes, the data were presented as standardized mean differences (SMDs) for continuous outcomes. Effect measures for continuous outcomes of included studies were calculated by the means and standard deviations of post-intervention values. The findings of every possible therapy derived from the NMA were presented as summary relative effect sizes. The ranking probabilities for all possible levels of therapy per intervention were also estimated (Salanti et al., 2011).
Dealing with missing data
Corresponding authors were contacted for more information about missing data. If there was no reply, two reviewers (YJX and YC) attempted to measure the data using the available coefficients. These missing data may potentially influence the results of the review; this was determined using a sensitivity analysis.
Assessment of clinical assumptions
The variability of participants, continuation of outcome measures, and intervention protocols and curative effects can result in multiple heterogeneities in the included studies, such as clinical, methodological, and statistical heterogeneity. Clinical and methodological heterogeneity within each pairwise comparison was evaluated by qualitatively comparing the study and population characteristics across the eligible studies.
The term “transitivity” means that there are no differences between two experiments regarding the distribution of effect modifiers (Salanti, 2012). The elementary presumption underlying NMAs is the assumption of transitivity, which needs to be carefully evaluated. The believability of transitivity can be appraised by originally assessing the resemblance of the competitive interventions in different design studies, and then comparing the distribution of the possible effect modifiers in different direct comparisons (Jansen and Naci, 2013). Quantitative synthesis may not be conducted when various comparisons exist in intransitivity, with considerable variation on effect modifiers (Rouse et al., 2017).
Statistical analysis
Methods for direct treatment comparisons
Standard pairwise meta-analysis was conducted using a random-effects model to calculate the direct relative effects of the competitive interventions in R version 3.6.1 (https://www.r-project.org/) (Salanti et al., 2011; Shim et al., 2019) using the “meta” package. Effect sizes were represented as SMD to determine whether the mean effect size was significant. For all statistical analyses, P < 0.05 implies that the effect size is significant. SMDs with 95% confidence intervals (CIs) were used to indicate the mean effect. A mean effect size of 0 was taken to indicate no effect (unchanged), while 0.2, 0.5, and 0.8 indicated small, medium, and large effects, respectively. For each subsequent treatment comparison, direct and indirect evidence was integrated into a single summary estimate to synthesize the evidence from the network of trials.
Methods for indirect and mixed comparisons
Bayesian network analysis using the Markov chain Monte Carlo algorithm was conducted to investigate the effectiveness of different rTMS protocol interventions (Madden et al., 2016). This requires convergence of the Markov chain Monte Carlo chain to its stationary distribution (Toft et al., 2007; Warren et al., 2017). The burn-in period was defined as 5000 simulations for each chain, and posterior summaries are based on 200 000 subsequent simulations. Convergence of chains was verified visually by observing trace plots and inspecting diagnostic statistics, as well as potential scale reduction factors obtained from Brooks–Gelman–Rubin plots (Brooks and Gelman, 1998; van Valkenhoef et al., 2012). When potential scale reduction factors are closer to 1, the simulated observations are closer to the target distribution. The deviance information criterion was then selected to determine the model fitness. A model with a lower deviance information criterion is considered a better fit and is the preferred choice (Dias et al., 2013). The results from NMA were indicated as SMD with 95% credible intervals (CrIs) (Roever and Biondi-Zoccai, 2016), which were presented as a league table (Mavridis et al., 2015). The square matrix contained all of the data about relative efficacy and their ambiguities for all probable interventions. Statistical analysis was performed using GeMTC (version 0.14.3) and R (version 3.6.1) software (Salanti et al., 2011; Shim et al., 2019) using the “gemtc” and “rjags” package (Neupane et al., 2014).
Assessment of statistical heterogeneity and inconsistency
The I2 statistics were used to assess statistical heterogeneity in standard pairwise meta-analysis. Effect sizes were calculated using the fixed-effects model when various independent studies maintained homogeneity (P > 0.05 or I2 < 50%) (Fleiss, 1993). Notwithstanding, when there was heterogeneity between eligible studies (P < 0.05 or I2 > 50%), we conducted a sensitivity analysis or stratified analysis to analyze the source of heterogeneity. The random-effects model was used for the analysis when the included studies remained non-homogeneous after the heterogeneity analysis (Borenstein et al., 2010). The heterogeneity variance parameter (t2) was used to assess the statistical heterogeneity of the NMA models (Turner et al., 2012). Presuming the comparability of direct and indirect evidence in NMAs may engender incorrect conclusions when there is prevailing statistically significant inconsistency (White et al., 2012). The node-splitting method was used to locally check inconsistency between direct and indirect evidence (Bucher et al., 1997; Dias et al., 2010). If inconsistency was identified, the potential effect modifiers of included studies within inconsistent loops came under scrutiny by fitting network meta-regression models and/or conducting sensitivity analyses to exclude studies that may possess sources of inconsistency (Higgins et al., 2012; Rouse et al., 2017).
Additional analyses and small study effects
If the necessary data were available, we conducted a subgroup analysis for different outcome measures. Funnel plots and the Egger’s test were used for estimating publication bias. Within the outcomes of motor function and cortical excitability, we performed a sensitivity analysis to determine whether our results changed. Studies with lower quality, no blind evaluation, or a dropout rate of more than 10% were excluded.
Results
Study selection
Of 4432 identified references, 4217 articles were excluded after systematically screening the titles and abstracts and removing duplicates. The full texts of 215 articles were retrieved for further exploration. Of these, 189 articles were omitted for several reasons, as described in Figure 1. Finally, 26 studies (Chang et al., 2010; Wang et al., 2012, 2016, 2019; Kakuda et al., 2013; Cha et al., 2014; Chieffo et al., 2014; Elkholy et al., 2014; Ji et al., 2014; Kim et al., 2014b; Cha and Kim, 2015; Ji and Kim, 2015; Lin et al., 2015, 2019; Choi et al., 2016; Du et al., 2016; Rastgoo et al., 2016; Cha and Kim, 2017; Forogh et al., 2017; Guan et al., 2017; Meng and Song, 2017; Chen, 2018; Huang et al., 2018; Zhao et al., 2018; Koch et al., 2019; Liu et al., 2019) were included (24 two-arm and 2 three-arm trials) in the quantitative synthesis, providing information on 30 comparisons among five different rTMS interventions (Figure 1).
Figure 1.
Flow diagram of the study selection.
Study characteristics and assessment of clinical assumptions
Of the 26 enrolled studies, 22 (Chang et al., 2010; Wang et al., 2012; Cha et al., 2014; Elkholy et al., 2014; Ji et al., 2014; Kim et al., 2014b; Cha and Kim, 2015; Ji and Kim, 2015; Lin et al., 2015, 2019; Du et al., 2016; Wang et al., 2016; Cha and Kim, 2017; Forogh et al., 2017; Guan et al., 2017; Meng and Song, 2017; Chen, 2018; Huang et al., 2018; Zhao et al., 2018; Koch et al., 2019; Liu et al., 2019; Wang et al., 2019) were RCTs, while the remaining studies were crossover trials (Kakuda et al., 2013; Chieffo et al., 2014; Choi et al., 2016; Rastgoo et al., 2016). Overall, 943 participants (aged 57.17 ± 11.95 years; 610 [65%] men) were randomized to treatment. The baseline characteristics were equivalent between competing treatments. Among 18 studies (Chang et al., 2010; Wang et al., 2012, 2016, 2019; Chieffo et al., 2014; Elkholy et al., 2014; Lin et al., 2015, 2019; Du et al., 2016; Rastgoo et al., 2016; Forogh et al., 2017; Guan et al., 2017; Meng and Song, 2017; Chen, 2018; Huang et al., 2018; Zhao et al., 2018; Koch et al., 2019; Liu et al., 2019) that reported the FMA as the primary outcome measure, the group comparing LF-rTMS versus sham was the most accepted comparison (Figure 2). Additionally, 11 studies (Wang et al., 2012, 2019; Kakuda et al., 2013; Chieffo et al., 2014; Elkholy et al., 2014; Ji et al., 2014; Kim et al., 2014b; Cha and Kim, 2015, 2017; Ji and Kim, 2015; Lin et al., 2019) used speed as a measure of lower extremity function, while 12 studies (Cha et al., 2014; Elkholy et al., 2014; Kim et al., 2014b; Choi et al., 2016; Rastgoo et al., 2016; Wang et al., 2016; Forogh et al., 2017; Chen, 2018; Huang et al., 2018; Zhao et al., 2018; Koch et al., 2019; Lin et al., 2019) also reported balance function. Cortical excitability (MEP amplitude) was assessed in six studies (Wang et al., 2012, 2019; Cha et al., 2014; Du et al., 2016; Cha and Kim, 2017; Huang et al., 2018). The principal characteristics of the included studies are listed in Tables 1 and 2. There were no significant discrepancies concerning baseline characteristics or total intervention sessions among the direct comparisons. This finding indicates a strong possibility that the underlying assumption of transitivity is correct in this review.
Figure 2.
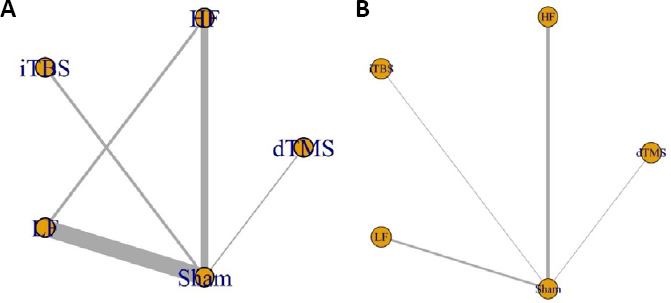
Network diagrams of treatments in patients with stroke.
(A) Network diagram for the Fugl-Meyer assessment. (B) Network diagram for speed. The nodes were linked by a line when the treatments were directly comparable. The width of each line is proportional to the number of randomized controlled trials, and the size of each node is proportional to the number of patients (sample size). dTMS: Deep transcranial magnetic stimulation; HF: high-frequency repetitive transcranial magnetic stimulation; iTBS: intermittent theta-burst stimulation; LF: low-frequency repetitive transcranial magnetic stimulation.
Table 1.
Characteristics of participants in the included studies
| Study | Study design | Sample size (E/C) | Age (yr) | Gender (M/F) | Onset time | Hemiparesis (R/L) | Stroke type (infarction/hemorrhage) |
| Cha et al. (2014) | RCT | 12/12 | 53.08±7.65 | 11/13 | 2.92±1.31/3.58±0.90 mon | 10/14 | 9/15 |
| Cha and Kim (2015) | RCT | 15/15 | 60.72±12.36 | 13/27 | 14.45±3.14/14.13±1.55 mon | 12/18 | 10/20 |
| Cha and Kim (2017) | RCT | 10/10 | 54.8±14.56 | 10/10 | 3.90±1.59/4.20±1.22 mon | 11/9 | 7/13 |
| Chang et al. (2010) | RCT | 10/18 | 56.61±12.21 | 11/17 | 12.9±5.2/14.4±5.9 d | 13/15 | NR |
| Chen (2018) | RCT | 70/70 | 53.25±11.92 | 105/35 | 31.6±17.9/27.6±19.3 mon | 72/68 | 97/41 |
| Chieffo et al. (2014) | Crossover | 10 | 62.2±9.70 | NR | 212±6.91 d | 6/4 | 5/5 |
| Choi et al. (2016) | Crossover | 30 | 67.9±4.59 | 3/27 | 49.6±28.3/44.0±29.9 mon | 15/15 | 30/0 |
| Du et al. (2016) | RCT | 23/23/23 | 55.72±11.6 | 45/24 | 7(4–16)/6(5–12)/8(3–24) d# | NR | 69/0 |
| Elkholy et al. (2014) | RCT | 30/15 | 44.59±3.93 | 23/22 | 2.53±0.52/2.53±0.52 mon | 0/45 | NR |
| Forogh et al. (2017) | RCT | 13/13 | 53–79* | 10/16 | NR | 8/18 | NR |
| Guan et al. (2017) | RCT | 21/21 | 58.55±10.93 | 12/30 | 3.8±3.4/4.8±4.1 d | 19/23 | 42/0 |
| Huang et al. (2018) | RCT | 18/20 | 61.67±9.76 | 23/15 | 31.3±25.5/25.9±18.1 d | 17/21 | 25/13 |
| Ji et al. (2014) | RCT | 15/14 | 46.68±10.01 | 12/17 | 6.26±2.65/6.35±2.97 mon | 14/15 | 15/14 |
| Ji and Kim (2015) | RCT | 20/19 | 56±9.58 | 23/16 | 1.9±0.72/1.68±0.58 mon | 20/19 | 12/27 |
| Kakuda et al. (2013) | Crossover | 18 | 52.1±11.9 | 5/13 | 52.8±30.7 mon | 12/6 | 5/13 |
| Kim et al. (2014b) | RCT | 10/22 | 66.59±9.08 | 17/15 | 16.2±13.0/15.1±5.1 d | NR | 32/0 |
| Koch et al. (2019) | RCT | 17/17 | 64±11.3 | 21/13 | 13.06±16.94 mon | 20/14 | 34/0 |
| Lin et al. (2015) | RCT | 16/16 | 60.3±11.26 | 11/21 | 40.6±29.1/33.5±23.8 d | 15/17 | 10/22 |
| Lin et al. (2019) | RCT | 10/10 | 60.95±8.7 | 3/17 | 359±171/384±270 d | 9/11 | 4/16 |
| Liu et al. (2019) | RCT | 18/18/18 | 59.33±6.52 | 37/17 | NR | NR | 54/0 |
| Meng and Song (2017) | RCT | 10/10 | 65±9.35 | 3/17 | NR | NR | 20/0 |
| Rastgoo et al. (2016) | Crossover | 20 | 52.15±11.51 | 4/16 | 30.2±18.3/27.4±20.1 mon | 7/13 | 5/15 |
| Wang et al. (2012) | RCT | 12/12 | 63.9±11.44 | 9/15 | 1.84±1.16/2.00±1.23 yr | 10/14 | NR |
| Wang et al. (2016) | RCT | 15/15 | 64.6±14.32 | 16/14 | 2.05±1.35/1.98±1.12 yr | NR | NR |
| Wang et al. (2019) | RCT | 8/6 | 54.01±12.6 | 11/3 | 31.8±24.0/25.3±15.7 mon | 8/6 | 6/8 |
| Zhao et al. (2018) | RCT | 36/39 | 55.14±12.06 | 47/28 | 4.0±2.0/4.3±3.1 mon | 36/39 | 38/37 |
Data are expressed as the mean ± SD for age and onset time, while other data are expressed as numbers. *Age range. #Mean (range). C: Control group; E: experimental group; F: female; M: male; NR: not reported; RCT: randomized controlled trial.
Table 2.
Characteristics of rTMS variables in included studies
| Study | Coil type | rTMS site | rTMS frequency (Hz) | Intensity (%) | No. of pulses | Treatment duration | Outcome measures |
| Cha et al. (2014) | F8 | Ipsi-hotspot/contra-hotspot | 10/1 | 90 RMT/90 RMT | 2000×20/1200×20 | 4 wk | BBS, MEP |
| Cha and Kim (2015) | F8 | Vertex | 10 | 90 RMT | 2000×20 | 4 wk | Speed |
| Cha and Kim (2017) | F8 | Ipsi-M1 | 10 | 90 RMT | 1000×40 | 8 wk | Speed, MEP |
| Chang et al. (2010) | F8 | Ipsi-M1 | 10 | 90 RMT | 1000×10 | 10 d | FMA, BI |
| Chen (2018) | F8 | Contra-M1-LL | 1 | 90 RMT | 1000×5 | 5 d | FMA, TUG |
| Chieffo et al. (2014) | H | Vertex | 20 | 90 RMT | 1500×11 | 3 wk | FMA, speed |
| Choi et al. (2016) | F8 | Trunk motor spot | 10 | 90 RMT | 1000×10 | 2 wk | BBS |
| Du et al. (2016) | F8 | Ipsi/contra | 3/1 | 80–90 RMT/110–120 RMT | 1200×5/1200×5 | 5 d | FMA, BI |
| Elkholy et al. (2014) | NR | Ipsi | 1 | 2 G | NR×18 | 6 wk | TUG, FMA, speed |
| Forogh et al. (2017) | F8 | Contra-M1 | 1 | 90 RMT | 1200×5 | 5 d | FMA, BBS |
| Guan et al. (2017) | F8 | Ipsi-M1 | 5 | 120 MT | 2000×10 | 10 d | FMA, BI |
| Huang et al. (2018) | Double-cone | Contra-M1 | 1 | 120 AMT | 900×15 | 3 wk | TUG, FMA, BI |
| Ji et al. (2014) | F8 | Ipsi-hotspot | 10 | NR | 1500×18 | 6 wk | Speed |
| Ji and Kim (2015) | F8 | Ipsi-hotspot | 10 | NR | 2000×20 | 4 wk | Speed |
| Kakuda et al. (2013) | Double-cone | Bi-M1-LL | 10 | 90 RMT | 2000×2 | 2 d | Speed |
| Kim et al. (2014b) | F8 | Ipsi-cerebellar | 1 | 100 RMT | 900×5 | 5 d | Speed, BBS |
| Koch et al. (2019) | F8 | Contra-cerebellar | iTBS | 80 AMT | 1200×15 | 3 wk | BBS, FMA, BI, speed, MEP |
| Lin et al. (2015) | F8 | Contra-M1-LL | 1 | 130 MT | 900×15 | 15 d | BI, TUG, FMA, BI |
| Lin et al. (2019) | F8 | Bi-M1-LL | iTBS | 100 MT | 1200×10 | 5 wk | BBS, TUG, speed, FMA, BI |
| Liu et al. (2019) | NR | Contra-M1/Ipsi-M1 | 0.5/10 | 80 MT/80 MT | 600×15/12000×15 | 3 wk | FMA, MEP |
| Meng and Song (2017) | F8 | Contra-M1 | 1 | 90 MT | 1800×14 | 14 d | BI, FMA |
| Rastgoo et al. (2016) | F8 | Contra-M1-LL | 1 | 90 MT | 1000×5 | 5 d | FMA, TUG |
| Wang et al. (2012) | F8 | Contra-M1-LL | 1 | 90 RMT | 600×10 | 2 wk | FMA, MEP, speed, |
| Wang et al. (2016) | F8 | Contra-M1 | 1 | 80 RMT | 900×20 | 4 wk | FML, BBS |
| Wang et al. (2019) | F8 | Vertex | 5 | 90 RMT | 900×9 | 3 wk | FMA, speed, MEP |
| Zhao et al. (2018) | F8 | Contra-M1 | 1 | 80–120 RMT | 1000×20 | 20 d | FMA, BBS |
AMT: Active motor threshold; BBS: Berg Balance Scale; BI: Barthel Index; bi: bilateral; contra: contralateral; F8: figure of 8; FMA: Fugl-Meyer assessment; H: H-coil; ipsi: ipsilateral; iTBS: intermittent theta-burst stimulation; M1: primary motor cortex; M1-LL: primary motor cortex of lower limb; MEP: motor evoked potential; MT: motor threshold; NR: not reported; RMT: resting motor threshold; rTMS: repetitive transcranial magnetic stimulation; TUG: Timed Up and Go Test.
Quality assessment
The quality levels of all involved studies were appraised as good to excellent according to the PEDro results (Additional Table 1). All included studies specified eligibility criteria, and a majority of the studies were determined to be at low risk of bias for random allocation, blinding of outcome assessment, and incomplete outcome data, although they had a high risk of bias for concealed allocation. Of all included studies, 50% presented blinding of participants and outcome assessments, 88% reported adequate random sequence generation, and 77% performed intention-to-treat analysis, showing a low risk of bias. Only 30% presented blinding of therapists, and 27% presented allocation concealment.
Additional Table 1.
Risk of bias assessment according to Physiotherapy Evidence Database scale
| Study | Eligibility criteria specified (0/1) | Random allocation (0/1) | Concealed allocation (0/1) | Comparable at baseline (0/1) | Blinded subjects (0/1) | Blinded therapists (0/1) | Blinded assessors (0/1) | Adequate follow-up (0/1) | Intention-totreat analysis (0/1) | Between group comparisons (0/1) | Point estimates and variability (0/1) | Summary |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cha et al. (2014) | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 8 |
| Cha and Kim (2015) | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Cha and Kim (2017) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Chang et al. (2010) | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 9 |
| Chen (2018) | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 7 |
| Chieffo et al. (2014) | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 6 |
| Choi et al. (2016) | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Du et al. (2016) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| Elkholy et al. (2014) | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Forogh et al. (2017) | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Guan et al. (2017) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| Huang et al. (2018) | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 8 |
| Ji et al.(2014) | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Ji and Kim (2015) | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Kakuda et al. (2013) | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Kim et al. (2014b) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Koch et al. (2019) | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 8 |
| Lin et al.(2015) | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 7 |
| Lin et al. (2019) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| Liu et al. (2019) | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 8 |
| Meng and Song (2017) | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Rastgoo et al. (2016) | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Wang et al. (2012) | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 9 |
| Wang et al. (2016) | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Wang et al.(2019) | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 10 |
| Zhao et al. (2018) | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
0 indicates the criterion was not satisfied; 1 the criterion was satisfied.
According to the GRADE system, the FMA was assessed as being high-quality evidence, while speed outcome was classified as moderate-quality evidence. In contrast, MEP amplitude and BI outcomes were classified as low-quality evidence (Additional Table 2).
Additional Table 2.
Levels of evidence according to Grades of Recommendation, Assessment, Development, and Evaluation scale
| Certainty assessment | No. of patients | Effect | Certainty | Importance | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | TMS | TMS Sham | Relative (95% CI) | Absolute (95% CI) | |||||||
| Motor function (follow up: range 1 weeks to 3 months; assessed with: Fugl-Meyer assessment) | |||||||||||||||||
| 18 | Randomised trials | Seriousa | Not serious | Not serious | Not serious | All plausible residual confounding would reduce the demonstrated effect | 431 | 411 | - | SMD 0.27 SD higher (0.09 higher to 0.45 higher) | ⊕⊕⊕⊕HIGH | CRITICAL | |||||
| Speed (follow up: range 1 months to 3 months; assessed with: walking speed) | |||||||||||||||||
| 11 | Randomised trials | Seriousa | Not serious | Not serious | Not serious | None | 169 | 138 | - | SMD 0.63 SD higher (0.3 higher to 0.96 higher) | ⊕⊕⊕ΟMODERATE | IMPORTANT | |||||
| Balance (assessed with: Berg Balance scale and Timed up and go test) | |||||||||||||||||
| 12 | Randomised trials | Seriousa | Seriousb | Not serious | Not serious | None | 360 | 338 | - | SMD 0.39 SD higher (0.11 higher to 0.67 higher) | ⊕⊕ΟΟ LOW | IMPORTANT | |||||
| Motor evoked potential amplitude (assessed with: motor evoked potential) | |||||||||||||||||
| 6 | Randomised trials | Seriousa | Seriousc | Not serious | Not serious | None | 83 | 83 | - | SMD 0.32 SD higher (0.02 lower to 0.67 higher) | ⊕⊕ΟΟ LOW | IMPORTANT | |||||
| Barthel Index (assessed with: Barthel Index) | |||||||||||||||||
| 8 | Randomised | Seriousa | Seriousd | Not serious | Not serious | None | 133 | 127 | - | SMD 0.87 | ⊕⊕ΟΟ | IMPORTANT | |||||
| trials | SD higher (0.39 higher to 1.36 higher) | LOW | |||||||||||||||
a. In general, the studies had poor methodological rigor, with few blinded studies. b. I-squared is equal to 61%. c. I-squared is equal to 66%. d. I-squared is equal to 74%. CI: Confidence interval; SMD: standardised mean difference; SD: standard deviation.
Relative effects and relative rankings of interventions
Details regarding convergence and priors are given in Additional Figure 1 (3.8MB, tif) .
FMA
The NMA for lower extremity motor recovery included 18 RCTs. According to the direct evidence, only LF-rTMS was significantly more effective than sham simulation with respect to motor function (SMD, 0.34; 95% CI, 0.11 to 0.58) (Additional Table 3). In contrast, dTMS, HF-rTMS, and iTBS appeared to have no better efficacy than sham stimulation (SMD, 0.01 [95% CI, –0.91 to 0.94]; SMD, 0.16 [95% CI, –0.29 to 0.61]; and SMD, 0.40 [95% CI, –0.67 to 1.47]; respectively).
Additional Table 3.
Pairwise meta-analysis results of Fugl-Meyer assessment.
| Study | SMD (95%CI) |
|---|---|
| dTMS vs. Sham | |
| Chieffo et al. (2014) | 0.01 (-0.91,0.94) |
| HF vs. LF | |
| Duetal. (2016) | -0.03 (-0.60, 0.55) |
| Liuetal. (2019) | 0.06 (-0.59,0.71) |
| Summary | 0.01 (-0.42, 0.45) |
| HF vs. Sham | τ2=0.13,I2=49% |
| Chang et al. (2010) | -0.05 (-0.82, 0.72) |
| Duetal. (2016) | 0.34 (-0.25, 0.92) |
| Guan et al. (2017) | -0.48 (-1.09, 0.14) |
| Liuetal. (2019) | 0.77(0.09, 1.45) |
| Wang et al. (2019) | 0.32 (-0.75, 1.38) |
| Summary | 0.16 (-0.29, 0.61) |
| iTBS vs. Sham | τ2=0.43, I2=72% |
| Kochetal. (2019) | 0.91 (0.20, 1.62) |
| Linetal. (2019) | -0.18 (-1.06, 0.70) |
| Summary | 0.40 (-0.67, 1.47) |
| LF vs. Sham | τ2=0.07, I2=46% |
| Chen (2018) | 0.50 (0.26, 0.74) |
| Duetal. (2016) | 0.34 (-0.25, 0.92) |
| Elkholy et al. (2014) | 1.07 (0.41, 1.74) |
| Forogh et al. (2017) | -0.99 (-1.82,-0.17) |
| Huang etal. (2018) | -0.14 (-0.77, 0.50) |
| Linetal. (2015) | 0.13 (-0.56, 0.83) |
| Linetal. (2019) | 0.71 (0.03, 1.38) |
| Meng and Song (2017) | 0.61 (-0.29, 1.51) |
| Rastgoo et al. (2016) | 0.38 (-0.25, 1.00) |
| Wang et al. (2012) | 0.16 (-0.64, 0.96) |
| Wang et al. (2016) | 0.44 (-0.28, 1.17) |
| Zhao etal. (2018) | 0.42 (-0.04, 0.88) |
| Summary | 0.34 (0.11,0.58) |
Heterogeneity standard deviation (τ2) has been estimated using the methods of moments and is reported only for comparisons for which is estimable and larger than 0. CI: Confidence interval; dTMS: deep transcranial magnetic stimulation; HF: high-frequency repetitive transcranial magnetic stimulation; iTBS: intermittent theta-burst stimulation; LF: low-frequency repetitive transcranial magnetic stimulation; SMD: standardized mean difference.
On the basis of the NMA results, there were no significant differences between interventions on motor function. After carefully scanning the quality of the included studies, we conducted a sensitivity analysis by excluding one study (Forogh et al., 2017) whose baseline characteristics were significantly different between the experimental and control groups. Results from the NMA then indicated that LF-rTMS (SMD, 2.28; 95% CrI, 0.92 to 3.62) was more effective than sham, which was consistent with the direct evidence. In contrast, HF-rTMS (SMD, 0.85; 95% CrI, –0.98 to 2.80), iTBS (SMD, 2.53; 95% CrI, –1.46 to 6.68), and dTMS (SMD, 0.55; 95% CrI, –6.48 to 7.04) were not more effective than sham (Table 3), similar to the results from the direct evidence. Additionally, no active interventions appeared to exert a better effect than any other intervention group.
Table 3.
Relative effects estimated from the network meta-analysis and from a sensitivity analysis comparing every pair of the five interventions with respect to the Fugl-Meyer assessment
| HF | 1.02 (–1.72, 3.64) | –0.44 (–2.87, 1.96) | –0.39 (–5.88, 4.84) | 2.32 (–3.05, 7.74) |
| –1.48 (–3.52, 0.79) | LF | –1.46 (–3.27, 0.28) | –1.45 (–6.38, 3.71) | 1.30 (–3.73, 6.53) |
| 0.85 (–0.98, 2.80) | 2.28 (0.92, 3.62) | Sham | –0.00 (–4.70, 4.86) | 2.79 (–1.95, 7.65) |
| 0.34 (–6.47, 7.65) | 1.81 (–4.93, 8.94) | –0.55 (–7.04, 6.48) | dTMS | 2.70 (–3.92, 9.71) |
| –1.63 (–6.17, 2.75) | –0.19 (–4.66, 4.04) | –2.53 (–6.68, 1.46) | –2.23 (–10.04, 5.89) | iTBS |
Upper triangle: network meta-analysis; lower triangle: sensitivity analysis. dTMS: Deep transcranial magnetic stimulation; HF: high-frequency repetitive transcranial magnetic stimulation; iTBS: intermittent theta-burst stimulation; LF: low-frequency repetitive transcranial magnetic stimulation.
Speed
For the secondary outcome of speed, 11 RCTs (307 participants) were included. The outcomes from the pairwise meta-analysis suggested a significant difference between HF-rTMS and sham stimulation (SMD, 0.70; 95% CI, 0.37 to 1.03). However, dTMS, LF-rTMS, and iTBS were not significantly more effective than sham stimulation (SMD, 0.15 [95% CI, –0.77 to 1.08]; SMD, 0.91 [95% CI, –0.01 to 1.83]; and SMD, –0.36 [95% CI, –1.24 to 0.53]; respectively) (Additional Table 4). The NMA for speed indicated that there were no significant differences between the different interventions (Additional Table 5).
Additional Table 4.
Pairwise meta-analysis results of speed.
| Study | SMD (95%CI) |
|---|---|
| dTMS vs. Sham | |
| Chieffo et al. (2014) | 0.15 (-0.77,1.08) |
| HF vs. Sham | |
| Cha and Kim (2017) | 0.95 (0.02,1.89) |
| Cha and Kim (2015) | 0.75 (0.01,1.49) |
| Ji et al. (2014) | 0.77(0.01,1.53) |
| Ji et al. (2015) | 0.75 (0.10,1.41) |
| Kakudaetal. (2013) | 0.28 (-0.65,1.21) |
| Wang et al. (2019) | 0.57 (-0.52,1.66) |
| Summary | 0.70 (0.37,1.03) |
| iTBS vs. Sham | |
| Linetal. (2019) | -0.36 (-1.24,0.53) |
| LF vs. Sham | τ2=0.51,τ2=77% |
| Elkholy et al. (2014) | 1.76 (1.03,2.48) |
| Kim et al. (2014b) | 0.74 (-0.09,1.58) |
| Wang et al. (2012) | 0.22 (-0.53, 0.97) |
| Summary | 0.91 (-0.01,1.83) |
Heterogeneity standard deviation (τ2)has been estimated using the methods of moments and is reported only for comparisons for which is estimable and larger than 0. CI: Confidence interval; dTMS: deep transcranial magnetic stimulation; HF: high-frequency repetitive transcranial magnetic stimulation; iTBS: intermittent theta-burst stimulation; LF: low-frequency repetitive transcranial magnetic stimulation; SMD: standardized mean difference.
Additional Table 5.
Relative effects estimated from the network meta-analysis with respect to speed and motor evoked potential amplitude
| HF | -0.27 (-0.58, -0.04) | -0.19 (-0.47, 0.00) | ||
| -0.24 (-1.04,0.54) | LF | 0.07 (-0.15,0.30) | ||
| 0.00 (-0.39,0.41) | 0.25 (-0.44, 0.93) | Sham | ||
| -0.66 (-3.66,2.66) | -0.42 (-3.43,2.96) | -0.66 (-3.60,2.69) | dTMS | |
| 3.42 (-3.64, 13.88) | 3.63 (-3.44, 14.13) | 3.41 (-3.68, 13.89) | 4.19 (-3.96,15.49) | |
| iTBS |
Upper triangle: Network meta-analysis. Lower triangle: Sensitivity analysis. Heterogeneity standard deviation (τ2) has been estimated using the methods of moments and is reported only for comparisons for which is estimable and larger than 0. dTMS: Deep transcranial magnetic stimulation; HF: high-frequency repetitive transcranial magnetic stimulation; iTBS: intermittent theta-burst stimulation; LF: low-frequency repetitive transcranial magnetic stimulation.
Balance
For the investigation of balance, 13 RCTs were included. Direct evidence indicated that LF-rTMS was significantly more effective than both HF-rTMS (SMD, 1.23; 95% CI, 0.34 to 2.12) and sham (SMD, 0.28; 95% CI, 0.03 to 0.52) (Additional Table 6). Furthermore, the NMA suggested that HF-rTMS was more effective than LF-rTMS in the inconsistency model (SMD, 8.54; 95% CrI, 0.72 to 17.40). There was no significant difference in the improvement of balance between HF-rTMS, LF-rTMS, or iTBS compared with sham (SMD, 3.31 [95% CrI, –3.41 to 10.43]; SMD, 2.07 [95% CrI, –0.75 to 4.22]; and SMD, 5.59 [95% CrI, –0.58 to 10.06]; respectively) (Additional Table 7).
Additional Table 6.
Pairwise meta-analysis results of balance
| Study | SMD (95%CI) |
|---|---|
| LF vs. HF | |
| Cha et al. (2014) | 1.23 (0.34,2.12) |
| HF vs. Sham | |
| Choietal. (2016) | 0.14 (-0.58, 0.86) |
| iTBS vs. Sham | τ2=1.05,I2=85% |
| Kochetal. (2019) | 1.73 (0.93,2.54) |
| Linetal. (2019) | -0.08 (-0.95, 0.80) |
| Linetal. (2019) | -0.20 (-1.08,0.68) |
| Summary | 0.50 (-0.76, 1.76) |
| LF vs. Sham | τ2=0.04, I2=37% |
| Chen (2018) | 0.01 (-0.22, 0.25) |
| Elkholy et al. (2014) | 0.93 (0.28,1.58) |
| Forogh et al. (2017) | 0.38 (-0.39, 1.16) |
| Huang etal. (2018) | 0.22 (-0.42, 0.86) |
| Kim et al. (2014b) | -0.11 (-0.86,0.64) |
| Rastgoo et al. (2016) | 0.04 (-0.58, 0.66) |
| Wang et al. (2016) | 0.48 (-0.25, 1.21) |
| Zhao etal. (2018) | 0.55 (0.09,1.01) |
| Summary | 0.28 (0.03,0.52) |
Heterogeneity standard deviation (τ2) has been estimated using the methods of moments and is reported only for comparisons for which is estimable and larger than 0. CI: Confidence interval; dTMS: deep transcranial magnetic stimulation; HF: high-frequency repetitive transcranial magnetic stimulation; iTBS: intermittent theta-burst stimulation; LF: low-frequency repetitive transcranial magnetic stimulation; SMD: standardized mean difference.
Additional Table 7.
Relative effects estimated from the network meta-analysis with respect to balance and Barthel Index
| HF | 6.32 (-8.39,23.60) | -7.16 (-14.81, 0.55) | 19.18 (-1.01, 38.45) |
| 8.54 (0.72, 17.40) | LF | -3.55 (-12.76,2.89) | 13.22 (-2.78, 23.37) |
| 3.31 (-3.41, 10.43) | 2.07 (-0.75, 4.22) | Sham | 16.77(5.47, 25.33) |
| 5.26 (-4.26, 15.63) | -3.62 (-8.62,2.62) | -5.59 (-10.06,0.58) | iTBS |
Upper triangle: Network meta-analysis with respect to Barthel Index. Lower triangle: Network meta-analysis with respect to balance. Heterogeneity standard deviation (τ2) has been estimated using the methods of moments and is reported only for comparisons for which is estimable and larger than 0. HF: High-frequency repetitive transcranial magnetic stimulation; iTBS: intermittent theta-burst stimulation; LF: low-frequency repetitive transcranial magnetic stimulation.
The results of the node-splitting analysis suggested an inconsistency in the NMA relative to balance (Additional Table 8). The direct and indirect evidence between HF-rTMS versus LF-rTMS, HF-rTMS versus sham, and LF-rTMS versus sham were not in agreement (inconsistency factor, 1.24 [95% CrI, 0.14 to 5.07]; 1.31 [95% CrI, 0.11 to 4.87]; and 1.43 [95% CrI, 0.24 to 5.30]; respectively). We therefore closely examined the potential effect modifiers of the included studies, and conducted a subgroup analysis by separating the BBS and TUG results. This analysis revealed no significant associations with balance of HF-rTMS, LF-rTMS, or iTBS relative to sham, for either BBS or TUG.
Additional Table 8.
Node-splitting approach for balance
| Name | Direct effect | Indirect effect | Overall | Median | P-value |
|---|---|---|---|---|---|
| HF, LF | -11.12 (-19.49,-2.10) | 1.57 (-5.88, 7.77) | -3.47 (-10.89, 1.59) | 1.24 (0.14,5.07) | 0.03 |
| HF, Sham | -1.04 (-7.50,5.00) | -14.40 (-23.10, -5.36) | -5.11 (-11.88, 0.15) | 1.31 (0.11,4.87) | 0.03 |
| LF, Sham | -2.22 (-4.31,0.25) | 10.78 (-0.26,21.60) | -1.62 (-4.04, 1.35) | 1.43 (0.24,5.30) | 0.03 |
HF: High-frequency repetitive transcranial magnetic stimulation; LF: low-frequency repetitive transcranial magnetic stimulation.
MEP amplitude
The NMA for the MEP of corticospinal excitability contained five RCTs. The NMA model of the competing interventions for MEP amplitude suggested that HF-rTMS was significantly more effective than LF-rTMS (SMD, 0.27; 95% CrI, 0.04 to 0.58) and sham (SMD, 0.19; 95% CrI, 0.00 to 0.47) (Additional Table 5). The direct relative effects indicated that HF-rTMS performed better than LF-rTMS (SMD, 0.77; 95% CI, 0.15 to 1.38) (Additional Table 9), which is in accordance with the results of the NMA.
Additional Table 9.
Pairwise meta-analysis results of motor evoked potential amplitude
| Study | SMD (95%CI) |
|---|---|
| HF vs. LF | τ=0.17,I2=59% |
| Chaetal. (2014) | 1.45 (0.53,2.37) |
| Duetal. (2016) | 0.82 (0.21, 1.42) |
| Duetal. (2016) | 0.27 (-0.31,0.85) |
| Summary | 0.77 (0.15, 1.38) |
| HF vs. Sham | τ2=0.25,I2=59% |
| Chaand Kim (2017) | 1.72 (0.66,2.78) |
| Duetal. (2016) | 0.85 (0.25, 1.46) |
| Duetal. (2016) | 0.22 (-0.36, 0.80) |
| Wang et al. (2019) | 0.00 (-1.06, 1.06) |
| Wang et al. (2019) | -0.19 (-1.25, 0.87) |
| Summary | 0.52 (-0.06, 1.10) |
| LF vs. Sham | τ2=0.13, I2=52% |
| Duetal. (2016) | 0.19 (-0.39, 0.77) |
| Duetal. (2016) | -0.10 (-0.67,0.48) |
| Huang et al. (2018) | 0.56 (-0.26, 1.38) |
| Wang et al. (2012) | -1.02 (-1.88, -0.16) |
| Wang et al. (2012) | -0.32 (-0.96, 0.32) |
| Summary | -0.11 (-0.55,0.33) |
Heterogeneity standard deviation (τ2) has been estimated using the methods of moments and is reported only for comparisons for which is estimable and larger than 0. CI: Confidence interval; HF: high-frequency repetitive transcranial magnetic stimulation; LF: low-frequency repetitive transcranial magnetic stimulation; SMD: standardized mean difference.
BI
Direct evidence suggested that HF-rTMS and LF-rTMS were more effective than sham for improving BI (SMD, 0.83 [95% CI, 0.09 to 1.56] and SMD, 0.63 [95% CI, 0.28 to 0.97], respectively). In contrast, iTBS was not significantly different compared with sham (SMD, 1.55; 95% CI, –1.36 to 4.46) (Additional Table 10).
Additional Table 10.
Pairwise meta-analysis results of Barthel Index
| Study | SMD (95%CI) |
|---|---|
| HF vs. Sham | τ=0.29, I2=70% |
| Chang et al. (2010) | 1.56 (0.67,2.45) |
| Duetal. (2016) | 0.90 (0.29, 1.51) |
| Guan et al. (2017) | 0.18 (-0.42, 0.79) |
| Summary | 0.83 (0.09, 1.56) |
| iTBS vs. Sham | τ4.17, I2=95% |
| Kochetal. (2019) | 3.05 (2.03,4.07) |
| Linetal. (2019) | 0.08 (-0.80, 0.96) |
| Summary | 1.55 (-1.36,4.46) |
| LF vs. Sham | |
| Duetal. (2016) | 0.90 (0.29, 1.51) |
| Huang et al. (2018) | 0.31 (-0.33, 0.96) |
| Linetal. (2015) | 0.46 (-0.25, 1.16) |
| Meng and Song (2017) | 0.95 (0.01, 1.88) |
| Summary | 0.63 (0.28, 0.97) |
Heterogeneity standard deviation (τ2) has been estimated using the methods of moments and is reported only for comparisons for which is estimable and larger than 0. CI: Confidence interval; HF: high-frequency repetitive transcranial magnetic stimulation; iTBS: intermittent theta-burst stimulation; LF: low-frequency repetitive transcranial magnetic stimulation; SMD: standardized mean difference.
The NMA for activities of daily life contained eight RCTs, and the results indicated that iTBS was significantly more effective than sham (SMD, 16.77; 95% CrI, 5.47 to 25.33). There was no evidence to suggest that other active treatments were more powerful than sham stimulation (Additional Table 7).
Assessment of statistical heterogeneity and inconsistency
According to the results of the node-splitting method for statistical inconsistency, the direct evidence and indirect evidence were not significantly consistent for balance, but they were consistent for motor function, speed, MEP amplitude, and activities of daily life.
Small study effects
No publication bias was observed among the included studies for the FMA using the Egger’s test (P = 0.159) (Additional Figure 2A (3.4MB, tif) ). The comparison-adjusted funnel plots appeared symmetrical for both speed (P = 0.248) and balance (P = 0.132), suggesting that small studies had similar effects compared with large studies regarding speed and balance functions (Additional Figure 2B (3.4MB, tif) and C (3.4MB, tif) ).
Discussion
To the best of our knowledge, this is the first NMA to explore the effects of TMS on lower extremity motor function, and it is currently the most comprehensive review. This systematic review and NMA of TMS for patients with stroke included data from 26 RCTs, including 943 participants who were randomized to one of four rTMS interventions (deep, high-frequency, low-frequency, and intermittent theta-burst rTMS) or sham stimulation. Only LF-rTMS was superior to sham stimulation for motor function improvement, as measured by the FMA. Although direct evidence suggested that HF-rTMS was more effective than sham stimulation for speed, this result was not replicated in the NMA. In addition, HF-rTMS appeared to be more effective than LF-rTMS for MEP amplitudes.
The quality of the evidence used for the primary outcome was typically categorized as high quality. Nonetheless, the summary treatment effect estimates were imprecise for most comparisons. Furthermore, there was large uncertainty regarding novel treatments or those with little or no sham-controlled trials. Therefore, there was no conclusive evidence of the superiority of any particular intervention.
LF-rTMS was superior to sham stimulation with respect to motor function. Evidence-based guidelines on the therapeutic use of rTMS also recommend LF-rTMS, applied to the contralesional motor cortex, in the chronic phase of stroke recovery (Lefaucheur et al., 2014). This intervention can theoretically reduce contralesional cortical excitability and thus increase ipsilesional activity under the mechanism of long-term depression. Ueda et al. (2019) reported that intra-voxel directional coherence was greatly increased in some white matter structures bordering on lesioned regions after intervention, suggesting that white matter participates in the motor recovery of stroke patients after LF-rTMS interventions (Takeuchi et al., 2008; Bolognini and Ro, 2010).
Previous standardized meta-analyses have drawn contradictory conclusions regarding balance function and the lower extremity subscale of the FMA in patients with stroke. Tung et al. (2019) revealed the benefit of rTMS on walking speed but not on balance function, which was consistent with previous results (Li et al., 2018). Moreover, Tung et al. (2019) demonstrated improvement in the lower extremity subscale of the FMA after rTMS intervention. In contrast, Ghayour-Najafabadi et al. (2019) reported that rTMS improved balance function but did not have a positive effect on the lower extremity subscale of the FMA. In addition, none of these meta-analyses provided an overall treatment hierarchy of the different modalities of rTMS. The findings of the present NMA in terms of our primary outcome—motor function measured by the FMA—are in line with a previous systematic review and meta-analysis examining the effects of rTMS on walking and balance function after stroke (Li et al., 2018). Furthermore, we found that LF-rTMS was superior to other rTMS interventions. However, the findings regarding speed in the present NMA do not support those of previous standard meta-analyses, which might stem from an insufficient number of studies exploring the effects of novel forms of TMS.
Direct evidence demonstrated the superiority of HF-rTMS versus LF-rTMS for MEP amplitude, and network evidence revealed that HF-rTMS was also preferable to sham. The magnitude of MEP amplitude is considered to be a measure of corticospinal excitability, which denotes the strength or physiological integrity of the corticospinal pathway (Rothwell et al., 1999). Typically, the MEP amplitude of stroke patients lacking voluntary motor control is smaller than that of healthy individuals. Nevertheless, the MEP is an inherently variable measure, and can be affected by the subtle position of the coil or by multiple converging inhibitory and excitatory inputs (Maeda et al., 2000; Carroll et al., 2001). Larger high-quality RCTs are therefore needed to further explore the effectiveness of HF-rTMS. In future studies, the disruption of experimental operations should be minimized by maintaining a consistency of the participants’ characteristics and a stable position of the coil.
In the present study, there was statistical inconsistency in the network for balance function, which suggests an inappropriate combination of different measurements (BBS and TUG). Moreover, there were inadequate numbers of studies to explore the efficacy of TMS on balance, and more RCTs are needed to confirm its effectiveness. In the future, researchers should unify the outcome measurements used for balance. We did not investigate the ranking probability of these outcomes here because only a few trials reported these data.
TBS, a novel form of TMS that lasts approximately 5 minutes only, is potentially useful because of its short session duration and induction of neuroplasticity. However, few studies have investigated the efficacy of TBS. Cerebellar iTBS might be a potential treatment to improve balance and gait functions in patients with stroke because the cerebellum is involved in motor control (Popa et al., 2010; Manto et al., 2012) through the disynaptic cerebello-thalamocortical pathway (Bostan et al., 2013). The results of our NMA suggested that iTBS may be beneficial for improving the activities of daily life; this finding merits further clinical investigation.
Finally, dTMS was not more effective than sham stimulation, according to our statistical approach. dTMS is delivered using the Hesed coil, which can effectively stimulate deep brain regions without bringing about greater stimulation of the superficial cortical regions (Roth et al., 2002; Zangen et al., 2005). Various experiments have explored the efficacy of dTMS in recent years (Kranz et al., 2010; Harel et al., 2011; Levkovitz et al., 2011). Nonetheless, the exact coil configuration and placement may vary with different applications, and there is scarce evidence to demonstrate its effectiveness in patients with stroke. More high-quality RCTs investigating the efficacy of dTMS are needed in the near future.
Our study had several limitations. Most studies presented an unclear risk of bias on allocation concealment, which is a renowned methodological drawback in rTMS interventions. Properly assigning concealment, preventing contamination bias, and reporting all results would have improved the included studies. Furthermore, some nodes were not well connected, which may lead to the inaccurate estimation of relative effects, especially when comparing different active interventions. Nevertheless, iTBS and dTMS lacked sufficient evidence to support their effects in stroke patients, and more controlled studies should be conducted to confirm their effectiveness.
The available data suggested that there are differences in clinical effects between the different rTMS modalities, but this was unable to be confirmed. Our findings suggest that LF-rTMS can improve motor function and that HF-rTMS can increase MEP amplitude. Moreover, this finding implies that LF-rTMS is superior to other interventions for stroke rehabilitation. Novel forms of rTMS interventions (dTMS and iTBS) were not more effective than sham stimulation. However, there is little available evidence for rTMS interventions other than LF-rTMS and HF-rTMS. Hence, new high-quality RCTs for novel rTMS interventions are needed to establish their efficacy with higher reliability. In the future, we encourage clinical therapists to include LF-rTMS as a supplementary therapy for stroke rehabilitation in clinical practice.
Additional files:
Additional file 1: PRISMA 2009 checklist.
Additional file 2 (93.3KB, pdf) : Search strategy.
Search strategy
Additional file 3: Open peer reviewer reports 1 (127.9KB, pdf) and 2 (96.5KB, pdf) .
Additional Table 1: Risk of bias assessment according to Physiotherapy Evidence Database scale.
Additional Table 2: Levels of evidence according to Grades of Recommendation, Assessment, Development, and Evaluation scale.
Additional Table 3: Pairwise meta-analysis results of Fugl-Meyer assessment.
Additional Table 4: Pairwise meta-analysis results of speed.
Additional Table 5: Relative effects estimated from the network meta-analysis with respect to speed and motor evoked potential amplitude.
Additional Table 6: Pairwise meta-analysis results of balance.
Additional Table 7: Relative effects estimated from the network meta-analysis with respect to balance and Barthel Index.
Additional Table 8: Node-splitting approach for balance.
Additional Table 9: Pairwise meta-analysis results of motor evoked potential amplitude.
Additional Table 10: Pairwise meta-analysis results of Barthel Index.
Additional Figure 1 (3.8MB, tif) : Trace plot and density plot.
Trace plot and density plot.
The potential scale reduction factor (PSRF) were 1.00, 1.32 and 1.01 separately for Fugl-Meyer assessment, speed and balance. The PSRF is less than 1.2 can be acceptable, which means simulated observations are close to the target distribution. Heterogeneity standard deviation (τ2) has been estimated using the methods of moments and is reported only for comparisons for which is estimable and larger than 0. dTMS: Deep transcranial magnetic stimulation; HF: high-frequency repetitive transcranial magnetic stimulation; iTBS: intermittent theta-burst stimulation; LF: low-frequency repetitive transcranial magnetic stimulation; sd: standard deviation
Additional Figure 2 (3.4MB, tif) : Comparison-adjusted funnel plot of Fugl-Meyer assessment (A), speed (B), and balance (C).
Comparison-adjusted funnel plot of Fugl-Meyer assessment (A), speed (B), and balance (C).
Heterogeneity standard deviation (τ2) has been estimated using the methods of moments and is reported only for comparisons for which is estimable and larger than 0.
Footnotes
P-Reviewers: Arya K, Bai Y; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Gatdner B, Yu J, Song LP; T-Editor: Jia Y
Conflicts of interest: None declared.
Financial support: This work was supported by the 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University, China, No. 2020HXFH051 (to QG). The funding source had no role in study conception and design, data analysis or interpretaton,paper writing or deciding to submit this paper for publicaton.
Reporting statement: This work followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Kamal Arya, Institute for the Physically Handicapped, Occupational Therapy India; Yulong Bai, Huashan Hospital, Fudan University, China.
Funding: This work was supported by the 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University, China, No. 2020HXFH051 (to QG).
References
- 1.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O’Connell D, Oxman AD, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benussi A, Dell’Era V, Cantoni V, Turrone R, Pilotto A, Alberici A, Cotelli MS, Rizzetti C, Padovani A, Borroni B. Stimulation over the cerebellum with a regular figure-of-eight coil induces reduced motor cortex inhibition in patients with progressive supranuclear palsy. Brain Stimul. 2019;12:1290–1297. doi: 10.1016/j.brs.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Bolognini N, Ro T. Transcranial magnetic stimulation: disrupting neural activity to alter and assess brain function. J Neurosci. 2010;30:9647–9650. doi: 10.1523/JNEUROSCI.1990-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 5.Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013;17:241–254. doi: 10.1016/j.tics.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brignardello-Petersen R, Mustafa RA, Siemieniuk RAC, Murad MH, Agoritsas T, Izcovich A, Schünemann HJ, Guyatt GH. GRADE approach to rate the certainty from a network meta-analysis: addressing incoherence. J Clin Epidemiol. 2019;108:77–85. doi: 10.1016/j.jclinepi.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Brignardello-Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B, Hazlewood GS, Alhazzani W, Mustafa RA, Murad MH, Puhan MA, Schünemann HJ, Guyatt GH. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. 2018;93:36–44. doi: 10.1016/j.jclinepi.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434–455. [Google Scholar]
- 9.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 10.Calautti C, Leroy F, Guincestre JY, Baron JC. Dynamics of motor network overactivation after striatocapsular stroke: a longitudinal PET study using a fixed-performance paradigm. Stroke. 2001;32:2534–2542. doi: 10.1161/hs1101.097401. [DOI] [PubMed] [Google Scholar]
- 11.Carroll TJ, Riek S, Carson RG. Reliability of the input-output properties of the cortico-spinal pathway obtained from transcranial magnetic and electrical stimulation. J Neurosci Methods. 2001;112:193–202. doi: 10.1016/s0165-0270(01)00468-x. [DOI] [PubMed] [Google Scholar]
- 12.Cha HG, Kim MK. The effects of repetitive transcranial magnetic stimulation integrated mirror therapy on the gait of chronic stroke patients. J Magn. 2015;20:133–137. [Google Scholar]
- 13.Cha HG, Kim MK. Effects of strengthening exercise integrated repetitive transcranial magnetic stimulation on motor function recovery in subacute stroke patients: A randomized controlled trial. Technol Health Care. 2017;25:521–529. doi: 10.3233/THC-171294. [DOI] [PubMed] [Google Scholar]
- 14.Cha HG, Kim MK, Nam HC, Ji SG. Effects of high frequency repetitive transcranial magnetic stimulation on function in subacute stroke patients. J Magn. 2014;19:192–196. [Google Scholar]
- 15.Chang WH, Kim YH, Bang OY, Kim ST, Park YH, Lee PK. Long-term effects of rTMS on motor recovery in patients after subacute stroke. J Rehabil Med. 2010;42:758–764. doi: 10.2340/16501977-0590. [DOI] [PubMed] [Google Scholar]
- 16.Chen YJ. Effects of repetitive transcranial magnetic stimulation on spasm and motor function of lower limbs in patients with stroke. Chongqing Yixue. 2018;47:3292-3295,3298. [Google Scholar]
- 17.Chieffo R, Prezzo SD, Houdayer E, Nuara A, Straffi L, Spagnolo F, Libera DD, Maggio GD, Coppi E, Ferrari L, Sessa M, Comola M, Zangen A, Comi G, Leocani L. 56, Effects of deep repetitive transcranial magnetic stimulation (rTMS) on motor function of paretic lower limb in chronic sub-cortical stroke: A pilot study. Clin Neurophysiol. 2013;124:e201. [Google Scholar]
- 18.Chieffo R, De Prezzo S, Houdayer E, Nuara A, Di Maggio G, Coppi E, Ferrari L, Straffi L, Spagnolo F, Velikova S, Sessa M, Comola M, Zangen A, Comi G, Leocani L. Deep repetitive transcranial magnetic stimulation with H-coil on lower limb motor function in chronic stroke: a pilot study. Arch Phys Med Rehabil. 2014;95:1141–1147. doi: 10.1016/j.apmr.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 19.Chinese Society of Neuroscience and Chinese Neurosurgical Society (1996) Diagnosis of various cerebrovascular diseases. Zhonghua Shenjingke Zazhi. 29:379–380. [Google Scholar]
- 20.Choi CM, Kim JH, Lee JK, Lee BY, Kee HS, Jung KI, Yoon SR. Effects of repetitive transcranial magnetic stimulation over trunk motor spot on balance function in stroke patients. Ann Rehabil Med. 2016;40:826–834. doi: 10.5535/arm.2016.40.5.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55:129–133. doi: 10.1016/s0004-9514(09)70043-1. [DOI] [PubMed] [Google Scholar]
- 22.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 23.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33:607–617. doi: 10.1177/0272989X12458724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du J, Tian L, Liu W, Hu J, Xu G, Ma M, Fan X, Ye R, Jiang Y, Yin Q, Zhu W, Xiong Y, Yang F, Liu X. Effects of repetitive transcranial magnetic stimulation on motor recovery and motor cortex excitability in patients with stroke: a randomized controlled trial. Eur J Neurol. 2016;23:1666–1672. doi: 10.1111/ene.13105. [DOI] [PubMed] [Google Scholar]
- 25.Elkholy SH, Atteya AA, Hassan WA, Sharaf M, Gohary AME. Low rate repetitive transcranial magnetic stimulation (rTMS) and gait rehabilitation after stroke. Int J Neurorehabilitation. 2014;1:275–280. [Google Scholar]
- 26.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2:121–145. doi: 10.1177/096228029300200202. [DOI] [PubMed] [Google Scholar]
- 27.Forogh B, Ahadi T, Nazari M, Sajadi S, Abdul Latif L, Akhavan Hejazi SM, Raissi G. The effect of repetitive transcranial magnetic stimulation on postural stability after acute stroke: a clinical trial. Basic Clin Neurosci. 2017;8:405–411. doi: 10.18869/nirp.bcn.8.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghayour-Najafabadi M, Memari AH, Hosseini L, Shariat A, Cleland JA. Repetitive transcranial magnetic stimulation for the treatment of lower limb dysfunction in patients poststroke: a systematic review with meta-analysis. J Stroke Cerebrovasc Dis. 2019;28:104412. doi: 10.1016/j.jstrokecerebrovasdis.2019.104412. [DOI] [PubMed] [Google Scholar]
- 29.Gu P, Zhang ZX, Ma QY, Geng Y, Wang YY, Zhang LN, Wang MW. Transcranial magnetic stimulation promotes proliferation of endogenous neural stem cells of Parkinson’s disease model mice. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:7939–7946. [Google Scholar]
- 30.Guan YZ, Li J, Zhang XW, Wu S, Du H, Cui LY, Zhang WH. Effectiveness of repetitive transcranial magnetic stimulation (rTMS) after acute stroke: A one-year longitudinal randomized trial. CNS Neurosci Ther. 2017;23:940–946. doi: 10.1111/cns.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hankey GJ. Stroke. Lancet. 2017;389:641–654. doi: 10.1016/S0140-6736(16)30962-X. [DOI] [PubMed] [Google Scholar]
- 32.Harel EV, Zangen A, Roth Y, Reti I, Braw Y, Levkovitz Y. H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World J Biol Psychiatry. 2011;12:119–126. doi: 10.3109/15622975.2010.510893. [DOI] [PubMed] [Google Scholar]
- 33.Harrington A, Hammond-Tooke GD. Theta burst stimulation of the cerebellum modifies the TMS-evoked N100 potential, a marker of GABA inhibition. PLoS One. 2015;10:e0141284. doi: 10.1371/journal.pone.0141284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions, Version 5.10 Cochrane Collaboration. 2011 [Google Scholar]
- 35.Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3:98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang YZ, Lin LF, Chang KH, Hu CJ, Liou TH, Lin YN. Priming with 1-Hz repetitive transcranial magnetic stimulation over contralesional leg motor cortex does not increase the rate of regaining ambulation within 3 months of stroke: a randomized controlled trial. Am J Phys Med Rehabil. 2018;97:339–345. doi: 10.1097/PHM.0000000000000850. [DOI] [PubMed] [Google Scholar]
- 37.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 38.Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis. It all depends on the distribution of effect modifiers. BMC Med. 2013;11:159. doi: 10.1186/1741-7015-11-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji SG, Kim MK. The effects of repetitive transcranial magnetic stimulation on the gait of acute stroke patients. J Magn. 2015;20:129–132. [Google Scholar]
- 40.Ji SG, Cha HG, Kim KJ, Kim MK. Effects of motor imagery practice in conjunction with repetitive transcranial magnetic stimulation on stroke patients. J Magn. 2014;19:181–184. [Google Scholar]
- 41.Kakuda W, Abo M, Nakayama Y, Kiyama A, Yoshida H. High-frequency rTMS using a double cone coil for gait disturbance. Acta Neurol Scand. 2013;128:100–106. doi: 10.1111/ane.12085. [DOI] [PubMed] [Google Scholar]
- 42.Kim H, Lee G, Song C. Effect of functional electrical stimulation with mirror therapy on upper extremity motor function in poststroke patients. J Stroke Cerebrovasc Dis. 2014a;23:655–661. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 43.Kim WS, Jung SH, Oh MK, Min YS, Lim JY, Paik NJ. b Effect of repetitive transcranial magnetic stimulation over the cerebellum on patients with ataxia after posterior circulation stroke: A pilot. J Rehabil Med. 2014b;46:418–423. doi: 10.2340/16501977-1802. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 45.Koch G, Bonnì S, Casula EP, Iosa M, Paolucci S, Pellicciari MC, Cinnera AM, Ponzo V, Maiella M, Picazio S, Sallustio F, Caltagirone C. Effect of cerebellar stimulation on gait and balance recovery in patients with hemiparetic stroke: a randomized clinical trial. JAMA Neurol. 2019;76:170–178. doi: 10.1001/jamaneurol.2018.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kranz G, Shamim EA, Lin PT, Kranz GS, Hallett M. Transcranial magnetic brain stimulation modulates blepharospasm: a randomized controlled study. Neurology. 2010;75:1465–1471. doi: 10.1212/WNL.0b013e3181f8814d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lavados PM, Hennis AJ, Fernandes JG, Medina MT, Legetic B, Hoppe A, Sacks C, Jadue L, Salinas R. Stroke epidemiology, prevention, and management strategies at a regional level: Latin America and the Caribbean. Lancet Neurol. 2007;6:362–372. doi: 10.1016/S1474-4422(07)70003-0. [DOI] [PubMed] [Google Scholar]
- 48.Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, Devanne H, Di Lazzaro V, Filipovic SR, Hummel FC, Jääskeläinen SK, Kimiskidis VK, Koch G, Langguth B, Nyffeler T, Oliviero A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin Neurophysiol. 2014;125:2150–2206. doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 49.Leucht S, Chaimani A, Cipriani AS, Davis JM, Furukawa TA, Salanti G. Network meta-analyses should be the highest level of evidence in treatment guidelines. Eur Arch Psychiatry Clin Neurosci. 2016;266:477–480. doi: 10.1007/s00406-016-0715-4. [DOI] [PubMed] [Google Scholar]
- 50.Levkovitz Y, Rabany L, Harel EV, Zangen A. Deep transcranial magnetic stimulation add-on for treatment of negative symptoms and cognitive deficits of schizophrenia: a feasibility study. Int J Neuropsychopharmacol. 2011;14:991–996. doi: 10.1017/S1461145711000642. [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Fan J, Yang J, He C, Li S. Effects of repetitive transcranial magnetic stimulation on walking and balance function after stroke: a systematic review and meta-analysis. Am J Phys Med Rehabil. 2018;97:773–781. doi: 10.1097/PHM.0000000000000948. [DOI] [PubMed] [Google Scholar]
- 52.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin LF, Chang KH, Huang YZ, Lai CH, Liou TH, Lin YN. Simultaneous stimulation in bilateral leg motor areas with intermittent theta burst stimulation to improve functional performance after stroke: a feasibility pilot study. Eur J Phys Rehabil Med. 2019;55:162–168. doi: 10.23736/S1973-9087.18.05245-0. [DOI] [PubMed] [Google Scholar]
- 54.Lin YN, Hu CJ, Chi JY, Lin LF, Yen TH, Lin YK, Liou TH. Effects of repetitive transcranial magnetic stimulation of the unaffected hemisphere leg motor area in patients with subacute stroke and substantial leg impairment: A pilot study. J Rehabil Med. 2015;47:305–310. doi: 10.2340/16501977-1943. [DOI] [PubMed] [Google Scholar]
- 55.Liu CM, Meng Y, Li HH, Zhang GQ. Effects of repetitive transcranial magnetic stimulation on motor function and serum levels of MMP-9/hs-CRP of ischemic stroke patients. Tianjin Yiyao. 2019;47:184–188. [Google Scholar]
- 56.Madden LV, Piepho HP, Paul PA. Statistical models and methods for network meta-analysis. Phytopathology. 2016;106:792–806. doi: 10.1094/PHYTO-12-15-0342-RVW. [DOI] [PubMed] [Google Scholar]
- 57.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133:425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- 58.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–721. [PubMed] [Google Scholar]
- 59.Manto M, Bower JM, Conforto AB, Delgado-García JM, da Guarda SN, Gerwig M, Habas C, Hagura N, Ivry RB, Mariën P, Molinari M, Naito E, Nowak DA, Oulad Ben Taib N, Pelisson D, Tesche CD, Tilikete C, Timmann D. Consensus paper: roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2012;11:457–487. doi: 10.1007/s12311-011-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mavridis D, Giannatsi M, Cipriani A, Salanti G. A primer on network meta-analysis with emphasis on mental health. Evid Based Ment Health. 2015;18:40–46. doi: 10.1136/eb-2015-102088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meng ZY, Song WQ. Low frequency repetitive transcranial magnetic stimulation improves motor dysfunction after cerebral infarction. Neural Regen Res. 2017;12:610–613. doi: 10.4103/1673-5374.205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ. 2013;346:f2914. doi: 10.1136/bmj.f2914. [DOI] [PubMed] [Google Scholar]
- 63.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 65.Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta-analysis using R: a review of currently available automated packages. PLoS One. 2014;9:e115065. doi: 10.1371/journal.pone.0115065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009;23:641–656. doi: 10.1177/1545968309336661. [DOI] [PubMed] [Google Scholar]
- 67.Peters DM, Fridriksson J, Stewart JC, Richardson JD, Rorden C, Bonilha L, Middleton A, Gleichgerrcht E, Fritz SL. Cortical disconnection of the ipsilesional primary motor cortex is associated with gait speed and upper extremity motor impairment in chronic left hemispheric stroke. Hum Brain Mapp. 2018;39:120–132. doi: 10.1002/hbm.23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Popa T, Russo M, Meunier S. Long-lasting inhibition of cerebellar output. Brain Stimul. 2010;3:161–169. doi: 10.1016/j.brs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 69.Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, Kessels AG, Guyatt GH. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 70.Rastgoo M, Naghdi S, Nakhostin Ansari N, Olyaei G, Jalaei S, Forogh B, Najari H. Effects of repetitive transcranial magnetic stimulation on lower extremity spasticity and motor function in stroke patients. Disabil Rehabil. 2016;38:1918–1926. doi: 10.3109/09638288.2015.1107780. [DOI] [PubMed] [Google Scholar]
- 71.Robinson CA, Shumway-Cook A, Matsuda PN, Ciol MA. Understanding physical factors associated with participation in community ambulation following stroke. Disabil Rehabil. 2011;33:1033–1042. doi: 10.3109/09638288.2010.520803. [DOI] [PubMed] [Google Scholar]
- 72.Roever L, Biondi-Zoccai G. Network meta-analysis to synthesize evidence for decision making in cardiovascular research. Arq Bras Cardiol. 2016;106:333–337. doi: 10.5935/abc.20160052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roth Y, Zangen A, Hallett M. A coil design for transcranial magnetic stimulation of deep brain regions. J Clin Neurophysiol. 2002;19:361–370. doi: 10.1097/00004691-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 74.Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology Electroencephalogr. Clin Neurophysiol Suppl. 1999;52:97–103. [PubMed] [Google Scholar]
- 75.Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med. 2017;12:103–111. doi: 10.1007/s11739-016-1583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3:80–97. doi: 10.1002/jrsm.1037. [DOI] [PubMed] [Google Scholar]
- 77.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 78.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 79.Shim SR, Kim SJ, Lee J, Rücker G. Network meta-analysis: application and practice using R software. Epidemiol Health. 2019;41:e2019013. doi: 10.4178/epih.e2019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Strzalkowski NDJ, Chau AD, Gan LS, Kiss ZHT. Both 50 and 30 Hz continuous theta burst transcranial magnetic stimulation depresses the cerebellum. Cerebellum. 2019;18:157–165. doi: 10.1007/s12311-018-0971-0. [DOI] [PubMed] [Google Scholar]
- 81.Takeuchi N, Tada T, Toshima M, Chuma T, Matsuo Y, Ikoma K. Inhibition of the unaffected motor cortex by 1 Hz repetitive transcranical magnetic stimulation enhances motor performance and training effect of the paretic hand in patients with chronic stroke. J Rehabil Med. 2008;40:298–303. doi: 10.2340/16501977-0181. [DOI] [PubMed] [Google Scholar]
- 82.Toft N, Innocent GT, Gettinby G, Reid SW. Assessing the convergence of Markov Chain Monte Carlo methods: an example from evaluation of diagnostic tests in absence of a gold standard. Prev Vet Med. 2007;79:244–256. doi: 10.1016/j.prevetmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Tung YC, Lai CH, Liao CD, Huang SW, Liou TH, Chen HC. Repetitive transcranial magnetic stimulation of lower limb motor function in patients with stroke: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2019;33:1102–1112. doi: 10.1177/0269215519835889. [DOI] [PubMed] [Google Scholar]
- 84.Turner L, Shamseer L, Altman DG, Schulz KF, Moher D. Does use of the CONSORT Statement impact the completeness of reporting of randomised controlled trials published in medical journals. A Cochrane review Syst Rev. 2012;1:60. doi: 10.1186/2046-4053-1-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ueda R, Yamada N, Abo M, Senoo A. White matter changes follow low-frequency repetitive transcranial magnetic stimulation plus intensive occupational therapy for motor paralysis after stroke: a DTI study using TBSS. Acta Neurol Belg. 2019 doi: 10.1007/s13760-019-01150-2. doi: 101007/s13760-019-01150-2. [DOI] [PubMed] [Google Scholar]
- 86.van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012;3:285–299. doi: 10.1002/jrsm.1054. [DOI] [PubMed] [Google Scholar]
- 87.Wang RY, Wang FY, Huang SF, Yang YR. High-frequency repetitive transcranial magnetic stimulation enhanced treadmill training effects on gait performance in individuals with chronic stroke: A double-blinded randomized controlled pilot trial. Gait Posture. 2019;68:382–387. doi: 10.1016/j.gaitpost.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 88.Wang RY, Tseng HY, Liao KK, Wang CJ, Lai KL, Yang YR. rTMS combined with task-oriented training to improve symmetry of interhemispheric corticomotor excitability and gait performance after stroke: a randomized trial. Neurorehabil Neural Repair. 2012;26:222–230. doi: 10.1177/1545968311423265. [DOI] [PubMed] [Google Scholar]
- 89.Wang XW, Liang JF, Wen ZY, Chen HB, Feng CR, Zhang XF. The effect of repetitive transcranial magnetic stimulation combined with functional electrical stimulation on the lower extremity function of hemiplegia after stroke. Shijie Zuixin Yixue Xinxi Wenzhai. 2016;16:58. [Google Scholar]
- 90.Warren DL, Geneva AJ, Lanfear R. RWTY (R We There Yet): An R package for examining convergence of bayesian phylogenetic analyses. Mol Biol Evol. 2017;34:1016–1020. doi: 10.1093/molbev/msw279. [DOI] [PubMed] [Google Scholar]
- 91.White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3:111–125. doi: 10.1002/jrsm.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, Deruyter F, Eng JJ, Fisher B, Harvey RL, Lang CE, MacKay-Lyons M, Ottenbacher KJ, Pugh S, Reeves MJ, Richards LG, Stiers W, Zorowitz RD. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:e98-e169. doi: 10.1161/STR.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 93.Yang YW, Pan WX, Xie Q. Combined effect of repetitive transcranial magnetic stimulation and physical exercise on cortical plasticity. Neural Regen Res. 2020;15:1986–1994. doi: 10.4103/1673-5374.282239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zangen A, Roth Y, Voller B, Hallett M. Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin Neurophysiol. 2005;116:775–779. doi: 10.1016/j.clinph.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 95.Zappasodi F, Tecchio F, Marzetti L, Pizzella V, Di Lazzaro V, Assenza G. Longitudinal quantitative electroencephalographic study in mono-hemispheric stroke patients. Neural Regen Res. 2019;14:1237–1246. doi: 10.4103/1673-5374.251331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao XL, Liu TL, Zhou YX, Zhang LX. The effect of repetitive transcranial magnetic stimulation on dyskinesia in stroke patients. Zhongguo Kangfu Yixue Zazhi. 2018;33:800–805. [Google Scholar]
- 97.Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, Li X, Wang L, Wang L, Liu Y, Liu J, Zhang M, Qi J, Yu S, Afshin A, Gakidou E, Glenn S, Krish VS, Miller-Petrie MK, Mountjoy-Venning WC, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394:1145–1158. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy
Trace plot and density plot.
The potential scale reduction factor (PSRF) were 1.00, 1.32 and 1.01 separately for Fugl-Meyer assessment, speed and balance. The PSRF is less than 1.2 can be acceptable, which means simulated observations are close to the target distribution. Heterogeneity standard deviation (τ2) has been estimated using the methods of moments and is reported only for comparisons for which is estimable and larger than 0. dTMS: Deep transcranial magnetic stimulation; HF: high-frequency repetitive transcranial magnetic stimulation; iTBS: intermittent theta-burst stimulation; LF: low-frequency repetitive transcranial magnetic stimulation; sd: standard deviation
Comparison-adjusted funnel plot of Fugl-Meyer assessment (A), speed (B), and balance (C).
Heterogeneity standard deviation (τ2) has been estimated using the methods of moments and is reported only for comparisons for which is estimable and larger than 0.


