Keywords: ascorbic acid, axon, macrophage, myelin, peripheral nerve injury, phagocytosis, Schwann cell, Wallerian degeneration
Abstract
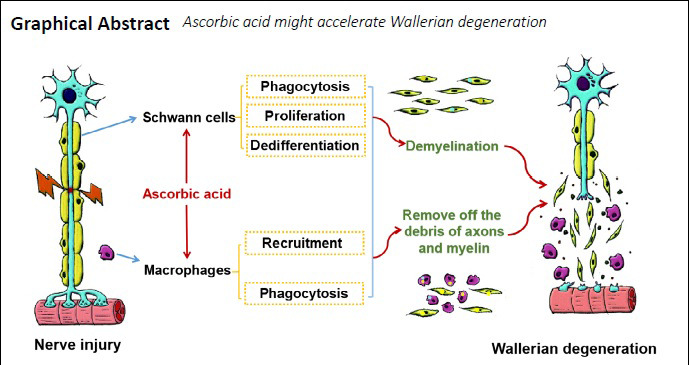
Wallerian degeneration occurs after peripheral nerve injury and provides a beneficial microenvironment for nerve regeneration. Our previous study demonstrated that ascorbic acid promotes peripheral nerve regeneration, possibly through promoting Schwann cell proliferation and phagocytosis and enhancing macrophage proliferation, migration, and phagocytosis. Because Schwann cells and macrophages are the main cells involved in Wallerian degeneration, we speculated that ascorbic acid may accelerate this degenerative process. To test this hypothesis, 400 mg/kg ascorbic acid was administered intragastrically immediately after sciatic nerve transection, and 200 mg/kg ascorbic acid was then administered intragastrically every day. In addition, rat sciatic nerve explants were treated with 200 μM ascorbic acid. Ascorbic acid significantly accelerated the degradation of myelin basic protein-positive myelin and neurofilament 200-positive axons in both the transected nerves and nerve explants. Furthermore, ascorbic acid inhibited myelin-associated glycoprotein expression, increased c-Jun expression in Schwann cells, and increased both the number of macrophages and the amount of myelin fragments in the macrophages. These findings suggest that ascorbic acid accelerates Wallerian degeneration by accelerating the degeneration of axons and myelin in the injured nerve, promoting the dedifferentiation of Schwann cells, and enhancing macrophage recruitment and phagocytosis. The study was approved by the Southern Medical University Animal Care and Use Committee (approval No. SMU-L2015081) on October 15, 2015.
Chinese Library Classification No. R453; R722.14+4; Q564
Introduction
Wallerian degeneration refers to a progressive anterograde disintegration of axons and accompanying demyelination, and results from injury to nerve fibers in both the peripheral and central nervous systems (Chang et al., 2017). In the first stages of peripheral nerve injury, axonal and myelin debris are produced as myelin ovoids in the cytoplasm and paranodal loops (Tricaud and Park, 2017; Lin et al., 2019). In addition, Schwann cells dedifferentiate into an immature state (Jessen and Mirsky, 2008), which results in the downregulated expression of myelin-associated proteins, such as myelin-associated glycoprotein (MAG), peripheral myelin protein 22, and myelin protein zero (Parkinson et al., 2008). Schwann cell dedifferentiation also leads to the upregulated expression of c-Jun, sex determining region Y-box 2, and p75 neurotrophin receptor, among other proteins (Wen et al., 2018; Nocera and Jacob, 2020). In the later stages of peripheral nerve injury, hematogenous macrophages are recruited to the injured nerve (Tricaud and Park, 2017), where they collaborate with Schwann cells to remove myelin and axon debris; this produces an advantageous microenvironment for nerve regeneration (Yu et al., 2016). Subsequently, Schwann cells proliferate and migrate in line to form bands of Büngner, secrete neurotrophins to promote axonal regrowth, and remyelinate the regenerated axons (Arthur-Farraj et al., 2012; Jessen et al., 2015; Nocera and Jacob, 2020). Many studies have reported that accelerating Wallerian degeneration can promote repair after peripheral nerve injury (Elberg et al., 2019; Loring and Thompson, 2020); thus, accelerating Wallerian degeneration is regarded as a promising strategy for nerve regeneration.
Ascorbic acid is an essential dietary micronutrient (Granger and Eck, 2018) that has long been used clinically for treating colds, cancer, and many other diseases. Recent studies have indicated that ascorbic acid has many unexpected novel biological functions, including the promotion of reprogramming and differentiation in stem cells (Wang et al., 2011; Cimmino et al., 2018), the prevention of sperm DNA damage and male infertility (Ilic et al., 2018), and the treatment of Charcot-Marie-Tooth disease type 1 (Verhamme et al., 2009). We recently reported that ascorbic acid can promote the functional repair of the injured peripheral nerve (Li et al., 2019), mainly by promoting axonal regeneration and remyelination, thus accelerating both nerve conduction and motor and sensory function recovery, as well as alleviating myoatrophy in the targeted muscles. Furthermore, ascorbic acid treatment enhanced the proliferation and phagocytosis capabilities of Schwann cells and macrophages in this previous study. Considering that both Schwann cells (Ji et al., 2019; Gu et al., 2020) and macrophages (Liu et al., 2019) play important roles in Wallerian degeneration, we hypothesized that ascorbic acid might accelerate Wallerian degeneration. Herein, we used an in vivo rat model of sciatic nerve transection injury and an in vitro model of sciatic nerve explants to obtain experimental data to verify this hypothesis.
Materials and Methods
Animals
The study was approved by the Southern Medical University Animal Care and Use Committee (approval No. SMU-L2015081) on October 15, 2015. In this study, 32 specific-pathogen-free female Sprague-Dawley rats, weighing 200–250 g and aged 7–8 weeks, were provided by the Animal Center of Southern Medical University, China (license No. SCXK (Yue) 2016-0041). The experimental animals were housed under a 12-hour light/dark cycle and given free access to water and food. Of the 32 rats, 24 were used for the in vivo experiments of sciatic nerve transection, while the other 8 rats were used for sciatic nerve collection and nerve explant culture.
Sciatic nerve transection surgery and drug treatment
The 24 rats were randomly divided into the saline and ascorbic acid groups (n = 12/group). All rats underwent sciatic nerve transection surgery based on previously published protocols (Qian et al., 2018; Zhou et al., 2018). Briefly, the rats were intraperitoneally injected with 12 mg/mL tribromoethanol (180 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) as an anesthetic. The sciatic nerve was exposed and then cut at 5 mm below the piriformis muscle. Immediately after surgery, the rats in the ascorbic acid group were intragastrically administered a solution of ascorbic acid (400 mg/kg suspended in saline at a concentration of 20 mg/mL; Baiyunshan Pharmaceuticals Company, Guangzhou, China). They were then administered ascorbic acid at a dose of 200 mg/kg per day for 5 or 8 days. The animals in the saline group were administered the same volume (2 mL) of saline as a control. The drug administration method strictly complied with previous protocols (Liu et al., 2016; Li et al., 2019). At 5 or 8 days post-injury (dpi), the rats were sacrificed and the distal trunks of the injured nerves were collected for immunohistochemistry and western blot assay experiments.
In vitro culture of nerve explants
The in vitro cultures of nerve explants were performed according to previous publications (Shin et al., 2013; Park et al., 2015), with minor modifications. Briefly, after rats were anesthetized with tribromoethanol (180 mg/kg by intraperitoneal injection), the sciatic nerves were collected under aseptic conditions and cut into segments with lengths of 5 mm. Next, the nerve segments were explanted in a culture dish and incubated with basic medium, which consisted of Dulbecco’s modified Eagle’s medium/F12 (Corning, New York, NY, USA) containing 3% fetal bovine serum (Corning), 10 ng/mL heregulin (PeproTech, Rocky Hill, NJ, USA), 3 mM forskolin (Sigma-Aldrich), and 100 mg/mL penicillin-streptomycin (Gibco, Grand Island, NY, USA). The explant cultures were divided into the ascorbic acid group and the control group. In the ascorbic acid group, ascorbic acid was added to the basic culture medium with a final concentration of 200 μM. The same volume of solvent used for the ascorbic acid solution was added into the basic medium of the control group. After being cultured for 5 or 8 days in vitro (div), the explants were collected for immunohistochemistry, western blot assay, and transmission electron microscopy experiments.
Transmission electron microscopy
Sciatic nerve explants cultured for 5 or 8 div (n = 3 per group) were selected for assessment using transmission electron microscopy as previously described (Pan et al., 2017; Wen et al., 2017). Briefly, the sciatic nerve explants were immersed in 2.5% glutaraldehyde for 24 hours, postfixed in 1% osmium tetroxide for 2 hours at 4°C, dehydrated in a graded acetone series, and embedded in Spurr’s resin (Sigma-Aldrich). After ultrathin sectioning (70 nm), the sections were stained with 2% uranyl acetate and lead citrate, and images were captured under a transmission electron microscope (H-7500; Hitachi, Tokyo, Japan). Based on the protocol described by Jang et al. (2016), the numbers of primary ovoid (P)-type nerve fibers and demyelination (D)-type fibers in each image were counted.
Western blot assay
Both the sciatic nerves (with length 1 cm), dissected from the distal trunk of the transected nerves (n = 3 for each group and each time point), and the cultured sciatic nerve explants (n = 5 for each group and each time point) were quickly frozen in liquid nitrogen for 30 seconds before being pulverized and lysed in radioimmunoprecipitation assay buffer (Sigma-Aldrich) with 1% protease inhibitor cocktail (Cell Signaling, Danvers, MA, USA). The proteins were resolved using sodium dodecyl sulfate-polyacrylamide gels (10%) and transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA). After being blocked with 5% bovine serum albumin for 1 hour, the membranes were incubated with the following primary antibodies at 4°C overnight: mouse anti-myelin basic protein (MBP; a marker for myelin; 1:2000; Cat# SMI-99P; BioLegend, San Diego, CA, USA), rabbit anti-neurofilament 200 (NF200; a marker for axon 1:1000; Cat# N4142; Sigma-Aldrich), mouse anti-MAG (a marker for mature Schwann cell; 1:2000; Cat# ab89780; Abcam, Cambridge, MA, USA), mouse anti-c-Jun (a marker for immature Schwann cell; 1:1000; Cat# 630126; BD Biosciences, San Jose, CA, USA), and rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (1:1000; Cat# CW0101M; Cwbiotech, Beijing, China). Next, the blots were incubated with horseradish peroxidase-conjugated anti-rabbit IgG (1:2000; Cat# 31460; Molecular Probes, Eugene, OR, USA) or anti-mouse IgG (1:2000; Cat# 31430; Molecular Probes) at room temperature for 2 hours, and were then visualized using enhanced chemiluminescence. Finally, the images were captured and the band densities were calculated using Image-Pro Plus software (Media Cybernetics, Silver Springs, MD, USA).
Immunofluorescent staining
The nerve explants after 5 or 8 div (n = 10/group at each time point) or the collected distal trunk of the injured nerves from the perfused animals (n = 6/group at each time point) were fixed in 4% paraformaldehyde for 24 hours and treated with 30% sucrose overnight. They were then sectioned into 10 μm slices with a cryostat (Leica, Wetzlar, Germany). Every tenth section was selected for routine immunohistochemistry. First, the sections were permeabilized with 0.5% Triton X-100 (Sigma-Aldrich) for 30 minutes and then blocked with 5% bovine serum albumin (Sigma-Aldrich) for 1 hour at room temperature. Next, the sections were incubated at 4°C overnight with primary antibodies, and then incubated at room temperature for 2 hours with secondary antibodies (goat anti-mouse secondary antibody conjugated to Alexa Fluor® 488, 1:500, Cat# A-11001, Molecular Probes; goat anti-mouse secondary antibody conjugated to Alexa Fluor® 568, 1:500, Cat# A-11004, Molecular Probes; goat anti-rabbit secondary antibody conjugated to Alexa Fluor® 568, 1:500, Cat# A-11011, Molecular Probes). The nuclei were then counterstained with 4’,6-diamidino-2-phenylindole (1:5000; Sigma-Aldrich) for 2 minutes. The following primary antibodies were used: mouse anti-MBP (1:200; Cat# SMI-99P; BioLegend), rabbit anti-NF200 (1:500; Cat# N4142; Sigma), mouse anti-ED1 (CD68; a marker for macrophage; 1:200; Cat# MAC341GA; AbD Serotec, Kidlington, UK), mouse anti-MAG (1:200; Cat# ab89780; Abcam), and rabbit anti-c-Jun (1:400; Cat#630126; BD Biosciences). To investigate macrophage phagocytosis in the injured nerve, ED1-immunostained sections were counterstained with oil red O (ORO; Sigma-Aldrich) solution at room temperature for 15 minutes, followed by routine mounting. The ORO solution was a mixture of three volumes of 0.3% ORO (dissolved in 60% 2-propanol) and two volumes of deionized water (Martella et al., 2014).
Image analysis
After the immunostaining, images of each section were captured under a fluorescence microscope (Leica). Different quantification methods were then performed on the images, as follows. The myelin segment length was taken as the distance between the beginning and the end of the myelin ovoid, discriminated using the MBP immunofluorescence staining. The axonal segment length was labeled with NF200. For quantification, the myelin segment lengths and axonal segment lengths were measured in five visual fields (100 μm × 100 μm) from each section (the center and four quadrants) (Catenaccio et al., 2017). All measurements were performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA) and calibrated microscope images. The numbers of P-type and D-type fibers in degenerating nerves at 5 and 8 div were counted from 10–12 randomly selected transmission electron microscopy images (3000×, 55 μm × 30 μm, n = 3) as previously described (Jang et al., 2016). The numbers of MAG- and c-Jun-positive cells were counted in transverse sections from each sample, 0.5 mm distal to the injury site, from 10–15 photomicrographs per animal; these micrographs were obtained with a light microscope (Nikon, Tokyo, Japan) using a 10× objective (Taniguchi et al., 2009). The numbers of ED1-positive cells in each section were counted at the transection site using ImageJ software. The density of macrophages was defined as the mean number of ED1-positive cells per 0.315 mm2 area (Ito et al., 2014). The number of engulfed myelin debris (ORO-positive) droplets per macrophage (ED1-positive) was also counted (Brosius Lutz et al., 2017). Immunoreactive cells were counted in a blinded manner by two investigators.
Statistical analysis
All obtained data were statistically analyzed using SPSS 23.0 (IBM, Armonk, NY, USA). The data are stated as the mean ± the standard error of the mean (SEM). Independent samples t-tests were used to analyze differences between the two groups. Differences were considered statistically significant when P < 0.05.
Results
Ascorbic acid treatment accelerates axonal and myelin fragmentation in the injured nerve
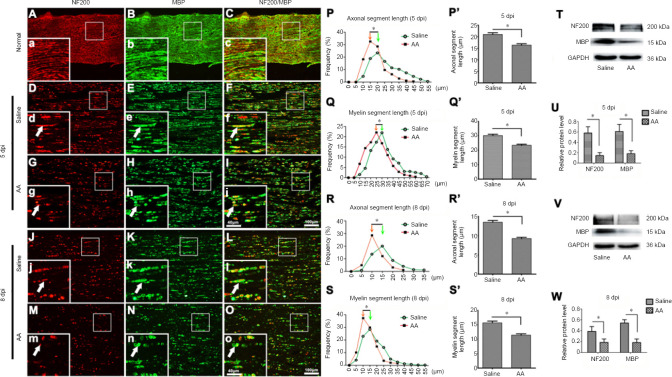
To investigate the fragmentation of axons and myelin—the two main structures of nerve fibers—in the injured nerve, double immunofluorescence staining for NF200 (a specific marker for axons) (Bombeiro et al., 2020) and MBP (a specific marker for myelin) (Liu et al., 2019) was performed on the distal trunk of transected nerves. Under normal conditions, the axon structure of the peripheral nerve is well distributed, and the myelin sheath structure is complete and tightly surrounds the axon (Figure 1A–C). In contrast, both NF200-positive axons and MBP-positive myelin in the injured nerve had disintegrated into discontinuous segments. Moreover, the axonal and myelin fragments in the 8 dpi sections were significantly shorter than in the 5 dpi sections, and the amount of remaining axons and myelin was markedly decreased at 8 dpi compared with 5 dpi (P < 0.05; Figure 1D–O). Compared with the saline group, the ascorbic acid group exhibited much more severe degradation. To quantify the differences between the saline and ascorbic acid groups, the distributions of the axonal and myelin segment lengths were measured as described previously (Catenaccio et al., 2017). These quantification data demonstrated that the lengths of the axonal and myelin segments in the ascorbic acid group showed a significant shift toward lower values compared with the saline group (i.e., the axonal and myelin segments in the ascorbic acid group were significantly shorter) at both 5 and 8 dpi (P < 0.05; Figure 1P–S & P’–S’). These data indicated that ascorbic acid treatment accelerated the fragmentation of axons and myelin in injured nerves. Western blotting was also used to detect the protein levels of NF200 and MBP (Figure 1T–W), revealing that ascorbic acid treatment enhanced the disintegration of NF200 and MBP proteins (P < 0.05).
Figure 1.
Ascorbic acid (AA) enhances the fragmentation of axons and myelin in the injured nerve.
Immediately after sciatic nerve transection surgery, rats were intragastrically administered 400 mg/kg AA, which was followed by 200 mg/kg AA per day for 5 or 8 days. (A–C) Immunohistochemistry showing the axons (NF200, red, Alexa Fluor 568) and myelin sheaths (MBP, green, Alexa Fluor 488) in longitudinal sections of the normal sciatic nerve. (D–O) Immunohistochemistry showing the fragmented axons (NF200, red, Alexa Fluor 568) and myelin sheaths (MBP, green, Alexa Fluor 488) (white arrows) in longitudinal sections of the distal trunk of transected sciatic nerves at 5 or 8 dpi. The lengths of axonal and myelin fragments in the AA group were significantly shorter than in the saline group. (a-o) Higher magnification images of the boxes in A-O. Scale bars: 100 μm in A–O, 40 μm in a–o. (P–S, P’–S’) Quantification data of the length distributions of axons and myelin segments at 5 or 8 dpi. (T–W) Western blot illustrating the relative expression of MBP and NF200 protein at 5 or 8 dpi. The relative expression levels of NF200 and MBP were normalized to GAPDH expression. Data are expressed as the mean ± SEM (n = 3 per group). *P < 0.05 (independent samples t-tests). dpi: Days post-injury; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; MBP: myelin basic protein; NF200: neurofilament 200.
Ascorbic acid promotes Schwann cell dedifferentiation in the injured nerve
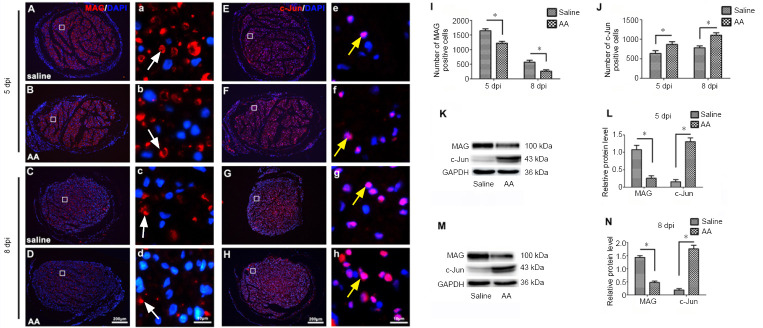
Because the dedifferentiation of Schwann cells plays an important role in Wallerian degeneration after nerve injury (Park et al., 2019), we wanted to investigate whether ascorbic acid treatment was able to alter Schwann cell dedifferentiation in the injured nerve. We therefore performed immunohistochemistry for MAG (a marker of mature Schwann cells) (Elazar et al., 2019) and c-Jun (a marker of immature Schwann cells) (Elazar et al., 2019) on transverse sections of injured nerves from 5 mm distal to the lesion site. As shown in Figure 2A–J, ascorbic acid treatment significantly decreased the density of MAG-positive cells and increased the density of c-Jun-positive cells at both 5 and 8 dpi (P < 0.05). The protein level results from the western blot assays confirmed these findings. Both the blots and statistical analysis revealed that ascorbic acid suppressed MAG expression and promoted c-Jun expression in the injured nerve (P < 0.05; Figure 2K–N).
Figure 2.
Ascorbic acid (AA) enhances Schwann cell dedifferentiation in the injured nerve.
Immediately after sciatic nerve transection surgery, rats were intragastrically administered 400 mg/kg AA, which was followed by 200 mg/kg AA per day for 5 or 8 days. (A–H) Immunochemistry images showing MAG- (white arrows) or c-Jun (yellow arrows)-positive Schwann cells (red, Alexa Fluor 568) in the injured nerves at 5 or 8 dpi. The number of MAG-positive Schwann cells in the AA group was markedly lower than in the saline group, while the number of c-Jun-positive Schwann cells in the AA group was significantly higher than in the saline group. (a-h) Higher magnification images of the boxes in A-H. Scale bars: 200 μm in A–H, 10 μm in a–h. (I, J) Quantification of MAG- or c-Jun-positive cells in sciatic nerve cross sections from each group at 5 or 8 dpi. (K–N) Western blot analysis illustrating MAG and c-Jun protein levels at 5 or 8 dpi. The relative expression levels of MAG and c-Jun were normalized to GAPDH expression. Data are expressed as the mean ± SEM (n = 3 per group). *P < 0.05 (independent samples t-tests). DAPI: 4’,6-Diamidino-2-phenylindole; dpi: days post-injury; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; MAG: myelin-associated glycoprotein.
Ascorbic acid promotes axonal and myelin fragmentation in cultured nerve explants
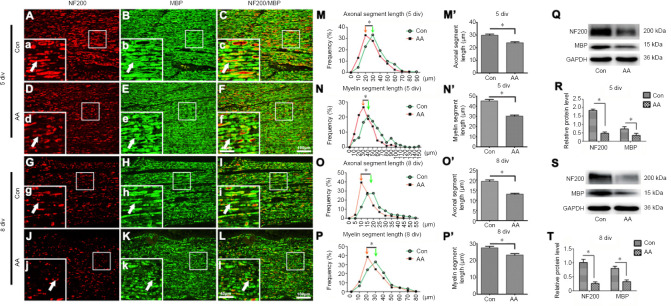
Recently, nerve explant cultures have been demonstrated to be a reliable in vitro model for Wallerian degeneration research, because this model can eliminate unpredictable influences in in vivo experiments (Shin et al., 2013; Park et al., 2015). We therefore tested the outcomes of ascorbic acid treatment on axonal and myelin fragmentation in cultured nerve explants to confirm the results obtained in vivo. Based on the immunofluorescence staining results (Figure 3A–L), the distributions of axon and myelin fragments in the ascorbic acid-treated nerve explants also shifted toward significantly lower values compared with the control group (P < 0.05; Figure 3M–P & M’–P’). Furthermore, western blotting revealed that the levels of MBP and NF200 protein were significantly lower in the ascorbic acid group than in the control group (P < 0.05; Figure 3Q–T). These data confirmed that ascorbic acid can speed up the disintegration of axons and myelin not only in vivo, but also in vitro.
Figure 3.
Ascorbic acid (AA) enhances myelin and axonal fragmentation in cultured nerve explants.
The nerve explants were cultured with 200 μM AA for 5 or 8 days. (A–L) Immunohistochemistry showing fragmented axons (NF200, red, Alexa Fluor 568) and myelin sheaths (MBP, green, Alexa Fluor 488) (white arrows) in longitudinal sections of the distal trunk of transected sciatic nerves at 5 or 8 div. The lengths of axonal and myelin fragments were significantly shorter in the AA group than in the control (Con) group. (a-l) Higher magnification images of the boxes in A-L. Scale bars: 100 μm in A–L, 40 μm in a–l. (M–P, M’–P’) Quantification data showing the length distributions of axons and myelin at 5 or 8 div. (Q–T) Western blot analysis illustrating the MBP and NF200 protein levels at 5 or 8 div. The relative expression levels of NF200 and MBP were normalized to GAPDH expression. Data are expressed as the mean ± SEM (n = 3 per group). *P < 0.05 (independent samples t-tests). div: Days in vitro; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; MBP: myelin basic protein; NF200: neurofilament 200.
Previous reports have suggested that primary ovoid formation may occur after nerve injury (Jang et al., 2016). First, primary ovoids with deformed myelin sheaths occur in nerve fibers, which are termed P-type fibers. As Wallerian degeneration proceeds, the myelin is severely folded or densely packed and enlarged. Fibers in this deteriorated stage are termed D-type fibers (Jang et al., 2016) Figure 4A, B, E, and F). In the present study, the number of P- and D-type nerve fibers were counted and statistically analyzed between the two groups (Figure 4C, D, G, and H). There were fewer P-type nerve fibers (P < 0.05; (Figure 4C) and more D-type nerve fibers in the ascorbic acid group than in the control group at 5 div (P < 0.05; Figure 4D). Moreover, at 8 div, there were also fewer P-type nerve fibers (P < 0.05; Figure 4G) and more D-type nerve fibers (P < 0.05; Figure 4H) in the ascorbic acid group than in the control group. These results further suggest that ascorbic acid can accelerate the fragmentation of myelin during the progression of Wallerian degeneration.
Figure 4.
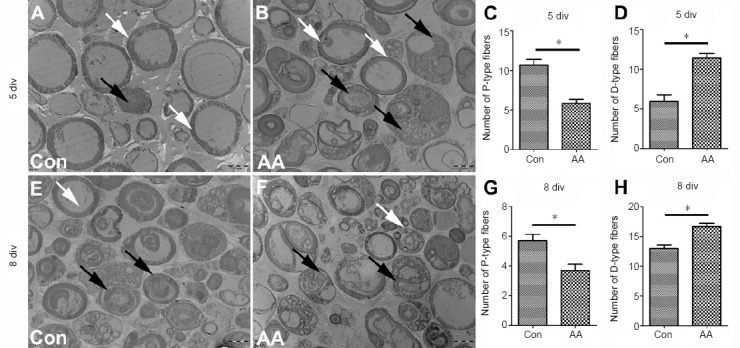
Effects of ascorbic acid (AA) on the primary ovoid (P)- and demyelinating (D)-types of degenerating nerve fibers in cultured nerve explants, detected using transmission electron microscopy.
The nerve explants were cultured with 200 μM AA for 5 or 8 days. (A, B) Transmission electron microscopy images of the nerve explants at 5 div. The number of P-type nerve fibers in the AA group was observably lower than in the control (Con) group, while the number of D-type nerve fibers in the AA group was markedly higher than in the Con group. (C, D) The number of P-type nerve fibers (C) and D-type nerve fibers (D) in sciatic nerve explants at 5 div in a single field at 3000× magnification. (E, F) Transmission electron microscopy images of the sciatic nerve at 8 div. The number of P-type nerve fibers (white arrows) in the AA group was observably lower than in the Con group, while the numbers of D-type nerve fibers (black arrows) in the AA group were markedly higher than in the Con group. Scale bars: 5 μm. (G, H) Statistical analysis of the numbers of P-type nerve fibers (G) and D-type nerve fibers (H) in the sciatic nerve explants at 8 div in each image. Data are expressed as the mean ± SEM (n = 3 per group).*P < 0.05 (independent samples t-tests). div: Days in vitro.
Ascorbic acid promotes Schwann cell dedifferentiation in cultured nerve explants
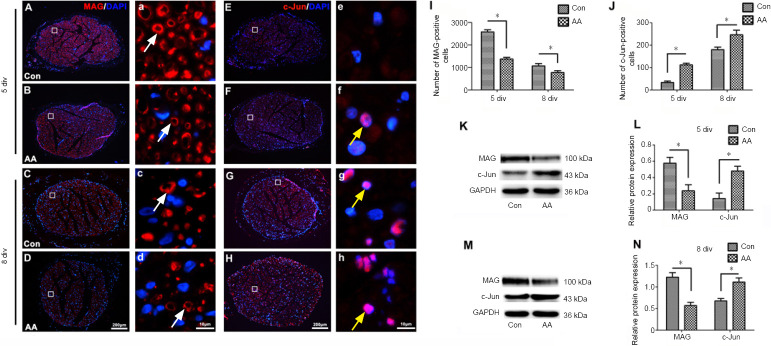
Schwann cell dedifferentiation in the cultured nerve explants was also detected by immunohistochemistry and western blot (Figure 5). Immunofluorescence staining illustrated that MAG-positive cells were significantly decreased and c-Jun-positive cells were significantly increased in the ascorbic acid group compared with the control group (P < 0.05; Figure 5A–J). In addition, western blot assays demonstrated that MAG protein levels were markedly lower in the nerve explants of the ascorbic acid group compared with the control group, while c-Jun protein levels were significantly higher compared with the control group (P < 0.05; Figure 5K–N).
Figure 5.
Ascorbic acid (AA) enhances Schwann cell dedifferentiation in nerve explants.
The nerve explants were cultured with 200 μM AA for 5 or 8 days. (A–H) Immunochemistry images showing MAG- (white arrows) or c-Jun (yellow arrows)-positive Schwann cells (red, Alexa Fluor 568) in the cultured nerve explants at 5 or 8 div. The numbers of MAG-positive Schwann cells in the AA group were markedly lower than in the control (Con) group, while the numbers of c-Jun-positive Schwann cells in the AA group were significantly higher than in the Con group. (a-h) Higher magnification images of the boxes in A-H. Scale bars: 200 μm in A–H, 10 μm in a–h. (I, J) Quantification of MAG- or c-Jun-positive cells in sciatic nerve cross sections from each group at 5 and 8 div. (K–N) Western blot analysis of MAG and c-Jun protein expression at 5 and 8 div. The relative expression levels of MAG and c-Jun were normalized to GAPDH expression. Data are expressed as the mean ± SEM (n = 3 per group). *P < 0.05 (independent samples t-tests). DAPI: 4’,6-Diamidino-2-phenylindole; div: days in vitro; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; MAG: myelin-associated glycoprotein.
Ascorbic acid promotes the infiltration and phagocytosis of macrophages in the injured nerve
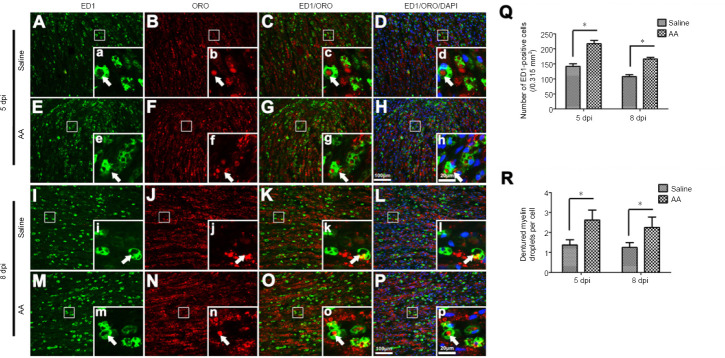
In addition to Schwann cells, macrophages also participate in Wallerian degeneration (Martini et al., 2008; Stratton and Shah, 2016; Zigmond and Echevarria, 2019). Following nerve injury, the dedifferentiated Schwann cells release multiple chemoattractants, including leukemia inhibitory factor, chemokine (C–C motif) ligand 2, pancreatitis-associated protein III, and interleukin-1α and -1β. These factors can recruit macrophages to injured nerves to remove the debris of degenerated axons and myelin (Chen et al., 2015). To detect whether ascorbic acid can affect the infiltration and phagocytosis capabilities of macrophages, sections of injured sciatic nerves were immunostained with anti-ED1 antibodies to illustrate macrophages and were counterstained with ORO to visualize denatured myelin. We were thus able to observe many ED1-positive macrophages scattered throughout the injured nerve; most of these macrophages engulfed ORO-positive myelin debris (Figure 6A–P). With quantification, which was performed based on the protocol by Ito et al. (Ito et al., 2014), we revealed that the numbers of macrophages in the ascorbic acid group were significantly higher than in the saline group, both at 5 and 8 dpi (P < 0.05; Figure 6Q). Meanwhile, macrophage phagocytosis was assessed by the number of ORO-positive myelin debris droplets in each ED1-positive macrophage (Brosius Lutz et al., 2017), and was also higher in the ascorbic acid group than in the saline group at both 5 and 8 dpi (P < 0.05; Figure 6R).
Figure 6.
Ascorbic acid (AA) enhances the infiltration and phagocytosis of macrophages in the injured nerve.
Immediately after sciatic nerve transection surgery, rats were intragastrically administered 400 mg/kg AA, which was followed by 200 mg/kg AA per day for 5 or 8 days. (A–P) ED1 immunofluorescence and ORO counterstaining show macrophages (white arrows) and denatured myelin (white arrows) in the injured nerves at 5 and 8 dpi. The numbers of ED1-positive cells and droplets of denatured myelin per macrophage in the AA group were significantly higher than in the saline group. (a-p) Higher magnification images of the boxes in A-P. Scale bars: 100 μm in A–P, 20 μm in a–p. (Q, R) The numbers of macrophages and droplets of denatured myelin per macrophage at 5 and 8 dpi. Data are expressed as the mean ± SEM (n = 3 per group). *P < 0.05 (independent samples t-tests). dpi: Days post-injury; ED1: CD68; ORO: oil red O.
Discussion
We have recently reported that ascorbic acid treatment promotes the morphological and functional recovery of injured peripheral nerves (Li et al., 2019). Because Wallerian degeneration is essential for nerve regeneration after peripheral nerve injury, we hypothesized that ascorbic acid might play a role in Wallerian degeneration to enhance nerve regeneration. In the present study, the fragmentation of axon and myelin, via the expression of MBP, NF200, c-Jun, and MAG, was used to indicate the level of Wallerian degeneration. We know that axonal regeneration occurs in the crush-injured nerve as early as 3 dpi, even though Wallerian degeneration is still occurring. Regenerating axons and myelin result in the upregulation of NF200, MBP, and MAG, which mean that it is difficult to analyze Wallerian degeneration based on the degradation of these proteins. Therefore, we did not use a nerve crush injury model in the present study, but instead used an in vivo peripheral nerve injury model of sciatic nerve transection and an in vitro model of nerve explant culture. Based on preliminary experiments, we selected 5 and 8 dpi for the assessment of morphological and protein level changes in the in vivo and in vitro samples. Overall, the present data illustrated that ascorbic acid promotes the fragmentation of axons and myelin and the degradation of key axon and myelin proteins (NF200 and MBP).
Following nerve injury, Wallerian degeneration can trigger Schwann cell dedifferentiation (Park et al., 2019). The dedifferentiated Schwann cells can then facilitate Wallerian degeneration by initiating phagocytosis to clear the debris of fragmented axons and myelin (Gomez-Sanchez et al., 2015), and by secreting chemoattractants to recruit macrophages to enhance debris clearance (Chen et al., 2015). The results from the present study demonstrated that ascorbic acid administration increased c-Jun-positive cells and c-Jun protein levels in the injured nerve, whereas MAG levels were decreased. It is well known that c-Jun is highly expressed both in immature Schwann cells during development and in dedifferentiated Schwann cells after nerve injury; however, it is hardly detectable in adult myelinated Schwann cells (Jessen and Mirsky, 2016). In contrast, MAG is a key protein in compact myelin and is widely used as a specific marker for mature Schwann cells. In nerve degeneration and demyelination, MAG is downregulated in dedifferentiated Schwann cells (Nocera and Jacob, 2020). Therefore, the present results from the c-Jun and MAG assessments indicate that ascorbic acid facilitates Schwann cell dedifferentiation in the injured nerve.
Wallerian degeneration is a progressive process in the breakdown of the distal portion of injured nerves. The clearance of axonal and myelin debris is a prerequisite for the subsequent axonal regeneration and remyelination (Namgung, 2014). In the early stage of peripheral nerve injury, removal of axonal and myelin debris is mainly performed by Schwann cells. However, in the later stage, macrophages are the major cells that remove the debris as well as myelin-derived fat (Gaudet et al., 2011; Chen et al., 2015). Because resident macrophages in adult nerves rarely proliferate, the majority of macrophages in injured nerves are recruited from circulating monocytes (Chen et al., 2019). In the present study, ED1 immunostaining revealed that there were more macrophages in the injured nerves with ascorbic acid administration than in the controls, which indicates that ascorbic acid can enhance the migration and infiltration abilities of macrophages. This view is consistent with our previous findings, that adding ascorbic acid to culture medium results in more macrophages migrating through the Transwells (Li et al., 2019). In this previous study, we also detected the phagocytotic capacity of cultured macrophages using a microsphere assay, which indicated that ascorbic acid-treated macrophages can engulf many more microspheres than control-treated cells (Li et al., 2019). In the current study, to investigate whether ascorbic acid can also promote macrophages to clear debris in the injured nerve, we used ORO staining to label degraded and denatured myelin-derived fat. We thus revealed that ED1-positive macrophages in the ascorbic acid group engulfed many more ORO-positive droplets. Interestingly, the present data indicated that degeneration of the injured peripheral nervous system is more rapid in vivo than in explants in vitro when comparing the lengths of segments. We believe that this may be because no exogenous macrophages can be recruited into the in vitro cultured nerve explants to accelerate the Wallerian degeneration.
The methods used were generally appropriate, although relatively superficial. This was particularly true for the quantification of axon and myelin degeneration and for the quantification of macrophage phagocytosis, where more in-depth methods could have been used (such as 3D reconstruction and volume-based techniques, or even stereological methods).
Together, the overall results of the present study indicate that ascorbic acid administration can accelerate Wallerian degeneration in the injured peripheral nerve. This might be attributed to the enhancement by ascorbic acid of the dedifferentiation of Schwann cells and of the infiltration and phagocytosis capabilities of macrophages. The data from the current study can partially explain the mechanisms by which ascorbic acid promotes morphological and functional recovery after sciatic nerve injury in rats. However, a major limitation of this study is that the present data do not thoroughly reveal the underlying mechanisms by which ascorbic acid can accelerate Wallerian degeneration. Further studies need to be carried out in primates to investigate the prospect of using ascorbic acid to treat peripheral nerve injuries in the clinic.
Additional file:
Additional file 1 (527.4KB, pdf) : Original data of the experiment.
Footnotes
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Gardner D, Yu J, Song LP; T-Editor: Jia Y
Funding: This study was supported by the National Natural Science Foundation of China, Nos. 81870982 & 81571182; the Program for Changjiang Scholars and Innovative Research Team in Universities of China, No. IRT-16R37; the National Key Basic Research Program of China, No. 2014CB542202; the Science and Technology Project of Guangdong Province of China, No. 2015A020212024; Key Research & Development Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory of China, No. 2018GZR110104008; the Natural Science Foundation of Guangdong Province of China, No. 2017A030312009, and Research Grant of Guangdong Province Key Laboratory of Psychiatric Disorders of China, No. N201904 (all to JG).
Conflicts of interest: The authors declare that they have no conflicts of interest.
Financial support: This study was supported by the National Natural Science Foundation of China, Nos. 81870982 & 81571182; the Program for Changjiang Scholars and Innovative Research Team in Universities of China, No. IRT-16R37; the National Key Basic Research Program of China, No. 2014CB542202; the Science and Technology Project of Guangdong Province of China, No. 2015A020212024; Key Research & Development Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory of China, No. 2018GZR110104008; the Natural Science Foundation of Guangdong Province of China, No. 2017A030312009, and Research Grant of Guangdong Province Key Laboratory of Psychiatric Disorders of China, No. N201904 (all to JG). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: All procedures involving animals were carried out with the approval of the Southern Medical University Animal Care and Use Committee in accordance with the guidelines for the ethical treatments of animals (approval No. SMU-L2015081) on October 15, 2015. All efforts were made to minimize the number of animals used and their suffering.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bombeiro AL, Pereira BTN, Bonfanti AP, Oliveira ALR. Immunomodulation by dimethyl fumarate treatment improves mouse sciatic nerve regeneration. Brain Res Bull. 2020;160:24–32. doi: 10.1016/j.brainresbull.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Brosius Lutz A, Chung WS, Sloan SA, Carson GA, Zhou L, Lovelett E, Posada S, Zuchero JB, Barres BA. Schwann cells use TAM receptor-mediated phagocytosis in addition to autophagy to clear myelin in a mouse model of nerve injury. Proc Natl Acad Sci U S A. 2017;114:E8072–8080. doi: 10.1073/pnas.1710566114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catenaccio A, Llavero Hurtado M, Diaz P, Lamont DJ, Wishart TM, Court FA. Molecular analysis of axonal-intrinsic and glial-associated co-regulation of axon degeneration. Cell Death Dis. 2017;8:e3166. doi: 10.1038/cddis.2017.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang B, Quan Q, Sun X, Liu RX, Wang Y, Lu SB, Peng J. Wallerian degeneration after peripheral nerve injury: research advance in nerve conduits. Zhongguo Zuzhi Gongcheng Yanjiu. 2017;21:1596–1603. [Google Scholar]
- 6.Chen G, Luo X, Wang W, Wang Y, Zhu F, Wang W. Interleukin-1β promotes schwann cells de-differentiation in wallerian degeneration via the c-JUN/AP-1 pathway. Front Cell Neurosci. 2019;13:304. doi: 10.3389/fncel.2019.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen P, Piao X, Bonaldo P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015;130:605–618. doi: 10.1007/s00401-015-1482-4. [DOI] [PubMed] [Google Scholar]
- 8.Cimmino L, Neel BG, Aifantis I. Vitamin C in stem cell reprogramming and cancer. Trends Cell Biol. 2018;28:698–708. doi: 10.1016/j.tcb.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elazar N, Vainshtein A, Rechav K, Tsoory M, Eshed-Eisenbach Y, Peles E. Coordinated internodal and paranodal adhesion controls accurate myelination by oligodendrocytes. J Cell Biol. 2019;218:2887–2895. doi: 10.1083/jcb.201906099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elberg G, Liraz-Zaltsman S, Reichert F, Matozaki T, Tal M, Rotshenker S. Deletion of SIRPa (signal regulatory protein-a) promotes phagocytic clearance of myelin debris in Wallerian degeneration, axon regeneration, and recovery from nerve injury. J Neuroinflammation. 2019;16:277. doi: 10.1186/s12974-019-1679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Sanchez JA, Carty L, Iruarrizaga-Lejarreta M, Palomo-Irigoyen M, Varela-Rey M, Griffith M, Hantke J, Macias-Camara N, Azkargorta M, Aurrekoetxea I, De Juan VG, Jefferies HB, Aspichueta P, Elortza F, Aransay AM, Martínez-Chantar ML, Baas F, Mato JM, Mirsky R, Woodhoo A, et al. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J Cell Biol. 2015;210:153–168. doi: 10.1083/jcb.201503019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granger M, Eck P. Dietary vitamin C in human health. Adv Food Nutr Res. 2018;83:281–310. doi: 10.1016/bs.afnr.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Gu XK, Li XR, Lu ML, Xu H. Lithium promotes proliferation and suppresses migration of Schwann cells. Neural Regen Res. 2020;15:1955–1961. doi: 10.4103/1673-5374.280324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilic BS, Kolarevic A, Kocic G, Šmelcerovic A. Ascorbic acid as DNase I inhibitor in prevention of male infertility. Biochem Biophys Res Commun. 2018;498:1073–1077. doi: 10.1016/j.bbrc.2018.03.120. [DOI] [PubMed] [Google Scholar]
- 16.Ito T, Kaneko T, Yamanaka Y, Shigetani Y, Yoshiba K, Okiji T. M2 macrophages participate in the biological tissue healing reaction to mineral trioxide aggregate. J Endod. 2014;40:379–383. doi: 10.1016/j.joen.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Jang SY, Shin YK, Park SY, Park JY, Lee HJ, Yoo YH, Kim JK, Park HT. Autophagic myelin destruction by Schwann cells during Wallerian degeneration and segmental demyelination. Glia. 2016;64:730–742. doi: 10.1002/glia.22957. [DOI] [PubMed] [Google Scholar]
- 18.Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- 19.Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol. 2016;594:3521–3531. doi: 10.1113/JP270874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jessen KR, Mirsky R, Lloyd AC. Schwann cells: development and role in nerve repair. Cold Spring Harb Perspect Biol. 2015;7:a020487. doi: 10.1101/cshperspect.a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji XM, Wang SS, Cai XD, Wang XH, Liu QY, Wang P, Cheng ZC, Qian TM. Novel, miR-sc14, promotes Schwann cell proliferation and migration. Neural Regen Res. 2019;14:1651–1656. doi: 10.4103/1673-5374.255996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Li Y, Fan Z, Wang X, Li Z, Wen J, Deng J, Tan D, Pan M, Hu X, Zhang H, Lai M, Guo J. Ascorbic acid facilitates neural regeneration after sciatic nerve crush injury. Front Cell Neurosci. 2019;13:108. doi: 10.3389/fncel.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin YF, Xie Z, Zhou J, Yin G, Lin HD. Differential gene and protein expression between rat tibial nerve and common peroneal nerve during Wallerian degeneration. Neural Regen Res. 2019;14:2183–2191. doi: 10.4103/1673-5374.262602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu GM, Xu K, Li J, Luo YG. Curcumin upregulates S100 expression and improves regeneration of the sciatic nerve following its complete amputation in mice. Neural Regen Res. 2016;11:1304–1311. doi: 10.4103/1673-5374.189196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu P, Peng J, Han GH, Ding X, Wei S, Gao G, Huang K, Chang F, Wang Y. Role of macrophages in peripheral nerve injury and repair. Neural Regen Res. 2019;14:1335–1342. doi: 10.4103/1673-5374.253510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loring HS, Thompson PR. Emergence of SARM1 as a potential therapeutic target for wallerian-type diseases. Cell Chem Biol. 2020;27:1–13. doi: 10.1016/j.chembiol.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martella E, Bellotti C, Dozza B, Perrone S, Donati D, Lucarelli E. Secreted adiponectin as a marker to evaluate in vitro the adipogenic differentiation of human mesenchymal stromal cells. Cytotherapy. 2014;16:1476–1485. doi: 10.1016/j.jcyt.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Martini R, Fischer S, López-Vales R, David S. Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia. 2008;56:1566–1577. doi: 10.1002/glia.20766. [DOI] [PubMed] [Google Scholar]
- 29.Namgung U. The role of Schwann cell-axon interaction in peripheral nerve regeneration. Cells Tissues Organs. 2014;200:6–12. doi: 10.1159/000370324. [DOI] [PubMed] [Google Scholar]
- 30.Nocera G, Jacob C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell Mol Life Sci. 2020 doi: 10.1007/s00018-020-03516-9. doi:101007/s00018-020-03516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan M, Wang X, Chen Y, Cao S, Wen J, Wu G, Li Y, Li L, Qian C, Qin Z, Li Z, Tan D, Fan Z, Wu W, Guo J. Tissue engineering with peripheral blood-derived mesenchymal stem cells promotes the regeneration of injured peripheral nerves. Exp Neurol. 2017;292:92–101. doi: 10.1016/j.expneurol.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Park BS, Kim HW, Rhyu IJ, Park C, Yeo SG, Huh Y, Jeong NY, Jung J. Hydrogen sulfide is essential for Schwann cell responses to peripheral nerve injury. J Neurochem. 2015;132:230–242. doi: 10.1111/jnc.12932. [DOI] [PubMed] [Google Scholar]
- 33.Park HT, Kim JK, Tricaud N. The conceptual introduction of the “demyelinating Schwann cell” in peripheral demyelinating neuropathies. Glia. 2019;67:571–581. doi: 10.1002/glia.23509. [DOI] [PubMed] [Google Scholar]
- 34.Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L, Behrens A, Mirsky R, Jessen KR. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181:625–637. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian C, Tan D, Wang X, Li L, Wen J, Pan M, Li Y, Wu W, Guo J. Peripheral nerve injury-induced astrocyte activation in spinal ventral horn contributes to nerve regeneration. Neural Plast. 2018;2018:8561704. doi: 10.1155/2018/8561704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin YH, Lee SJ, Jung J. Extracellular ATP inhibits Schwann cell dedifferentiation and proliferation in an ex vivo model of Wallerian degeneration. Biochem Biophys Res Commun. 2013;430:852–857. doi: 10.1016/j.bbrc.2012.11.057. [DOI] [PubMed] [Google Scholar]
- 37.Stratton JA, Shah PT. Macrophage polarization in nerve injury: do Schwann cells play a role. Neural Regen Res. 2016;11:53–57. doi: 10.4103/1673-5374.175042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taniguchi Y, Amazaki M, Furuyama T, Yamaguchi W, Takahara M, Saino O, Wada T, Niwa H, Tashiro F, Miyazaki J, Kogo M, Matsuyama T, Inagaki S. Sema4D deficiency results in an increase in the number of oligodendrocytes in healthy and injured mouse brains. J Neurosci Res. 2009;87:2833–2841. doi: 10.1002/jnr.22124. [DOI] [PubMed] [Google Scholar]
- 39.Tricaud N, Park HT. Wallerian demyelination: chronicle of a cellular cataclysm. Cell Mol Life Sci. 2017;74:4049–4057. doi: 10.1007/s00018-017-2565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhamme C, de Haan RJ, Vermeulen M, Baas F, de Visser M, van Schaik IN. Oral high dose ascorbic acid treatment for one year in young CMT1A patients: a randomised, double-blind, placebo-controlled phase II trial. BMC Med. 2009;7:70. doi: 10.1186/1741-7015-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang T, Chen K, Zeng X, Yang J, Wu Y, Shi X, Qin B, Zeng L, Esteban MA, Pan G, Pei D. The histone demethylases Jhdm1α/1b enhance somatic cell reprogramming in a vitamin-C-dependent manner. Cell Stem Cell. 2011;9:575–587. doi: 10.1016/j.stem.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Wen J, Tan D, Li L, Wang X, Pan M, Guo J. RhoA regulates Schwann cell differentiation through JNK pathway. Exp Neurol. 2018;308:26–34. doi: 10.1016/j.expneurol.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Wen J, Qian C, Pan M, Wang X, Li Y, Lu Y, Zhou Z, Yan Q, Li L, Liu Z, Wu W, Guo J. Lentivirus-mediated RNA interference targeting rhoa slacks the migration, proliferation, and myelin formation of Schwann cells. Mol Neurobiol. 2017;54:1229–1239. doi: 10.1007/s12035-016-9733-5. [DOI] [PubMed] [Google Scholar]
- 44.Yu J, Gu X, Yi S. Ingenuity pathway analysis of gene expression profiles in distal nerve stump following nerve injury: insights into Wallerian degeneration. Front Cell Neurosci. 2016;10:274. doi: 10.3389/fncel.2016.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou XB, Liu N, Wang D, Zou DX, Wei CW, Zhou JL. Neuroprotective effect of ischemic postconditioning on sciatic nerve transection. Neural Regen Res. 2018;13:492–496. doi: 10.4103/1673-5374.228733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zigmond RE, Echevarria FD. Macrophage biology in the peripheral nervous system after injury. Prog Neurobiol. 2019;173:102–121. doi: 10.1016/j.pneurobio.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.