Keywords: cognitive training, magnetic resonance imaging, multiple sclerosis, neuroplasticity, neuropsychology, rehabilitation, salience network
Abstract
Cognitive impairments are commonly observed in patients with multiple sclerosis and are associated with lower levels of quality of life. No consensus has been reached on how to tackle effectively cognitive decline in this clinical population non-pharmacologically. This exploratory case-control study aims to investigate the effectiveness of a hypothesis-based cognitive training designed to target multiple domains by promoting the synchronous co-activation of different brain areas and thereby improve cognition and induce changes in functional connectivity in patients with relapsing-remitting multiple sclerosis. Forty-five patients (36 females and 9 males, mean age 44.62 ± 8.80 years) with clinically stable relapsing-remitting multiple sclerosis were assigned to either a standard cognitive training or to control groups (sham training and non-active control). The standard training included twenty sessions of computerized exercises involving various cognitive functions supported by distinct brain networks. The sham training was a modified version of the standard training that comprised the same exercises and number of sessions but with increased processing speed load. The non-active control group received no cognitive training. All patients underwent comprehensive neuropsychological and magnetic resonance imaging assessments at baseline and after 5 weeks. Cognitive and resting-state magnetic resonance imaging data were analyzed using repeated measures models. At reassessment, the standard training group showed significant cognitive improvements compared to both control groups in memory tasks not specifically targeted by the training: the Buschke Selective Reminding Test and the Semantic Fluency test. The standard training group showed reductions in functional connectivity of the salience network, in the anterior cingulate cortex, associated with improvements on the Buschke Selective Reminding Test. No changes were observed in the sham training group. These findings suggest that multi-domain training that stimulates multiple brain areas synchronously may improve cognition in people with relapsing-remitting multiple sclerosis if sufficient time to process training material is allowed. The associated reduction in functional connectivity of the salience network suggests that training-induced neuroplastic functional reorganization may be the mechanism supporting performance gains. This study was approved by the Regional Ethics Committee of Yorkshire and Humber (approval No. 12/YH/0474) on November 20, 2013.
Chinese Library Classification No. R493; R741
Introduction
Cognitive impairment afflicts the majority of people with multiple sclerosis (MS) and is increasingly recognized as a major determinant of quality of life (Mortensen et al., 2020). Its management has captured the attention of clinicians and researchers, but various medications have been only marginally effective (Mitolo et al., 2015). A number of non-pharmacological interventions for cognitive impairment have been proposed mainly for people with relapsing remitting MS (RRMS) and only more recently also applied to secondary progressive MS (Messinis et al., 2020). However, evidence of their efficacy has been hindered by several methodological concerns, among which sample selection and appropriate choice of a comparator arm (Mhizha-Murira et al., 2017). As a result, the debate on cognitive training efficacy in MS and on the best approach is still open (Mitolo et al., 2015). Processing speed (PS) deficits have long been observed to have a wider impact on cognition in people with MS (Manca et al., 2018) and a recently published pilot study showed PS training transfer to verbal short-term memory and timed instrumental activities of daily living (Chiaravalloti et al., 2018).
Neuroimaging, in particular magnetic resonance imaging (MRI), has been used as a sensitive biomarker to assess response to cognitive training in MS. Whilst no training effects were found when structural parameters were used as outcome measures (Filippi et al., 2012; Campbell et al., 2017), post-training increases in task-related brain activation have been observed in the cerebellum (Sastre-Garriga et al., 2010; Cerasa et al., 2013), parietal cortex (Cerasa et al., 2013; Campbell et al., 2017), the posterior default mode network, and the left dorsolateral prefrontal cortex (Filippi et al., 2012). Increases in resting-state functional connectivity (rs-FC), i.e. spontaneous patterns of synchronised activity at rest in sets of brain areas that support different functions, were also noted in the posterior default mode network (Filippi et al., 2012; Bonavita et al., 2015) and in the salience network (SN) (Filippi et al., 2012; Parisi et al., 2014a). Moreover, variations in rs-FC were observed across other networks involved in cognitive (Filippi et al., 2012; De Giglio et al., 2016; Pareto et al., 2018) as well as sensory functions (Pareto et al., 2018).
Due to the limited number of studies in this field that used MRI outcome measures, the significance of these results remains uncertain, both regarding the clarification of mechanisms of action and the potential impact on future training design. Patient samples in these studies were small, no active control group was included and in general, no a priori hypotheses were put forward about the mechanisms of action fostered by the interventions. Therefore, no clear conclusions can be drawn on whether cognitive training yields consistent therapeutic effects in people with MS.
The aim of this experimental study was to apply multi-modality MRI to assess the effects of a novel hypothesis-based cognitive training program and to characterize the associated neuroplastic changes in a sample of people affected by RRMS. Our research question stemmed from the hypothesis that disruption of rs-FC might be the main neural mechanism underlying cognitive decline in MS (Schoonheim et al., 2015) and we hypothesized that multidomain training exercises would improve cognitive performance and promote neural plasticity in people with RRMS. Multidomain training was favored over training focused on a single domain, usually at the basis of symptomatic interventions, since previous findings suggest that this approach may induce the strongest effects compared to other interventions and over longer periods of time in people with neurodegenerative conditions (Gates and Sachdev, 2014) and healthy older adults (Cheng et al., 2012). Moreover, multidomain training was shown to maintain or improve functional integration within and across different brain networks (Cao et al., 2016).
Subjects and Methods
Subjects
Forty-five right-handed patients who fulfilled the modified McDonald diagnostic criteria and the Lublin classification for RRMS (Polman et al., 2010) were identified and recruited from the MS clinic at Sheffield Teaching Hospitals NHS Foundation Trust (Sheffield, UK) between April 2014 and December 2017. Inclusion criteria were: age between 25 and 65 years; Expanded Disability Status Scale (EDSS) (Kurtzke, 1983) ≤ 6; self-reported cognitive symptoms; objective cognitive impairment defined as a score of 2 standard deviations below normative values in at least one of the neuropsychological tests used for cognitive profiling (see the section of “Neuropsychological assessment”); stable disease status and drug treatment for at least 3 months prior to recruitment; visual acuity within the normal range with provision of visual aids enabling patients to read off a computer screen (≥ 6/6) (Davis et al., 2009). Exclusion criteria were: history of major psychiatric disorders; presence of other concomitant neurological diseases; participation in cognitive rehabilitation in the year prior to recruitment; upper limb motor impairment; inability to undergo MRI. Ethical approval was obtained from the Regional Ethics Committee of Yorkshire and Humber (approval No. 12/YH/0474) on November 20, 2013 (Additional file 1 (176.7KB, pdf) ). The study conformed to the principles stated in the Declaration of Helsinki and the reporting guidelines of the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement (Additional file 2). Written informed consent was obtained from all participants in this study (Additional file 3 (142.3KB, pdf) ).
STROBE Statement—Checklist of items that should be included in reports of case-control studies
| Item No | Recommendation | Page No | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study's design with a commonly used term in the title or the abstract | 2 |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 2-3 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 4-5 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 5 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 6-7 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 6-7, 11 |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls | 6-7 |
| (b) For matched studies, give matching criteria and the number of controls per case | 7 | ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 6, 11-12 |
| Data sources/ measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 11-12 |
| Bias | 9 | Describe any efforts to address potential sources of bias | 6 |
| Study size | 10 | Explain how the study size was arrived at | 7 |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 14-15 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 14 |
| (b) Describe any methods used to examine subgroups and interactions | 14 | ||
| (c) Explain how missing data were addressed | n.a. (no missing data) | ||
| (d) If applicable, explain how matching of cases and controls was addressed | 7 | ||
| (e) Describe any sensitivity analyses | n.a. | ||
| Results | |||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 7 |
| (b) Give reasons for non-participation at each stage | 6-7 | ||
| (c) Consider use of a flow diagram | Not applicable | ||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | 15, 31 |
| (b) Indicate number of participants with missing data for each variable of interest | Not applicable (no missing data) | ||
| Outcome data | 15* | Report numbers in each exposure category, or summary measures of exposure | 15, 35 |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 15-17 |
| (b) Report category boundaries when continuous variables were categorized | Not applicable | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | Not applicable | ||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | 16-17, 34, 41 |
| Discussion | |||
| Key results | 18 | Summarise key results with reference to study objectives | 18 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 21 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 19-20 |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 21 |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 22 (no funding) |
*Give information separately for cases and controls.
Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine athttp://www.annals.org/, and Epidemiology athttp://www.epidem.com/). Information on the STROBE Initiative is available at http://www.strobe-statement.org.
Study design
All participants meeting the inclusion criteria were approached within the same clinic following the same procedure in order to minimize a possible selection bias. Patients willing to take part in this case-control study were provided with written information material and contacted via telephone after 1 week to confirm their willingness to participate. The main reasons for not taking part in the study reported by patients were almost exclusively time commitment and daily commuting to hospital (study site), while only one patient refused due to claustrophobia and consequent inability to undergo MRI. At the first appointment, participants gave written consent and underwent a comprehensive neuropsychological assessment. An MRI scanning session was carried out within 3 days from baseline assessment. Subsequently, from week 2 to week 5, patients were allocated (unconcealed allocation) to either the standard cognitive training (n = 15) or to one of two control groups, sham training (n = 15) or non-active (n = 15), matched for demographic characteristics, in order to minimize the influence of confounding factors on cognitive performance, i.e., age, education and disease severity. Neuropsychological and MRI assessments were repeated the week following the end of the training program or, for the non-active control group, after 4 weeks of care as usual. The sample size for this exploratory study was based on previous work by our team investigating the effects of a similar protocol on patients with mild cognitive impairment (De Marco et al., 2018).
Cognitive training protocol
Both the standard and sham training groups included twenty sessions: 5 days a week for 4 consecutive weeks. Participants were allowed to catch up with any missed sessions at the end of the 4 weeks. Session lasted for about 1 hour under the supervision of a neuropsychologist.
All tasks were administered through the E-Prime Software, Version 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA, USA). The standard training exercises were adapted from previously published methodology (De Marco et al., 2016, 2018) with three clusters of exercises tapping the main cognitive domains involving the most prominent distributed functions: PS/attention, semantic knowledge and logical reasoning.
Four tasks were designed to exercise PS and sustained attention abilities considered to be fundamentally affected by MS (Costa et al., 2017) and related to cognitive efficiency (Rypma and Prabhakaran, 2009). Indeed, it has been reported that faster and more efficient individuals exhibit less activation in prefrontal regions and higher activations in parietal cortices (Rypma and Prabhakaran, 2009). The tasks used were the following:
Verbal simple reaction time: an initial fixation cross was presented for 1000 ms and then a blue capital A was displayed at the centre of the computer screen for 150 ms. Participants were instructed to press the “0” key as fast as they could any time they saw the stimulus.
Visual simple reaction time: this task was the same as the previous one, but the stimulus presented was a blue square (1 cm × 1 cm).
Verbal choice reaction time: an initial fixation cross was presented for 1000 ms and then either a blue capital A or a blue capital B (counterbalanced) was displayed at the center of the computer screen for 150 ms and participants were instructed to press the “1” key as fast as they could in response to A and to press “2” in response to B.
Visual choice reaction time: this task was equivalent to the previous one but stimuli presented were either a red or a blue squares (same as in the simple reaction time task). The “1” key was to be pressed in response to red squares while “2” in response to the blue ones.
For all these tasks an inter-stimulus interval of 10 seconds was set in order to allow participants to respond to each stimulus. However, the delivery of a new stimulus was triggered by each response.
Four tasks were aimed to exercise semantic retrieval and control processes widely accepted to be reliant on a network of associative areas scattered across temporal, frontal and parietal lobes (Binder et al., 2009; Lambon Ralph et al., 2017). Lexical-semantic processing served as a scaffolding to develop integrated tasks that required working memory and inhibitory processes (Figure 1), since deficits in such functions have been extensively documented in MS (Henry and Beatty, 2006). Therefore, these tasks were meant to facilitate exchange and integration of information across multiple cognitive brain networks:
Figure 1.
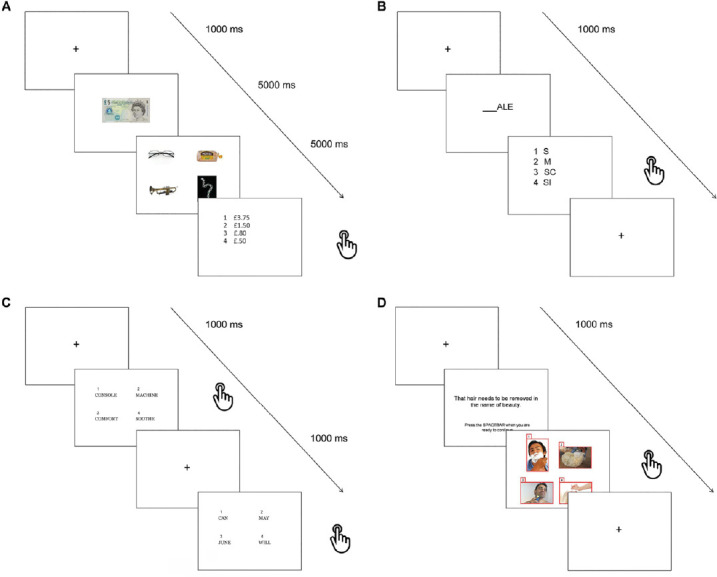
Lexical-semantic tasks.
Sample trials included in: (A) Change calculation, (B) Lexical odd one out, (C) Semantic odd one out, (D) Semantic inhibition tasks. Used images have been selected from a publicly a freely available database that can be found at http://www.freedigitalphotos.net/.
Change calculation: first a fixation cross was presented for 1000 ms, followed by the image of a banknote or a coin to remember (5000 ms). Then the images of four different items were presented for 5000 ms and participants had to identify the only item that could be bought with the amount of money previously presented (no answer required). Finally, a list of four amounts of money were presented and the participants had to choose (by pressing the key of the corresponding number 1 to 4) which one represented the change most likely to be received after paying for the affordable item with the note/coin originally presented.
Lexical odd one out: a fixation cross was presented for 1000 ms at first and then the ending of a word was displayed for 5000 ms, followed by a list of four possible word beginnings, only three of which could make a word if completed with the previously given ending. The participants had to press the key (1 to 4) associated with the only option resulting in a non-word.
Semantic odd one out: after a fixation cross lasting 1000 ms four words were displayed simultaneously, three of which belonged to one semantic category. Participants were instructed to find the only word which did not fit the target category. However, one of the other three words represented a distractor being semantically related to the odd one.
Semantic inhibition: in each trial after a fixation cross displayed for 1000 ms, a sentence was presented with no time limit to allow the participants to read it carefully and memorise it. As instructed, when they felt ready they had to press the space bar to show four images on the screen: three of them semantically related to the sentence and one not. The aim was to report the latter.
Similarly, also logical reasoning tasks were created based on semantic material that could train higher order cognitive functioning on both verbal and visual material (Figure 2). Indeed, complex and sequential cognitive computations are performed during exercises of deductive reasoning (Fangmeier et al., 2006), thus engaging a variety of brain networks:
Figure 2.
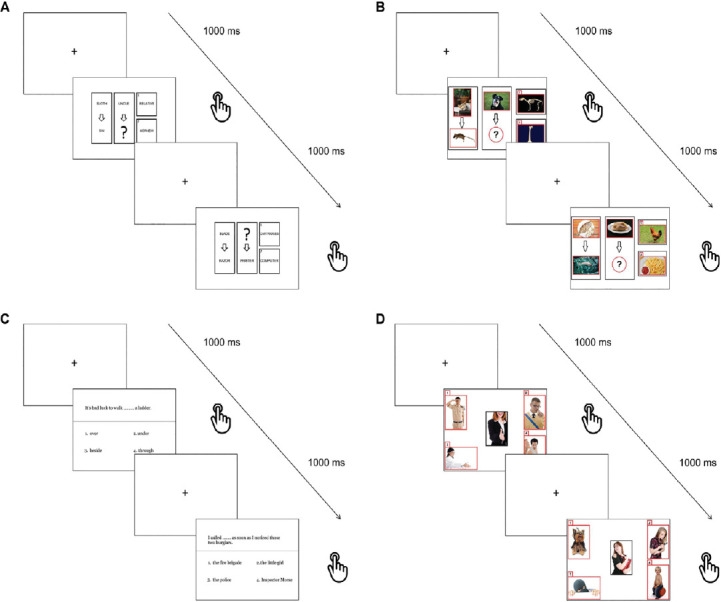
Reasoning tasks.
Sample trials included in: (A) Verbal sequence completion, (B) Visual sequence completion, (C) Sentence completion, (D) Scene completion tasks.Used images have been selected from a publicly a freely available database that can be found at http://www.freedigitalphotos.net/.
Verbal sequence completion: after an initial presentation of a fixation cross (1000 ms), a set of different words was displayed in each trial with two on the left side of the screen being connected by an arrow and related to one another. In the middle of the screen a word only was presented with an arrow and a question mark for a word to be picked from two options on the right side of the screen so that the same relationship of the first couple was reproduced. However, both options were semantically related to the words in the middle of the screen, so that inhibitory processes had to be used in order to detect the most relevant one in the given context.
Visual sequence completion: this test was similar to the previous one apart from the fact that images were presented instead of words.
Sentence completion: after an initial presentation of a fixation cross (1000 ms), a sentence was presented on the screen with a missing word and four options below it. The participants had to select the most appropriate word to obtain semantically correct sentences.
Scene completion: in each trial an array of five images was presented, one in the middle and four smaller ones at the corners of the computer screen. The participants were instructed to select the image most logically/semantically associated with the target one placed in the middle of the computer screen.
The sham training included the same tasks of the standard form but modified by setting a maximum amount of time to respond to each trial. The allocated time in each individual task was equivalent to the median reaction time obtained from a pilot study comprising seven people with RRMS recruited from the same clinic to ensure that patients could respond correctly to at least 75% of the responses in each task. Moreover, from session 6 onwards, the maximum response time allocated to each task was progressively reduced of 100 ms in each session with the aim of challenging participants’ PS ability gradually. Time was not adjusted to individual PS capacity in order for each participant to receive the same sham training. The initial maximum response times (sessions 1 to 5) were as follows: 4500 ms for Change calculation, 5500 ms for Lexical odd one out, 5500 ms for Semantic odd one out, 5000 ms for Semantic inhibition, 5500 ms for Verbal sequence completion, 5000 ms for Visual sequence completion, 5500 ms for Sentence completion and 5000 ms for Scene completion.
In contrast, the non-active control group underwent no cognitive training, but continued to receive all usual treatments.
Neuropsychological assessment
A comprehensive battery of tests (core tests are described in a study by Wakefield et al. (2014)) and questionnaires were used to evaluate cognitive functioning of participants. This battery included: the 3” and 2” versions of the Paced Auditory Serial Addition Test (Gronwall, 1977); Digit Span Test (Wakefield et al., 2014); Logical Memory Test (Wechsler, 2008); Buschke Selective Reminding Test (BSRT) (Buschke, 1973); Phonemic and Semantic Fluency tasks (Wakefield et al., 2014); Corsi Block-Tapping Test (Corsi, 1972); Rey-Osterreith Complex Figure Test (Wakefield et al., 2014); Digit Cancellation Test (Wakefield et al., 2014); Trail Making Test (Armitage, 1946); Stroop test (Wakefield et al., 2014); Digit Symbol Coding Test (Wechsler, 2008); Modified Fatigue Impact Scale (Fisk et al., 1994); Patient Health Questionnaire (Spitzer et al., 1999); Generalized Anxiety Disorder scale (Spitzer et al., 2006); and Multiple Sclerosis Quality of Life Questionnaire (Vickrey et al., 1995). Testers were not blind to which experimental group participants belonged to.
MRI protocol and preprocessing
MRI was performed at baseline and post-training. Images were acquired using a 3T system (Ingenia 3T, Philips Healthcare, Best, The Netherlands) equipped with a 32-channel radiofrequency receive-only head coil. The MRI protocol is reported in Table 1.
Table 1.
Acquisition parameters for the sequences included in the MRI protocol
| MRI sequence | Acquisition parameters |
|---|---|
| Sagittal 3-dimensional (3D) T1-weighted magnetization prepared rapid acquisition gradient-echo | Repetition time = 8.1 ms, echo time = 3.7 ms, matrix size = 240 × 222, field of view = 240 × 240 mm2 |
| Sagittal 3D T2-weighted fluid attenuated inversion recovery | Repetition time = 4800 ms, echo time = 289 ms, inversion time = 1000 ms, matrix size = 224 × 224, field of view = 250 × 250 mm2 |
| Axial 2D T2*-weighted, single-shot, echo planar imaging | Repetition time = 2600 ms, echo time = 35 ms, time points = 200, matrix size = 96 × 94, field of view = 230 × 230 mm2. Prior to the acquisition of this dataset, patients were instructed to close their eyes and rest without falling asleep. |
2D: 2-Dimensional; 3D: 3-dimensional; MRI: magnetic resonance imaging.
Structural and resting-state scan analyses were carried out by means of the Statistical Parametric Mapping software (SPM12, Wellcome Centre for Human Neuroimaging, London, UK) running on MATLAB R2008a, version 7.6.0 (The Mathworks, Natick, MA, USA).
First, T1-weighted and FLAIR scans were reoriented to the bicommissural line. Secondly, total lesion volume quantified in millilitres by means of automatic white matter lesion segmentation on the reoriented T1-weighted and FLAIR images. The lesion growth algorithm of the Lesion Segmentation Toolbox v1.2.3 was used with a threshold of k = 0.3 (Schmidt et al., 2011). The T1-weighted images were segmented into gray matter, white matter and cerebrospinal fluid. The MATLAB function “get_totals” was used to extract the volume of the three tissue classes and the total intracranial volume was calculated as their sum.
All resting-state scans were first slice-time corrected in order to compensate for the difference in time of acquisition between slices of each brain volume. Second, each session was realigned independently by using the 4th Degree B-Spline Interpolation option to correct for possible head movements occurred in between the acquisition of different volumes. Graphical reports were visually inspected to ensure linear and rotational head movements would not exceed ± 3 mm and ± 3°. Third, realigned images were normalized using the first realigned volume of the first session as source image to match the default EPI template and voxel size was isotropied at 2 × 2 × 2 mm3 to account for differences in head size and shape. Fourth, a band-pass filter was applied to normalized scans with the aim of removing non-neural noise signal by using the REST toolbox (Song et al., 2011). A low-pass filter was set at 0.1 Hz to eliminate frequencies generated by physiological mechanisms and a high-pass filter was set at 0.008 Hz to remove low-frequency scanner drifts (Fox and Raichle, 2007). Finally, filtered volumes were spatially smoothed with a 6 mm3 full-width at half maximum Gaussian kernel to improve signal-to-noise ratio.
Subsequently, a group-level independent component analysis was performed on resting-state scans using the GIFT toolbox (GIFT v1.3i; mialab.mrn.org/software/gift) (Calhoun et al., 2001) to identify several sample-specific functional networks. The Infomax algorithm was chosen and the number of components to be extracted was set at twenty to detect the main known functional brain networks and avoids excessive dissociation of signal sources (Wang and Li, 2015). Finally, reconstruction of participant-specific spatial maps of each component was performed. This study focused on six different networks already investigated in the literature: four extensively involved in cognitive processes, i.e. the default mode network, the SN, the right and left fronto-parietal networks, and two control networks associated with sensory and motor functions particularly affected in MS, i.e. the visual network and the sensorimotor network. The z-score spatial maps of these networks were visually identified and extracted from all individual sets of components for statistical analysis.
Statistical analysis
The Shapiro-Wilk test was used to check whether demographic and clinical variables were normally distributed. Homogeneity of variance was assessed using the Levene’s test. Analysis of variance and the Kruskal-Wallis test were used to investigate differences across the three patient groups in normally and non-normally distributed variables, respectively. Descriptive statistics reported were means and standard deviation, for normally distributed variables, and medians and interquartile ranges, for non-normally distributed variables. Analysis of covariance was used to check for differences at baseline in cognitive performance controlling for demographic/clinical variables that resulted significantly different across groups. A Bonferroni correction for multiple comparisons was applied to the significance threshold P < 0.05/25 = 0.002. Additionally, the Mann-Whitney U test was used to investigate differences in performance accuracy in the first and last training sessions on all those tasks to which an increased PS load was applied. Chi-squared was used to compare numbers of female/male participants in the three groups.
The effects of the cognitive training programs on cognition and functional connectivity were evaluated using three repeated measures models to test for interaction effects between two factors, namely time (within-group factor) and training group (between-group factor). In particular, two groups at a time were investigated: 1) standard training vs. non-active control; 2) sham training vs. non-active control; 3) standard training vs. sham training. This choice was made to avoid the reduction in the degrees of freedom in the analysis, in consideration of the limited sample size of the patient groups. Partial η2 was also calculated to quantify the effect size of the observed cognitive changes.
Post-hoc analyses were carried out to investigate whether changes in cognition were associated with changes in within-network rs-FC induced by the standard training. For those networks on which a significant effect of the training was observed, subtraction maps were calculated by means of the SPM function ImCalc: baseline network maps were subtracted, for each individual who underwent the standard cognitive training, from the post-training maps. Similarly, difference scores (post-training – baseline) were created for the Semantic Fluency and the BSRT and regression models were run to investigate any association between cognitive difference scores and the subtraction maps (FWE P < 0.05).
Results
Baseline clinical characteristics
Analyses on demographic and clinical characteristics of the participants revealed that only educational levels significantly differed across groups (Table 2). Dunn’s pairwise tests showed that patients allocated to the control group had significantly fewer years of education than those in the sham group (test statistics = –15.27, P = 0.004 adjusted with Bonferroni correction), but not than those in the standard training group. For this reason education was included as a covariate in subsequent analyses.
Table 2.
Clinical and demographic characteristics of the included patients
| Characteristic | Standard training | Sham training | Non-active control | F-value | P-value |
|---|---|---|---|---|---|
| Demographic | |||||
| Age (yr) | 45.40±10.55 | 45.73±8.61 | 42.73±7.27 | 0.51 | 0.604 |
| Age at onset (yr) | 36.80±9.92 | 37.13±8.40 | 32.73±6.86 | 1.25 | 0.297 |
| Education (yr) | 14.00±5.00 | 17.00±3.00 | 12.00±2.00 | 10.68† | 0.005 |
| Sex (F/M) | 13/2 | 10/5 | 13/2 | 2.50‡ | 0.287 |
| Clinical | |||||
| Disease duration (yr)* | 8.00 (7.00) | 6.00 (7.00) | 8.00 (13.00) | 0.31† | 0.856 |
| Relapses (n in last 12 mon)* | 0.00 (1.00) | 0.00 (4.00) | 0.00 (2.00) | 3.09† | 0.213 |
| EDSS* | 3.50 (2.50) | 3.00 (3.00) | 4.00 (2.50) | 5.22† | 0.074 |
| GMV (mL) | 659.19±84.94 | 607.21±60.54 | 624.71±94.44 | 1.59 | 0.216 |
| WMV (mL) | 404.24±150.18 | 418.88±62.73 | 438.43±152.47 | 0.27 | 0.768 |
| TLV (mL)* | 3.26 (13.66) | 7.27 (12.55) | 5.91 (6.33) | 0.36† | 0.837 |
Values are means ± standard deviations. * Median and interquartile range; † Kruskal-Wallis test; ‡ Pearson chi-square test. n = 15 in each group. EDSS: Expanded Disability Status Scale; F: female; GMV: grey matter volume; M: male; TLV: total lesion volume; WMV: white matter volume.
Cognitive results
No between-group differences on cognitive or self-reported variables were observed after applying Bonferroni correction to the results of the analysis of covariance (Table 3).
Table 3.
Cognitive and self-reported characteristics of the sample and changes resulting from the three group-by-time repeated measures models
| Characteristic | Standard training | Sham training | Non-active control | Standard vs. non-active | Sham vs. non-active | Standard vs. Sham | ||||||
| Baseline | Post-training | Baseline | Post-training | Baseline | Post-training | F-value | P-value | F-value | P-value | F-value | P-value | |
| Verbal working memory | ||||||||||||
| PASAT 3”† | 35.93±17.79 | 41.87±16.35 | 36.87±19.90 | 39.80±16.33 | 39.53±14.41 | 43.07±13.90 | 0.30 | 0.587 | 0.92 | 0.347 | 2.87 | 0.102 |
| PASAT 2”† | 21.40±14.98 | 26.93±16.00 | 23.73±12.90 | 26.40±14.96 | 23.40±15.51 | 28.33±15.83 | 0.35 | 0.558 | 2.43 | 0.13 | 2.36 | 0.136 |
| DS -F† | 6.00±0.84 | 6.60±0.99 | 6.47±1.68 | 6.53±1.19 | 6.33±0.98 | 6.07±0.70 | 7.61 | 0.01 | 0.01 | 0.914 | 1.98 | 0.17 |
| DS -B† | 4.53±1.06 | 4.93±1.49 | 4.93±1.53 | 5.27±1.33 | 4.73±1.10 | 4.60±0.91 | 6.35 | 0.018 | 0.28 | 0.599 | 1.49 | 0.233 |
| Verbal long term memory | ||||||||||||
| LMT -IR† | 13.40±3.52 | 14.27±3.61 | 13.93±3.90 | 13.73±4.46 | 12.73±3.26 | 11.40±3.68 | 3.13 | 0.088 | 0.81 | 0.377 | 1.34 | 0.256 |
| LMT -DR† | 14.87±3.89 | 17.73±3.08 | 17.13±3.54 | 16.33±4.27 | 15.00±2.90 | 14.20±5.28 | 9.40 | 0.005 | 0.01 | 0.918 | 4.78 | 0.038 |
| BSRT -total† | 90.33±17.43 | 115.80±12.76 | 100.93±13.30 | 110.60±15.44 | 102.33±15.87 | 107.73±15.37 | 19.21* | < 0.001 | 2.34 | 0.138 | 12.22* | 0.002 |
| BSRT -DR† | 8.13±2.53 | 8.93±2.37 | 6.80±2.76 | 8.73±2.55 | 7.47±2.56 | 8.13±2.26 | 8.88 | 0.006 | 0.23 | 0.637 | 3.77 | 0.063 |
| PF† | 28.40±9.17 | 38.80±10.53 | 38.93±9.58 | 43.73±12.03 | 32.07±9.14 | 36.87±10.49 | 9.42 | 0.005 | 0.54 | 0.469 | 2.9 | 0.1 |
| SF† | 43.73±9.47 | 55.93±13.91 | 52.87±9.22 | 55.73±8.78 | 43.47±9.65 | 44.67±8.68 | 18.96* | < 0.001 | 0.05 | 0.82 | 13.03* | 0.001 |
| Visuo-spatial memory | ||||||||||||
| CT -span† | 4.87±1.19 | 5.47±1.24 | 4.73±1.22 | 5.00±1.36 | 4.93±1.16 | 5.40±0.91 | 0.27 | 0.608 | 0.22 | 0.642 | 3.94 | 0.057 |
| CT -SU† | 25.00±3.82 | 27.04±1.94 | 25.64±3.46 | 25.76±3.66 | 26.11±2.68 | 27.50±1.42 | 0.09 | 0.769 | 1.64 | 0.211 | 1.76 | 0.196 |
| RF -copy† | 34.80±1.37 | 35.00±1.00 | 33.47±2.53 | 34.20±2.31 | 34.80±1.37 | 34.93±1.03 | 0.05 | 0.824 | 0.29 | 0.594 | 0.01 | 0.93 |
| RF -DR† | 14.53±4.26 | 22.47±4.94 | 18.27±6.38 | 18.80±6.90 | 18.16±3.77 | 22.20±5.17 | 6.69 | 0.015 | 1.64 | 0.212 | < 0.01 | 0.988 |
| PS/visuo-spatial attention | ||||||||||||
| DCT† | 50.87±7.38 | 55.07±5.24 | 52.00±6.00 | 54.47±5.89 | 54.27±4.83 | 54.47±5.36 | 3.00 | 0.095 | 2.4 | 0.133 | 0.27 | 0.605 |
| TMT-A (s) | 44.33±14.45 | 35.67±14.36 | 36.00±9.88 | 33.67±9.55 | 36.47±16.91 | 34.00±13.89 | 3.55 | 0.071 | 1.31 | 0.262 | 2.30 | 0.141 |
| SS (s) | 17.83±3.87 | 15.50±2.72 | 17.53±2.65 | 16.63±3.12 | 16.33±3.07 | 17.13±3.80 | 6.3 | 0.018 | 2.55 | 0.122 | 0.65 | 0.428 |
| DSCT† | 62.20±16.56 | 69.07±16.92 | 62.60±13.24 | 67.00±15.29 | 64.20±16.87 | 70.87±21.75 | 0.03 | 0.86 | 0.15 | 0.704 | 0.60 | 0.446 |
| Executive functions | ||||||||||||
| SI (s) | 16.27±8.63 | 12.10±5.46 | 16.10±5.99 | 14.97±5.47 | 15.40±7.62 | 13.87±4.68 | 3.90 | 0.059 | 0.1 | 0.758 | 0.79 | 0.381 |
| TMT-B-A (s) | 43.87±24.03 | 35.53±23.48 | 45.73±27.61 | 39.87±19.82 | 36.53±21.07 | 29.60±12.45 | 0.28 | 0.602 | 0.02 | 0.894 | 0.17 | 0.686 |
| Self-reported measures | ||||||||||||
| MFIS‡ | 44.00±16.67 | 32.40±15.98 | 53.07±14.23 | 45.13±17.92 | 48.27±17.13 | 49.53±17.91 | 7.12 | 0.013 | 1.56 | 0.222 | 1.85 | 0.185 |
| PHQ-9‡ | 8.93±5.65 | 6.53±3.96 | 10.87±5.79 | 8.60±5.34 | 8.40±3.60 | 8.73±4.03 | 4.42 | 0.045 | 3.79 | 0.062 | < 0.01 | 0.987 |
| GAD-7‡ | 6.87±6.08 | 4.33±3.31 | 6.13±4.53 | 5.87±5.07 | 7.20±5.06 | 5.27±3.86 | 0.03 | 0.86 | 0.02 | 0.894 | 0.21 | 0.647 |
| MSQoL-54 -P‡ | 51.68±20.25 | 61.80±21.21 | 44.81±16.63 | 44.45±17.81 | 52.02±19.79 | 50.38±22.08 | 10.66 | 0.003 | 0.17 | 0.679 | 4.74 | 0.038 |
| MSQoL-54 -M‡ | 58.64±17.52 | 66.58±19.87 | 53.38±18.83 | 56.03±24.42 | 64.53±19.79 | 59.30±19.28 | 4.97 | 0.034 | 2.04 | 0.164 | 0.01 | 0.914 |
Values are means ± standard deviations. n = 15 in each group. * (in bold) Tests that survived Bonferroni correction: P < 0.002; † Total number of correct items; ‡ Total score n a Likert-like scale. BSRT: Buschke Selective Reminding Test; CT: Corsi Test; DCT: Digit Cancellation Test (total number of correct items detected on the three matrices in 45 seconds); DR: Delayed recall; DS: Digit Span-B/F (B: backward, F: forward); DSCT: Digit Symbol Coding Test (total number of correct items reported in 120 seconds); GAD-7: 7-item Generalized Anxiety Disorder; IR: Immediate recall; LMT: Logical Memory Test; MFIS: Modified Fatigue Impact Scale; MSQoL-54-M/P: 54-item Multiple Sclerosis Quality of Life (M: mental, P: physical); PASAT: Paced Auditory Serial Addition Test; PF: Phonemic Fluency; PHQ-9: 9-item Patient Health Questionnaire; PS: Processing speed; RF: Rey Figure; SF: Semantic Fluency; SI: Stroop inhibition (difference between completion time on colour-word inhibition trial and average completion time on word reading and colour naming trials); SS: Stroop speed (average of completion time for word reading and colour naming trials); SU: supraspan; TMT-A/B-A: Trail Making Test (A: part A, A–B: part B–part A difference).
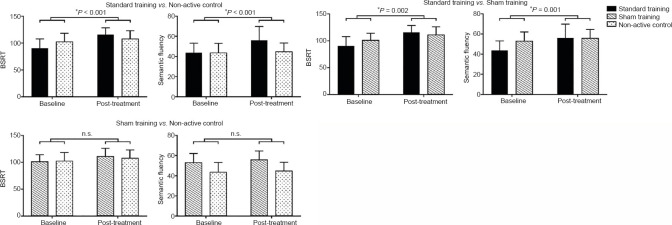
Pairwise repeated measures models were used to investigate differential effects of the training programs on cognitive performance. These models showed significant interactions between trainings and time. In particular, the standard training induced significantly stronger improvements than care as usual on two measures of verbal memory (Table 3): total recall on the BSRT and the Semantic Fluency test. The effect size was high for both tests as shown by a partial η2 index of 0.42 and 0.41 respectively.
No significant differences in cognitive variables were seen when comparing the sham training and the non-active control groups. Finally, the direct contrast between the standard and sham training groups showed results consistent with those reported above. In fact, participants who received the standard training showed an increase in cognitive performance that was significantly higher than that observed in those who received the sham training exactly on the same tests (Table 3 and Figure 3), i.e. the total recall on the BSRT and the Semantic Fluency test. The effect sizes for both tests were high with observed partial η2 values of 0.31 and 0.33 respectively.
Figure 3.
Cognitive performance.
Cognitive changes on the Buschke Selective Reminding Test (BSRT) and the Semantic Fluency Test resulting from the three group-by-time repeated measures analysis of covariance comparing two experimental groups at a time (n = 15 for each group). Values are expressed as the mean ± standard error. n.s.: Not significant.
Since trend towards a reduction of fatigue levels were observed in the standard training group and in consideration of the fact that fatigue is thought to play a role in cognitive dysfunction in MS, a stepwise regression model was used to test whether cognitive changes (BSRT and Semantic Fluency) were predicted by changes in fatigue (post-training – baseline scores) and education.
Cognitive improvements observed in the standard training group were not significantly explained by either changes in fatigue levels alone (Model 1) or by the combination of such changes and education (Model 2) (Table 4).
Table 4.
Results of stepwise regression: association between cognitive improvements and changes in fatigue in the standard training group
| Variable | Regression coefficient | P-value |
|---|---|---|
| BSRT change | ||
| Model 1 (adjusted R2 = 0.005) | ||
| MFIS change | –0.256 | 0.321 |
| Model 2 (adjusted R2 = –0.062) | ||
| MFIS change | –0.302 | 0.299 |
| Education | –0.531 | 0.679 |
| Semantic fluency change | ||
| Model 1 (adjusted R2 = 0.072) | ||
| MFIS change | 0.044 | 0.811 |
| Model 2 (adjusted R2 = –0.123) | ||
| MFIS change | 0.093 | 0.647 |
| Education | 0.573 | 0.532 |
BSRT: Buschke Selective Reminding Test; MFIS: Modified Fatigue Impact Scale.
Testing for between-group differences in performance on the training tasks showed that accuracy was significantly lower in the sham group compared to the standard group on most of the exercises that differed in PS load across the two experimental groups. In particular, these differences were seen in most tasks, both on the first and last training sessions (Table 5).
Table 5.
Results of Mann-Whitney U test: differences in accuracy of performance between the standard and sham training groups in the first and last training sessions
| Variable | Standard training | Sham training | U-value | P-value |
| 1st session | ||||
| Change calculation | 0.83 (1.00) | 0.33 (0.67) | 31.5 | 0.001* |
| Lexical odd one out | 0.88 (0.50) | 0.38 (1.00) | 29.0 | 0.001* |
| Semantic odd one out | 0.90 (0.40) | 0.70 (0.70) | 29.0 | 0.001* |
| Sematic inhibition | 0.67 (0.67) | 0.67 (0.67) | 89.5 | 0.505 |
| Verbal sequence completion | 0.90 (0.40) | 0.70 (0.80) | 38.5 | 0.003* |
| Visual sequence completion | 0.86 (0.29) | 0.57 (0.43) | 29.5 | 0.001* |
| Sentence completion | 1.00 (0.20) | 0.70 (0.70) | 24.5 | < 0.001* |
| Scene completion | 0.88 (0.50) | 0.75 (0.88) | 31.5 | 0.001* |
| 20th session | ||||
| Change calculation | 0.50 (0.66) | 0.42 (0.66) | 83.0 | 0.354 |
| Lexical odd one out | 1.00 (0.62) | 0.50 (0.75) | 30.5 | 0.001* |
| Semantic odd one out | 1.00 (0.50) | 0.50 (0.75) | 26.0 | 0.001* |
| Sematic inhibition | 0.50 (1.00) | 0.00 (1.00) | 52.0 | 0.020* |
| Verbal sequence completion | 1.00 (0.50) | 0.75 (1.00) | 56.0 | 0.033* |
| Visual sequence completion | 0.75 (0.50) | 0.50 (1.00) | 48.5 | 0.012* |
| Sentence completion | 0.75 (0.50) | 0.50 (1.00) | 45.0 | 0.008* |
| Scene completion | 1.00 (1.00) | 0.50 (1.00) | 32.0 | 0.001* |
Values are medians and interquartile ranges. *P < 0.05
Functional connectivity results
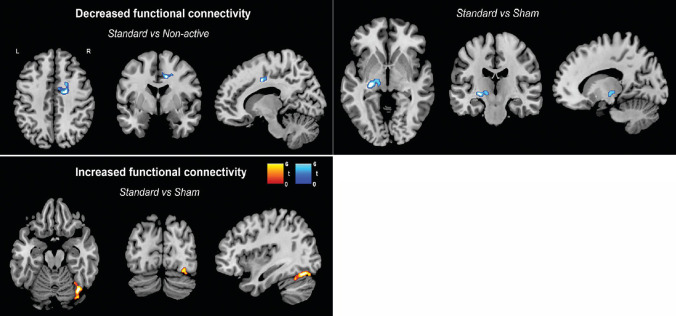
Significant changes in the resting-state topography of the SN were observed when comparing the standard training group with the non-active control and the sham training groups. These changes were distinct between the two comparator groups and characterized by both increases and decreases (Table 6). Participants undergoing the standard training showed a decrease in rs-FC in the anterior cingulate when compared to the control group. Instead, the comparison between the standard and sham training groups showed decreased rs-FC in the left putamen and thalamus, but increased rs-FC in temporo-occipital areas (Figure 4). No significant changes were detected for any of the other functional networks included in the analysis.
Table 6.
Significant changes in resting-state functional connectivity within the salience network resulting from the repeated measures models (PFWE < 0.05)
| FC changes | Cluster extent | Side | Brain region (Brodmann Area) | t-value | MNI coordinates (mm) | ||
| x | y | z | |||||
| Standard vs. Non-active | |||||||
| Decrease | 110 | R | Anterior cingulate (24) | 4.9 | 20 | –6 | 42 |
| R | Anterior cingulate (24) | 4.81 | 12 | –2 | 40 | ||
| R | Anterior cingulate (32) | 4.03 | 18 | 6 | 42 | ||
| Standard vs. Sham | |||||||
| Decrease | 119 | L | Putamen | 5.3 | –28 | –22 | –2 |
| L | Thalamus | 3.81 | –16 | –16 | –2 | ||
| Increase | 160 | R | Fusiform gyrus (19) | 4.79 | 36 | –70 | –18 |
| R | Fusiform gyrus (37) | 4.37 | 34 | –58 | –24 | ||
| R | Middle occipital gyrus (18) | 4.06 | 30 | –82 | –12 | ||
FC: Functional connectivity; FEW: Family Wise Error; L: left; MNI: Montreal Neurological Institute and Hospital; R: right.
Figure 4.
Functional connectivity of the salience network.
Decreases (blue) and increases (red) in resting-state functional connectivity of the salience network resulting from the three group-by-time repeated measures analysis of covariance comparing two experimental groups at a time in SPM12 (n = 15 for each group). P < 0.05 Family Wise Error corrected. L: Left; R: right.
In the standard training group, improvement on the BSRT was associated with decreased rs-FC within the SN (r = –0.789, P = 0.002) (Figure 5). This was specifically detected within the anterior cingulate cortex (Brodmann Areas 32/24), hub of the SN, and in the medial frontal gyrus (Brodmann Area 9). No significant results were found for the Semantic Fluency test.
Figure 5.
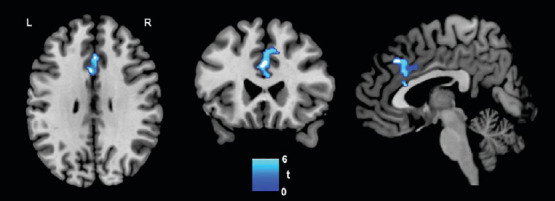
Association between cognitive and functional connectivity changes.
Negative association between decreased resting-state functional connectivity (post-training – baseline difference maps) within the salience network and increased cognitive performance on the Buschke Selective Reminding Test (post-training – baseline difference score) in the Standard training group (n = 15). P < 0.05 Family Wise Error corrected. L: Left; R: right.
Discussion
This study investigated the effects on cognition and within-network rs-FC of an experimental cognitive training designed to stimulate the coactivation of brain networks supporting PS/attention, lexical-semantic processes, and logical reasoning in patients with RRMS. Cognitive improvements in semantic processing and verbal long-term memory and changes in the resting-state topography of the SN were observed only in the experimental group that completed the standard version of the training compared to both a sham training, i.e., with high PS demands, and to a non-active control group.
Effects on semantic and learning functions have been observed in previous studies that prevalently used a symptomatic approach to training and generally found no transfer effects (Filippi et al., 2012; Bonavita et al., 2015; Rilo et al., 2016). Improvements in memory, a cognitive domain often impaired in MS (Lafosse et al., 2013), may represent a consequence of improved efficiency of information processing since long-term learning was not specifically trained in this study. Indeed, mnestic functions are supported not only by hippocampal structures, but by integration of information across a wider neural network (Paul et al., 2016). Similarly, improvements were also seen in some aspects of semantic memory that depends on a distributed neural system and, thus, possibly prone to benefits from more efficient cross-region communications (Lambon Ralph et al., 2017).
Whilst the same cognitive improvements were detected for the standard group when compared to either the non-active control or the sham training, the latter did not elicit greater changes than those detected in the non-active control group. We argue this may have occurred because the high PS load made the tasks too challenging for patients with more severe PS deficits since no adaptations were applied on the basis of individual PS abilities and, thus, prevented any potential benefit of the sham training. Indeed, both in the first and last session the sham group performed less accurately than the standard group on most tasks.
Of particular note was the comparison between the two training groups who performed the same tasks across the same number of sessions and interacted with the researcher in analogous ways, thus making the sham condition a more appropriate comparator in this complex training. The consistent gains seen in the standard training group when compared to the two control groups suggest that these results were not a consequence of the personal attention received while engaging with the trial. Indeed, there were no changes in self-reported levels of depression and anxiety across groups. Additional analyses on changes in self-reported fatigue levels in the standard training group showed that these were not significant predictors of cognitive improvements, thus ruling out a possible confounding effect of fatigue on the observed results.
In line with the cognitive findings, the standard cognitive training induced changes in rs-FC within the SN compared to both the sham training and the non-active control groups. The comparison between the standard and sham training groups showed an increase in synchronicity of resting-state activity in occipito-temporal associative areas that have been previously seen to be over-recruited in patients with MS (Loitfelder et al., 2011). This may indicate that the SN, which is thought to facilitate access of stimuli to fronto-parietal systems for further processing (Uddin, 2015), may recruit additional areas as a post-training compensatory change aimed at improving information processing. The rational behind this speculation is the hypothesis that resting-state synchronicity in sets of brain areas may be reflective of frequent coactivation during performance of goal-directed tasks (Martínez et al., 2013). If this were the case, patterns of coactivations observed after training could predispose future task-related processes. Additionally, in the standard training group rs-FC decreases were found in a left-lateralized cluster comprising the thalamus and the putamen. Both these deep gray matter nuclei are believed to be part of the SN (Seeley, 2019) and usually undergo shrinkage in MS (Eshaghi et al., 2018). The severity of atrophy of these structures was also consistently found associated with cognitive impairment in people with MS (Bisecco et al., 2017). Hence, the effects observed may be interpreted as a more efficient reshaping of the SN.
Findings from the comparison between the standard training and the two control groups are consistent with this hypothesis, since a similar decrease in rs-FC was observed in an important hub of the SN: the anterior cingulate cortex (Seeley, 2019). Moreover, this post-training decrease correlated with improved performance on the BSRT, supporting an interpretation of such changes in rs-FC as compensatory. The anterior cingulate appears to support a range of cognitive functions including memory (Sestieri et al., 2014), but this correlational finding may reflect a reduction in the cognitive effort required to perform challenging memory tasks. In fact, activation in the anterior cingulate cortex has been previously found to be associated with cognitively demanding (Allen et al., 2007) as well as inaccurate long-term memory performance of healthy people (Dhanjal and Wise, 2014).
In contrast, other studies on cognitive training for people with MS reported opposite changes in the anterior cingulate (Filippi et al., 2012; Parisi et al., 2014b), that were associated with maintenance of cognitive improvements at follow-up (Parisi et al., 2014a). This dicrepancy may be due to differences across studies in the functions trained, training exposure and intensity, sample characteristics and neuroimaging analyses.
A first limitation of this study is the relatively small sample size of the groups, partially due to the willingness of patients to attend several hospital visits to undergo daily sessions. Indeed, home-based trainings may favour recruitment of larger patient groups, even in more severe disease stages (Messinis et al., 2020). Moreover, this might have also induced a selection bias towards more educated and motivated participants than would be expected in the general population. Second, the group of patients was carefully selected to exclude patients with any comorbidities, especially depression. Although this choice was necessary to control for the influence of confounding variables on the effects of the training, this may limit the generalisation of these findings to all people affected by RRMS. Third, a longer follow-up assessment (6–12 months after treatment) was not included, thus it is not possible to determine whether the observed effects of training on functional connectivity and cognition are solid and long-lasting. Fourth, this exploratory case-control study is essentially an experimental proof of concept and testers were not blind to the experimental conditions participants were allocated to. Future large randomized controlled trials including multiple follow-ups over a longer period of time are needed to ascertain the effectiveness of the proposed approach.
In conclusion, significant effects of the standard training were found in both cognitive performance and rs-FC within the SN. The comparison between the two training groups provided additional robust evidence on the benefits of the standard training and showed that the observed improvements were related to the training material and not to non-specific placebo effects. Overall, this study provides objective evidence that multidomain cognitive training designed to induce functional neuroplastic changes (Schoonheim et al., 2015) may improve cognition in people with stable RRMS.
Additional files:
Additional file 1 (176.7KB, pdf) : Ethical Approval Documentation.
Additional file 2: STROBE checklist.
Additional file 3 (142.3KB, pdf) : Model consent form.
Acknowledgments:
This is a summary of independent research carried out at the NIHR Sheffield Biomedical Research Centre (Translational Neuroscience). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The authors would like to acknowledge Dr Sian Price for her contribution to participants’ recruitment. The authors also thank Matteo De Marco for making the original training stimuli available for adaptation for this study.
Footnotes
C-Editors: Zhao M, Li CH; T-Editor: Jia Y
Conflicts of interest: The authors declare that they have no conflicts of interest.
Financial support: The authors received no funding for the research reported in this paper.
Institutional review board statement: The study was approved by the the Regional Ethics Committee of Yorkshire and Humber (approval No. 12/YH/0474) on November 20, 2013.
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the forms the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Reporting statement: This study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by a statistical expert at the Department of Neuroscience of the University of Sheffield in UK.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: The conditions of our ethics approval do not permit the sharing of any data supporting this study with any individual outside the authors’ core team under any circumstances.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Rhonda R. Voskuhl, David Geffen School of Medicine, University of California, USA; Anuska V. Andjelkovic, University of Michigan, USA.
References
- 1.Allen MD, Bigler ED, Larsen J, Goodrich-Hunsaker NJ, Hopkins RO. Functional neuroimaging evidence for high cognitive effort on the Word Memory Test in the absence of external incentives. Brain Inj. 2007;21:1425–1428. doi: 10.1080/02699050701769819. [DOI] [PubMed] [Google Scholar]
- 2.Armitage SG. An analysis of certain psychological tests used for the evaluation of brain injury. Psychol Monogr. 1946;60:1–48. [Google Scholar]
- 3.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system. A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisecco A, Stamenova S, Caiazzo G, d’Ambrosio A, Sacco R, Docimo R, Esposito S, Cirillo M, Esposito F, Bonavita S, Tedeschi G, Gallo A. Attention and processing speed performance in multiple sclerosis is mostly related to thalamic volume. Brain Imaging Behav. 2017;12:20–28. doi: 10.1007/s11682-016-9667-6. [DOI] [PubMed] [Google Scholar]
- 5.Bonavita S, Sacco R, Della Corte M, Esposito S, Sparaco M, d’Ambrosio A, Docimo R, Bisecco A, Lavorgna L, Corbo D, Cirillo S, Gallo A, Esposito F, Tedeschi G. Computer-aided cognitive rehabilitation improves cognitive performances and induces brain functional connectivity changes in relapsing remitting multiple sclerosis patients: An exploratory study. J Neurol. 2015;262:91–100. doi: 10.1007/s00415-014-7528-z. [DOI] [PubMed] [Google Scholar]
- 6.Buschke H. Selective reminding for analysis of memory and learning. J Verbal Learning Verbal Behav. 1973;12:543–550. [Google Scholar]
- 7.Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell J, Langdon D, Cercignani M, Rashid W. A randomised controlled trial of efficacy of cognitive rehabilitation in multiple sclerosis: A cognitive, behavioural, and MRI study. Neural Plast. 2017;2016:4292585. doi: 10.1155/2016/4292585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao W, Cao X, Hou C, Li T, Cheng Y, Jiang L, Luo C, Li C, Yao D. Effects of cognitive training on resting-state functional connectivity of default mode, salience, and central executive networks. Front Aging Neurosci. 2016;8:70. doi: 10.3389/fnagi.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerasa A, Valentino P, Chiriaco C, Pirritano D, Nisticò R, Gioia CM, Trotta M, Del Giudice F, Tallarico T, Rocca F, Augimeri A, Bilotti G, Quattrone A. MR imaging and cognitive correlates of relapsing-remitting multiple sclerosis patients with cerebellar symptoms. J Neurol. 2013;260:1358–1366. doi: 10.1007/s00415-012-6805-y. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Y, Wu W, Feng W, Wang J, Chen Y, Shen Y, Li Q, Zhang X, Li C. The effects of multi-domain versus single-domain cognitive training in non-demented older people: a randomized controlled trial. BMC Med. 2012;10:30. doi: 10.1186/1741-7015-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiaravalloti ND, Goverover Y, Costa SL, DeLuca J. A pilot study examining speed of processing training (SPT) to improve processing speed in persons with multiple sclerosis. Front Neurol. 2018;9:685. doi: 10.3389/fneur.2018.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corsi PM. Human memory and the medial temporal region of the brain. Dis Abstr Intl. 1972;34:819B. [Google Scholar]
- 14.Costa SL, Genova HM, DeLuca J, Chiaravalloti ND. Information processing speed in multiple sclerosis: present, and future. Mult Scler. 2017;23:772–789. doi: 10.1177/1352458516645869. [DOI] [PubMed] [Google Scholar]
- 15.Davis AS, Hertza J, Williams RN, Gupta AS, Ohly JG. The influence of corrected visual acuity on visual attention and incidental learning in patients with multiple sclerosis. Appl Neuropsychol. 2009;16:165–168. doi: 10.1080/09084280903098497. [DOI] [PubMed] [Google Scholar]
- 16.De Giglio L, Tona F, De Luca F, Petsas N, Prosperini L, Bianchi V, Pozzilli C, Pantano P. Multiple sclerosis: changes in thalamic resting-state functional connectivity induced by a home-based cognitive rehabilitation program. Radiology. 2016;280:202–211. doi: 10.1148/radiol.2016150710. [DOI] [PubMed] [Google Scholar]
- 17.De Marco M, Meneghello F, Pilosio C, Rigon J, Venneri A. Up-regulation of DMN connectivity in mild cognitive impairment via network-based cognitive training. Curr Alzheimer Res. 2018;15:578–589. doi: 10.2174/1567205015666171212103323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Marco M, Meneghello F, Duzzi D, Rigon J, Pilosio C, Venneri A. Cognitive stimulation of the default-mode network modulates functional connectivity in healthy aging. Brain Res Bull. 2016;121:26–41. doi: 10.1016/j.brainresbull.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Dhanjal NS, Wise RJ. Frontoparietal cognitive control of verbal memory recall in Alzheimer’s disease. Ann Neurol. 2014;76:241–251. doi: 10.1002/ana.24199. [DOI] [PubMed] [Google Scholar]
- 20.Eshaghi A, et al. Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann Neurol. 2018;83:210–222. doi: 10.1002/ana.25145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fangmeier T, Knauff M, Ruff CC, Sloutsky V. FMRI evidence for a three-stage model of deductive reasoning. J Cogn Neurosci. 2006;18:320–334. doi: 10.1162/089892906775990651. [DOI] [PubMed] [Google Scholar]
- 22.Filippi M, Riccitelli G, Mattioli F, Capra R, Stampatori C, Pagani E, Valsasina P, Copetti M, Falini A, Comi G, Rocca MA. Multiple sclerosis: Effects of cognitive rehabilitation on structural and functional MR imaging measures--an explorative study. Radiology. 2012;262:932–940. doi: 10.1148/radiol.11111299. [DOI] [PubMed] [Google Scholar]
- 23.Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci. 1994;21:9–14. [PubMed] [Google Scholar]
- 24.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 25.Gates NJ, Sachdev P. Is cognitive training an effective treatment for preclinical and early Alzheimer’s disease. J Alzheimers Dis. 2014;42(Suppl 4):S551–559. doi: 10.3233/JAD-141302. [DOI] [PubMed] [Google Scholar]
- 26.Gronwall DM. Paced auditory serial-addition task: A measure of recovery from concussion. Percept Mot Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- 27.Henry JD, Beatty WW. Verbal fluency deficits in multiple sclerosis. Neuropsychologia. 2006;44:1166–1174. doi: 10.1016/j.neuropsychologia.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 29.Lafosse JM, Mitchell SM, Corboy JR, Filley CM. The nature of verbal memory impairment in multiple sclerosis: A list-learning and meta-analytic study. J Int Neuropsychol Soc. 2013;19:995–1008. doi: 10.1017/S1355617713000957. [DOI] [PubMed] [Google Scholar]
- 30.Lambon Ralph MA, Jefferies E, Patterson K, Rogers TT. The neural and computational bases of semantic cognition. Nat Rev Neurosci. 2017;18:42–55. doi: 10.1038/nrn.2016.150. [DOI] [PubMed] [Google Scholar]
- 31.Loitfelder M, Fazekas F, Petrovic K, Fuchs S, Ropele S, Wallner-Blazek M, Jehna M, Aspeck E, Khalil M, Schmidt R, Neuper C, Enzinger C. Reorganization in cognitive networks with progression of multiple sclerosis: insights from fMRI. Neurology. 2011;76:526–533. doi: 10.1212/WNL.0b013e31820b75cf. [DOI] [PubMed] [Google Scholar]
- 32.Manca R, Sharrack B, Paling D, Wilkinson ID, Venneri A. Brain connectivity and cognitive processing speed in multiple sclerosis: A systematic review. J Neurol Sci. 2018;388:115–127. doi: 10.1016/j.jns.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Martínez K, Solana AB, Burgaleta M, Hernández-Tamames JA, Alvarez-Linera J, Román FJ, Alfayate E, Privado J, Escorial S, Quiroga MA, Karama S, Bellec P, Colom R. Changes in resting-state functionally connected parietofrontal networks after videogame practice. Hum Brain Mapp. 2013;34:3143–3157. doi: 10.1002/hbm.22129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messinis L, Kosmidis MH, Nasios G, Konitsiotis S, Ntoskou A, Bakirtzis C, Grigoriadis N, Patrikelis P, Panagiotopoulos E, Gourzis P, Malefaki S, Papathanasopoulos P. Do secondary progressive multiple sclerosis patients benefit from computer-based cognitive neurorehabilitation. A randomized sham controlled trial. Mult Scler Relat Disord. 2020;39:101932. doi: 10.1016/j.msard.2020.101932. [DOI] [PubMed] [Google Scholar]
- 35.Mhizha-Murira JR, Drummond A, Klein OA, dasNair R. Reporting interventions in trials evaluating cognitive rehabilitation in people with multiple sclerosis: A systematic review. Clin Rehabil. 2017;32:243–254. doi: 10.1177/0269215517722583. [DOI] [PubMed] [Google Scholar]
- 36.Mitolo M, Venneri A, Wilkinson ID, Sharrack B. Cognitive rehabilitation in multiple sclerosis: A systematic review. J Neurol Sci. 2015;354:1–9. doi: 10.1016/j.jns.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Mortensen GL, Theódórsdóttir Á, Sejbæk T, Illes Z. Patient attitudes to routine cognitive testing in multiple sclerosis. Patient Prefer Adherence. 2020;14:693–704. doi: 10.2147/PPA.S245623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pareto D, Sastre-Garriga J, Alonso J, Galán I, Arévalo MJ, Renom M, Montalban X, Rovira À. Classic block design “pseudo”-resting-state fMRI changes after a neurorehabilitation program in patients with multiple sclerosis. J Neuroimaging. 2018;28:313–319. doi: 10.1111/jon.12500. [DOI] [PubMed] [Google Scholar]
- 39.Parisi L, Rocca MA, Valsasina P, Panicari L, Mattioli F, Filippi M. Cognitive rehabilitation correlates with the functional connectivity of the anterior cingulate cortex in patients with multiple sclerosis. Brain Imaging Behav. 2014a;8:387–393. doi: 10.1007/s11682-012-9160-9. [DOI] [PubMed] [Google Scholar]
- 40.Parisi L, Rocca MA, Mattioli F, Copetti M, Capra R, Valsasina P, Stampatori C, Filippi M. Changes of brain resting state functional connectivity predict the persistence of cognitive rehabilitation effects in patients with multiple sclerosis. Mult Scler. 2014b;20:686–694. doi: 10.1177/1352458513505692. [DOI] [PubMed] [Google Scholar]
- 41.Paul LK, Erickson RL, Hartman JA, Brown WS. Learning and memory in individuals with agenesis of the corpus callosum. Neuropsychologia. 2016;86:183–192. doi: 10.1016/j.neuropsychologia.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Hávrdová E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2010;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rilo O, Peña J, Ojeda N, Rodríguez-Antigüedad A, Mendibe-Bilbao M, Gómez-Gastiasoro A, DeLuca J, Chiaravalloti N, Ibarretxe-Bilbao N. Integrative group-based cognitive rehabilitation efficacy in multiple sclerosis: a randomized clinical trial. Disabil Rehabil. 2016;40:208–216. doi: 10.1080/09638288.2016.1250168. [DOI] [PubMed] [Google Scholar]
- 44.Rypma B, Prabhakaran V. When less is more and when more is more: The mediating roles of capacity and speed in brain-behavior efficiency. Intelligence. 2009;37:207–222. doi: 10.1016/j.intell.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sastre-Garriga J, Alonso J, Renom M, Arevalo MJ, Gonzalez I, Galan I, Montalban X, Rovira A. A functional magnetic resonance proof of concept pilot trial of cognitive rehabilitation in multiple sclerosis. Mult Scler. 2010;17:457–467. doi: 10.1177/1352458510389219. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt P, Gaser C, Arsic M, Buck D, Forschler A, Berthele A, Hoshi M, Ilg R, Schmid VJ, Zimmer C, Hemmer B, Muhlau M. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage. 2011;59:3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 47.Schoonheim MM, Meijer KA, Geurts JJ. Network collapse and cognitive impairment in multiple sclerosis. Front Neurol. 2015;6:82. doi: 10.3389/fneur.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seeley WW. The salience network: A neural system for perceiving and responding to homeostatic demands. J Neurosci. 2019;39:9878–9882. doi: 10.1523/JNEUROSCI.1138-17.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sestieri C, Corbetta M, Spadone S, Romani GL, Shulman GL. Domain-general signals in the cingulo-opercular network for visuospatial attention and episodic memory. J Cogn Neurosci. 2014;26:551–568. doi: 10.1162/jocn_a_00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. REST: A toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. Primary care evaluation of mental disorders Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 52.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 53.Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- 54.Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW. A health-related quality of life measure for multiple sclerosis. Qual Life Res. 1995;4:187–206. doi: 10.1007/BF02260859. [DOI] [PubMed] [Google Scholar]
- 55.Wakefield SJ, McGeown WJ, Shanks MF, Venneri A. Differentiating normal from pathological brain ageing using standard neuropsychological tests. Curr Alzheimer Res. 2014;11:765–772. doi: 10.2174/156720501108140910121631. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Li TQ. Dimensionality of ICA in resting-state fMRI investigated by feature optimized classification of independent components with SVM. Front Hum Neurosci. 2015;9:259. doi: 10.3389/fnhum.2015.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wechsler D. San Antonio: NCS Pearson; 2008. Wechsler adult intelligence scale–Fourth Edition (WAIS–IV) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.