Keywords: apoptosis, cerebral ischemia and reperfusion injury, microRNA, miR-670, neurological function, neuron, non-coding RNA, pathway
Abstract
Apoptosis is an important programmed cell death process involved in ischemia/reperfusion injury. MicroRNAs are considered to play an important role in the molecular mechanism underlying the regulation of cerebral ischemia and reperfusion injury. However, whether miR-670 can regulate cell growth and death in cerebral ischemia/reperfusion and the underlying mechanism are poorly understood. In this study, we established mouse models of transient middle artery occlusion and Neuro 2a cell models of oxygen-glucose deprivation and reoxygenation to investigate the potential molecular mechanism by which miR-670 exhibits its effects during cerebral ischemia/reperfusion injury both in vitro and in vivo. Our results showed that after ischemia/reperfusion injury, miR-670 expression was obviously increased. After miR-670 expression was inhibited with an miR-670 antagomir, cerebral ischemia/reperfusion injury-induced neuronal death was obviously reduced. When miR-670 overexpression was induced by an miR-670 agomir, neuronal apoptosis was increased. In addition, we also found that miR-670 could promote Yap degradation via phosphorylation and worsen neuronal apoptosis and neurological deficits. Inhibition of miR-670 reduced neurological impairments after cerebral ischemia/reperfusion injury. These results suggest that microRNA-670 aggravates cerebral ischemia/reperfusion injury through the Yap pathway, which may be a potential target for treatment of cerebral ischemia/reperfusion injury. The present study was approved by the Institutional Animal Care and Use Committee of China Medical University on February 27, 2017 (IRB No. 2017PS035K).
Chinese Library Classification No. R446; R741; Q522
Introduction
Cerebrovascular disease is currently the most common central nervous system disease and increases the death and disability rates among Chinese older adults (Guan et al., 2017). Because of the increasing proportion of the population aged older than 65 years, the incidence of cerebrovascular disease has increased each year. Beginning at 25 years of age, the risk of cerebrovascular disease throughout the lifespan is greater than 25% (GBD 2016 Lifetime Risk of Stroke Collaborators et al., 2018). The situation is even worse in East Asia (Micha et al., 2015). Every year approximately one-third of cerebrovascular disease patients die, mainly because of ischemic stroke (Campbell, 2017). Patients may suffer from hemiplegia, aphasia, or disturbance of consciousness caused by impaired blood circulation and secondary hypoxia in the focal brain tissue. Timely thrombolytic treatments can recanalize the blocked cerebral vessel and relieve clinical symptoms. Nevertheless, the outcomes are not satisfying because of the additional subsequent cerebral damage induced by ischemia and reperfusion (I/R) injury.
MicroRNAs (miRNAs) are non-coding RNAs 20–22 nucleotides in length. Evidence has shown that miRNAs participate in multiple biological processes, such as cell growth, development, differentiation, senescence, and apoptosis (Li et al., 2017; Saliminejad et al., 2019; Zhang et al., 2020). MiRNAs can destabilize targeted mRNAs and repress their translation. MiR-696 overexpression was shown to inhibit myoblast proliferation and myofiber formation during myogenesis (Wang et al., 2017). MiR-570 could promote epithelial cell senescence in airway inflammatory reactions (Baker et al., 2019). In addition, accumulating studies have focused on the roles of miRNAs in cerebral vascular diseases. MiR-181c has been shown to play a neuroprotective role by inhibiting apoptosis in intracerebral hemorrhage via the phosphatase and tensin homolog/phosphatidylinositol-3-kinase/protein kinase B pathway (Lu et al., 2020). According to our previous study, miR-200a participates in autophagy regulation during I/R injury in cerebral ischemic stroke, targeting its downstream transcriptional factor forkhead box O-3 (Yu et al., 2019). MiR-670 is involved in regulating proliferation in hepatic neoplasms (Shi and Xu, 2016). Additionally, miR-670 modulates RNA methylation during mouse embryo development (Hao et al., 2020). Therefore, we questioned whether miR-670 could regulate cell growth and death in cerebral I/R injury and thus analyzed the expression levels of miR-670 after I/R injury.
The Hippo pathway acts as a pivotal signaling cascade to maintain the balance among cell proliferation, differentiation, and apoptosis (Yu and Guan, 2013). Yes-associated protein (Yap) is a core transcription co-activator of the Hippo axis that regulates biological activities (Moon et al., 2019). During liver regeneration, Yap expression is greatly upregulated (Bai et al., 2012), and inhibition of Yap exerts an anti-tumor effect in gastric cancer (Zhang et al., 2019). Specific knockout of Yap in mice suppressed myocardial regeneration (Xin et al., 2013). Furthermore, activation of the Hippo/Yap pathway facilitated liver repairment after hepatic I/R injury (Liu et al., 2019). On the basis of these findings, we aimed to examine the impact of the Yap pathway in cerebral I/R injury. A transient middle cerebral artery occlusion (tMCAO) mouse model and oxygen-glucose deprivation and reoxygenation (OGD/R) cell model were established to mimic I/R injury both in vivo and in vitro. Then, the downstream Hippo signaling pathway was explored to reveal the potential molecular regulation mechanism of cerebral I/R injury.
Materials and Methods
Experimental animals
This study was performed in total compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal experiments were approved by the Institutional Animal Care and Use Committee of China Medical University on February 27, 2017 (IRB No. 2017PS035K). The experiments were designed to reduce the stress and pain experienced by animals. Male C57BL/6J mice aged 8–10 weeks and weighing 22–25 g were purchased from Beijing HFK Bioscience Cooperation, China (License No. SCXK (Jing) 2014-0004). All animals were kept in a specific-pathogen-free environment with 70% humidity, a temperature of 22 ± 2°C, and a 12-hour light and dark cycle before surgery. To verify tMCAO modeling, mice were randomly divided into sham and tMCAO groups (n = 15/group). To explore the mechanism of miR-670 in cerebral I/R injury, another set of mice was randomly assigned to three groups: I/R, I/R + pre-miR-670, and I/R + anti-miR-670 (n = 17/group).
tMCAO
A mouse model of tMCAO was used to mimic cerebral I/R injury in vivo as described in our previous study (Yu et al., 2019). The rectal temperature of mice was kept at approximately 37°C during surgery. Anesthesia was induced and maintained with 3% and 1.5% isoflurane (Sigma, St. Louis, MO, USA), respectively, through a facemask in an acrylic chamber containing 70% nitrogen and 30% oxygen. Then, the left carotid artery was exposed. The 6-0 silicone nylon monofilaments with rubber coating used in the procedure were produced by Beijing Cinontech Co. Ltd. (Beijing, China). A monofilament was inserted through the external carotid artery to the middle cerebral artery. After 1 hour of occlusion, the monofilament was withdrawn to allow brain reperfusion. The sham group underwent the same surgery without monofilament occlusion.
Evaluation of impaired brain tissue
Brain tissue was obtained 24 hours after reperfusion. Then, 2% 2,3,5-triphenyltetrazolium chloride (Sigma) was applied to stain brain tissue sections. The relative volume of impaired brain tissue (%) was calculated as follows: (volume of the contralateral hemisphere - volume of the nonischemic ipsilateral hemisphere)/volume of the contralateral hemisphere × 100.
Intracerebroventricular injection
Lentivirus (5 μL, 1 × 108 TU/mL) was injected into the lateral ventricles through a Hamilton microsyringe (Hamilton Co., Reno, NV, USA) 2 weeks before the surgical procedure. The detailed procedure was the same as that used in our previous study (Yu et al., 2019) and included cutting the skin and removing the local bone flap. A brain stereotaxic device was used to fix the injection site as follows: anteroposterior –0.3 mm, mediolateral 1.0 mm, and dorsoventral –2.2 mm.
Neurological deficit evaluation
Neurological deficits were assessed by the modified neurological severity scores using a 10-point scale. Two investigators who were blinded to the study groups recorded the modified neurological severity scores at 1, 3, 7, and 14 days after I/R injury. The severity of injury is quantitatively reflected as an increased modified neurological severity score (mild injury, 1–4; moderate injury, 5–9; severe injury, 10–14).
Behavioral tests
All behavioral tests were conducted at 1, 3, 7, and 14 days after I/R injury by two investigators who were blinded to the experimental groups. A rotarod test was conducted to estimate mouse sensorimotor coordination. Mice were placed on a rotating rod with an accelerating speed from 4 to 100 r/min over 5 minutes (Yang et al., 2017). The latency until the mouse fell was recorded for each trial. All experiments were repeated three times. Tactile reactions and sensorimotor asymmetries were assessed by an adhesive removal test. Both forepaws were tested with 2 × 3 mm adhesive tape separately (Yang et al., 2017). The time to contact and remove the tape was recorded for analysis.
Cell culture and OGD/R treatment
The Neuro 2a (N2a) mouse neuroblastoma cell line was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Dulbecco’s modified Eagle medium/high glucose with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA) was used as the culture medium. Experiments were performed using cells at passages 8–10. N2a cells in the OGD/R group were cultured in glucose-free Dulbecco’s modified Eagle medium without oxygen for 6 hours. Then, the medium was replaced with normal medium for a 24 hour-reoxygenation period as previously described (Yu et al., 2019).
Cell transfection
N2a cells were pre-plated 24 hours before transfection. An agomir (pre-miR-670) and an antagomir (anti-miR-670) of miR-670 and their corresponding non-sense sequences (pre-NC and anti-NC) were generated by GenePharma (Shanghai, China). After cells reached approximately 75% confluence, plasmids (0.78 μg/μL) were transfected using Lipofectamine reagents (Cat# L3000008; Invitrogen, Carlsbad, CA, USA).
Reverse transcription polymerase chain reaction analysis
The TRIzol reagent was applied to extract total RNA from brain tissue and N2a cells (Life Technologies Corporation, Carlsbad, CA, USA). Quantitative reverse transcription polymerase chain reaction (PCR) assays were conducted to assess Yap mRNA expression using the One Step SYBR Prime Script RT-PCR kit (Cat# RR064A; Takara Bio, Inc., Shiga, Japan) according to the manufacturer’s instructions. The working concentration for primers was 10 μM. The mmu-miR-670 expression level was detected using a TaqMan MicroRNA Reverse Transcription kit, TaqMan microRNA assay, and TaqMan Universal Master Mix II (Cat# 4366596, Cat# 4427975, Cat# 4440045; Applied Biosystems, Foster City, CA, USA). The relative expression of genes was assessed by the 2–ΔΔCt method and normalized to the corresponding endogenous control. The primer sequences were as follows: mmu-Yap forward: 5’-CCA CCA GAT CTC ATT ATA GAT GG-3’; mmu-Yap reverse: 5’-TGA CCC TTT GAC CAT GAA TGA TCC-3’; Mmu-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward: 5’-CCT CGT CCC GTA GAC AAA ATG-3’; Mmu-GAPDH reverse: 5’-TGA GGT CAA TGA AGG GGT CGT-3’; U6 control sequence: 5’-GTG CTC GCT TCG GCA GCA CAT ATA CTA AAA TTG GAA CGA TAC AGA GAA GAT TAG CAT GGC CCC TGC GCA AGG ATG ACA CGC AAA TTC GTG AAG CGT TCC ATA TTT T-3’.
Cell counting kit 8 analysis
The cell viability of different groups was evaluated by a cell counting kit 8 (Cat# CK04-01; Dojindo, Kumamoto, Japan). N2a cells were subjected to OGD/R injury as described in our previous study (Yu et al., 2019). After 6 hours of oxygen and glucose deprivation, cells were re-oxygenated for another 24 hours. Cell counting kit 8 reagents were mixed with the cell culture medium following the manufacturer’s instructions. Then, cells were incubated at 37°C for another 2 hours. A microplate reader (BioTek, Winooski, VT, USA) was used to record the absorbance at 450 nm.
Transferase-mediated nick end labeling assay
The In Situ Cell Death Detection kit used for the transferase-mediated nick end labeling (TUNEL) assay in N2a cells was purchased from Roche (Cat# 12156792910; Mannheim, Germany). 4’,6-Diamidino-2-phenylindole was applied to display the nucleus. The TUNEL-positive cell percentage was measured in five random 30-μm fields.
Flow cytometry
Flow cytometry was conducted to evaluate the apoptosis rate of N2a cells. Annexin V/7-AAD (Cat# 559763; Becton Dickinson, San Jose, CA, USA) was used to stain cells based on the manufacturer’s protocol. After incubation at room temperature, single-cell suspensions were detected by flow cytometry (FACSCalibur; Becton Dickinson) to determine the percentage of apoptosis.
Western blot assay
Brain tissue and N2a cells were lysed in a radioimmunoprecipitation assay buffer with protease inhibitors on ice to extract total proteins. Electrophoresis was conducted to separate proteins on a sodium dodecyl sulfate-polyacrylamide gel. Then, proteins were transferred to polyvinylidene difluoride membranes and blocked for 2 hours in 5% non-fat milk. The membranes were incubated with primary antibodies at 4°C overnight, including rabbit anti-Yap (Cat# 14074T; 1:1000; Cell Signaling Technology, Beverly, MA, USA), rabbit anti-phospho-(p-)YAP (Cat# 13619S; 1:1000; Cell Signaling Technology), and mouse anti-GAPDH (Cat# sc-365062; 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After incubation for 2 hours with horseradish peroxidase-conjugated secondary antibodies (goat anti-mouse IgG, Cat# SA00001-1 or goat anti-rabbit IgG, Cat# SA00001-2; 1:10,000; Proteintech, Chicago, IL, USA), immunoreactive bands were visualized by enhanced chemiluminescence (Cat# WBKLS0500; Millipore, Billerica, MA, USA). GAPDH was used as an endogenous control. The integrated density values were calculated using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data are reported as the mean ± standard deviation (SD) of three repeated experiments. Student’s t-test was applied to analyze the significance of differences between groups using SPSS 23.0 software (IBM, Armonk, NY, USA). Kaplan-Meier analysis was performed to generate survival curves. A value of P < 0.05 was regarded as statistically significant.
Results
MiR-670 expression is increased after I/R injury
To detect miR-670 expression after I/R injury in vivo, we generated a tMCAO mouse model. An infarct volume of approximately 40–50% was generated in mouse brain tissue (P < 0.05; Figure 1A). Relative expression of miR-670 was upregulated in the tMCAO group (approximately 2.2-fold) (P < 0.05; Figure 1B). Moreover, we examined miR-670 mRNA expression in vitro using an OGD/R injury model. OGD/R injury increased miR-670 levels in N2a cells compared with the control group (approximately 1.8-fold, P < 0.05; Figure 1C). To explore the regulatory function of miR-670 in I/R injury, N2a cell lines with overexpression or knockdown of miR-670 were established (P < 0.05; Figure 1D). Cell viability was decreased after OGD/R treatment, and overexpression of miR-670 further decreased cell viability. However, miR-670 knockdown could rescue the decreased cell viability caused by OGD/R (P < 0.05; Figure 1E).
Figure 1.
MiR-670 is notably upregulated after cerebral ischemia and reperfusion injury.
(A) Infarct volume after cerebral ischemia and reperfusion (I/R) injury (n = 6). (B) Quantitative polymerase chain reaction (qPCR) assay of miR-670 in I/R injured mouse brain tissue (n = 9). (C) qPCR assay of miR-670 in Neuro 2a cells (n = 3) exposed to oxygen-glucose deprivation and reoxygenation-induced (OGD/R). (D) qPCR assay of miR-670 after transfection (n = 3). The relative expression of miR-670 was calculated as the optical density ratio to that of the endogenous control (GAPDH). (E) Cell counting kit 8 assay of cell viability with altered miR-670 expression in I/R-injured Neuro 2a cells. Data are presented as the mean ± SD. †P < 0.05 vs. sham group; *P < 0.05, vs. control group; #P < 0.05, vs. OGD/R + pre-NC group; $P < 0.05, vs. OGD/R + anti-NC group (Student’s t-test). anti-miR-670: An miR-670 antagomir; anti-NC: a non-sense sequence of the miR-670 antagomir; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; I/R: ischemia and reperfusion; OGD/R: oxygen-glucose deprivation and reoxygenation; pre-miR-670: an miR-670 agomir; pre-NC: a non-sense sequence of the miR-670 agomir; qPCR: quantitative polymerase chain reaction.
MiR-670 promotes neuronal apoptosis in I/R injury
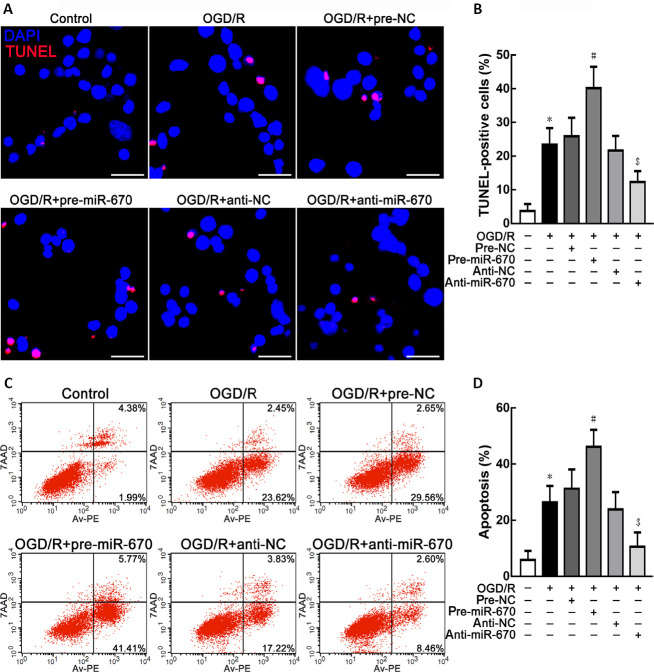
In addition to the cell viability assay, neuronal apoptosis was observed using TUNEL staining. Overexpression of miR-670 could accentuate apoptosis in N2a cells. However, knockdown of miR-670 could repress apoptosis after OGD/R treatment (P < 0.05; Figure 2A and B). The flow cytometry analysis results, shown in Figure 2C and D revealed that miR-670 could promote OGD/R-induced neuronal apoptosis.
Figure 2.
MiR-670 promotes neuronal apoptosis in Neuro 2a cells after ischemia and reperfusion injury.
(A, B) Transferase-mediated nick end labeling (TUNEL) staining of Neuro 2a cells with altered miR-670 expression after ischemia and reperfusion (I/R) injury. Apoptotic cells are labeled in red. 4’,6-Diamidino-2-phenylindole (DAPI) was used to display the nucleus (blue). Scale bars: 30 μm. At least 20 visual fields were counted in each experiment. (C, D) Flow cytometry analysis of Neuro 2a cells with altered miR-670 expression after I/R injury. Data are presented as the mean ± SD (n = 3). *P < 0.05, vs. control group; #P < 0.05, vs. OGD/R + pre-NC group; $P < 0.05, vs. OGD/R + anti-NC group (Student’s t-test). anti-miR-670: An miR-670 antagomir; anti-NC: a non-sense sequence of the miR-670 antagomir; DAPI: 4’,6-diamidino-2-phenylindole; I/R: ischemia and reperfusion; OGD/R: oxygen-glucose deprivation and reoxygenation; pre-miR-670: an miR-670 agomir; pre-NC: a non-sense sequence of the miR-670 agomir; TUNEL: transferase-mediated nick end labeling.
MiR-670 aggravates apoptosis by inhibiting downstream Yap in I/R injury
Next, we explored the underlying molecular mechanism by which miR-670 regulated apoptosis in I/R injury. The Yap pathway was evaluated because of its close relationship with apoptosis (Moon et al., 2019). Both quantitative PCR and western blot analysis were performed to evaluate Yap expression by measuring mRNA and protein levels in N2a cells (P < 0.05; Figure 3A and B). A robust decline in the Yap level was detected when miR-670 was overexpressed after OGD/R treatment. However, Yap expression was upregulated when miR-670 was knocked down (P < 0.05; Figure 3A–C). Because phosphorylation can inhibit the transcriptional activity of Yap (Flinn et al., 2020), p-Yap was further examined. A significant increase in p-Yap was detected in the miR-670 overexpression group after OGD/R injury. However, p-Yap expression was inhibited after knockdown of miR-670 (P < 0.05; Figure 3B–E). These results suggested that miR-670 inhibits Yap expression via phosphorylation-induced degradation.
Figure 3.
MiR-670 regulates downstream Yap expression in Neuro 2a cells after ischemia and reperfusion injury.
(A) Quantitative polymerase chain reaction (qPCR) assay of Yap expression with altered miR-670 expression after ischemia and reperfusion (I/R) injury. Relative expression of Yap was calculated as the optical density ratio to that of the endogenous control (GAPDH). (B–E) Western blot assay of Yap and p-Yap expression in cells with altered expression of miR-670 after I/R injury. Data are presented as the mean ± SD (n = 3). *P < 0.05, vs. control group; #P < 0.05, vs. OGD/R + pre-NC group; $P < 0.05, vs. OGD/R + anti-NC group (Student’s t-test). anti-miR-670: An miR-670 antagomir; anti-NC: a non-sense sequence of the miR-670 antagomir; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; I/R: ischemia and reperfusion; OGD/R: oxygen-glucose deprivation and reoxygenation; pre-miR-670: an miR-670 agomir; pre-NC: a non-sense sequence of the miR-670 agomir; p-Yap: phosphor-yes-associated protein; qPCR: quantitative polymerase chain reaction; Yap: yes-associated protein.
MiR-670 exacerbates neurological impairments of mice after I/R injury
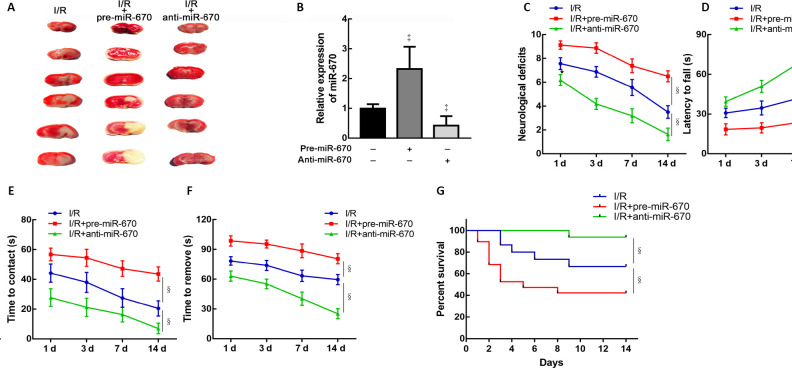
To verify the influence of miR-670 on neurological function after I/R injury in vivo, mice were intracerebroventricularly injected with pre-miR-670 or anti-miR-670 lentivirus 2 weeks before the tMCAO operation. The cerebral infarct volume was increased after miR-670 overexpression (Figure 4A). The transfection efficiency was determined by a quantitative PCR assay (P < 0.05; Figure 4B). Mice overexpressing miR-670 exhibited more severe neurological deficits than the I/R group at 14 days after tMCAO (P < 0.05; Figure 4C). The latency to fall in the rotarod test was greatly increased in miR-670 knockdown mice (P < 0.05; Figure 4D). This result implied an improvement of motor coordination when miR-670 was inhibited. Adhesive removal tests revealed that both the time to contact and removal were shortened in the miR-670 knockdown group. The results indicate that inhibition of miR-670 could alleviate sensorimotor deficits in I/R injury (P < 0.05; Figure 4E and F). In addition, the miR-670 knockdown group had a longer survival time after I/R injury (P < 0.05; Figure 4G).
Figure 4.
MiR-670 exacerbates neurological deficits induced by ischemia and reperfusion injury.
(A) 2,3,5-Triphenyltetrazolium chloride stain of mouse brain tissue slices. (B) Quantitative polymerase chain reaction (qPCR) assay of miR-670 in brain tissue after transfection (n = 9). Relative expression of miR-670 was calculated as the optical density ratio to that of the endogenous control (GAPDH). (C) Neurological deficits were evaluated by modified neurological severity scores in mice with altered miR-670 expression after cerebral I/R injury (n = 9). (D) Rotarod tests of mice with altered miR-670 expression (n = 9). (E, F) Adhesive removal tests of mice with altered miR-670 expression (n = 9). (G) Survival time of mice with altered miR-670 expression (n = 15–17). Data are presented as the mean ± SD. ‡P < 0.05, vs. I/R group; §P < 0.05 (Student’s t-test). The survival curves were generated by Kaplan–Meier analysis. anti-miR-670: An miR-670 antagomir; anti-NC: a non-sense sequence of the miR-670 antagomir; I/R: ischemia and reperfusion; pre-miR-670: an miR-670 agomir; pre-NC: a non-sense sequence of the miR-670 agomir; qPCR: quantitative polymerase chain reaction.
Discussion
Our study showed for the first time that miR-670 was upregulated after cerebral I/R injury. tMCAO and OGD/R models were used to explore the underlying molecular mechanism of I/R injury. The results showed that miR-670 could participate in the regulation of neuronal apoptosis via modulation of Yap expression. Furthermore, Yap phosphorylation was enhanced with increased miR-670 expression. Increased miR-670 levels aggravated neurological impairments and shortened survival time.
Cerebral ischemic stroke can result in many neurological deficits that severely influence the quality of daily life and even threaten the lives of individuals (GBD 2013 Mortality and Causes of Death Collaborators, 2015; Mo et al., 2020). As the most common complication in thrombolytic treatment of cerebral ischemic stroke, I/R injury can lead to aggravated clinical manifestations (Hill et al., 2020). Evidence has indicated that many pathophysiological processes are involved in I/R injury, including endoplasmic reticulum stress (Shu et al., 2018), apoptosis (Tang et al., 2020), autophagy (Yu et al., 2019), and inflammation (Wu et al., 2017). Apoptosis is a crucial process in programmed cell death, and endoplasmic reticulum stress and mitochondria-associated apoptosis pathways have been reported to be of great importance in cerebral I/R injury (Gong et al., 2017). Inhibition of oxidative stress and neuronal apoptosis could impede brain damage after I/R injury (Dai et al., 2018). Additionally, selective brain hypothermia could repress Fis1 expression to reduce apoptosis and act as a neuroprotective factor (Tang et al., 2020). Moreover, salvianolic acid could protect the central nervous system against I/R injury by inhibiting neuronal apoptosis (Hou et al., 2016). In our study, we focused on modulation of apoptosis to further explore the possible factors that influence of I/R injury.
MiRNAs have been confirmed to exert a complex influence on regulation of I/R injury. A previous study suggested that inhibition of miR-106b-5p could repress apoptosis and oxidative stress and alleviate cerebral I/R injury (Li et al., 2017). MiR-431 could negatively regulate the downstream Rho/Rho-kinase signaling pathway and prevent neuron apoptosis after I/R injury (Han et al., 2018). MiR-182-5p was reported to function as an anti-inflammatory factor to protect against I/R-induced cerebral injury via binding with toll-like receptor 4 (Wang et al., 2018). MiR-670 is a non-coding RNA that was verified to induce cell proliferation and migration in hepatocellular carcinoma (Shi and Xu, 2016; Fu et al., 2019). Furthermore, miR-670 could target downstream prospero homeobox 1 by binding to its 3’ untranslated region and inhibit its transcription. Additionally, reduction of miR-670 could inhibit cell proliferation in hepatocellular carcinoma (Shi and Xu, 2016). Another study revealed that miR-670 could act as a competing endogenous RNA of circular RNA ATP binding cassette subfamily B member 10 to regulate downstream high mobility group 20A expression, affecting hepatocellular carcinoma progression (Fu et al., 2019). Furthermore, endometrial cancer patients with downregulated expression of miR-670 and potassium voltage-gated channel modifier subfamily S member 1 had a decreased survival time (Xu et al., 2019). However, little is known about the role of miR-670 in regulation of cerebral I/R injury. In the present study, we confirmed that miR-670 expression was upregulated after I/R injury. Overexpression of miR-670 could increase the apoptosis rate of neurons. In contrast, apoptosis was reduced after miR-670 expression was repressed. These findings indicate a neuroprotective effect of miR-670 inhibition.
Yap is known as a critical regulator in many biological processes. Activation of the anti-oxidative response, which is also regulated by Yap, could relieve damage caused by cardiac injury (Tao et al., 2016). The Hippo-Yap-dependent signaling pathway was also shown to modulate blood-brain barrier restoration and ameliorate neurological impairments (Gong et al., 2019). In addition, the Hippo-Yap pathway was activated to promote optic atrophy 1-associated mitochondrial fusion, preventing the brain reperfusion stress reaction (Wei et al., 2019). Our results suggested that miR-670 might aggravate I/R-induced neurological deficits and neuronal apoptosis by repressing downstream Yap expression. In addition, phosphorylation of Yap was confirmed to enhance Yap ubiquitination by generating a complex, which was degraded by proteasomes (Nguyen and Kugler, 2018). Therefore, we further examined p-Yap expression after I/R treatment of cells with altered miR-670 levels. The results indicated that p-Yap was upregulated by miR-670 overexpression, which implied that miR-670 might accelerate apoptosis after I/R injury by promoting Yap degradation.
However, there are some limitations of our study. Apoptosis may have been induced by oxidative responses and endoplasmic reticulum stress. In addition, other pathways could also participate in the modulation of I/R injury. More specific studies are needed to determine the molecular regulation mechanism of cerebral I/R injury and the role of crosstalk in the future. Based on the pivotal functions of miR-670 in mice and cell models of I/R injury, further clinical studies are needed to explore its underlying roles in patients.
In conclusion, our findings ascertained the expression changes and possible function of miR-670 in cerebral I/R injury for the first time. The underlying molecular mechanism was also explored, and a better understanding of this process was obtained in the present study. MiR-670 was upregulated in brain tissue and neurons subjected to I/R injury and could affect neuronal apoptosis and cerebral damage. Inhibition of miR-670 could increase cell viability and relieve neurological deficits by reducing phosphorylation of downstream Yap and inhibiting Yap degradation. Yap may be a potential candidate for treatment and prognosis prediction of cerebral ischemic stroke.
Additional files:
Additional file 1: Open peer review report 1 (91.2KB, pdf) .
Additional file 2 (110.2KB, pdf) : Original data of the experiment.
Footnotes
P-Reviewer: Neuhaus W; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
Funding: This work was supported by the National Natural Science Foundation of China, Nos. 81771271 (to JF), 81902537 (to MJY), 82001475 (to SJY); a Scientific Fund of Shengjing Hospital of China Medical University, No. M0124 (to SJY); the “345 Talent Project” from Shengjing Hospital of China Medical University (to SJY); the Natural Science Foundation of Liaoning Province of China, No. 20180550913 (to MJY).
Conflicts of interest: All authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Financial support: This work was supported by the National Natural Science Foundation of China, Nos. 81771271 (to JF), 81902537 (to MJY), 82001475 (to SJY); a Scientific Fund of Shengjing Hospital of China Medical University, No. M0124 (to SJY); the “345 Talent Project” from Shengjing Hospital of China Medical University (to SJY); the Natural Science Foundation of Liaoning Province of China, No. 20180550913 (to MJY). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: Experiments were approved by the Institutional Animal Care and Use Committee of China Medical University on February 27, 2017 (IRB No. 2017PS035K). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Winfried Neuhaus, AIT-Austrian Institute of Technology GmbH, Austria.
References
- 1.Bai H, Zhang N, Xu Y, Chen Q, Khan M, Potter JJ, Nayar SK, Cornish T, Alpini G, Bronk S, Pan D, Anders RA. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology. 2012;56:1097–1107. doi: 10.1002/hep.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker JR, Vuppusetty C, Colley T, Hassibi S, Fenwick PS, Donnelly LE, Ito K, Barnes PJ. MicroRNA-570 is a novel regulator of cellular senescence and inflammaging. FASEB J. 2019;33:1605–1616. doi: 10.1096/fj.201800965R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell BC. Thrombolysis and thrombectomy for acute ischemic stroke: strengths and synergies. Semin Thromb Hemost. 2017;43:185–190. doi: 10.1055/s-0036-1585078. [DOI] [PubMed] [Google Scholar]
- 4.Dai Y, Zhang H, Zhang J, Yan M. Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-κB pathway. Chem Biol Interact. 2018;284:32–40. doi: 10.1016/j.cbi.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Flinn MA, Link BA, O’Meara CC. Upstream regulation of the Hippo-Yap pathway in cardiomyocyte regeneration. Semin Cell Dev Biol. 2020;100:11–19. doi: 10.1016/j.semcdb.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu Y, Cai L, Lei X, Wang D. Circular RNA ABCB10 promotes hepatocellular carcinoma progression by increasing HMG20A expression by sponging miR-670-3p. Cancer Cell Int. 2019;19:338. doi: 10.1186/s12935-019-1055-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.GBD 2013 Mortality and Causes of Death Collaborators (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GBD 2016 Lifetime Risk of Stroke Collaborators. Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, Abajobir AA, Abate KH, Abd-Allah F, Abejie AN, Abyu GY, Ademi Z, Agarwal G, Ahmed MB, Akinyemi RO, Al-Raddadi R, Aminde LN, Amlie-Lefond C, Ansari H, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018;379:2429–2437. doi: 10.1056/NEJMoa1804492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong L, Tang Y, An R, Lin M, Chen L, Du J. RTN1-C mediates cerebral ischemia/reperfusion injury via ER stress and mitochondria-associated apoptosis pathways. Cell Death Dis. 2017;8:e3080. doi: 10.1038/cddis.2017.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong P, Zhang Z, Zou C, Tian Q, Chen X, Hong M, Liu X, Chen Q, Xu Z, Li M, Wang J. Hippo/YAP signaling pathway mitigates blood-brain barrier disruption after cerebral ischemia/reperfusion injury. Behav Brain Res. 2019;356:8–17. doi: 10.1016/j.bbr.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan T, Ma J, Li M, Xue T, Lan Z, Guo J, Shen Y, Chao B, Tian G, Zhang Q, Wang L, Liu Y. Rapid transitions in the epidemiology of stroke and its risk factors in China from 2002 to 2013. Neurology. 2017;89:53–61. doi: 10.1212/WNL.0000000000004056. [DOI] [PubMed] [Google Scholar]
- 12.Han XR, Wen X, Wang YJ, Wang S, Shen M, Zhang ZF, Fan SH, Shan Q, Wang L, Li MQ, Hu B, Sun CH, Wu DM, Lu J, Zheng YL. Protective effects of microRNA-431 against cerebral ischemia-reperfusion injury in rats by targeting the Rho/Rho-kinase signaling pathway. J Cell Physiol. 2018;233:5895–5907. doi: 10.1002/jcp.26394. [DOI] [PubMed] [Google Scholar]
- 13.Hao J, Hu H, Jiang Z, Yu X, Li C, Chen L, Xia Y, Liu D, Wang D. microRNA-670 modulates Igf2bp1 expression to regulate RNA methylation in parthenogenetic mouse embryonic development. Sci Rep. 2020;10:4782. doi: 10.1038/s41598-020-61816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill MD, Goyal M, Menon BK, Nogueira RG, McTaggart RA, Demchuk AM, Poppe AY, Buck BH, Field TS, Dowlatshahi D, van Adel BA, Swartz RH, Shah RA, Sauvageau E, Zerna C, Ospel JM, Joshi M, Almekhlafi MA, Ryckborst KJ, Lowerison MW, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020;395:878–887. doi: 10.1016/S0140-6736(20)30258-0. [DOI] [PubMed] [Google Scholar]
- 15.Hou S, Zhao MM, Shen PP, Liu XP, Sun Y, Feng JC. Neuroprotective effect of salvianolic acids against cerebral ischemia/reperfusion injury. Int J Mol Sci. 2016;17:1190. doi: 10.3390/ijms17071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li P, Shen M, Gao F, Wu J, Zhang J, Teng F, Zhang C. An antagomir to microRNA-106b-5p ameliorates cerebral ischemia and reperfusion injury in rats via inhibiting apoptosis and oxidative stress. Mol Neurobiol. 2017;54:2901–2921. doi: 10.1007/s12035-016-9842-1. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Lu T, Zhang C, Xu J, Xue Z, Busuttil RW, Xu N, Xia Q, Kupiec-Weglinski JW, Ji H. Activation of YAP attenuates hepatic damage and fibrosis in liver ischemia-reperfusion injury. J Hepatol. 2019;71:719–730. doi: 10.1016/j.jhep.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu X, Zhang HY, He ZY. MicroRNA-181c provides neuroprotection in an intracerebral hemorrhage model. Neural Regen Res. 2020;15:1274–1282. doi: 10.4103/1673-5374.272612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Micha R, Khatibzadeh S, Shi P, Andrews KG, Engell RE, Mozaffarian D Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (NutriCoDE) Global, regional and national consumption of major food groups in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys worldwide. BMJ Open. 2015;5:e008705. doi: 10.1136/bmjopen-2015-008705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mo Y, Sun YY, Liu KY. Autophagy and inflammation in ischemic stroke. Neural Regen Res. 2020;15:1388–1396. doi: 10.4103/1673-5374.274331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon S, Lee OH, Lee S, Lee J, Park H, Park M, Chang EM, Park KH, Choi Y. STK3/4 expression is regulated in uterine endometrial cells during the estrous cycle. Cells. 2019;8:1643. doi: 10.3390/cells8121643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen TH, Kugler JM. Ubiquitin-dependent regulation of the mammalian hippo pathway: therapeutic implications for cancer. Cancers (Basel) 2018;10:121. doi: 10.3390/cancers10040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234:5451–5465. doi: 10.1002/jcp.27486. [DOI] [PubMed] [Google Scholar]
- 24.Shi C, Xu X. MiR-670-5p induces cell proliferation in hepatocellular carcinoma by targeting PROX1. Biomed Pharmacother. 2016;77:20–26. doi: 10.1016/j.biopha.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 25.Shu Q, Fan H, Li SJ, Zhou D, Ma W, Zhao XY, Yan JQ, Wu G. Protective effects of Progranulin against focal cerebral ischemia-reperfusion injury in rats by suppressing endoplasmic reticulum stress and NF-κB activation in reactive astrocytes. J Cell Biochem. 2018;119:6584–6597. doi: 10.1002/jcb.26790. [DOI] [PubMed] [Google Scholar]
- 26.Tang YN, Zhang GF, Chen HL, Sun XP, Qin WW, Shi F, Sun LX, Xu XN, Wang MS. Selective brain hypothermia-induced neuroprotection against focal cerebral ischemia/reperfusion injury is associated with Fis1 inhibition. Neural Regen Res. 2020;15:903–911. doi: 10.4103/1673-5374.268973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao G, Kahr PC, Morikawa Y, Zhang M, Rahmani M, Heallen TR, Li L, Sun Z, Olson EN, Amendt BA, Martin JF. Pitx2 promotes heart repair by activating the antioxidant response after cardiac injury. Nature. 2016;534:119–123. doi: 10.1038/nature17959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Shi L, Liang T, Wang B, Wu W, Su G, Wei J, Li P, Huang R. MiR-696 regulates C2C12 cell proliferation and differentiation by targeting CNTFRa. Int J Biol Sci. 2017;13:413–425. doi: 10.7150/ijbs.17508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Xu Z, Chen X, Li Y, Chen C, Wang C, Zhu J, Wang Z, Chen W, Xiao Z, Xu R. MicroRNA-182-5p attenuates cerebral ischemia-reperfusion injury by targeting Toll-like receptor 4. Biochem Biophys Res Commun. 2018;505:677–684. doi: 10.1016/j.bbrc.2018.09.165. [DOI] [PubMed] [Google Scholar]
- 30.Wei N, Pu Y, Yang Z, Pan Y, Liu L. Therapeutic effects of melatonin on cerebral ischemia reperfusion injury: Role of Yap-OPA1 signaling pathway and mitochondrial fusion. Biomed Pharmacother. 2019;110:203–212. doi: 10.1016/j.biopha.2018.11.060. [DOI] [PubMed] [Google Scholar]
- 31.Wu R, Li X, Xu P, Huang L, Cheng J, Huang X, Jiang J, Wu LJ, Tang Y. TREM2 protects against cerebral ischemia/reperfusion injury. Mol Brain. 2017;10:20. doi: 10.1186/s13041-017-0296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu X, Liu T, Wang Y, Fu J, Yang Q, Wu J, Zhou H. miRNA-mRNA associated with survival in endometrial cancer. Front Genet. 2019;10:743. doi: 10.3389/fgene.2019.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Liu H, Zhang H, Ye Q, Wang J, Yang B, Mao L, Zhu W, Leak RK, Xiao B, Lu B, Chen J, Hu X. ST2/IL-33-dependent microglial response limits acute ischemic brain injury. J Neurosci. 2017;37:4692–4704. doi: 10.1523/JNEUROSCI.3233-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu S, Yu M, He X, Wen L, Bu Z, Feng J. KCNQ1OT1 promotes autophagy by regulating miR-200a/FOXO3/ATG7 pathway in cerebral ischemic stroke. Aging Cell. 2019;18:e12940. doi: 10.1111/acel.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang JW, Wang X, Li GC, Wang D, Han S, Zhang YD, Luo CH, Wang HW, Jiang WJ, Li CX, Li XC. MiR-30a-5p promotes cholangiocarcinoma cell proliferation through targeting SOCS3. J Cancer. 2020;11:3604–3614. doi: 10.7150/jca.41437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Xin C, Qiu J, Wang Z. Essential oil from pinus koraiensis pinecones inhibits gastric cancer cells via the HIPPO/YAP signaling pathway. Molecules. 2019;24:3851. doi: 10.3390/molecules24213851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.