Abstract
Mice use ultrasonic vocalizations (USVs) to communicate each other and to convey their emotional state. USVs have been greatly characterized in specific life phases and contexts, such as mother isolation-induced USVs for pups or female-induced USVs for male mice during courtship. USVs can be acquired by means of specific tools and later analyzed on the base of both quantitative and qualitative parameters. Indeed, different ultrasonic call categories exist and have already been defined. The understanding of different calls meaning is still missing, and it will represent an essential step forward in the field of USVs. They have long been studied in the ethological context, but recently they emerged as a precious instrument to study pathologies characterized by deficits in communication, in particular neurodevelopmental disorders (NDDs), such as autism spectrum disorders. This review covers the topics of USVs characteristics in mice, contexts for USVs emission and factors that modulate their expression. A particular focus will be devoted to mouse USVs in the context of NDDs. Indeed, several NDDs murine models exist and an intense study of USVs is currently in progress, with the aim of both performing an early diagnosis and to find a pharmacological/behavioral intervention to improve patients’ quality of life.
Keywords: autism spectrum disorders, behavioral phenotyping, emotional state, environmental modulation, maternal immune activation, mouse models, neurodevelopmental disorders, social context, ultrasonic communication, vocalizations classification
Introduction
Mouse communication occurs both in the audible and ultrasonic range of sound frequencies (Ehret and Bernecker, 1986; Zippelius and Schleidt, 1956). Mice predominantly communicate using the ultrasonic vocalizations (USVs) with a frequency from 30 to 110 kHz (Holy and Guo, 2005). Three types of USVs have been largely studied in laboratory mice: - isolation-induced USVs in pups, - interaction-induced USVs in juvenile mice and - interaction-induced USVs in adult mice (Figure 1). For the first time, Zippelius and Schleidt described USVs emitted by pups during separation from the mother and the littermates and they referred to USVs as “whistles of loneliness” able to elicit mother retrieval (Zippelius and Schleidt, 1956). Pups USVs represent an early communicative behavior of the mother-pup dyad; indeed USVs trigger maternal care and facilitate communication between mother and offspring (D’Amato et al., 2005; Hernandez-Miranda et al., 2017). In addition, alterations in the pups USVs features reveal modifications in emotional states of pups and therefore in arousal states of mother (D’Amato et al., 2005; Lahvis et al., 2011). Indeed, different types of calls are produced in response to particular conditions such as the presence of odor of an unfamiliar, potentially infanticidal, adult male (Branchi et al., 1998). Moreover, experiments using mice with alleles linked to social bonding and separation distress, like oxytocin or mu-opioid receptor, demonstrated that isolation-induced USVs are emitted in response to affective variations (Winslow et al., 2000; Moles et al., 2004). In addition, it is also interesting the idea that emotional state and responsiveness of mother influence the emission of pups USVs. D’Amato and colleagues reported an increased number of isolation calls in pups born from mothers with a lower maternal responsiveness such as BALB/c females in comparison with C57BL/6 mothers (D’Amato et al., 2005). The second type of USVs concerns vocalizations of juvenile mice during social interactions and it is correlated with social bonding and motivational level of mice (Panskepp et al., 2007; Peleh et al., 2019). Also adult mice produce USVs in different situations such as courtship, mating and social interaction. The most characterized adult mice USVs are those emitted during male-female interactions and/or in presence of female odor cues/urine. This type of calls is primarily attributed to males to attract females and has an important role in social/sexual behaviors (Egnor and Seagraves, 2016). Using devocalizing males, no USVs were detected during male-female interactions and this supports the idea that is male mice that emitted vocalizations (Sugimoto et al., 2011). However, other authors do not exclude the possibility of USVs production by female mice because during interaction it is not easy to distinguish the animal that produces vocalizations. With the use of new technologies such as a microphone array system and sound source localization method, it is possible to localize and assign USVs to individual mice during a social context (Neunuebel et al., 2015; Heckman et al., 2017; Warren et al., 2018a). This permitted to demonstrate that also female mice vocalize during interaction with male mice, even if further research is needed in this field. Finally, USVs can be detected during male-male and female-female social interactions. Different authors proposed some functions for these vocalizations such as to mediate competition over social status for male (Nyby et al., 1976; D’Amato, 1991; Zala et al., 2017) and to have affiliative purposes for female (Maggio and Withney, 1985; Moles et al., 2007; Zala et al., 2017).
Figure 1.
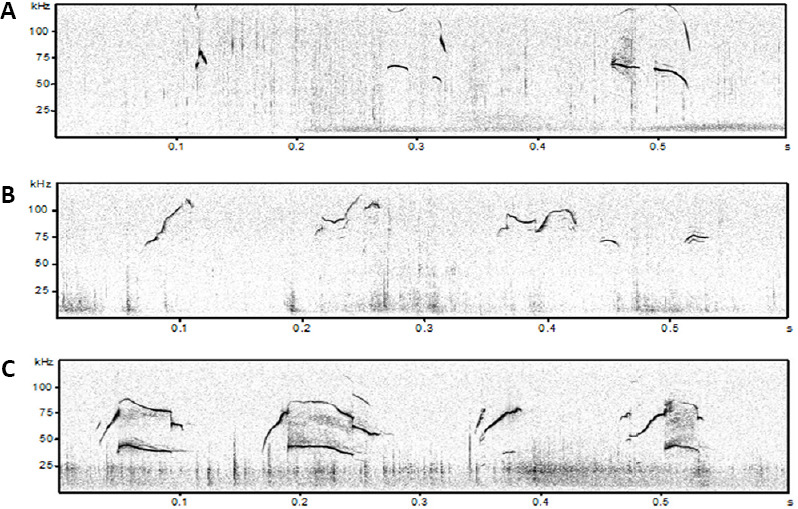
Examples of isolation-induced USVs emitted by B6;129PF2 pups (A), male-female interaction-induced USVs produced by juveniles (B) and adult B6;129PF2 mice (C).
In y axis is reported frequency expressed in kHz and in x axis time expressed in seconds (s). The figures were created exporting images from software Avisoft Bioacoustics. USVs: Ultrasonic vocalizations.
The nature of mice USVs is another interesting theme in this area of research that is still under investigation. Are vocalizations innate or learned? This is a debate yet ongoing. Supporting innate nature of vocalizations there is the fact that mouse pups when born are deaf and theirs hearing starts to develop after ten days of pups life (Ehret, 1975). Therefore, mice cannot be vocal learners during their infancy. Nevertheless, Holy and Guo suggested that USVs produced by adult male mice have features similar to songs of birds that are vocal learners (Holy and Guo, 2005). This has opened the debate on the idea that mice can be a model for vocal learning. Imitation of another species calls is essential to demonstrate vocal learning. Kikusui et al. (2011) demonstrated the incapacity of mice to imitate USVs of other strains during cross-fostering experiments. This is another evidence for innate nature of USVs. In addition, auditory experience during development is not necessary to produce normal vocalizations in mice (Hammerschmidt et al., 2012). Indeed, USVs of hearing and deaf mice were qualitatively similar (Mahrt et al., 2013). For these reasons, USVs seems to be innate and not learned. Other recent studies reported limited vocal plasticity in mice linked to the absence or limited presence of a forebrain pathway that it is very developed in vocal learners such as birds and humans (Arriaga and Jarvis, 2013). On the contrary, to demonstrate vocal learning in mice, several experiments focused on acoustic changes of USVs in different contexts were done. In particular, recent studies found evidences for learned nature of murine USVs due to modifications of USVs features during development (Grimsley et al., 2011), after isolation (Chabout et al., 2012) and in a competitive social condition (Arriaga et al., 2012). Therefore, the argument about the innate or learned nature of USVs is still totally open and under study.
Regarding specific features that describe mice USVs types, there are several temporal spectral components such as vocalization rate, frequency and duration that depend from different factors including age and genetic background of mice. In particular, the USVs rate induced by maternal isolation increases during the first week of pup life, reaching a peak that depends from genetic strain of mice, and it decreases until the end of the second week of pup life (Elwood and Keeling, 1982). Then, the emission of USVs from mouse pups is interrupted for a period that corresponds to the insurgence of social stimuli aging as trigger for USVs. Later, vocalizations rate increases again during social interactions especially between males and females (Warburton et al., 1989). Furthermore, the vocal repertoire concerning different types of vocalizations (as the syllable vocabulary) is unvaried by age of mice. Indeed, both pups and adults are able to emit all types of USVs except noisy syllables (Grimsley et al., 2011). Nevertheless, the proportion of USVs different types changes by age and also the other acoustic features, such as the duration and the frequency of USVs types, decrease with mice advancing age (Heckman et al., 2016; Peleh et al., 2019). In particular, with the increase of age, pups emit calls with more complex features. This pattern continues in juvenile mice with a decreased number and duration of calls (Peleh et al., 2019). Pups produce many calls with a frequency above 100 kHz unlike adult mice that emit calls with a lower frequency. Other differences in calls features concern duration of intervals between syllables with a longer inter-syllable intervals in younger than adult mice (Grimsley et al., 2011). All these features of vocalizations together with others, such as amplitude and bandwidth (i.e., the range of frequencies that a signal spanned), can be detected recording USVs with an ultrasound sensitive microphone and can be quantitatively analyzed by specific software (e.g., Avisoft Bioacoustics, Metris Sonotrack and Noldus UltraVox XT). Each vocalization can be also qualitatively classified into different categories based on several criteria. One of the most used classifications for mice is described by Scattoni et al. (2008) including 10 categories that are: complex, harmonics, two-syllable, upward, downward, chevron, short, composite, frequency steps and flat calls. Examples of spectrograms of these 10 different calls typologies emitted by mice are reported in Figure 2. In addition, also other qualitative classification methods exist in the literature (Holy and Guo, 2005; Gaub et al., 2016; Grimsley et al., 2016). To date, specific meaning of calls categories is still unknown and it will be very interesting to find out more on this topic in the future. Finally, some automated systems can be used to deeply analyze USVs of rodents with standardized methods of machine learning (van Segbroeck et al., 2017; Coffey et al., 2019; Vogel et al., 2019).
Figure 2.
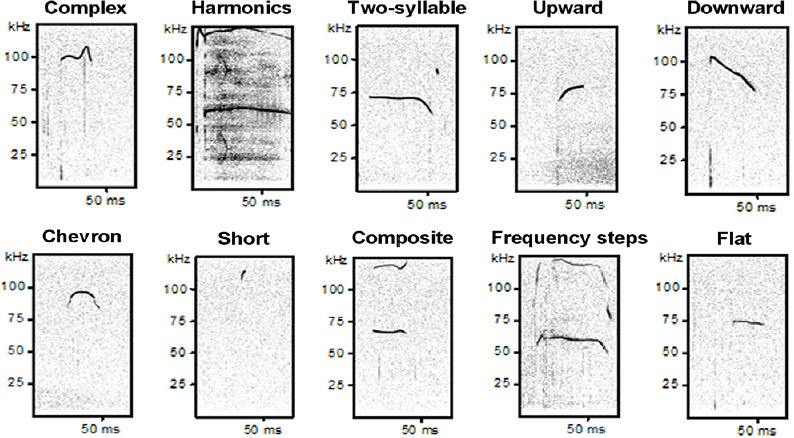
Examples of spectrograms of different USVs categories emitted by B6;129PF2 mice.
In y axis is reported frequency expressed in kHz and in x axis is time interval expressed in milliseconds (ms). The figures were created exporting images from software Avisoft Bioacoustics. USVs: Ultrasonic vocalizations.
Data Source
Studies cited in this review were found on the PubMed database, between April and August 2020, using the following keywords: ultrasonic communication in mice, neurodevelopmental disorders, autism spectrum disorders, mouse models, environmental modulation, drug, immune system and various combinations of the above terms.
Relevance of Ultrasonic Vocalizations Study
Mice produce USVs to convey information related to positive or negative emotional states and to mediate social interactions. Communication is intensely linked to social behavior and for these reasons, USVs study has become a valid assay in behavioral readout and monitoring in this context (Granon et al., 2018). For several decades, USVs have been extensively analyzed from an ethological point of view but in recent times the USVs study has acquired large importance in the field of psychiatric and neurological disorders, starting from those characterized by communication and social interaction deficits such as neurodevelopmental disorders (NDDs) and in particular autism spectrum disorders (ASD) (Moy and Nadler, 2008; Scattoni et al., 2009; Fischer and Hammerschmidt, 2011; Simola and Granon, 2018). Quantitative and qualitative alterations in USVs are reported in numerous mouse models of NDDs and ASD. USVs produced from several genetic strains of mice are different, being influenced by genetic background of animal (Sugimoto et al., 2011). In particular, variations in number, duration, frequency and other quantitative parameters have been found. In addition, also qualitative differences in calls typology were detected between control and NDDs/ASD mouse models (Roy et al., 2012; Hodges et al., 2017; Premoli et al., 2019). It is very interesting to analyze USVs from a qualitative point of view because it permits to investigate the meaning and potential functions of vocalizations in order to use them as a valid tool in NDDs and ASD field. Regarding this, experiments were performed to assign specific USV calls to different behaviors during social interactions (Warren et al., 2018b). In addition, Sangiamo and colleagues demonstrated that distinct ultrasonic vocalizations are associated with different types of murine social behavior highlighting important role of communication in social contexts (Sangiamo et al., 2020). Furthermore, since communication impairment is one of the core symptoms of ASD, altered calling patterns in mice can offer a useful assay for the diagnosis of ASD and to study the effects of pharmacological/behavioral interventions. Therefore, mouse ultrasonic communication analysis is a fundamental tool to investigate the mechanisms at the basis of these brain disorders and to test the efficacy of pharmacological treatments for these pathologies.
This review provides an overview of different contexts in which it is important to study mouse ultrasonic communication and highlights the relevance of USVs use in NDDs and ASD paradigms.
Ultrasonic Vocalizations and Environmental Modulation
Between factors that can induce USV modifications, in addition to genetic background of mice, several stimuli can be grouped as “environmental stimuli”. Since mice emit USVs to communicate their emotional state or to coordinate social interaction (Simola and Granon, 2018), these environmental stimuli affect these fields. They can be split in two main categories: social context, and immunity.
Ultrasonic vocalizations and social context
Social context can constitute a positive stimulus for mice emotional state, such as social enrichment, or negative, such as social stress or segregation. All these situations significantly affect mice vocalizations (Table 1).
Table 1.
Environmental modulation effect on ultrasonic communication
| Effect of environmental modulation on USVs | USVs features | Strains | References |
|---|---|---|---|
| Social context | |||
| Pups | ↓ Number when exposed to adult male odor | Shank1 KO | Sungur et al. (2016) |
| ↓ Number when exposed to nest odor vs. clean bedding | WT, µ-opioid receptor KO, serotonin receptor 1A KO | D’Amato and Cabib (1987); Moles et al. (2004); Zanettini et al. (2010) | |
| ↓ Number and duration when exposed to soiled vs. clean bedding | WT and BTBR | Wöhr (2015) | |
| ↑ Number when exposed to pheromones of adult male | WT | Kessler et al. (2011) | |
| ↓ Number when exposed to maternal enrichment | WT and Fmr1 KO | Oddi et al. (2015) | |
| ↑ Number when separated from mother vs. standard-facility reared | Balb/c and WT | Feifel et al. (2017) | |
| Adults | Gender effect on number, frequency and amplitude | WT | Zala et al. (2017) |
| ↓ Number when exposed to communal nesting vs. standard nesting | WT | Gracceva et al. (2009) | |
| ↓ Number when grouped vs. isolated | WT | Chabout et al. (2012) | |
| ↓ Number when isolated vs. exposed to other mice | CBA/CaJ | Burke et al. (2018) | |
| Male audience effect on number and acoustic features | SWR/J | Seagraves et al. (2016) | |
| ↓ Number in socially safe environment | WT and neuronal nicotinic acetylcholine receptor KO | Chabout et al. (2013) | |
| ↑ Number when emitted by dominant male vs. subordinate male mice | DBA/2J | Nyby et al. (1976) | |
| ↑ Number of restrain-induced USVs by isolation and presence of a social congener; ↑ Number of free moving-induced USVs by isolation | WT | Lefebvre et al. (2020) | |
| MIA context | MIA vs. vehicle group | ||
| Pups | ↑ Or ↓ number | LPS and Poly I:C models | Jouda et al. (2019) |
| ↓ Number and duration on PND 8, 10 and 12 | Poly I:C model | Malkova et al. (2012) | |
| ↑ Number on PND 8 in BTBR and ↑ number on PND 10 in BTBR and WT | Poly I:C model | Schwartzer et al. (2013) | |
| ↓ Or ↑ number | Poly I:C model; LPS model | Carlezon et al. (2019) | |
| ↑ Number | Poly I:C model | Choi et al. (2016); Kim et al. (2017) | |
| ↓ Number | LPS model | Schaafsma et al. (2017) | |
| ↑ Number and duration | LPS model | Aria et al. (2020) | |
| Adults | ↓ Number | Poly I:C models | Malkova et al. (2012); Jouda et al. (2019) |
KO: Knock-out; MIA: maternal immune activation; USVs: ultrasonic vocalizations; WT: wild-type.
In pups, besides developmental factors, social odors play an important role in modulating USVs emission (Branchi et al., 1998). This is particularly relevant for ASD mouse models, as ASD is characterized by deficits in the processing of social context information (American Psychiatric Association, 2013). In the context of maternal separation, mouse pups emit fewer USVs when exposed to nest odor than to clean bedding (D’Amato and Cabib, 1987; Moles et al., 2004; Wöhr, 2015). Typically, the presence of odors from mothers and littermates produce a calming response of the isolated mouse pup, and hence to a reduction in isolation-induced USVs emission (Moles et al., 2004; Zanettini et al., 2010). Interestingly, in BTBR mice, a mouse model of ASD, it was observed that this social context produces a reduction in isolation-induced USVs, but, contrary to the control wild-type (WT) pups, that emitted USV with shorter call durations and lower levels of frequency modulation, it had no effect on acoustic call features (Wöhr, 2015). Furthermore, the exposure to pheromones of an adult male induces a significant increase in pups USVs emission, as a result of anxiety related behavior (Kessler et al., 2011). In another model of ASD, Shank1 knock-out (KO) pups, this adverse social context (adult male odor) caused a significant reduction in USVs emission, compared to WT pups, so exacerbating communication deficits in the ASD mice (Sungur et al., 2016).
Early social enrichment, in particular housing pups until weaning with the mother and an additional female, resulted to decrease both number and duration of USVs emitted during maternal separation in postnatal day (PND) 8 pups (Oddi et al., 2015). This effect was observed both in WT and in a murine model of ASD, Fmr1 KO mice with FVB background and suggests a weaker emotional response to maternal separation. In another study, communal nesting was used as procedure to induce early social enrichment and it provoked decreased USVs emission rate in the social recognition test in adult female offspring (Gracceva et al., 2009).
Another important stimulus for pup vocalizations is early life stress. It was demonstrated that mice, both Balb/c and C57BL/6, emit significantly more isolation-induced USVs in the first PNDs after infant maternal separation (a common stressful stimulus) compared to standard-facility reared pups (Feifel et al., 2017).
In adult mice, social context is a key factor for USVs emission and regulation. Mice emit the highest number of calls with the largest diversity of call types during social interaction. In particular, it has been proven that mice of both sexes emit vocalizations at a higher rate and higher frequencies during opposite-sex compared to same-sex interaction and that male mice emit USVs with higher amplitude in presence of a male mouse compared to a female mouse stimulus, thus suggesting an USVs modulation depending on the gender of potential receiver (Zala et al., 2017).
Furthermore, housing conditions (isolation or grouped), that act as a modulator factor for social motivation, significantly influence vocal behavior during social interaction (Chabout et al., 2012). In addition, Burke and colleagues demonstrated that prior social experiences deeply influence USVs production (Burke et al., 2018). They found significant differences in USVs number and call types that isolated mice (CBA/CaJ strain, both genders) produce across exposure conditions (following period of isolation or an exposure of a same- or opposite-sex mouse).
Also, a complex social environment such as the presence of additional listeners (male mice) during male-female mice courtship, alters vocal behavior (Seagraves et al., 2016). In particular, Seagraves et al. (2016) demonstrated the existence of an “audience effect” in mice: male vocal behavior elicited by female odor is affected by the presence of a male audience, with changes in vocalizations count, acoustic structure and syllable complexity. This effect requests multiple cues, indeed a single sensory cue (odor or vocalizations), indicating the presence of a male audience, was not sufficient to elicit an effect; probably there is the need for an audience to be apparent. Moreover, a socially safe environment (in the experiment consisting in the three chamber apparatus, without physical threat) dramatically reduces communication between two adult mice competing for a natural reward, compared to a more uncertain environment (in the experiment consisting in two mice freely interacting in a large and novel cage) (Chabout et al., 2013). A relevant factor for male mice vocalization is also social status; indeed, Nyby and colleagues (1976) demonstrated that when interacting with a female, dominant males produce more USVs compared to subordinate males. Dominant males also emit wider vocal repertoire than mice avoiding social interactions (Sangiamo et al., 2020).
Finally, adult mice emit USVs also in non-social context, such as exploration of a novel environment and restrain stress, also if much less compared to social context, and only during exploration task social motivation has been demonstrated to significantly modulate vocal behavior (Chabout et al., 2012).
Stress is another key factor modulating USVs in adult mice. A commonly used behavioral paradigm to induce stress is restrain (immobilization in a small container for few minutes), as it causes anxiety-like behavior and increase in corticosterone levels. Lefebvre and colleagues demonstrated that restrain induces low-frequency USVs (≤ 60 kHz) in mice and these calls emission is increased by both a previous period of social isolation and the presence during restrain of a social congener (Lefebvre et al., 2020). Free exploration of a novel environment is also considered a stressful situation, as it induces anxiety. This behavioral task induced low-frequency USVs that resulted to be quantitatively decreased by a previous period of social isolation (Lefebvre et al., 2020).
Ultrasonic vocalizations and maternal immune activation
Most of studies that found a robust effect of immune system status on USVs in mice were performed in maternal immune activation (MIA) models. Indeed, stimulation of immune system of pregnant dams, by means of both bacterial or viral agents, causes significant changes in offspring vocalizations (Jouda et al., 2019). Final effect of infection on USVs differs between different experiments due to the stimulus used, the dose, gestational time, offspring gender and frequency of injection. Activation of the maternal immune system during pregnancy causes profound behavioral alterations in the offspring, resembling behavioral deficits typical of certain neuropsychiatric disorders, including a significant impairment in ultrasonic communication (Malkova et al., 2012; Carlezon et al., 2019). In brief, injection of poly I:C, a viral stimulus, in C57BL/6J female mice at 10.5, 12.5 and 14.5 gestational days caused a reduction of isolation-induced USVs in pups from PND 8, and qualitatively differences in comparison with USVs of pups born from saline-injected mothers (fewer harmonics, more complex and short syllables). Adult male offspring also presented altered vocalizations, with fewer USVs in response to social encounters (Malkova et al., 2012). Carlezon and colleagues (2019) studied both the effect of MIA (by poly I:C injection at gestational day 12.5) and the effect of early life immune system activation (EIA, by the injection of a bacterial agent named lipopolysaccharide, LPS, in the offspring at PND 9) on C57BL/6J mice communication. They found that MIA significantly reduced maternal separation-induced USVs compared to vehicle at PND 10, specifically in male pups, whereas EIA led to an increase in USVs in both males (at PND 14) and females (at PND 12); furthermore the two-hits (MIA+EIA) induced a significant increase in USVs compared to vehicle-treated pups in both female and male pups at PND 12, 14 and 16. Concerning USVs quality, they found that at PND 12, EIA and two-hits induced in both genders an increase in calls under 75 hertz frequency compared to vehicle pups vocalizations (Carlezon et al., 2019). In another study, a single poly I:C injection at embryonic day 12.5 caused no significant changes in maternal isolation-induced USVs number at PND 8 in C57BL/6J strain pups, but only in the BTBR strain pups; instead, calls emitted at PND 10 resulted significantly increased in pups of both strains compared to control pups (Schwarzer et al., 2013). Also Choi and colleagues recorded increased isolation-induced USVs number in pups at PND 9 in the same MIA model, demonstrating a direct link with the pro-inflammatory interleukin 17 (Choi et al., 2016; Kim et al., 2017). In addition, in a study published in 2016, Cntnap2 mice were prenatally exposed to LPS (0.3 mg/kg at embryonic day 7) and this provoked a significant decrease in maternal separation-induced vocalizations at PND 3. Furthermore, the MIA dependent effect on USVs resulted to be the strongest hit, when compared to other two hits: genotype (KO versus WT) and sex (male versus female) (Schaafsma et al., 2017). Finally, Aria and colleagues also used LPS as MIA inducing stimulus (0.3 mg/kg chronic administration during pregnancy) in B6;129PF2 mice, and this caused a significant increase in both number and duration of isolation-induced ultrasonic calls in pups from PND 4 to 8 (Aria et al., 2020). Therefore, perturbation of the immune system during brain development, both in the pre-natal and the post-natal periods, causes dramatic and long-lasting effects on mice communication, with some specific differences between genders, that sometimes exacerbate the mice social behavior impairment.
Ultrasonic Vocalizations and Murine Models of Neurodevelopmental Disorders
NDDs are a heterogeneous group of neurobehavioral disorders with an early onset during development characterized by alterations in social interactions, communication, cognition and motor behaviors (Homberg JR et al., 2016; Silverman and Ellegood, 2018; Mossa and Manzini, 2019). Several genes have been associated with increased risk for NDDs, and rodent models are very useful for research in this field. Murine models with a high construct and face validity are able to reproduce molecular and behavioral modifications typical of human pathologies (Foxe et al., 2018; Vogel et al., 2019). In NDDs mouse models, it is possible to test social communication deficits recording and analyzing USVs. In this review, analysis of ultrasonic communication in some models of NDDs and in particular in ASD models will be discussed.
Fmr1 knock-out mice
Fragile X syndrome (FXS) is a NDD caused by the expansion of a CGG triplet in the X-linked fragile X mental retardation gene (FMR1) resulting in the absence of the FMR protein (FMRP) (Pieretti et al., 1991) that modulates mRNA trafficking, dendritic maturation and synaptic plasticity (Greenough et al., 2001). FXS is the most common inherited form of intellectual disability and monogenic cause of ASD (Rogers et al., 2001). Models of Fmr1 KO mice were generated and they are very useful to study both FXS and autistic disorders. Indeed, Fmr1 KO mice display many of the symptoms typical of FXS and ASD patients, including cognitive deficits, altered social interaction and communication, repetitive behaviors, seizures and also cortical cytoarchitecture deficits (Gaudissard et al., 2017; Hodges et al., 2017; Sarè et al., 2019). Regarding ultrasonic communication, several authors have studied USVs in Fmr1 KO mice (Table 2). Lai and colleagues (2014) investigated features of USV spectrograms emitted by Fmr1 KO pups with FVB/NJ background, and they found an increased number of calls, especially frequency jump calls on PND 7 in comparison with WT pups. In addition, a shift in USVs temporal distribution was found in Fmr1 KO pups (Lai et al., 2014). Another analysis of ultrasonic communication has been performed by Reynolds and colleagues (2016), showing a reduction in frequency and duration of USVs in Fmr1 KO with FVB/NJ background in comparison with WT pups from PND 9 to 14. Furthermore, gender differences were found in calling pattern of this model (Reynolds et al., 2016). On the contrary, no quantitative differences in number or duration of USVs, but only a decreased proportion of downward calls, were detected in Fmr1 KO with B6 background on PND 8 from Roy’s study (Roy et al., 2012). Finally, in a study on Fmr1 KO pups with B6 background it emerged that they emitted a similar USVs rate, but with longer duration than WT pups (Gaudissard et al., 2017). USVs analysis has revealed more calls emitted by adolescent than adult mice but without genotype differences (Gaudissard et al., 2017). Other papers described the study of ultrasonic communication in Fmr1 KO adult mice. For example, Rotschafer reported a reduced rate of USVs in Fmr1 KO with FVB background compared to WT adult male during mating behavior (Rotschafer et al., 2012). Instead, no differences in USVs number but only a different proportion of calls typologies were recorded in another courtship paradigm including high level of upward syllables for KO with B6 background and harmonics calls for WT adult males (Belagodu et al., 2016). Lastly, the effect of exposition to female urine on USVs features was evaluated, resulting in higher frequency, lower amplitude and duration of vocalizations in Fmr1 KO adult male with FVB background in comparison with control mice as well as a different USVs repertoire (Hodges et al., 2017).
Table 2.
Altered ultrasonic communication in Fmr1 KO mice
| Mouse model of ASD | USVs features vs. WT mice | References | |
|---|---|---|---|
| Quantitative parameters | Qualitative parameters | ||
| Fmr1 KO (FVB background) | |||
| Pups | ↑ Number on PND 7 | ↑ Frequency steps calls on PND 7 | Lai et al. (2014) |
| ↓ Number and duration on PND 9–14 | Reynolds et al. (2016) | ||
| Adults | ↓ Duration and amplitude, ↑ peak frequency | ↑ Complex, chevron and flat; ↓ composite and frequency steps calls | Hodges et al. (2017) |
| ↓ Number | Rotschafer et al. (2012) | ||
| Fmr1 KO (B6 background) | |||
| Pups | No differences in number but ↑ duration on PND 4, 6, 8, 10 and 12 | Gaudissard et al. (2017) | |
| No differences in number and duration on PND 8 | ↓ Downward calls on PND 8 | Roy et al. (2012) | |
| Adolescents | No differences in number and duration | Gaudissard et al. (2017) | |
| Adults | No differences in number and duration | ↑ Upward calls | Belagodu et al. (2016); Gaudissard et al. (2017) |
ASD: Autism spectrum disorders; KO: knock-out; PND: postnatal day; USVs: ultrasonic vocalizations; WT: wild-type.
BTBR mice
The BTBR T+tf/J (BTBR) mouse strain was bred for insulin resistance studies, diabetes-induced nephropathy and phenylketonuria (Clee et al., 2005) but recently, it has been associated also to ASD. Indeed, it is one of the most known idiopathic models of ASD because it displays core symptoms typical of ASD patients such as social deficit, impaired communication and repetitive stereotype behaviors as well as neuroanatomical abnormalities (Meyza and Blanchard, 2017; Faraji et al., 2018). Analyses of ultrasonic communication in this model (Table 3) have revealed a higher number of isolation-induced USVs with a longer duration than WT pups. BTBR pups emitted louder USVs with a lower frequency in comparison with WT pups on PND 6 and 8. From a qualitative point of view, specific alterations of calls typologies were found such as high level of harmonics, two-syllable and composite calls on PND 8 (Scattoni et al., 2008). This unusual calling pattern in BTBR pups suggests the translational value of pups USVs with human babies cry in ASD context (Esposito et al., 2017).
Table 3.
Altered ultrasonic communication in BTBR mice
| Mouse model of ASD (BTBR) | USVs features vs. WT mice | References | |
|---|---|---|---|
| Quantitative parameters | Qualitative parameters | ||
| Pups | ↑ Number, duration and peak amplitude; ↓ peak frequency on PND 8 | ↑ Harmonics, two-syllable and composite calls on PND 8 | Scattoni et al. (2008) |
| Adolescents | ↓ Number | Scattoni et al. (2013) | |
| Adults | ↓ Number | Different USVs repertoire on basis of different social interaction test | Scattoni et al. (2011); Wöhr et al. (2011a); Yang et al. (2013) |
ASD: Autism spectrum disorders; PND: postnatal day; USVs: ultrasonic vocalizations; WT: wild-type.
Communication deficit continues during adolescence and adulthood with a decreased USVs number in BTBR than control mice (Scattoni et al., 2011, 2013). Indeed, reduced USVs rate was detected in BTBR adult mice during male-male, female-female and male-female social interactions. In particular, BTBR males emitted fewer chevron, two-syllable and frequency steps in presence of other males. Females displayed a narrow repertoire of USVs that included upward and short calls when they interacted with other females with a reduction of complex, chevron and frequency steps calls. Instead, during male-female interactions, BTBR mice emitted fewer short and frequency steps calls than control mice (Scattoni et al., 2011). Furthermore, Yang et al. (2013) studied interaction-induced USVs, founding a distinct calling scheme of BTBR males after the removal of females (Yang et al., 2013). Finally, ultrasonic communication deficit was found in BTBR adult male mice also in presence of female mice urine (Wöhr et al., 2011a).
Cntnap2 knock-out mice and Nlgn mutant mice
Mutations in genes coding for synaptic cell adhesion molecules, such as neuroligins, neurexins and contacting-associated proteins have been associated to NDDs and ASD (Penagarikano et al., 2012; El-kordi et al., 2013). Contactin associated protein-like 2 (Cntnap2) KO mice display a reduced ultrasonic communication (Table 4) as well as other ASD core behavioral features and neurophysiological alterations (Penagarikano et al., 2011; 2015, Mohrle et al., 2020). In particular, Cntnap2 KO pups emit less USVs than WT pups when they are isolated from the mother (Penagarikano et al., 2011). USVs production remains altered in this model also during adulthood in presence of female mice urine (Brunner et al., 2015). Also in another model of ASD, the neuroligins (Nlgn) mutant mouse, ultrasonic communication was analyzed. Reduced isolation-induced USVs were found in Nlgn2 KO pups but without differences in other USVs parameters in comparison with their control pups (Wöhr et al., 2013). Conflicting results were detected in Nlgn4 KO mice. Indeed, a reduction of USVs number was reported in female juvenile mice of this model (Ju et al., 2014) and during interaction of adult males with adult females (Jamain et al., 2008). On the contrary, Ey and colleagues did not find alterations in USVs pattern in pups and adults of this model (Ey et al., 2012).
Table 4.
Altered ultrasonic communication in Cntnap2 KO mice and Nlgn mutant mice
| Mouse model of ASD | USVs features vs. WT mice | References |
| Quantitative parameters | ||
| Cntnap2 KO | ||
| Pups | ↓ Number on PND 3, 6 and 9 | Peñagarikano et al. (2015) |
| Adults | ↓ Number | Brunner et al. (2015) |
| Nlgn2 KO | ||
| Pups | ↓ Number and total calling time on PND 7 | Wöhr et al. (2013) |
| Nlgn4 KO | ||
| Pups | No differences in number and duration | Ey et al. (2012) |
| Adolescents | ↓ Number and duration and ↑ latency to first call | Ju et al. (2014) |
| Adults | ↓ Number | Jamain et al. (2008) |
| No differences in number and duration | Ey et al. (2012) |
ASD: Autism spectrum disorders; KO: knock-out; PND: postnatal day; USVs: ultrasonic vocalizations; WT: wild-type.
Shank models
SHANK proteins are a family of scaffolding proteins that connect the actin cytoskeleton of the dendritic spine with proteins of the postsynaptic membrane, promoting synapse formation and spine maturation (Monteiro and Feng, 2017). Several studies have reported a correlation between mutations in SHANK genes and autism (Sato et al, 2012; Bai et al, 2018). Three Shank mouse models for ASD have been generated and all of them have some characteristic abnormalities of this neurodevelopmental disorder (for Shank1: Sungur et al., 2016, 2017, 2018; for Shank2: Eltokhi et al, 2018; for Shank3: Wang et al., 2011; Balaan et al., 2019).
Shank1 knock-out
Four studies on ultrasonic communication analysis in this model have been conducted (Table 5). At first, Wöhr and colleagues (2011) reported that Shank1 KO pups emitted less USV induced by maternal separation and spent less time calling in comparison with WT pups on PND 8. Furthermore, their USVs peak frequency was higher and frequency modulation was lower than those of WT pups. This calling pattern was present only in females and not in males pups (Wöhr et al., 2011b). Wöhr found two groups of USVs (e.g. the first between 50 and 80 kHz and the second between 80 and 100 kHz) present in both genotypes but with a different distribution. Indeed, the majority of WT USVs belonged to the first group, whereas Shank1 KO USVs were distributed similarly between the two groups. Comparing the two genotypes, reduced number of KO USVs was related to the first group of vocalizations (Wöhr, 2014). Other authors have confirmed reduced USVs in Shank1 KO pups extending analysis of USVs to several days of pups development and evidenced the fact that ultrasonic communication deficits were more marked in presence of a social odor (Sungur et al., 2016).
Table 5.
Altered ultrasonic communication in Shank models
| Mouse model of ASD | USVs features vs. WT mice | References | |
|---|---|---|---|
| Quantitative parameters | Qualitative parameters | ||
| Shank1 KO | |||
| Pups | ↓ Number and frequency modulation, ↑ peak frequency | Different distribution of USVs in two groups (50–80 kHz and 80–100 kHz) on PND 8 | Wöhr (2014); Wöhr et al. (2011b); Sungur et al. (2016) |
| Adolescents | No differences in number | Sungur et al. (2017) | |
| Adults | No differences in number | Wöhr et al. (2011b) | |
| Shank2 KO (exon 7) | |||
| Pups | ↑ Number in female on PND 4 and 10 | Schmeisser et al. (2012) | |
| ↓ Number on PND 6 | Ey et al. (2013) | ||
| Adults | ↓ Number in female-female social interaction test; no differences in number in male-female interaction social test | Schmeisser et al. (2012); Ey et al. (2013) | |
| Shank2 KO (exon 6–7) | |||
| Adults | ↓ Number | Won et al. (2012) | |
| Shank3 heterozygous | |||
| Adults | ↓ Number and ↑ latency to call | Bozdagi et al. (2010) | |
| Shank3 KO | |||
| Pups | No differences in number and duration | Yang et al. (2012); Balaan et al. (2019) | |
| Adults | ↓ Number, duration and frequency modulation in female-female social interaction test; ↑ number, ↓ duration and frequency modulation in male-female social interaction test | Wang et al. (2011) | |
| No differences in number in presence of female urine | Yang et al. (2012) | ||
ASD: Autism spectrum disorders; KO: knock-out; PND: postnatal day; USVs: ultrasonic vocalizations; WT: wild-type.
Regarding interaction induced USVs in juveniles, no genotype differences were detected (Sungur et al., 2017). At adulthood, the number of USVs produced by males during female urine exposition did not vary between genotypes. However, calling pattern of WT changed on the basis of their previous exposure to female but not in Shank1 KO male mice (Wöhr et al., 2011b).
Shank2 knock-out
Shank2 KO models display alterations in ultrasonic communication (Table 5). Schmeisser and colleagues observed increased number of USVs in female, and not in male, Shank2 KO pups with deletion of exon 7 on PND 4 and 10 (Schmeisser et al., 2012). Another study was conducted on this model but no differences between male and female mice were detected (Ey et al., 2013). In addition, Shank2 KO pups lost their typical inverted U-shape developmental pattern with calls peak on PND 6, leading to a reduced number of calls in this day (Ey et al., 2013). During female-female social interaction test, ultrasonic vocalizations were recorded obtaining a reduced calls rate in Shank2 KO adult female mice (Schmeisser et al., 2012; Ey et al., 2013). Longer latency to emit the first USV during male-female social interactions was found in Shank2 KO in comparison with WT adult mice but no change in number of calls was noticed (Schmeisser et al., 2012; Ey et al., 2013). In other Shank2 KO model (with deletion of exon 6 and 7), Won and colleagues reported a reduced emission of USVs in adult male mice in presence of a female (Won et al., 2012).
Shank3 knock-out
Analysis of ultrasonic communication in Shank3 KO pups (Table 5) did not reveal genotype effects on calling rate and duration of USVs (Yang et al., 2012; Balaan et al., 2019). On the contrary, adult Shank3 KO females emitted fewer calls but they produced more calls with a shorter duration than those of WT mice during social interaction with female. Also their USVs frequency modulation was decreased in comparison with WT mice (Wang et al., 2011). Opposite results were obtained in Shank3 KO male mice, including an increased USVs rate compared to WT mice in one study (Wang et al., 2011) and no genotype change in USV emission founded in another study (Yang et al., 2012). Finally, reduced USVs emission pattern with an increased latency to call was detected in adult heterozygous males during social interaction with females (Bozdagi et al., 2010).
p50 knock-out mice
p50 KO mice have a deletion of the NF?B1 gene coding for the NF-κB p50 subunit. Nuclear factor-κB (NF-κB) pathway is involved in many processes such as neuron plasticity, neurogenesis, cell proliferation, apoptosis and immune system regulation (Kaltschmidt and Kaltschidt, 2009; Gutierrez and Davies, 2011). p50 KO mice display immune responses defects, hyperactivity, reduced anxiety behavior and altered hippocampal neurogenesis associated with cognitive deficit in spatial short-term memory (Sha et al., 1995; Kassed and Herkenham, 2004; Denis Donini et al., 2008). Recently, they have been characterized as a model of NDDs because they have cortical structural abnormalities and some behavioral alterations typical of neurodevelopmental disorders, such as social interaction deficit and ultrasonic vocalizations communication impairment (Bonini et al., 2016; Mastinu et al., 2018; Premoli et al., 2019). Our research group analyzed ultrasonic communication in this model, founding a higher number and duration of maternal isolation-induced USVs compared to WT pups. On the contrary, during adulthood, p50 KO emitted less USVs than WT adult males in presence of adult females. A detailed qualitative analysis has revealed specific changes in calling pattern involving only two USVs categories, such as two-syllable and frequency steps, that are altered both during infancy and adulthood in p50 KO mice (Premoli et al., 2019; Table 6).
Table 6.
Altered ultrasonic communication in p50 KO mice
| Mouse model of ASD (p50 KO) | USVs features vs. WT mice | References | |
|---|---|---|---|
| Quantitative parameters | Qualitative parameters | ||
| Pups | ↑ Number and duration | ↑ Two-syllable and frequency steps calls on PND 6 | Premoli et al. (2019) |
| Adults | ↓ Number | ↓ Two-syllable and frequency steps calls | Premoli et al. (2019) |
ASD: Autism spectrum disorders; KO: knock-out; PND: postnatal day; USVs: ultrasonic vocalizations; WT: wild-type.
Pharmacological/Behavioral Ultrasonic Vocalizations Modulation
Mouse vocalizations can be modulated by both pharmacological and behavioural interventions (Table 7). One example of the pharmacological treatment that resulted effective in USVs modulation is minocycline. Rotschafer and colleagues (2012) demonstrated that a 4-week minocycline treatment (from PND 0 to PND 28) was able to reverse Fmr1 KO mice (FVB background) vocalization deficits (in terms of calling rate) during mating. In another study, the effect of several anxiolytics (citalopram, escitalopram, R-citalopram, paroxetine, fluoxetine and venlafaxine) were tested on Carworth Farms Webster mouse pups (7 days old) and maternal-separation USVs were recorded; acute treatment (45 minutes before USVs recording) with all these drugs induced a reduction in USVs emission compared to vehicle treated pups (Fish et al., 2004). Veronesi and colleagues (2017) studied the effect of an anti-inflammatory drug (dypirone) administered to dams during lactation; they found that it induced increase number of USVs in male pups, but not females, compared to vehicle pups, suggesting a greater vulnerability of male pups to anti-inflammatory treatment during lactation. An interesting toxicological study concerned the impact of dioxin exposure on USVs emission in infant mice born to dams treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin on gestational day 12.5. Total USVs and mean call durations in infant mice exposed to 3 μg/kg dioxin were shorter than those in the control mice. In addition, the percentages of complicated call types (i.e., chevron and wave) in dioxin-exposed mice were decreased compared to control mice (Kimura and Tohyama, 2018).
Table 7.
Pharmacological/behavioral modulation effect on ultrasonic communication
| Effect of pharmacological/behavioral modulation on USVs | USVs features | Strains | References |
| pups | Minocycline treatment effect on number | WT and Fmr1 KO | Rotschafer et al. (2011) |
| Anxiolytics treatment effect on number | Carworth Farms Webster | Fish et al. (2004) | |
| Dypirone treatment effect on number | WT | Veronesi et al. (2017) | |
| Dioxin exposure effect on number, duration and call types | WT | Kimura and Tohyama (2018) |
KO: Knock-out; USVs: ultrasonic vocalizations; WT: wild-type.
An example of behavioral intervention able to act on mice vocalizations is early social enrichment; a maternal social enrichment on Fmr1 KO pups resulted in a weaker emotional response to maternal separation. Indeed, enriched-KO pups at PND 8 emitted less USVs compared to KO pups during maternal separation (Oddi et al., 2015).
Hence, USVs analysis is a useful behavioral endpoint in developmental, pharmacological and toxicological assessment.
Conclusions and Future Perspectives
In recent years, the relevance of USVs has been consolidated as a valid tool for behavioral analysis of mice, both in the context of ethological studies and in the context of medical/pathological studies, especially in NDDs field. Altered ultrasonic communication is found in several mouse models of NDDs and currently it is emerged the evidence that the study of USVs can provide additional value to NDDs models.
Different open questions on the communicative function and the meaning of USVs in mice are still under investigation. An important role is exerted by the emotional state and the motivation that can influence social communication in mice and modulate some specific USVs features such as number and typology (Granon et al., 2018). So, one of the main challenge for the future will be to better understand the meaning of USVs different categories emitted by mice and/or to associate the type of USV to a proper emotional state of the animal. As soon as this key topic will be unravelled, the study of ultrasonic communication will become even more important also in order to modulate, both pharmacologically and with behavioral therapies, the state of health of mice, and so the translational value of USVs will dramatically increase.
Footnotes
C-Editors: Zhao M, Qiu Y; T-Editor: Jia Y
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This work was supported by Research Grant from the University of Brescia (to Memo M).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by Research Grant from the University of Brescia (to Memo M).
References
- 1.Aria F, Bonini SA, Cattaneo V, Premoli M, Mastinu A, Maccarinelli G, Memo M. Brain structural and functional alterations in mice prenatally exposed to LPS are only partially rescued by anti-inflammatory treatment. Brain Sci. 2020;10:E620. doi: 10.3390/brainsci10090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arriaga G, Jarvis ED. Mouse vocal communication system: are ultrasounds learned or innate. Brain Lang. 2013;124:96–116. doi: 10.1016/j.bandl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arriaga G, Zhou EP, Jarvis ED. Of, birds, and men: the mouse ultrasonic song system has some features similar to humans and song-learning birds. PLoS One. 2012;7:e46610. doi: 10.1371/journal.pone.0046610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai Y, Qiu S, Li Y, Li Y, Zhong W, Shi M, Zhu X, Jiang H, Yu Y, Cheng Y, Liu Y. Genetic association between SHANK2 polymorphisms and susceptibility to autism spectrum disorder. IUBMB Life. 2018;70:763–776. doi: 10.1002/iub.1876. [DOI] [PubMed] [Google Scholar]
- 5.Balaan C, Corley MJ, Eulalio T, Leite-Ahyo K, Pang APS, Fang R, Khadka VS, Maunakea AK, Ward MA. Juvenile Shank3b deficient mice present with behavioral phenotype relevant to autism spectrum disorder. Behav Brain Res. 2019;356:137–147. doi: 10.1016/j.bbr.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belagodu AP, Johnson AM, Galvez R. Characterization of ultrasonic vocalizations of Fragile X mice. Behav Brain Res. 2016;310:76–83. doi: 10.1016/j.bbr.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Bonini SA, Mastinu A, Maccarinelli G, Mitola S, Premoli M, La Rosa LC, Ferrari-Toninelli G, Grilli M, Memo M. Cortical structure alterations and social behavior impairment in p50-deficient mice. Cereb Cortex. 2016;26:2832–2849. doi: 10.1093/cercor/bhw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Yang M, Katz AM, Scattoni ML, Harris MJ, Saxena R, Silverman JL, Crawley JN, Zhou Q, Hof PR, Buxbaum JD. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic, social interaction, and social communication. Mol Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branchi I, Santucci D, Vitale A, Alleva E. Ultrasonic vocalizations by infant laboratory mice: a preliminary spectrographic characterization under different conditions. Dev Psychobiol. 1998;33:249–256. doi: 10.1002/(sici)1098-2302(199811)33:3<249::aid-dev5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Brunner D, Kabitzke P, He D, Cox K, Thiede L, Hanania T, Sabath E, Alexandrov V, Saxe M, Peles E, Mills A, Spooren W, Ghosh A, Feliciano P, Benedetti M, Clayton AL, Biemans B., 3 Comprehensive analysis of the, 16p11.2 deletion and null Cntnap2 mouse models of autism spectrum disorder. PLoS One. 2015;10:e0134572. doi: 10.1371/journal.pone.0134572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke K, Screven LA, Dent ML. CBA/CaJ mouse ultrasonic vocalizations depend on prior social experience. PLoS One. 2018;13:e0197774. doi: 10.1371/journal.pone.0197774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlezon WA, Jr, Kim W, Missig G, Finger BC, Landino SM, Alexander AJ, Mokler EL, Robbins JO, Li Y, Bolshakov VY, McDougle CJ, Kim KS. Maternal and early postnatal immune activation produce sex-specific effects on autism-like behaviors and neuroimmune function in mice. Sci Rep. 2019;9:16928. doi: 10.1038/s41598-019-53294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chabout J, Serreau P, Ey E, Bellier L, Aubin T, Bourgeron T, Granon S. Adult male mice emit context-specific ultrasonic vocalizations that are modulated by prior isolation or group rearing environment. PLoS One. 2012;7:e29401. doi: 10.1371/journal.pone.0029401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chabout J, Cressant A, Hu X, Edeline JM, Granon S. Making choice between competing rewards in uncertain vs. safe social environment: role of neuronal nicotinic receptors of acetylcholine. Front Hum Neurosci. 2013;7:468. doi: 10.3389/fnhum.2013.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clee SM, Nadler ST, Attie AD. Genetic and genomic studies of the BTBR ob/ob mouse model of type 2 diabetes. Am J Ther. 2005;12:491–498. doi: 10.1097/01.mjt.0000178781.89789.25. [DOI] [PubMed] [Google Scholar]
- 17.Coffey KR, Marx RG, Neumaier JF. DeepSqueak: a deep learning-based system for detection and analysis of ultrasonic vocalizations. Neuropsychopharmacology. 2019;44:859–868. doi: 10.1038/s41386-018-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Amato FR, Cabib S. Chronic exposure to a novel odor increases pups’ vocalizations, maternal care, and alters dopaminergic functioning in developing mice. Behav Neural Biol. 1987;48:197–205. doi: 10.1016/s0163-1047(87)90738-2. [DOI] [PubMed] [Google Scholar]
- 19.D’Amato FR. Courtship ultrasonic vocalizations and social status in mice. Anim Behav. 1991;41:875–885. [Google Scholar]
- 20.D’Amato FR, Scalera E, Sarli C, Moles A. Pups call, mothers rush: does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups. Behav Genet. 2005;35:103–112. doi: 10.1007/s10519-004-0860-9. [DOI] [PubMed] [Google Scholar]
- 21.Denis-Donini S, Dellarole A, Crociara P, Francese MT, Bortolotto V, Quadrato G, Canonico PL, Orsetti M, Ghi P, Memo M, Bonini SA, Ferrari-Toninelli G, Grilli M. Impaired adult neurogenesis associated with short-term memory defects in NF-kappaB p50-deficient mice. J Neurosci. 2008;28:3911–3919. doi: 10.1523/JNEUROSCI.0148-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egnor SR, Seagraves KM. The contribution of ultrasonic vocalizations to mouse courtship. Curr Opin Neurobiol. 2016;38:1–5. doi: 10.1016/j.conb.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Ehret G. Frequency and intensity difference limens and non-linearities in the ear of the house mouse (Mus musculus) J Comp Physiol. 1975;102:321–336. [Google Scholar]
- 24.Ehret G, Bernecker C. Low-frequency sound communication by mouse pups (Mus musculus): Wriggling calls release maternal behaviour. Animal Behaviour. 1986;34:821-830. [Google Scholar]
- 25.El-Kordi A, Winkler D, Hammerschmidt K, Kastner A, Krueger D, Ronnenberg A, Ritter C, Jatho J, Radyushkin K, Bourgeron T, Fischer J, Brose N, Ehrenreich H. Development of an autism severity score for mice using Nlgn4 null mutants as a construct-valid model of heritable monogenic autism. Behav Brain Res. 2013;251:41–49. doi: 10.1016/j.bbr.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Eltokhi A, Rappold G, Sprengel R. Distinct phenotypes of Shank2 mouse models reflect neuropsychiatric spectrum disorders of human patients with SHANK2 variants. Front Mol Neurosci. 2018;11:240. doi: 10.3389/fnmol.2018.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elwood RW, Keeling F. Temporal organization of ultrasonic vocalizations in infant mice. Dev Psychobiol. 1982;15:221–227. doi: 10.1002/dev.420150306. [DOI] [PubMed] [Google Scholar]
- 28.Esposito G, Hiroi N, Scattoni ML. Cry, baby, cry: Expression of distress as a biomarker and modulator in autism spectrum disorder. Int J Neuropsychopharmacol. 2017;20:498–503. doi: 10.1093/ijnp/pyx014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ey E, Yang M, Katz AM, Woldeyohannes L, Silverman JL, Leblond CS, Faure P, Torquet N, Le Sourd AM, Bourgeron T, Crawley JN. Absence of deficits in social behaviors and ultrasonic vocalizations in later generations of mice lacking neuroligin4. Genes Brain Behav. 2012;11:928–941. doi: 10.1111/j.1601-183X.2012.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ey E, Torquet N, Le Sourd AM, Leblond CS, Boeckers TM, Faure P, Bourgeron T. The Autism ProSAP1/Shank2 mouse model displays quantitative and structural abnormalities in ultrasonic vocalisations. Behav Brain Res. 2013;256:677–689. doi: 10.1016/j.bbr.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 31.Faraji J, Karimi M, Lawrence C, Mohajerani MH, Metz GAS. Non-diagnostic symptoms in a mouse model of autism in relation to neuroanatomy: the BTBR strain reinvestigated. Transl Psychiatry. 2018;8:234. doi: 10.1038/s41398-018-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feifel AJ, Shair HN, Schmauss C. Lasting effects of early life stress in mice: interaction of maternal environment and infant genes. Genes Brain Behav. 2017;16:768–780. doi: 10.1111/gbb.12395. [DOI] [PubMed] [Google Scholar]
- 33.Fischer J, Hammerschmidt K. Ultrasonic vocalizations in mouse models for speech and socio-cognitive disorders: insights into the evolution of vocal communication. Genes Brain Behav. 2011;10:17–27. doi: 10.1111/j.1601-183X.2010.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fish EW, Faccidomo S, Gupta S, Miczek KA. Anxiolytic-like effects of, citalopram, and R-citalopram in maternally separated mouse pups. J Pharmacol Exp Ther. 2004;308:474–80. doi: 10.1124/jpet.103.058206. [DOI] [PubMed] [Google Scholar]
- 35.Foxe JJ, Molholm S, Baudouin SJ, Wallace MT. Explorations and perspectives on the neurobiological bases of autism spectrum disorder. Eur J Neurosci. 2018;47:488–496. doi: 10.1111/ejn.13902. [DOI] [PubMed] [Google Scholar]
- 36.Gaub S, Fisher SE, Ehret G. Ultrasonic vocalizations of adult male Foxp2-mutant mice: behavioral contexts of arousal and emotion. Genes Brain Behav. 2016;15:243–259. doi: 10.1111/gbb.12274. [DOI] [PubMed] [Google Scholar]
- 37.Gaudissard J, Ginger M, Premoli M, Memo M, Frick A, Pietropaolo S. Behavioral abnormalities in the Fmr1-KO2 mouse model of fragile X syndrome: The relevance of early life phases. Autism Res. 2017;10:1584–1596. doi: 10.1002/aur.1814. [DOI] [PubMed] [Google Scholar]
- 38.Gracceva G, Venerosi A, Santucci D, Calamandrei G, Ricceri L. Early social enrichment affects responsiveness to different social cues in female mice. Behav Brain Res. 2009;196:304–309. doi: 10.1016/j.bbr.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 39.Granon S, Faure A, Chauveau F, Cressant A, Ey E. Why should my mouse call me? Acoustic communication in mouse models of social disorders: ultrasonic vocalizations as index of emotional and motivational states. In: Brudzynski SM, editor. Handbook of Behavioral Neuroscience. London: Elsevier; 2018. pp. 423–431. [Google Scholar]
- 40.Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ. Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci U S A. 2001;98:7101–7106. doi: 10.1073/pnas.141145998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grimsley JM, Monaghan JJ, Wenstrup JJ. Development of social vocalizations in mice. PLoS One. 2011;6:e17460. doi: 10.1371/journal.pone.0017460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grimsley JMS, Sheth S, Vallabh N, Grimsley CA, Bhatall J, Latsko M, Jasnow A, Wenstrup JJ. Contextual modulation of vocal behavior in mouse: newly identified 12 kHz “mid-frequency” vocalization emitted during restraint. Front Behav Neurosci. 2016;10:38. doi: 10.3389/fnbeh.2016.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutierrez H, Davies AM. Regulation of neural process, elaboration and structural plasticity by NF-κB. Trends Neurosci. 2011;34:316–325. doi: 10.1016/j.tins.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hammerschmidt K, Reisinger E, Westekemper K, Ehrenreich L, Strenzke N, Fischer J. Mice do not require auditory input for the normal development of their ultrasonic vocalizations. BMC Neurosci. 2012;13:40. doi: 10.1186/1471-2202-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heckman J, McGuinness B, Celikel T, Englitz B. Determinants of the mouse ultrasonic vocal structure and repertoire. Neurosci Biobehav Rev. 2016;65:313–325. doi: 10.1016/j.neubiorev.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 46.Heckman JJ, Proville R, Heckman GJ, Azarfar A, Celikel T, Englitz B. High-precision spatial localization of mouse vocalizations during social interaction. Sci Rep. 2017;7:3017. doi: 10.1038/s41598-017-02954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernandez-Miranda LR, Ruffault PL, Bouvier JC, Murray AJ, Morin-Surun MP, Zampieri N, Cholewa-Waclaw JB, Ey E, Brunet JF, Champagnat J, Fortin G, Birchmeier C. Genetic identification of a hindbrain nucleus essential for innate vocalization. Proc Natl Acad Sci U S A. 2017;114:8095–8100. doi: 10.1073/pnas.1702893114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hodges SL, Nolan SO, Reynolds CD, Lugo JN. Spectral and temporal properties of calls reveal deficits in ultrasonic vocalizations of adult Fmr1 knockout mice. Behav Brain Res. 2017;332:50–58. doi: 10.1016/j.bbr.2017.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biol. 2005;3:1–10. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Homberg JR, Kyzar EJ, Nguyen M, Norton WH, Pittman J, Poudel MK, Gaikwad S, Nakamura S, Koshiba M, Yamanouchi H, Scattoni ML, Ullman JF, Diamond DM, Kaluyeva AA, Parker MO, Klimenko VM, Apryatin SA, Brown RE, Song C, Gainetdinov RR, et al. Understanding autism and other neurodevelopmental disorders through experimental translational neurobehavioral models. Neurosci Biobehav Rev. 2016;65:292–312. doi: 10.1016/j.neubiorev.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 51.Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jouda J, Wöhr M, Del Rey A. Immunity and ultrasonic vocalization in rodents. Ann N Y Acad Sci. 2019;1437:68–82. doi: 10.1111/nyas.13931. [DOI] [PubMed] [Google Scholar]
- 53.Ju A, Hammerschmidt K, Tantra M, Krueger D, Brose N, Ehrenreich H. Juvenile manifestation of ultrasound communication deficits in the neuroligin-4 null mutant mouse model of autism. Behav Brain Res. 2014;270:159–164. doi: 10.1016/j.bbr.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 54.Kaltschmidt B, Kaltschmidt C. NF-kappaB in the nervous system. Cold Spring Harb Perspect Biol. 2009;1:a001271. doi: 10.1101/cshperspect.a001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kassed CA, Herkenham M. NF-kappaB p50-deficient mice show reduced anxiety-like behaviors in tests of exploratory drive and anxiety. Behav Brain Res. 2004;154:577–584. doi: 10.1016/j.bbr.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 56.Kessler MS, Bosch OJ, Bunck M, Landgraf R, Neumann ID. Maternal care differs in mice bred for high vs. low trait anxiety: impact of brain vasopressin and cross-fostering. Soc Neurosci. 2011;6:156–168. doi: 10.1080/17470919.2010.495567. [DOI] [PubMed] [Google Scholar]
- 57.Kikusui T, Nakanishi K, Nakagawa R, Nagasawa M, Mogi K, Okanoya K. Cross fostering experiments suggest that mice songs are innate. PLoS One. 2011;6:e17721. doi: 10.1371/journal.pone.0017721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, Longman RS, Honda K, Littman DR, Choi GB, Huh JR. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017;549:528–532. doi: 10.1038/nature23910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kimura E, Tohyama C. Vocalization as a novel endpoint of atypical attachment behavior in 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-exposed infant mice. Arch Toxicol. 2018;92:1741–1749. doi: 10.1007/s00204-018-2176-1. [DOI] [PubMed] [Google Scholar]
- 60.Lahvis GP, Alleva E, Scattoni ML. Translating mouse vocalizations: prosody and frequency modulation. Genes Brain Behav. 2011;10:4–16. doi: 10.1111/j.1601-183X.2010.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lai JK, Sobala-Drozdowski M, Zhou L, Doering LC, Faure PA, Foster JA. Temporal and spectral differences in the ultrasonic vocalizations of fragile X knock out mice during postnatal development. Behav Brain Res. 2014;259:119–130. doi: 10.1016/j.bbr.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 62.Lefebvre E, Granon S, Chauveau F. Social context increases ultrasonic vocalizations during restraint in adult mice. Anim Cogn. 2020;23:351–359. doi: 10.1007/s10071-019-01338-2. [DOI] [PubMed] [Google Scholar]
- 63.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26:607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maggio JC, Withney G. Ultrasonic Vocalizing by Adult Female Mice (Mus musculus) J Comp Psychol. 1985;99:420–436. [PubMed] [Google Scholar]
- 65.Mahrt EJ, Perkel DJ, Tong L, Rubel EW, Portfors CV. Engineered deafness reveals that mouse courtship vocalizations do not require auditory experience. J Neurosci. 2013;33:5573–5583. doi: 10.1523/JNEUROSCI.5054-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mastinu A, Premoli M, Maccarinelli G, Grilli M, Memo M, Bonini SA. Melanocortin 4 receptor stimulation improves social deficits in mice through oxytocin pathway. Neuropharmacology. 2018;133:366–374. doi: 10.1016/j.neuropharm.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 67.Meyza KZ, Blanchard DC. The BTBR mouse model of idiopathic autism - Current view on mechanisms. Neurosci Biobehav Rev. 2017;76:99–110. doi: 10.1016/j.neubiorev.2016.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Möhrle D, Fernández M, Peñagarikano O, Frick A, Allman B, Schmid S. What we can learn from a genetic rodent model about autism. Neurosci Biobehav Rev. 2020;109:29–53. doi: 10.1016/j.neubiorev.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 69.Moles A, Kieffer BL, D’Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- 70.Moles A, Costantini F, Garbugino L, Zanettini C, D’Amato FR. Ultrasonic vocalizations emitted during dyadic interactions in female mice: a possible index of sociability. Behav Brain Res. 2007;182:223–230. doi: 10.1016/j.bbr.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 71.Monteiro P, Feng SHANK proteins: roles at the synapse and in autism spectrum disorder. Nat Rev Neurosci. 2017;18:147–157. doi: 10.1038/nrn.2016.183. [DOI] [PubMed] [Google Scholar]
- 72.Mossa A, Manzini MC. Molecular causes of sex-specific deficits in rodent models of neurodevelopmental disorders. J Neurosci Res. 2019 doi: 10.1002/jnr.24577. doi:101002/jnr24577 online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moy SS, Nadler JJ. Advances in behavioral genetics: mouse models of autism. Mol Psychiatry. 2008;13:4–26. doi: 10.1038/sj.mp.4002082. [DOI] [PubMed] [Google Scholar]
- 74.Nyby J, Dizinno GA, Whitney G. Social status and ultrasonic vocalizations of male mice. Behavioral biology. 1976;18:285–289. doi: 10.1016/s0091-6773(76)92198-2. [DOI] [PubMed] [Google Scholar]
- 75.Neunuebel JP, Taylor AL, Arthur BJ, Egnor SE. Female mice ultrasonically interact with males during courtship displays. Elife. 2015;4:e06203. doi: 10.7554/eLife.06203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oddi D, Subashi E, Middei S, Bellocchio L, Lemaire-Mayo V, Guzman M, Crusio WE, D’Amato FR, Pietropaolo S. Early social enrichment rescues adult behavioral and brain abnormalities in a mouse model of fragile X syndrome. Neuropsychopharmacology. 2015;40:1113–1122. doi: 10.1038/npp.2014.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Panskepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peleh T, Eltokhi A, Pitzer C. Longitudinal analysis of ultrasonic vocalizations in mice from infancy to adolescence: Insights into the vocal repertoire of three wild-type strains in two different social contexts. PLoS One. 2019;14:e0220238. doi: 10.1371/journal.pone.0220238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, Golshani P, Trachtenberg JT, Peles E, Geschwind DH. Absence of CNTNAP2 leads to, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peñagarikano O, Geschwind DH. What does CNTNAP2 reveal about autism spectrum disorder. Trends Mol Med. 2012;18:156–163. doi: 10.1016/j.molmed.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peñagarikano O, Lázaro MT, Lu XH, Gordon A, Dong H, Lam HA, Peles E, Maidment NT, Murphy NP, Yang XW, Golshani P, Geschwind DH. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7:271ra8. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- 83.Premoli M, Bonini SA, Mastinu A, Maccarinelli G, Aria F, Paiardi G, Memo M. Specific profile of ultrasonic communication in a mouse model of neurodevelopmental disorders. Sci Rep. 2019;9:15912. doi: 10.1038/s41598-019-52378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reynolds CD, Nolan SO, Jefferson T, Lugo JN. Sex-specific and genotype-specific differences in vocalization development in FMR1 knockout mice. Neuroreport. 2016;27:1331–1335. doi: 10.1097/WNR.0000000000000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr. 2001;22:409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 86.Rotschafer SE, Trujillo MS, Dansie LE, Ethell IM, Razak KA. Minocycline treatment reverses ultrasonic vocalization production deficit in a mouse model of Fragile X Syndrome. Brain Res. 2012;1439:7–14. doi: 10.1016/j.brainres.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 87.Roy S, Watkins N, Heck D. Comprehensive analysis of ultrasonic vocalizations in a mouse model of fragile X syndrome reveals limited, call type specific deficits. PLoS One. 2012;7:e44816. doi: 10.1371/journal.pone.0044816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sangiamo DT, Warren MR, Neunuebel JP. Ultrasonic signals associated with different types of social behavior of mice. Nat Neurosci. 2020;23:411–422. doi: 10.1038/s41593-020-0584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saré RM, Figueroa C, Lemons A, Loutaev I, Beebe Smith C. Comparative Behavioral Phenotypes of Fmr1 KO, Fxr2, Het, and Fmr1 KO/Fxr2 Het Mice. Brain Sci. 2019;9:13. doi: 10.3390/brainsci9010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sato D, Lionel AC, Leblond CS, Prasad A, Pinto D, Walker S, O’Connor I, Russell C, Drmic IE, Hamdan FF, Michaud JL, Endris V, Roeth R, Delorme R, Huguet G, Leboyer M, Rastam M, Gillberg C, Lathrop M, Stavropoulos DJ, et al. SHANK1 deletions in males with autism spectrum disorder. Am J Hum Genet. 2012;90:879–887. doi: 10.1016/j.ajhg.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T + tf/J mouse model of autism. PLoS One. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33:508–515. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scattoni ML, Martire A, Cartocci G, Ferrante A, Ricceri L. Reduced social interaction, behavioural flexibility and BDNF signalling in the BTBR T+ tf/J strain, a mouse model of autism. Behav Brain Res. 2013;251:35–40. doi: 10.1016/j.bbr.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 95.Schaafsma SM, Gagnidze K, Reyes A, Norstedt N, Månsson K, Francis K, Pfaff DW. Sex-specific gene-environment interactions underlying ASD-like behaviors. Proc Natl Acad Sci U S A. 2017;114:1383–1388. doi: 10.1073/pnas.1619312114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A, Janssen AL, Udvardi PT, Shiban E, Spilker C, Balschun D, Skryabin BV, Dieck St, Smalla KH, Montag D, Leblond CS, Faure P, Torquet N, Le Sourd AM, Toro R, et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486:256–260. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- 97.Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, Ashwood P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl Psychiatry. 2013;3:e240. doi: 10.1038/tp.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seagraves KM, Arthur BJ, Egnor SE. Evidence for an audience effect in mice: male social partners alter the male vocal response to female cues. J Exp Biol. 2016;219:1437–1448. doi: 10.1242/jeb.129361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 100.Silverman JL, Ellegood J. Behavioral and neuroanatomical approaches in models of neurodevelopmental disorders: opportunities for translation. Curr Opin Neurol. 2018;31:126–133. doi: 10.1097/WCO.0000000000000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simola N, Granon S. Ultrasonic vocalizations as a tool in studying emotional states in rodent models of social behavior and brain disease. Neuropharmacology. 2018;159:107420. doi: 10.1016/j.neuropharm.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 102.Sugimoto H, Okabe S, Kato M, Koshida N, Shiroishi T, Mogi K, Kikusui T, Koide T. A role for strain differences in waveforms of ultrasonic vocalizations during male-female interaction. PLoS One. 2011;6:e22093. doi: 10.1371/journal.pone.0022093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sungur AÖ, Schwarting RK, Wöhr M. Early communication deficits in the Shank1 knockout mouse model for autism spectrum disorder: Developmental aspects and effects of social context. Autism Res. 2016;9:696–709. doi: 10.1002/aur.1564. [DOI] [PubMed] [Google Scholar]
- 104.Sungur AÖ, Jochner MCE, Harb H, Kiliç A, Garn H, Schwarting RKW, Wöhr M. Aberrant cognitive phenotypes and altered hippocampal BDNF expression related to epigenetic modifications in mice lacking the post-synaptic scaffolding protein SHANK1: Implications for autism spectrum disorder. Hippocampus. 2017;27:906–919. doi: 10.1002/hipo.22741. [DOI] [PubMed] [Google Scholar]
- 105.Sungur AÖ, Schwarting RKW, Wöhr M. Behavioral phenotypes and neurobiological mechanisms in the Shank1 mouse model for autism spectrum disorder: A translational perspective. Behav Brain Res. 2018;352:46–61. doi: 10.1016/j.bbr.2017.09.038. [DOI] [PubMed] [Google Scholar]
- 106.van Segbroeck M, Knoll AT, Levitt P, Narayanan S. MUPET-mouse ultrasonic profile ExTraction: a signal processing tool for rapid and unsupervised analysis of ultrasonic vocalizations. Neuron. 2017;94:465–485. doi: 10.1016/j.neuron.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Veronesi VB, Batista TH, Ribeiro AC, Giusti-Paiva A, Vilela FC. Maternal dipyrone treatment during lactation in mice reduces maternal behavior and increases anxiety-like behavior in offspring. Int J Dev Neurosci. 2017;58:74–81. doi: 10.1016/j.ijdevneu.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 108.Vogel AP, Tsanas A, Scattoni ML. Quantifying ultrasonic mouse vocalizations using acoustic analysis in a supervised statistical machine learning framework. Sci Rep. 2019;9:8100. doi: 10.1038/s41598-019-44221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang X, McCoy PA, Rodriguiz RM, Pan Y, Je HS, Roberts AC, Kim CJ, Berrios J, Colvin JS, Bousquet-Moore D, Lorenzo I, Wu G, Weinberg RJ, Ehlers MD, Philpot BD, Beaudet AL, Wetsel WC, Jiang Y. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet. 2011;20:3093–3108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Warburton VL, Sales GD, Milligan SR. The emission and elicitation of mouse ultrasonic vocalizations: the effects of, sex and gonadal status. Physiol Behav. 1989;45:41–47. doi: 10.1016/0031-9384(89)90164-9. [DOI] [PubMed] [Google Scholar]
- 111.Warren MR, Sangiamo DT, Neunuebel JP. a High channel count microphone array accurately and precisely localizes ultrasonic signals from freely-moving. J Neurosci Methods. 2018a;297:44–60. doi: 10.1016/j.jneumeth.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Warren MR, Spurrier MS, Roth ED, Neunuebel JP. b Sex differences in vocal communication of freely interacting adult mice depend upon behavioral. PLoS One. 2018b;13:e0204527. doi: 10.1371/journal.pone.0204527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- 114.Wöhr M, Roullet FI, Crawley JN. a Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of. Genes Brain Behav. 2011a;10:35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wöhr M, Roullet FI, Hung AY, Sheng M, Crawley JN. b Communication impairments in mice lacking Shank1: reduced levels of ultrasonic vocalizations and scent marking. PLoS One. 2011b;6:e20631. doi: 10.1371/journal.pone.0020631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wöhr M, Silverman JL, Scattoni ML, Tuner SM, Harris MJ, Saxena R, Crawley JN. Developmental delays and reduced pup ultrasonic vocalizations but normal sociability in mice lacking the postsynaptic cell adhesion protein neuroligin2. Behav Brain Res. 2013;251:50–64. doi: 10.1016/j.bbr.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wöhr M. Ultrasonic vocalizations in Shank mouse models for autism spectrum disorders: detailed spectrographic analyses and developmental profiles. Neurosci Biobehav Rev. 2014;43:199–212. doi: 10.1016/j.neubiorev.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 118.Wöhr M. Effect of social odor context on the emission of isolation-induced ultrasonic vocalizations in the BTBR T+tf/J mouse model for autism. Front Neurosci. 2015;9:73. doi: 10.3389/fnins.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Won H, Lee HR, Gee HY, Mah W, Kim J, Lee J, Ha S, Chung C, Jung ES, Cho YS, Park S, Lee J, Lee K, Kim D, Bae YC, Kaang B, Lee MG, Kim E. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–265. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- 120.Yang M, Bozdagi O, Scattoni ML, Wöhr M, Roullet FI, Katz AM, Abrams DN, Kalikhman D, Simon H, Woldeyohannes L, Zhang JY, Harris MJ, Saxena R, Silverman JL, Buxbaum JD, Crawley JN. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J Neurosci. 2012;32:6525–6541. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang M, Loureiro D, Kalikhman D, Crawley JN. Male mice emit distinct ultrasonic vocalizations when the female leaves the social interaction arena. Front Behav Neurosci. 2013;7:159. doi: 10.3389/fnbeh.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zala SM, Reitschmidt D, Noll A, Balazs P, Penn DJ. Sex-dependent modulation of ultrasonic vocalizations in house mice (Mus musculus musculus) PLoS One. 2017;12:e0188647. doi: 10.1371/journal.pone.0188647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zanettini C, Carola V, Lo Iacono L, Moles A, Gross C, D’Amato FR. Postnatal handling reverses social anxiety in serotonin receptor 1A knockout mice. Genes Brain Behav. 2010;9:26–32. doi: 10.1111/j.1601-183X.2009.00531.x. [DOI] [PubMed] [Google Scholar]
- 124.Zippelius HM, Schleidt WM. Ultraschall-laute bei jungen Mause. Naturwissenschaften. 1956;43:502. [Google Scholar]


