Abstract
Acute central nervous system injuries are among the most common causes of disability worldwide, with widespread social and economic implications. Motor tract injury accounts for the majority of this disability; therefore, there is impetus to understand mechanisms underlying the pathophysiology of injury and subsequent reorganization of the motor tract that may lead to recovery. After acute central nervous system injury, there are changes in the microenvironment and structure of the motor tract. For example, ischemic stroke involves decreased local blood flow and tissue death from lack of oxygen and nutrients. Traumatic injury, in contrast, causes stretching and shearing injury to microstructures, including myelinated axons and their surrounding vessels. Both involve blood-brain barrier dysfunction, which is an important initial event. After acute central nervous system injury, motor tract reorganization occurs in the form of cortical remapping in the gray matter and axonal regeneration and rewiring in the white matter. Cortical remapping involves one cortical region taking on the role of another. cAMP-response-element binding protein is a key transcription factor that can enhance plasticity in the peri-infarct cortex. Axonal regeneration and rewiring depend on complex cell-cell interactions between axons, oligodendrocytes, and other cells. The RhoA/Rho-associated coiled-coil containing kinase signaling pathway plays a central role in axon growth/regeneration through interactions with myelin-derived axonal growth inhibitors and regulation of actin cytoskeletal dynamics. Oligodendrocytes and their precursors play a role in myelination, and neurons are involved through their voltage-gated calcium channels. Understanding the pathophysiology of injury and the biology of motor tract reorganization may allow the development of therapies to enhance recovery after acute central nervous system injury. These include targeted rehabilitation, novel pharmacotherapies, such as growth factors and axonal growth inhibitor blockade, and the implementation of neurotechnologies, such as central nervous system stimulators and robotics. The translation of these advances depends on careful alignment of preclinical studies and human clinical trials. As experimental data mount, the future is one of optimism.
Keywords: corticospinal tract, myelin-axon interaction, recovery, remapping, reorganization, RhoA/ROCK, translation
Introduction
Acute central nervous system (CNS) injury, including stroke and traumatic brain/spinal cord injury, is among the most important health concerns worldwide (Chauhan, 2014; Regenhardt et al., 2020; Rupp, 2020). Acute CNS injury represents a common medico-social and economic problem that affects all ages, leading to chronic functional disability with long-term social support implications (Christensen et al., 2009; Farhad et al., 2013; Feigin et al., 2014). Among the various neurofunctional disturbances caused by acute CNS injury, motor impairment may represent the largest burden to patients (Bolay and Dalkara, 1998). While the CNS may have some tolerability or reserve against insult, restorative capacities in the adult are limited. Moreover, motor neurons and long tracts are vulnerable, especially to mechanical (traumatic), hypoxic (ischemic) or oxidative stress. Directly or indirectly, CNS insult results in damage to cells, fibers and/or surrounding vasculatures via many mechanisms, including stretching, oxidation, and alternation of cell membranes. Furthermore, the corticospinal tract (CST) may represent a particularly high risk structure anatomically, given it is extremely dense with prototypical long axons extending from the cerebral cortex to the spinal cord. A primary hurdle in translating advances in the science of stroke and traumatic CNS injury is to understand the spatiotemporally complex and dynamic pathophysiology involved. Furthermore, spontaneous repair processes, which may represent targets for future therapies, may be different depending on injury severity, brain regions involved, or cause of injury (Regenhardt et al., 2020). Understanding processes following acute CNS injury of the motor tract and its surroundings is crucial to enhance reorganization/recovery and improve clinical outcomes.
In this review, we summarize critical events and molecular mechanisms underlying acute CNS injury in both clinical and experimental studies. We address blood-brain barrier (BBB) dysregulation as an important initial event after acute CNS injury. Then, we focus on the molecular mechanisms related to CST reconstruction, including cortical remapping in gray matter and motor tract reorganization in white matter. Within the white matter microenvironment, we describe molecular mechanisms of myelin-axon cell-cell interactions that play a central role in the inhibition of axonal regeneration. Interactions among other cell types, such as astrocytes, microglia and endothelial cells, during the reorganization of the motor pathway, have been reviewed in greater detail elsewhere (Toy and Namgung, 2013). Lastly, we discuss potential strategies and therapeutic interventions to promote motor tract reorganization and improve functional recovery after acute CNS injury.
Search Strategy and Selection Criteria
The articles reviewed in this manuscript were retrieved by electric search in Pubmed (https://www.ncbi.nlm.nih.gov/pubmed/) for literatures focused on motor tract reorganization after ischemic or traumatic brain injury. Keywords for searching were motor tract, axonal regeneration, rewiring, reorganization, repair, white matter, spinal cord, ischemia, stroke, traumatic brain injury, spinal cord injury, rehabilitation, cortical mapping, microenvironment. Date of searching was 2019.
Changes in Microenvironment and Structure of the Motor Tract after Acute CNS Injury
The principal system for fine and skilled motor control in primates, including humans, is the CST. Therefore, damage to the CST caused by acute brain or spinal cord injury results in decreased quality of life through motor impairment. The two major forms of acute CNS injury are stroke and traumatic brain/spinal cord injury. While they are initiated by different processes, they share a key pathologic feature of BBB or blood-spinal cord barrier dysfunction (Prakash and Carmichael, 2015). Once this barrier is disrupted, there is significant progression of tissue damage (Liebner et al., 2018). Therefore, it is important to understand the early molecular, cellular and tissue events that affect the BBB after acute CNS injury. Developing therapies to stabilize the BBB acutely (e.g., by blocking hypoxia inducible factor-1α (HIF-1α) initiated processes or protecting endothelial cells) may prevent a malignant cycle of deleterious events across injury mechanisms. Indeed, recent preclinical studies have shown HIF-1α blockade exerts protective effects by regulating several downstream processes (Shen et al., 2018; Wang et al., 2019). However, later events after BBB disruption, while the CNS is transitioning from injury to recovery, are also crucial for the development of potential clinical interventions. Interestingly, despite the different initial causes of injury from stroke and traumatic brain injury (TBI), a similar biphasic pattern of early and late BBB impairment is seen (Prakash and Carmichael, 2015).
Ischemic stroke, which comprises 80–90% of all human strokes, is characterized by a sudden reduction in local cerebral blood flow (Prakash and Carmichael, 2015). Although the pathophysiology of ischemic stroke is extremely heterogeneous and may differ between experimental models, vessel pathologies, and infarct sizes/locations, a biphasic time course of BBB dysregulation is believed to be a common underlying mechanism consisting of early hyperpermeability (4–6 hours after ischemic onset) and delayed BBB opening (2–3 days) (Kuroiwa et al., 1985; Rosenberg et al., 1998). In the early phase, cerebral blood flow is decreased by arterial narrowing or occlusion. The hypoxic condition and adenosine triphosphate (ATP) loss at the cellular and tissue level induce hypoxia inducible factor-1α (HIF-1α), leading to increased activated matrix metalloproteinase-2. This causes transient and reversible BBB opening and vasogenic edema in the first hours (Belayev et al., 1996; Asahi et al., 2000, 2001; Candelario-Jalil et al., 2009; Yang and Rosenberg, 2011). After this initial BBB opening, ischemia-reperfusion-related metabolites, such as free radicals and reactive oxygen species, also damage endothelial cells leading to further BBB injury. Subsequently, inflammatory cytokines, such as tumor necrosis factor-a and interleukin-1β, secreted from activated microglia in the ischemic core or the peri-infarct area induce activated matrix metalloproteinase-9 leading to secondary BBB damage (Asahi et al., 2000; Seo et al., 2012; Maki et al., 2013). This secondary damage after stroke is often more severe than the initial injury and is believed to be irreversible (Shi et al., 2016).
In contrast to stroke, the acute phase of TBI involves a traumatic force applied to microstructures, including myelinated axons and their surrounding vessels. As structures are stretched, there is shearing injury, disruption of endothelial junctions, and enlarging of intercellular spaces. These mechanical injuries lead to further damage of the brain microenvironment, often resulting in micro- or macro-bleeding which can further compress the microvasculature and decrease focal cerebral blood flow (Shlosberg et al., 2010). These vascular changes last only several hours in the acute phase; however, they can occur in vessels of various sizes and result in further variable BBB dysregulation. Secondary injuries involving inflammation, edema, and cellular hyper-excitability occur several days after TBI. These changes in the perineuronal (periaxonal) or microvascular environment result in the dysregulation of normal neurovascular coupling and neuronal function (Shlosberg et al., 2010).
Oxygen and nutrient deprivation, especially after stroke, causes inhibition of ATP production and inactivation of ATP dependent ion transport. BBB disruption-derived molecular processes primarily and secondarily induce other various pathological processes, such as edema, immune responses, and genetic changes (Lo et al., 2005; Simard et al., 2007). There is an increase of intracellular calcium concentration, resulting in cellular over-excitation, cytotoxic edema, and apoptotic cell death. Activation of these pathways within primary motor cortex adversely affects motor performance in preclinical models and humans (Bolay and Dalkara, 1998; Cirstea et al., 2011). Neurons exist in a variety of sizes and express a large spectrum of properties. These differences likely imply unique energy demands. Large pyramidal cells in the primary motor cortex, which must maintain energy-consuming processes such as ion pumping across membranes and axonal transport along significant distances, have considerably larger energy requirements than local inter neurons. Thus, motor neurons are especially sensitive to decreases in ATP supply (Squire et al., 2002). Efficient ATP supply or metabolic modification in the motor tract during the acute phase of injury may be helpful to protect the tract and may have implications for its reorganization.
It is important to note there are several differences and similarities between the brain and spinal cord regarding the damage and recovery of motor axons. Epidemiologically, stroke and trauma affect both the brain and spinal cord, but stroke is less common in the spinal cord due to its extensive vascular collateral system (Weidauer et al., 2015; Romi and Naess, 2016). At the molecular level, several types of voltage-gated calcium channels (VGCCs) are involved in motor neuron injury and axonal regeneration both in the brain and spinal cord (Mahar and Cavalli, 2018). However, recent studies indicate a particular subunit of VGCCs may play a key role in motor neuron plasticity within the spinal cord (Tedeschi et al., 2016; Marcantoni et al., 2020; Sun et al., 2020). The brain and spinal cord also share other inhibitory mechanisms, such as mitogen-activated protein kinase- or myelin-derived axonal-growth inhibitor (MAI)-related pathways.
Types of Motor Tract Reorganization after Acute CNS Injury
Very severe motor disturbances following large cortical strokes may not show meaningful recovery (Biernaskie et al., 2005). However, clinical and experimental studies have shown that most motor disturbances do show spontaneous recovery within days to weeks. One mechanism of recovery is remapping primary motor cortex if the cortical lesion is limited (Liepert et al., 2000; Starkey et al., 2012). Interestingly, even if cortical damage is relatively large, gene expression is altered to promote axonal regeneration and prevent degeneration, which may enhance motor recovery via compensational axon sprouting from the non-denervated side in the white matter or spinal cord (Takase et al., 2017). Thus, there are two primary processes important for plasticity in motor function: (a) cortical remapping in the gray matter, and (b) axonal regeneration and rewiring of motor pathway in the white matter and spinal cord.
Cortical remapping
Cortical remapping, also referred to as cortical reorganization, was originally described in the 1970s within the sensory system. This compensatory process involves a change in the functional map of the cortex whereby cortical function can be relocated to a different cortical region after damage (Paul et al., 1972). Subsequently, cortical remapping of somatosensory inputs was investigated in other studies, including phantom limb pain and tinnitus investigations (Liepert et al., 2000). In turn, cortical remapping for outputs (motor function) was examined (Liepert et al., 2000). Motor pathway cortical remapping is less understood given its more complicated interpretation. Cortical upper motor neurons do not directly activate muscles but synapse on lower motor neurons in the brain stem and spinal cord. Changes in these regions are challenging to assess in vivo (Wittenberg, 2010). Furthermore, there is evidence to suggest remapping of the primary motor cortex may be restricted in adults compared to the sensory cortex. This may be related to primary motor cortex neurons’ dependence on single long axons to the cord, while sensory neurons have several potential pathways for remapping before signals reach the sensory cortex in the opposite direction (Wittenberg, 2010).
Cortical remapping may be related to changes in excitability. After injury, excitability within the motor cortex is reduced. Studies suggest this reduction is associated with disadvantageous reorganization and impaired motor function (Liepert et al., 2000). Among the limited mechanistic findings associated with motor cortical remapping to date, cAMP-response-element binding protein (CREB) appears to be a key transcription factor involved with changes in excitability. Increasing CREB by utilizing lentiviral transfection to rodent motor neurons in the peri-infarct has been shown to enhance cortical circuit plasticity and motor recovery after small cortical stroke (Caracciolo et al., 2018). Other rodent studies have demonstrated that distant areas, such as premotor (Frost et al., 2003) and contralateral primary motor cortex (Biernaskie et al., 2005), may play a role in plasticity after large cortical strokes. One study showed that cortical remapping may occur even after spinal cord injury (SCI) via Wnt-Ryk signaling (Hollis et al., 2016). Within the peri-infarct and other regions of plasticity, other proteins have been reported to be related to axonal growth and synaptogenesis, such as growth associated protein 43, thrombospondins 1 and 2 and additional growth factors (Stroemer et al., 1995; Liauw et al., 2008).
Despite limited evidence of cortical remapping in humans, several propose that rehabilitation may be efficacious through the induction of a use-dependent reorganization that counteracts adverse brain function changes (Weiller et al., 1993; Duncan, 1997; Traversa et al., 1997; Liepert et al., 2000). Future studies must consider how preclinical models relate to the human condition as there are differences in functional anatomy between species. Importantly, fine motor control is less direct with more opportunities for remapping in rodents or even non-human primates compared to humans. Distal limb movements in humans rely less on the supplementary motor area and the premotor cortex (Wittenberg, 2010).
White matter reorganization: axonal regeneration and rewiring
During development, axon generation, changes in synaptic connections, and axonal “wiring” are normal phenomena. However, in the adult CNS plasticity is restricted by several inhibiting signals, such as acute cellular actions related to calcium influx or slower injury signaling mediated by mitogen-activated protein kinase (as introduced above) (Mahar and Cavalli, 2018). Among them, perhaps the most promising examples in translation are MAIs, such as Nogo-A, myelin-associated glycoprotein, and oligodendrocyte-myelin glycoprotein (Kempf and Schwab, 2013). These are secreted from mature oligodendrocytes after acute CNS injury and negatively affect axonal regeneration and reorganization of the motor pathway via RhoA/Rho-associated coiled-coil containing kinase (ROCK) signaling. This process is associated with inflammatory cytokines and degenerative factors from glial scar that forms after acute ischemic/traumatic injury. Animal studies have demonstrated that immunotherapy or gene modification targeting these myelin-derived inhibitors (or their neuronal receptor complexes: NgR1, p75, TROY and LINGO1) can lead to motor tract reorganization after CNS injury by enhancing axonal projection from the contra-lesional motor tract to ipsi-lesional nuclei in the brainstem or from the non-denervated side to the denervated side of the spinal cord (Tsai et al., 2011; Takase et al., 2017; Ueno et al., 2020). This injury-inducible compensatory motor recovery is robustly observed in rodents and to a greater extent with larger stroke and more severe TBI (Gonzalez et al., 2004; Zhang et al., 2010; Takase et al., 2017). The MAIs/RhoA/ROCK signaling pathway may be involved in plasticity within other brain structures, such as the hippocampus (Kempf and Schwab, 2013; Jitsuki et al., 2016). A phase 1 clinical trial examining a recombinant human antibody against Nogo-A has shown the antibody therapy to be safe and well tolerated (Zorner and Schwab, 2010; Kucher et al., 2018). A phase 2 trial is ongoing for patients with acute cervical SCI (https://clinicaltrials.gov/ct2/show/NCT03935321).
RhoA/ROCK signaling also plays a critical role regulating actin cytoskeletal dynamics by phosphorylating diverse downstream targets and affecting many intracellular processes (Sladojevic et al., 2017). ROCKs, normally inactive in the cytoplasm, can be activated by stresses-induced Rho and arachidonic acid. Activated ROCKs regulate phosphorylation of myosin light chain. Phosphorylated myosin light chain accelerates the actin-myosin interaction to induce stress fiber formation, which results in growth cone collapse, neurite retraction and even smooth muscle contraction. While direct evidence of motor tract reorganization by RhoA/ROCK inhibition after CNS injury has not been sufficiently demonstrated, there are data demonstrating improved motor outcomes (Mulherkar et al., 2017).
Overactivation of ROCKs during the acute phase of injury also stimulates the inflammatory response and reduces endothelial nitric oxide (NO) synthase (eNOS) (Rikitake et al., 2005). Phosphorylated (functional) eNOS has protective functions by producing NO, which regulates the vascular tone and has antithrombotic effects (Sawada and Liao, 2009). In stroke and SCI, ROCK activation stimulates neutrophil/leukocyte infiltration through reactive oxygen species production. Moreover, ROCK activation in microglia stimulates cytokine production. Importantly, these neuroinflammatory reactions also negatively affect BBB regulation, leading to vascular contraction and increased permeability or hemorrhagic transformation after stroke(Ishiguro et al., 2012). Although some ROCKs may have dual roles in cell survival and apoptosis (Julian and Olson, 2014), therapy with ROCK inhibitors, such as Fasudil, has been shown to reduce infarct volume and improve neurological outcome after SCI in experimental animals (Fournier et al., 2003). Furthermore, early clinical trials suggest improved neurological function in humans (Shibuya et al., 2005). In addition to phase 2/3 trials for the treatment of SCI, drug repurposing studies using statins to inhibit the RhoA/ROCK pathway are also ongoing (Fehlings et al., 2018).
In addition, oligodendrogenesis and remyelination are crucial for motor tract reorganization in white matter, as axonal regeneration alone is insufficient for recovery after acute CNS injury. Among many functions, oligodendrocytes support and regulate axons by producing myelin for increased action potential conduction, providing metabolic support, and stabilizing the axon cytoskeleton (Greer et al., 2011; Meyer et al., 2018). Conversely, axons also regulate oligodendrocytes and their precursor cells via the AMPA-receptor subunit GluA2 (Chen et al., 2018). An in vitro study demonstrated that oligodendrocytes preferentially myelinate electrically active axons (Wake et al., 2015). This axon-oligodendrocyte crosstalk modulates the signal conduction speed in an activity-dependent manner and highlights the role of oligodendrocytes as plasticity enhancers. While injury to white matter can be devastating given these complex interactions, there are potent self-repairing processes after CNS injury. With acute traumatic injury in a rodent model, there is active proliferation of oligodendrocyte precursor cells (OPCs) located in the white matter and derived from neural stem cells in the subventricular zone (Takase et al., 2018). In humans, increased OPCs at sites of ischemic brain pathology have been observed postmortem. However, only a fraction of proliferating OPCs become mature functional oligodendrocytes and contribute to remyelination (rewiring). Furthermore, there is an age-related decline in normal oligodendrogenesis. Interestingly, a recent study demonstrated the possibility of exercise to increase OPCs in white matter as a potent therapy for neural circuit remodeling (Ohtomo et al., 2019). More recent advances have been made understanding the role of VGCCs on neurons. VGCCs are widely expressed in excitable cells and involved in an array of various cellular processes. The a2d subunit type of VGCCs is specifically expressed in neurons. One of the four a2d subunits, a2d2, has been shown in adult mouse neurons to suppress axonal growth and regeneration in a novel and robust study of large-scale transcriptome sequencing (Tedeschi et al., 2016). Furthermore, administration of a2d ligands, such as clinically used pregabalin and gabapentin, can block this channel and promote regeneration of not only ascending sensory axons but also descending CST projections after SCI (Sun et al., 2020). Since the role of VGCC a2d subunits has not been well studied in other CNS injuries, such as stroke or TBI, future studies are warranted.
Rehabilitation and Other Factors Enhancing Motor Tract Reorganization
Previous studies have shown motor tract reorganization and recovery after acute CNS injury with various therapies, such as blocking axonal-growth inhibitors, administration of growth factors for axonal regeneration, electrical CNS stimulation, transplantation of stem cells, and post-injury rehabilitation (Regenhardt et al., 2020). Among them, rehabilitation may be more important than ever. Several studies examining rehabilitative training after ischemic or traumatic injury suggest there is significant benefit and describe potential mechanisms that may contribute to CST remodeling. Importantly, unlike pharmacotherapies there are few side effects and minimal risk with rehabilitation. Moreover, rehabilitation therapies are often tailor-made for each patient (Fukuda et al., 2016). The molecular underpinnings of its effects are gradually becoming elucidated. Okabe et al. (2017) suggested a possible mechanism may include therapy-induced increases in neural activity that increases production of trophic factors, such as brain-derived neurotrophic factor or ciliary neurotrophic factor. These factors may render the microenvironment more suitable for axonal remodeling (Okabe et al., 2017). A rodent model of intracerebral hemorrhage showed constraint induced movement therapy (CIMT), a routinely used clinical therapy originally developed in non-human primates (Taub et al., 1999), demonstrated motor-related reorganization from the ipsi-lesional hemisphere (Ishida et al., 2016). A more recent study utilizing a rat model of ischemic stroke revealed that CIMT in the subacute phase leads to MRI changes in the internal capsule consistent with augmentation of white matter remodeling and accelerated motor recovery of the phenotype (Hu et al., 2019). Regarding molecular mechanism, another rodent study of cerebral ischemia demonstrated significant decreases in p-ERK by CIMT (Zhang et al., 2015). While interpretation is challenging since p-ERK has both beneficial and detrimental effects, this example illustrates the complex interplay between rehabilitation, structural CNS remodeling, and molecular changes.
Translational Perspective and Conclusion
In conclusion, stroke and traumatic brain/spinal cord injury are among the most common causes of disability worldwide, having an extensive impact on quality of life for affected patients, social support systems, and the economics of healthcare (Christensen et al., 2009; Farhad et al., 2013; Feigin et al., 2014). Most of this disability is related to motor tract injury. Therefore, understanding the acute changes in the motor tract and its reorganization after injury is paramount to develop therapies to enhance motor tract reorganization and promote recovery (Figure 1).
Figure 1.
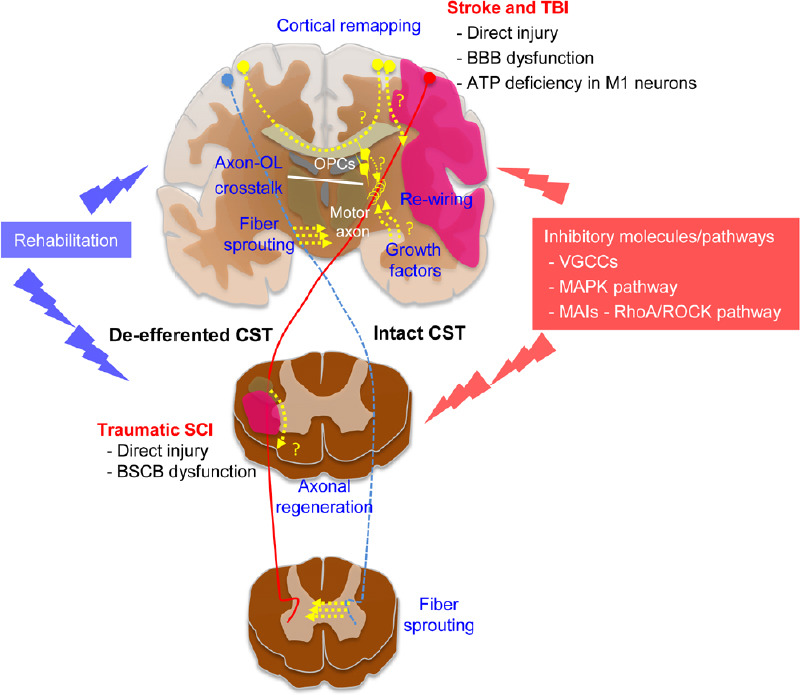
Schematic illustration of beneficial and inhibitory factors in CST reorganization after acute CNS injury.
ATP: Adenosine triphosphate; BBB: blood-brain barrier; BSCB: blood-spinal cord barrier; CST: corticospinal tract; MAIs: myelin-associated inhibitors; MAPK: mitogen-activated protein kinase; OL: oligodendrocytes; OPCs: oligodendrocyte precursor cells; RhoA: Ras homolog family member A; ROCK: Rho-associated coiled-coil containing kinase; SCI: spinal cord injury; TBI: traumatic brain injury; VGCCs: voltage-gated calcium channels.
The microenvironment and structure of the motor tract are altered depending on injury mechanism. After ischemic stroke tissue injury occurs secondary to decreased blood flow and oxygen/nutrient deprivation. After traumatic injury tissue is injured stretching and shearing of microstructures, including myelinated axons and their surrounding vessels. While different, there are similarities in injury pathophysiology, and these similarities may serve as ideal targets for novel therapeutics. One such similarity is BBB dysregulation. This is a key step in the pathophysiology of both stroke and traumatic injury. Developing therapies to stabilize the BBB, especially acutely, may prevent a chain reaction of deleterious events across injury mechanisms. As described above, HIF-1α blockade exerts protective effects by regulating several downstream processes and is currently under investigation for translation (Shen et al., 2018; Wang et al., 2019).
Treatments with the potential for pleiotropic effects should be prioritized for future studies, such as agents to modulate both cortical remapping in the gray matter and axonal rewiring in the white matter. CREB enhances cortical excitability (Dong et al., 2006; Han et al., 2006), cortical re-mapping, and recovery (Caracciolo et al., 2018), but it also regulates axonal outgrowth (Xu et al., 2011; Clarkson et al., 2015). Moreover, RhoA/ROCK signaling is involved in inhibitory pathways of axonal regeneration, but also has a critical role in cytoskeletal dynamics, inflammation, and BBB regulation (Ishiguro et al., 2012). Agents with multiple target effects (e.g., NgR1 blocking decoy for multiple inhibitory ligands of axonal regeneration) may be more likely to translate into highly efficacious therapies for patients.
In addition to pharmacotherapies, improved understanding of these biological processes can also aid in the development of improved rehabilitation strategies. Translation of new therapies developed in preclinical models has precedent with CIMT. To improve understanding of mechanisms underlying rehabilitation, reverse translation approaches to model rehabilitation in the lab are under study. Using a rodent model of stroke, extensive reach training therapy has been shown to promote recovery when started early but not when started late. However, if a second stroke is induced this appears to re-open a time limited window of heightened plasticity (Zeiler et al., 2016). Furthermore, the translation of other novel neurotechnology to augment recovery is under study (Regenhardt et al., 2020). The utility of transcranial magnetic stimulation and other CNS stimulators is being explored with mixed data (Hsu et al., 2012; Hao et al., 2013).
While there are many promising avenues of ongoing study, there are important hurdles to overcome for the successful translation of therapies into clinical practice. Timing is key; agents may have strong beneficial effects but can be deleterious if administered too early or ineffective if too late. Furthermore, a thorough understanding of each disease model’s biology must be carefully compared to the human condition for effective translation. Anatomy, physiology, lesion size/location, inflammatory/immunological responses, gene expression profiles, and transcription factors vary across species and disease models (Courtine et al., 2007). Among them, less developed white matter in rodents is a key consideration for CNS reorganization. There are many experimental design considerations to enhance alignment between preclinical studies and human trials (Corbett et al., 2017). With diligent reflections on past lessons and mounting preclinical data, this new decade holds much optimism for the translation of novel therapies.
Additional file: Open peer review reports 1 (92.7KB, pdf) and 2 (97.4KB, pdf) .
Footnotes
P-Reviewers: Zhao C, Kukley M; C-Editors: Zhao M, Qiu Y; T-Editor: Jia Y
Conflicts of interest: Both authors have no conflicts of interest to declare.
Financial support: This work was supported in part by JSPS “KAKENHI” Grant-in-Aid for Early-Career Scientists, Grant No. 18K16566 (to HT); Research Abroad from the Japan Brain Foundation (to HT); Mochida Memorial Foundation for Medical and Pharmaceutical Research of Japan (to HT); the Rotary Foundation Global Scholarship Grants, Grant Nos. GG1759314, GG1876795) (to HT); and the National Institute of Neurological Disorders and Stroke of USA, No. R25 NS065743 (to RWR).
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Chao Zhao, Fudan University, China; Maria Kukley, Eberhard Karls Universitat Tubingen, Germany.
Funding: This work was supported in part by JSPS “KAKENHI” Gxsrant-in-Aid for Early-Career Scientists, Grant No. 18K16566 (to HT); Research Abroad from the Japan Brain Foundation (to HT); Mochida Memorial Foundation for Medical and Pharmaceutical Research of Japan (to HT); the Rotary Foundation Global Scholarship Grants, Grant Nos. GG1759314, GG1876795) (to HT); and the National Institute of Neurological Disorders and Stroke of USA, No. R25 NS065743 (to RWR).
References
- 1.Asahi M, Sumii T, Fini ME, Itohara S, Lo EH. Matrix metalloproteinase 2 gene knockout has no effect on acute brain injury after focal ischemia. Neuroreport. 2001;12:3003–3007. doi: 10.1097/00001756-200109170-00050. [DOI] [PubMed] [Google Scholar]
- 2.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- 4.Biernaskie J, Szymanska A, Windle V, Corbett D. Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. Eur J Neurosci. 2005;21:989–999. doi: 10.1111/j.1460-9568.2005.03899.x. [DOI] [PubMed] [Google Scholar]
- 5.Bolay H, Dalkara T. Mechanisms of motor dysfunction after transient MCA occlusion: persistent transmission failure in cortical synapses is a major determinant. Stroke. 1998;29:1988–1994. doi: 10.1161/01.str.29.9.1988. [DOI] [PubMed] [Google Scholar]
- 6.Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caracciolo L, Marosi M, Mazzitelli J, Latifi S, Sano Y, Galvan L, Kawaguchi R, Holley S, Levine MS, Coppola G, Portera-Cailliau C, Silva AJ, Carmichael ST. CREB controls cortical circuit plasticity and functional recovery after stroke. Nat Commun. 2018;9:2250. doi: 10.1038/s41467-018-04445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauhan NB. Chronic neurodegenerative consequences of traumatic brain injury. Restor Neurol Neurosci. 2014;32:337–365. doi: 10.3233/RNN-130354. [DOI] [PubMed] [Google Scholar]
- 9.Chen TJ, Kula B, Nagy B, Barzan R, Gall A, Ehrlich I, Kukley M. In vivo regulation of oligodendrocyte precursor cell proliferation and differentiation by the AMPA-receptor subunit GluA2. Cell Rep. 2018;25:852–861e7. doi: 10.1016/j.celrep.2018.09.066. [DOI] [PubMed] [Google Scholar]
- 10.Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet. 2009;373:1105–1110. doi: 10.1016/S0140-6736(09)60214-2. [DOI] [PubMed] [Google Scholar]
- 11.Cirstea CM, Brooks WM, Craciunas SC, Popescu EA, Choi IY, Lee P, Bani-Ahmed A, Yeh HW, Savage CR, Cohen LG, Nudo RJ. Primary motor cortex in stroke: a functional MRI-guided proton MR spectroscopic study. Stroke. 2011;42:1004–1009. doi: 10.1161/STROKEAHA.110.601047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarkson AN, Parker K, Nilsson M, Walker FR, Gowing EK. Combined ampakine and BDNF treatments enhance poststroke functional recovery in aged mice via AKT-CREB signaling. J Cereb Blood Flow Metab. 2015;35:1272–1279. doi: 10.1038/jcbfm.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbett D, Carmichael ST, Murphy TH, Jones TA, Schwab ME, Jolkkonen J, Clarkson AN, Dancause N, Wieloch T, Johansen-Berg H, Nilsson M, McCullough LD, Joy MT. Enhancing the alignment of the preclinical and clinical stroke recovery research pipeline: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable translational working group. Int J Stroke. 2017;12:462–471. doi: 10.1177/1747493017711814. [DOI] [PubMed] [Google Scholar]
- 14.Courtine G, Bunge MB, Fawcett JW, Grossman RG, Kaas JH, Lemon R, Maier I, Martin J, Nudo RJ, Ramon-Cueto A, Rouiller EM, Schnell L, Wannier T, Schwab ME, Edgerton VR. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans. Nat Med. 2007;13:561–566. doi: 10.1038/nm1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, Malenka RC. CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci. 2006;9:475–477. doi: 10.1038/nn1661. [DOI] [PubMed] [Google Scholar]
- 16.Duncan PW. Synthesis of intervention trials to improve motor recovery following stroke. Top Stroke Rehabil. 1997;3:1–20. doi: 10.1080/10749357.1997.11754126. [DOI] [PubMed] [Google Scholar]
- 17.Farhad K, Khan HM, Ji AB, Yacoub HA, Qureshi AI, Souayah N. Trends in outcomes and hospitalization costs for traumatic brain injury in adult patients in the United States. J Neurotrauma. 2013;30:84–90. doi: 10.1089/neu.2011.2283. [DOI] [PubMed] [Google Scholar]
- 18.Fehlings MG, Kim KD, Aarabi B, Rizzo M, Bond LM, McKerracher L, Vaccaro AR, Okonkwo DO. Rho inhibitor VX-210 in acute traumatic subaxial cervical spinal cord injury: design of the SPinal Cord Injury Rho INhibition InvestiGation (SPRING) clinical trial. J Neurotrauma. 2018;35:1049–1056. doi: 10.1089/neu.2017.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feigin VL, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003;89:3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda H, Morishita T, Ogata T, Saita K, Hyakutake K, Watanabe J, Shiota E, Inoue T. Tailor-made rehabilitation approach using multiple types of hybrid assistive limb robots for acute stroke patients: a pilot study. Assist Technol. 2016;28:53–56. doi: 10.1080/10400435.2015.1080768. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez CL, Gharbawie OA, Williams PT, Kleim JA, Kolb B, Whishaw IQ. Evidence for bilateral control of skilled movements: ipsilateral skilled forelimb reaching deficits and functional recovery in rats follow motor cortex and lateral frontal cortex lesions. Eur J Neurosci. 2004;20:3442–3452. doi: 10.1111/j.1460-9568.2004.03751.x. [DOI] [PubMed] [Google Scholar]
- 24.Greer JE, McGinn MJ, Povlishock JT. Diffuse traumatic axonal injury in the mouse induces, c-Jun activation, and axonal outgrowth in the axotomized neuronal population. J Neurosci. 2011;31:5089–5105. doi: 10.1523/JNEUROSCI.5103-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han MH, Bolanos CA, Green TA, Olson VG, Neve RL, Liu RJ, Aghajanian GK, Nestler EJ. Role of cAMP response element-binding protein in the rat locus ceruleus: regulation of neuronal activity and opiate withdrawal behaviors. J Neurosci. 2006;26:4624–4629. doi: 10.1523/JNEUROSCI.4701-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hao Z, Wang D, Zeng Y, Liu M. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database Syst Rev. 2013;2013:CD008862. doi: 10.1002/14651858.CD008862.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollis ER, 2nd, Ishiko N, Yu T, Lu CC, Haimovich A, Tolentino K, Richman A, Tury A, Wang SH, Pessian M, Jo E, Kolodkin A, Zou Y. Ryk controls remapping of motor cortex during functional recovery after spinal cord injury. Nat Neurosci. 2016;19:697–705. doi: 10.1038/nn.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu WY, Cheng CH, Liao KK, Lee IH, Lin YY. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta-analysis. Stroke. 2012;43:1849–1857. doi: 10.1161/STROKEAHA.111.649756. [DOI] [PubMed] [Google Scholar]
- 29.Hu J, Li C, Hua Y, Zhang B, Gao BY, Liu PL, Sun LM, Lu RR, Wang YY, Bai YL. Constrained-induced movement therapy promotes motor function recovery by enhancing the remodeling of ipsilesional corticospinal tract in rats after stroke. Brain Res. 2019;1708:27–35. doi: 10.1016/j.brainres.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Ishida A, Isa K, Umeda T, Kobayashi K, Kobayashi K, Hida H, Isa T. Causal link between the cortico-rubral pathway and functional recovery through forced impaired limb use in rats with stroke. J Neurosci. 2016;36:455–467. doi: 10.1523/JNEUROSCI.2399-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishiguro M, Kawasaki K, Suzuki Y, Ishizuka F, Mishiro K, Egashira Y, Ikegaki I, Tsuruma K, Shimazawa M, Yoshimura S, Iwama T, Hara H. A Rho kinase (ROCK) inhibitor, fasudil, prevents matrix metalloproteinase-9-related hemorrhagic transformation in mice treated with tissue plasminogen activator. Neuroscience. 2012;220:302–312. doi: 10.1016/j.neuroscience.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Jitsuki S, Nakajima W, Takemoto K, Sano A, Tada H, Takahashi-Jitsuki A, Takahashi T. Nogo receptor signaling restricts adult neural plasticity by limiting synaptic AMPA receptor delivery. Cereb Cortex. 2016;26:427–439. doi: 10.1093/cercor/bhv232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Julian L, Olson MF. Rho-associated coiled-coil containing kinases (ROCK): regulation, and functions. Small GTPases. 2014;5:e29846. doi: 10.4161/sgtp.29846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kempf A, Schwab ME. Nogo-A represses anatomical and synaptic plasticity in the central nervous system. Physiology (Bethesda) 2013;28:151–163. doi: 10.1152/physiol.00052.2012. [DOI] [PubMed] [Google Scholar]
- 35.Kucher K, Johns D, Maier D, Abel R, Badke A, Baron H, Thietje R, Casha S, Meindl R, Gomez-Mancilla B, Pfister C, Rupp R, Weidner N, Mir A, Schwab ME, Curt A. First-in-man intrathecal application of neurite growth-promoting anti-Nogo-A antibodies in acute spinal cord injury. Neurorehabil Neural Repair. 2018;32:578–589. doi: 10.1177/1545968318776371. [DOI] [PubMed] [Google Scholar]
- 36.Kuroiwa T, Ting P, Martinez H, Klatzo I. The biphasic opening of the blood-brain barrier to proteins following temporary middle cerebral artery occlusion. Acta Neuropathol. 1985;68:122–129. doi: 10.1007/BF00688633. [DOI] [PubMed] [Google Scholar]
- 37.Liauw J, Hoang S, Choi M, Eroglu C, Choi M, Sun GH, Percy M, Wildman-Tobriner B, Bliss T, Guzman RG, Barres BA, Steinberg GK. Thrombospondins 1 and 2 are necessary for synaptic plasticity and functional recovery after stroke. J Cereb Blood Flow Metab. 2008;28:1722–1732. doi: 10.1038/jcbfm.2008.65. [DOI] [PubMed] [Google Scholar]
- 38.Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 2018;135:311–336. doi: 10.1007/s00401-018-1815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 40.Lo EH, Moskowitz MA, Jacobs TP. Exciting, radical, suicidal: how brain cells die after stroke. Stroke. 2005;36:189–192. doi: 10.1161/01.STR.0000153069.96296.fd. [DOI] [PubMed] [Google Scholar]
- 41.Mahar M, Cavalli V. Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci. 2018;19:323–337. doi: 10.1038/s41583-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maki T, Hayakawa K, Pham LD, Xing C, Lo EH, Arai K. Biphasic mechanisms of neurovascular unit injury and protection in CNS diseases. CNS Neurol Disord Drug Targets. 2013;12:302–315. doi: 10.2174/1871527311312030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcantoni M, Fuchs A, Low P, Bartsch D, Kiehn O, Bellardita C. Early delivery and prolonged treatment with nimodipine prevents the development of spasticity after spinal cord injury in mice. Sci Transl Med. 2020;12:eaay0167. doi: 10.1126/scitranslmed.aay0167. [DOI] [PubMed] [Google Scholar]
- 44.Meyer N, Richter N, Fan Z, Siemonsmeier G, Pivneva T, Jordan P, Steinhauser C, Semtner M, Nolte C, Kettenmann H. Oligodendrocytes in the mouse corpus callosum maintain axonal function by delivery of glucose. Cell Rep. 2018;22:2383–2394. doi: 10.1016/j.celrep.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 45.Mulherkar S, Firozi K, Huang W, Uddin MD, Grill RJ, Costa-Mattioli M, Robertson C, Tolias KF. RhoA-ROCK inhibition reverses synaptic remodeling and motor and cognitive deficits caused by traumatic brain injury. Sci Rep. 2017;7:10689. doi: 10.1038/s41598-017-11113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohtomo R, Kinoshita K, Ohtomo G, Takase H, Hamanaka G, Washida K, Islam MR, Wrann CD, Katsuki H, Iwata A, Lok J, Lo EH, Arai K. Treadmill exercise suppresses cognitive decline and increases white matter oligodendrocyte precursor cells in a mouse model of prolonged cerebral hypoperfusion. Transl Stroke Res. 2020;11:496–502. doi: 10.1007/s12975-019-00734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okabe N, Narita K, Miyamoto O. Axonal remodeling in the corticospinal tract after stroke: how does rehabilitative training modulate it. Neural Regen Res. 2017;12:185–192. doi: 10.4103/1673-5374.200792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paul RL, Goodman H, Merzenich M. Alterations in mechanoreceptor input to Brodmann’s areas 1 and 3 of the postcentral hand area of Macaca mulatta after nerve section and regeneration. Brain Res. 1972;39:1–19. doi: 10.1016/0006-8993(72)90782-2. [DOI] [PubMed] [Google Scholar]
- 49.Prakash R, Carmichael ST. Blood-brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Curr Opin Neurol. 2015;28:556–564. doi: 10.1097/WCO.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Regenhardt RW, Takase H, Lo EH, Lin DJ. Translating concepts of neural repair after stroke: structural and functional targets for recovery. Restor Neurol Neurosci. 2020;38:67–92. doi: 10.3233/RNN-190978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, Moskowitz MA, Liao JK. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36:2251–2257. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romi F, Naess H. Spinal cord infarction in clinical neurology: a review of characteristics and long-term prognosis in comparison to cerebral infarction. Eur Neurol. 2016;76:95–98. doi: 10.1159/000446700. [DOI] [PubMed] [Google Scholar]
- 53.Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- 54.Rupp R. Spinal cord lesions. Handb Clin Neurol. 2020;168:51–65. doi: 10.1016/B978-0-444-63934-9.00006-8. [DOI] [PubMed] [Google Scholar]
- 55.Sawada N, Liao JK. Targeting eNOS and beyond: emerging heterogeneity of the role of endothelial Rho proteins in stroke protection. Expert Rev Neurother. 2009;9:1171–1186. doi: 10.1586/ern.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seo JH, Guo S, Lok J, Navaratna D, Whalen MJ, Kim KW, Lo EH. Neurovascular matrix metalloproteinases and the blood-brain barrier. Curr Pharm Des. 2012;18:3645–3648. doi: 10.2174/138161212802002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen Y, Gu J, Liu Z, Xu C, Qian S, Zhang X, Zhou B, Guan Q, Sun Y, Wang Y, Jin X. Inhibition of HIF-1αlpha reduced blood brain barrier damage by regulating MMP-2 and VEGF during acute cerebral ischemia. Front Cell Neurosci. 2018;12:288. doi: 10.3389/fncel.2018.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi Y, Zhang L, Pu H, Mao L, Hu X, Jiang X, Xu N, Stetler RA, Zhang F, Liu X, Leak RK, Keep RF, Ji X, Chen J. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat Commun. 2016;7:10523. doi: 10.1038/ncomms10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shibuya M, Hirai S, Seto M, Satoh S, Ohtomo E Fasudil Ischemic Stroke Study G. Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. J Neurol Sci. 2005;238:31–39. doi: 10.1016/j.jns.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 60.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sladojevic N, Yu B, Liao JK. ROCK as a therapeutic target for ischemic stroke. Expert Rev Neurother. 2017;17:1167–1177. doi: 10.1080/14737175.2017.1395700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Squire L, Berg D, Bloom F, du Lac S, Ghosh A, Squire L, Spitzer N, Bloom F, McConnell S, Roberts J, Spitzer N, Zigmond M. San Diego, CA: Academic Press; 2002. Fundamental Neuroscience 2nd Edition. [Google Scholar]
- 64.Starkey ML, Bleul C, Zorner B, Lindau NT, Mueggler T, Rudin M, Schwab ME. Back seat driving: hindlimb corticospinal neurons assume forelimb control following ischaemic stroke. Brain. 2012;135:3265–3281. doi: 10.1093/brain/aws270. [DOI] [PubMed] [Google Scholar]
- 65.Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26:2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- 66.Sun W, Larson MJ, Kiyoshi CM, Annett AJ, Stalker WA, Peng J, Tedeschi A. Gabapentinoid treatment promotes corticospinal plasticity and regeneration following murine spinal cord injury. J Clin Invest. 2020;130:345–358. doi: 10.1172/JCI130391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takase H, Kurihara Y, Yokoyama TA, Kawahara N, Takei K. LOTUS overexpression accelerates neuronal plasticity after focal brain ischemia in mice. PLoS One. 2017;12:e0184258. doi: 10.1371/journal.pone.0184258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takase H, Washida K, Hayakawa K, Arai K, Wang X, Lo EH, Lok J. Oligodendrogenesis after traumatic brain injury. Behav Brain Res. 2018;340:205–211. doi: 10.1016/j.bbr.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 69.Taub E, Uswatte G, Pidikiti R. Constraint-induced movement therapy: a new family of techniques with broad application to physical rehabilitation--a clinical review. J Rehabil Res Dev. 1999;36:237–251. [PubMed] [Google Scholar]
- 70.Tedeschi A, Dupraz S, Laskowski CJ, Xue J, Ulas T, Beyer M, Schultze JL, Bradke F. The calcium channel subunit alpha2delta2 suppresses axon regeneration in the adult CNS. Neuron. 2016;92:419–434. doi: 10.1016/j.neuron.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 71.Toy D, Namgung U. Role of glial cells in axonal regeneration. Exp Neurobiol. 2013;22:68–76. doi: 10.5607/en.2013.22.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Traversa R, Cicinelli P, Bassi A, Rossini PM, Bernardi G. Mapping of motor cortical reorganization after stroke .A brain stimulation study with focal magnetic pulses. Stroke. 1997;28:110–117. doi: 10.1161/01.str.28.1.110. [DOI] [PubMed] [Google Scholar]
- 73.Tsai SY, Papadopoulos CM, Schwab ME, Kartje GL. Delayed anti-nogo-a therapy improves function after chronic stroke in adult rats. Stroke. 2011;42:186–190. doi: 10.1161/STROKEAHA.110.590083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ueno R, Takase H, Suenaga J, Kishimoto M, Kurihara Y, Takei K, Kawahara N, Yamamoto T. Axonal regeneration and functional recovery driven by endogenous Nogo receptor antagonist LOTUS in a rat model of unilateral pyramidotomy. Exp Neurol. 2020;323:113068. doi: 10.1016/j.expneurol.2019.113068. [DOI] [PubMed] [Google Scholar]
- 75.Wake H, Ortiz FC, Woo DH, Lee PR, Angulo MC, Fields RD. Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat Commun. 2015;6:7844. doi: 10.1038/ncomms8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Shen Y, Liu Z, Gu J, Xu C, Qian S, Zhang X, Zhou B, Jin Y, Sun Y. Dl-NBP (Dl-3-N-Butylphthalide) treatment promotes neurological functional recovery accompanied by the upregulation of white matter integrity and HIF-1αlpha/VEGF/Notch/Dll4 expression. Front Pharmacol. 2019;10:1595. doi: 10.3389/fphar.2019.01595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weidauer S, Nichtweiss M, Hattingen E, Berkefeld J. Spinal cord ischemia: aetiology, clinical syndromes and imaging features. Neuroradiology. 2015;57:241–257. doi: 10.1007/s00234-014-1464-6. [DOI] [PubMed] [Google Scholar]
- 78.Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol. 1993;33:181–189. doi: 10.1002/ana.410330208. [DOI] [PubMed] [Google Scholar]
- 79.Wittenberg GF. Experience, cortical, remapping, and recovery in brain disease. Neurobiol Dis. 2010;37:252–258. doi: 10.1016/j.nbd.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu Y, Zhang HT, O’Donnell JM. Phosphodiesterases in the central nervous system: implications in mood and cognitive disorders. Handb Exp Pharmacol. 2011:447–485. doi: 10.1007/978-3-642-17969-3_19. [DOI] [PubMed] [Google Scholar]
- 81.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zeiler SR, Hubbard R, Gibson EM, Zheng T, Ng K, O’Brien R, Krakauer JW. Paradoxical motor recovery from a first stroke after induction of a second stroke: reopening a postischemic sensitive period. Neurorehabil Neural Repair. 2016;30:794–800. doi: 10.1177/1545968315624783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang B, He Q, Li YY, Li C, Bai YL, Hu YS, Zhang F. Constraint-induced movement therapy promotes motor function recovery and downregulates phosphorylated extracellular regulated protein kinase expression in ischemic brain tissue of rats. Neural Regen Res. 2015;10:2004–2010. doi: 10.4103/1673-5374.172319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y, Xiong Y, Mahmood A, Meng Y, Liu Z, Qu C, Chopp M. Sprouting of corticospinal tract axons from the contralateral hemisphere into the denervated side of the spinal cord is associated with functional recovery in adult rat after traumatic brain injury and erythropoietin treatment. Brain Res. 2010;1353:249–257. doi: 10.1016/j.brainres.2010.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zorner B, Schwab ME. Anti-Nogo on the go: from animal models to a clinical trial. Ann N Y Acad Sci 1198 Suppl. 2010;1:E22–34. doi: 10.1111/j.1749-6632.2010.05566.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


