Abstract
The mammalian retina displays incomplete intrinsic regenerative capacities; therefore, retina degeneration is a major cause of irreversible blindness such as glaucoma, age-related macular degeneration and diabetic retinopathy. These diseases lead to the loss of retinal cells and serious vision loss in the late stage. Stem cell transplantation is a great promising novel treatment for these incurable retinal degenerative diseases and represents an exciting area of regenerative neurotherapy. Several suitable stem cell sources for transplantation including human embryonic stem cells, induced pluripotent stem cells and adult stem cells have been identified as promising target populations. However, the retina is an elegant neuronal complex composed of various types of cells with different functions. The replacement of these different types of cells by transplantation should be addressed separately. So far, retinal pigment epithelium transplantation has achieved the most advanced stage of clinical trials, while transplantation of retinal neurons such as retinal ganglion cells and photoreceptors has been mostly studied in pre-clinical animal models. In this review, we opine on the key problems that need to be addressed before stem cells transplantation, especially for replacing injured retinal ganglion cells, may be used practically for treatment. A key problem we have called the Switchboard Dilemma is a major block to have functional retinal ganglion cell replacement. We use the public switchboard telephone network as an example to illustrate different difficulties for replacing damaged components in the retina that allow for visual signaling. Retinal ganglion cell transplantation is confronted by significant hurdles, because retinal ganglion cells receive signals from different interneurons, integrate and send signals to the correct targets of the visual system, which functions similar to the switchboard in a telephone network – therefore the Switchboard Dilemma.
Keywords: cell transplantation, optic nerve regeneration, photoreceptors, retina degeneration, retinal ganglion cells, stem cells
Introduction
The eye, the organ of vision, is our window to the world and provides us with information which describes the outside environment. Responsible for the perception and integration of sensory information is the retina, which converts light signals to electrical signals. The retinas of teleost fish and amphibians are continuously growing throughout their lifetime owing to regenerating retina stem and progenitor cells located in the ciliary marginal zone (CMZ) at the rim of the retina. The CMZ gradually diminished during vertebrate evolution - being present in the neonatal but not in the adult bird and absent in mammals. The CMZ is the major source of regenerated peripheral but not of the central retina (Stenkamp et al., 2001). Muller cells are the source of central retina regeneration, including retinal ganglion cells (RGCs), in the fish (Nagashima, 2013; Lahne and Hyde, 2016). However, the mammalian Muller cells lack these regenerative abilities, as Muller cells do not spontaneously re-enter the cell cycle after retinal damage or loss. Several studies have shown that mammalian Muller cells may reprogram to a proliferative progenitor-like state by different intervention methods, such as by regulation of the Hippo signaling pathway in the mouse (Rueda et al., 2019).
The mammalian retina is a complex and elegant tissue consisting of a multi-layered complexly interconnected neural network: an outer nuclear layer, an outer plexiform layer, an inner nuclear layer, an inner plexiform layer and the retinal ganglion cell layer and is supported by an outer layer of retinal pigment epithelium (RPE) cells. As with other components of the central nervous system, the mature mammalian retina has limited regenerative capacity. Thus, retinal degeneration causes irreversible damage of retinal neurons and constitutes a major cause of serious vision loss worldwide. Among the most common of these sights threatening ophthalmic diseases are glaucoma, age-related macular degeneration (AMD) and diabetic retinopathy. These eye diseases are associated with degeneration of retinal ganglion cells, photoreceptor cells and/or RPE cells. Until now, available therapies for these diseases can slow disease progression or relieve symptoms, but currently no effective treatments to restore lost vision are satisfactory.
Glaucoma is one of the leading causes of irreversible blindness and the rate of vison loss due to glaucoma will nearly double by 2040 unless glaucoma can be effectively treated or prevented (Tham et al., 2014). While the primary site of injury in glaucoma is not well understood, this disease leads to a progressive degeneration of the RGCs and subsequent visual field loss. Currently, the only effective treatment is lowering intraocular pressure (IOP) since elevated IOP is the only proven risk factor for glaucomatous damage. However, glaucoma can occur at normal IOPs and lowering IOP may be insufficient to prevent vision loss in some patients (Realini et al., 2002; Sit, 2014). The increasing incidence of irreversible blindness because of an aging population is a cause of growing impactful social and economic burden, which highlight the importance of finding treatments that can stop and reverse vision loss and restore or improve vision.
Stem cell transplantation offers promising therapeutic strategies for patients suffering from brain neurodegenerative and retinal degeneration diseases. The eye is an excellent target for stem cell transplantation, because (a) the eye is an immune privileged environment, (b) the eye is easily accessible for cell delivery and able to be non-invasively monitored after transplantation, (c) the eye is a relatively small organ which requires only small numbers of stem cells and (d) the eye is a restricted environment that does not allow stem cells to move freely or systemically. Stem cell transplantation can physically replace unhealthy or degenerated retina cells and theoretically restore vision. Alternatively, stem cells might also provide nutritional support to surrounding cells by secreting trophic, paracrine factors, which can delay neurodegenerative progression and protect RGCs from dying or improve their function. Current research focuses on the identification of suitable stem cell sources for transplantation including human embryonic stem cells (hESC), induced pluripotent stem (iPS) cells and adult stem cells (such as Muller stem cells and mesenchymal stem cells) as promising cellular transplant target populations.
Search Strategy
Studies cited in this review were searched on the Medline database using the following keywords: retina degeneration, stem cell, cell transplantation, photoreceptor transplantation, RGC transplantation, optic nerve regeneration and Boolean combinations of the above terms. The results were further screened by title and abstract to exclude those studies which have relatively low relevance to our topic. We set year publication searching from 2005 to 2020.
Transplanted Photoreceptors Are Able to Structurally and Functionally Integrate into Host Retina
The vision system is complex and elegantly converts an image into a set of electrical signals and conducts these signals to the visual cortex and other regions of the brain through the optic nerve and other neural pathways. We can compare the visual system to a public switchboard telephone network (PSTN). The photoreceptors are analogous to our telephone at home, the former converting light signal to electrical signals while the latter converting sound signals to electrical signals (Figure 1). Rods and cones are afferent sensory neurons which send a proportional response synaptically to bipolar cells. These photoreceptors are also cross-linked by horizontal cells and amacrine cells, which modify the synaptic signal before it reaches the ganglion cells. The photoreceptors do not create action potentials and they are not involved in complex signal processing, so it might seem relatively easy to achieve integration of transplanted photoreceptor cells into a native neuronal network. If one has a broken telephone, one can easily replace the telephone with a new one and not bother calling the telephone company for a replacement or to fix the problem. In comparison, replacing the retinal equivalent is difficult, because one would have to replace all the dysfunctional or dead photoreceptors and their connections at the same time instead of only replacing one. To date, several successes in photoreceptor transplantation in both structural and functional have been made.
Figure 1.
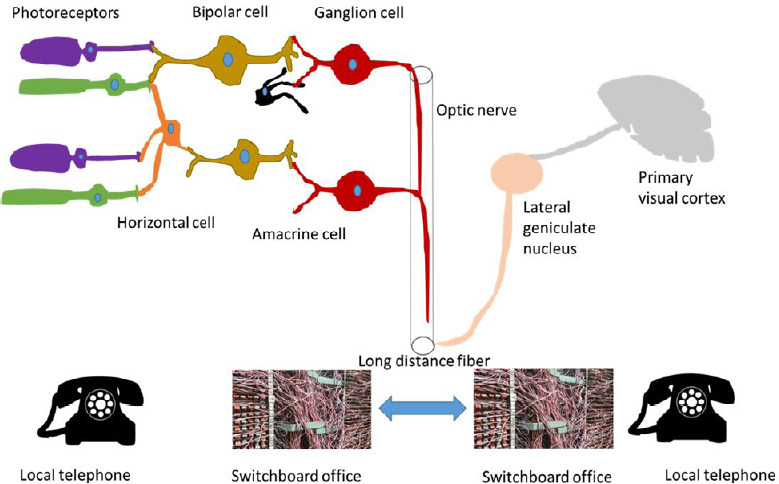
Visual neuro-system connects similarly to a public switched telephone network to produce an image versus a conversation.
Photoreceptors and local telephone convert light and sound signal to electrical signal, respectively, and form only one-direction connections. Retinal ganglion cell is comparable to switchboard office, which receiving, integrating and transmitting information. The optic nerve is the long-distance fiber cable in the visual system. The challenge of stem cell-based therapies to treat retinal degeneration diseases by replacing injured or dead ganglion cells is one of making the correction connections to produce a true image versus a series of mis-placed bright spots because of mis-guided complex connections with inappropriate targets, which we call “Switchboard Dilemma”. This would be analogous to transmitting a garbled conversation to the wrong person.
Protocols have been published demonstrating the stepwise differentiation of hESCs into retinal progenitors which express photoreceptor markers in vitro (Barnea-Cramer et al., 2016). iPS cells have been differentiated into photoreceptors, since iPS cells are better choices for producing patient-specific transplanted cells. Sequential treatment with Wingless-related integration site (Wnt) and Nodal antagonists, retinoic acid and taurine can induce human iPS cells to express photoreceptor markers (Hirami et al., 2009). Alternative protocols using bone morphogenetic protein (BMP)-4 and Wnt antagonists with insulin-like growth factor 1 (IGF-1) can facilitate the expression of photoreceptor markers in human iPS cells (Lamba et al., 2010). Recent work has shown that a set of five small molecules can directly induce fibroblasts to become functional photoreceptor-like cells without the need for pluripotent cells or viral transcriptional factors (Mahato et al., 2020). These chemically induced photoreceptor-like cells are a promising cell-replacement candidate, because of its relatively simple conversion process.
Compared to retina ganglion cells, it is much easier for transplanted photoreceptor cells to integrate into the local retinal environment and form correct connections. Transplanted mouse embryonic stem cell (ESC)-derived neural precursors can integrate into most layers of the retinal tissue and enhance survival of host photoreceptors in motor neuron degeneration mice, which have complete retinal degeneration by six months of age (Santos-Ferreira et al., 2016). Not limited to rodent models, primate models have also demonstrated success of stem cell therapy. Grafted hESCs can differentiate into photoreceptor cells, develop into a structured outer nuclear layer, and form host-graft synaptic connections (Shirai et al., 2016).
When delivered into the sub-retinal space of Crx-deficient mouse, which are a model of Leber’s Congenital Amaurosis, transplanted hESCs can integrate into the host outer nuclear layer and restore the b wave of the flash electroretinogram (Lamba et al., 2009). Similarly, transplanted mouse iPSC-derived photoreceptor precursors can integrate into the retina outer nuclear layer and improve electroretinography responses when injected into a retinal degeneration mouse model (Tucker et al., 2011). Besides 2D cell culture, 3D retinal organoid derived photoreceptors can survive in the sub-retinal space and differentially integrate into the retina of a mouse model with cone-rod degeneration (Santos-Ferreira et al., 2016). Recent reports also demonstrate improved visual function detected by optokinetic head tracking when human iPSC-derived photoreceptor precursors were transplanted into the sub-retinal space in different rodent models of retina regeneration (Barnea-Cramer et al., 2016).
The RPE is situated between the neural retina and choroid and plays a vital role both in supporting visual function and in providing structural support to the retina. The RPE itself does not form any synaptic connections with the photoreceptors and works similarly to the material shell for the home telephone which protects the electrical elements inside. Issues with RPE transplantation are less compared to neural retina components, because RPE cells are autonomous and do not rely upon neural retinal interconnections for function. Therefore, the development of RPE transplantation is currently at the most advanced stage of retinal clinical trials.
The first-generation human ESC-RPE cell line (MA09-hRPE) was tested in three key Phase1/2 clinical trials in parallel. These trials were started in April 2011 and completed in 2017 in the United States, United Kingdom, and South Korea (ClinicalTrial.gov identifiers: UK-SMD: NCT01469832; US-SMD: NCT01345006; US-AMD: NCT01344993). These clinical trials demonstrated good clinical safety profiles (Schwartz et al., 2015, 2016; Song et al., 2015). Several phase I/II clinical trials are still ongoing using various types of transplanted cells: human embryonic stem cell (hESC)-derived RPE (MA09) (ClinicalTrials.gov identifiers: NCT01344993, NCT02563782, NCT02463344, NCT03178149 and NCT01674829), hESC-RPE in suspension (McGill et al., 2017) (ClinicalTrials.gov identifiers: NCT02286089, NCT02749734, NCT03046407 and NCT02755428), hRPE on synthetic membrane patch (da Cruz et al., 2018; Kashani et al., 2018) (ClinicalTrials.gov identifiers: NCT01691261, NCT03102138), induced pluripotent stem cell (iPSC)-derived hRPE in either suspension or monolayer-sheet (Mandai et al., 2017) (ClinicalTrials.gov identifiers: UMIN000011929), bone marrow-derived SCs (Li et al., 2007; Qi et al., 2017) (ClinicalTrials.gov identifiers: NCT02016508, NCT01736059 and NCT01518127) and fetal human RPE (ClinicalTrials.gov identifiers: NCT02868424).
Retina Ganglion Cell Transplantation Faces Many Challenges
Unlike photoreceptors, RGCs are projection neurons that need to integrate into a complex synaptic network, extend long processes down the optic nerve and form spatially correct connections to targets of the visual system to achieve visual function and an appropriate image. The RGC layer works like a local switchboard office in the PSTN, receiving signals from different local telephones, integrating and sending signal out to other connections in the PSTN (Figure 1). Compared to only replacing the local telephone, it is much more complex to fix a broken switchboard. One needs to know where signals are coming from and where these signals should be sent. Another challenge of RGC transplantation is regrowth of RGC axons, which form the optic nerve. RGCs must extend long axons and re-establish functional and corresponding connections to their specific targets in the brain, whereas photoreceptor cells only require establishing short connections with underlying horizontal and bipolar cells. One can imagine the difficulties of building a new long fiber from a switchboard office to another switchboard office if one does even do not know the accurate address out of many possible connections. Compared to successes in functional and anatomic photoreceptor transplantation, RGC transplantation remains at an early stage of preclinical study, because of the challenges described above.
Various studies have demonstrated that both murine and hESC can differentiate into cells with RGC characteristics. Using various growth and differentiation factors, murine ESCs can be induced to express RGC markers such as Thy-1, Isl-1, Brn3b and Ath5 (Lamba et al., 2010). Some of these induced cells may even have RGC electrophysiological characteristics (Tabata et al., 2004). Efficient protocols have been published to induce human ESCs to differentiate into retinal precursors and yield a high percentage of inner retinal neurons with RGC characteristics (Lamba et al., 2006). Recently, a clustered regularly interspaced short palindromic repeats (CRISPR) engineered reporter cell line was shown to induce human ESCs to differentiate into retinal ganglion cells, providing a potential source of gene-edited transplanted RGCs (Sluch et al., 2015). Compared to ESCs, induced pluripotent cells have been investigated as potential transplantation targets since they provide the possibility of autologous transplantation, thereby minimizing immune rejection and yet maintain many characteristics of ESCs. Several protocols have proven successful for inducing iPS cells from reprogrammed fibroblasts to differentiate into RGC-like cells, which have expression of specific RGC markers such as Brn3b, Islet-1 and Thy1 (Ji and Tang, 2019).
Another cell transplantation target is Muller stem cells. Mammalian Muller cells have very limited regeneration capabilities. However, overexpression of Ascl1 and inhibiting histone deacetylase could enable mice to generate neurons from Muller glia cells after retinal injury (Jorstad et al., 2017). Adult mice Muller cells lose Muller glia identity and regain a proliferative, progenitor-like cellular state by repressing the Hippo signaling pathway (Rueda et al., 2019). Neurogenin2 induces lineage conversion of post-natal rodent Muller glia cells into RGC-like neurons in vitro and resume generation of this neuronal type from late progenitors in the retina in vivo (Guimarães et al., 2018). Inhibition of Notch1 has been shown to promote human Muller stem cells differentiate into cell populations with a phenotype resembling that of RGC precursors with both neural morphology and expression of RGC specific genes (Singhal et al., 2012). The differentiation of retinal progenitor cells to retinal ganglion cell could be increased by the application of retinal ganglion cell-conditioned medium, implicating the importance of the host environment in cell transplantation (Dai et al., 2018).
While we now benefit from a wide array of methods to generate bona fide retinal neurons, most of these remain inefficient for generating large numbers of specific retinal ganglion cells other than photoreceptors (Aparicio et al., 2017; Chao et al., 2017; Teotia et al., 2017; DiStefano et al., 2018). The yield range of stem cell-derived RGCs has been estimated to be 0.1–30% of the cells in the culture, which is much lower than that of photoreceptors. Most protocols that promote RGC differentiation mimic what is occurring during normal retina development. Therefore, this yield is similar to the normal percentage of RGCs in the retina at different developmental stages and the number of photoreceptors is significantly larger than RGCs in mouse and human retina. Although, purification methods, such as immunopanning and fluorescence-activated cell sorting, exist to produce highly pure RGC cultures, most unfortunately do not address the low production yield of RGCs in vitro (Sluch et al., 2015; Aparicio et al., 2017; Kobayashi et al., 2018). Furthermore, there methods are expensive, time-consuming and challenging to scale-up for clinical use.
If one gains success in promoting RGC differentiation from various stem cell sources and attaining relatively high yields, the next hurdle one has to overcome in order to achieve effective RGC replacement therapy, is to have transplanted RGCs survive and integrate into the host retinal environment and develop normal visual function. Once transplanted RGCs have been delivered to a host vitreal space, RGC cells have to overcome physical barriers preventing them from integrating and surviving within the host retina. Both the inner limiting membrane and the nerve fiber layer are likely to physically inhibit the engraftment of transplanted cells. Replacing a damaged or dead RGC with a newly transplanted RGC is similar to fixing a malfunctioning or broken switchboard with a new computer processor and router. However, this new chip has to make the correct connections to all the upstream and downstream components to create a correct communication. Newly grafted RGCs are required to establish correct synapses in a one to one correspondence with bipolar cells and the lateral geniculate nucleus of the thalamus to enable functional recovery. Otherwise, transplanted cells make projections which create bright pixels that are in the wrong spatial location, thereby resulting in a non-corresponding image.
After intravitreal injection, iPSC-derived RGCs do not typically integrate into the 5-week-old RGC-injured mouse retina, although the cells can survive in the vitreous on top of the retina (Chen et al., 2010). The internal limiting membrane is a significant physical barrier to cells entering and integrating into the retina. When using ESC-derived RGCs, very limited numbers of transplanted cells can integrate into the host retina ganglion cell layer (Jagatha et al., 2009). Besides rewiring of the retina network within the retina, re-establishment of connectivity between the newly integrated RGCs and the brain must be addressed. Interestingly, although in vitro hiPSC derived RGCs display target-specific responses when co-cultured with different targeted brain regions, non-specific connections have been reported in the same study (Teotia et al., 2017). Different from in vitro experiments, stem cell derived RGCs are required to extend long distance fibers to reach their targeted brain region when they are transplanted in vivo.
The regrowth of the optic nerve from RGCs is another challenge of stem cell transplantation. Both long-distance extension of new RGC axons and the correct corresponding synapses need to appropriately form in the lateral geniculate nucleus. Considerable published work demonstrates different methods promote the regeneration of existing mature RGC axons after injury. The lessons learned from these experiments provide significant guidance in facilitating optic nerve regeneration from transplanted stem cells. Previous studies demonstrate that upregulation of ciliary neurotrophic factor or activation of downstream signal transducer and activator of transcription 3 (STAT3) in adult RGC after injury was sufficient to promote robust axonal regeneration in vivo (Li et al., 2017). Intravitreal delivery of calcium channel inhibitors and human NgR-Fc decoys can promote optic nerve regeneration in crush and glaucoma rat models, respectively (Wang et al., 2015; Ribas et al., 2017). Deletion of two upstream repressors - phosphatase and tensin homolog (PTEN) and tuberous sclerosis 1/2 - selectively in retinal ganglion cells after optic nerve crush strongly stimulate axonal regeneration up to four mm length (Park et al., 2008; Liu et al., 2010). The induction of long-distance axon regeneration has been achieved by combination treatment involving elevated cyclic adenosine monophosphate and application of oncomodulin in a PTEN knockout environment (Kurimoto et al., 2010). Half of the regenerated axons pass beyond the optic chiasm with a small subset reaching the thalamus. The same group even reported full-length axon regeneration which built new synaptic connectivity within the brain (de Lima et al., 2012).
However, we wonder whether regeneration will be regulated with this robust level of local growth stimulation. Unexpected regeneration patterns of axons were observed in the injured optic nerve. The path reversal of many new axonal fibers toward the retina and pronounced axonal misrouting at the optic chiasm was observed. Nearly 40% of newly regenerated axons showed U-turns toward the retina upon activation of STAT3 and co-activation of STAT3 with mammalian target of rapamycin after injury (Luo et al., 2013; Pernet et al., 2013). More than half the axonal fibers joined the ipsilateral tract in PTEN and suppressor of cytokine signaling 3 double-deletion mice, whereas the percentage should be fewer than 5% in normal mice (Luo et al., 2013). To restore useful visual function that represents a true image, re-innervation of correct corresponding region in the visual cortex with appropriate types of retina ganglion cell axons has to be achieved with the correct corresponding spatial region in the visual cortex along with its appropriate interneurons.
Functional vision may be worse or even dangerous if neuronal synapses are made to a wrong target and present a false spatial image. A person may believe a car is coming from the right when a true dangerous object is coming from the left and the person walks into instead of away from a dangerous object. One may have a high intensity signal (from the transplanted RGC) but lack spatial or appropriate intensity without fidelity and map to an incorrect target in the visual cortex. To date, newly regenerated axons cannot be targeted region at high accuracy, if they were even able to have their RGC somas engraft and function and sprout new axons.
Conclusion
To conclude, development of stem cell-based therapies has been the focus of intense research and has gained huge successes and even more hope and excitement. Stem-cell replacement therapy may benefit large numbers of patients with visual impairment caused by retina degeneration in the years ahead. Restoring visual function is similar to fixing the malfunctioning PSTN, requiring specific protocols to target specific components of the phone system to specific locations with high fidelity. Retinal pigment epithelium cells do not form neural connections with other parts of vision system and probably provide an environment for successful RGC and photoreceptor function, which lower the difficulty of successful RPE transplantation. Photoreceptor cells are required to establish one-direction connection with bipolar cells, same as the local telephone in the PSTN, thereby increasing the difficulties of cellular transplantation similar to the issues for RGC transplantation.
A major issue for stem cell transplantation is determining if transplanted neuronal cells can integrate into host nuclear layers and form correct synapses with targeted inter-neuronal cells (i.e. bipolar, amacrine, and horizontal) and how these cells help a transplanted cell survive and function appropriately. Transplanted cell in the retina do not function on their own but in the context of a highly regulated and synchronized neural network that is highly inter-dependent.
Progress in photoreceptor production and visual function improvement after transplantation in animal studies suggests that photoreceptor may be the next candidate entering clinical studies, similar to chip-based retina prostheses which send electrical signals as photodetectors or the pixels in a television screen. RGC production and neuronal transplantation into an established neuro-network poses significant challenges because of lack of complete RGC integration, the difficulties of establishing high fidelity, accurate connections and intrinsic and extrinsic effects for axon growth and synaptogenesis. RGC are comparable to a switchboard office - receiving, integrating, and sending information to re-create a conversation. With early successes in promoting axon regeneration, a large challenge we face is how to guide transplanted RGCs to build correct synapses, meaning that new grafted RGC needs to know where the signal coming from and where they should be sent to create a real image. We call this problem the “Switchboard Dilemma”. While significant challenges remain to be solved, based upon the progress seen over the past decades, we remain confident stem cell-based therapies will be part of the clinical arsenal to combat vision loss disorders in the future although the types of retinal cells that may be successful are yet to be determined.
Footnotes
C-Editors: Zhao M, Qiu Y; T-Editor: Jia Y
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This work was supported by the NIH Center Core Grant, No. P30EY014801 (to Bascom Palmer Eye Institute) and a Research to Prevent Blindness Unrestricted Grant (to Bascom Palmer Eye Institute); the Walter G. Ross Foundation (to RKL).
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by the NIH Center Core Grant, No. P30EY014801 (to Bascom Palmer Eye Institute) and a Research to Prevent Blindness Unrestricted Grant (to Bascom Palmer Eye Institute); the Walter G. Ross Foundation (to RKL).
References
- 1.Aparicio JG, Hopp H, Choi A, Mandayam Comar J, Liao VC, Harutyunyan N, Lee TC. Temporal expression of CD184(CXCR4) and CD171(L1CAM) identifies distinct early developmental stages of human retinal ganglion cells in embryonic stem cell derived retina. Exp Eye Res. 2017;154:177–189. doi: 10.1016/j.exer.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnea-Cramer AO, Wang W, Lu SJ, Singh MS, Luo C, Huo H, McClements ME, Barnard AR, MacLaren RE, Lanza R. Function of human pluripotent stem cell-derived photoreceptor progenitors in blind mice. Sci Rep. 2016;6:29784. doi: 10.1038/srep29784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao JR, Lamba DA, Klesert TR, Torre A, Hoshino A, Taylor RJ, Jayabalu A, Engel AL, Khuu TH, Wang RK, Neitz M, Neitz J, Reh TA. Transplantation of human embryonic stem cell-derived retinal cells into the subretinal space of a non-human primate. Transl Vis Sci Technol. 2017;6:4. doi: 10.1167/tvst.6.3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen M, Chen Q, Sun X, Shen W, Liu B, Zhong X, Leng Y, Li C, Zhang W, Chai F, Huang B, Gao Q, Xiang AP, Zhuo Y, Ge J. Generation of retinal ganglion-like cells from reprogrammed mouse fibroblasts. Invest Ophthalmol Vis Sci. 2010;51:5970–5978. doi: 10.1167/iovs.09-4504. [DOI] [PubMed] [Google Scholar]
- 5.da Cruz L, Fynes K, Georgiadis O, Kerby J, Luo YH, Ahmado A, Vernon A, Daniels JT, Nommiste B, Hasan SM, Gooljar SB, Carr AF, Vugler A, Ramsden CM, Bictash M, Fenster M, Steer J, Harbinson T, Wilbrey A, Tufail A, et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. 2018;36:328–337. doi: 10.1038/nbt.4114. [DOI] [PubMed] [Google Scholar]
- 6.Dai M, Zhang Q, Zheng Z, Wang J. Retinal ganglion cell-conditioned medium and surrounding pressure alters gene expression and differentiation of rat retinal progenitor cells. Mol Med Rep. 2018;17:7177–7183. doi: 10.3892/mmr.2018.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lima S, Koriyama Y, Kurimoto T, Oliveira JT, Yin Y, Li Y, Gilbert HY, Fagiolini M, Martinez AM, Benowitz L. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Natl Acad Sci U S A. 2012;109:9149–9154. doi: 10.1073/pnas.1119449109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiStefano T, Chen HY, Panebianco C, Kaya KD, Brooks MJ, Gieser L, Morgan NY, Pohida T, Swaroop A. Accelerated and improved differentiation of retinal organoids from pluripotent stem cells in rotating-wall vessel bioreactors. Stem Cell Reports. 2018;10:300–313. doi: 10.1016/j.stemcr.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guimarães RPM, Landeira BS, Coelho DM, Golbert DCF, Silveira MS, Linden R, de Melo Reis RA, Costa MR. Evidence of Müller glia conversion into retina ganglion cells using neurogenin2. Front Cell Neurosci. 2018;12:410. doi: 10.3389/fncel.2018.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirami Y, Osakada F, Takahashi K, Okita K, Yamanaka S, Ikeda H, Yoshimura N, Takahashi M. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458:126–131. doi: 10.1016/j.neulet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 11.Jagatha B, Divya MS, Sanalkumar R, Indulekha CL, Vidyanand S, Divya TS, Das AV, James J. In vitro differentiation of retinal ganglion-like cells from embryonic stem cell derived neural progenitors. Biochem Biophys Res Commun. 2009;380:230–235. doi: 10.1016/j.bbrc.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 12.Ji SL, Tang SB. Differentiation of retinal ganglion cells from induced pluripotent stem cells: a review. Int J Ophthalmol. 2019;12:152–160. doi: 10.18240/ijo.2019.01.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorstad NL, Wilken MS, Grimes WN, Wohl SG, VandenBosch LS, Yoshimatsu T, Wong RO, Rieke F, Reh TA. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature. 2017;548:103–107. doi: 10.1038/nature23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashani AH, Lebkowski JS, Rahhal FM, Avery RL, Salehi-Had H, Dang W, Lin CM, Mitra D, Zhu D, Thomas BB, Hikita ST, Pennington BO, Johnson LV, Clegg DO, Hinton DR, Humayun MS. A bioengineered retinal pigment epithelial monolayer for, dry age-related macular degeneration. Sci Transl Med. 2018;10:eaao4097. doi: 10.1126/scitranslmed.aao4097. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi W, Onishi A, Tu HY, Takihara Y, Matsumura M, Tsujimoto K, Inatani M, Nakazawa T, Takahashi M. Culture systems of dissociated mouse and human pluripotent stem cell-derived retinal ganglion cells purified by two-step immunopanning. Invest Ophthalmol Vis Sci. 2018;59:776–787. doi: 10.1167/iovs.17-22406. [DOI] [PubMed] [Google Scholar]
- 16.Kurimoto T, Yin Y, Omura K, Gilbert HY, Kim D, Cen LP, Moko L, Kügler S, Benowitz LI. Long-distance axon regeneration in the mature optic nerve: contributions of, cAMP, and pten gene deletion. J Neurosci. 2010;30:15654–15663. doi: 10.1523/JNEUROSCI.4340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahne M, Hyde DR. Interkinetic nuclear migration in the regenerating retina. Adv Exp Med Biol. 2016;854:587–593. doi: 10.1007/978-3-319-17121-0_78. [DOI] [PubMed] [Google Scholar]
- 18.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4:73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5:e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li HJ, Sun ZL, Yang XT, Zhu L, Feng DF. Exploring optic nerve axon regeneration. Curr Neuropharmacol. 2017;15:861–873. doi: 10.2174/1570159X14666161227150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Atmaca-Sonmez P, Schanie CL, Ildstad ST, Kaplan HJ, Enzmann V. Endogenous bone marrow derived cells express retinal pigment epithelium cell markers and migrate to focal areas of RPE damage. Invest Ophthalmol Vis Sci. 2007;48:4321–4327. doi: 10.1167/iovs.06-1015. [DOI] [PubMed] [Google Scholar]
- 23.Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo X, Salgueiro Y, Beckerman SR, Lemmon VP, Tsoulfas P, Park KK. Three-dimensional evaluation of retinal ganglion cell axon regeneration and pathfinding in whole mouse tissue after injury. Exp Neurol. 2013;247:653–662. doi: 10.1016/j.expneurol.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahato B, Kaya KD, Fan Y, Sumien N, Shetty RA, Zhang W, Davis D, Mock T, Batabyal S, Ni A, Mohanty S, Han Z, Farjo R, Forster MJ, Swaroop A, Chavala SH. Pharmacologic fibroblast reprogramming into photoreceptors restores vision. Nature. 2020;581:83–88. doi: 10.1038/s41586-020-2201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, Fujihara M, Akimaru H, Sakai N, Shibata Y, Terada M, Nomiya Y, Tanishima S, Nakamura M, Kamao H, Sugita S, Onishi A, Ito T, Fujita K, Kawamata S, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376:1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 27.McGill TJ, Bohana-Kashtan O, Stoddard JW, Andrews MD, Pandit N, Rosenberg-Belmaker LR, Wiser O, Matzrafi L, Banin E, Reubinoff B, Netzer N, Irving C. Long-term efficacy of GMP grade xeno-free hESC-derived RPE cells following transplantation. Transl Vis Sci Technol. 2017;6:17. doi: 10.1167/tvst.6.3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagashima M, Barthel LK, Raymond PA. A self-renewing division of zebrafish Müller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development. 2013;140:4510–4521. doi: 10.1242/dev.090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pernet V, Joly S, Jordi N, Dalkara D, Guzik-Kornacka A, Flannery JG, Schwab ME. Misguidance and modulation of axonal regeneration by Stat3 and Rho/ROCK signaling in the transparent optic nerve. Cell Death Dis. 2013;4:e734. doi: 10.1038/cddis.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi X, Pay SL, Yan Y, Thomas J, Jr, Lewin AS, Chang LJ, Grant MB, Boulton ME. Systemic injection of RPE65-programmed bone marrow-derived cells prevents progression of chronic retinal degeneration. Mol Ther. 2017;25:917–927. doi: 10.1016/j.ymthe.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Realini T, Barber L, Burton D. Frequency of asymmetric intraocular pressure fluctuations among patients with and without glaucoma. Ophthalmology. 2002;109:1367–1371. doi: 10.1016/s0161-6420(02)01073-4. [DOI] [PubMed] [Google Scholar]
- 33.Ribas VT, Koch JC, Michel U, Bähr M, Lingor P. Attenuation of axonal degeneration by calcium channel inhibitors improves retinal ganglion cell survival and regeneration after optic nerve crush. Mol Neurobiol. 2017;54:72–86. doi: 10.1007/s12035-015-9676-2. [DOI] [PubMed] [Google Scholar]
- 34.Rueda EM, Hall BM, Hill MC, Swinton PG, Tong X, Martin JF, Poché RA. The Hippo pathway blocks mammalian retinal Müller glial cell reprogramming. Cell Rep. 2019;27:1637–1649. doi: 10.1016/j.celrep.2019.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos-Ferreira T, Völkner M, Borsch O, Haas J, Cimalla P, Vasudevan P, Carmeliet P, Corbeil D, Michalakis S, Koch E, Karl MO, Ader M. Stem cell-derived photoreceptor transplants differentially integrate into mouse models of cone-rod dystrophy. Invest Ophthalmol Vis Sci. 2016;57:3509–3520. doi: 10.1167/iovs.16-19087. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz SD, Tan G, Hosseini H, Nagiel A. Subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium for the treatment of macular degeneration: an assessment at 4 years. Invest Ophthalmol Vis Sci. 2016;57:ORSFc1-9. doi: 10.1167/iovs.15-18681. [DOI] [PubMed] [Google Scholar]
- 38.Shirai H, Mandai M, Matsushita K, Kuwahara A, Yonemura S, Nakano T, Assawachananont J, Kimura T, Saito K, Terasaki H, Eiraku M, Sasai Y, Takahashi M. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci U S A. 2016;113:E81-E90. doi: 10.1073/pnas.1512590113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singhal S, Bhatia B, Jayaram H, Becker S, Jones MF, Cottrill PB, Khaw PT, Salt TE, Limb GA. Human Muller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem Cells Transl Med. 2012;1:188–199. doi: 10.5966/sctm.2011-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sit AJ. Intraocular pressure variations: causes and clinical significance. Can J Ophthalmol. 2014;49:484–488. doi: 10.1016/j.jcjo.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Sluch VM, Davis CH, Ranganathan V, Kerr JM, Krick K, Martin R, Berlinicke CA, Marsh-Armstrong N, Diamond JS, Mao HQ, Zack DJ. Differentiation of human ESCs to retinal ganglion cells using a CRISPR engineered reporter cell line. Sci Rep. 2015;5:16595. doi: 10.1038/srep16595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song WK, Park KM, Kim HJ, Lee JH, Choi J, Chong SY, Shim SH, Del Priore LV, Lanza R. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Reports. 2015;4:860–872. doi: 10.1016/j.stemcr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stenkamp DL, Powers MK, Carney LH, Cameron DA. Evidence for two distinct mechanisms of neurogenesis and cellular pattern formation in regenerated goldfish retinas. J Comp Neurol. 2001;431:363–381. doi: 10.1002/1096-9861(20010319)431:4<363::aid-cne1076>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 44.Tabata Y, Ouchi Y, Kamiya H, Manabe T, Arai K, Watanabe S. Specification of the retinal fate of mouse embryonic stem cells by ectopic expression of Rx/rax, a homeobox gene. Mol Cell Biol. 2004;24:4513–4521. doi: 10.1128/MCB.24.10.4513-4521.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teotia P, Chopra DA, Dravid SM, Van Hook MJ, Qiu F, Morrison J, Rizzino A, Ahmad I. Generation of functional human retinal ganglion cells with target specificity from pluripotent stem cells by chemically defined recapitulation of developmental mechanism. Stem Cells. 2017;35:572–585. doi: 10.1002/stem.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Tucker BA, Park IH, Qi SD, Klassen HJ, Jiang C, Yao J, Redenti S, Daley GQ, Young MJ. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS One. 2011;6:e18992. doi: 10.1371/journal.pone.0018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Lin J, Arzeno A, Choi JY, Boccio J, Frieden E, Bhargava A, Maynard G, Tsai JC, Strittmatter SM. Intravitreal delivery of human NgR-Fc decoy protein regenerates axons after optic nerve crush and protects ganglion cells in glaucoma models. Invest Ophthalmol Vis Sci. 2015;56:1357–1366. doi: 10.1167/iovs.14-15472. [DOI] [PMC free article] [PubMed] [Google Scholar]


