Abstract
Background & objectives:
With increased isolation of Burkholderia cepacia complex (Bcc) and Stenotrophomonas maltophilia from clinical specimens, knowledge of their antimicrobial susceptibility trend will aid in better patient management. This study provides a comprehensive picture of this trend over a decade.
Methods:
A retrospective analysis of laboratory records over 10 years for antimicrobial susceptibility pattern of Bcc and S. maltophilia was carried out. The susceptibility pattern to commonly used antimicrobials was determined using disk diffusion and compared at the beginning, mid and end of the study period.
Results:
Five hundred and thirty Bcc and 665 S. maltophilia isolated over the past 10 yr were included in the study. Over the years, susceptibility of Bcc for co-trimoxazole varied as 80, 70 and 89 per cent at the beginning, middle and end of the study, respectively. Susceptibility to tetracycline was 43 per cent at the beginning of the study and that to minocycline was 100 per cent mid-study and 74 per cent at the end. Susceptibility to ceftazidime varied as 83, 60 and 65 per cent, respectively, and to meropenem, increased during the first half of the study and decreased in the second half, as 60, 70 and 43 per cent, respectively. Bcc susceptibility to levofloxacin decreased from 84 (in 2014) to 76 per cent (in 2016). S. maltophilia susceptibility to co-trimoxazole varied as 90, 82 and 87 per cent, respectively, whereas that to levofloxacin was 80, 100 and 94 per cent, respectively, during the start, mid and end of the study. Susceptibility to minocycline decreased from 100 per cent mid-study to 96 per cent at the end. Susceptibility of S. maltophilia to ceftazidime increased from 24 (in 2012) to 37 per cent (in 2016). All variations among the three phases of the study were significant for all antimicrobials tested for both the organisms.
Interpretation & conclusions:
While Bcc showed increased resistance to ceftazidime, meropenem and minocycline, S. maltophilia maintained >80 per cent susceptibility to minocycline, levofloxacin and co-trimoxazole throughout the decade. By 2016, Bcc was most susceptible to co-trimoxazole, whereas S. maltophilia was most susceptible to minocycline and levofloxacin.
Keywords: Antimicrobial resistance, Burkholderia cepacia complex, co-trimoxazole, minocycline, Stenotrophomonas maltophilia
Burkholderia cepacia complex (Bcc) and Stenotrophomonas maltophilia are two important opportunistic non-fermenting Gram-negative bacilli (NFGNBs) causing rampant infections in immunocompromised patients1. Bcc is an established pulmonary pathogen in patients with cystic fibrosis (CF) and chronic granulomatous disease2. Both Bcc and S. maltophilia can cause bacteraemia (particularly in patients with indwelling catheters), septic arthritis, urinary tract infection, peritonitis, cellulitis/myositis, osteomyelitis, meningitis, endophthalmitis/keratitis, endocarditis, etc3. S. maltophilia is also emerging as an opportunistic pathogen causing respiratory infections in humans3. Both these organisms are increasingly being isolated from various clinical samples in routine diagnostic laboratories as lysine-positive NFGNBs or with the aid of matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry (MALDI-TOF MS)4,5. Management of patients infected with these NFGNBs is challenging as both Bcc and S. maltophilia are prone to develop resistance to commonly used antibiotics. Monitoring their susceptibility trend over the years has thus become important. Only a handful of studies have documented the antibiogram data of Bcc and S. maltophilia from Indian hospitals1,6,7. These studies either give a cross-sectional view of the contemporary susceptibility patterns or not included a sufficient number of Bcc and S. maltophilia isolates. Misidentification of these less common isolates in the laboratory can lead to wrong institution of high-end antimicrobials to the patients which could further make these bugs multidrug resistant. This study was, therefore, planned to provide a comprehensive picture of the trend of susceptibility patterns of Bcc and S. maltophilia over a decade from a high-throughput laboratory in North India, as an extension to our previously reported study7 that included isolates from 2007 to 2012.
Material & Methods
Study design: Retrospective data were collected from samples processed in the Clinical Bacteriology Section of the department of Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh, India from January 2007 to December 2016 (10 yr). NFGNBs isolated from blood, sterile body fluids, pus and respiratory samples were included in the study. Before 2014, Bcc and S. maltophilia were identified by lysine positivity and other conventional biochemical reactions along with molecular identification and typing of Bcc using recA polymerase chain reaction–restriction fragment length polymorphism, as described previously4. After 2014, identification was done by MALDI-TOF MS (Bruker Daltonik GmbH, Germany).
Antimicrobial susceptibility testing (ABST): ABST was performed with Kirby–Bauer disk diffusion test using commercially available disks (HiMedia, Mumbai). Different antimicrobials tested were co-trimoxazole (1.25 μg/23.75 μg), tetracycline (30 μg) or minocycline (30 μg) (since 2012), levofloxacin (5 μg) and ceftazidime (30 μg) for S. maltophilia and in addition meropenem (10 μg) for Bcc. For quality control, Escherichia coli (ATCC® 25922), Staphylococcus aureus (ATCC® 25923) and Pseudomonas aeruginosa (ATCC® 27853) were also subjected to ABST. Results of zone diameters were interpreted according to contemporary Clinical and Laboratory Standards Institute (CLSI) guidelines over the decade, and breakpoints remained the same during these years8. Isolates with intermediate levels of resistance in disk diffusion were included in the resistant organisms for final analysis of isolates. Z-test for proportions was used to analyze data.
Statistical analysis: The Z-test for proportions was used to compare the mean susceptibilities of the isolates. A P value of <0.05 was considered statistically significant and depicted as *P<0.05, **P<0.01 and ***P<0.001.
Results
An analysis of antimicrobial susceptibility profile of Bcc (total: 530) and S. maltophilia (total: 665) isolates from all types of samples, i.e., blood, sterile body fluids, pus and respiratory samples, from 2007 to 2016 (10 yr period) is presented. In 2013, the susceptibility of Bcc and S. maltophilia could not be analyzed due to less number of the isolates (<30 both). The year-wise isolation of Bcc and S. maltophilia is depicted in Table.
Table.
Year-wise isolation of Burkholderia cepacia complex and Stenotrophomonas maltophilia
| Years | Bcc (n) | Stenotrophomonas maltophilia (n) |
|---|---|---|
| 2007-2009 | 63 | 38 |
| 2010-2011 | 89 | 54 |
| 2012 | 34 | 33 |
| 2013 | 28 | 26 |
| 2014 | 48 | 131 |
| 2015 | 110 | 184 |
| 2016 | 158 | 199 |
| Total | 530 | 665 |
Bcc, Burkholderia cepacia complex
ABST trend of Burkholderia cepacia complex (Bcc) over the decade: During the study period, the susceptibility of Bcc to co-trimoxazole varied from 80 per cent at the start, 70 per cent by mid-term to 89 per cent by the end. The trend of ceftazidime susceptibility varied as 83, 60 and 65 per cent through the start, mid and end of the study, respectively. The susceptibility to meropenem varied as 60 per cent at the start, increased to 70 per cent by mid-study and decreased to 43 per cent at the end. All these variations in the trend of susceptibility during the three phases of the study were statistically significant (P<0.001). Susceptibility to minocycline decreased from 100 per cent in 2012 to 74 per cent in 2016 (P<0.001) and that to levofloxacin decreased from 84 per cent in 2014 to 76 per cent in 2016 (P<0.001). By 2016, Bcc isolates showed the highest susceptibility to co-trimoxazole (89%) followed by levofloxacin (76%) and minocycline (74%) (Fig. 1).
Fig. 1.
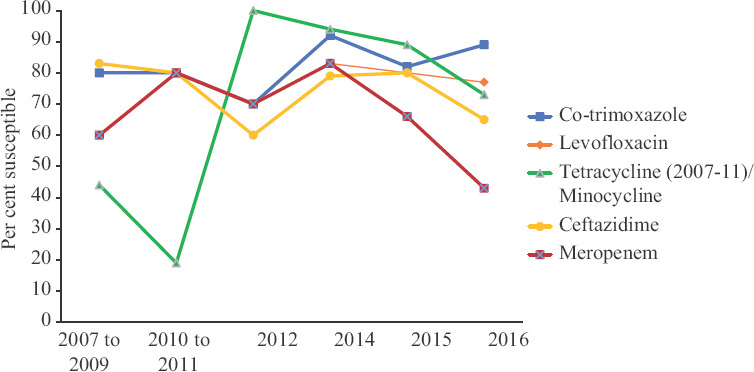
Year-wise antibiotic susceptibility pattern of Burkholderia cepacia complex.
ABST trend of Stenotrophomonas maltophilia over the decade: The susceptibility of S. maltophilia isolates to levofloxacin increased during the first half of the study (80-100%) and declined during the second half (100-94%) (P<0.001). Susceptibility to co-trimoxazole decreased during the first half of the study (90-82%) and increased during the second half (82-87%) (P<0.001). Susceptibility to minocycline decreased from 100 per cent in 2012 to 96 per cent in 2016 (P<0.001) and that to ceftazidime increased from 24 per cent in 2012 to 37 per cent in 2016 (P<0.001). By 2016, S. maltophilia were most susceptible to minocycline (96%), followed by levofloxacin (94%) and co-trimoxazole (87%) (Fig. 2).
Figure 2.
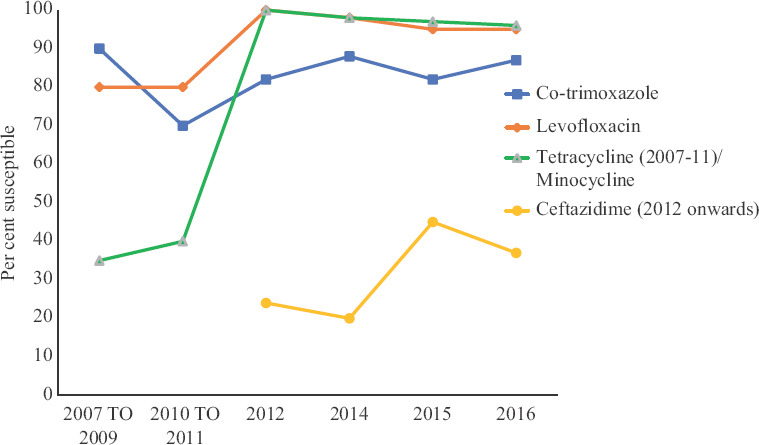
Year-wise antibiotic susceptibility pattern of Stenotrophomonas maltophilia.
Discussion
S. maltophilia and Bcc have been recognized as the third and the fourth most common NFGNBs worldwide, respectively, after Pseudomonas aeruginosa and Acinetobacter baumannii3. In our previous studies, Bcc was observed to be the third most common NFGNB followed by S. maltophilia, but when analyzed cumulatively over a period of 10 years, S. maltophilia (n=665) were more than Bcc (n=530)9,10,11.
The intrinsic resistance of Bcc to various antibiotics, including penicillin, first- and second-generation cephalosporins, aminoglycosides, fosfomycin and polymyxins, leaves the clinician with limited options for treatment2. Further, selectively permeable outer membrane, efflux pumps and/or production of inducible beta-lactamases enable Bcc to develop resistance de novo12. Based on the findings of our study, co-trimoxazole could be considered as the best therapeutic choice for Bcc infection among all antimicrobials tested as this drug showed the highest susceptibility along with an increase in susceptibility (80-89%) over the decade. Ceftazidime also showed a 5 per cent increase in susceptibility from mid-study to the end. However, susceptibility to other drugs, i.e., minocycline, levofloxacin and meropenem, has shown a progressive decline over the past decade. If we analyze the susceptibility pattern mentioned in different studies, Chien et al13 also found co-trimoxazole to be the most active agent with a susceptibility of 87 per cent for Bcc isolates from Taiwan. However, unlike our findings, the same study reported a higher susceptibility of 87, 78 and 44 per cent to meropenem, ceftazidime and levofloxacin, respectively. Similar to our study, Fehlberg et al14 also documented higher susceptibilities of Bcc to levofloxacin (96.3%), minocycline (94%), meropenem (94%) and ceftazidime (93.9%) and lower susceptibility to co-trimoxazole (71.9%) in their Brazilian isolates by disk diffusion method. Interestingly, susceptibility to co-trimoxazole was reportedly higher, i.e., 97.6 per cent, when broth microdilution method of susceptibility was employed on the same isolates in their study. Based on these findings, it was concluded that the methodology employed for susceptibility testing (broth or disk diffusion) has a bearing on the result interpretation. This finding is similar to our prior observation; upon testing some of the Bcc isolates, using agar dilution and disk diffusion methods. Another study from a trauma centre in North India showed lower susceptibilities to ceftazidime (44.6%) and co-trimoxazole (42.2%) with meropenem (53%) being the most active agent15. Although different studies have reported varying susceptibility ranges to different antibiotics, co-trimoxazole still remains the most commonly used antimicrobial option against Bcc infections14,16.
S. maltophilia has been reported to develop resistance to multiple antimicrobials, including extended-spectrum penicillins, third-generation cephalosporins, aminoglycosides, quinolones and carbapenems17. According to our observation, minocycline could be the drug of choice as it showed the highest susceptibility at the conclusion of the study (96%). Among other agents tested, levofloxacin susceptibility increased during the first half and decreased during the second half of the study. The susceptibility to ceftazidime increased over the decade and that to co-trimoxazole increased during the last five years of the study period. In a study from 2007 to 2011, minocycline showed better efficacy compared to tetracycline against S. maltophilia18. However, in literature, co-trimoxazole remains as a first-line agent for treatment as more than 90 per cent of S. maltophilia isolates are susceptible to co-trimoxazole19. In our study, co-trimoxazole susceptibility was almost comparable (87%) to other studies. However, there are scattered reports showing that resistance to co-trimoxazole is increasing. Declining susceptibility report (57%) has been made by Sun et al20 from China. It has been documented that up to 10 per cent of Indian isolates and up to 24 per cent of British isolates are resistant to co-trimoxazole21,22. In one report from New York, S. maltophilia isolates from the sputum samples of cystic fibrosis patients showed exceptionally high resistance of 84 per cent to co-trimoxazole23. S. maltophilia usually expresses variable susceptibility pattern to fluoroquinolones also3. Chang et al24 reported a decrease in susceptibility to levofloxacin, from 83 per cent (2003-2008) to 77 per cent (2011) among their S. maltophilia isolates, whereas Sun et al20, 83 per cent of S. maltophilia isolates from China were susceptible to levofloxacin in 2014. In the present study also, susceptibility of S. maltophilia was second highest for levofloxacin (94%) among the isolates from North Indian patients. A similar observation was made in South India also where 91 per cent of S. maltophilia were susceptible to levofloxacin21. With respect to ceftazidime, different geographical regions have reported varying susceptibility for S. maltophilia20,21,25.
Interestingly, increased susceptibility to both co-trimoxazole and ceftazidime was observed in the last five years of this study for both Bcc and S. maltophilia. It could possibly be due to discontinuation of the use of these antimicrobials that lead to re-emergence of susceptibility, as noticed earlier in Salmonella spp26. Loss of plasmids conferring resistance to co-trimoxazole and ceftazidime over time could also be responsible for increasing susceptibility towards these antimicrobials27. Increased susceptibility of S. maltophilia have been reported in Europe as well28.
The present study is unique in incorporating a comprehensive collection of two important NFGNBs and the trend of their antimicrobial susceptibility profile over a time frame of 10 years. However, the limitation of the study is that although broth microdilution is considered the gold standard method for antimicrobial susceptibility testing, the same could not be pursued in the present study owing to large number of samples processed in our high-throughput laboratory.
Correct and timely identification of the multidrug-resistant isolates of Bcc and S. maltophilia in a routine microbiology laboratory is a herculean task as these need to be differentiated from P. aeruginosa as both have inherently contrasting susceptibility pattern to that of P. aeruginosa4. Further, their misidentification has a direct bearing on patient care as these organisms are intrinsically resistant to the antibiotics that are used as the last resort agents against P. aeruginosa and most of the other GNBs such as carbapenems and polymyxins. Therefore, accurate identification of Bcc and S. maltophilia isolates along with the antibiogram comes to the forefront in optimal management of patients infected with these NFGNBs and also in preventing development of multidrug-resistant strains. Overall, our data suggest that co-trimoxazole should be the preferred drug of choice for Bcc, whereas minocycline or levofloxacin should be the preferred drug of choice for S. maltophilia.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Samanta P, Gautam V, Thapar R, Ray P. Emerging resistance of non-fermenting gram negative bacilli in a tertiary care centre. Indian J Pathol Microbiol. 2011;54:666–7. doi: 10.4103/0377-4929.85150. [DOI] [PubMed] [Google Scholar]
- 2.Gautam V, Shafiq N, Singh M, Ray P, Singhal L, Jaiswal NP, et al. Clinical and in vitro evidence for the antimicrobial therapy in Burkholderia cepacia complex infections. Expert Rev Anti Infect Ther. 2015;13:629–63. doi: 10.1586/14787210.2015.1025056. [DOI] [PubMed] [Google Scholar]
- 3.LiPuma JJ, Currie BJ, Lum GD, Vandamme PAR. Burkholderia, Stenotrophomonas, Ralstonia, Cupriavidus, Pandoraea, Brevundimonas, Comamonas and Acidovorax. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of clinical microbiology. 9th ed. Washington, DC: ASM Press; 2007. pp. 749–69. [Google Scholar]
- 4.Gautam V, Ray P, Vandamme P, Chatterjee SS, Das A, Sharma K, et al. Identification of lysine positive non-fermenting gram negative bacilli (Stenotrophomonas maltophilia and Burkholderia cepacia complex) Indian J Med Microbiol. 2009;27:128–33. doi: 10.4103/0255-0857.49425. [DOI] [PubMed] [Google Scholar]
- 5.Gautam V, Sharma M, Singhal L, Kumar S, Kaur P, Tiwari R, et al. MALDI-TOF mass spectrometry: An emerging tool for unequivocal identification of non-fermenting Gram-negative bacilli. Indian J Med Res. 2017;145:665–72. doi: 10.4103/ijmr.IJMR_1105_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaul S, Brahmadathan KN, Jagannati M, Sudarsanam TD, Pitchamuthu K, Abraham OC, et al. One year trends in the gram-negative bacterial antibiotic susceptibility patterns in a medical intensive care unit in South India. Indian J Med Microbiol. 2007;25:230–5. doi: 10.4103/0255-0857.34764. [DOI] [PubMed] [Google Scholar]
- 7.Gautam V, Kumar S, Kaur P, Deepak T, Singhal L, Tewari R, et al. Antimicrobial susceptibility pattern of Burkholderia cepacia complex Stenotrophomonas maltophilia over six years (2007-2012) Indian J Med Res. 2015;142:492–4. doi: 10.4103/0971-5916.169225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 27th informational supplement. CLSI document M100-27. Wayne, USA: CLSI; 2017. [Google Scholar]
- 9.Gautam V, Singhal L, Ray P. Burkholderia cepacia complex: Beyond Pseudomonas and acinetobacter. Indian J Med Microbiol. 2011;29:4–12. doi: 10.4103/0255-0857.76516. [DOI] [PubMed] [Google Scholar]
- 10.Gautam V, Ray P, Puri GD, Sharma K, Vandamme P, Madhup SK, et al. Investigation of Burkholderia cepacia complex in septicaemic patients in a tertiary care hospital, India. Nepal Med Coll J. 2009;11:222–4. [PubMed] [Google Scholar]
- 11.Gautam V, Arora A, Madhup SK, Das A, Vandamme P, Sharma K, et al. Burkholderia cepacia complex in septicaemic non-cystic fibrosis cases from two tertiary care hospitals in North India. Indian J Med Res. 2010;131:829–32. [PubMed] [Google Scholar]
- 12.Gautam V, Kumar S, Patil PP, Meletiadis J, Patil PP, Mouton JW, et al. Exploring the Interplay of Resistance Nodulation Division Efflux Pumps, Amp C and Opr D in Antimicrobial Resistance of Burkholderia cepacia Complex in Clinical Isolates. Microb Drug Resist. 2020;26:1144–52. doi: 10.1089/mdr.2019.0102. [DOI] [PubMed] [Google Scholar]
- 13.Chien YC, Liao CH, Sheng WH, Chien JY, Huang YT, Yu CJ, et al. Clinical characteristics of bacteremia caused by Burkholderia cepacia complex species and antimicrobial susceptibility of the isolates in a medical center in Taiwan. Int J Antimicrob Agents. 2018;51:357–64. doi: 10.1016/j.ijantimicag.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Fehlberg LC, Nicoletti AG, Ramos AC, Rodrigues-Costa F, de Matos AP, Girardello R, et al. In vitro susceptibility of Burkholderia cepacia complex isolates: Comparison of disk diffusion, Etest®, agar dilution, and broth microdilution methods. Diagn Microbiol Infect Dis. 2016;86:422–7. doi: 10.1016/j.diagmicrobio.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Rajkumari N, Mathur P, Gupta AK, Sharma K, Misra MC. Epidemiology and outcomes of Stenotrophomonas maltophilia and Burkholderia cepacia infections among trauma patients of India: A five year experience. J Infect Prev. 2015;16:103–10. doi: 10.1177/1757177414558437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aggarwal N, Garg S, Pannu HS, Kler TS. Fatal Burkholderia cepacia early prosthetic valve endocarditis: A very rare case and a review of the literature. J Heart Valve Dis. 2005;14:271–4. [PubMed] [Google Scholar]
- 17.McGowan JE Jr. Resistance in nonfermenting gram-negative bacteria: Multidrug resistance to the maximum. Am J Infect Control. 2006;34:S29–37. doi: 10.1016/j.ajic.2006.05.226. [DOI] [PubMed] [Google Scholar]
- 18.Castanheira M, Mendes RE, Jones RN. Update on Acinetobacter species: Mechanisms of antimicrobial resistance and contemporary in vitro activity of minocycline and other treatment options. Clin Infect Dis. 2014;59(Suppl 6):S367–73. doi: 10.1093/cid/ciu706. [DOI] [PubMed] [Google Scholar]
- 19.Al Johani SM, Akhter J, Balkhy H, El-Saed A, Younan M, Memish Z. Prevalence of antimicrobial resistance among gram-negative isolates in an adult intensive care unit at a tertiary care center in Saudi Arabia. Ann Saudi Med. 2010;30:364–9. doi: 10.4103/0256-4947.67073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun E, Liang G, Wang L, Wei W, Lei M, Song S, et al. Antimicrobial susceptibility of hospital acquired Stenotrophomonas maltophilia isolate biofilms. Braz J Infect Dis. 2016;20:365–73. doi: 10.1016/j.bjid.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhavana MV, Joshi S, Adhikary R, Beena HB. Antibiotic susceptibility pattern of Burkholderia cepacia complex and Stenotrophomonas maltophilia: A 5-year analysis. Indian J Med Microbiol. 2017;35:318–9. doi: 10.4103/ijmm.IJMM_16_236. [DOI] [PubMed] [Google Scholar]
- 22.Milne KEN, Gould IM. Combination antimicrobial susceptibility testing of multidrug-resistant Stenotrophomonas maltophilia from cystic fibrosis patients. Antimicrob Agents Chemother. 2012;56:4071–7. doi: 10.1128/AAC.00072-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.San Gabriel P, Zhou J, Tabibi S, Chen Y, Trauzzi M, Saiman L. Antimicrobial susceptibility and synergy studies of Stenotrophomonas maltophilia isolates from patients with cystic fibrosis. Antimicrob Agents Chemother. 2004;48:168–71. doi: 10.1128/AAC.48.1.168-171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang YT, Lin CY, Chen YH, Hsueh PR. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol. 2015;6:893. doi: 10.3389/fmicb.2015.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sader HS, Flamm RK, Jones RN. Tigecycline activity tested against antimicrobial resistant surveillance subsets of clinical bacteria collected worldwide (2011) Diagn Microbiol Infect Dis. 2013;76:217–21. doi: 10.1016/j.diagmicrobio.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Britto CD, Wong VK, Dougan G, Pollard AJ. A systematic review of antimicrobial resistance in Salmonella enterica serovar Typhi, the etiological agent of typhoid. PLoS Negl Trop Dis. 2018;12:E0006779. doi: 10.1371/journal.pntd.0006779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chand HJ, Rijal KR, Neupane B, Sharma VK, Jha B. Re-emergence of susceptibility to conventional first line drugs in Salmonella isolates from enteric fever patients in Nepal. J Infect Dev Ctries. 2014;8:1483–7. doi: 10.3855/jidc.4228. [DOI] [PubMed] [Google Scholar]
- 28.Gales AC, Seifert H, Gur D, Castanheira M, Jones RN, Sader HS. Antimicrobial Susceptibility of Acinetobacter calcoaceticus-Acinetobacter baumannii complex and Stenotrophomonas maltophilia clinical isolates: Results from the SENTRY antimicrobial surveillance program (1997-2016) Open Forum Infect Dis. 2019;6:S34–46. doi: 10.1093/ofid/ofy293. [DOI] [PMC free article] [PubMed] [Google Scholar]


