Abstract
Vascular endothelial growth factor (VEGF) inhibitors are used in the treatment of various cancers as well as diabetic retinopathy. The systemic use of these drugs has been associated with adverse effects such as worsening hypertension, proteinuria, and renal function. There have been some reported cases of worsening hypertension, thrombotic microangiopathy, or glomerular disease after intravitreal injections of bevacizumab, aflibercept, and ranibizumab. We present a case of a patient who was taking intravitreal bevacizumab injections for diabetic retinopathy and was hospitalized with worsening renal function, high blood pressure, and nephrotic-range proteinuria in the setting of tight glycemic control.
Keywords: Diabetic nephropathy, hypertension, intravitreal bevacizumab
Vascular endothelial growth factor (VEGF) inhibitors have been associated with adverse effects such as hypertension, proteinuria, and thrombotic microangiopathy.1 These adverse effects are more commonly seen with systemic use of these drugs.
CASE DESCRIPTION
A 41-year-old man with a past medical history of chronic kidney disease stage 3, diabetes mellitus type 2, diabetic retinopathy, and hypertension presented to the emergency department with shortness of breath for a month. He reported persistent lower-extremity swelling for a year despite taking furosemide. The physical exam was remarkable for bilateral lower-extremity pitting edema and lung crackles. The patient’s blood pressure on presentation was 219/117 mm Hg; he reported systolic blood pressure in the 160s to 170s before hospitalization. Blood workup revealed normal troponin, pro-brain natriuretic peptide of 5759 pg/mL, D-dimer of 0.72 mg/L, and creatinine of 2.15 mg/dL (baseline 1.5–1.9 mg/dL). Chest x-ray showed bibasilar opacities. Renal ultrasound was unremarkable. Echocardiography showed a left ventricular ejection fraction of 60%. His home medications were furosemide 80 mg twice a day, metoprolol, metformin, and Levemir. He had been receiving intravitreal bevacizumab injections for diabetic retinopathy in both eyes every 6 weeks for 1 year. He was given intravenous furosemide with improvement in shortness of breath. Urinalysis revealed proteinuria (>1000 mg/dL), microscopic hematuria, and granular casts. Hemoglobin A1c was 5.9% (improved from 8.3% 4 months prior to admission). Further workup revealed normal complement 3 and 4 levels, negative antineutrophil and cytoplasmic antineutrophil antibodies, unremarkable serum protein electrophoresis, negative serum cryoglobulin, elevated kappa-lambda ratio of 2.66, urine immunofixation negative for monoclonal protein, and urine protein/creatinine ratio of 11.2 (normal <0.2) consistent with nephrotic syndrome. Given his worsening renal function, microscopic hematuria, and substantial proteinuria, the patient underwent nontargeted left kidney biopsy. The biopsy findings were consistent with diabetic nephropathy class 3 by Tervaert classification (Figures 1–3).
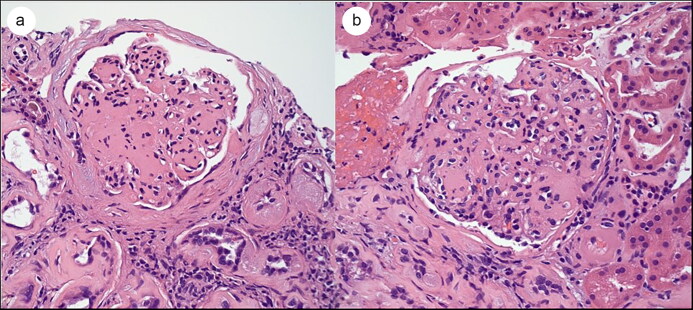
Figure 1.
Light microscopy showing nodular sclerosis (Kimmelstiel-Wilson nodules) with mild mesangial proliferation. Hematoxylin and eosin (H&E) stain, 400× magnification.
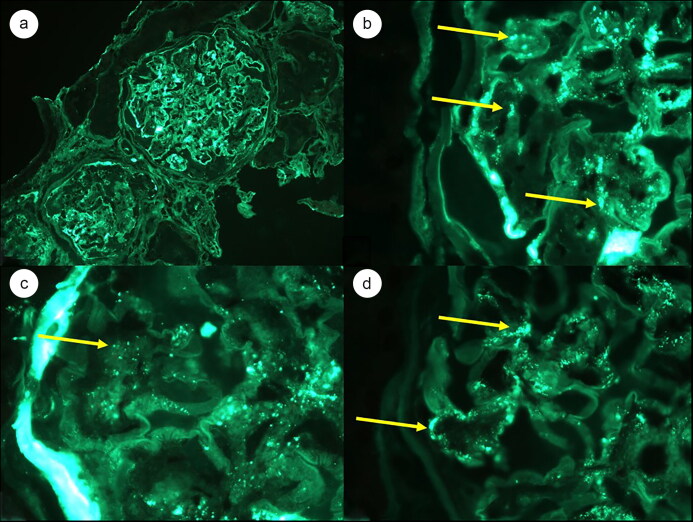
Figure 2.
Immunofluorescence microscopy showing scattered staining for anti-IgA along the basement membranes at (a) 200× magnification and (b) 1000× magnification. Similar staining patterns for (c) anti-C3 and (d) lambda light chain were seen (1000× magnification).
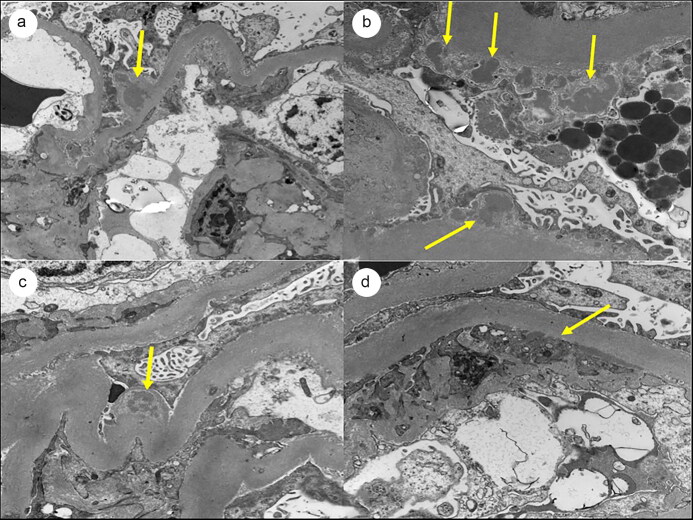
Figure 3.
Electron microscopy showing electron dense (a, b, c) subepithelial and (d) subendothelial deposits. There are changes consistent with diabetic nephropathy, including thickening of the basement membranes and mesangial expansion.
The patient’s blood pressure remained difficult to control. He was discharged on Bumex 2 mg twice a day, amlodipine 10 mg, carvedilol 25 mg twice a day, and hydralazine 100 mg three times a day; furosemide, metoprolol, and metformin were discontinued. His creatinine remained between 2.15 and 2.76 mg/dL in the hospital. He was not started on angiotensin-converting enzyme inhibitor/angiotensin receptor blocker due to a decline in the glomerular filtration rate. He eventually became dialysis dependent.
DISCUSSION
Bevacizumab is a VEGF inhibitor used off label intravitreally, while aflibercept and ranibizumab are approved by the Food and Drug Administration. Some cases have been reported of worsening hypertension, thrombotic microangiopathy, or glomerular disease after intravitreal injections of VEGF inhibitors.2 Bevacizumab is associated with proteinuria as a side effect at a frequency of 21% and with hypertension at a frequency of 64%.3
The systemic adverse effects reported with the use of VEGF inhibitors include thromboembolic events, stroke, and kidney disease. There is growing evidence that intravitreal use of these agents can contribute to hypertension and proteinuria.4 These agents are thought to be absorbed systemically in a significant amount5 that is likely the reason for these adverse effects. The possible mechanism of bevacizumab-induced hypertension is the inhibition of vasodilation and the altered kidney function leading to sodium and water retention. A case of bevacizumab-related immunoglobulin A vasculitis with nephritis has also been reported.6 Bevacizumab may cause thrombotic microangiopathy that is characterized histologically by mesangiolysis and double contours of the glomerular basement membrane.7 Our patient had IgA deposits on biopsy that could be from diabetes or bevacizumab; however, the absence of thrombotic microangiopathy on biopsy argues against bevacizumab as the cause of worsening renal function. Studies have shown a strong relationship between hemoglobin A1c levels and microalbuminuria in type 2 diabetes patients.8 The United Kingdom Prospective Diabetes Study has shown that improved glucose control reduces the risk of diabetic complications. In that study, each 1% reduction in hemoglobin A1c was associated with a 37% decrease in risk for microvascular complications and a 21% decrease in the risk of any endpoint or death related to diabetes.9 In our case, the patient’s hemoglobin A1c had improved to 5.9% from 8.3%, but his urine protein to creatinine ratio was very high at 11.2. This shows that bevacizumab could be the cause of the high level of proteinuria. However, the reduced insulin clearance due to a low glomerular filtration rate could also be the reason for lower hemoglobin A1c.
This case illustrates that intravitreal bevacizumab injection may be related to worsening proteinuria, renal function, and hypertension. More research is needed on this topic, and the disease course of diabetes and chronic kidney disease should be considered when interpreting this association.
ACKNOWLEDGMENT
The authors acknowledge the contributions of Saad Ur Rahman.
References
- 1.Hanna RM, Lopez E, Wilson J, Barathan S, Cohen AH.. Minimal change disease onset observed after bevacizumab administration. Clin Kidney J. 2016;9(2):239–244. doi: 10.1093/ckj/sfv139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanna RM, Lopez EA, Hasnain H, et al. Three patients with injection of intravitreal vascular endothelial growth factor inhibitors and subsequent exacerbation of chronic proteinuria and hypertension. Clin Kidney J. 2019;12(1):92–100. doi: 10.1093/ckj/sfy060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358(11):1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasier R, Artunay O, Yuzbasioglu E, Sengul A, Bahcecioglu H.. The effect of intravitreal bevacizumab (Avastin) administration on systemic hypertension. Eye. 2009;23(8):1714–1718. doi: 10.1038/eye.2008.360. [DOI] [PubMed] [Google Scholar]
- 5.Avery RL, Castellarin AA, Steinle NC, et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina. 2017;37(10):1847–1858. doi: 10.1097/IAE.0000000000001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endo Y, Negishi K, Hirayama K, Suzuki H, Shimizu A.. Bevacizumab-induced immunoglobulin A vasculitis with nephritis: a case report. Medicine (Baltimore). 2019;98(45):e17870 doi: 10.1097/MD.0000000000017870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izzedine H, Escudier B, Lhomme C, et al. Kidney diseases associated with anti-vascular endothelial growth factor (VEGF): an 8-year observational study at a single center. Medicine. 2014;93(24):333–339. doi: 10.1097/MD.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habib MB, Akbar NS.. Association of microalbuminuria with HbA1c in patients of type II diabetes mellitus in different age groups and genders. Diabetes Case Rep. 2018;3(3):137. [Google Scholar]
- 9.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7251):405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]