Abstract
The prevalence and seroconversion rate of SARS-CoV-2 infection among asymptomatic health care workers in the US is unclear. Our study utilized real-time polymerase chain reaction (RT-PCR) SARS-CoV-2 testing and serological evaluation to detect IgG antibodies specific to SARS-CoV-2 antigens in asymptomatic health care workers. A total of 197 subjects with a mean age of 35 years were recruited into the study. While most (67%) reported prolonged contact with known COVID-19 patients, only 8 (4.2%) tested positive on RT-PCR and 23 (11.7%) had detectable levels of IgG antibody to SARS-CoV-2. Out of 19 subjects with detectable IgG antibody at week 1, 11 (57.9%) lost their antibody response by week 3. No statistically significant difference was found in baseline characteristics or exposure status between subjects with positive and negative results on RT-PCR or antibody positivity. In conclusion, we found a low incidence of PCR positivity for SARS-CoV-2 in a high-risk group. This likely demonstrates the effectiveness of proper personal protective equipment use and low transmission risk in health care settings. The detectable IgG antibody titer was low, and a significant portion of subjects lost their antibody response on repeat testing. This may mean that antibody response in asymptomatic patients is categorically different than in symptomatic hospitalized patients with COVID-19.
Keywords: Antibody, COVID-19, healthcare workers, PCR testing, SARS-CoV-2, serology
The disease caused by the coronavirus SARS-CoV-2 (COVID-19) has presented primarily with respiratory symptoms and has spread globally since first being identified in Wuhan, China, in December 2019.1 Multiple studies have suggested that asymptomatic patients may propagate SARS-CoV-2 infection, most notably in the presymptomatic stage.2,3 Among the most important factions of the population to identify as asymptomatic carriers of SARS-CoV-2 are health care workers (HCWs). However, little data exist to describe the carrier status of HCWs in the US who have had either unprotected exposure to patients with COVID-19 or who have participated in the direct care of COVID-19 patients in the setting of appropriate protective equipment. In this study, we report the results of serial testing performed in high-risk asymptomatic HCWs with positive real-time reverse transcription PCR (RT-PCR) and IgG serological testing for SARS-CoV-2 along with details about their exposure status.
METHODS
This study was conducted as a substudy from an ongoing trial (trial registration number NCT04333225) assessing the efficacy of hydroxychloroquine on prevention of SARS-CoV-2 infection in HCWs. Regulatory and institutional review board approval was obtained along with consent from each patient. All study subjects were employed by and worked at Baylor University Medical Center in Dallas, Texas. The study was publicized to hospital departments with personnel in direct contact with potential COVID-19 patients (i.e., emergency departments, COVID-19 intensive care units). To be included, subjects had to have no signs or symptoms of COVID-19 infection. A total of 197 subjects enrolled, with 96% completing at least two serological tests at the time of analysis for this study. Subjects filled out a questionnaire at the start of the study detailing their exposure status to COVID-19 patients (see Supplemental Material).
The Roche Cobas test, conducted weekly by trained personnel, was utilized to detect nucleic acids from SARS-CoV-2 in nasopharyngeal swab samples from study participants.4 If the result of a swab test demonstrated the presence of SARS-CoV-2, the subject was directed to immediately receive appropriate medical care and to discontinue study treatment and procedures; however, the sponsor followed the subject until their condition resolved. Medical management following a positive test during the study was not directed by study procedures and was at the discretion of the subject’s physician and occupational health.
The serology enzyme-linked immunosorbent assay was developed by our immunology department using standard accepted practices. A detailed description of serology test development is presented in the Supplemental Material. The SARS-CoV-2 antigens used were the receptor-binding domain (RBD) of the spike glycoprotein and the nucleocapsid phosphoprotein (N). A sample was considered positive if the IgG antibody titer was detectable above our set threshold for either antigen.
Optical density (OD) values of the samples were compared with seven in-plate negative control samples (collected pre-COVID-19) to calculate P values using Crawford and Howell’s adjusted t test.5 Additionally, percentage of OD change was defined as (Sample OD – mean [negative control ODs])/(positive control OD − mean [negative control ODs]) × 100%. A sample was considered positive if two criteria were satisfied: (a) the sample OD was significantly greater than the mean of negative controls at the 0.001 level and (b) the percentage of OD change was above 10%. N protein and RBD data were analyzed separately, and a test was considered positive as long as one of the antigens was positive. To evaluate the sensitivity and specificity of the assay, we also collected and analyzed 76 pre-COVID-19 donors as independent negative controls and 11 samples from hospitalized patients with confirmed SARS-CoV-2 infection as positive controls. Using a P value threshold of 0.001 and a 10% percentage cutoff, the specificities for N protein (1:500 dilution) and RBD (1:250 dilution) were 97.36% and 96.05%, respectively. The sensitivities for N protein and RBD were both 100%.
We used descriptive statistics including mean, standard deviation, frequency, and percentage to describe the study population. Body mass index and number of hours worked in high-risk units were first explored using histogram and boxplot. Their normality was then checked utilizing Q-Q plot and Shapiro-Wilk test. The results suggested no significant departures from the normal distribution for both variables. Thus, we used Student’s t test to compare the study groups with regard to body mass index and length of time working in the high-risk units during the past week. Categorical variables were compared between antibody-positive and -negative individuals using chi-square test and Fisher exact test depending on which was more appropriate.
We used Student’s paired t test to assess the temporal changes in OD values. Additionally, we drew 10,000 samples to obtain the bootstrap estimate of the standard error of the mean difference. We found the bias to be negligible for both antigens. Furthermore, we confirmed the results of the paired t test with that of Wilcoxon signed-rank test. All statistical analysis was performed using SAS statistical software 9.4, and the significance level was considered 0.05 throughout the analysis.
RESULTS
Of the 197 study participants, 165 (83%) were white and 148 (75%) were women (Table 1). Their average age was 35.2 years old, with only four individuals older than 65. The study sample predominantly consisted of nurses (56%) followed by physicians or advanced practice providers (30%). While most worked in either the emergency department (41%) or in the intensive care unit (30%), there were 56 (28%) enrollees from other hospital departments. Comorbidities were uncommon in our younger skewed population, with depression/anxiety/attention deficit disorder being the most common. The median number of hours worked in the emergency/intensive care/COVID-19 unit in the last 7 days prior to enrollment was 36. Nearly 52% of subjects reported being present in a room during a procedure likely to generate higher concentrations of respiratory secretions or aerosols. Most study individuals (67%) reported prolonged close contact with a suspected/confirmed COVID-19–positive patient (within 6 feet for >15 minutes cumulatively in one or more shifts). Only 3.5% of subjects noted any exposure to COVID-19 patients without proper protective equipment. While some participants were critical of their protective equipment, most (75%) reported feeling safe with their personal protective gear.
Table 1.
Baseline and exposure characteristics of health care workers, overall and broken down by antibody result
| Variable | Total (n = 197) | Antibody positive (n = 23)* | Antibody negative (n = 174)* | P value |
|---|---|---|---|---|
| Age (years) | 35.2 ± 10.8 | 33.3 ± 10.7 | 35.4 ± 10.8 | 0.38 |
| Race | ||||
| White | 165 (84%) | 20 (87%) | 145 (83%) | 0.73 |
| Black | 7 (4%) | 0 (0%) | 7 (42%) | |
| Other | 25 (13%) | 3 (13%) | 22 (13%) | |
| Hispanic | 39 (20%) | 7 (30%) | 32 (18%) | 0.17 |
| Female | 148 (75%) | 18 (78%) | 130 (75%) | 0.71 |
| Department | ||||
| Emergency department (ED) | 81 (41%) | 10 (43%) | 71 (41%) | 0.59 |
| Intensive care unit (ICU) | 60 (30%) | 5 (22%) | 55 (32%) | |
| Other | 56 (28%) | 8 (35%) | 48 (28%) | |
| Job title | ||||
| Physician/nurse practitioner/physician assistant | 60 (30%) | 4 (17%) | 56 (32%) | 0.065 |
| Nurse | 111 (56%) | 18 (78%) | 93 (53%) | |
| Tech/coordinator/other | 26 (13%) | 1 (4%) | 25 (14%) | |
| Body mass index (kg/m2) | 25.9 ± 4.9 | 25.6 ± 5.0 | 26.0 ± 4.9 | 0.71 |
| Diabetes | 2 (1%) | 0 (0%) | 2 (1%) | 1.00 |
| Hypertension | 16 (8%) | 0 (0%) | 16 (9%) | 0.22 |
| Hypothyroidism | 14 (7%) | 3 (13%) | 11 (6%) | 0.21 |
| Anxiety/depression/attention deficit disorder | 34 (17%) | 5 (22%) | 29 (17%) | 0.56 |
| Lung disease | 8 (4%) | 0 (0%) | 8 (5%) | 0.60 |
| Hours worked in ED/ICU/COVID-19 unit during last 7 days** | 39.7 ± 17.7 | 40.8 ± 15.2 | 39.6 ± 18.0 | 0.77 |
| In the room for procedure likely to generate higher concentrations of respiratory secretions or aerosols | 102 (52%) | 11 (48%) | 91 (52%) | 0.69 |
| Had extensive body contact with a COVID-19–positive patient (e.g. rolling the patient) | 95 (48%) | 12 (52%) | 83 (48%) | 0.68 |
| Had prolonged close contact with a suspected/confirmed COVID-19–positive patient (within 6 feet for >15 min cumulatively in one or more shift) | 131 (67%) | 17 (74%) | 114 (66%) | 0.42 |
| Had unprotected exposures (e.g. incomplete protective equipment for any duration with a COVID-19 patient)*** | 7 (4%) | 2 (9%) | 5 (3%) | 0.16 |
n (%) or mean ± SD.
Two individuals in the serology-positive group and four in the serology-negative group did not report their total number of hours worked in ED/ICU.
Three individuals in the serology-positive group and four in the serology-negative group did not report whether they had an unprotected exposure to a COVID-19 patient.
Eight subjects (4.2%) tested positive for SARS-CoV-2 via RT-PCR testing, one of whom did not complete serological testing. Of the seven PCR-positive subjects who completed serological testing, only four had detectable IgG antibodies to SARS-CoV-2. All positive PCR tests occurred during the first week of testing and none occurred in the subsequent 3 weeks of testing. All PCR-positive subjects were taken out of the study protocol and did not receive any further PCR testing. No significant differences in baseline characteristics or exposure status was noted in comparing PCR-positive with PCR-negative subjects, but the low sample number of PCR-positive subjects precluded any meaningful statistical analysis between the groups (see Supplemental Table 1).
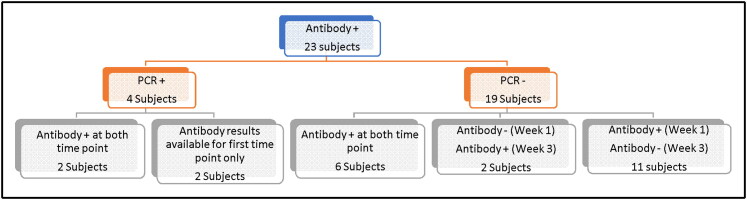
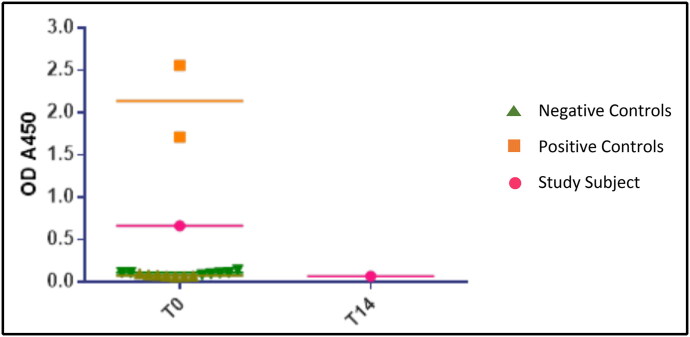
Overall, 23 subjects (11.7%) had detectable IgG antibodies to SARS-CoV-2 in our study (Table 1, Figure 1). Almost all of these subjects demonstrated weak signals of antibody positivity relative to the positive control samples obtained from hospitalized patients with COVID-19 (Figure 2). Of those 23 subjects with positive IgG antibody, only 4 were also positive by PCR and the remaining 19 tested and remained negative on serial PCR testing. Since those who tested positive on PCR were taken out of the study protocol and quarantined for at least 14 days, their repeat serology testing did not follow the serology protocol for the study (i.e., week 1 and week 3) and was done as time/schedule allowed. Two of the four subjects with positive PCR had detectable IgG antibodies to SARS-CoV-2 at both time points. The other two were unable to get repeat serological testing.
Figure 1.
A flowchart of patients with detectable IgG antibody to SARS-CoV-2 in the context of PCR positivity as well as when the antibody test became positive and if it was sustained.
Figure 2.
A subject’s detectable antibody to RBD antigen signal (in pink) on the first week of testing (T0) and the loss of signal on testing 2 weeks later. Two different sets of negative controls (light and dark green) and a positive control (orange) are shown for comparison. The signals from positive control samples were considerably higher than those of the study subjects.
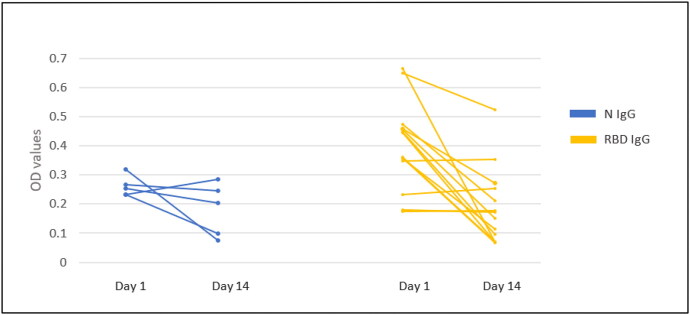
Of the 19 IgG-positive but PCR-negative subjects, only six had positive serology at both testing time points (week 1 and week 3). Eleven subjects tested positive in week 1 but lost their antibody response in week 3 testing. In the subjects who lost their IgG antibody titers, the decrease in the mean value of the OD of the N antigen at the second time point was not statistically significant (P = 0.20), while the decrease in the mean OD of the RBD antigen was statistically significant (P < 0.0006) (Figure 3). Two subjects tested negative at week 1 but positive at week 3. There was no statistically significant difference in baseline characteristics or exposure status between patients with detectable IgG antibodies to SARS-CoV-2 vs those with no detectable IgG antibodies (Table 1).
Figure 3.
A plot of patients who had detectable IgG antibody (plotted according to either N or RBD IgG positivity) at two different time points. Most patients had a weak positive signal to begin with, which decreased or was lost at testing 2 weeks later.
DISCUSSION
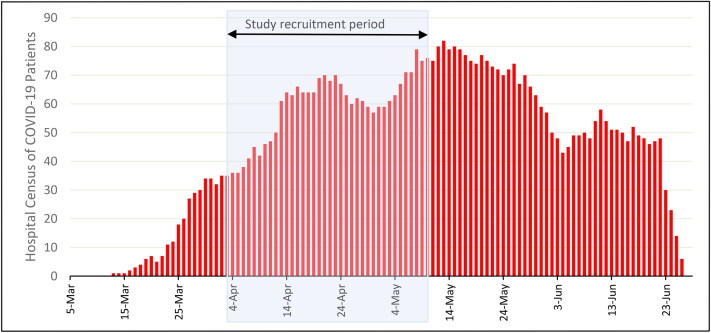
The 4.2% prevalence of RT-PCR–positive subjects was lower than expected in our asymptomatic but high-risk group of HCWs. In addition, the fact that no positive results occurred after the first week of testing was also unexpected considering the significant amount of reported exposure our subjects had to COVID-19 patients during our study enrollment time (Figure 4). Our results were similar to those of Treibel et al.6 They reported only a slightly higher rate of PCR positivity at 7.1% and a nearly sixfold drop in PCR-positive detection in subsequent serial testing in HCWs at a London hospital.6 These findings could be a result of efficacy of appropriate infection control measures and protective equipment donning in our subjects. Of note, our institution had implemented strict personal protective equipment utilization and infection control guidelines, as well as isolation and testing of potential exposed HCWs, thus further reducing the risk of transmission.
Figure 4.
Weekly hospital census of COVID-19 patients at Baylor University Medical Center with the time of study recruitment noted.
Lastly, it is also possible that participation bias could have played a role, where enrolled subjects might have paid extra attention to infection control measures and use of protective equipment. At the time of enrollment of our study, the Dallas metropolitan area had not yet seen the spike of SARS-CoV-2 infection. According to the New York Times, the 7-day moving average of new cases in the beginning of April 2020 was 80 in Dallas County, which implies an approximate incidence rate of 3 new cases per 100,000 individuals. The low incidence rate in the community could also contribute to the low infection rates seen in our study.
The accuracy of PCR testing in this population must also be scrutinized. We are unaware of any reported sensitivity of PCR testing in asymptomatic COVID-19 patients, although data may be emerging as the pandemic continues and further studies are done in asymptomatic subjects.7 False-positive PCR tests may explain the three subjects who tested positive on PCR but did not have any detectable IgG antibodies to SARS-CoV-2 on serial testing. It may also be that their infection was in an acute phase and antibodies had not yet developed or that their antibody response was too weak to be categorized as positive by our threshold.
While there is a paucity of data on the serological status of asymptomatic HCWs, multiple studies have noted seroconversion of asymptomatic HCWs.7–9 Our seroconversion rate of asymptomatic HCWs of 13.7% was higher than the reported rate of 6.4% in the earlier noted study of asymptomatic HCWs in Belgium. A key question that has remained largely unanswered is the extent and duration of antibody response in asymptomatic patients. To that end, our study illustrated two crucial points. First, the IgG SARS-CoV-2 antibody response in asymptomatic subjects tended to be of lower magnitude than in those who were hospitalized with COVID-19. Second, the duration of this weak antibody response appeared to be short lived in most asymptomatic subjects in our study. This was demonstrated by the loss of IgG antibody signal to SARS-CoV-2 in 11 of the 19 subjects with detectable IgG to SARS-CoV-2 in week 1 testing. These results are supported by a recent study of 37 asymptomatic patients with PCR-confirmed SARS-CoV-2 infection with waning immunity. Long et al noted a significantly weaker IgG antibody signal in those 37 asymptomatic subjects as well as loss of IgG antibody detection in 40% of their subjects at serological testing done at 8 weeks.10 This trend was observed in our study as well. These findings have broad implications for societal-level immunity strategies and demonstrate why population-level screening and “immunity passport” strategies may need to be reconsidered.
Our study is limited by our small sample size and the lower rate of positive PCR and serological tests. Our population sample was also young, relatively healthy, and predominantly female, so our results may not be generalizable. Other studies in HCWs have had a similar demographic. Our trial started enrolling at a time when commercial SARS-CoV-2 serology testing was not widely available, so we developed serological testing in conjunction with our immunology department. As such, our serology test has not yet been approved by the US Food and Drug Administration but meets and often exceeds the sensitivity and specificity performance of the currently available serological testing, as demonstrated by validation studies further detailed in the supplemental material.11
In conclusion, our study suggests that the SARS-CoV-2 antibody response in asymptomatic health care workers tends to be short term and different from that of hospitalized COVID-19 patients. However, these hypotheses need to be further confirmed by larger studies.
Supplementary Material
References
- 1.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He X, Lau EH, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. medRxiv. 2020; 2020.03.15.20036707. doi: 10.1101/2020.03.15.20036707. [DOI] [PubMed] [Google Scholar]
- 4.Poljak M, Korva M, Gašper NK, et al. Clinical evaluation of the cobas SARS-CoV-2 test and a diagnostic platform switch during 48 hours in the midst of the COVID-19 pandemic. J Clin Microbiol. 2020;58(6):1–7. doi: 10.1128/JCM.00599-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford JR, Howell DC.. Comparing an individual’s test score against norms derived from small samples. Clin Neuropsychol. 1998;12(4):482–486. doi: 10.1076/clin.12.4.482.7241. [DOI] [Google Scholar]
- 6.Treibel TA, Manisty C, Burton M, et al. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet. 2020;395(10237):1608–1610. doi: 10.1016/S0140-6736(20)31100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stubblefield WB, Talbot HK, Feldstein LR, et al. Seroprevalence of SARS-CoV-2 among frontline healthcare personnel during the first month of caring for COVID-19 patients—Nashville, Tennessee. Clin Infect Dis. 2020;70:ciaa936. doi: 10.1093/cid/ciaa936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(2):195–197. doi: 10.1001/jama.2020.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korth J, Wilde B, Dolff S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 11.Whitman JD, Hiatt J, Mowery CT, et al. Test performance evaluation of SARS-CoV-2 serological assays. Preprint. medRxiv. 2020; 2020.04.25.20074856. doi: 10.1101/2020.04.25.20074856. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.