A large-scale screen to target SARS-CoV-2
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) genome is initially expressed as two large polyproteins. Its main protease, Mpro, is essential to yield functional viral proteins, making it a key drug target. Günther et al. used x-ray crystallography to screen more than 5000 compounds that are either approved drugs or drugs in clinical trials. The screen identified 37 compounds that bind to Mpro. High-resolution structures showed that most compounds bind at the active site but also revealed two allosteric sites where binding of a drug causes conformational changes that affect the active site. In cell-based assays, seven compounds had antiviral activity without toxicity. The most potent, calpeptin, binds covalently in the active site, whereas the second most potent, pelitinib, binds at an allosteric site.
Science, this issue p. 642
A repurposed drug-library screen reveals two allosteric drug binding sites of the SARS-CoV-2 main protease.
Abstract
The coronavirus disease (COVID-19) caused by SARS-CoV-2 is creating tremendous human suffering. To date, no effective drug is available to directly treat the disease. In a search for a drug against COVID-19, we have performed a high-throughput x-ray crystallographic screen of two repurposing drug libraries against the SARS-CoV-2 main protease (Mpro), which is essential for viral replication. In contrast to commonly applied x-ray fragment screening experiments with molecules of low complexity, our screen tested already-approved drugs and drugs in clinical trials. From the three-dimensional protein structures, we identified 37 compounds that bind to Mpro. In subsequent cell-based viral reduction assays, one peptidomimetic and six nonpeptidic compounds showed antiviral activity at nontoxic concentrations. We identified two allosteric binding sites representing attractive targets for drug development against SARS-CoV-2.
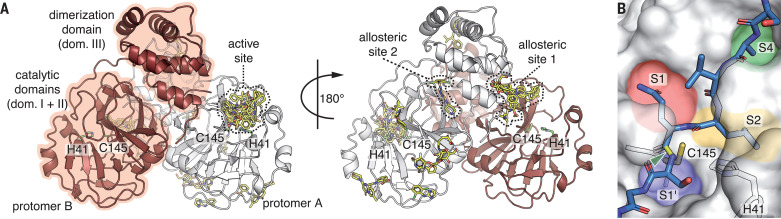
Infection of host cells by SARS-CoV-2 is governed by the complex interplay of molecular factors from both the host and the virus (1, 2). Coronaviruses are RNA viruses with a genome of approximately 30,000 nucleotides. The viral open reading frames are expressed as two overlapping large polyproteins which must be separated into functional subunits for replication and transcription activity (1). This proteolytic cleavage is primarily accomplished by the main protease (Mpro), also known as 3C-like protease 3CLpro or nsp5. Mpro cleaves the viral polyprotein pp1ab at 11 distinct sites. The core cleavage motif is Leu-Gln↓(Ser/Ala/Gly) (1). Mpro possesses a chymotrypsin-like fold appended with a C-terminal helical domain and harbors a catalytic dyad comprised of Cys145 and His41 in its active site, which is formed by four major pockets that are labeled according to their position relative to the scissile bond of the substrate (Fig. 1) (1). The active site is located in a cleft between the two N-terminal domains of the three-domain structure of the monomer, whereas the C-terminal helical domain is involved in regulation and dimerization of the enzyme (Fig. 1A). Because of its central involvement in virus replication, Mpro is recognized as a prime target for antiviral drug discovery and compound screening activities aiming to identify and optimize drugs which can tackle coronavirus infections (3). Indeed, a number of recent publications confirm the potential of targeting Mpro for inhibition of virus replication (1, 2, 4).
Fig. 1. The x-ray screening of drug-repurposing libraries reveals compound binding sites distributed across the complete Mpro surface.
(A) Schematic drawing of Mpro dimer structure. Protomer A is shown in white, and protomer B is in red. For clarity, the 29 binding compounds (yellow sticks) are only depicted on one of the two protomers. Catalytic residues His41 (H41) and Cys145(C145), the active site, and two allosteric drug binding sites are highlighted. (B) Close-up view of the active site with peptide substrate bound (blue sticks), modeled after SARS-CoV Mpro (PDB 2Q6G). The scissile bond is indicated in yellow and with the green arrowhead. Substrate binding pockets S1ʹ, S1, S2, and S4 are indicated by colored regions.
In order to find drug candidates against SARS-CoV-2, we performed a large-scale x-ray crystallographic screen of Mpro against two repurposing libraries containing 5953 compounds from the Fraunhofer IME Repurposing Collection and the Safe-in-man library from Dompé Farmaceutici S.p.A. (5).
In contrast to crystallographic fragment screening experiments, compounds in repurposing libraries are chemically more complex (fig. S1A) (6, 7). Thus, these compounds likely bind more specifically and with higher affinity (8). Because of the higher molecular weights, we performed cocrystallization experiments at a physiological pH of 7.5 instead of compound soaking into native crystals (9).
From the 5953 compounds in our screen, we obtained x-ray diffraction datasets for 2381 compounds, which we subjected to automated structure refinement followed by cluster analysis (10) and pan dataset density analysis (PanDDA) (11) (table S1). We observed additional electron density, indicating binding to Mpro, for 43 compounds, which were classified as hits, representing 37 distinct compounds (tables S1, S2, and S3). From these, the binding mode could be unambiguously determined for 29 molecules (Fig. 1A and table S4). The majority of hits were found in the active site of the enzyme. Of the 16 active site binders, six covalently bind as thioethers to Cys145, one compound binds covalently as a thiohemiacetal to Cys145, one is zinc-coordinated, and eight bind noncovalently. The remaining 13 compounds bind outside the active site at various locations (Fig. 1A).
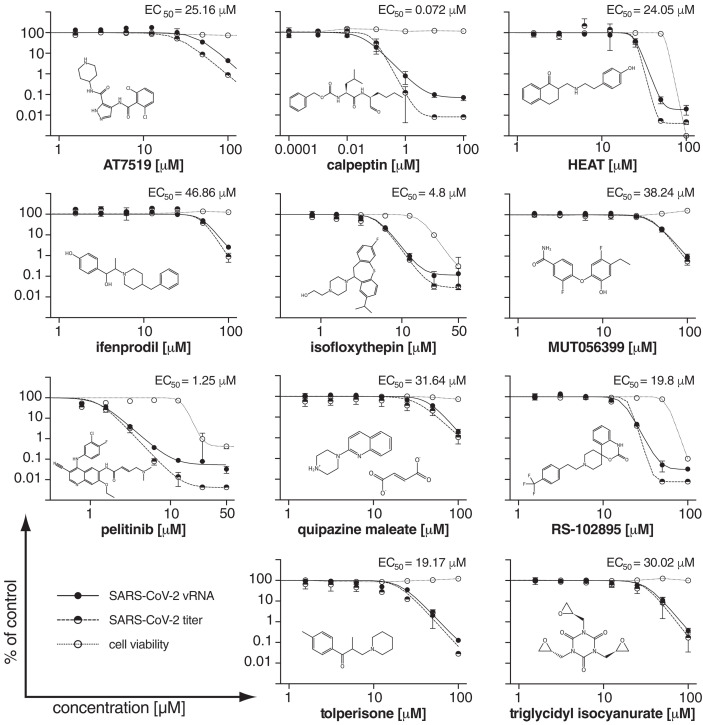
Of the 43 hits from our x-ray screen, 37 compounds were available in quantities required for testing their antiviral activity against SARS-CoV-2 in cell assays (table S2). Nine compounds that reduced viral RNA (vRNA) replication by at least two orders of magnitude in Vero E6 cells (fig. S2) were further evaluated to determine the effective concentrations that reduced not only vRNA but also SARS-CoV-2 infectious particles by 50% (EC50) (Fig. 2). Additionally, AT7519 and ifenprodil, which showed slightly lower vRNA level reduction, were included because of their distinct binding sites outside of the active site. From these 11, seven compounds (AT7519, calpeptin, ifenprodil, MUT056399, pelitinib, tolperisone, and triglycidyl isocyanurate) exhibited a ≥100-fold reduction in infectious particles in combination with either a selectivity index [SI; calculated as the 50% cytotoxic concentration (CC50) divided by the EC50] of >5 or no cytotoxicity in the tested concentration range and are considered antivirally active (table S5).
Fig. 2. Effect of selected compounds on SARS-CoV-2 replication in Vero E6 cells.
The vRNA yield (solid circles), viral titers (half-solid circles), and cell viability (empty circles) were determined by reverse transcription–quantitative polymerase chain reaction, immunofocus assays, and the CCK-8 method, respectively. EC50 for the viral titer reduction is shown. Individual data points represent means ± SD from three independent replicates in one experiment.
Here, we focus on a more detailed description of the 11 compounds analyzed in the secondary screen, which are grouped according to their different binding sites. The remaining hits are described in the supplementary text and figs. S3 to S5.
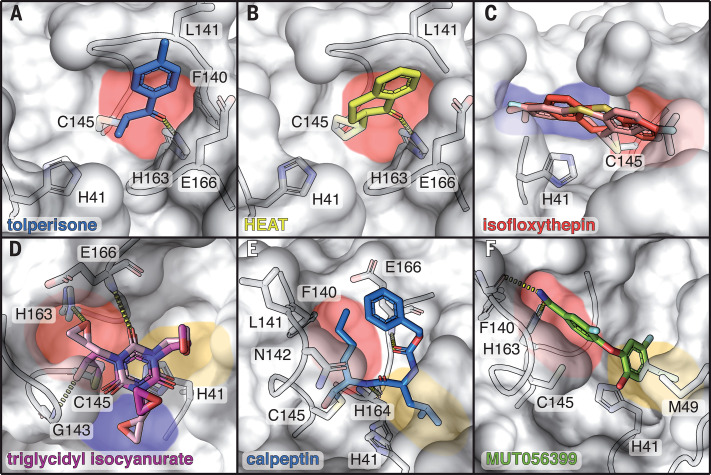
Tolperisone, 2-[β-(4-hydroxyphenyl)-ethylaminomethyl]-tetralone (HEAT), and isofloxythepin bind covalently to the active site. Tolperisone is antivirally active (EC50 = 19.17 μM) and shows no cytotoxicity (CC50 > 100 μM) (Fig. 2), whereas HEAT (EC50 = 24.05 μM, CC50 = 55.42 μM) and isofloxythepin (EC50 = 4.8 μM, CC50 = 17 μM) show unfavorable cytotoxicity. For all three compounds, only breakdown products are observed in the active site. Tolperisone and HEAT are β-aminoketones, but we only observe the part of the drug containing the ketone (2,4′-dimethylpropiophenone and 2-methyl-1-tetralone), whereas the remaining part with the amine group is missing. The breakdown product binds as a Michael acceptor to the thiol of Cys145, independently confirmed for HEAT by mass spectrometry (fig. S6 and table S6). The decomposition of tolperisone and HEAT was detected in both the crystallization and cell culture conditions (fig. S7) and is reported to be pH dependent (12). The parent compounds can be regarded as prodrugs (13, 14). In the x-ray structures the aromatic ring systems of tolperisone (Fig. 3A) and HEAT (Fig. 3B) protrude into the S1 pocket and form van der Waals contacts with the backbone of Phe140 and Leu141 and the side chain of Glu166. In addition, the keto group accepts a hydrogen bond from the imidazole side chain of His163. Tolperisone is used as a skeletal muscle relaxant (15). The x-ray structure suggests that isofloxythepin binds similarly as a fragment to Cys145 (Fig. 3C).
Fig. 3. Covalent and noncovalent binders in the active site of Mpro.
Bound compounds are depicted as colored sticks, and the surface of Mpro is shown in gray with selected interacting residues shown as sticks. Substrate binding pockets are colored as in Fig. 1. Hydrogen bonds are depicted by dashed lines. (A) Tolperisone. (B) HEAT. (C) Isofloxythepin. (D) Triglycidyl isocyanurate. (E) Calpeptin. (F) MUT056399.
Triglycidyl isocyanurate has antiviral activity (EC50 = 30.02 μM, CC50 > 100 μM) and adopts covalent and noncovalent binding modes to the active site. In both modes, the compound’s central ring sits on top of the catalytic dyad (His41, Cys145), and its three epoxypropyl substituents reach into subsites S1′, S1, and S2. The noncovalent binding mode is stabilized by hydrogen bonds to the main chain of Gly143 and Gln166 and to the side chain of His163. In the covalently bound form, one oxirane ring is opened by nucleophilic attack of Cys145, forming a thioether (Fig. 3D). Triglycidyl isocyanurate has been tested as an antitumor agent (16).
Calpeptin shows the highest antiviral activity in the screen (EC50 = 72 nM, CC50 > 100 μM). It binds covalently via its aldehyde group to Cys145, forming a thiohemiacetal. This peptidomimetic inhibitor occupies substrate pockets S1 to S3, similar to the peptidomimetic inhibitors GC-376 (17, 18), calpain inhibitors (19), N3 (2), and the α-ketoamide 13b (1). The peptidomimetic backbone forms hydrogen bonds to the main chain of His164 and Glu166, whereas the norleucine side chain maintains van der Waals contacts with the backbone of Phe140, Leu141, and Asn142 (Fig. 3E). Calpeptin has known activity against SARS-CoV-2 Mpro in enzymatic assays (17). The structure is highly similar to the common protease inhibitor leupeptin (fig. S3A), which served as a positive control in our x-ray screen but was not tested further in antiviral assays. In silico docking experiments also suggested calpeptin as a possible Mpro binding molecule (table S7). Calpeptin also inhibits cathepsin L (20), and dual targeting of cathepsin L and Mpro is suggested as an attractive path for SARS-CoV-2 inhibition (19).
MUT056399 binds noncovalently to the active site (EC50 = 38.24 μM, CC50 > 100 μM). The diphenyl ether core of MUT056399 blocks access to the catalytic site, which consists of Cys145 and His41. The terminal carboxamide group occupies pocket S1 and forms hydrogen bonds to the side chain of His163 and the backbone of Phe140 (Fig. 3F). The ethyl phenyl group of the molecule reaches deep into pocket S2, which is enlarged by a shift of the side chain of Met49 out of the substrate binding pocket. MUT056399 was developed as an antibacterial agent against multidrug-resistant Staphylococcus aureus strains (21).
Quipazine maleate showed moderate antiviral activity (EC50 = 31.64 μM, CC50 > 100 μM). In the x-ray structure, only the maleate counterion is observed covalently bound as a thioether (supplementary text and fig. S3B). Maleate is observed in structures of six other compounds showing no antiviral activity. The observed antiviral activity is thus likely caused by an off-target effect of quipazine.
In general, the enzymatic activity of Mpro relies on the architecture of the active site, which critically depends on the dimerization of the enzyme and the correct relative orientation of the subdomains. This could allow ligands that bind outside of the active site to affect activity. In fact, we identified two such allosteric binding sites of Mpro.
Five compounds of our x-ray screen bind in a hydrophobic pocket in the C-terminal dimerization domain (Fig. 4, A and B), located close to the oxyanion hole in pocket S1 of the substrate binding site. One of these showed strong antiviral activity (Fig. 2). Another compound binds between the catalytic and dimerization domains of Mpro.
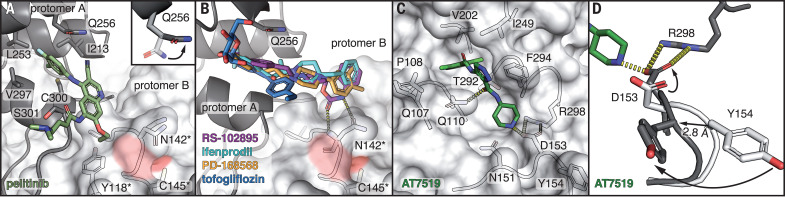
Fig. 4. Screening hits at allosteric sites of Mpro.
(A) Close-up view of the binding site in the dimerization domain (protomer A, gray cartoon representation), close to the active site of the second protomer (protomer B, surface representation) in the native dimer. Residues forming the hydrophobic pocket are indicated. Pelitinib (dark green) binds to the C-terminal α-helix at Ser301 and pushes against Asn142 and the β-turn of the pocket S1 of protomer B (residues marked with an asterisk). The inset shows the conformational change of Gln256 (gray sticks) compared with the Mpro apo structure (white sticks). (B) RS-102895 (purple), ifenprodil (cyan), PD-168568 (orange), and tofogliflozin (blue) occupy the same binding pocket as pelitinib. (C) AT7519 occupies a deep cleft between the catalytic and dimerization domain of Mpro. (D) Conformational changes in the AT7519-bound Mpro structure (gray) compared with those in the apo structure (white).
Central to the first allosteric binding site is a hydrophobic pocket formed by Ile213, Leu253, Gln256, Val297, and Cys300 within the C-terminal dimerization domain (Fig. 4A). Pelitinib, ifenprodil, RS-102895, PD-168568, and tofogliflozin all exploit this site by inserting an aromatic moiety into this pocket.
Pelitinib shows the second highest antiviral activity in our screen (EC50 = 1.25 μM, CC50 = 13.96 μM). Its halogenated benzene ring binds to the hydrophobic groove in the helical domain, which becomes accessible by movement of the Gln256 side chain (Fig. 4A). The central 3-cyanoquinoline moiety interacts with the end of the C-terminal helix (Ser301). The ethyl ether substituent pushes against Tyr118 and Asn142 (from loop 141–144 of the S1 pocket) of the opposing protomer within the native dimer. The integrity of this pocket is crucial for enzyme activity (22). Pelitinib is an amine-catalyzed Michael acceptor (23) and was developed as an anticancer agent to bind to a cysteine in the active site of the tyrosine kinase epidermal growth factor receptor inhibitor (24). However, from its observed binding position, it is impossible for it to reach into the active site, and no evidence for covalent binding to Cys145 is found in the electron density maps.
Ifenprodil and RS-102895 bind to the same hydrophobic pocket in the dimerization domain as pelitinib (Fig. 4B; fig. S4, A and B; and supplementary text). Only ifenprodil (EC50 = 46.86 μM, CC50 > 100 μM) shows moderate activity. RS-102895 (EC50 = 19.8 μM, CC50 = 54.98 μM) interacts, similar to pelitinib, with the second protomer by forming two hydrogen bonds to the side and main chains of Asn142, whereas the other compounds exhibit weaker or no interaction with the second protomer. PD-168568 and tofogliflozin bind the same site but are inactive (Fig. 4B and fig. S4, C and D).
The second allosteric site is formed by the deep groove between the catalytic domains and the dimerization domain. AT7519 is the only compound in our screen that we identified bound to this site (Fig. 4C). Though it has only moderate activity, we discuss it here because this site may be a target. The chlorinated benzene ring is engaged in various van der Waals interactions to loop 107-110, Val202, and Thr292. The central pyrazole has van der Waals contacts to Ile249 and Phe294, and its adjacent carbonyl group forms a hydrogen bond to the side chain of Gln110. The terminal piperidine sits on top of Asn151 and forms hydrogen bonds to the carboxylate of Asp153. This results in a displacement of loop 153-155, slightly narrowing the binding groove. The Cα atom of Tyr154 moves 2.8 Å, accompanied by a conformational change of Asp153 (Fig. 4D). This allows hydrogen bonding to the compound and the formation of a salt bridge to Arg298. Arg298 is crucial for dimerization (25). The mutation Arg298Ala causes a reorientation of the dimerization domain relative to the catalytic domain, leading to changes in the oxyanion hole and destabilization of the S1 pocket by the N terminus. AT7519 was evaluated for treatment of human cancers (26). The potential of allosteric inhibition of Mpro through modulation of Arg298 has been independently demonstrated by mass spectrometry (27).
Our x-ray screen revealed 43 compounds binding to Mpro, with seven compounds showing antiviral activity against SARS-CoV-2. We present structural evidence for interaction of these compounds at active and allosteric sites of Mpro, although we cannot exclude that off-target effects played a role in the antiviral effect in cell culture, in particular for compounds with a low selectivity index. Conversely, an absence of antiviral activity of compounds binding clearly to Mpro in the crystal might be due to rapid metabolization in the cellular environment. Calpeptin and pelitinib showed strong antiviral activity with low cytotoxicity and are suitable for preclinical evaluation. In any case, all hit compounds are valuable lead structures with potential for further drug development, especially because drug-repurposing libraries offer the advantage of proven bioactivity and cell permeability (28).
The most active compound, calpeptin, binds in the active site similar to other members of the large class of peptide-based inhibitors that bind as thiohemi-acetals or -ketals to Mpro (29). In addition to this peptidomimetic inhibitor, we discovered several nonpeptidic inhibitors. Those compounds binding to the active site of Mpro contained new Michael acceptors based on β-aminoketones (tolperisone and HEAT). These compounds lead to the formation of thioethers and have not been described as prodrugs for viral proteases. We also identified a noncovalent binder, MUT056399, that blocked the active site. In addition to this common active site inhibition, we identified compounds that inhibit the enzyme through binding at two allosteric sites of Mpro.
The first allosteric site (dimerization domain) is in the direct vicinity of the S1 pocket of the adjacent monomer within the native dimer. The potential for antiviral inhibition through this site is demonstrated by pelitinib. The hydrophobic nature of the residues forming the main pocket is conserved in all human coronavirus Mpro (fig. S8). Consequently, potential drugs targeting this binding site may be effective against other coronaviruses. The potential of the second allosteric site as a druggable target is demonstrated by the observed moderate antiviral activity of AT7519.
Acknowledgments
We acknowledge Deutsches Elektronen-Synchrotron (DESY; Hamburg, Germany), a member of the Helmholtz Association HGF, for the provision of experimental facilities. Parts of this research were carried out at PETRA III at beamline P11. Further MX data were collected at beamline P13 and P14 operated by EMBL. We thank the DESY machine group, in particular M. Wunderlich, K. Heuck, A. Brinkmann, O. Goldbeck, J. Haar, T. Schulz, G. Priebe, M. Holz, B. Lemcke, K. Knaack, O. Seebauer, P. Willanzheimer, R. Jonas, and N. Engling. We thank T. Dietrich, S. Geile, F. Guicking, H. Noei, and T. Pakendorf from DESY and B. Di Fabrizio and S. Kühn from BNITM for assistance. This research was supported in part through the Maxwell computational resources operated at DESY. We acknowledge the use of the XBI biological sample preparation laboratory at European XFEL, enabled by the XBI User Consortium. Funding: We acknowledge financial support from the EXSCALATE4CoV EU-H2020 Emergency Project (101003551), the Cluster of Excellence “Advanced Imaging of Matter” of the Deutsche Forschungsgemeinschaft (DFG), EXC 2056 project ID 390715994, the Helmholtz Association Impulse and Networking funds (projects ExNet-0002 and InternLabs-0011 “HIR3X”), the Federal Ministry of Education and Research (BMBF) via projects 05K16GUA, 05K19GU4, 05K20BI1, 05K20FL1, 16GW0277, and 031B0405D, and the Joachim-Herz-Stiftung Hamburg (project Infecto-Physics). C.E. and M.R. acknowledge financial support from grant HIDSS-0002 DASHH (Data Science in Hamburg, HELMHOLTZ, Graduate School for the Structure of Matter). R.C. is supported by DFG grants INST 187/621-1 and INST 187/686-1. D.T. is supported by the Slovenian Research Agency (research program P1-0048, Infrastructural program IO-0048). B.S. was supported by an Exploration Grant from the Boehringer Ingelheim Foundation. The Heinrich Pette Institute, Leibniz Institute for Experimental Virology was supported by the Free and Hanseatic City of Hamburg and the Federal Ministry of Health. C.U. and B.K. were supported by EU Horizon 2020 ERC StG-2017 759661, BMBF RTK Struktur 01KI20391, BMBF Visavix 05K16BH1, and the Leibniz Association SAW-2014-HPI-4 grant. Author contributions: Se.G., P.Y.A.R., Y.F.-G., W.B., P.G., A.R.B., R.C., D.T., A.Z., H.N.C., A.R.P., C.B., and A.M. designed the research. Se.G., P.Y.A.R., T.J.L., W.H., H.N.C., A.R.P., C.B., and A.M. wrote the manuscript. Se.G., P.Y.A.R., J.L., F.H.M.K., S.M., W.B., I.D., B.S., H.Gie., B.N.-B., M.B., P.L.X., N.W., H.A., N.U., S.F., B.A.F., M.S., H.B., J.K., G.E.P.-M., A.R.M., P.G., V.H., P.F., M.W., E.-C.S., P.M., H.T., and T.B. participated in sample preparation. P.Y.A.R., performed crystallization experiments. Se.G., P.Y.A.R., J.L., T.J.L., O.Y., S.S., A.T., M.Gr., H.F., F.T., M.Ga., Y.G., C.L., S.A., A.P., G.B., D.v.S., G.P., T.R.S., I.B., and S.P. performed x-ray data collection. T.J.L., H.M.G., D.O., O.Y., L.G., M.D., T.A.W., F.S., C.R., D.M., J.J.Z.-D., I.K., C.S., R.S., H.H., and D.C.F.M. contributed to x-ray data management. Se.G., P.Y.A.R., J.L., T.J.L., H.M.G., F.H.M.K., W.E., D.O., A.H., V.S., J.H., J.M., J.B., J.W., C.G.F., M.S.W., A.C., D.T., W.H., and A.M. performed x-ray data analysis. K.L., B.K., C.U., and R.C. performed and analyzed mass spectrometry experiments. Y.F.-G., B.E.-P., and St.G. performed and analyzed antiviral activity assays. P.G., B.E., M.K., M.M.G.-A., S.N., C.G., L.Z., X.S., K.K., A.U., J.L., and R.H. performed and analyzed ligand binding studies and protein activity assays. C.E., J.P.-Z., and M.R. performed computational binding studies. Competing interests: M.R. is a stakeholder of BioSolveIT GmbH, licensor of the software HYDE. Data and materials availability: The coordinates and structure factors for all described crystal structures of SARS-CoV-2 Mpro in complex with compounds are deposited in the PDB with accession codes 6YNQ, 6YVF, 7A1U, 7ABU, 7ADW, 7AF0, 7AGA, 7AHA, 7AK4, 7AKU, 7AMJ, 7ANS, 7AOL, 7AP6, 7APH, 7AQE, 7AQI, 7AQJ, 7AR5, 7AR6, 7ARF, 7AVD, 7AWR, 7AWS, 7AWU, 7AWW, 7AX6, 7AXM, 7AXO, 7AY7, 7B83, and 7NEV. Code used in this analysis has been previously published (10). The code for forcing adherence to the Wilson distribution is included in the Vagabond refinement package (https://vagabond.hginn.co.uk/) under a GPLv3 license. Compounds from the Fraunhofer IME Repurposing Collection were obtained from the Fraunhofer Institute for Molecular Biology and Applied Ecology under a material transfer agreement. Compounds from the Safe-in-man Library were kindly provided by Dompé Farmaceutici S.p.A. Other materials are available from Se.G. or A.M. upon request. This work is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/. This license does not apply to figures/photos/artwork or other content included in the article that is credited to a third party; obtain authorization from the rights holder before using such material.
Supplementary Materials
science.sciencemag.org/content/372/6542/642/suppl/DC1
Materials and Methods
Supplementary Text
Figs. S1 to S9
Tables S1 to S7
MDAR Reproducibility Checklist
References and Notes
- 1.Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R., Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 368, 409–412 (2020). 10.1126/science.abb3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L. W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H., Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 582, 289–293 (2020). 10.1038/s41586-020-2223-y [DOI] [PubMed] [Google Scholar]
- 3.Hilgenfeld R., From SARS to MERS: Crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 281, 4085–4096 (2014). 10.1111/febs.12936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiao J., Li Y.-S., Zeng R., Liu F.-L., Luo R.-H., Huang C., Wang Y.-F., Zhang J., Quan B., Shen C., Mao X., Liu X., Sun W., Yang W., Ni X., Wang K., Xu L., Duan Z.-L., Zou Q.-C., Zhang H.-L., Qu W., Long Y.-H.-P., Li M.-H., Yang R.-C., Liu X., You J., Zhou Y., Yao R., Li W.-P., Liu J.-M., Chen P., Liu Y., Lin G.-F., Yang X., Zou J., Li L., Hu Y., Lu G.-W., Li W.-M., Wei Y.-Q., Zheng Y.-T., Lei J., Yang S., SARS-CoV-2 Mpro inhibitors with antiviral activity in a transgenic mouse model. Science 371, 1374–1378 (2021). 10.1126/science.abf1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuzikov M., Costanzi E., Reinshagen J., Esposito F., Vangeel L., Wolf M., Ellinger B., Claussen C., Geisslinger G., Corona A., Iaconis D., Talarico C., Manelfi C., Cannalire R., Rossetti G., Gossen J., Albani S., Musiani F., Herzog K., Ye Y., Giabbai B., Demitri N., Jochmans D., Jonghe S. D., Rymenants J., Summa V., Tramontano E., Beccari A. R., Leyssen P., Storici P., Neyts J., Gribbon P., Zaliani A., Identification of inhibitors of SARS-CoV-2 3CL-Pro enzymatic activity using a small molecule in vitro repurposing screen. ACS Pharmacol. Transl. Sci. 10.1021/acsptsci.0c00216 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wollenhaupt J., Metz A., Barthel T., Lima G. M. A., Heine A., Mueller U., Klebe G., Weiss M. S., F2X-Universal and F2X-Entry: Structurally Diverse Compound Libraries for Crystallographic Fragment Screening. Structure 28, 694–706.e5 (2020). 10.1016/j.str.2020.04.019 [DOI] [PubMed] [Google Scholar]
- 7.Cox O. B., Krojer T., Collins P., Monteiro O., Talon R., Bradley A., Fedorov O., Amin J., Marsden B. D., Spencer J., von Delft F., Brennan P. E., A poised fragment library enables rapid synthetic expansion yielding the first reported inhibitors of PHIP(2), an atypical bromodomain. Chem. Sci. 7, 2322–2330 (2016). 10.1039/C5SC03115J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hann M. M., Leach A. R., Harper G., Molecular complexity and its impact on the probability of finding leads for drug discovery. J. Chem. Inf. Comput. Sci. 41, 856–864 (2001). 10.1021/ci000403i [DOI] [PubMed] [Google Scholar]
- 9.Ehrmann F. R., Stojko J., Metz A., Debaene F., Barandun L. J., Heine A., Diederich F., Cianférani S., Reuter K., Klebe G., Soaking suggests “alternative facts”: Only co-crystallization discloses major ligand-induced interface rearrangements of a homodimeric tRNA-binding protein indicating a novel mode-of-inhibition. PLOS ONE 12, e0175723 (2017). 10.1371/journal.pone.0175723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginn H. M., Pre-clustering data sets using cluster4x improves the signal-to-noise ratio of high-throughput crystallography drug-screening analysis. Acta Crystallogr. D Biol. Crystallogr. 76, 1134–1144 (2020). 10.1107/S2059798320012619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearce N. M., Krojer T., Bradley A. R., Collins P., Nowak R. P., Talon R., Marsden B. D., Kelm S., Shi J., Deane C. M., von Delft F., A multi-crystal method for extracting obscured crystallographic states from conventionally uninterpretable electron density. Nat. Commun. 8, 15123 (2017). 10.1038/ncomms15123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simplício A. L., Clancy J. M., Gilmer J. F., β-aminoketones as prodrugs with pH-controlled activation. Int. J. Pharm. 336, 208–214 (2007). 10.1016/j.ijpharm.2006.11.055 [DOI] [PubMed] [Google Scholar]
- 13.Rautio J., Meanwell N. A., Di L., Hageman M. J., The expanding role of prodrugs in contemporary drug design and development. Nat. Rev. Drug Discov. 17, 559–587 (2018). 10.1038/nrd.2018.46 [DOI] [PubMed] [Google Scholar]
- 14.Altmeyer M., Amtmann E., Heyl C., Marschner A., Scheidig A. J., Klein C. D., Beta-aminoketones as prodrugs for selective irreversible inhibitors of type-1 methionine aminopeptidases. Bioorg. Med. Chem. Lett. 24, 5310–5314 (2014). 10.1016/j.bmcl.2014.09.047 [DOI] [PubMed] [Google Scholar]
- 15.Quasthoff S., Möckel C., Zieglgänsberger W., Schreibmayer W., Tolperisone: A typical representative of a class of centrally acting muscle relaxants with less sedative side effects. CNS Neurosci. Ther. 14, 107–119 (2008). 10.1111/j.1527-3458.2008.00044.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccart M., Rozencweig M., Dodion P., Cumps E., Crespeigne N., Makaroff O., Atassi G., Kisner D., Kenis Y., Phase I clinical trial with alpha 1,3,5- triglycidyl-s-triazinetrione (NSC-296934). Eur. J. Cancer Clin. Oncol. 17, 1263–1266 (1981). 10.1016/0014-2964(81)90006-2 [DOI] [PubMed] [Google Scholar]
- 17.Ma C., Sacco M. D., Hurst B., Townsend J. A., Hu Y., Szeto T., Zhang X., Tarbet B., Marty M. T., Chen Y., Wang J., Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 30, 678–692 (2020). 10.1038/s41422-020-0356-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vuong W., Khan M. B., Fischer C., Arutyunova E., Lamer T., Shields J., Saffran H. A., McKay R. T., van Belkum M. J., Joyce M. A., Young H. S., Tyrrell D. L., Vederas J. C., Lemieux M. J., Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat. Commun. 11, 4282 (2020). 10.1038/s41467-020-18096-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacco M. D., Ma C., Lagarias P., Gao A., Townsend J. A., Meng X., Dube P., Zhang X., Hu Y., Kitamura N., Hurst B., Tarbet B., Marty M. T., Kolocouris A., Xiang Y., Chen Y., Wang J., Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against Mpro and cathepsin L. Sci. Adv. 6, eabe0751 (2020). 10.1126/sciadv.abe0751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki T., Kishi M., Saito M., Tanaka T., Higuchi N., Kominami E., Katunuma N., Murachi T., Inhibitory effect of di- and tripeptidyl aldehydes on calpains and cathepsins. J. Enzyme Inhib. 3, 195–201 (1990). 10.3109/14756369009035837 [DOI] [PubMed] [Google Scholar]
- 21.Escaich S., Prouvensier L., Saccomani M., Durant L., Oxoby M., Gerusz V., Moreau F., Vongsouthi V., Maher K., Morrissey I., Soulama-Mouze C., The MUT056399 inhibitor of FabI is a new antistaphylococcal compound. Antimicrob. Agents Chemother. 55, 4692–4697 (2011). 10.1128/AAC.01248-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan J., Verschueren K. H. G., Anand K., Shen J., Yang M., Xu Y., Rao Z., Bigalke J., Heisen B., Mesters J. R., Chen K., Shen X., Jiang H., Hilgenfeld R., pH-dependent conformational flexibility of the SARS-CoV main proteinase (Mpro) dimer: Molecular dynamics simulations and multiple x-ray structure analyses. J. Mol. Biol. 354, 25–40 (2005). 10.1016/j.jmb.2005.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wissner A., Overbeek E., Reich M. F., Floyd M. B., Johnson B. D., Mamuya N., Rosfjord E. C., Discafani C., Davis R., Shi X., Rabindran S. K., Gruber B. C., Ye F., Hallett W. A., Nilakantan R., Shen R., Wang Y.-F., Greenberger L. M., Tsou H.-R., Synthesis and structure-activity relationships of 6,7-disubstituted 4-anilinoquinoline-3-carbonitriles. The design of an orally active, irreversible inhibitor of the tyrosine kinase activity of the epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor-2 (HER-2). J. Med. Chem. 46, 49–63 (2003). 10.1021/jm020241c [DOI] [PubMed] [Google Scholar]
- 24.Erlichman C., Hidalgo M., Boni J. P., Martins P., Quinn S. E., Zacharchuk C., Amorusi P., Adjei A. A., Rowinsky E. K., Phase I study of EKB-569, an irreversible inhibitor of the epidermal growth factor receptor, in patients with advanced solid tumors. J. Clin. Oncol. 24, 2252–2260 (2006). 10.1200/JCO.2005.01.8960 [DOI] [PubMed] [Google Scholar]
- 25.Shi J., Sivaraman J., Song J., Mechanism for controlling the dimer-monomer switch and coupling dimerization to catalysis of the severe acute respiratory syndrome coronavirus 3C-like protease. J. Virol. 82, 4620–4629 (2008). 10.1128/JVI.02680-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyatt P. G., Woodhead A. J., Berdini V., Boulstridge J. A., Carr M. G., Cross D. M., Davis D. J., Devine L. A., Early T. R., Feltell R. E., Lewis E. J., McMenamin R. L., Navarro E. F., O’Brien M. A., O’Reilly M., Reule M., Saxty G., Seavers L. C. A., Smith D.-M., Squires M. S., Trewartha G., Walker M. T., Woolford A. J.-A., Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H-pyrazole-3-carboxamide (AT7519), a novel cyclin dependent kinase inhibitor using fragment-based X-ray crystallography and structure based drug design. J. Med. Chem. 51, 4986–4999 (2008). 10.1021/jm800382h [DOI] [PubMed] [Google Scholar]
- 27.El-Baba T. J., Lutomski C. A., Kantsadi A. L., Malla T. R., John T., Mikhailov V., Bolla J. R., Schofield C. J., Zitzmann N., Vakonakis I., Robinson C. V., Allosteric Inhibition of the SARS-CoV-2 Main Protease: Insights from Mass Spectrometry Based Assays*. Angew. Chem. Int. Ed. 59, 23544–23548 (2020). 10.1002/anie.202010316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pushpakom S., Iorio F., Eyers P. A., Escott K. J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., McNamee C., Norris A., Sanseau P., Cavalla D., Pirmohamed M., Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 18, 41–58 (2019). 10.1038/nrd.2018.168 [DOI] [PubMed] [Google Scholar]
- 29.Liu Y., Liang C., Xin L., Ren X., Tian L., Ju X., Li H., Wang Y., Zhao Q., Liu H., Cao W., Xie X., Zhang D., Wang Y., Jian Y., The development of Coronavirus 3C-Like protease (3CLpro) inhibitors from 2010 to 2020. Eur. J. Med. Chem. 206, 112711 (2020). 10.1016/j.ejmech.2020.112711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Arcy A., Bergfors T., Cowan-Jacob S. W., Marsh M., Microseed matrix screening for optimization in protein crystallization: What have we learned? Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 70, 1117–1126 (2014). 10.1107/S2053230X14015507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kabsch W., XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010). 10.1107/S0907444909047337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vonrhein C., Flensburg C., Keller P., Sharff A., Smart O., Paciorek W., Womack T., Bricogne G., Data processing and analysis with the autoPROC toolbox. Acta Crystallogr. D Biol. Crystallogr. 67, 293–302 (2011). 10.1107/S0907444911007773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.I. J. Tickle, C. Flensburg, P. Keller, W. Paciorek, A. Sharff, C. Vonrhein, G. Bricogne, STARANISO. (Global Phasing, 2018). [Google Scholar]
- 34.Beilsten-Edmands J., Winter G., Gildea R., Parkhurst J., Waterman D., Evans G., Scaling diffraction data in the DIALS software package: Algorithms and new approaches for multi-crystal scaling. Acta Crystallogr. D Struct. Biol. 76, 385–399 (2020). 10.1107/S2059798320003198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winter G., xia2: An expert system for macromolecular crystallography data reduction. J. Appl. Cryst. 43, 186–190 (2010). 10.1107/S0021889809045701 [DOI] [Google Scholar]
- 36.Liebschner D., Afonine P. V., Baker M. L., Bunkóczi G., Chen V. B., Croll T. I., Hintze B., Hung L.-W., Jain S., McCoy A. J., Moriarty N. W., Oeffner R. D., Poon B. K., Prisant M. G., Read R. J., Richardson J. S., Richardson D. C., Sammito M. D., Sobolev O. V., Stockwell D. H., Terwilliger T. C., Urzhumtsev A. G., Videau L. L., Williams C. J., Adams P. D., Macromolecular structure determination using X-rays, neutrons and electrons: Recent developments in Phenix. Acta Crystallogr. D Biol. Struct. 75, 861–877 (2019). 10.1107/S2059798319011471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Browse xia2pipe/xia2pipe DESY Stash; https://stash.desy.de/projects/X2P/repos/xia2pipe/browse.
- 38.Murshudov G. N., Skubák P., Lebedev A. A., Pannu N. S., Steiner R. A., Nicholls R. A., Winn M. D., Long F., Vagin A. A., REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 67, 355–367 (2011). 10.1107/S0907444911001314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turk D., MAIN software for density averaging, model building, structure refinement and validation. Acta Crystallogr. D Biol. Crystallogr. 69, 1342–1357 (2013). 10.1107/S0907444913008408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emsley P., Lohkamp B., Scott W. G., Cowtan K., Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010). 10.1107/S0907444910007493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sommer K., Friedrich N.-O., Bietz S., Hilbig M., Inhester T., Rarey M., UNICON: A Powerful and Easy-to-Use Compound Library Converter. J. Chem. Inf. Model. 56, 1105–1111 (2016). 10.1021/acs.jcim.6b00069 [DOI] [PubMed] [Google Scholar]
- 42.Bietz S., Rarey M., SIENA: Efficient Compilation of Selective Protein Binding Site Ensembles. J. Chem. Inf. Model. 56, 248–259 (2016). 10.1021/acs.jcim.5b00588 [DOI] [PubMed] [Google Scholar]
- 43.Bietz S., Urbaczek S., Schulz B., Rarey M., Protoss: A holistic approach to predict tautomers and protonation states in protein-ligand complexes. J. Cheminform. 6, 12 (2014). 10.1186/1758-2946-6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flachsenberg F., Meyder A., Sommer K., Penner P., Rarey M., A Consistent Scheme for Gradient-Based Optimization of Protein-Ligand Poses. J. Chem. Inf. Model. 60, 6502–6522 (2020). 10.1021/acs.jcim.0c01095 [DOI] [PubMed] [Google Scholar]
- 45.Schneider N., Lange G., Hindle S., Klein R., Rarey M., A consistent description of HYdrogen bond and DEhydration energies in protein-ligand complexes: Methods behind the HYDE scoring function. J. Comput. Aided Mol. Des. 27, 15–29 (2013). 10.1007/s10822-012-9626-2 [DOI] [PubMed] [Google Scholar]
- 46.van den Heuvel R. H. H., van Duijn E., Mazon H., Synowsky S. A., Lorenzen K., Versluis C., Brouns S. J. J., Langridge D., van der Oost J., Hoyes J., Heck A. J. R., Improving the performance of a quadrupole time-of-flight instrument for macromolecular mass spectrometry. Anal. Chem. 78, 7473–7483 (2006). 10.1021/ac061039a [DOI] [PubMed] [Google Scholar]
- 47.Mogi M., Ito T., Matsuki Y., Kurata Y., Nambara T., Determination of isofloxythepin in biological fluids by gas chromatography-mass spectrometry. J. Chromatogr. 399, 234–250 (1987). 10.1016/S0021-9673(00)96126-0 [DOI] [PubMed] [Google Scholar]
- 48.Aoyagi T., Miyata S., Nanbo M., Kojima F., Matsuzaki M., Ishizuka M., Takeuchi T., Umezawa H., Biological activities of leupeptins. J. Antibiot. (Tokyo) 22, 558–568 (1969). 10.7164/antibiotics.22.558 [DOI] [PubMed] [Google Scholar]
- 49.Kneller D. W., Galanie S., Phillips G., O’Neill H. M., Coates L., Kovalevsky A., Malleability of the SARS-CoV-2 3CL Mpro Active-Site Cavity Facilitates Binding of Clinical Antivirals. Structure 28, 1313–1320.e3 (2020). 10.1016/j.str.2020.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fisch F., Fleites C. M., Delenne M., Baudendistel N., Hauer B., Turkenburg J. P., Hart S., Bruce N. C., Grogan G., A covalent succinylcysteine-like intermediate in the enzyme-catalyzed transformation of maleate to fumarate by maleate isomerase. J. Am. Chem. Soc. 132, 11455–11457 (2010). 10.1021/ja1053576 [DOI] [PubMed] [Google Scholar]
- 51.Hong C. R., Dickson B. D., Jaiswal J. K., Pruijn F. B., Hunter F. W., Hay M. P., Hicks K. O., Wilson W. R., Cellular pharmacology of evofosfamide (TH-302): A critical re-evaluation of its bystander effects. Biochem. Pharmacol. 156, 265–280 (2018). 10.1016/j.bcp.2018.08.027 [DOI] [PubMed] [Google Scholar]
- 52.Hsu J. T.-A., Kuo C.-J., Hsieh H.-P., Wang Y.-C., Huang K.-K., Lin C. P.-C., Huang P.-F., Chen X., Liang P.-H., Evaluation of metal-conjugated compounds as inhibitors of 3CL protease of SARS-CoV. FEBS Lett. 574, 116–120 (2004). 10.1016/j.febslet.2004.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee C.-C., Kuo C.-J., Ko T.-P., Hsu M.-F., Tsui Y.-C., Chang S.-C., Yang S., Chen S.-J., Chen H.-C., Hsu M.-C., Shih S.-R., Liang P.-H., Wang A. H.-J., Structural basis of inhibition specificities of 3C and 3C-like proteases by zinc-coordinating and peptidomimetic compounds. J. Biol. Chem. 284, 7646–7655 (2009). 10.1074/jbc.M807947200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.te Velthuis A. J. W., van den Worm S. H. E., Sims A. C., Baric R. S., Snijder E. J., van Hemert M. J., Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLOS Pathog. 6, e1001176 (2010). 10.1371/journal.ppat.1001176 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
science.sciencemag.org/content/372/6542/642/suppl/DC1
Materials and Methods
Supplementary Text
Figs. S1 to S9
Tables S1 to S7
MDAR Reproducibility Checklist