Abstract
AIM:
Opioid-targeted vaccines are under consideration as candidate Opioid Use Disorder medications. We recently reported that a fentanyl-targeted vaccine produced a robust and long-lasting attenuation of fentanyl-vs-food choice in rats. In the current study, we evaluated an optimized fentanyl-targeted vaccine in rhesus monkeys to determine whether vaccine effectiveness to attenuate fentanyl choice translated to a species with greater phylogenetic similarity to humans.
METHODS:
Adult male (2) and female (3) rhesus monkeys were trained to respond under a concurrent schedule of food (1g pellets) and intravenous fentanyl (0, 0.032–1 μg/kg/injection) reinforcement during daily 2h sessions. Fentanyl choice dose-effect functions were determined daily and 7-day buprenorphine treatments (0.0032–0.032 mg/kg/h IV; n=4–5) were determined for comparison to vaccine effects. Subsequently, a fentanyl-CRM197 conjugate vaccine was administered at week 0, 3, 8, 15 over a 29-week experimental period during which fentanyl choice dose-effect functions continued to be determined daily.
RESULTS:
Buprenorphine significantly decreased fentanyl choice and reciprocally increased food choice. Vaccination eliminated fentanyl choice and increased food choice in four-of-the-five monkeys. A transient and less robust vaccine effect was observed in the fifth monkey. Fentanyl-specific antibody concentrations peaked after the third vaccination to approximately 50 μg/mL while anti-fentanyl antibody affinity increased to a sustained low nanomolar level.
CONCLUSION:
These results translate fentanyl vaccine effectiveness from rats to rhesus monkeys to decrease fentanyl-vs-food choice, albeit with greater individual differences observed in monkeys. These results support the potential and further clinical evaluation of this fentanyl-targeted vaccine as a candidate Opioid Use Disorder medication.
Keywords: opioid vaccine, rhesus monkey, drug self-administration, fentanyl, choice
1. Introduction
Fatal overdoses involving fentanyl and other synthetic opioids totaled 31,892 in the United States in 2018, a 33-fold increase relative to 1999 estimates (Hedegaard et al. 2020). This alarming increase in overdose deaths may be attributable to unintentional exposure to fentanyl, as drug seizures have uncovered fentanyl-adulterated heroin, methamphetamine, and cocaine, as well as fentanyl-laced counterfeit pills/tablets intended to resemble prescription opioids and benzodiazepines (Mars et al. 2018; Pichini et al. 2018). The mu-opioid receptor (MOR) antagonist naltrexone and the low efficacy MOR agonist buprenorphine are approved in the United States for the treatment of Opioid Use Disorder in non-opioid dependent (naltrexone, buprenorphine) or mildly opioid dependent (buprenorphine) individuals. Both of these medications can antagonize fentanyl effects through competitive interactions at MORs. However, the poor patient compliance associated with naltrexone (Jarvis et al. 2018) and the restricted availability of buprenorphine contribute to an inadequate level of protection against fentanyl overdose, at least at a population level. Furthermore, those that primarily misuse non-opioid drugs of abuse (e.g., cocaine, methamphetamine, benzodiazepines) would be expected to have even lesser access to Opioid Use Disorder medications, further limiting the likelihood of pharmacological protection against the adulteration of non-opioid drugs of abuse with fentanyl. Therefore, public health efforts to prevent opioid overdose would benefit from new strategies to combat the complex issues associated with the fentanyl overdose crisis.
One strategy under consideration to address this public health crisis is the development of fentanyl-targeted vaccines (Bremer et al. 2016; Miller et al. 1975; Raleigh et al. 2019). Active immunization against fentanyl can be accomplished by administering a conjugate vaccine, comprised of a fentanyl-like hapten linked to an immunogenic carrier protein. Once administered, the vaccine elicits antibody production by the host’s immune system. These antibodies circulate outside of the central nervous system and are hypothesized to not interact with receptors, proteins, or structurally dissimilar medications (Anton and Leff 2006; Schlosburg et al. 2013; Stowe et al. 2011). Following subsequent fentanyl exposure, these antibodies bind to fentanyl, generating a fentanyl + antibody complex that is unable to pass through the blood-brain barrier or initiate centrally-mediated fentanyl effects. Fentanyl-targeted vaccines may address some of the aforementioned limitations of naltrexone and buprenorphine. Specifically, fentanyl-targeted vaccines have been shown to remain effective for several months in both rhesus monkeys and rats (Tenney et al. 2019; Townsend et al. 2019). These results suggest that once fentanyl vaccine effectiveness is established, “booster” vaccinations may be scheduled infrequently to increase patient compliance over currently available monthly depot naltrexone and buprenorphine formulations. In addition, there has been no evidence of abuse liability identified with opioid-targeted vaccines or their antibodies, suggesting that a clinically available fentanyl-targeted vaccine may have fewer restrictions than buprenorphine. Finally, fentanyl vaccine-elicited antibodies display a high degree of specificity and affinity for fentanyl and fentanyl analogs (e.g., (Bremer et al. 2016; Hwang et al. 2018b; Tenney et al. 2019; Townsend et al. 2020a)). Thus, structurally dissimilar OUD treatments such as naltrexone and buprenorphine could theoretically be used as adjuncts to opioid-targeted vaccines and/or confer protection during the antibody induction period, which could require weeks to months.
Research with vaccine formulations using a previously described hapten (Bremer et al. 2016) has reported vaccine-elicited antibodies to produce robust, long lasting, and selective decreases in the thermal antinociceptive potency of fentanyl in mice (Bremer et al. 2016; Hwang et al. 2018b), rats (Townsend et al. 2019; Townsend et al. 2020a), and rhesus monkeys (Tenney et al. 2019). In addition, administration of a similar fentanyl vaccine produced a 22-fold reduction in the reinforcing potency of fentanyl under a fentanyl-versus-food choice procedure in male and female rats (Townsend et al. 2019). This finding is particularly encouraging, as the ultimate goal of an Opioid Use Disorder medication is to reduce/eliminate opioid self-administration while also increasing behaviors maintained by adaptive non-opioid reinforcers (Townsend et al. 2020b). However, one remaining hurdle in the development of this vaccine is a demonstration of its effectiveness to attenuate fentanyl reinforcement in non-human primates. Non-human primates and humans share considerable genetic and physiologic similarities, establishing non-human primates as the most predictive whole-organism model of human immunology and responsiveness to vaccination (Palermo et al. 2013). The present study evaluated the effectiveness, timecourse, and selectivity of a fentanyl-CRM (cross reacting material) conjugate vaccine on intravenous (IV) fentanyl self-administration using a fentanyl-vs-food choice self-administration procedure in male and female rhesus monkeys. Fentanyl vaccine effectiveness was compared to continuous 7-day buprenorphine treatment, because this medication is FDA approved for Opioid Use Disorder in non-opioid dependent or mildly opioid dependent individuals, and because a previous study reported on effectiveness of naltrexone maintenance to decrease opioid-vs-food choice in rhesus monkeys (Townsend et al. 2020c).
2. Methods
2.1. Subjects
A total of five adult rhesus monkeys (Macaca mulatta) of Indian origin (two males, three females) served as experimental subjects. Monkeys were surgically implanted with silicone double-lumen venous catheters (i.d. = 0.76 mm, o.d. = 2.36 mm, durometer = 60; Reiss Manufacturing, Blackstone, VA) inserted into a major vein (i.e. femoral or jugular). The three female monkeys (1525F, 1540F, 1541F) had prior exposure to tetanus toxoid and opioid self-administration, one male had a prior cocaine and methamphetamine self-administration history (1533M), and the other male monkey had prior opioid exposure, but was naïve to operant procedures (1536M). The monkeys’ diet consisted of food biscuits (Teklad Global Diet, 2050, 20 % Protein Primate Diet) and fresh fruit or vegetables delivered in the afternoons after behavioral sessions. Water was available continuously in each monkey’s home chamber, which also served as the experimental chamber. A 12-h light/dark cycle was in effect (lights on from 6AM to 6PM). Environmental enrichment was provided after behavioral sessions and included foraging devices loaded with nuts, seeds, or diced vegetables, as well as movies displayed on a monitor in the housing room. Facilities were licensed by the United States Department of Agriculture and accredited by AAALAC International. The Institutional Animal Care and Use Committee approved all experimental and enrichment protocols. Animal research and husbandry were conducted according to the 8th edition of the Guide for the Care and Use of Laboratory Animals.
2.2. Fentanyl-vs-food choice procedure
Each housing chamber was equipped with a customized operant response panel, which had two keys that could be transilluminated red, yellow, or green, as well as a pellet dispenser (Med Associates, ENV- 203–1000, St. Albans, VT) that delivered food pellets to a receptacle below the operant panel. The externalized portion of the IV catheter was routed through a custom jacket and tether system connected to a dual-channel fluid swivel (Lomir Biomedical, Malone, NY) on the top of the chamber, which connected to two safety syringe pumps (Med Associates, PHM-108), one for each lumen of the double-lumen catheter. One pump (the “self-administration” pump) was used to deliver contingent IV fentanyl injections through one lumen of the catheter. The second pump (the “treatment” pump) was used to deliver non-contingent IV saline or buprenorphine (0.0032–0.032 mg/kg/h) injections through the second lumen at a programmed rate of 0.1 mL injections every 20 min from 12PM each day until 11AM the next morning. Catheter patency was periodically evaluated with IV ketamine (Vedco, St. Joseph, MO) administration and after any treatment that produced a rightward shift in the fentanyl choice dose-effect function. The catheter was considered patent if IV ketamine administration produced loss of muscle tone within 10 s.
Daily experimental sessions were conducted from 9AM to 11AM in each monkey’s home chamber as described previously for heroin-, cocaine-, and methamphetamine-versus-food choice studies (Banks and Blough 2015; Banks et al. 2011; Negus 2006; Townsend et al. 2020c). The terminal choice schedule consisted of five 20-min components separated by 5-min inter-component intervals during which responding had no scheduled consequences. During each component, the right, fentanyl-associated key was transilluminated green, and completion of the FR requirement (FR10) resulted in delivery of the unit fentanyl dose available during that component. The unit fentanyl doses available during each of the five successive components were 0 (i.e., no fentanyl), 0.032, 0.1, 0.32, and 1 μg/kg/injection, respectively, and dose was varied by changing the injection volume (0, 0.032, 0.1, 0.32, and 1.0 ml/injection, respectively). Stimulus lights on the fentanyl-associated key were flashed on and off in 3 s cycles, and longer flashes were associated with larger fentanyl doses and the light was not illuminated when fentanyl was not available. In addition, the left, food-associated key was transilluminated red, and completion of the fixed-ratio (FR) requirement resulted in delivery of a food pellet (1-g banana-flavored pellets; 5TUR Grain-based Precision Primate Tablets; Test Diets, Richmond, IL). The food FR requirement was individually adjusted in each monkey (FR 10 in 1536M, FR 100 in the other monkeys) to produce primarily food choice at lower fentanyl doses and primarily fentanyl choice at higher fentanyl doses to confer sensitivity to treatment-induced leftward or rightward shifts in fentanyl choice dose-effect curves. Ratio requirement completion on either key initiated a 3 s timeout, during which all stimulus lights were turned off, and responding had no scheduled consequences. A maximum of 10 total injection or food deliveries was available during each component, and if all 10 deliveries were obtained before the 20-min component had elapsed, that all stimulus lights were extinguished and responding had no scheduled consequences for the remainder of that component. Choice behavior was considered to be stable when the lowest unit fentanyl dose maintaining at least 80 % fentanyl choice varied by ≤0.5 log units for three consecutive days.
2.3. Effects of buprenorphine treatment on fentanyl choice
Once fentanyl-vs-food choice was stable, fentanyl was retained in the self-administration pump, and either saline or buprenorphine (0.0032–0.032 mg/kg/h, IV) solutions were placed into the treatment pump and each dose was tested for seven consecutive days. At the conclusion of each 7-day test period, saline solutions were reinstated if necessary in the treatment pumps, and responding was monitored for at least 4 days and until the aforementioned stability criteria were met. Following a buprenorphine treatment wherein a rightward or downward shift of the fentanyl-choice function was observed, the food-associated light was not illuminated during the last two components of the subsequent baseline-recovery sessions to promote behavioral allocation to the fentanyl-associated key. This was generally implemented during the session immediately following the largest buprenorphine dose. However, some monkeys required additional retraining, likely owing to the relatively long duration of action of buprenorphine and its sustained antagonism of the reinforcing effects of fentanyl. Subsequently, fentanyl-versus-food choice sessions during saline maintenance were conducted until the aforementioned stability criteria were met. The largest dose of buprenorphine (0.032 mg/kg/h) was evaluated in 4 monkeys, and saline and the smaller buprenorphine doses (0.0032, 0.01 mg/kg/h) were evaluated in all 5 monkeys. The buprenorphine dosing order was counterbalanced across subjects.
2.4. Effects of fentanyl vaccine on fentanyl choice
Once buprenorphine testing was complete and stable choice behavior was observed, monkeys were administered a fentanyl-targeted vaccine (see Drugs and Vaccine below) intramuscularly, and daily fentanyl-vs-food choice sessions continued for 29 weeks. Additional “booster” vaccine injections were administered 3, 8, and 15 weeks after the initial vaccine injection (Figure 2). Venous blood samples were collected at weeks 3, 8, 12, 15, 22, and 29 in all monkeys for antibody affinity and titer analysis. Blood samples were also collected at week 56 in the three female monkeys. Although subjects 1533M and 1540F maintained catheter patency throughout the entirety of the 29-week experiment, subject 1525F lost patency between weeks 9 and 14, subject 1541F lost patency at week 22, and subject 1536M lost patency at week 28.
Figure 2:
Panel Experimental timeline for vaccine administration and blood collection. Upward arrows denote weeks that vaccine was administered. “B” denotes weeks that venous blood samples were collected.
For monkeys 1540F and 1525F, the food-associated discriminative stimuli were extinguished by the experimenter during the final two components of the choice session 44 days after the initial vaccine administration. For monkey 1533M, the pellet dispenser was inoperable during the choice session 127 days after the initial vaccine administration. For monkey 1536M, the food light failed to illuminate during the choice session 169 days after the initial vaccine administration. The effects of these temporary modifications to the experimental parameters on fentanyl choice are shown in Supplementary Figure 2.
2.5. Effects of fentanyl vaccine on heroin choice
A pre-vaccination determination of heroin-vs-food choice behavior was established in the three female monkeys prior to the training of fentanyl-vs-food choice (Townsend et al. 2020c). The parameters of the heroin-vs-food choice procedure were identical to those of the fentanyl-vs-food choice procedure with the following exceptions: 1) unit doses of heroin (0.001–0.032 mg/kg/injection) were available for self-administration, and 2) the drug-associated key was illuminated yellow instead of green. Heroin-vs-food choice was then re-determined in these female monkeys at experimental week 30. During the first heroin-substitution session, the food light was not illuminated during the final two components to facilitate responding on the heroin-associated key. The food light remained illuminated during all components of the three subsequent choice sessions, and the data from these sessions were used for subsequent analysis. Heroin choice was not assessed in the male subjects because a pre-vaccination baseline was not collected.
2.6. Antibody titer levels, affinity, and selectivity
Samples were run on a Nicoya OpenSPR instrument. Amine sensors were coated with fentanyl-BSA (fen-BSA) preactivated with EDC and NHS (channel 1) followed by preactivated non-conjugated BSA (channels 1+2). Serum samples were diluted 1:500 in running buffer (PBS-T+0.1% BSA) and flowed across both channels, subtracting the average channel 1 signal from channel 2 signal at equilibrium to give fentanyl binding in response units (RU). Following each sample injection, the sensor was regenerated with 10 mM HCl. Serum antibody concentrations were interpolated using a standard curve generated with known concentrations of an anti-fentanyl monoclonal antibody. Serum analysis by ELISA (data not shown) indicated that the IgG antibody isotype was the only detectable anti-fentanyl isotype present. Fentanyl IC50 values were determined from a four-point fentanyl dilution series in which serum samples were incubated with fentanyl for 30 min prior to injection. Week 22 samples were subjected to a six-point fentanyl dilution series to obtain more accurate IC50 values. In both cases, RU data was normalized and applied a variable-slope IC50 curve in GraphPad Prism 8 to obtain fentanyl competitive IC50 values. Pooled serum from week 22 was incubated with naloxone, buprenorphine, hydrocodone, acetylfentanyl and carfentanil prior to injection to obtain selectivity data.
2.7. Data analysis
The primary dependent measure was percent opioid choice (either fentanyl or heroin), defined as (number of ratio requirements, or ‘choices,’ completed on the opioid-associated key/total number of ratio requirements completed on both the opioid- and food- associated keys)*100. For the buprenorphine-treatment experiment, mean data from the last 3 days of each 7-day treatment were averaged for each individual monkey and then averaged across monkeys. Percent fentanyl choice was then plotted as a function of the unit fentanyl dose and analyzed using a two-way repeated-measures ANOVA with buprenorphine dose and unit fentanyl dose as the fixed main effects (Prism 8.0.1 for macOS, GraphPad Software, San Diego, CA). Additional dependent measures collected during each session were total choices, total food choices, and total fentanyl choices. To analyze the time course of 0.032 mg/kg/h buprenorphine treatment effects, total percent fentanyl choice (i.e., irrespective of unit fentanyl dose) for the session preceding treatment onset and the 7 treatment days were plotted as a function of day and analyzed using a one-way ANOVA. Total percent fentanyl choice data were also plotted for the seven days after termination of buprenorphine treatment but were not analyzed statistically. For the fentanyl-vaccine experiment, mean data from the last three days preceding vaccination were averaged for each individual monkey and then averaged across monkeys to serve as a baseline. At 7-week intervals of this portion of the experiment (i.e., weeks 7, 14, 21, and 28), data from the seven sessions of these selected weeks were plotted as a function of the unit fentanyl dose and analyzed using a two-way mixed-effects analysis with experimental week and fentanyl unit dose as the fixed main effects. To analyze vaccine time course effects, total percent fentanyl choice of the pre-vaccination baseline as well as the data from each week were plotted as a function of week and analyzed using a one-way mixed-effects analysis. To evaluate fentanyl vaccine selectivity, the final three pre-vaccination heroin-vs-food choice sessions were averaged and compared to the average of the three heroin-vs-food choice sessions collected during week 30 of the vaccination experiment using a two-way ANOVA, with vaccination status and heroin unit dose as the fixed main effects. Following a significant interaction, a Dunnett’s test was performed to compare treatment effects to baseline within a fentanyl unit dose or treatment day. If a significant main effect of time was detected in the absence of an interaction within a two-way ANOVA, a repeated measures one-way ANOVA was used for analysis of percent fentanyl choice data irrespective of the unit fentanyl dose. The criterion for significance was set a priori at the 95% confidence level (p < 0.05).
2.8. Drugs and vaccine
Fentanyl HCl, buprenorphine HCl, and heroin HCl were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD) and dissolved in sterile saline. All solutions were passed through a 0.22-μm sterile filter (Millex GV, Millipore Sigma, Burlington, MA) before administration. All drug doses were expressed as the salt forms listed above and delivered as milligrams per kilogram based on weights collected at least every four weeks. Drug molecules for the SPR selectivity assay were obtained as certified reference material from Cayman Chemical. The fentanyl hapten was synthesized and conjugated to CRM197 (Fina Biosolutions, Rockville, MD) as previously described (Bremer et al. 2016) and characterized by MALDI-TOF to reveal hapten to protein ratios of 18:1 for fentanyl-CRM197. Conjugates were formulated at approximately 2 mg/ml protein in 15% w/v trehalose PBS and passed through a 0.22 μm filter prior to adjuvant addition. Each vaccine formulation contained 200 μg of fentanyl-CRM197, 630 μg of CpG ODN 2006 (Eurofins Genomics, Louisville, KY), and 1 mg of Alhydrogel (Invivogen, San Diego, CA). Premeasured vaccine formulations were stored in lyophilized form at room temperature as previously described (Hwang et al. 2018a). Reconstitution of the vaccine only required the addition of sterile water 15–20 min prior to injection, yielding injection volumes of approximately 0.75 ml.
3. Results
3.1. Baseline fentanyl choice
Under baseline conditions, monkeys responded primarily on the food-associated key when fentanyl was not available (0 μg/kg/injection) or when the unit dose of fentanyl was small (0.032 μg/kg/injection). Responding on both the food- and fentanyl-associated keys was observed when the intermediate unit dose of fentanyl (0.1 μg/kg/injection) was available, and monkeys responded almost exclusively on the fentanyl-associated key when the largest unit doses of fentanyl (0.32 – 1 μg/kg/injection) were available (Figures 1A, 3A). When these baseline data were plotted irrespective of the unit dose of fentanyl, overall percent fentanyl choice was approximately 50% across the session (Figures 1C, 3C). In addition, monkeys generally completed all ten of the possible ratio requirements for each of the five components under baseline conditions (50 total choices), and the total number of completed ratio requirements was not affected by buprenorphine or vaccine administration (Figures 1B, 3B). No somatic signs of opioid withdrawal were observed following buprenorphine treatment, vaccination, or otherwise, suggesting that the monkeys were non-opioid dependent.
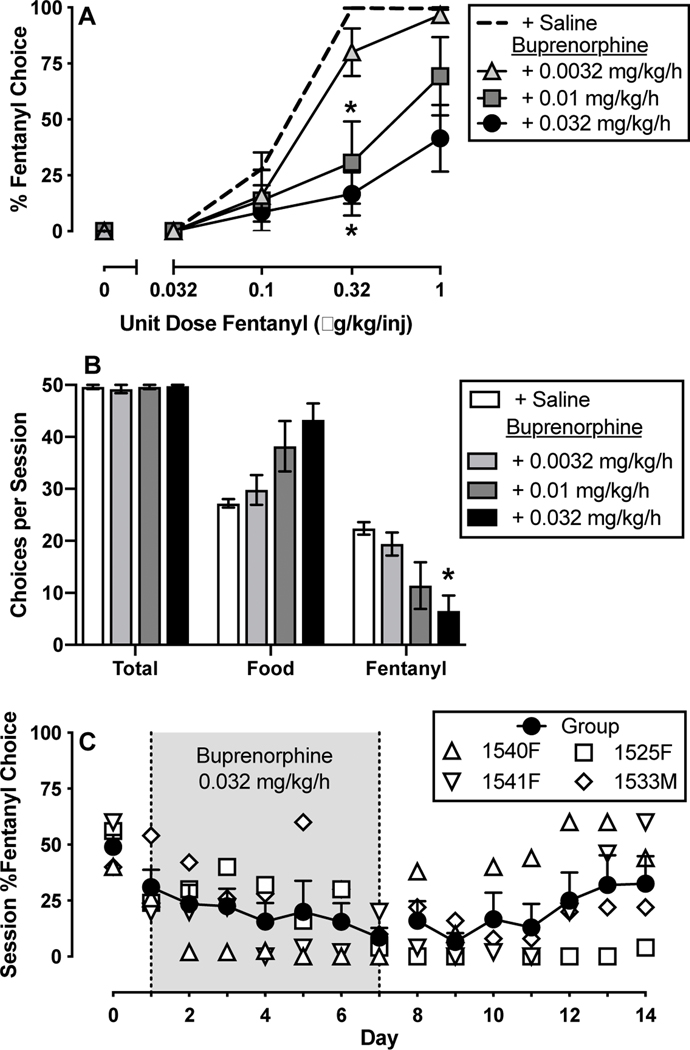
Figure 1:
Effects of 7-day intravenous buprenorphine (0.0032–0.032 mg/kg/h) treatment on choice between fentanyl and food in rhesus monkeys (n=4–5). Top ordinate (A): percent fentanyl choice. Top abscissa: unit fentanyl dose in micrograms per kilogram per injection. Middle ordinate (B): choices per session. Middle abscissa: Reinforcer type. Bottom ordinate (C): session percent fentanyl choice. Bottom abscissa: experimental day. Points and bars in panels A and B represent mean ±SEM obtained during days 5–7 of each 7-day treatment period. For panel C, filled symbols represent mean ±SEM and open symbols represent individual subject data. Asterisks denote significantly different from saline treatment (p<0.05).
Figure 3:
Effectiveness and time course of a fentanyl vaccine to attenuate choice between fentanyl and food in rhesus monkeys (n=3–5). Panel A ordinate: percent fentanyl choice. Panel A abscissa: unit fentanyl dose in micrograms per kilogram per injection. Panel B ordinate: choices per session. Panel B abscissa: Reinforcer type. Bottom ordinate (C): session percent fentanyl choice. Bottom abscissa: experimental week. Points and bars in panels A and B represent mean ±SEM obtained during days 5–7 of each 7-day treatment period. For panel C, filled symbols represent mean ±SEM and open symbols represent individual subject data. Asterisks denote significantly different from saline treatment “baseline” (p<0.05).
3.2. Effects of buprenorphine on fentanyl choice
Figure 1A shows average fentanyl-vs-food choice during the last three days of each 7-day continuous buprenorphine treatment (0.0032–0.032 mg/kg/h). Both 0.01 and 0.032 mg/kg/h buprenorphine significantly decreased percent fentanyl choice at the 0.32 ug/kg/injection unit fentanyl dose (Fig. 1A: fentanyl unit dose: F2,8 = 55.3, p < 0.0001; buprenorphine dose: F1.3,5.4 = 7.9, p = 0.03; interaction: F2.6,9.1 = 7.0, p = 0.012). This 0.032 mg/kg/h buprenorphine dose also significantly decreased total session fentanyl choices and trended toward increasing food choices without disrupting total session choices (Fig. 1B: dependent measure: F1.1,4.3 = 116.7, p = 0.0003; interaction: F1.5,5.2 = 10.2, p = 0.02). A time course of 0.032 mg/kg/h buprenorphine treatment on overall percent fentanyl choice across the seven-day treatment period is shown in Figure 1C. There was a trend for fentanyl choice to decline over time, but this effect did not achieve the criterion for statistical significance (F1.9,5.7 = 3.7, p = 0.09).
3.3. Effects of a fentanyl-targeted vaccine on fentanyl choice
Figure 2 shows a timeline of vaccine injections and blood-collection dates. When the effects of vaccination were assessed at seven-week intervals during the experiment, significant main effects of unit fentanyl dose and time-since-vaccination were detected in the absence of a significant interaction (Fig. 3A: fentanyl unit dose: F1.2,4.7 = 7.2, p = 0.04; time: F1.1,4.5 = 20.2, p = 0.008; interaction: F1.5,4.7 = 5.1, p = 0.07). A subsequent one-way ANOVA did not detect a significant difference between the selected weeks-since-vaccination (F1.1,4.5 = 4.8 p = 0.09). Figure 3B shows that the fentanyl vaccine significantly increased the number of food choices and decreased the number of fentanyl choices without altering total session choices at weeks 7, 21, and 28 (dependent measure: F1,4.1 = 52, p = 0.002; interaction: F1.6,5.5 = 26.1, p = 0.002). The time course of vaccine effects on overall percent fentanyl choice is shown in Figure 3C. The fentanyl vaccine significantly decreased overall percent fentanyl choice as a function of time (Fig. 3C: F2.0,7.0 = 7.4, p = 0.02), with statistically detectable decreases beginning at week 11 and re-emerging weeks 17–27.
3.4. Antibody titer levels, affinities, and selectivity
As shown in Figure 4A, concentrations of fentanyl-specific antibody titers increased steadily with each vaccination until peak levels were reached at week 12 following the third vaccination. While titers gradually decreased thereafter, subsequent booster injections certainly prolonged the titer decline. Concurrently, fentanyl affinity increased with vaccination, quickly reaching <10 nM IC50 in all monkeys by week 12 through the rest of the study. Individual IC50 values determined using a 6-point fentanyl-dilution curve from blood samples collected during week 22 are shown in Table 1. Notably, the antibody-titer concentrations of subject 1541F were so low relative to the other monkeys that an accurate IC50 by SPR could not be determined at any timepoint besides week 12, where antibody-titer concentrations were highest for this monkey. However, an ELISA-based analysis with a lower limit of antibody detection revealed anti-fentanyl antibody affinities for subject 1541F that were comparable to the other monkeys at all timepoints (data not shown). Regarding selectivity, analysis of serum antibody selectivity revealed a lower binding relative to fentanyl (IC50 = 1–5 nM) of approximately 5–10 fold for acetylfentanyl, 50–100 fold for carfentanil and >10,000-fold for naloxone, buprenorphine, and hydrocodone (Table 2).
Figure 4:
Time course of fentanyl antibody levels and affinity measures in rhesus monkeys (n=5). Panel A shows fentanyl antibody levels in the blood in micrograms per milliliter as a function of experimental week. Panel B shows fentanyl antibody affinity (IC50 values, nM) measures as a function of experimental week. Filled symbols represent mean ±SEM and open symbols represent individual subject data.
Table 1.
Individual IC50 values (nM) determined using a 6-point fentanyl dilution curve from blood samples collected during week 22 of the vaccination experiment.
| Subject ID | 1540F | 1541F | 1525F | 1533M | 1536M |
| nM IC50 (SEM) | 4.67 (0.4) | N/A | 7.54 (1.26) | 1.45 (0.51) | 1.76 (0.33) |
Table 2.
Selectivity of antibodies for fentanyl, fentanyl analogues, and non-synthetic opioids. Analysis performed on serum antibody samples collected during week 22 of the vaccination experiment.
| Competitor Drug | [Competitor] (nM) | % Binding | Fen-BSA Estimated (nM) | IC50 Fold Selectivity |
|---|---|---|---|---|
| None | 0 | 100.0 | N/A | N/A |
| Fentanyl | 10 | 0.0 | 3 | 1 |
| Acetylfentanyl | 100 | 4.1 | 9 | 3 |
| Acetylfentanyl | 10 | 47.0 | 9 | 3 |
| Carfentanil | 100 | 43.1 | 43 | 14 |
| Carfentanil | 10 | 61.9 | 43 | 14 |
| Hydrocodone | 1000 | 99.2 | >10000 | >10000 |
| Naloxone | 1000 | 101.4 | >10000 | >10000 |
| Buprenorphine | 1000 | 104.0 | >10000 | >10000 |
3.5. Effects of a fentanyl-targeted vaccine on heroin choice
The effects of the fentanyl-targeted vaccine on choice of the structurally dissimilar MOR agonist heroin were determined in the three female monkeys, and results are shown in Figure 5. Heroin-vs-food choice was not significantly altered by fentanyl vaccine administration (Fig. 5A). The fentanyl vaccine also did not significantly alter total session, food, or heroin choices (Fig. 5B).
Figure 5:
Stability of heroin-vs-food choice pre- and post-fentanyl vaccine administration in rhesus monkeys (n=3). Top ordinate (A): percent heroin choice. Top abscissa: unit heroin dose in milligrams per kilogram per injection. Bottom ordinate (B): choices per session. Bottom abscissa: Reinforcer type. Points and bars in panels B and C represent mean ±SEM.
4. Discussion
The present study determined the effectiveness of a fentanyl-CRM197 conjugate vaccine to attenuate fentanyl self-administration in male and female rhesus monkeys over a 29-week experimental period. Vaccination significantly decreased fentanyl choice. The magnitude of this effect was similar to that produced by continuous 7-day treatment with a large dose of buprenorphine (0.032 mg/kg/h, similar to approximately 50 mg per day in a 70kg human). Although the onset of vaccine effectiveness to attenuate fentanyl self-administration was slower than the onset of buprenorphine effects, the vaccine displayed a longer duration of action once effectiveness was achieved. Furthermore, the fentanyl vaccine did not significantly alter heroin-vs-food choice, providing in-vivo evidence for vaccine selectivity, and corroborated by in vitro SPR experiments showing no serum antibody recognition of non-synthetic opioids. Given the immunogenic similarities between rhesus monkeys and humans (Palermo et al. 2013), these results suggest that similar vaccine effects may be achievable in humans, supporting the continued development and promotion of this fentanyl vaccine to clinical testing.
4.1. Effects of buprenorphine treatment on fentanyl choice
Because pharmacotherapies for Opioid Use Disorder are currently approved and available, new candidate Opioid Use Disorder medications should be compared to a current clinical standard of care to facilitate interpretation. We described the effectiveness of naltrexone maintenance to decrease heroin-vs-food choice in a previous study (Townsend et al. 2020c), and the present study compared a fentanyl vaccine effectiveness to continuous 7-day buprenorphine treatment. Following buprenorphine treatment, fentanyl choice decreased and there was a trend toward increased food choice, consistent with a previous rhesus monkey study evaluating repeated buprenorphine treatment effects on heroin-vs-food choice (Negus 2006). Furthermore, the present results are also consistent with human-laboratory studies and clinical trials demonstrating that buprenorphine maintenance decreases opioid choice over an alternative monetary reinforcer (Comer et al. 2002; Comer et al. 2005; Greenwald et al. 2002; Mello et al. 1982; Nasser et al. 2016). Collectively, these consistent results between preclinical and clinical evaluations of buprenorphine effects on choice between an opioid and an alternative non-drug reinforcer provide an empirical benchmark for interpreting effects of candidate Opioid Use Disorder medications, such as a fentanyl-targeted vaccine.
4.2. Vaccine effects on fentanyl choice
Active fentanyl vaccination decreased fentanyl choice and increased food choice in male and female rhesus monkeys. These results are consistent with recent evidence of a similar fentanyl-targeted vaccine eliminating fentanyl choice while increasing food choice in male and female rats (Townsend et al. 2019), as well as decreasing the antinociceptive potency of fentanyl in mice, rats, and rhesus monkeys (Bremer et al. 2016; Hwang et al. 2018b; Tenney et al. 2019; Townsend et al. 2019; Townsend et al. 2020a). Below we discuss the present results related to the magnitude of the vaccine effect, its time course, and its selectivity for the targeted opioid.
Regarding the magnitude of vaccine effect, a downward and rightward shift of the fentanyl-versus-food choice function was observed. Fentanyl choice was eliminated at week 23, (after initial vaccination and three follow-up boosters) in the four monkeys that maintained catheter patency. Previous research suggests that opioid-targeted vaccines decrease the potency of the targeted opioid (Townsend and Banks 2020), which would manifest as a parallel rightward shift of the dose-response function of the targeted opioid. To minimize the risk of fentanyl overdose, the current study did not evaluate whether higher fentanyl unit doses might surmount vaccine effectiveness. This decision limited our ability to precisely calculate the magnitude of this potency shift. Nevertheless, the magnitude of the vaccine effect was qualitatively similar to continuous treatment with 0.032 mg/kg/h buprenorphine in monkeys that were responsive to the fentanyl vaccine.
Although the magnitude of vaccine effects on fentanyl choice was similar to that of 0.032 mg/kg/h buprenorphine, the time courses of these treatment effects were different. For example, a significant decrease in group fentanyl choice following fentanyl vaccine administration was not detected until week 11. In contrast, 7 days of treatment with 0.032 mg/kg/h buprenorphine were sufficient to produce a significant decrease in fentanyl choice. This relatively lengthy induction period for the fentanyl vaccine suggests that the clinical deployment of opioid-targeted vaccines might require adjunctive medication (e.g., buprenorphine, naltrexone), at least during the induction period. However, whereas buprenorphine effects dissipated within days after treatment termination, fentanyl choice remained decreased in 4/5 of the monkeys for at least 14 weeks following administration of the final vaccine booster. In addition, high-affinity antibodies remained detectable up to week 56 in the three monkeys tested, although it is unclear whether these antibody levels would be sufficient to attenuate fentanyl reinforcement.
Regarding selectivity, the fentanyl-targeted vaccine did not significantly decrease heroin-vs-food choice in the three monkeys tested, demonstrating in vivo selectivity. This agrees with other evidence for in vivo selectivity for this vaccine. For example, in vitro binding experiments revealed good cross reactivity of serum antibodies to related fentanyl analogues but no detectable recognition of structurally unrelated opioids. This degree of selectivity can be argued to be both a limitation and a strength of opioid-targeted vaccines as Opioid Use Disorder medications. For example, an individual could theoretically circumvent the effects of a fentanyl-targeted vaccine by consuming other MOR agonists that do not share a similar structure to fentanyl (e.g., heroin, oxycodone, etc.). On the other hand, antibodies elicited by the fentanyl vaccine would not be expected to affect the potency of methadone, buprenorphine, or naltrexone for treatment of Opioid Use Disorder or of other clinically approved opioids for treatment of other conditions such as pain (Bremer et al. 2016). For example, an Opioid Use Disorder patient receiving methadone-maintenance therapy could be administered a fentanyl-targeted vaccine without impacting methadone effectiveness or presumably precipitating opioid withdrawal, which would be expected following naltrexone or buprenorphine administration. Thus, the fentanyl vaccine evaluated in the present study could be used as a harm reduction therapy to mitigate the risk of fentanyl-induced overdose during a relapse event. Nevertheless, we and other groups are also investigating the feasibility of vaccinating against multiple opioids of abuse (e.g., heroin, fentanyl) (Hwang et al. 2018a; Hwang et al. 2018b; Natori et al. 2019; Pravetoni et al. 2012), which could theoretically confer protection against several illicit opioids while also permitting the clinical use of structurally dissimilar opioids for the management of pain or the treatment of Opioid Use Disorder. However, a technical challenge to vaccinating against multiple opioids is that it appears to be more difficult to block the behavioral effects of opioids that are less potent than fentanyl (e.g., heroin, morphine, oxycodone, hydrocodone), as a higher titer of high-affinity antibodies is required to sequester a behaviorally relevant dose of these less potent opioids.
Individual differences in fentanyl vaccine responsiveness were observed between monkeys. For example, the most rapid vaccine response was observed in monkey 1540F with fentanyl choice eliminated by week 6 after just one booster. In contrast, total percent fentanyl choice never decreased below 25% during the 29-week experiment in monkey 1541F. Consistent with these individual differences in behavior, serum antibody concentrations were generally highest in monkey 1540F and lowest in monkey 1541F. Moreover, 1540F was also more sensitive to buprenorphine treatment than 1541F. Overall, these results highlight the utility of nonhuman primates to evaluate individual differences in candidate medication responsiveness and support the concept of individualized medicine approaches (Nader 2016).
4.3. Conclusions
Published research on this vaccine across the last four years have consistently demonstrated its effectiveness to attenuate fentanyl-mediated effects across multiple endpoints, species, and laboratories. Adding to these encouraging data, the current study provides evidence that this vaccine can confer buprenorphine-like protection against the reinforcing effects of fentanyl, albeit with a slower onset and, importantly, a longer duration of action than buprenorphine. These findings are particularly encouraging because they were observed in male and female rhesus monkeys, the most concordant preclinical model of human immunology available for such studies. Collectively, these results support the clinical evaluation of this vaccine as a strategy to combat the synthetic opioid crisis.
Supplementary Material
Article Highlights.
A fentanyl-targeted vaccine decreases choice of intravenous fentanyl injections over an alternative food reinforcer in male and female rhesus monkeys.
The effectiveness of the fentanyl-targeted vaccine to attenuate fentanyl-vs.-food choice was similar to continuous buprenorphine treatment, although the duration of action was greater for the vaccine.
This vaccine formulation was stored at room temperature without any apparent degradation in its effectiveness.
Acknowledgments
Funding Statement: Research was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Numbers UH3DA041146 and F32DA047026. The content is the sole responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosures: Research was supported by the National Institutes of Health grants [F32DA047026, UH3DA041146]. The National Institute on Drug Abuse had no role in the writing or decision to submit the manuscript for publication. The manuscript content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest Disclosure
PTB and NTJ are employees of Cessation Therapeutics LLC. Cessation has a licensed a fentanyl conjugate vaccine patent from Scripps Research Institute for which KDJ is the inventor.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anton B, Leff P (2006) A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents Vaccine 24:3232–3240 doi: 10.1016/j.vaccine.2006.01.047 [DOI] [PubMed] [Google Scholar]
- Banks ML, Blough BE (2015) Effects of environmental maniuplations and bupropion and risperidone treatments on choice between methamphetamine and food in rhesus monkeys Neuropsychoharmacology 40:2198–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS (2011) Effects of monoamine releasers with varying selectivity for releasing dopamine/norepinephrine versus serotonin on choice between cocaine and food in rhesus monkeys Behav Pharmacol 22:824–836 doi: 10.1097/FBP.0b013e32834d63ac [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer PT, Kimishima A, Schlosburg JE, Zhou B, Collins KC, Janda KD (2016) Combatting Synthetic Designer Opioids: A Conjugate Vaccine Ablates Lethal Doses of Fentanyl Class Drugs Angew Chem Int Ed Engl 55:3772–3775 doi: 10.1002/anie.201511654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fischman MW (2002) Depot naltrexone: long-lasting antagonism of the effects of heroin in humans Psychopharmacology (Berl) 159:351–360 doi: 10.1007/s002130100909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Walker EA, Collins ED (2005) Buprenorphine/naloxone reduces the reinforcing and subjective effects of heroin in heroin-dependent volunteers Psychopharmacology (Berl) 181:664–675 doi: 10.1007/s00213-005-0023-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Schuh KJ, Hopper JA, Schuster CR, Johanson CE (2002) Effects of buprenorphine sublingual tablet maintenance on opioid drug-seeking behavior by humans Psychopharmacology (Berl) 160:344–352 doi: 10.1007/s00213-001-0975-0 [DOI] [PubMed] [Google Scholar]
- Hedegaard H, Minino AM, Warner M (2020) Drug Overdose Deaths in the United States, 1999–2018 NCHS Data Brief:1–8 [PubMed] [Google Scholar]
- Hwang CS et al. (2018a) Enhancing Efficacy and Stability of an Antiheroin Vaccine: Examination of Antinociception, Opioid Binding Profile, and Lethality Mol Pharm 15:1062–1072 doi: 10.1021/acs.molpharmaceut.7b00933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CS, Smith LC, Natori Y, Ellis B, Zhou B, Janda KD (2018b) Improved Admixture Vaccine of Fentanyl and Heroin Hapten Immunoconjugates: Antinociceptive Evaluation of Fentanyl-Contaminated Heroin ACS Omega 3:11537–11543 doi: 10.1021/acsomega.8b01478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis BP, Holtyn AF, Subramaniam S, Tompkins DA, Oga EA, Bigelow GE, Silverman K (2018) Extended-release injectable naltrexone for opioid use disorder: a systematic review Addiction 113:1188–1209 doi: 10.1111/add.14180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars SG, Ondocsin J, Ciccarone D (2018) Sold as Heroin: Perceptions and Use of an Evolving Drug in Baltimore, MD J Psychoactive Drugs 50:167–176 doi: 10.1080/02791072.2017.1394508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC (1982) Buprenorphine effects on human heroin self-administration: an operant analysis J Pharmacol Exp Ther 223:30–39 [PubMed] [Google Scholar]
- Miller CH, Torten M, Benjamini E (1975) Prevention of the analgesic effect of fentanyl by immunologic manipulations Proc West Pharmacol Soc 18:192–195 [PubMed] [Google Scholar]
- Nader MA (2016) Animal models for addiction medicine: From vulnerable phenotypes to addicted individuals Prog Brain Res 224:3–24 doi: 10.1016/bs.pbr.2015.07.012 [DOI] [PubMed] [Google Scholar]
- Nasser AF et al. (2016) Sustained-Release Buprenorphine (RBP-6000) Blocks the Effects of Opioid Challenge With Hydromorphone in Subjects With Opioid Use Disorder J Clin Psychopharmacol 36:18–26 doi: 10.1097/JCP.0000000000000434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natori Y, Hwang CS, Lin L, Smith LC, Zhou B, Janda KD (2019) A chemically contiguous hapten approach for a heroin-fentanyl vaccine Beilstein J Org Chem 15:1020–1031 doi: 10.3762/bjoc.15.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS (2006) Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone J Pharmacol Exp Ther 317:711–723 doi: 10.1124/jpet.105.095380 [DOI] [PubMed] [Google Scholar]
- Palermo RE, Tisoncik-Go J, Korth MJ, Katze MG (2013) Old world monkeys and new age science: the evolution of nonhuman primate systems virology ILAR J 54:166–180 doi: 10.1093/ilar/ilt039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichini S, Solimini R, Berretta P, Pacifici R, Busardo FP (2018) Acute Intoxications and Fatalities From Illicit Fentanyl and Analogues: An Update Ther Drug Monit 40:38–51 doi: 10.1097/FTD.0000000000000465 [DOI] [PubMed] [Google Scholar]
- Pravetoni M et al. (2012) Co-administration of morphine and oxycodone vaccines reduces the distribution of 6-monoacetylmorphine and oxycodone to brain in rats Vaccine 30:4617–4624 doi: 10.1016/j.vaccine.2012.04.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh MD et al. (2019) A Fentanyl Vaccine Alters Fentanyl Distribution and Protects against Fentanyl-Induced Effects in Mice and Rats J Pharmacol Exp Ther 368:282–291 doi: 10.1124/jpet.118.253674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE et al. (2013) Dynamic vaccine blocks relapse to compulsive intake of heroin Proc Natl Acad Sci U S A 110:9036–9041 doi: 10.1073/pnas.1219159110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe GN et al. (2011) A vaccine strategy that induces protective immunity against heroin J Med Chem 54:5195–5204 doi: 10.1021/jm200461m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney RD et al. (2019) Vaccine blunts fentanyl potency in male rhesus monkeys Neuropharmacology 158:107730 doi: 10.1016/j.neuropharm.2019.107730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Banks ML (2020) Preclinical Evaluation of Vaccines to Treat Opioid Use Disorders: How Close are We to a Clinically Viable Therapeutic? CNS Drugs 34:449–461 doi: 10.1007/s40263-020-00722-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA et al. (2019) Conjugate vaccine produces long-lasting attenuation of fentanyl vs. food choice and blocks expression of opioid withdrawal-induced increases in fentanyl choice in rats Neuropsychopharmacology doi: 10.1038/s41386-019-0385-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA et al. (2020a) Evaluation of a Dual Fentanyl/Heroin Vaccine on the Antinociceptive and Reinforcing Effects of a Fentanyl/Heroin Mixture in Male and Female Rats ACS Chem Neurosci 11:1300–1310 doi: 10.1021/acschemneuro.0c00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Negus SS, Banks ML (2020b) Medications Development for Treatment of Opioid Use Disorder Cold Spring Harb Perspect Med doi: 10.1101/cshperspect.a039263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Negus SS, Poklis JL, Banks ML (2020c) Lorcaserin maintenance fails to attenuate heroin vs. food choice in rhesus monkeys Drug Alcohol Depend 208:107848 doi: 10.1016/j.drugalcdep.2020.107848 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.