Introduction
Chimeric antigen receptor (CAR) T cell therapy is a paradigm shift in the management of B cell non-Hodgkin lymphomas (NHL) 1. Two CAR T cell products, axicabtagene ciloleucel (axi-cel, Kite, Gilead) and tisagenlecleucel (Novartis), have shown significant responses in relapsed/ refractory B cell NHL and are now FDA approved for this indication 2, 3. Neither of the pivotal trials included patients who underwent CAR T cell therapy following allogeneic hematopoietic cell transplantation (alloHCT). We hereby share our experience in four patients who received CAR T cell therapy with axi-cel, following standard lymphodepletion with fludarabine and cyclophosphamide, for relapsed NHL after alloHCT, where T cells were harvested from the recipient following relapse.
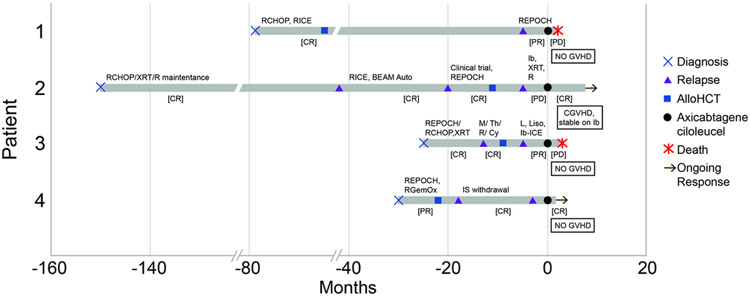
Written informed consent for treatment was obtained, and approval for this retrospective review was obtained from the Institutional Review and Privacy Board. The details of CAR T cell therapy and alloHCT are outlined in Table 1 and timeline of events is illustrated in Figure 1. All responses used Deauville criteria.
Table 1:
Patient and Treatment Characteristics*
| Patient | Age at CAR T/ Gender |
Diagnosis | Donor Type | Graft source |
Conditioning regimen with alloHCT |
GVHD prophylaxis/ GVHD at CAR T |
Interval/ lines of therapy between alloHCT and CAR T (days) |
Donor chimerism at the time of mononuclear apheresis |
CRS/ Neurotoxicity |
Response at 1 month |
B cell recovery |
Status at last follow up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38/ M | T cell rich DLBCL | 10/10 MUD | Peripheral blood | Flu, Cy, Thio, 400cGy TBI | Tacrolimus, Methotrexate/ none | 1,914/ one | T cell chimerism 100% donor | Grade 2/ Grade 2 | PR | Not achieved until last follow up | Dead (106 days post CAR T) from disease |
| 2 | 55/ M | Non-GCB DLBCL | MMRD (haploidentical from cousin) | Bone marrow | Flu Mel, Thiotepa | Post-transplant Cy, Tacrolimus, Mycophenolate/ chronic GVHD stable on ibrutinib | 269/ two | Whole blood 100% donor (T cell subset chimerism not available) | None/ none | CR | B cell recovery at 9 months | Alive with CR (270 days post CAR T) |
| 3 | 56/ M | Non-GCB DLBCL | 10/10 MUD | Peripheral blood | Thio, Bu, Cy, Rituximab | Tacrolimus, Alemtuzumab/ none | 291/ three | T cell chimerism 18% donor | None/ none | POD | Not achieved until last follow up | Dead (77 days post CAR T) from disease |
| 4 | 66/ F | Double hit DLBCL | 9/10 MMUD | Peripheral blood | Flu, Mel | Tacrolimus, Methotrexate/ none | 694/ none | Bone marrow (unsorted) 100% donor | Grade 2/ none | CR | Not available | Alive with CR (112 days post CAR T) |
All patients received Flu/ Cy lymphodepletion and axicabtagene ciloleucel (2 x 108 cells)
alloHCT, Allogeneic hematopoietic cell transplantation; Bu, Busulfan; CAR-T, Chimeric Antigen Receptor T cell therapy; CR, complete response; CRS, Cytokine release syndrome; Cy, Cyclophosphamide; DLBCL, Diffuse large B cell lymphoma; F, Female; Flu, Fludarabine; GCB, Germinal Center B cell like; GVHD, Graft versus host disease; M, Male; Mel, Melphalan; MMRD, Mismatched related donor; MMUD, Mismatched unrelated donor; MUD, Matched unrelated donor; POD, Progression of disease; PR, Partial response; TBI, Total Body Irradiation; Thio, thiotepa
Figure 1: Timeline of events for all patients.
CGVHD, chronic graft versus host disease; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; CR, complete response; CY, cyclophosphamide; EPOCH, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; Gem, gemcitabine; GVHD, graft versus host disease; lb, ibrutinib; ICE, ifosfamide, carboplatin and etoposide; IS, immunosuppression therapy; L, lenalidomide; Liso, Lisocabtagene Maraleucel; M, methotrexate; Ox, oxaliplatin; PR, partial response; PD, progressive disease; R, rituximab; Th, Thiotepa; XRT, radiation therapy
Case Descriptions
Patient 1:
A 33-year-old man was diagnosed with T cell rich B cell lymphoma with significant supra- and infra-diaphragmatic lymphadenopathy, splenomegaly and B-symptoms. He was treated with rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP) [progression of disease (POD)], rituximab, ifosfamide, carboplatin and etoposide (R-ICE) [complete response (CR)] and then alloHCT from a matched unrelated donor 14 months after diagnosis [CR]. Post-alloHCT course was complicated by moderate chronic graft versus host disease (GVHD) managed with corticosteroids and tacrolimus. He relapsed 5 years later (off immunosuppression by then) with diffuse lymphadenopathy, and received rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin (R-EPOCH) [partial response (PR)], followed by axi-cel. He developed grade 2 cytokine release syndrome (CRS) and grade 2 neurotoxicity (ASBMT consensus grading4) that resolved with tocilizumab and dexamethasone, respectively. He had a PR on day +30, but day +60 imaging revealed POD and he died day +108 after CAR T cell infusion. There was no reappearance of GVHD following CAR T cells.
Patient 2:
A 45-year-old man was diagnosed with right submandibular diffuse large B cell lymphoma (DLBCL) Ann Arbor Stage 1A, non-germinal center cell of origin (non-GCB). He was treated with R-CHOP and radiation to the right neck [CR] followed by rituximab maintenance for 2 years. Late local relapse was noted 9 years after initial presentation, managed with high-dose therapy and autologous HCT [CR]. Over a year later, relapse was noted with diffuse lymphadenopathy treated with R-EPOCH [CR], followed by haploidentical alloHCT with post-transplant cyclophosphamide. The course was complicated by stage 2 lower gastrointestinal GVHD and later, chronic GVHD of the oral cavity, skin, joints, and liver. This was managed with systemic corticosteroids, tacrolimus, and later ibrutinib, with resolution of symptoms. Ibrutinib was started around the time of relapse, 6 months after alloHCT, with an aim to target both the disease and GVHD. PET imaging showed uptake in a lacrimal gland mass causing diplopia (radiated), and diffuse lymphadenopathy. He had systemic POD two months after initiation of ibrutinib and received rituximab [POD] as a bridge to axi-cel. Ibrutinib continued for a total 3 months until the initiation of lymphodepleting chemotherapy. He did not experience CRS or neurotoxicity. Ibrutinib was resumed two weeks post-CAR T cells due to fluctuations in transaminases, without elevation in bilirubin, attributed to azole anti-fungal agents or possible GVHD. Day +30 disease evaluation post-CAR T cell revealed no evidence of disease (Supplementary Figure 1) and he remains disease-free at 9 months post treatment. Liver transaminases have normalized on ibrutinib and low dose tacrolimus. Other GVHD symptoms remain completely resolved.
Patient 3:
A 56-year-old man was diagnosed with DLBCL, non-GCB subtype, with a conglomerate mass in the abdomen, treated with R-EPOCH subsequently changed to R-CHOP followed by radiation to the small residual abdominal mass [CR]. CNS relapse, a year later, was treated with rituximab, methotrexate, cytarabine and thiotepa [CR]. This was followed by alloHCT from an unrelated donor, but he relapsed with a PET avid abdominal mass 4 months later. No GVHD was reported in this interval. He received lenalidomide [POD] followed by fludarabine and cyclophosphamide lymphodepletion with CD19 CAR T cells (Lisocabtagene Maraleucel) on a clinical trial (NCT03483103) [POD]. He was then treated with ibrutinib with ICE [PR] followed by treatment with axi-cel within 2 months. Ibrutinib was given for over 1 month and discontinued prior to leukapheresis. No CRS or neurotoxicity was reported. Day +30 PET showed POD, and he died on day +77 post-CAR T cells, due to sepsis in the setting of rapid POD. No GVHD was reported after CAR T cell infusion.
Patient 4:
A 63-year-old woman was diagnosed with myc-rearranged DLBCL, transformed from a prior marginal zone lymphoma, with diffuse lymphadenopathy, splenomegaly and bone marrow involvement. She was treated with R-EPOCH [CR] but relapsed within 3 months of treatment with multiple PET avid subcutaneous nodules and inguinal lymphadenopathy. Treatment was initiated with rituximab, gemcitabine and oxaliplatin [PR]. This was followed by unmodified mismatched unrelated donor alloHCT complicated by upper gastrointestinal GVHD, resolved with budesonide. Relapse was noted with another subcutaneous nodule four months later, that responded to withdrawal of immunosuppression without worsening GVHD. However, 15 months later, relapse was noted with subcutaneous nodules on bilateral upper extremities, treated with axi-cel nearly two years after alloHCT. This was complicated by grade 2 CRS requiring tocilizumab but no new GVHD symptoms were reported. CR was noted at day +30 and she remains in remission four months after CAR T cell infusion.
Discussion
The question of safety of CAR T cells following prior alloHCT is clinically important but remains relatively under-studied so far. Herein we report our experience with recipient-derived CAR T cell therapy with axi-cel in patients with relapsed DLBCL following alloHCT. While ours is the first report describing safety of recipient derived (or “pseudo donor-derived”) CAR T cells post alloHCT in DLBCL, there are reports of donor-derived CAR T cells administered in various hematological malignancies (Table 2). Brudno et al. reported use of a single dose of allogeneic CAR T cells derived from the patient’s alloHCT donor 5. Eight out of 20 patients responded, including two CRs, without any new onset acute GVHD, despite 14 having had had GVHD following alloHCT. In another study, 19 patients received planned adjuvant donor derived CAR T cells after alloHCT, generated using the sleeping beauty transposon 6. Three patients developed acute GVHD. In a third report, donor-derived, virus-specific T cells engineered to express CD19-targeted CAR showed no GVHD in patients who relapsed after alloHCT 7. These data, along with our report, collectively suggest safety and feasibility to use CAR T cell therapy following alloHCT. A recent report studying factors associated with durable remission after CAR T cell therapy for NHL, included patients with a prior alloHCT, but did not discuss GVHD occurrence 8.
Interestingly, the 4 CAR T cell constructs reported above demonstrating safety of CAR T cells following a prior alloHCT [REF], including our series, used CD28 as co-stimulation domain. In a pre-clinical mouse model, Ghosh et al. showed that donor derived CD19 CAR T cells co-stimulated by CD28 can exert anti-tumor effect without developing GVHD 9. This is possible due to cumulative CAR and alloreactive T cell receptor signaling, resulting in exhaustion and hence, deletion of alloreactive CAR T cells, while the non-alloreactive CAR T cells retain activity against CD19 targets. In contrast, in another report from the NCI, CD28 based donor derived CD19 CAR T cells studied in immunocompetent murine models showed leukemia responses but also the potential for lethal GVHD, especially in the presence of active leukemia 10. Whether this translates into similar clinical findings in humans remains to be studied.
It should be noted that patients with B cell acute lymphoblastic leukemia with prior alloHCT have been included in CD19 CAR T cell studies using constructs with co-stimulation with 4-1BB or CD28 11-13, without any evidence of development or worsening of GVHD.
Patient 2 in our series has an ongoing response, 9 months after CAR T cells in the setting of prior haploidentical alloHCT. This patient had also received ibrutinib to address chronic GVHD following alloHCT and showed stabilization of chronic GVHD 14. Although not seen in this patient, ibrutinib has been shown to induce disease responses in 37% of patients with non-GCB subtype of DLBCL with higher responses in patients with concomitant BCR and MYD88 mutations 15. Additionally, he received ibrutinib until collection of mononuclear cells for CAR T cell production. This strategy, in vitro, has been shown to improve CAR T expansion in a chronic lymphocytic leukemia model, associated with decreased PD1 on T cells and decreased CD200 on B cells 16. Additionally, in xenograft models of mantle cell lymphoma, combined treatment with ibrutinib and CD19 CAR T cells has shown more durable responses compared to CAR T alone17. This patient has an ongoing response after CAR T cells while being on ibrutinib for chronic GVHD, with a prior progression on ibrutinib. Whether ibrutinib along with CAR T cells would augment efficacy or survival is currently being studied in clinical studies.
While this report is limited by a small sample size, our experience thus far suggests that use of CAR T cells after alloHCT is generally safe and doesn’t appear to worsen GVHD. Given the sample size, we are unable to analyze factors associated with response. When standard risk factors were analyzed in patients treated in the ZUMA-1 trial, none were identified as associated with disease response, including disease bulk 2. However, we cannot exclude low T cell donor chimerism at the time of apheresis or immune rejection as a mechanism of failure for Patient 3 who received two CAR T cell constructs using the same scFv. Concerns about T cell function, persistence and exhaustion post-alloHCT, especially in the setting immunosuppression therapy, remain to be addressed. Larger prospective studies will be needed to confirm these findings and careful monitoring should be pursued until more data becomes available.
Supplementary Material
Supplementary Figure 1: PET Images for Diffuse Large B Cell Lymphoma responses with Chimeric Antigen Receptor T cell Therapy in Patient 2
Table 2:
Studies with CAR-T cell after allogeneic hematopoietic cell transplantation including B cell lymphoma patients
Acknowledgments
Funding: This research was supported in part by NIH/NCI Cancer Center Support Grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- alloHCT
Allogeneic hematopoietic cell transplantation
- axi-cel
axicabtagene ciloleucel
- CAR
Chimeric Antigen Receptor
- CR
Complete response
- CRS
Cytokine release syndrome
- DLBCL
Diffuse large B cell lymphoma
- GCB
Germinal center B cell
- GVHD
Graft versus host disease
- NHL
Non Hodgkin’s lymphoma
- POD
Progression of disease
- PR
Partial Response
- R-CHOP
Rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone
- R-EPOCH
Rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin
- R-ICE
Rituximab, ifosfamide, carboplatin and etoposide
Footnotes
Disclosures:
Tania Jain – No conflict of interest
Craig Sauter - Consultant on advisory boards for: Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Precision Biosciences, Kite, a Gilead Company and GSK. Research funds for investigator-initiated trials from: Juno Therapeutics and Sanofi-Genzyme.
Gunjan Shah - Research funding from Janssen and Amgen
Molly Maloy – No conflict of interest
Jason Chan – No conflict of interest
Michael Scordo – Consultancy: Angiocrine Bioscience, Inc., McKinsey & Company
Scott Avecilla - Honoraria for presenting a project in partnership with Abbott Laboratories
Yakup Batlevi – No conflict of interest
Parastoo Dahi – No conflict of interest
Connie Batlevi – Research funding from Epizyme, Novartis, Janssen, BMS, Miragen, Mediimmune; Consultancy from Defined Health, GLG, Guidepoint Global; Honoraria from Dava Oncology
M Lia Palomba – Advisory Board for Celgene, Consultant for Merck and Pharmacyclics
Sergio Giralt – Advisory Board for Amgen, Actinuum, Celgene, Johnson & Johnson, Jazz pharmaceutical, Takeda, Novartis, Kite, Spectrum Pharma; Research funding from Amgen, Actinuum, Celgene, Johnson & Johnson, Miltenyi, Takeda
Miguel-Angel Perales - Honoraria from Abbvie, Bellicum, Bristol-Myers Squibb, Incyte, Merck, Novartis, Nektar Therapeutics, and Takeda. He serves on DSMBs for Servier and Medigene, and the scientific advisory boards of MolMed and NexImmune. Research support for clinical trials from Incyte, Kite (Gilead) and Miltenyi Biotec.
References:
- 1.Perales MA, Kebriaei P, Kean LS, Sadelain M. Building a Safer and Faster CAR: Seatbelts, Airbags, and CRISPR. Biol Blood Marrow Transplant 2018. January; 24(1): 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017. December 28; 377(26): 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak O, et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N Engl J Med 2017. December 28; 377(26): 2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASBMT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant 2018. December 25. [DOI] [PubMed] [Google Scholar]
- 5.Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. J Clin Oncol 2016. April 1; 34(10): 1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kebriaei P, Singh H, Huls MH, Figliola MJ, Bassett R, Olivares S, et al. Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. J Clin Invest 2016. September 1; 126(9): 3363–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood 2013. October 24; 122(17): 2965–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirayama AV, Gauthier J, Hay KA, Voutsinas JM, Wu Q, Gooley T, et al. The response to lymphodepletion impacts PFS in aggressive non-Hodgkin lymphoma patients treated with CD19 CAR-T cells. Blood 2019. February 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh A, Smith M, James SE, Davila ML, Velardi E, Argyropoulos KV, et al. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat Med 2017. February; 23(2): 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacoby E, Yang Y, Qin H, Chien CD, Kochenderfer JN, Fry TJ. Murine allogeneic CD19 CAR T cells harbor potent antileukemic activity but have the potential to mediate lethal GVHD. Blood 2016. March 10; 127(10): 1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015. February 7; 385(9967): 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 2018. February 1; 378(5): 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med 2018. February 1; 378(5): 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 2017. November 23; 130(21): 2243–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 2015. August; 21(8): 922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng Z, Barrett DM, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood 2016. March 3; 127(9): 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruella M, Kenderian SS, Shestova O, Fraietta JA, Qayyum S, Zhang Q, et al. The Addition of the BTK Inhibitor Ibrutinib to Anti-CD19 Chimeric Antigen Receptor T Cells (CART19) Improves Responses against Mantle Cell Lymphoma. Clin Cancer Res 2016. June 1; 22(11): 2684–2696. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: PET Images for Diffuse Large B Cell Lymphoma responses with Chimeric Antigen Receptor T cell Therapy in Patient 2