Abstract
Non-conductive olfactory dysfunction (OD) is an important extra-pulmonary manifestation of coronavirus disease 2019 (COVID-19). Olfactory bulb (OB) volume loss and olfactory network functional connectivity (FC) defects were identified in two patients suffering from prolonged COVID-19-related OD. One patient received olfactory treatment (OT) by the combination of oral vitamin A and smell training via the novel electronic portable aromatic rehabilitation (EPAR) diffusers. After four-weeks of OT, clinical recuperation of smell was correlated with interval increase of bilateral OB volumes [right: 22.5 mm3 to 49.5 mm3 (120%), left: 37.5 mm3 to 42 mm3 (12%)] and improvement of mean olfactory FC [0.09 to 0.15 (66.6%)].
Keywords: COVID-19, olfactory dysfunction, vitamin A, smell training, resting-state fMRI
1. Introduction
Non-conductive olfactory dysfunction (OD) is an important extra-pulmonary manifestation of coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Local experience and multiple cross-sectional studies revealed that up to 60% of COVID-19 patients suffer from OD, with a female predominance [1,2]. The severity and duration of COVID-19-related OD varies but olfactory chemosensory disturbances may persist beyond four weeks [3].
The neuroinvasive properties of SARS-CoV-2 at the olfactory epithelium (OE) has been confirmed in human autopsy specimens [4,5,6]. Furthermore, the neurotropic properties of SARS-CoV-2 have been demonstrated in multiple biological models, such as U251 human glioblastoma cell line, Tuj1+ Pax6+Nestin+ human brain organoids, and golden Syrian hamster models [7,8,9]. Direct invasion of SARS-CoV-2 at the delicate neural-mucosal interface between the olfactory and central nervous systems (CNS) may be the underlying pathophysiological cause of COVID-19-related OD.
OE is a pseudostratified columnar epithelium which consists of olfactory sensory neurons, non-neuronal supporting cells (for example: sustentacular cells, Bowman’s glands and ducts), as well as progenitor and stem cells. The OE is maintained continuously by globose basal cells (GBCs) throughout life. After substantial tissue injury, OE regeneration is further supported by the differentiation of multipotent horizontal basal cells (HBCs) [10,11]. Therefore, the regenerative potentials and neuroplasticity of OE may be harnessed in the treatment against COVID-19-related OD.
In this case report, one patient received oral vitamin A (VitA) in combination with smell training (ST) as olfactory treatment (OT) against prolonged COVID-19-related OD. VitA supplement was selected as a metabolic enhancer for OE regeneration based on previous studies [12,13]. In addition, the novel electronic portable aromatic rehabilitation (EPAR) diffuser (SOVOS, Hong Kong, China) technology was adopted for superior olfactory stimulation as an enhancement to conventional ST [14,15].
2. Case Presentation
In this case study, two patients diagnosed with prolonged COVID-19-related OD were recruited to undergo structural and resting-state functional MRI (rs-fMRI) brain scans. Two non-clinical staff were invited to participate as controls (Supplementary Table S1). COVID-19 patients received nasoendoscopic assessments. Detailed functional olfactory evaluations are outlined in Supplementary Tables S2 and S4. SARS-CoV-2 virologic assessments can be found in Supplementary Note S1.
All participants underwent MRI brain scans using a 1.5 T MR scanner (SIGNA; GE Healthcare, Chicago, IL, USA). Structural and three-dimensional (3D) arterial spin labeling images were acquired. Volumetric analyses of the olfactory bulbs (OB) were performed. MR spectroscopy was performed using the single voxel point resolved spectroscopy (PRESS) at the gyrus rectus (GR) and superior frontal cortex (SFC).
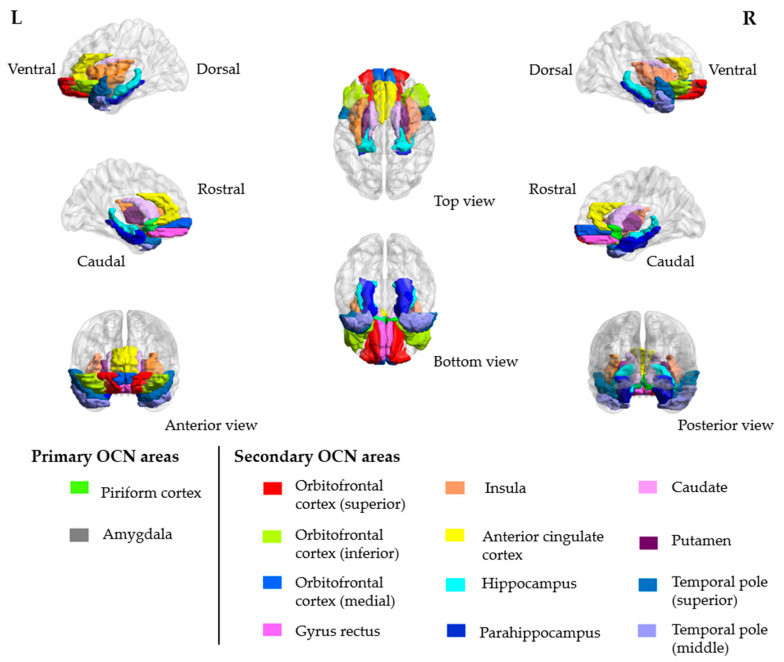
rs-fMRI brain scans were collected using a gradient-echo echo-planar sequence sensitive to blood-oxygen-level-dependent (BOLD) contrast. Hypothesis-driven region of interest (ROI) approach was applied [16,17,18]. The olfactory network seed regions were defined at the caudate nuclei [19,20]. With reference to the automated anatomical labeling template, rs-fMRI data were segmented into 90 regions, of which 28 out of 90 regions were associated with the functional olfactory cortical networks (OCN; Figure 1). The functional connectivity (FC) between seed regions and OCN ROIs were obtained by extracting the average time series from individual ROIs and calculating the correlation. Detailed methodology of the MRI data acquisition can be found in Supplementary Note S2.
Figure 1.
Three-dimensional (3D) representations of the primary and secondary olfactory cortical network (OCN) areas.
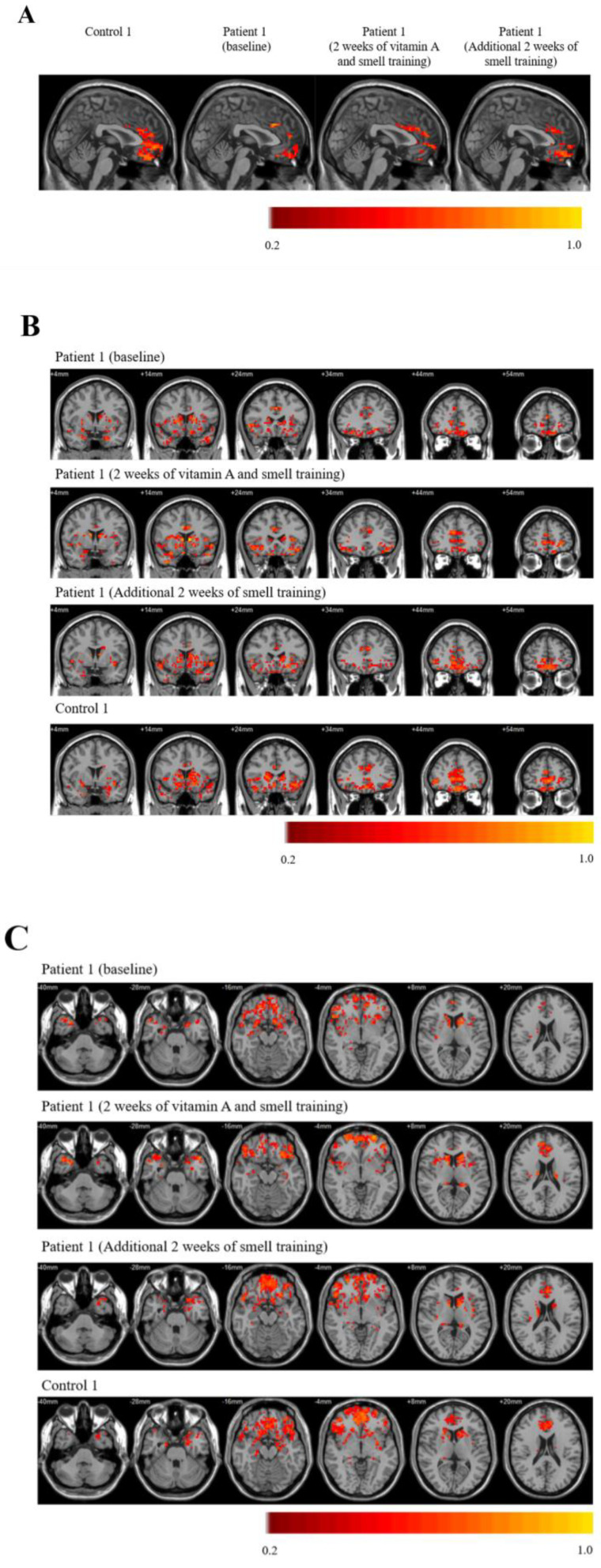
Serial rs-fMRI FC images of the olfactory network are shown (Figure 2A–C). Olfactory network FC were reduced in COVID-19 patients when compared to controls. FC were homogenously decreased in the right and left caudate seed regions for patient 1 (P1) and patient 2 (P2), respectively (Supplementary Table S5).
Figure 2.
Olfactory network functional connectivity (FC) images using the left caudate as the seed region, as represented in the (A) sagittal, (B) coronal, and (C) axial planes.
Structural MRI brain scans showed OB volume defects in COVID-19 patients (Supplementary Table S6) when compared to healthy controls. MR spectroscopy confirmed neuronal loss (Supplementary Figure S1C), as evident by reduction of N-acetylaspartate levels at the GR and SFC [21].
After baseline rs-fMRI assessments, OT was initiated for patient 1 (P1). Oral VitA 25,000 IU [7500 µg retinol activity equivalents (RAE); Carlson Laboratories, Arlington Heights, IL, USA] soft gels were prescribed daily for two weeks in combination with smell training (ST) thrice daily for four weeks via the novel EPAR diffusers. Methodological details of ST can be found in Supplementary Note S3 and Supplementary Figure S2A.
Clinical improvements in olfaction were documented serially by subjective questionnaires (Supplementary Tables S2 and S3) and objective olfactory quantitation (Supplementary Table S1) for P1 after OT. Butanol threshold test (BTT) revealed improvement in olfactory sensitivity from more than 4% to 1%. Smell identification test (SIT) confirmed categorical improvement in olfaction from anosmia to severe microsmia. Notably, there were measurable increase in the bilateral OB volumes [Supplementary Table S6; right OB: 22.5 mm3 to 49.5 mm3 (120%), left OB: 37.5 mm3 to 42 mm3 (12%)] in P1 after OT.
Olfactory recovery in P1 was correlated with significant improvements in the mean olfactory FC [0.09 to 0.15 (66.6%); Supplementary Table S5], when compared with pre-OT baseline scans. FC improvements were documented in both primary [left piriform cortex (PC), and right amygdala] and secondary (bilateral GR, and medial orbitofrontal cortices) OCN areas (Figure 2A–C). Importantly, there were corresponding increase in regional cerebral blood flow at the bilateral PC (left: 17.43%, right: 10.99%) and multiple secondary OCN regions (Supplementary Table S7), demonstrating robust neurovascular coupling.
3. Discussion
In this case report, COVID-19-related OD was correlated with OB volume loss and abnormal MR spectroscopy findings, indicating neuronal destruction in the CNS secondary to COVID-19 infection. Furthermore, rs-fMRI demonstrated olfactory FC impairments, thereby providing further insights into the underlying neuropathological process of COVID-19-related OD.
In the treatment for prolonged COVID-19-related OD, P1 was successfully challenged with oral VitA and ST, as demonstrated by (1) interval improvements in olfactory function; (2) structural restoration in OB volumes; (3) olfactory network recovery; and (4) corresponding regional increase in cerebral perfusion.
The long-term outcomes of patients suffering from prolonged COVID-19-related OD remain unknown [22]. Extended longitudinal follow-up studies will be needed to determine the prognosis of olfactory chemosensory deficits for these patients. The therapeutic efficacy of oral VitA and ST in the treatment against prolonged COVID-19-related OD should also be validated in large scale randomized–controlled trials, which would differentiate between spontaneous natural recovery and treatment cure.
The integrity of the mammalian OE is preserved by the mitotically active GBCs and dormant HBCs [10,11]. The dormancy of HBCs is maintained by Notch1 signaling which is correlated with the expression of transcription factor protein 63 (ΔNp63α) [23,24]. During extensive OE injury, Notch1 signals and ΔNp63α expressions are downregulated, leading to HBCs activation and differentiation.
We hypothesize that VitA is an important metabolic substrate for robust neurogenesis at the olfactory apparatus, as retinoic acid (RA; the active metabolite of VitA), has been shown to reduce ΔNp63α expression in HBCs, thereby promoting the differentiation of multipotent Sox2+ and Pax6+ progenitors via the canonical Wnt signaling pathway in the OE [10,25,26]. Furthermore, the neurophysiological importance of RA signaling in the OE has also been demonstrated in the maintenance and survival of Ascl1+ GBCs [27,28].
Within the CNS, RA machineries have been identified in the murine and human brains, especially at the hippocampus and dentate gyrus [29,30,31]. RA increases neurogenesis in the rodent subventricular zone (SVZ)–OB pathway, as demonstrated by increased bromodeoxyuridine-positive (BrdU+, proliferating cell marker) cells in SVZ neurospheres and altered cellular migration to the olfactory bulbs [32]. In addition, depletion of doublecortin-positive (DCX+, immature neuronal marker) cells in the adult murine dentate gyrus was evident in the retinoid–deficient mouse model, indicating the crucial role of RA in the survival of neural progenitors [33].
Olfactory stimulation via ST, as the mainstay of non-pharmaceutical intervention for post-infectious OD, was used to complement the therapeutic effects of oral VitA [14,15,34]. MRI studies in ST-treated patients have demonstrated functional network improvements and structural increase of cortical thickness in the frontal cortex, where the olfactory apparatus is located [19,20,35]. The exact therapeutic mechanism of ST is unknown; however, olfactory stimulations remain essential to the neuro-rehabilitation processes [36]. Reversible and irreversible olfactory occlusion experiments in murine models have shown that olfactory stimuli were contributory to olfactory neurogenesis and glomeruli maturation [37]. In this report, ST was delivered by the novel EPAR diffusers, which utilized ultrasonification to generate aerosolized essential oils for olfactory stimulation. The EPAR diffuser technology enhances the ST experience by facilitating the physiological penetration of aromatic molecules through the olfactory meatus to the OE at the roof of the nasal cavity, therefore providing potent olfactory stimulation and neurosensory rehabilitation.
4. Conclusions
In conclusion, structural and functional olfactory defects were identified in patients presenting with prolonged COVID-19-related OD. Preliminary evidence suggested that combination treatment by oral VitA and ST may facilitate the recovery of olfaction, restore OB volumes, and improve functional olfactory network connectivity. The therapeutic efficacy of oral VitA and ST for prolonged COVID-19-related OD should be validated in large scale randomized–controlled trials.
Acknowledgments
We would like to thank SOVOS (Hong Kong, China) for their non-monetary donations of essential oils and EPAR diffusers for the purpose of smell training in the olfactory treatment.
Supplementary Materials
Supplementary materials are available online at https://www.mdpi.com/article/10.3390/brainsci11060686/s1. Supplementary Notes–Note S1: Olfactory evaluations and SARS-CoV-2 virologic assessments; Note S2: Neuroradiological magnetic resonance imaging (MRI) data acquisition; Note S3: Smell training via the electronic portable aromatic rehabilitation (EPAR) diffusers. Supplementary Tables–Table S1: Demographic and clinical characteristics of COVID-19 patients and controls; Table S2: Sino-nasal outcome test (SNOT-22) of patient 1, before olfactory treatment; Table S3: Sino-nasal outcome test (SNOT-22) of patient 1, after 4-weeks of olfactory treatment; Table S4: Sino-nasal outcome test (SNOT-22) of patient 2; Table S5: Functional connectivity (FC) of the left and right caudate and the olfactory cortical network (OCN) regions; Table S6: Volume of the olfactory bulb and tract; Table S7: Cerebral blood flow (CBF) in the olfactory cortical network (OCN) regions; Table S8: Butanol threshold test (BTT). Supplementary Figures–Figures S1 | Magnetic resonance (MR) spectroscopy of the gyrus rectus and superior frontal cortex. (A) The position of the single voxel Point Resolved Spectroscopy (PRESS) was placed at the gyrus rectus and superior frontal cortex. (B) Healthy control. (C) Patient 2. Cho, choline; Cr, creatine; mI, myo-inositol; NAA, N-acetylaspartate; Figures S2 | Electronic portable aromatic rehabilitation (EPAR) diffuser. (A) Correct usage, stream of aerosolised essential oil directed vertically upwards, away from the user’s face. (B) Incorrect usage, stream of aerosolised essential oil directed at the user’s face, which may cause transient irritation to the skin and mucosal surfaces. Supplementary Item–Item S1: Olfactory function questionnaire. Supplementary References.
Author Contributions
T.W.-H.C., I.F.-N.H., and H.K.-F.M. conceived the study. T.W.-H.C., H.Z. and H.K.-F.M. designed the experiments and wrote the manuscript draft. T.W.-H.C. and F.K.-C.W. performed the olfactory assessments. H.Z. and H.K.-F.M. conducted the rs-fMRI scans and analyses. T.W.-H.C. and K.-H.C. performed SARS-CoV-2 RT-PCR and serology tests and analyses. S.S., V.C.-C.C., I.F.-N.H., and K.-Y.Y. were involved in the patient recruitment, data analysis, and critically reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Shaw Foundation Hong Kong; Michael Seak-Kan Tong; Richard Yu and Carol Yu; May Tam Mak Mei Yin; Jessie & George Ho Charitable Foundation; Perfect Shape Medical Limited; Respiratory Viral Research Foundation; Hui Ming, Hui Hoy and Chow Sin Lan Charity Fund Limited; Sanming Project of Medicine in Shenzhen, China (SZSM201911014); High Level-Hospital Program, Health Commission of Guangdong Province, China; Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases and Research Capability on Antimicrobial Resistance for the Department of Health of the Hong Kong Special Administrative Region Government; Theme-Based Research Scheme (T11/707/15) of the Research Grants Council of the Hong Kong Special Administrative Region Government, China. The funding sources played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the manuscript for publication.
Institutional Review Board Statement
This study was approved by the institutional review board (IRB) of the University of Hong Kong/Hospital Authority–UW 20-454. Written informed consents were obtained.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in the manuscript text and Supplementary Materials of this article.
Conflicts of Interest
S.S. has received speaker’s honoraria from Sanofi-Aventis Hong Kong Limited and Abbott laboratories Limited. The other authors declare no conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chung T.W., Sridhar S., Zhang A.J., Chan K.H., Li H.L., Wong F.K., Ng M.Y., Tsang R.K., Lee A.C., Fan Z., et al. Olfactory Dysfunction in Coronavirus Disease 2019 Patients: Observational Cohort Study and Systematic Review. Open Forum Infect. Dis. 2020;7:ofaa199. doi: 10.1093/ofid/ofaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meng X., Deng Y., Dai Z., Meng Z. COVID-19 and anosmia: A review based on up-to-date knowledge. Am. J. Otolaryngol. 2020;41:102581. doi: 10.1016/j.amjoto.2020.102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paderno A., Mattavelli D., Rampinelli V., Grammatica A., Raffetti E., Tomasoni M., Gualtieri T., Taboni S., Zorzi S., Del Bon F., et al. Olfactory and Gustatory Outcomes in COVID-19: A Prospective Evaluation in Nonhospitalized Subjects. Otolaryngol. Head Neck Surg. 2020;163:1144–1149. doi: 10.1177/0194599820939538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., Laue M., Schneider J., Brünink S., Greuel S., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 5.Solomon I.H., Normandin E., Bhattacharyya S., Mukerji S.S., Keller K., Ali A.S., Adams G., Hornick J.L., Padera R.F., Sabeti P. Neuropathological Features of Covid-19. N. Engl. J. Med. 2020;383:989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., Chilla S., Heinemann A., Wanner N., Liu S., et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu H., Chan J.F., Yuen T.T., Shuai H., Yuan S., Wang Y., Hu B., Yip C.C., Tsang J.O., Huang X., et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: An observational study. Lancet Microbe. 2020;1:e14–e23. doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang B.Z., Chu H., Han S., Shuai H., Deng J., Hu Y.F., Gong H.R., Lee A.C., Zou Z., Yau T., et al. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020;30:928–931. doi: 10.1038/s41422-020-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang A.J., Lee A.C., Chu H., Chan J.F., Fan Z., Li C., Liu F., Chen Y., Yuan S., Poon V.K., et al. SARS-CoV-2 infects and damages the mature and immature olfactory sensory neurons of hamsters. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher R.B., Das D., Gadye L., Street K.N., Baudhuin A., Wagner A., Cole M.B., Flores Q., Choi Y.G., Yosef N., et al. Deconstructing Olfactory Stem Cell Trajectories at Single-Cell Resolution. Cell Stem Cell. 2017;20:817–830.e818. doi: 10.1016/j.stem.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung C.T., Coulombe P.A., Reed R.R. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat. Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- 12.Reden J., Lill K., Zahnert T., Haehner A., Hummel T. Olfactory function in patients with postinfectious and posttraumatic smell disorders before and after treatment with vitamin A: A double-blind, placebo-controlled, randomized clinical trial. Laryngoscope. 2012;122:1906–1909. doi: 10.1002/lary.23405. [DOI] [PubMed] [Google Scholar]
- 13.Hummel T., Whitcroft K.L., Rueter G., Haehner A. Intranasal vitamin A is beneficial in post-infectious olfactory loss. Eur Arch. Otorhinolaryngol. 2017;274:2819–2825. doi: 10.1007/s00405-017-4576-x. [DOI] [PubMed] [Google Scholar]
- 14.Addison A.B., Wong B., Ahmed T., Macchi A., Konstantinidis I., Huart C., Frasnelli J., Fjaeldstad A.W., Ramakrishnan V.R., Rombaux P., et al. Clinical Olfactory Working Group consensus statement on the treatment of postinfectious olfactory dysfunction. J. Allergy Clin. Immunol. 2021;147:1704–1719. doi: 10.1016/j.jaci.2020.12.641. [DOI] [PubMed] [Google Scholar]
- 15.Hopkins C., Alanin M., Philpott C., Harries P., Whitcroft K., Qureishi A., Anari S., Ramakrishnan Y., Sama A., Davies E., et al. Management of new onset loss of sense of smell during the COVID-19 pandemic—BRS Consensus Guidelines. Clin. Otolaryngol. 2021;46:16–22. doi: 10.1111/coa.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Logothetis N.K., Pauls J., Augath M., Trinath T., Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 17.Attwell D., Buchan A.M., Charpak S., Lauritzen M., Macvicar B.A., Newman E.A. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Esposito M., Deouell L.Y., Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: A challenge for neuroimaging. Nat. Rev. Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- 19.Kollndorfer K., Fischmeister F.P., Kowalczyk K., Hoche E., Mueller C.A., Trattnig S., Schöpf V. Olfactory training induces changes in regional functional connectivity in patients with long-term smell loss. Neuroimage Clin. 2015;9:401–410. doi: 10.1016/j.nicl.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kollndorfer K., Kowalczyk K., Hoche E., Mueller C.A., Pollak M., Trattnig S., Schöpf V. Recovery of olfactory function induces neuroplasticity effects in patients with smell loss. Neural Plast. 2014;2014:140419. doi: 10.1155/2014/140419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oz G., Alger J.R., Barker P.B., Bartha R., Bizzi A., Boesch C., Bolan P.J., Brindle K.M., Cudalbu C., Dinçer A., et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270:658–679. doi: 10.1148/radiol.13130531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan R.Q., Li W.T.V., Shum W.Z., Chu S.C., Li H.L., Shea Y.F., Chung T.W. A systematic review and meta-analysis protocol examining the clinical characteristics and epidemiological features of olfactory dysfunction (OD) in coronavirus disease 2019 (COVID-19) Syst. Rev. 2021;10:73. doi: 10.1186/s13643-021-01624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnittke N., Herrick D.B., Lin B., Peterson J., Coleman J.H., Packard A.I., Jang W., Schwob J.E. Transcription factor p63 controls the reserve status but not the stemness of horizontal basal cells in the olfactory epithelium. Proc. Natl. Acad. Sci. USA. 2015;112:E5068–E5077. doi: 10.1073/pnas.1512272112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrick D.B., Lin B., Peterson J., Schnittke N., Schwob J.E. Notch1 maintains dormancy of olfactory horizontal basal cells, a reserve neural stem cell. Proc. Natl. Acad. Sci. USA. 2017;114:E5589–E5598. doi: 10.1073/pnas.1701333114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin B., Coleman J.H., Peterson J.N., Zunitch M.J., Jang W., Herrick D.B., Schwob J.E. Injury Induces Endogenous Reprogramming and Dedifferentiation of Neuronal Progenitors to Multipotency. Cell Stem Cell. 2017;21:761–774.e765. doi: 10.1016/j.stem.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson J., Lin B., Barrios-Camacho C.M., Herrick D.B., Holbrook E.H., Jang W., Coleman J.H., Schwob J.E. Activating a Reserve Neural Stem Cell Population In Vitro Enables Engraftment and Multipotency after Transplantation. Stem Cell Rep. 2019;12:680–695. doi: 10.1016/j.stemcr.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peluso C.E., Jang W., Dräger U.C., Schwob J.E. Differential expression of components of the retinoic acid signaling pathway in the adult mouse olfactory epithelium. J. Comp. Neurol. 2012;520:3707–3726. doi: 10.1002/cne.23124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paschaki M., Cammas L., Muta Y., Matsuoka Y., Mak S.S., Rataj-Baniowska M., Fraulob V., Dollé P., Ladher R.K. Retinoic acid regulates olfactory progenitor cell fate and differentiation. Neural Dev. 2013;8:13. doi: 10.1186/1749-8104-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fragoso Y.D., Shearer K.D., Sementilli A., de Carvalho L.V., McCaffery P.J. High expression of retinoic acid receptors and synthetic enzymes in the human hippocampus. Brain Struct. Funct. 2012;217:473–483. doi: 10.1007/s00429-011-0359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rioux L., Arnold S.E. The expression of retinoic acid receptor alpha is increased in the granule cells of the dentate gyrus in schizophrenia. Psychiatry Res. 2005;133:13–21. doi: 10.1016/j.psychres.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Stoney P.N., Fragoso Y.D., Saeed R.B., Ashton A., Goodman T., Simons C., Gomaa M.S., Sementilli A., Sementilli L., Ross A.W., et al. Expression of the retinoic acid catabolic enzyme CYP26B1 in the human brain to maintain signaling homeostasis. Brain Struct. Funct. 2016;221:3315–3326. doi: 10.1007/s00429-015-1102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang T.W., Zhang H., Parent J.M. Retinoic acid regulates postnatal neurogenesis in the murine subventricular zone-olfactory bulb pathway. Development. 2005;132:2721–2732. doi: 10.1242/dev.01867. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs S., Lie D.C., DeCicco K.L., Shi Y., DeLuca L.M., Gage F.H., Evans R.M. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc. Natl. Acad. Sci. USA. 2006;103:3902–3907. doi: 10.1073/pnas.0511294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damm M., Pikart L.K., Reimann H., Burkert S., Göktas Ö., Haxel B., Frey S., Charalampakis I., Beule A., Renner B., et al. Olfactory training is helpful in postinfectious olfactory loss: A randomized, controlled, multicenter study. Laryngoscope. 2014;124:826–831. doi: 10.1002/lary.24340. [DOI] [PubMed] [Google Scholar]
- 35.Al Aïn S., Poupon D., Hétu S., Mercier N., Steffener J., Frasnelli J. Smell training improves olfactory function and alters brain structure. Neuroimage. 2019;189:45–54. doi: 10.1016/j.neuroimage.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Mainland J.D., Bremner E.A., Young N., Johnson B.N., Khan R.M., Bensafi M., Sobel N. Olfactory plasticity: One nostril knows what the other learns. Nature. 2002;419:802. doi: 10.1038/419802a. [DOI] [PubMed] [Google Scholar]
- 37.Zou D.J., Feinstein P., Rivers A.L., Mathews G.A., Kim A., Greer C.A., Mombaerts P., Firestein S. Postnatal refinement of peripheral olfactory projections. Science. 2004;304:1976–1979. doi: 10.1126/science.1093468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the manuscript text and Supplementary Materials of this article.