Abstract
We aimed to identify plasma and urine metabolites altered by the Dietary Approaches to Stop Hypertension (DASH) diet in a post-hoc analysis of a pilot feeding trial. Twenty adult participants with un-medicated hypertension consumed a Control diet for one week followed by 2 weeks of random assignment to either Control or DASH diet. Non-missing fasting plasma (n = 56) and 24-h urine (n = 40) were used to profile metabolites using untargeted gas chromatography/mass spectrometry. Linear models were used to compare metabolite levels between the groups. In urine, 19 identifiable untargeted metabolites differed between groups at p < 0.05. These included a variety of phenolic acids and their microbial metabolites that were higher during the DASH diet, with many at false discovery rate (FDR) adjusted p < 0.2. In plasma, eight identifiable untargeted metabolites were different at p < 0.05, but only gamma-tocopherol was significantly lower on DASH at FDR adjusted p < 0.2. The results provide insights into the mechanisms of benefit of the DASH diet.
Keywords: nutrition, polyphenols, blood pressure, DASH, metabolomics
1. Introduction
The Dietary Approaches to Stop Hypertension (DASH) diet emphasizes consumption of fruits, vegetables, low-fat dairy, whole grains, fish, poultry, and nuts, while limiting red meat, sweets, and sugar-containing beverages [1]. In randomized clinical trials, DASH reduces systolic blood pressure (BP) by an average of 5.2 mmHg over durations of 2–24 weeks [2]. Observational studies suggest that DASH may also reduce the risks of atherosclerotic cardiovascular disease, stroke, diabetes, and kidney disease [3,4]. However, the mechanism of benefit of the DASH diet is poorly understood. Metabolomics phenotyping can help characterize the changes in body chemistry following a dietary intervention, such as DASH, and thus identify potential mechanisms of benefit [5]. Understanding the potential mechanisms of DASH using informative biomarkers could help to fine-tune implementation strategies. Thus, in this post-hoc analysis, we characterized changes in the plasma and urine metabolome among generally healthy patients with un-medicated stage 1 hypertension while consuming the DASH diet.
2. Materials and Methods
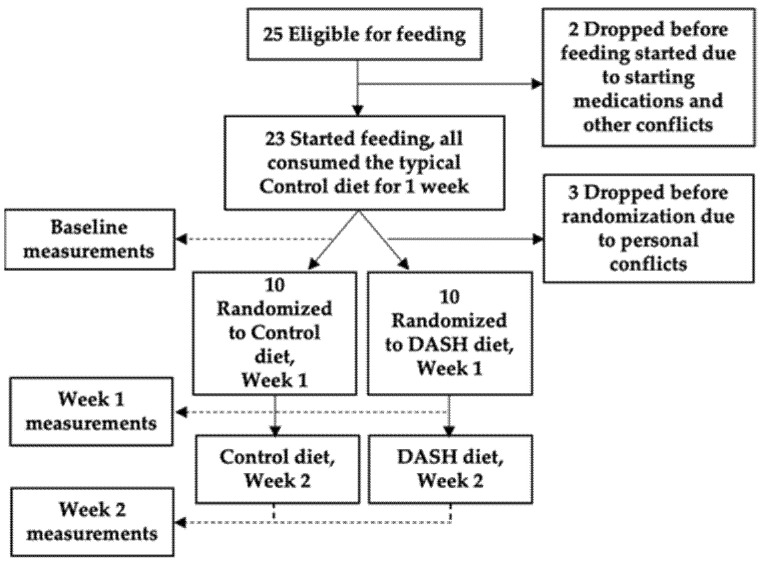
The DASH Mechanism Study (n = 20) was a single-center, 3-week controlled feeding randomized clinical trial designed to evaluate the mechanism of DASH as previously published [6]. All participants were 22 years of age or older, met the Joint National Commission (JNC) 7 criteria for stage 1 hypertension (systolic BP of 140–159 or diastolic BP of 90–99 mmHg) [7], and were not taking any anti-hypertensive therapies. Participants were excluded if they had a glomerular filtration rate (GFR) < 60 mL/min/1.73 m2, diabetes, active heart disease, or any other serious medical conditions. After screening, confirmation of eligibility and informed consent, all participants first consumed a Control diet representative of typical American intake for one week. Baseline sample collections including fasting plasma and 24-h urine were obtained at the end of this period as a baseline assessment. Immediately after the completion of the baseline period, participants were randomized 1:1 to 2 additional weeks of either the Control diet or the DASH diet with sample data collection at the end of each week (randomized period). Figure 1 shows the study flow chart. All recruitment occurred between 2007 and 2009 at Duke University and samples were stored at −80 °C until metabolomics testing in 2014.
Figure 1.
Study flow chart. Adapted from original published manuscript [6].
Adherence to the study diet was monitored daily at on-site feeding visits and with diet logs. When compared to the Control diet, the DASH diet is designed to be lower in total fat and saturated fat, but higher in both mono- and polyunsaturated fats. DASH is also higher in protein, fiber, potassium, magnesium, and calcium content. Although the original DASH diet was designed with the specific nutrient targets, these were later translated into food groups for practical implementation. Compared to the Control diet, the DASH diet is rich in fruits, vegetables, low-fat dairy, nuts and seeds, and lower in red meats, sweets and sugar-sweetened beverages.
To characterize the metabolic impacts of the DASH diet, we performed untargeted gas chromatography/mass spectrometry (GC/MS) on stored non-missing fasting plasma (n = 56 out of 60 time points were available) and 24-h urine, diluted in pure water to achieve constant creatinine concentration across samples (n = 40 out of 60 time points were available). Detailed GC/MS methods are published elsewhere [8]. All metabolites are quantified as the log2 peak areas. Within each untargeted dataset, metabolites were filtered out if they were detected in <50% of samples. Remaining untargeted data was imputed using k-nearest neighbor (k = 6). A generalized estimating equation model was implemented in R for each metabolite to compare levels in the DASH versus Control during the randomized weeks accounting for correlation of repeated measures within the same individual. The Benjamini-Hochberg false discovery rate (FDR) threshold <0.2 was used to account for multiple testing, while allowing discovery in this exploratory analysis. Metabolites with an unknown Chemical Abstracts Services number were not reported.
Analyses were performed following the initial Control diet period to evaluate any baseline imbalance between the two randomized groups. This was followed by models for the 2 randomized feeding weeks for the difference between individuals consuming DASH and Control. We also performed sensitivity analyses in R using linear mixed models to determine the within-subject change for each metabolite between baseline and the first randomized week, as well as between baseline and the second randomized week, focusing interpretation on metabolites identified in the primary approach. These change models were not selected as the primary analysis because of missing samples, which resulted in loss of participants from the model and reduced power.
3. Results
Overall, 20 participants completed the DASH Mechanism Study, as previously reported [6]. The average age range in participants of DASH Mechanism was 46.1 ± 9.0 years. The population included 13 females and had an average body mass index (BMI) of 33.9 ± 6.6 kg/m2 and average systolic BP of 144.2 ± 9.4 mmHg at screening. Table 1 shows baseline participant characteristics by randomized diet groups. Clinical parameters including changes in blood pressure, endothelial function, pulse wave velocity, and plasma nitric oxide levels were reported in the original study. Consumption of DASH diet resulted in significant reductions of blood pressure starting at the end of week one (both systolic and diastolic) and end of week two (systolic) [6].
Table 1.
Baseline participant characteristics by randomized diet groups.
| Characteristic (Mean ± SD or n (%)) | DASH Diet | Control Diet |
|---|---|---|
| N | 10 | 10 |
| Age (years) | 46.1 ± 9.0 | 42.4 ± 6.3 |
| Female Sex (%) | 7 (70.0) | 6 (60.0) |
| Race | ||
| African American (%) | 6 (60.0) | 8 (80.0) |
| Caucasian (%) | 3 (30.0) | 2 (20.0) |
| Other (%) | 1 (10.0) | 0 (0.0) |
| Body Weight (kg) | 85.0 ± 15.5 | 107.0 ± 18.5 |
| Body Mass Index (kg/m2) | 31.0 ± 5.9 | 36.9 ± 6.1 |
| Blood Pressure (BP) | ||
| Systolic BP (mmHg) | 142.8 ± 7.03 | 145.6 ± 11.5 |
| Diastolic BP (mmHg) | 88.8 ± 6.67 | 88.2 ± 5.68 |
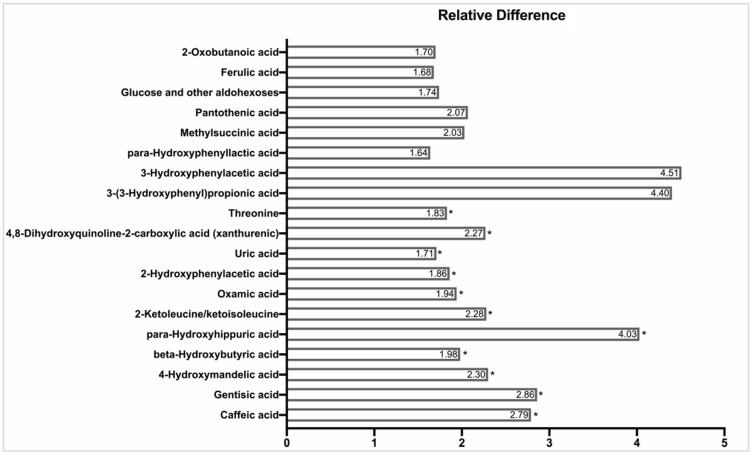
We found >150 unique identifiable metabolites in the urine. Five metabolites differed in the two groups at baseline with a nominal p-value of <0.05, reflecting modest baseline imbalance in profiles across groups. These metabolites included lower levels of octanoic acid (p = 0.02), adipic acid (p = 0.02), and cinnamoyl-glycine (p = 0.04) and higher levels of methyl-succinic acid (p = 0.03) and 2-hydroxyphenylacetic acid (p = 0.04). Nineteen urine metabolites differed between Control and DASH with a nominal p-value < 0.05 (Figure 2 and Table 2). Eleven of these metabolites had FDR adjusted p < 0.20, including caffeic acid, gentisic acid, 4-hydroxymandelic acid, beta-hydroxybutyric acid, para-hydroxy-hippuric acid, keto-leucine/keto-isoleucine, oxamic acid, 2-hydroxyphenylacetic acid, uric acid, 4,8-dihydroxyquinoline-2-carboxylic acid, and threonine.
Figure 2.
Relative Difference in Nominally Significant Metabolites from Untargeted Urine GC/MS during Randomized DASH and Control Feeding Periods. Relative difference represents the ratio of peak areas by GC/MS. Differences are tested using generalized estimating equation models evaluating the difference in log2 peak areas in non-missing samples obtained during randomized study weeks on either DASH or Control diet. Relative differences re-express absolute log2 difference coefficients (β) as 2β. Significance at a false discovery rate (FDR) adjusted p < 0.20 is indicated by an asterisk (*). Metabolites are ordered by statistical significance with the most statistically significant metabolites beginning at the bottom near the x-axis. Several metabolites had between 10–20 imputed values including caffeic acid, gentisic acid, uric acid and threonine.
Table 2.
Annotation of identified urine metabolites as candidates modified by DASH.
| Class | Metabolite | Sub-Class * | Example Sources * |
|---|---|---|---|
| Phenolic Acids | ¥ Caffeic acid | Hydroxycinnamic acids | Wine; whole grains; herbs; fruits; vegetables |
| Ferulic acid | Hydroxycinnamic acids | Whole grains; cocoa; fruits; vegetables; herbs | |
| ¥ 4-Hydroxymandelic acid | Hydroxyphenylacetic acids | Microbial phenol/polyphenol metabolite | |
| 3-Hydroxyphenylacetic acid | Hydroxyphenylacetic acids | Microbial phenol/polyphenol metabolite | |
| ¥ 2-Hydroxyphenylacetic acid | Hydroxyphenylacetic acids | Microbial phenol/polyphenol metabolite | |
| ¥ Para-Hydroxyhippuric acid | Hydroxybenzoic acids | Microbial phenol/polyphenol metabolite | |
| ¥ Gentisic Acid | Hydroxybenzoic acids | Aspirin; selected fruits; herbs | |
| 3-(3-Hydroxyphenyl)propionic acid | Hydroxyphenylpropanoic acid | Microbial phenol/polyphenol metabolite | |
| Para-Hydroxyphenyllactic acid | Hydroxyphenylpropanoic acid | Microbial phenol/polyphenol metabolite | |
| Quinolines | ¥ 4,8-Dihydroxyquinoline-2-carboxylic acid (xanthurenic acid) | Quinoline carboxylic acids | Metabolite of tryptophan, protein sources |
| Keto Acids and Derivatives | ¥ 2-Ketoleucine | Short-chain keto acids/ derivatives |
Branched chain amino acid degradation |
| 2-Oxobutanoic acid | Short-chain keto acids/ derivatives |
Amino acid degradation | |
| Fatty acyls | Methylsuccinic acid | Fatty acids and conjugates | Involved in isoleucine metabolism |
| Imidazopyrimidines | ¥ Uric acid | Purines and purine derivatives | Final product of purine metabolism; Protein sources |
| Organooxygen Compounds | Pantothenic acid (vitamin B5) | Alcohols and polyols | Whole grain; legumes; eggs; meat |
| Glucose and other aldohexoses | Carbohydrates | Many | |
| Carboxylic Acids and Derivatives | ¥ Oxamic acid | Carboxylic acid derivatives | Amine derivative of oxalate |
| Hydroxy Acid and Derivatives | ¥ β-Hydroxybutyric acid | β-Hydroxy acids | Ketone body |
| Amino Acids | ¥ Threonine | NA | Cottage cheese; poultry; fish; meat; lentils; black bean; sesame seeds |
* Sources: Human Metabolome Database (hmdb.ca), U.S. National Library of Medicine (www.pubchem.ncbi.nlm.nih.gov; access date: 18 November 2019), National Institute of Standards and Technology (webbook.nist.gov; access date: 18 November 2019), and Phenol-Explorer (www.phenol-explorer.eu; access date: 18 November 2019). ¥ Metabolites were identified at nominal p < 0.05 and FDR adjusted p < 0.20.
In plasma, we found >110 uniquely annotated metabolites of known identity. At the end of the first week of Control diet (baseline), 11 metabolites differed in the two groups, reflecting modest baseline imbalance in profiles. Unbalanced metabolites included 2-ketoleucine/keto-isoleucine (p = 0.01), beta-hydroxybutyric acid (p = 0.02), hydroxyprolines (p = 0.02), 2-ketovaline (p = 0.02), leucine (p = 0.03), valine (p = 0.04), oleic acid (p = 0.04), and pyruvic acid (p = 0.04). We found eight plasma metabolites that differed between Control and DASH during randomized weeks with a nominal p-value ≤ 0.05. These metabolites included lower gamma-tocopherol, hydroxyprolines, and methionine, and higher oleic acid, beta-hydroxybutyric acid, myoinositol, citric acid/iso-citric acid, and beta-sitosterol on DASH. We interpret these cautiously as several of these were imbalanced at baseline (hydroxyprolines, oleic acid, beta-hydroxybutyric acid). Only gamma-tocopherol remained different at an FDR adjusted p < 0.20, which was not expected given the higher fruit and vegetable composition of DASH.
In sensitivity analyses of within participant change, the two urine metabolites 3-(3-hydroxyphenyl)propionic acid and threonine increased more in DASH vs. Control at the end of the first randomized week (p < 0.05 and FDR-adjusted p < 0.2). No metabolites in plasma or urine changed at both a nominal and FDR-adjusted p-value between baseline and week 2, but power was limited in all analyses due to missing data.
4. Discussion
In this study, we used metabolomics to examine changes that occur with the consumption of the DASH diet in comparison to a typical American diet. Metabolomics can provide us with a ‘read out’ of the metabolic impact of DASH and identify potential objective biomarkers which could be used to further study and monitor favorable responses to DASH. In summary, we found several urine metabolites that were higher in DASH vs. Control participants during randomized feeding weeks. These primarily consisted of small organic acids of a variety of classes including phenols/polyphenols, carboxylic acids, and ketoacids, among others.
Phenols and their derivatives have antioxidant and anti-inflammatory properties [9,10,11] with potential BP lowering effects through mechanisms such as scavenging reactive oxygen species in the vasculature, inhibiting vascular smooth muscle cell proliferation, and enhancing nitric oxide (NO) availability [12]. Caffeic acid, ferulic acid and other related metabolites are known to be absorbed in the gastrointestinal tract and are abundant in coffee and tea [13,14,15]. While individuals in our study were allowed to consume coffee and tea up to a combination of three servings a day, it is unlikely that their intake would have differed significantly between Control and DASH to result in the observed differences in the metabolites. Thus, it is more likely that the high caffeic and ferulic acid content of fruits and vegetables is responsible for the noted differences in excretion [16]. Many of the phenolic acids identified are also products of microbial phenol catabolism, including 3-(3-hydroxyphenyl) propionic acid, 2-hydroxyphenylacetic acid, para-hydroxy-hippuric acid, and were elevated on DASH [17]. Although we did not directly characterize the microbiota, the higher excretion of microbial metabolites may suggest an impact of DASH on the microbiota itself as a mechanism of benefit.
We identified gentisic acid which is a metabolite of salicylic acid or aspirin. Although aspirin intake is a common source of these metabolites, in the context of this feeding study, this finding could be due to the abundance of salicylic acid and related botanical phenolic compounds in fruits and vegetables [18,19]. Although research on dietary salicylates is limited, aspirin and sodium salicylate have been shown to raise inducible NO synthase (iNOS) and enhance NO production [20,21]. While acetylsalicylate (aspirin) is not found in foods, the similar efficacy of aspirin and sodium salicylate indicate that the acetyl moiety is not a significant factor in the effect on NO [21,22]. Improvement in NO bioavailability was a candidate mechanism of action of DASH identified by the primary results of the DASH Mechanism Study [6]. Further studies are needed to understand if salicylates in foods contribute to this effect.
The results of our study are consistent with prior research. In another recent small study by Reisdorph et al. (n = 19), investigators evaluated metabolic profiles of 12 foods common in DASH-style diets. They evaluated urine LC-MS metabolomics and detected many of these food specific metabolites in urine samples from participants consuming the DASH diet, particularly urine phenolic compounds from fruits and vegetables [23]. This is consistent with our finding of a higher urine phenolic acids in the DASH group which contains higher levels of fruits and vegetables. Rebholz et al. evaluated serum metabolites using a combination of GC-MS and LC-MS in stored samples from the original DASH trial [24]. With a much larger sample size, they identified 96 serum metabolites more common in DASH. One of the top hits, chiro-inositol, was from the same chemical class as myoinositol, which was found in our plasma samples. Inositol can be found in both animal and plant cells with its derivatives most abundant in grains, beans, nuts, seeds, and fruits [24,25]. These observations are consistent with the high content of fruits, nuts, and seeds in the DASH diet.
Our study has a few limitations. Due to the small sample size, we could not adjust for baseline values and other clinical covariates in this hypothesis-generating analysis. Thus, it is possible that some of the differences we noted were chance differences in groups at baseline and effects of missing samples. We primarily utilized semi-quantitative, untargeted tools for metabolomics, which are an initial discovery step that needs further refinement before clinical application. Given that most of the urinary metabolites we observed belong to the class of phenolic acids, which may have antioxidant activities, future studies should also assess total antioxidant activity to further understand the mechanisms of both urinary and plasma metabolites of DASH diet. This study also has multiple strengths. According to a recent review by Kim et al. [26], there are about 17 studies which have investigated the metabolomics signatures of different dietary patterns, including the DASH diet. However, out of those studies, 12 have used cross-sectional designs, three have used data from behavioral intervention trials, and only two studies are controlled feeding trials. In the current study, we used a highly-controlled feeding protocol to ensure optimal delivery of the DASH diet and we investigated metabolites related to a dietary pattern rather than a single food or nutrient.
5. Conclusions
The results of this study demonstrate several metabolite differences comparing DASH to the typical American diet. Consumption of the DASH diet may increase urine levels of a set of bioactive phenolic compounds (e.g., caffeic acid) and their metabolites formed by the gastrointestinal microbiota (e.g., 3-(3-hydroxyphenyl) propionic acid; 2-hydroxyphenylacetic acid). The results of our study, along with results of more follow up studies, can be useful in understanding potential mechanisms of action of the DASH diet.
Acknowledgments
We gratefully acknowledge the invaluable contributions of participants in the DASH Mechanism Study, without whom this work would not be possible. We also acknowledge and thank Christopher Newgard, Director of the Duke Molecular Physiology Institute, for consultation. This work was reported in abstract form at the 2020 American Heart Association Epidemiology and Lifestyle Scientific Session.
Author Contributions
Author contributions to this manuscript include: designed research (L.P.S., P.-H.L., J.J.S.); conducted research measurements (L.P.S., J.R.B., M.J.M., O.I., P.-H.L.); analyzed data/statistical analysis (D.L.C., T.M.O., J.J.S.); data interpretation (all authors); drafted manuscript (M.N., S.P., J.J.S.); revised for important intellectual content (all authors). All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a Pilot and Feasibility Award to JS from the Duke O’Brien Center for Kidney Research (P30DK096493), a National Institute of Diabetes and Digestive and Kidney Diseases funded research Center. The DASH Mechanism Study was supported by the American Heart Association under award number 0755460U to PHL. This manuscript reflects the opinions of the authors and not the official views of the National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Diseases, or the American Heart Association.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Duke University (protocol code: Pro0001236 and date of approval: 26 June 2007) and registered on ClinicalTrials.gov (NCT01017484).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical and privacy restrictions.
Conflicts of Interest
J.J.S. has received consulting fees from Tricida. The authors declare no other conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vollmer W.M., Sacks F.M., Ard J., Appel L.J., Bray G.A., Simons-Morton D.G., Conlin P.R., Svetkey L.P., Erlinger T.P., Moore T.J. Effects of diet and sodium intake on blood pressure: Subgroup analysis of the DASH-sodium trial. Ann. Intern. Med. 2001;135:1019–1028. doi: 10.7326/0003-4819-135-12-200112180-00005. [DOI] [PubMed] [Google Scholar]
- 2.Siervo M., Lara J., Chowdhury S., Ashor A., Oggioni C., Mathers J.C. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: A systematic review and meta-analysis. Br. J. Nutr. 2015;113:1–15. doi: 10.1017/S0007114514003341. [DOI] [PubMed] [Google Scholar]
- 3.Salehi-Abargouei A., Maghsoudi Z., Shirani F., Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases–incidence: A systematic review and meta-analysis on observational prospective studies. Nutrition. 2013;29:611–618. doi: 10.1016/j.nut.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Rebholz C.M., Crews D.C., Grams M.E., Steffen L.M., Levey A.S., Miller I.I.I.E.R., Appel L.J., Coresh J. DASH (Dietary Approaches to Stop Hypertension) diet and risk of subsequent kidney disease. Am. J. Kidney Dis. 2016;68:853–861. doi: 10.1053/j.ajkd.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikolic S.B., Sharman J.E., Adams M.J., Edwards L.M. Metabolomics in hypertension. J. Hypertens. 2014;32:1159–1169. doi: 10.1097/HJH.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 6.Lin P.-H., Allen J.D., Li Y.-J., Yu M., Lien L.F., Svetkey L.P. Blood Pressure-Lowering Mechanisms of the DASH Dietary Pattern. J. Nutr. Metab. 2012;2012:472396. doi: 10.1155/2012/472396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenfant C., Chobanian A.V., Jones D.W., Roccella E.J. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) resetting the hypertension sails. Circulation. 2003;107:2993–2994. doi: 10.1161/01.CIR.0000080481.62058.03. [DOI] [PubMed] [Google Scholar]
- 8.Tyson C.C., Luciano A., Modliszewski J.L., Corcoran D.L., Bain J.R., Muehlbauer M., Ilkayeva O., Pourafshar S., Allen J., Bowman C., et al. Effect of bicarbonate on net acid excretion, blood pressure and metabolism in patients with and without CKD: The Acid Base Compensation in CKD Study. Am. J. Kidney Dis. 2021 doi: 10.1053/j.ajkd.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Touyz R.M., Briones A.M. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2011;34:5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 10.Li P.G., Xu J.W., Ikeda K., Kobayakawa A., Kayano Y., Mitani T., Ikami T., Yamori Y. Caffeic acid inhibits vascular smooth muscle cell proliferation induced by angiotensin II in stroke-prone spontaneously hypertensive rats. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2005;28:369–377. doi: 10.1291/hypres.28.369. [DOI] [PubMed] [Google Scholar]
- 11.Nollet L.M., Gutierrez-Uribe J.A., editors. Phenolic Compounds in Food: Characterization and Analysis. CRC Press; Boca Raton, FL, USA: 2018. [Google Scholar]
- 12.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lafay S., Morand C., Manach C., Besson C., Scalbert A. Absorption and metabolism of caffeic acid and chlorogenic acid in the small intestine of rats. Br. J. Nutr. 2006;96:39–46. doi: 10.1079/BJN20061714. [DOI] [PubMed] [Google Scholar]
- 14.Renouf M., Guy P.A., Marmet C., Fraering A.L., Longet K., Moulin J., Enslen M., Barron D., Dionisi F., Cavin C., et al. Measurement of caffeic and ferulic acid equivalents in plasma after coffee consumption: Small intestine and colon are key sites for coffee metabolism. Mol. Nutr. Food Res. 2010;54:760–766. doi: 10.1002/mnfr.200900056. [DOI] [PubMed] [Google Scholar]
- 15.Konishi Y., Kobayashi S. Transepithelial transport of chlorogenic acid, caffeic acid, and their colonic metabolites in intestinal caco-2 cell monolayers. J. Agric. Food Chem. 2004;52:2518–2526. doi: 10.1021/jf035407c. [DOI] [PubMed] [Google Scholar]
- 16.Manach C., Scalbert A., Morand C., Remesy C., Jimenez L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 17.Rechner A.R., Kuhnle G., Bremner P., Hubbard G.P., Moore K.P., Rice-Evans C.A. The metabolic fate of dietary polyphenols in humans. Free Radic. Biol. Med. 2002;33:220–235. doi: 10.1016/S0891-5849(02)00877-8. [DOI] [PubMed] [Google Scholar]
- 18.Siniorakis E., Arvanitakis S., Zarreas E., Saridakis M., Balanis A., Tzevelekos P., Bokos G., Limberi S. Mediterranean diet: Natural salicylates and other secrets of the pyramid. Int. J. Cardiol. 2013;166:538–539. doi: 10.1016/j.ijcard.2012.09.192. [DOI] [PubMed] [Google Scholar]
- 19.Duthie G.G., Wood A.D. Natural salicylates: Foods, functions and disease prevention. Food Funct. 2011;2:515–520. doi: 10.1039/c1fo10128e. [DOI] [PubMed] [Google Scholar]
- 20.Amin A.R., Vyas P., Attur M., Leszczynska-Piziak J., Patel I.R., Weissmann G., Abramson S.B. The mode of action of aspirin-like drugs: Effect on inducible nitric oxide synthase. Proc. Natl. Acad. Sci. USA. 1995;92:7926–7930. doi: 10.1073/pnas.92.17.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishio E., Watanabe Y. Aspirin and salicylate enhances the induction of inducible nitric oxide synthase in cultured rat smooth muscle cells. Life Sci. 1998;63:429–439. doi: 10.1016/S0024-3205(98)00292-6. [DOI] [PubMed] [Google Scholar]
- 22.Janssen P.L., Katan M.B., van Staveren W.A., Hollman P.C., Venema D.P. Acetylsalicylate and salicylates in foods. Cancer Lett. 1997;114:163–164. doi: 10.1016/S0304-3835(97)04650-8. [DOI] [PubMed] [Google Scholar]
- 23.Reisdorph N.A., Hendricks A.E., Tang M., Doenges K.A., Reisdorph R.M., Tooker B.C., Quinn K., Borengasser S.J., Nkrumah-Elie Y., Frank D.N., et al. Nutrimetabolomics reveals food-specific compounds in urine of adults consuming a DASH-style diet. Sci. Rep. 2020;10:1157. doi: 10.1038/s41598-020-57979-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rebholz C.M., Lichtenstein A.H., Zheng Z., Appel L.J., Coresh J. Serum untargeted metabolomic profile of the Dietary Approaches to Stop Hypertension (DASH) dietary pattern. Am. J. Clin. Nutr. 2018;108:243–255. doi: 10.1093/ajcn/nqy099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pintaudi B., Di Vieste G., Bonomo M. The Effectiveness of Myo-Inositol and D-Chiro Inositol Treatment in Type 2 Diabetes. Int. J. Endocrinol. 2016;2016:9132052. doi: 10.1155/2016/9132052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H., Rebholz C.M. Metabolomic Biomarkers of Healthy Dietary Patterns and Cardiovascular Outcomes. Curr. Atheroscler. Rep. 2021;23:1–2. doi: 10.1007/s11883-021-00921-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical and privacy restrictions.