Abstract
Dried blood spots (DBS) are commonly used for serologic testing for viruses and provide an alternative collection method when phlebotomy and/or conventional laboratory testing are not readily available. DBS collection could be used to facilitate widespread testing for SARS-CoV-2 antibodies to document past infection, vaccination, and potentially immunity. We investigated the characteristics of Roche’s Anti-SARS-CoV-2 (S) assay, a quantitative commercial assay for antibodies against the spike glycoprotein. Antibody levels were reduced relative to plasma following elution from DBS. Quantitative results from DBS samples were highly correlated with values from plasma (r2 = 0.98), allowing for extrapolation using DBS results to accurately estimate plasma antibody levels. High concordance between plasma and fingerpick DBS was observed in PCR-confirmed COVID-19 patients tested 90 days or more after the diagnosis (45/46 matched; 1/46 mismatched plasma vs. DBS). The assessment of antibody responses to SARS-CoV-2 using DBS may be feasible using a quantitative anti-S assay, although false negatives may rarely occur in those with very low antibody levels.
Keywords: SARS-CoV-2, COVID-19, dried blood spot, serology, antibody
1. Introduction
To date, the COVID-19 pandemic has resulted in more than 3 million deaths worldwide (https://coronavirus.jhu.edu/map.html, accessed on 19 May 2021). While physical distancing and stay-at-home measures play an important role, detection and monitoring of cases is crucial to the management of the pandemic. SARS-CoV-2 serology is useful for seroprevalence studies, identifying cases of negative/indeterminate molecular results despite high clinical suspicion of COVID-19, and diagnostic assessment for multi-system inflammatory syndrome in children [1]. In addition, plasma antibody levels may determine natural and/or vaccine-derived immunity to SARS-CoV-2 infection.
The development of serologic testing platforms has been rapid. This has included platforms for unique antibodies with combined isotypes against different viral targets, with qualitative and, more recently, quantitative platforms. However, sample collection for SARS-CoV-2 serological testing requires phlebotomy from a trained provider with access to sample processing and a central laboratory.
The use of dried blood spots (DBS), a collection method whereby capillary blood is collected onto filter paper, may facilitate more widespread testing by overcoming some of these obstacles [2,3]. Serological testing using DBS has already been validated and optimized for other viral infections including HIV and hepatitis B and C [4,5,6,7,8,9,10,11,12,13,14,15]. Compared to venous blood collection, DBS sample collection is less complex and offers the opportunity for peer or even self-collection, facilitating collection in rural and remote settings, as well as in populations who may experience geographic or structural barriers seeking healthcare in traditional settings. Collection kits could theoretically be mailed to patients who are then able to reliably collect their own samples and send them back to a testing facility [16]. This eliminates the need for person-to-person contact and healthcare visits; facilitates repetitive sampling and widespread surveillance; and allows for testing to reach populations that lack adequate testing resources, such as in rural areas and parts of low- and middle-income countries.
Since the beginning of the pandemic, several manufacturers have developed commercial high throughput assays for SARS-CoV-2 serology, as not all laboratories have the capacity to develop in-house assays. Commercial assays also allow for comparisons between labs, which is important as the medical and scientific community incorporate serology testing into various aspects of clinical care. These assays have demonstrated high specificity and sensitivity using serum/plasma. Although there have been preliminary studies evaluating DBS collection for anti-SARS-CoV-2 immunoassays, DBS serology tests have yet to be used on a widespread scale [2,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. Importantly, the majority of platforms evaluated have been for qualitative assays for antibodies against SARS-CoV-2, a potential gap should a quantitative option prove beneficial.
Here, we evaluated a commercial anti-SARS-CoV-2 S Total assay (Roche) using DBS prepared from plasma-spiked RBC. Following the characterization of the assay, we then completed a head-to-head real-world comparison of matched plasma and DBS collected by finger prick from individuals with a PCR-confirmed positive history of COVID-19.
2. Materials and Methods
2.1. Specimens
Residual plasma (Li-Heparin) samples from PCR-confirmed positive SARS-CoV-2 patients (n = 24, plasma collected 5–39 days after swab collection for PCR testing) and COVID-19-negative patients (n = 21, pre-COVID-19 plasma samples or samples from PCR-confirmed negative patient) were used for spiking red blood cells (Table S1). To prepare spiked blood samples, we mixed plasma with saline-washed (3×) O-negative red blood cells (1:1 ratio). A total of 50 µL of prepared whole blood was then added to completely fill 5 spots on the Whatman 903 Protein Saver cards (GE Healthcare, Chicago, IL, USA). The approach of spiking blood samples has been used previously in the validation of COVID-19 and non-COVID-19 DBS serology testing [14,31]. Filter cards were dried for 2–3 h at room temperature in the absence of any external source of heat and processed the same day.
Matched plasma and dried blood spots were also collected from 52 PCR-positive confirmed patients, 90 days or greater from diagnosis, and 11 healthy controls (Table S2). These 52 patients were recruited from a phase II randomized clinical trial of COVID-19 treatment [33]. Blood was collected by finger prick to fill the spots on a Whatman 903 Protein Saver card. All specimens were dried and stored at −80 °C until use. The use of residual samples was exempt from quality improvement (QI) review, and the use of both sets of patient samples was approved by Clinical Trials Ontario Research Ethics Board (REB) (Toronto, ON, Canada) on 9 September 2020 (CTO 2148).
2.2. Dried Blood Spots
DBS processing was modified from a previously published protocol [34,35]. Two punches per spot on the filter card, with a total of 10 punches per filter card, were prepared. Each punch size had a diameter of 6.35 mm, corresponding to an area of 31.65 mm2 (24.99% of each filter card circle area). Punched spots were placed in 750 µL of phosphate-buffered saline (plus 0.05% Tween 20 (v/v) and 0.08% sodium azide (w/v)) in 12-well plates (Thermo Scientific BioLite Multidishes and Microwell Plate, Thermo Fisher Scientific, Waltham, MA, USA) and incubated overnight on a shaker at room temperature. Eluates were then transferred to microcentrifuge tubes, centrifuged for 2 min at 10,500× g, and stored at −80 °C until use. Assuming 50 µL whole blood is applied and 45% hematocrit, we had an expected dilution of plasma after DBS elution of ×11.
2.3. SARS-CoV-2 Antibody Assay
The quantitative Roche Elecsys Anti-SARS-CoV-2 (S) assay measuring antibodies to spike (S) glycoprotein was chosen to evaluate the relationship between serum/plasma SARS-CoV-2 antibody testing and testing from DBS. The assay detects antibodies to RBD of S glycoprotein. Threshold for positivity is greater than or equal to 0.8 U/mL. Analytical measurement range is 0.4 to 250 U/mL, with dilution 0.4 to 2500 U/mL. Imprecision at 9.06 U/mL was 1.26% on the basis of quality control material. The assay was carried out according to the manufacturers’ instructions.
2.4. Data Analysis
The correlation between plasma and DBS quantitative results was determined using the Pearson correlation coefficient. All statistical analysis and graphing were performed using R (https://www.R-project.org, accessed on 19 May 2021) with the following packages: tidyverse, xlsx, ggpmisc, multcomp, irr, pROC, and epiR [36].
3. Results
3.1. Initial Validation of Quantitative Roche S Assay Using Prepared DBS Specimens
DBS were processed by removing two punches per spot on the filter card, with a total of 10 punches per filter card, into 750 µL of PBS overnight. The optimal procedure was chosen on the basis of the magnitude of decrease of the signal observed, linear correlation between plasma and DBS, and sufficient extraction volume for multiple testing, as well as ease of processing.
To determine the ability of commercial automated serology assay to detect antibody response to SARS-CoV-2 in DBS eluants, we mixed plasma samples from COVID-19-positive patients with a wide range of antibody titers (Table S1) with RBCs and applied them to filter cards. The samples were deliberately chosen to include both extremes of high and low signal samples to test the performance of DBS. The antibody signal obtained from eluants was compared to results from corresponding plasma samples.
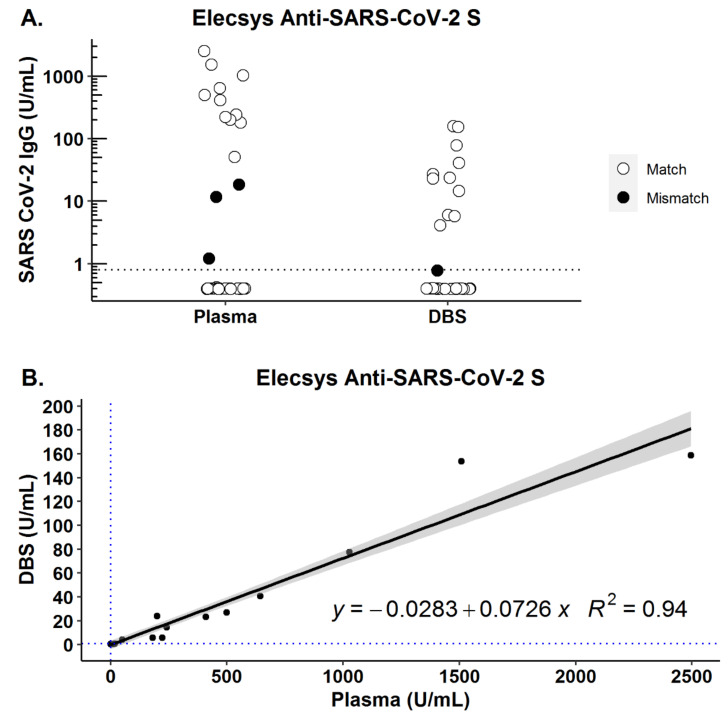
For all samples, the recommended threshold for detection in plasma/serum was used for both the plasma and DBS samples. A decrease in signal was observed after DBS elution; however, the correlation between plasma and DBS concentration was high (r2 = 0.94). There were no false positive DBS results, but 3/23 samples were positive in plasma but negative in DBS (Figure 1).
Figure 1.
RBC mixed with plasma (1:1) were applied to filter cards, dried, and eluted. The sera and corresponding eluants were analyzed using Roche Elecsys Anti-SARS-CoV-2 (S) assay (plasma used for spiking RBC: n = 24 COVID-19-positive (n = 23 serology positive) patients; n = 21 COVID-19-negative (n = 22 serology negative) patients). The horizontal line indicates assay threshold as quoted by manufacturer for plasma/serum. (A) Distribution of values of DBS samples prepared from spiked RBC and corresponding plasma used for spiking for mismatched samples (●) and matched samples (ο). (B) Dataset from (A) showing correlation between concentrations obtained from plasma vs. DBS.
3.2. Performance of Quantitative Roche S Assay in Matched Plasma/DBS Fingerprick Collection
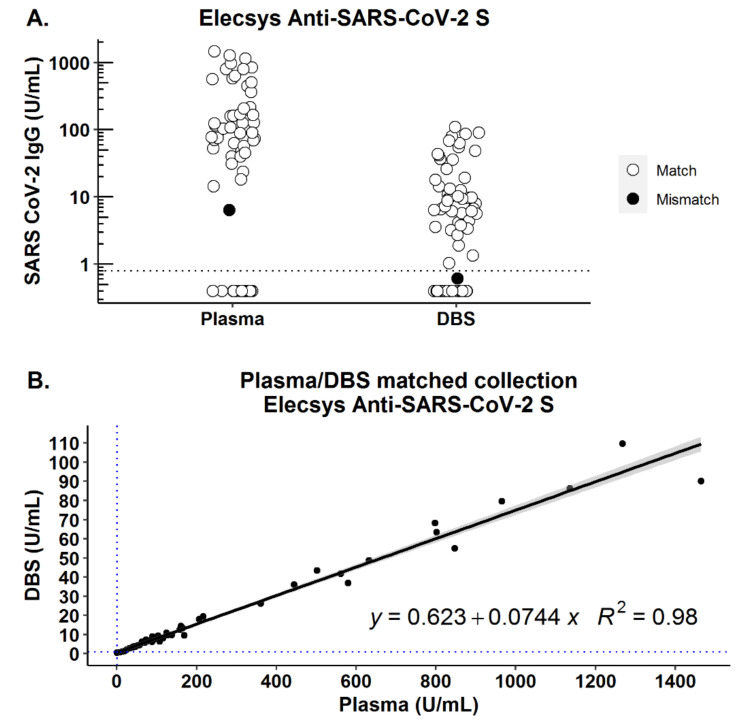
Plasma and DBS were collected from 52 patients 90 days or more after their initial positive PCR positive test (mean: 136, min: 90, max: 208 days; Table S2). Of the 46 patients with positive serology from plasma, 45 (97.8%) were detected by DBS. All 17 (6 patients and 11 negative controls) samples that tested negative for SARS-CoV-2 antibodies in plasma were also negative on the corresponding DBS sample (Figure 2A, Table 1). Using the quantitative Roche assay, we found that the correlation between quantitative results obtained on plasma and DBS was very high (r2 = 0.98, Figure 2B). Clinical sensitivity and specificity were not significantly different from plasma-based performance in this group of patients. DBS (using serum/plasma-based threshold) showed 98% (95% CI: 88–100%) sensitivity and 100% (95% CI: 80–100%) specificity relative to plasma (Table 1). Of note, not all individuals who were PCR-positive had persistent antibodies by plasma on day 90 (n = 6); these individuals were also negative by DBS (Table 1).
Figure 2.
Plasma and dried blood spot collection comparison. Plasma and DBS samples collected in parallel from the same participant were processed and analyzed by Roche Elecsys Anti-SARS-CoV-2 (S) assay (n = 52 COVID-19-positive, n = 11 COVID-19-negative). Horizontal line indicates assay threshold as quoted by manufacturer for plasma/serum. (A) Distribution of values in plasma and DBS samples for mismatched samples (●) and matched samples (ο). (B) Dataset from (A) showing correlation between concentrations obtained from plasma vs. DBS.
Table 1.
Clinical performance of DBS vs. plasma for PCR-confirmed COVID-19 detection.
| Comparisons | Threshold (U/mL) | True Negatives |
True Positives |
False Negatives |
False Positives |
Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| Plasma vs. PCR-confirmed COVID-19 | 0.8 | 11 | 46 | 6 | 0 | 0.88 (95% CI: 0.77, 0.96) | 1 (95% CI: 0.72, 1) |
| DBS vs. PCR-confirmed COVID-19 | 0.8 | 11 | 45 | 7 | 0 | 0.87 (95% CI: 0.74, 0.94) | 1 (95% CI: 0.72, 1) |
| DBS vs. plasma-positive COVID-19 | 0.8 | 17 | 45 | 1 | 0 | 0.98 (95% CI: 0.88, 1) | 1 (95% CI: 0.8, 1) |
4. Discussion
In this study, our aim was to determine the feasibility of using the commercial quantitative SARS-CoV-2 anti-S Roche assay, with no changes to assay configuration or threshold, in order to measure antibody levels from DBS specimens. We demonstrated that antibody measurement after DBS collection showed high concordance, both qualitatively and quantitatively, with standard plasma measurements, even though the antibody levels are predictably decreased on DBS.
We used plasma spiked with RBCs to mimic whole blood that is normally collected on DBS for initial assay optimization and comparisons; however, we found that the plasma-spiked DBS cards performed near identically to contemporaneously collected DBS and plasma samples, giving us confidence in the results observed (Figure S1). We found detectable antibodies in the plasma in 88% of people at least 3 months post-PCR positivity. The clinical performance of the different commercial assays, such as Abbott SARS-CoV-2 (N) IgG, DiaSorin SARS-CoV-2 S1/S2 IgG, and Roche Elecsys Anti-SARS-CoV-2 (N), have been investigated, and sensitivity greater than 80% and specificity greater than 95% 14 weeks after confirmed infection have been reported in hospitalized patients. Slightly lower sensitivity for antibodies has been observed in outpatient settings [37,38,39,40,41,42,43,44,45,46]. Roche Elecsys Anti-SARS-CoV-2 (S) is a quantitative assay that detects antibodies against the receptor-binding domain (RBD) with reported sensitivity of 84.0% 15–30 days post-PCR positivity, with no cross-reactivity leading to 100% specificity [47]. Compared to plasma, we found that antibodies detected from DBS using the Roche (S) assay performed well with a sensitivity of 98% and specificity of 100%. We also observed excellent correlation between quantitative plasma and DBS antibody levels with a predictable decrease in signal, allowing for prediction of the serum signal from the DBS signal, which would be important if thresholds to define immunity are identified. The performance of the Roche (S) assay we observed is consistent with recent research highlighting its high specificity and sensitivity with serum [47,48].
High concordance between antibody detection from serum/plasma versus DBS has been reported in studies using ELISA-type immunoassays and lateral flow immunoassays where sample dilution is adjusted for in DBS specimens to compensate for dilution due to filter card extraction [2,17,20,24,27,28]. One group analyzed DBS samples from 80 volunteers using an in-house-developed ELISA protocol. Detecting the presence of IgG, IgA, and IgM against the SARS-CoV-2 spike protein, they achieved a sensitivity and specificity of 98.1% and 100%, respectively, relative to serum [17]. In another study with 111 (31 COVID-19-positive) samples, a modified antibody detection by agglutination-PCR (ADAP) procedure was used with concentrated DBS eluant. They were able to achieve 100% sensitivity and specificity compared to serum/plasma for antibodies against the SARS-CoV-2 S1 spike protein subunit [18]. A recent study evaluated performance of DBS using Abbott SARS-CoV-2 IgG (N) and SARS-CoV-2 IgM (S) qualitative assays, but with sample volume modification to compensate for DBS elution, and showed high concordance between plasma and DBS samples [32].
However, while useful, these studies relied on in-house assays for antibody detection that were modified to improve performance with DBS. Most clinical laboratories rely on commercially available antibody detection tools with limited ability to adjust assay parameters, and thus we felt it was important to evaluate DBS using commercially available assays in order to understand how it might be used outside of a research context. The amount of dilution from the filter cards can be theoretically predicted and normalized; however, whether this leads to predictable decrease in antibody signal depends on assay linearity. Roche Elecsys SARS-CoV-2 S assay was shown to be linear [47]. The correlation between signal predicted from serum or plasma vs. observed DBS signal was high (Figure S2). A recent study tested the Roche assay (total N assay) and showed limited performance of DBS relative to serum. The sensitivity was only 44% relative to plasma if the plasma cut-offs were used or 89% if a derived cut-off was used, about 30× higher than the reported LOD. However, the size of the area eluted and elution buffer volume likely resulted in higher dilution of sample compared to the current study. The study also observed variation in the quality of DBS collected [29].
Several parameters affect the amount of predicted elution from the filter cards [26,28,49]. The number of circles spotted with blood, the punch size, and the number of punches will determine the size of the surface area exposed to elution buffer. The volume of elution buffer is also important. Our initial choice of elution procedure was driven by literature review of DBS testing of non-SARS-CoV-2 serology and focused on several parameters that could affect dilution of the final eluant. We found modifications of the standard procedure had limited impact on the performance of DBS, but overall, we found that 10 spots from 5 cards eluted in 750 µL of PBS gave the most consistent results. A recent study also showed the effect of DBS size and punch location on the observed DBS signal [26]. DBS specimens have been shown to be stable at room temperature, 4 °C and −20 °C, at ambient or low humidity conditions for at least 28 days [26]. An automated DBS extraction procedure is also being developed [23].
Our study has limitations. The assay we report does not specifically test for neutralizing antibodies. Some studies have reported potential use of DBS collection for neutralizing antibodies as well [50]. However, it has been found that high receptor binding domain-specific antibody titers may be indicative of the presence of neutralizing antibodies against SARS-CoV-2 [51,52,53,54]. Further validation of DBS collection may be required once the level of neutralizing antibodies that confers protection has been identified. We had a limited number of samples with very low antibody levels, the situation in which the sensitivity of DBS declines and there is a greater risk for misclassification; however, the clinical importance of this scenario is questionable given that very low levels of antibody may not be protective. Quantitative assays are yet to establish traceability to higher order reference material, which would facilitate comparison between different assays and establishment of universal thresholds of immunity if possible. Recently the WHO prepared an international standard and reference panel for SARS-CoV-2 immunoglobulins, which will hopefully help in assay standardization [55]. We used the same thresholds for DBS as for plasma. New cut-offs for DBS could potentially be developed; however, with the very small difference between the limit of detection (LOD) and the thresholds for positivity (e.g., 0.4 U/mL vs. 0.8 U/mL on Roche S assay), it may be difficult to find a DBS-specific cut-off. Furthermore, changing the cut-off on the basis of expected dilution resulted in cut-off values that fell below the limit of quantitation (LOQ). With these limitations in mind, it will be important for individual laboratories to conduct their own internal validation studies, as well as to evaluate diagnostic limitations while balancing clinical utility. This would include negative and positive controls, as well as evaluating for cross-reactivity for seasonal coronaviruses.
As SARS-CoV-2 vaccines continue to roll out, the feasibility of DBS serology testing can allow for widespread monitoring of vaccine responses. In addition, DBS opens the door to identifying unknown previous cases of COVID-19 (i.e., asymptomatic infections), which may be helpful in developing targeted vaccination strategies and understanding the overall burden and case number in the pandemic. The use of DBS for COVID-19 serology testing has been tested in neonates and in COVID-19 seroprevalence studies [22,25,30]. We show promising performance of quantitative Roche SARS-CoV-2 S assay on DBS samples, with high correlation with plasma antibody levels and concordance with serum-based positivity classification.
Acknowledgments
We thank Tony Mazzulli for his technical assistance, as well as the volunteers who participated in the study.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/v13060962/s1, Figure S1: Comparison between Anti-S concentration obtained from DBS prepared from capillary blood (“Direct DBS collection”) and DBS prepared from RBC spiked with corresponding plasma (“DBS from mixed plasma + RBC”). Figure S2: Correlation between antibody level measured in eluted DBS and predicted dilution of plasma. Table S1: Patient samples selected for initial DBS validation. Table S2: Patient samples selected for DBS SARS-CoV-2 serology validation using matched filter card/plasma collection, 90 days or more post-infection, and negative controls.
Author Contributions
Conceptualization, D.B., M.J.B., C.C., V.K., and J.J.F.; data curation, M.J.B., M.A.Z., and J.C.; formal analysis, D.B.; funding acquisition, C.C., V.K., and J.J.F.; investigation, H.S., D.S., and J.C.; methodology, D.B., M.J.B., H.S., M.A.Z., V.K., and J.J.F.; project administration, C.C. and J.J.F.; resources, C.C.; supervision, M.J.B., M.A.Z., V.K., and J.J.F.; validation, D.B., M.J.B., and H.S.; visualization, D.B.; writing—original draft, D.L., D.B., and M.J.B.; writing—review and editing, D.B., M.J.B., D.L., C.C., D.S., M.A.Z., J.C., V.K., and J.J.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the Toronto COVID-19 Action Initiative, the University of Toronto (72059280), the Ontario First COVID-19 Rapid Research Fund (C-224-2428560-FELD), the Toronto General Hospital Research Institute, and the Toronto General and Western Hospital Foundation.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Clinical Trials Ontario Research Ethics Board (REB) (Toronto, Canada) on 9 September 2020 (CTO 2148). The use of residual samples was exempt from quality improvement (QI) review.
Informed Consent Statement
In the case the use of residual samples, consent was waived as this study was exempt from quality improvement (QI) review. However, informed consent was obtained from all subjects involved in all other sample collection within the study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bailey D., Konforte D., Barakauskas V.E., Yip P.M., Kulasingam V., Abou E.H.M., Beach L.A., Blasutig I.M., Catomeris P., Dooley K.C., et al. Canadian Society of Clinical Chemists (CSCC) Interim Consensus Guidance for Testing and Reporting of SARS-CoV-2 Serology. Clin. Biochem. 2020;86:1–7. doi: 10.1016/j.clinbiochem.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnamurthy H.K., Jayaraman V., Krishna K., Rajasekaran K.E., Wang T., Bei K., Rajasekaran J.J., Yaskin I., Rai A.J., Choung R.S., et al. Antibody Profiling and Prevalence in US Patients during the SARS-CoV2 Pandemic. PLoS ONE. 2020;15:e0242655. doi: 10.1371/journal.pone.0242655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Protti M., Mandrioli R., Mercolini L. Quantitative Microsampling for Bioanalytical Applications Related to the SARS-CoV-2 Pandemic: Usefulness, Benefits and Pitfalls. J. Pharm. Biomed. Anal. 2020;191:113597. doi: 10.1016/j.jpba.2020.113597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimech W., Mulders M.N. A Review of Testing Used in Seroprevalence Studies on Measles and Rubella. Vaccine. 2016;34:4119–4122. doi: 10.1016/j.vaccine.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Villar L.M., Bezerra C.S., Cruz H.M., Portilho M.M., Flores G.L. Applicability of Oral Fluid and Dried Blood Spot for Hepatitis B Virus Diagnosis. Can. J. Gastroenterol. Hepatol. 2019;2019:5672795. doi: 10.1155/2019/5672795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villar L.M., de Oliveira J.C., Cruz H.M., Yoshida C.F.T., Lampe E., Lewis-Ximenez L.L. Assessment of Dried Blood Spot Samples as a Simple Method for Detection of Hepatitis B Virus Markers. J. Med. Virol. 2011;83:1522–1529. doi: 10.1002/jmv.22138. [DOI] [PubMed] [Google Scholar]
- 7.Flores G.L., Cruz H.M., Miguel J.C., Potsch D.V., Pilotto J.H., Lewis-Ximenez L.L., Lampe E., Villar L.M. Assessing Hepatitis B Immunity Using Dried Blood Spot Samples from HIV+ Individuals. J. Med. Virol. 2018;90:1863–1867. doi: 10.1002/jmv.25275. [DOI] [PubMed] [Google Scholar]
- 8.Croom H.A., Richards K.M., Best S.J., Francis B.H., Johnson E.I., Dax E.M., Wilson K.M. Commercial Enzyme Immunoassay Adapted for the Detection of Antibodies to Hepatitis C Virus in Dried Blood Spots. J. Clin. Virol. 2006;36:68–71. doi: 10.1016/j.jcv.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Mercader S., Featherstone D., Bellini W.J. Comparison of Available Methods to Elute Serum from Dried Blood Spot Samples for Measles Serology. J. Virol. Methods. 2006;137:140–149. doi: 10.1016/j.jviromet.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Cruz H.M., Cruz J.C.M., Da Silva E.F., Portilho M.M., Marques V.A., Lewis-Ximenez L.L., Lampe E., Villar L.M. Comparison of the Performance of Enzyme Immunoassays for Hepatitis B and C Detection in Dried Blood Spot. J. Immunoass. Immunochem. 2018;39:228–233. doi: 10.1080/15321819.2018.1470095. [DOI] [PubMed] [Google Scholar]
- 11.Villar L.M., Cruz H.M., Deodato R.M., Miguel J.C., Da Silva E.F., Flores G.L., Lewis-Ximenez L.L. Usefulness of Automated Assays for Detecting Hepatitis B and C Markers in Dried Blood Spot Samples. BMC Res. Notes. 2019;12:523. doi: 10.1186/s13104-019-4547-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross R.S., Stambouli O., Grüner N., Marcus U., Cai W., Zhang W., Zimmermann R., Roggendorf M. Detection of Infections with Hepatitis B Virus, Hepatitis C Virus, and Human Immunodeficiency Virus by Analyses of Dried Blood Spots--Performance Characteristics of the ARCHITECT System and Two Commercial Assays for Nucleic Acid Amplification. Virol. J. 2013;10:72. doi: 10.1186/1743-422X-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee C.E., Ponnampalavanar S.S., Omar S.F.S., Mahadeva S., Ong L.Y., Kamarulzaman A. Evaluation of the Dried Blood Spot (DBS) Collection Method as a Tool for Detection of HIV Ag/Ab, HBsAg, Anti-HBs and Anti-HCV in a Malaysian Tertiary Referral Hospital. Ann. Acad. Med. Singap. 2011;40:448–453. [PubMed] [Google Scholar]
- 14.Brandão C.P.U., Marques B.L.C., Marques V.A., Villela-Nogueira C.A., Do Ó K.M.R., de Paula M.T., Lewis-Ximenez L.L., Lampe E., Sá Ferreira J.A., Villar L.M. Simultaneous Detection of Hepatitis C Virus Antigen and Antibodies in Dried Blood Spots. J. Clin. Virol. 2013;57:98–102. doi: 10.1016/j.jcv.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Tuaillon E., Kania D., Pisoni A., Bollore K., Taieb F., Ngoyi E.N.O., Schaub R., Plantier J.-C., Makinson A., van de Perre P. Dried Blood Spot Tests for the Diagnosis and Therapeutic Monitoring of HIV and Viral Hepatitis B and C. Front. Microbiol. 2020;11:373. doi: 10.3389/fmicb.2020.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valentine-Graves M., Hall E., Guest J.L., Adam E., Valencia R., Shinn K., Hardee I., Sanchez T., Siegler A.J., Sullivan P.S. At-home Self-collection of Saliva, Oropharyngeal Swabs and Dried Blood Spots for SARS-CoV-2 Diagnosis and Serology: Post-collection Acceptability of Specimen Collection Process and Patient Confidence in Specimens. PLoS ONE. 2020;15:e0236775. doi: 10.1371/journal.pone.0236775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morley G.L., Taylor S., Jossi S., Perez-Toledo M., Faustini S.E., Marcial-Juarez E., Shields A.M., Goodall M., Allen J.D., Watanabe Y., et al. Sensitive Detection of SARS-CoV-2-Specific Antibodies in Dried Blood Spot Samples. Emerg. Infect. Dis. 2020;26:2970–2973. doi: 10.3201/eid2612.203309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karp D.G., Danh K., Espinoza N.F., Seftel D., Robinson P.V., Tsai C.-T. A Serological Assay to Detect SARS-CoV-2 Antibodies in At-home Collected Finger-prick Dried Blood Spots. Sci. Rep. 2020;10:20188. doi: 10.1038/s41598-020-76913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan P.S., Sailey C., Guest J.L., Guarner J., Kelley C., Siegler A.J., Valentine-Graves M., Gravens L., Del Rio C., Sanchez T.H. Detection of SARS-CoV-2 RNA and Antibodies in Diverse Samples: Protocol to Validate the Sufficiency of Provider-Observed, Home-Collected Blood, Saliva, and Oropharyngeal Samples. JMIR Public Health Surveill. 2020;6:e19054. doi: 10.2196/19054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amendola A., Bianchi S., Gori M., Barcellini L., Colzani D., Canuti M., Giacomet V., Fabiano V., Folgori L., Zuccotti G.V., et al. Back to school: Use of Dried Blood Spot for the Detection of SARS-CoV-2-specific Immunoglobulin G (IgG) among Schoolchildren in Milan, Italy. medRxiv. 2020 doi: 10.1101/2020.07.29.20164186. [DOI] [Google Scholar]
- 21.Amendola A., Bianchi S., Gori M., Barcellini L., Colzani D., Canuti M., Giacomet V., Fabiano V., Folgori L., Zuccotti G.V., et al. Dried Blood Spot as an Alternative to Plasma/Serum for SARS-CoV-2 IgG Detection, an Opportunity to Be Sized to Facilitate COVID-19 Surveillance Among Schoolchildren. Pediatr. Infect. Dis. J. 2021;40:e46–e47. doi: 10.1097/INF.0000000000002955. [DOI] [PubMed] [Google Scholar]
- 22.Demonbreun A.R., McDade T.W., Pesce L., Vaught L.A., Reiser N.L., Bogdanovic E., Velez M.E., Hsieh R.R., Klaisner C.-N., Simons L.M., et al. Patterns and Persistence of SARS-CoV-2 IgG Antibodies in a US Metropolitan Site. medRxiv. 2020 doi: 10.1101/2020.11.17.20233452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaugler S., Sottas P.-E., Blum K., Luginbühl M. Fully Automated Dried Blood Spot Sample Handling and Extraction for Serological Testing of SARS-CoV-2 Antibodies. Drug Test. Anal. 2021;13:223–226. doi: 10.1002/dta.2946. [DOI] [PubMed] [Google Scholar]
- 24.Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., Mills R., Teng E., Kamruzzaman M., Garcia-Beltran W.F., et al. Persistence and Decay of Human Antibody Responses to the Receptor Binding Domain of SARS-CoV-2 Spike Protein in COVID-19 Patients. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu F., Nguyen M., Vijayakumar P., Kaplan A., Meir A., Dai Y., Wang E., Walsh H., Ring A.M., Omer S.B., et al. Newborn Dried Blood Spots for Serologic Surveys of COVID-19. Pediatr. Infect. Dis. J. 2020;39:e454–e456. doi: 10.1097/INF.0000000000002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moat S.J., Zelek W.M., Carne E., Ponsford M.J., Bramhall K., Jones S., El-Shanawany T., Wise M.P., Thomas A., George C., et al. Development of a High-throughput SARS-CoV-2 Antibody Testing Pathway Using Dried Blood Spot Specimens. Ann. Clin. Biochem. 2020;58 doi: 10.1177/0004563220981106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thevis M., Knoop A., Schaefer M.S., Dufaux B., Schrader Y., Thomas A., Geyer H. Can Dried Blood Spots (DBS) Contribute to Conducting Comprehensive SARS-CoV-2 Antibody Tests? Drug Test. Anal. 2020;12:994–997. doi: 10.1002/dta.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zava T.T., Zava D.T. Validation of Dried Blood Spot Sample Modifications to Two Commercially Available COVID-19 IgG Antibody Immunoassays. Bioanalysis. 2021;13:13–28. doi: 10.4155/bio-2020-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulchandani R., Brown B., Brooks T., Semper A., Machin N., Linley E., Borrow R., Wyllie D., Taylor-Philips S., Jones H., et al. Use of Dried Blood Spot Samples for SARS-CoV-2 Antibody Detection Using the Roche Elecsys® High throughput Immunoassay. J. Clin. Virol. 2021;136:104739. doi: 10.1016/j.jcv.2021.104739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDade T.W., McNally E.M., Zelikovich A.S., D’Aquila R., Mustanski B., Miller A., Vaught L.A., Reiser N.L., Bogdanovic E., Fallon K.S., et al. High Seroprevalence for SARS-CoV-2 among Household Members of Essential Workers Detected Using a Dried Blood Spot Assay. PLoS ONE. 2020;15:e0237833. doi: 10.1371/journal.pone.0237833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDade T.W., McNally E.M., D’Aquila R., Mustanski B., Miller A., Vaught L.A., Reiser N.L., Bogdanovic E., Zelikovich A.S., Demonbreun A.R. Enzyme Immunoassay for SARS-CoV-2 Antibodies in Dried Blood Spot Samples: A Minimally-Invasive Approach to Facilitate Community- and Population-based Screening. medRxiv. 2020 doi: 10.1101/2020.04.28.20081844. [DOI] [Google Scholar]
- 32.Anderson M., Holzmayer V., Vallari A., Taylor R., Moy J., Cloherty G. Expanding Access to SARS-CoV-2 IgG and IgM Serologic Testing Using Fingerstick Whole Blood, Plasma, and Rapid Lateral Flow Assays. J. Clin. Virol. 2021:104855. doi: 10.1016/j.jcv.2021.104855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feld J.J., Kandel C., Biondi M.J., A Kozak R., Zahoor M.A., Lemieux C., Borgia S.M., Boggild A.K., Powis J., McCready J., et al. Peginterferon Lambda for the Treatment of Outpatients with COVID-19: A Phase 2, Placebo-controlled Randomised Trial. Lancet Respir. Med. 2021;9:498–510. doi: 10.1016/S2213-2600(20)30566-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grüner N., Stambouli O., Ross R.S. Dried Blood Spots-Preparing and Processing for Use in Immunoassays and in Molecular Techniques. J. Vis. Exp. 2015:e52619. doi: 10.3791/52619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biondi M.J., Van Tilborg M., Smookler D., Heymann G., Aquino A., Perusini S., Mandel E., Kozak R.A., Cherepanov V., Kowgier M., et al. Hepatitis C Core-Antigen Testing from Dried Blood Spots. Viruses. 2019;11:830. doi: 10.3390/v11090830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. R Core Team; Vienna, Austria: 2013. [(accessed on 19 May 2021)]. Available online: https://www.R-project.org/ [Google Scholar]
- 37.Wellinghausen N., Voss M., Ivanova R., Deininger S. Evaluation of the SARS-CoV-2-IgG Response in Outpatients by Five Commercial Immunoassays. GMS Infect. Dis. 2020;8:Doc22. doi: 10.3205/id000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber M.C., Risch M., Thiel S.L., Grossmann K., Nigg S., Wohlwend N., Lung T., Hillmann D., Ritzler M., Ferrara F., et al. Characteristics of Three Different Chemiluminescence Assays for Testing for SARS-CoV-2 Antibodies. Dis. Markers. Res. Artic. Hindawi. 2021 doi: 10.1155/2021/8810196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Catry E., Jacqmin H., Dodemont M., Saad Albichr I., Lardinois B., de Fays B., Delaere B., Closset M., Laurent T., Denis O., et al. Analytical and Clinical Evaluation of Four Commercial SARS-CoV-2 Serological Immunoassays in Hospitalized Patients and Ambulatory Individuals. J. Virol. Methods. 2021;289:114060. doi: 10.1016/j.jviromet.2020.114060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turbett S.E., Anahtar M., Dighe A.S., Beltran W.G., Miller T., Scott H., Durbin S.M., Bharadwaj M., Thomas J., Gogakos T.S., et al. Evaluation of Three Commercial SARS-CoV-2 Serologic Assays and Their Performance in Two-Test Algorithms. J. Clin. Microbiol. 2020;59 doi: 10.1128/JCM.01892-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan S.S., Saw S., Chew K.L., Wang C., Pajarillaga A., Khoo C., Wang W., Ali Z.M., Yang Z., Chan Y.H., et al. Comparative Clinical Evaluation of the Roche Elecsys and Abbott Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Serology Assays for Coronavirus Disease 2019 (COVID-19) Arch. Pathol. Lab. Med. 2021;145:32–38. doi: 10.5858/arpa.2020-0499-SA. [DOI] [PubMed] [Google Scholar]
- 42.Trabaud M.-A., Icard V., Milon M.-P., Bal A., Lina B., Escuret V. Comparison of Eight Commercial, High-throughput, Automated or ELISA Assays Detecting SARS-CoV-2 IgG or Total Antibody. J. Clin. Virol. 2020;132:104613. doi: 10.1016/j.jcv.2020.104613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan S.S., Saw S., Chew K.L., Huak C.Y., Khoo C., Pajarillaga A., Wang W., Tambyah P., Ong L., Jureen R., et al. Head-to-head Evaluation on Diagnostic Accuracies of Six SARS-CoV-2 Serological Assays. Pathology. 2020;52:770–777. doi: 10.1016/j.pathol.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charlton C.L., Kanji J.N., Johal K., Bailey A., Plitt S.S., MacDonald C., Kunst A., Buss E., Burnes L.E., Fonseca K., et al. Evaluation of Six Commercial Mid- to High-Volume Antibody and Six Point-of-Care Lateral Flow Assays for Detection of SARS-CoV-2 Antibodies. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.01361-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theel E.S., Harring J., Hilgart H., Granger D. Performance Characteristics of Four High-Throughput Immunoassays for Detection of IgG Antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.01243-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higgins V., Fabros A., Wang X.Y., Bhandari M., Daghfal D.J., Kulasingam V. Anti-SARS-CoV-2 IgM Improves Clinical Sensitivity Early in Disease Course. Clin. Biochem. 2021;90:1–7. doi: 10.1016/j.clinbiochem.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higgins V., Fabros A., Kulasingam V. Quantitative measurement of anti-SARS-CoV-2 antibodies: Analytical and clinical evaluation. J. Clin. Microbiol. 2021;59 doi: 10.1128/JCM.03149-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaffner A., Risch L., Aeschbacher S., Risch C., Weber M.C., Thiel S.L., Jüngert K., Pichler M., Grossmann K., Wohlwend N., et al. Characterization of a Pan-Immunoglobulin Assay Quantifying Antibodies Directed against the Receptor Binding Domain of the SARS-CoV-2 S1-Subunit of the Spike Protein: A Population-Based Study. J. Clin. Med. 2020;9:3989. doi: 10.3390/jcm9123989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mei J.V., Alexander J.R., Adam B.W., Hannon W.H. Use of Filter Paper for the Collection and Analysis of Human Whole Blood Specimens. J. Nutr. 2001;131:1631S–1636S. doi: 10.1093/jn/131.5.1631S. [DOI] [PubMed] [Google Scholar]
- 50.Iankov I., Viker K., Turgeon C., Matern D., Galanis E. Parameters of Immunoglobulin Extraction from Dried Blood Spot Cards and Immunoassays for Detection of Antibody Response to Pathogens Including the Novel SARS-CoV-2. J. Immunol. Methods. 2021;492:112996. doi: 10.1016/j.jim.2021.112996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suthar M.S., Zimmerman M.G., Kauffman R.C., Mantus G., Linderman S.L., Hudson W.H., Vanderheiden A., Nyhoff L., Davis C.W., Adekunle O., et al. Rapid Generation of Neutralizing Antibody Responses in COVID-19 Patients. Cell Rep. Med. 2020;1:100040. doi: 10.1016/j.xcrm.2020.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suhandynata R.T., A Hoffman M., Huang D., Tran J.T., Kelner M.J., Reed S.L., McLawhon R.W., E Voss J., Nemazee D., Fitzgerald R.L. Commercial Serology Assays Predict Neutralization Activity Against SARS-CoV-2. Clin. Chem. 2021;67:404–414. doi: 10.1093/clinchem/hvaa262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang M.S., Case J.B., Franks C.E., Chen R.E., Anderson N.W., Henderson J.P., Diamond M.S., Gronowski A.M., Farnsworth C.W. Association between SARS-CoV-2 Neutralizing Antibodies and Commercial Serological Assays. Clin. Chem. 2020;66:1538–1547. doi: 10.1093/clinchem/hvaa211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., Mills R., Teng E., Kamruzzaman M., Garcia-Beltran W.F., et al. Dynamics and Significance of the Antibody Response to SARS-CoV-2 Infection. MedRxiv. 2020 doi: 10.1101/2020.07.18.20155374. [DOI] [Google Scholar]
- 55.Mattiuzzo G., Bentley E.M., Hassall M., Routley S., Richardson S., Bernasconi V., Kristiansen P., Harvala H., Roberts D., Semple M.G., et al. WHO/BS.2020.2403 Establishment of the WHO International Standard and Reference Panel for Anti-SARS-CoV-2 Antibody. World Health Organization; Geneva, Switzerland: 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.