Abstract
Background
The scale-up of indoor residual spraying and long-lasting insecticidal nets, together with other interventions have considerably reduced the malaria burden in The Gambia. This study examined the biting and resting preferences of the local insecticide-resistant vector populations few years following scale-up of anti-vector interventions.
Method
Indoor and outdoor-resting Anopheles gambiae mosquitoes were collected between July and October 2019 from ten villages in five regions in The Gambia using pyrethrum spray collection (indoor) and prokopack aspirator from pit traps (outdoor). Polymerase chain reaction assays were performed to identify molecular species, insecticide resistance mutations, Plasmodium infection rate and host blood meal.
Results
A total of 844 mosquitoes were collected both indoors (421, 49.9%) and outdoors (423, 50.1%). Four main vector species were identified, including An. arabiensis (indoor: 15%, outdoor: 26%); An. coluzzii (indoor: 19%, outdoor: 6%), An. gambiae s.s. (indoor: 11%, outdoor: 16%), An. melas (indoor: 2%, outdoor: 0.1%) and hybrids of An. coluzzii-An. gambiae s.s (indoors: 3%, outdoors: 2%). A significant preference for outdoor resting was observed in An. arabiensis (Pearson X2 = 22.7, df = 4, P<0.001) and for indoor resting in An. coluzzii (Pearson X2 = 55.0, df = 4, P<0.001). Prevalence of the voltage-gated sodium channel (Vgsc)-1014S was significantly higher in the indoor-resting (allele freq. = 0.96, 95%CI: 0.78–1, P = 0.03) than outdoor-resting (allele freq. = 0.82, 95%CI: 0.76–0.87) An. arabiensis population. For An. coluzzii, the prevalence of most mutation markers was higher in the outdoor (allele freq. = 0.92, 95%CI: 0.81–0.98) than indoor-resting (allele freq. = 0.78, 95%CI: 0.56–0.86) mosquitoes. However, in An. gambiae s.s., the prevalence of Vgsc-1014F, Vgsc-1575Y and GSTe2-114T was high (allele freq. = 0.96–1), but did not vary by resting location. The overall sporozoite positivity rate was 1.3% (95% CI: 0.5–2%) in mosquito populations. Indoor-resting An. coluzzii had mainly fed on human blood while indoor-resting An. arabiensis fed on animal blood.
Conclusion
In this study, high levels of resistance mutations were observed that could be influencing the mosquito populations to rest indoors or outdoors. The prevalent animal-biting behaviour demonstrated in the mosquito populations suggest that larval source management could be an intervention to complement vector control in this setting.
Introduction
Successful implementation of indoor residual spraying (IRS) and long-lasting insecticidal nets (LLINs) has hugely contributed to the malaria decline observed in sub-Saharan Africa [1]. These interventions reduce transmission by primarily limiting human contact with human-feeding (anthropophagic), indoor-feeding (endophagic) and indoor-resting (endophilic) vectors [2]. Unfortunately, these measures also induce selection for physiological and behavioral resistance in vector populations, resulting in reduced mosquito susceptibility to most of the current insecticides used for LLINs and IRS [3], as well as increased exophilic behavioral phenotypes in primarily endophilic vectors [4]. Moreover, residual transmission partly driven by high LLINs and IRS use, is maintained by vectors with physiological and behavioral resistance [5]. Therefore, studying the behavioral dynamics of vector populations during the scale up of vector control interventions will assist in determining the appropriate response to emerging behavioral changes.
Malaria burden in The Gambia has declined significantly over the last decades with vector control approaches being a major component of intervention, coordinated and implemented by The Gambia National Malaria Control Program (GNMCP). Following the World Health Organization (WHO) Global Plan for Insecticide Resistance Management (GPIRM), the GNMCP has consistently implemented rotational use of different classes of insecticides for IRS, to curtail dichlorodiphenyltrichloroethane (DDT) and deltamethrin resistance. For IRS, DDT was replaced initially by deltamethrin and bendiocarb, and since 2017 by pirimiphos-methyl (actellic 300CS) [6]. Similarly, LLINs intervention has been stable over the years and Gambia has recorded successful LLINs coverage as high as 90% [7, 8].
Despite such successes, residual transmission has become increasingly spatially heterogeneous, with its intensity increasing from western to eastern Gambia, and could have been driven by specific vector population dynamics [7]. The major vector species, namely Anopheles arabiensis, An. coluzzii and An. gambiae sensu stricto (s.s.) are variably distributed throughout the country. An. arabiensis is most prevalent in the eastern Gambia while An. coluzzii and An. gambiae s.s. inhabit the western region [9, 10]. However, An. arabiensis has been recently found throughout the country [11], indicating possible replacement due to successful control of other sibling species [12, 13]. Moreover, the population prevalence of each vector species varies by season, whereby An. arabiensis and An. coluzzii are dominant throughout the rainy season, while An. gambiae s.s. become rarest early in the onset of dry season [9, 10]. DDT and pyrethroid resistance has been reported at various degrees in all vectors, that continue to be highly susceptible to carbamates and organophosphates [11, 14, 15].
Host seeking and resting behavior of vectors are important metrics to evaluate the impact of control and resistance management strategies [16]. Vector behavioral adaptation, resistance selection and persistent transmission could increase during extensive scale-up of interventions, and this information can only be captured by real-time surveillance [17, 18]. Hence, national malaria control programs should actively monitor behavioral dynamics in the local vector population, to inform decisions.
In the Gambia, DDT and pyrethroid resistance is widespread and associated with residual transmission [11, 14]. However, the effect of control activities on vectors feeding and resting behavior remains unclear. The biting and resting preferences of An. gambiae sensu lato (s.l.) populations was investigated in The Gambia following few years of intensive vector control interventions.
Materials and methods
Ethical clearance
Ethical approval was obtained from The Gambia Government/Medical Research Council (MRC) Unit The Gambia at London School of Hygiene and Tropical Medicine (LSHTM) Joint Ethics Committee. The permit Number was: SCC 1586.
Anopheles gambiae s.l. collection
Indoor and outdoor-resting adult mosquitoes were sampled from July to October 2019, during the malaria transmission season across five administrative regions in The Gambia, namely Central River Region (CRR), Lower River Region (LRR), North Bank Region (NBR), Upper River Region (URR) and West Coast Region (WCR) (Fig 1). WCR is a coastal area characterized by mangrove swamps. The remaining regions are mainly inland and have forest vegetation. Rice is mainly cultivated in CRR while cereals farming is common in all regions. Two villages were selected from each region and most of the villages are GNMCP surveillance sites with high LLIN and IRS coverage. Malaria transmission is highest in URR compared to other regions in The Gambia [7].
Fig 1. Map of The Gambia showing the study sites, comprising two villages each from the 5 administrative regions in the country.
Indoor-resting mosquitoes were collected from sleeping rooms using pyrethrum spray collection (PSC). Twenty houses per village, at least 50m apart from each other, were randomly selected. In each village, collections were done for two consecutive days, with ten houses sampled per day. Outdoor-resting mosquitoes were sampled from pit shelter traps using prokopak aspirator. Three pit shelter traps that were 10m away from the selected compounds, were placed at different parts in each village. Both indoor and outdoor collections were conducted from 06.00 am to 09.00am in every collection day.
Mosquito identification
Morphological identification of female An. gambiae s.l. was done using identification keys as described by Gillies & Coetzee [19]. Afterwards, mosquitoes were stored individually in 96% ethanol in 1.5ml Eppendorf tube until DNA extraction. DNA was extracted separately from abdomen and head/thoraces of individual mosquitoes using Qiagen QIAxtractor robot. Species-specific genotyping PCR to identify An. arabiensis, An. melas and An. gambiae was performed using specific primers to discriminate the species as previously done [20]. This was followed by restriction enzyme digestion to specifically identify An. coluzzii, An. gambiae s.s. and their hybrids (An. coluzzii-An. gambiae s.s.) [21].
Insecticide resistance markers identification
Screening for molecular markers of target-site resistance to carbamates, DDT, pyrethroids and organophosphates was done on all samples using a probe-based assay (TaqMan SNP genotyping) [22]. The following markers were investigated: voltage-gated sodium (Vgsc)-1014F, Vgsc-1014S and Vgsc-1575Y associated with target-site mutation to DDT and pyrethroids [23–25]. Acetylcholine esterase (Ace)-119S, marker for carbamate and organophosphate resistance [26] and glutathione-S-transferase epsilon 2 (Gste2)-114T, involved in metabolic resistance to DDT [27] were also assayed. The TaqMan allelic discrimination assay used is a multiplex real time PCR, where primers and probes specific for each insecticide target gene were employed to discriminate susceptible (wild type) and resistant (mutant) alleles based on probe fluorescence signals [28].
Plasmodium sporozoite detection
DNA extracted from mosquito head and thoraces was used to detect sporozoites of Plasmodium falciparum, P. ovale, P. malariae and P. vivax species, employing TaqMan SNP genotyping protocol [29] which enables discriminatory identification of circum-sporozoites (CSPs) of P. falciparum from P. ovale, P. malariae and P. vivax CSPs. Genomic DNA specific to each of these Plasmodium species were analyzed in each assay as positive controls.
Blood meal identification
Extracted DNA from engorged mosquito abdomens were amplified using modified multiplex PCRs with specific primers that amplify cytochrome B genes of human and animal hosts including chicken, cow, dog, donkey, goat, horse and pig [30, 31].
Statistical analyses
The proportion of each mosquito species in relation to the total number of mosquitoes captured from each region was calculated in percentage, as well as allele frequencies of indoor and outdoor-resting mosquitoes. Sporozoite positivity rate was the proportion of PCR positive mosquitoes among all mosquitoes tested. Human (HBI) and animal blood meal indices were estimated as the proportion of mosquitoes positive for human or animal hosts among those positive for all hosts. Mean differences between HBI and animal blood meal indices by vector species and resting locations were analyzed by ANOVA. Statistical analyses were done using Stata/IC 15.0 (2017 StataCorp LP).
Results
Anopheles species distribution and their resting behavior
A total of 844 An. gambiae s.l. mosquitoes were collected from the five regions. Four main vector species were identified, namely An. arabiensis (N = 350, 41%); An. coluzzii (N = 214, 25%), An. gambiae s.s. (N = 224, 27%) and An. melas (N = 17, 2%). Hybrids of An. coluzzii-An. gambiae s.s. were also detected (N = 39, 5%). Most mosquitoes were collected from URR (642, 76%), followed by LRR (97, 11%) and then the other regions (Fig 2).
Fig 2. Distribution of Anopheles gambiae s.l by region as collected indoors and outdoors.
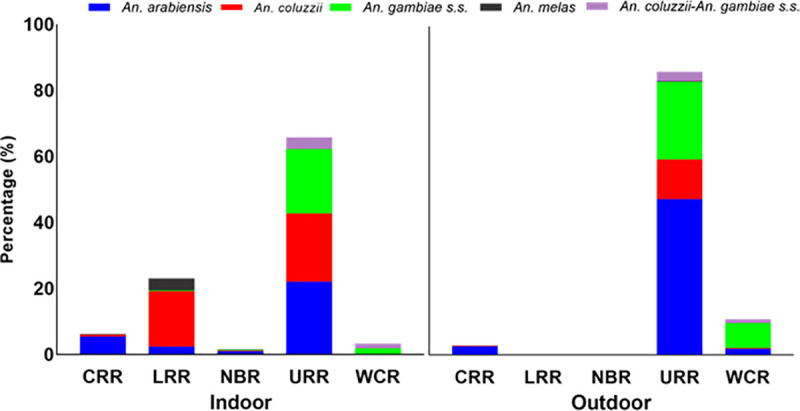
An. coluzzii-An. gambiae s.s. are the hybrids of An. coluzzii and An. gambiae s.s. Mosquitoes were collected from 5 regions: CRR- central river region, LRR-lower river region. NBR- north bank region. URR-upper river region and WCR- West coast region.
Overall, the number of mosquitoes resting indoors (421, 49.9%) and outdoors (423, 50.1%) were similar. Nevertheless, the resting preference varied by species. A significantly higher proportion of An. arabiensis were found outdoor (26.1%) than indoor (15.4%) (Pearson X2 = 22.7, df = 4, P<0.001) while both An. coluzzii (19.1% indoor and 6.3% outdoor, Pearson X2 = 55.0, df = 4, P<0.001) and An. melas (1.9% indoor and 0.1% outdoor, Pearson X2 = 13.3, df = 4, P<0.01) preferred resting indoor. For An. gambiae s.s. (10.9% indoor and 15.6% outdoor, Pearson X2 = 7.0, df = 4, P = 0.14) and An. coluzzii-An. gambiae s.s. hybrids (2.6% indoor and 2% outdoor, Pearson X2 = 0.7, df = 4, P = 0.45), there was no significant difference between resting indoor and outdoor. In URR, the region with the highest malaria transmission in The Gambia, An. arabiensis was most abundant vector (45.8%, 294) (indoor: 14.5%, outdoor: 31.3%), followed by An. gambiae s.s. (28.4%, 182) (indoor: 12.8%, outdoor: 15.6%) and An. coluzzii (21.5%, 138) (indoor: 13.6%, outdoor: 7.9%). No An. gambiae s.s. was collected in CRR while An. melas was mainly found in LRR (N = 15). All mosquitoes collected from LRR and NBR were resting indoors. The hybrids of An. coluzzii and An. gambiae s.s. were mainly found in URR (indoor: 2.3%, outdoor: 1.9%) and WCR (indoor: 10%, outdoor: 8.3%).
Distribution of voltage-gated sodium channel (Vgsc) mutation markers in the vectors
Vgsc point mutations associated with DDT and pyrethroid resistance were highly prevalent and detected at varying frequencies in all vector species across all regions. Overall, An. arabiensis was found resting indoors when resistance allele frequency was higher in the indoor population, whereas An. coluzzii were resting outdoors with higher outdoor resistance. No consistent resting preference was observed in An. gambiae in the presence of mutations.
Vgsc-1014S mutation was found predominantly in indoor-resting vector populations (Table 1). In An. arabiensis, the mutation was more frequent in the indoor-resting than outdoor-resting mosquitoes regardless of the region. Vgsc-1014S was also the only mutation identified in An. gambiae s.s. and An. melas when found resting indoors.
Table 1. Frequencies of insecticide resistance alleles on VGSC, GST and AChE loci in Anopheles gambiae s.l. populations from all study regions.
| Region | Anopheles species | Vgsc-1014F | Vgsc-1014S | Vgsc-1575Y | GSTe2-114T | Ace1-119S | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoor | Outdoor | Indoor | Outdoor | Indoor | Outdoor | Indoor | Outdoor | Indoor | Outdoor | ||
| URR | An. arabiensis | 0.05 | 0.02 | 0.91 | 0.82 | 0 | 0.004 | 0 | 0.01 | 0 | 0 |
| N = 5 | N = 4 | N = 85 | N = 164 | N = 1 | N = 1 | ||||||
| An. coluzzii | 0.74 | 0.92 | 0.25 | 0.04 | 0.68 | 0.9 | 0.78 | 0.9 | 0 | 0 | |
| N = 64 | N = 47 | N = 22 | N = 2 | N = 59 | N = 46 | N = 68 | N = 46 | ||||
| An. gambiae s.s. | 1 | 0.99 | 0 | 0.01 | 0.96 | 0.98 | 0.98 | 0.99 | 0.05 | 0.04 | |
| N = 82 | N = 99 | N = 1 | N = 79 | N = 98 | N = 80 | N = 99 | N = 4 | N = 4 | |||
| An. coluzzii-An. gam biae s.s | 0.93 | 1 | 0 | 0 | 0.87 | 1 | 0.87 | 0 | 0.07 | 0 | |
| N = 14 | N = 12 | N = 13 | N = 12 | N = 13 | N = 1 | ||||||
| LRR | An. arabiensis | 0 | - | 0.9 | - | 0 | - | 0.1 | - | 0 | - |
| N = 9 | N = 4 | N = 1 | |||||||||
| An. coluzzii | 0.66 | - | 0.3 | - | 0 | - | 0 | - | 0 | - | |
| N = 47 | N = 21 | ||||||||||
| An. gambiae s.s | 0 | - | 1 | - | 0 | - | 0 | - | 0 | - | |
| N = 1 | |||||||||||
| An. melas | 0 | - | 1 | - | 0 | - | 0 | - | 0 | - | |
| N = 15 | |||||||||||
| WCR | An. arabiensis | - | 0.13 | - | 0.88 | - | 0 | - | 0 | - | 0 |
| N = 1 | N = 7 | ||||||||||
| An. coluzzii | - | 1 | - | 0 | - | 0 | - | 0 | - | 0 | |
| An. gambiae s.s. | 0.25 | 0.13 | 0.75 | 0.84 | 0.13 | 0.06 | 0 | 0 | 0 | 0 | |
| N = 2 | N = 4 | N = 6 | N = 27 | N = 1 | N = 2 | ||||||
| An. coluzzii-An. gam biae s.s | 0 | 0 | 1 | 1 | 0 | 0 | 0.17 | 0 | 0 | - | |
| N = 6 | N = 5 | N = 1 | |||||||||
| CRR | An. arabiensis | 0 | 1 | 0.96 | 0 | 0 | 0 | 0.1 | 0.27 | 0 | 0 |
| N = 11 | N = 22 | N = 3 | N = 3 | ||||||||
| An. coluzzii | - | 1 | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| An. melas | 0 | - | 1 | - | 0 | - | 0 | - | - | 0 | |
Vgsc- voltage-gated sodium channel. GSTe2-glutathione-s-transferase epsilon 2. Ace1-Acetylcholine esterase1. N = number of mosquitoes positive for respective resistance marker.
In An. arabensis resting indoors in URR, Vgsc-1014S frequency was significantly higher (Z = 2.230, P = 0.03) in the indoor- (allele freq. = 0.91, 95%CI: 0.84–0.96) than outdoor-resting (allele freq. = 0.82, 95%CI: 0.76–0.87) mosquitoes. Moreover, Vgsc-1014S was the only mutation identified in this species when found resting indoors (allele freq. = 0.96, 95%CI: 0.78–1) in CRR. Whereas in An. coluzzii in URR, Vgsc-1014S mutation was higher in the indoor (allele freq. = 0.25, 95%CI: 0.17–0.36) than outdoor-resting mosquitoes (allele freq. = 0.04, 95%CI: 0.005–1.3) but this was not statistically significant (Z = 0.965, P = 0.33). In LRR, the mutation was found only in indoor-resting mosquitoes (allele freq. = 0.3, 95%CI: 0.19–0.42). In WCR, the mutation was common in An. gambiae s.s. and higher among outdoor- (allele freq. = 0.84, 95%CI: 0.67–0.95) than indoor-resting (allele freq. = 0.75, 95%CI: 0.35–0.97) mosquitoes, with no significant difference (Z = 0.510, P = 0.61).
Vgsc-1014F was almost fixed in most mosquitoes, except An. arabiensis. It was also more common in the outdoor- than indoor-resting mosquitoes. Specifically in URR, the mutation was found to be significantly higher (Z = 2.956, P = 0.003) in outdoor-resting (allele freq. = 0.92, 95%CI: 0.81–0.98) than the indoor-resting An. coluzzi population (allele freq. = 0.74, 95%CI: 0.63–0.82). Likewise, in the hybrid population of An. coluzzi and An. gambiae s.s., the mutation was fixed and higher in the outdoor-resting (allele freq. = 1, 95%CI: 0.74–1) than indoor-resting (allele freq. = 0.93, 95%CI: 0.80–1) mosquitoes but not statistically significant (Z = 1.027, P = 0.3). The mutation was similarly fixed in both the indoor (allele freq. = 1, 95%CI: 0.96–1) and outdoor (allele freq. = 0.99, 95%CI: 0.95–1) An. gambiae s.s. populations. Although in WCR, Vgsc-1014F was more frequent in An. gambiae s.s. resting indoors (allele freq. = 0.25, 95%CI: 0.03–0.65) than those outdoors (allele freq. = 0.13, 95%CI: 0.04–0.29), the difference was not statistically significant (Z = 0.688, P = 0.49). Whereas in LRR, where only mosquitoes resting indoors were caught, this mutation was most common in An. coluzzii (allele freq. = 0.66, 95%CI: 0.81–0.98).
Vgsc-1575Y and GSTe2-114T were found mostly in URR and were more frequent in outdoor-resting mosquitoes. The mutations were almost fixed in An. gambiae s.s. regardless of resting place (allele freq. = 0.96–1, 95% CI: 0.92–1.2). In An. coluzzii, these mutations were significantly higher (Vgsc-1575Y: Z = 3.343, P = 0.001. GSTe2-114T: Z = 1.948, P = 0.05) in those resting outdoors (allele freq: 0.9, 95% CI: 0.79–0.97) than in their indoor-resting counterpart (allele freqs: Vgsc-1575Y = 0.68, 95% CI: 0.57–0.77. GSTe2-114T = 0.78, 95% CI: 0.68–0.86). An. coluzzii -An. gambiae s.s. hybrids with higher and fixed Vgsc-1575Y mutation were equally found resting outdoors (allele freq. = 1, 95% CI: 0.74–1) while those found resting indoors were carrying only the GSTe2-114T mutation (allele freq. = 0.87, 95% CI: 0.60–0.98).
The carbamate and organophosphate resistance marker, acetylcholine esterase (Ace)-119S was detected only in 8 (4 indoor and 4 outdoor) An. gambiae s.s. and in one hybrid specimen in URR.
Sporozoite infection rate
Plasmodium falciparum sporozoites were detected in 11 out of 844 mosquitoes (Table 2), representing a 1.3% (95% CI: 0.5–2%) infection rate. All the infected mosquitoes were caught in URR, of which six were resting indoors and five resting outdoors. Outdoor-resting An. arabiensis were mostly infected (36%, 4/11), followed by indoor-resting An. gambiae s.s. (27%, 3/11) and indoor-resting An. arabiensis (18%, 2/11). One each of outdoor-resting An. coluzzii and An. coluzzii-An. gambiae s.s. hybrid were also infected.
Table 2. Sporozoite positivity rate in the eleven vector species that were infected based on their resting locations.
| An. arabiensis Proportion (n) | An. coluzzii Proportion (n) | An. gambiae s.s. Proportion (n) | An. coluzzii-An. gambiae s.s. proportion (n) | |
|---|---|---|---|---|
| Indoor | 0.18 (2) | 0.09 (1) | 0.27 (3) | 0 |
| Outdoor | 0.36 (4) | 0 | 0 | 0.09 (1) |
n = number of mosquitoes positive for sporozoite detection. Proportion = the number positive per species divided by overall positive (11).
Host blood meal preference
Host blood meal origin was determined in 251 randomly selected engorged mosquito abdomens. Overall, animal and human blood meal indices were higher for indoor- than outdoor-resting mosquitoes (Table 3). In all vector species, most blood meal (91%) were from animal origin. Indoor-resting An. coluzzii had the highest preference for human blood while indoor-resting An. arabiensis had most preference for animal blood. Regardless of their resting location, all vector species preferred cow and donkey blood meal compared to other animals. Most vectors rarely fed on chicken and horse.
Table 3. Human and animal blood meal preferences of the indoor and outdoor-resting vector species in combined study sites.
| An. arabiensis | An. coluzzii | An. gambiae s.s. | An. coluzzii-An. gambiae s.s. | |||||
|---|---|---|---|---|---|---|---|---|
| Indoor(n) | Outdoor(n) | Indoor(n) | Outdoor(n) | Indoor(n) | Outdoor(n) | Indoor(n) | Outdoor(n) | |
| Human | 0.01(3) | 0.004(1) | 0.03(7) | 0 | 0.008(2) | 0.008(2) | 0 | 0.004(1) |
| Cow | 0.09(22) | 0.02(6) | 0.06(14) | 0.03(8) | 0.09(22) | 0.08(21) | 0.03(7) | 0.02(4) |
| Chicken | 0 | 0 | 0 | 0 | 0 | 0.004(1) | 0 | 0 |
| Dog | 0.008(2) | 0.004(1) | 0 | 0.004(1) | 0 | 0.004(1) | 0 | 0 |
| Donkey | 0.11(27) | 0.12(31) | 0.06(14) | 0.04(11) | 0.02(6) | 0.03(8) | 0.004(1) | 0.008(2) |
| Goat | 0.02(4) | 0.008(2) | 0.008(2) | 0.008(2) | 0.004(1) | 0.004(1) | 0.004(1) | 0 |
| Horse | 0.004(1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Human + animals | 0.004(1) | 0.004(1) | 0.01(3) | 0 | 0.008(2) | 0 | 0 | 0 |
| Mixed animals | 0.008(2) | 0 | 0 | 0.004(1) | 0 | 0.004(1) | 0 | 0 |
| HBI | 2 | 0.8 | 4 | 0 | 1 | 0.8 | 0 | 0.4 |
| Animal blood indices | 23 | 16 | 12 | 9 | 12 | 13 | 4 | 2 |
Proportion (number). HBI = Human blood index.
Discussion
Insecticide resistance is currently widespread among malaria vectors in The Gambia [11, 14], resulting in insecticide rotation for IRS. Currently, actellic, an organophosphate insecticide is being used, whilst LLINs impregnated with deltamethrin and permethrin as recommended by WHO are distributed widely [32]. This study assessed how such vector interventions have influenced the feeding and resting behaviour, as well as malaria transmission dynamics of the vector population. Anopheles arabiensis showed a marked preference for outdoor resting and An. coluzzii and An. melas for indoor resting. An. gambiae s.s. and An. coluzzii-An. gambiae s.s. hybrid populations were found mostly resting outdoors, but with no statistical difference from those resting indoors. Moreover, local vectors had a marked preference for animal blood. The overall sporozoite infection rate was low and infectious mosquitoes were mainly outdoor-resting An. arabiensis.
In this study, high frequencies of Vgsc-1014F, Vgsc-1575Y and GSTe2-114T were recorded in the malaria vector populations resting indoors and outdoors. These mutations were particularly at saturation in both indoor and outdoor-resting An. gambiae s.s. populations in URR, as well as in most of the An. coluzzii-An. gambiae s.s. hybrid samples analyzed. This magnitude of the DDT and pyrethroid-associated resistance mutations [23, 24, 27] recorded in the vector populations is worrying. These might have been as a result of extensive coverage of IRS and LLINs across The Gambia [7, 8]. These findings suggest that effectiveness of LLINs may be compromised in the local vector populations as pyrethroids remain the only public health-approved insecticide for LLINs [32]. Selection for resistance could have resulted from the extensive use of pyrethroids for vector control [7, 8] and pest control in agriculture [33, 34] in this region. Moreover, the level of resistance found in the An. coluzzii-An. gambiae s.s. hybrid population suggests an extensive gene flow between An. gambiae and An. coluzzii [35–37] in these settings. These observations are a concern for a possible setback to the ongoing anti-vector efforts; thus deserve close monitoring.
A possible influence of genotypic resistance on the resting behaviour of the vector populations [38], was observed from this study. A higher proportion of An. arabiensis that was found resting indoors harboured the Vgsc-1014S mutation whereas An. coluzzii exhibited an outdoor-resting behavior when all mutation markers except Vgsc-1014S were higher in the outdoor than indoor population. Anopheles gambiae s.s. with extremely high mutations were also found resting both indoors and outdoors. These indicate that resistance could be driving An. coluzzii and An. gambiae s.s. from their usual indoor resting to outdoor resting behaviour [4, 39, 40], a trait that could promote residual transmission [5, 17, 40]. Indeed, indoor resting behaviour demonstrated in the resistant vectors despite the presence of IRS and LLINs suggests that resistance could be protecting these vectors against the effect of insecticides used indoors [41, 42]. This has been previously reported from studies in Kenya [43, 44], where An. gambiae s.s and An. arabiensis that had higher frequencies of Vgsc-1014F and Vgsc-1014S respectively, were predominantly found resting indoors. Similarly, association between genotypic resistance and outdoor-resting behavior was earlier found in An. coluzzii [45, 46]. Any resistance-driven resting behavior in these vectors could further limit the success of anti-vector interventions currently being scaled-up in these settings [47, 48].
The majority of the vector populations analyzed had a marked preference for animal than human blood meal. Anopheles arabiensis demonstrated predominant preference for animal blood meal than other vector species. This is not surprising as An. arabiensis is known to be highly zoophilic [49, 50]. However, the proportion of vectors resting indoors and that have taken a blood meal either from human and animal source, could be a concern for the effectiveness of vector control measures. As animals were found only in outdoor locations in the study sites, this finding shows that the vectors took blood meal from animals outdoors and later went indoors to rest regardless of the presence of IRS and LLINs, indicating that these vectors are resistant to the insecticides being used [51]. Furthermore, the observed choice of animal blood by majority of the vectors could lead to increase in vector population that may eventually resort to biting humans in the long run and become difficult to control. Notably, alternative vector control methods such as treatment of animals with endectocides [52] and larval source management [53] could be promising tools that could be adopted by the Gambia National Malaria Control Program.
This study recorded an overall low sporozoite rate in the vector populations. Given the current low malaria prevalence in The Gambia [7], a low sporozoite rate is expected. This may reflect the impact of the scaled-up in IRS and LLINs program in the study sites which seems to successfully limit mosquito access to human blood meal indoors and consequently reducing transmission, as previously reported [54, 55]. Indeed, the study sites benefitted from improved housing projects conducted in country which aimed at reducing mosquito survival as well as malaria transmission [56].
The composition of the vector species was consistent with previous studies in the Gambia where the most abundant vector was An. arabiensis, followed by An. gambiae s.s. and, An. coluzzii along with their hybrids [11, 14, 15]. Low density of An. melas found was as a result of our choice of villages in the West, which were not located in the coastal regions where this species breeds in salty water [57]. Remarkably, predominance of An. arabiensis could be as a result of its outdoor-resting preference to avoid insecticide used in IRS and LLINs [58, 59]. This leaves the highly anthropophilic and endophilic species more exposed to vector interventions, possibly leading to relative advantage that maintains the exophilic population and malaria transmission [59].
Conclusion
This study observed high levels of resistance mutations in the local vectors that could be influencing their resting behaviour. As The Gambia is in earnest preparation for pre-elimination phase of malaria, the magnitude of resistance mutations observed in the vectors in this study suggests that vectors could pose great challenges for their control using the present control paradigm. Therefore, use of larval source management as a complementary vector control measure is highly recommended.
Supporting information
(XLSX)
Acknowledgments
We thank Messrs Musa Jawara, and Mamlie Touray for their assistance in the field work for this study.
Data Availability
All relevant data are within the paper and its S1 File.
Funding Statement
This work was supported by funds from a Wellcome Trust DELTAS Africa grant (DEL-15-007: Awandare). Majidah Hamid-Adiamoh was supported by a WACCBIP-Wellcome Trust DELTAS PhD fellowship. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (107755/Z/15/Z: Awandare) and the UK government. Additional support was also provided by the H3Africa PAMGENe project, H3A/18/002, funded by the AAS; and the National Institutes of Health (D43 TW 011513).
References
- 1.WHO. World Malaria Report. 2020. Geneva: World Health Organization; 2020. [Google Scholar]
- 2.Killeen GF, Smith TA. Exploring the contributions of bed nets, cattle, insecticides and excitorepellency to malaria control: a deterministic model of mosquito host-seeking behaviour and mortality. Trans R Soc Trop Med Hyg. 2007;101:867–880. doi: 10.1016/j.trstmh.2007.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranson H, Guessan RN, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African Anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2010;1–8. doi: 10.1016/j.pt.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 4.Gatton ML, Chitnis N, Churcher T, Donnelly MJ, Ghani AC, Charles HJ, et al. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution. 2013;67(4):1218–1230. doi: 10.1111/evo.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J. 2014;13(1):330. doi: 10.1186/1475-2875-13-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gambia National Malaria Control Programme (GNMCP). Malaria Indicator Survey, Banjul. 2011.
- 7.Mwesigwa J, Okebe J, Affara M, Di Tanna GL, Nwakanma D, Janha O, et al. On-going malaria transmission in The Gambia despite high coverage of control interventions: A nationwide cross-sectional survey. Malar J. 2015;14(1):1–9. doi: 10.1186/s12936-015-0829-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinder M, Jawara M, Jarju LBS, Salami K, Jeffries D, Adiamoh M, et al. Efficacy of indoor residual spraying with dichlorodiphenyltrichloroethane against malaria in Gambian communities with high usage of long-lasting insecticidal mosquito nets: A cluster-randomised controlled trial. Lancet. 2015;385(9976):1436–46. doi: 10.1016/S0140-6736(14)61007-2 [DOI] [PubMed] [Google Scholar]
- 9.Caputo B, Nwakanma D, Jawara M, Adiamoh M, Dia I, Konate L, et al. Anopheles gambiae complex along the Gambia river, with particular reference to the molecular forms of An. gambiae s.s. Malar J. 2008; 7:182. doi: 10.1186/1475-2875-7-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jawara M, Pinder M, Drakeley CJ, Nwakanma DC, Jallow E, Bogh C, et al. Dry season ecology of Anopheles gambiae complex mosquitoes in the Gambia. Malar J. 2008;7:156. doi: 10.1186/1475-2875-7-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Opondo KO, Jawara M, Cham S, Jatta E, Jarju L, Camara M, et al. Status of insecticide resistance in Anopheles gambiae (s.l.) of the Gambia. Parasites and Vectors. 2019;12(1):1–8. doi: 10.1186/s13071-018-3256-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitau J, Oxborough RM, Tungu PK, Matowo J, Malima RC, Magesa SM, et al. Species shifts in the Anopheles gambiae complex: Do LLINs successfully control Anopheles arabiensis? PLoS One. 2012; 7:e31481. doi: 10.1371/journal.pone.0031481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sougoufara S, Harry M, Doucouré S, Sembène PM, Sokhna C. Shift in species composition in the Anopheles gambiae complex after implementation of long-lasting insecticidal nets in Dielmo, Senegal. Med Vet Entomol. 2016;30(3):365–368. doi: 10.1111/mve.12171 [DOI] [PubMed] [Google Scholar]
- 14.Opondo KO, Weetman D, Jawara M, Diatta M, Fofana A, Crombe F, et al. Does insecticide resistance contribute to heterogeneities in malaria transmission in the Gambia? Malar J. 2016;15(1):1–10. doi: 10.1186/s12936-016-1203-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson AL, Pinder M, Bradley J, Donnelly MJ, Hamid-Adiamoh M, Jarju LBS, et al. Emergence of knock-down resistance in the Anopheles gambiae complex in the Upper River Region, the Gambia, and its relationship with malaria infection in children. Malar J. 2018;17(1):205. doi: 10.1186/s12936-018-2348-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padonou G, Sezonlin M, Gbedjissi G, Ayi I, Azondekon R DA, et al. Biology of Anopheles gambiae and insecticide resistance: Entomological study for a large scale of indoor residual spraying in south east Benin. J Parasitol Vector Biol. 2011;3(4):59–68. [Google Scholar]
- 17.Shcherbacheva A, Haario H, Killeen G. Modeling host-seeking behavior of African malaria vector mosquitoes in the presence of long-lasting insecticidal nets. Math Biosci. 2017;295:36–47. doi: 10.1016/j.mbs.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 18.Govella NJ, Chaki PP, Killeen GF. Entomological surveillance of behavioural resilience and resistance in residual malaria vector populations. Malar Journal. 2013;12:124. doi: 10.1186/1475-2875-12-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillies MT, Coetzee M. A Supplement to the Anophelinae of the South of the Sahara (Afrotropical Region). Publications of the South African Institute for Medical Research. 1987; 55: 1–143. [Google Scholar]
- 20.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993; doi: 10.4269/ajtmh.1993.49.520 [DOI] [PubMed] [Google Scholar]
- 21.Fanello C, Santolamazza F, Della Torre A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med Vet Entomol. 2002; 16:461–464. doi: 10.1046/j.1365-2915.2002.00393.x [DOI] [PubMed] [Google Scholar]
- 22.Bass C, Nikou D, Donnelly MJ, Williamson MS, Ranson H, Ball A, et al. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: A comparison of two new high-throughput assays with existing methods. Malar J. 2007;6:111. doi: 10.1186/1475-2875-6-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones CM, Liyanapathirana M, Agossa FR, Weetman D, Ranson H, Donnelly MJ, et al. Footprints of positive selection associated with a mutation (N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc Natl Acad Sci. 2012;109:6614–6619. doi: 10.1073/pnas.1201475109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Bergé JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–184. doi: 10.1046/j.1365-2583.1998.72062.x [DOI] [PubMed] [Google Scholar]
- 25.Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9:491–497. doi: 10.1046/j.1365-2583.2000.00209.x [DOI] [PubMed] [Google Scholar]
- 26.Djogbénou L, Weill M, Hougard JM, Raymond M, Akogbéto M, Chandre F. Characterization of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae (Diptera: Culicidae): resistance levels and dominance. J Med Entomol. 2007;44(5):805–10. doi: 10.1603/0022-2585(2007)44[805:coiaai]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- 27.Mitchell SN, Rigden DJ, Dowd AJ, Lu F, Wilding CS, Weetman D, et al. Metabolic and target-site mechanisms combine to confer strong DDT resistance in Anopheles gambiae. PLoS One. 2014;9(3): e92662. doi: 10.1371/journal.pone.0092662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bass C, Nikou D, Vontas J, Williamson MS, Field LM. Development of high-throughput real-time PCR assays for the identification of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae. Pestic Biochem Physiol. 2010;96:80–85. [Google Scholar]
- 29.Bass C, Nikou D, Blagborough AM, Vontas J, Sinden RE, Williamson MS, et al. PCR-based detection of Plasmodium in Anopheles mosquitoes: A comparison of a new high-throughput assay with existing methods. Malar J. 2008;7:177. doi: 10.1186/1475-2875-7-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kent RJ, Thuma PE, Mharakurwa S, Norris DE. Seasonality, blood feeding behavior, and transmission of Plasmodium falciparum by Anopheles arabiensis after an extended drought in southern Zambia. Am J Trop Med Hyg. 2007;76:267–274. [PMC free article] [PubMed] [Google Scholar]
- 31.Kent RJ and Norris D E. Identification of Mammalian Blood Meals in Mosquitoes by a Multiplexed Polymerase Chain Reaction Targeting Cytochrome B. Am J Trop Med Hyg. 2005;73(2):336–342. [PMC free article] [PubMed] [Google Scholar]
- 32.WHO. Guidelines for malaria vector control 2019. Geneva: World Health Organization; 2019. [PubMed] [Google Scholar]
- 33.Nkya T, Poupardin R, Laporte F, Akhouayri I, Mosha F, Magesa S, et al. Impact of agriculture on the selection of insecticide resistance in the malaria vector Anopheles gambiae: a multigenerational study in controlled conditions. Parasit Vectors. 2014;7:480. doi: 10.1186/s13071-014-0480-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reid MC, McKenzie FE. The contribution of agricultural insecticide use to increasing insecticide resistance in African malaria vectors. Malar J. 2016;15:107. doi: 10.1186/s12936-016-1162-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dabiré KR, Diabaté A, Djogbenou L, Ouari A, N’Guessan R, Ouédraogo JB, et al. Dynamics of multiple insecticide resistance in the malaria vector Anopheles gambiae in a rice growing area in South-Western Burkina Faso. Malar J. 2008;7:188. doi: 10.1186/1475-2875-7-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tripet F, Dolo G, Lanzaro GC. Multilevel analyses of genetic differentiation in Anopheles gambiae s.s. reveal patterns of gene flow important for malaria-fighting mosquito projects. Genetics. 2005;169(1):313–24. doi: 10.1534/genetics.104.026534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Djogbénou L, Chandre F, Berthomieu A, Dabiré R, Koffi A, Alout H, et al. Evidence of introgression of the ace-1(R) mutation and of the ace-1 duplication in West African Anopheles gambiae s. s. PLoS One. 2008;3(5):e2172. doi: 10.1371/journal.pone.0002172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stump AD, Atieli FK, Vulule JM, Besansky NJ. Dynamics of the pyrethroid knockdown resistance allele in western Kenyan populations of Anopheles gambiae in response to insecticide-treated bed net trials. Am J Trop Med Hyg. 2004;70(6): 591–596. [PubMed] [Google Scholar]
- 39.Kreppel KS, Viana M, Main BJ, Johnson PCD, Govella NJ, Lee Y, et al. Emergence of behavioural avoidance strategies of malaria vectors in areas of high LLIN coverage in Tanzania. Sci Rep. 2020;10(1):14527. doi: 10.1038/s41598-020-71187-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyers JI, Pathikonda S, Popkin-Hall ZR, Medeiros MC, Fuseini G, Matias A, et al. Increasing outdoor host-seeking in Anopheles gambiae over 6 years of vector control on Bioko Island. Malar J. 2016; 26(15):239. doi: 10.1186/s12936-016-1286-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silva APB, Santos JMM, Martins AJ, Tadei W, Thatcher B, Santos J, et al. Mutations in the voltage-gated sodium channel gene of anophelines and their association with resistance to pyrethroids–a review. Parasit Vectors. 2014;7(1):450. doi: 10.1186/1756-3305-7-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kabula B, Kisinza W, Tungu P, Ndege C, Batengana B, Kollo D, et al. Co-occurrence and distribution of East (L1014S) and West (L1014F) African knock-down resistance in Anopheles gambiae sensu lato population of Tanzania. Trop Med Int Heal. 2014;19:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machani MG, Ochomo E, Amimo F, Kosgei J, Munga S, Zhou G, et al. Resting behaviour of malaria vectors in highland and lowland sites of western Kenya: Implication on malaria vector control measures. PLoS One. 2020;15:e0224718. doi: 10.1371/journal.pone.0224718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Owuor KO, Machani MG, Mukabana WR, Munga S, Yan G, Ochomo E, et al. Insecticide resistance status of indoor and outdoor resting malaria vectors in a highland and lowland site in Western Kenya. PLoS One. 2021;16(3):e0240771. doi: 10.1371/journal.pone.0240771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamid-Adiamoh M, Amambua-Ngwa A, Nwakanma D, D’Alessandro U, Awandare GA, Afrane YA. Insecticide resistance in indoor and outdoor-resting Anopheles gambiae in Northern Ghana. Malar J. 2020;19(1):314. doi: 10.1186/s12936-020-03388-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bamou R, Mbakop LR, Kopya E, Ndo C, Awono-Ambene P, Tchuinkam T, et al. Changes in malaria vector bionomics and transmission patterns in the equatorial forest region of Cameroon between 2000 and 2017. Parasit Vectors. 2018;11:464. doi: 10.1186/s13071-018-3049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Churcher TS, Lissenden N, Griffin JT, Worrall E, Ranson H. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. Elife. 2016;5:e16090. doi: 10.7554/eLife.16090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ranson H, Lissenden N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016;32(3):187–196. doi: 10.1016/j.pt.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 49.Main BJ, Lee Y, Ferguson HM, Kreppel KS, Kihonda A, Govella NJ, et al. The Genetic Basis of Host Preference and Resting Behavior in the Major African Malaria Vector, Anopheles arabiensis. PLoS Genet. 2016;12(9):e1006303. doi: 10.1371/journal.pgen.1006303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asale A, Duchateau L, Devleesschauwer B, Huisman G, Yewhalaw D. Zooprophylaxis as a control strategy for malaria caused by the vector Anopheles arabiensis (Diptera: Culicidae): A systematic review. Infect Dis Poverty. 2017;6(1):160. 54. doi: 10.1186/s40249-017-0366-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Machani MG, Ochomo E, Sang D, Bonizzoni M, Zhou G, Githeko AK, et al. Influence of blood meal and age of mosquitoes on susceptibility to pyrethroids in Anopheles gambiae from Western Kenya. Malar J. 2019;18(1):1–9. doi: 10.1186/s12936-018-2635-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaccour C, Killeen GF. Mind the gap: Residual malaria transmission, veterinary endectocides and livestock as targets for malaria vector control. Malar J. 2016;15:24.55. doi: 10.1186/s12936-015-1063-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tusting LS, Thwing J, Sinclair D, Fillinger U, Gimnig J, Bonner KE, et al. Mosquito larval source management for controlling malaria. Cochrane Database Syst Rev. 2013;2013(8):CD008923. doi: 10.1002/14651858.CD008923.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Musiime AK, Smith DL, Kilama M, Rek J, Arinaitwe E, Nankabirwa JI, et al. Impact of vector control interventions on malaria transmission intensity, outdoor vector biting rates and Anopheles mosquito species composition in Tororo, Uganda. Malar J. 2019;18:445. doi: 10.1186/s12936-019-3076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abong’o B, Gimnig JE, Torr SJ, Longman B, Omoke D, Muchoki M, et al. Impact of indoor residual spraying with pirimiphos-methyl (Actellic 300CS) on entomological indicators of transmission and malaria case burden in Migori County, western Kenya. Sci Rep. 2020; 10:4518. doi: 10.1038/s41598-020-61350-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lindsay SW, Jawara M, Mwesigwa J, Achan J, Bayoh N, Bradley J, et al. Reduced mosquito survival in metal-roof houses may contribute to a decline in malaria transmission in sub-Saharan Africa. Sci Rep. 2019;9(1):7770. doi: 10.1038/s41598-019-43816-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bryan JH, Petrarca V, Di Deco MA, Coluzzi M. Adult behaviour of members of the Anopheles gambiae complex in the Gambia with special reference to An. melas and its chromosomal variants. Parassitologia. 1987;29: 221–249. [PubMed] [Google Scholar]
- 58.Durnez L, Coosemans M. Residual transmission of Malaria: An Old Issue for New Approaches. Anopheles mosquitoes—New insights into Malar vectors. 2013;671–704. [Google Scholar]
- 59.Killeen GF, Govella NJ, Lwetoijera DW, Okumu FO. Most outdoor malaria transmission by behaviourally-resistant Anopheles arabiensis is mediated by mosquitoes that have previously been inside houses. Malar J. 2016;15(1):1–10. doi: 10.1186/s12936-016-1280-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its S1 File.


