Abstract
Background
Patients with haematologic malignancies are increasingly treated by oral anticancer medications, heightening the challenge of ensuring optimal adherence to treatment. However, except for chronic myelogenous leukaemia or acute lymphoid leukaemia, the extent of non-adherence has rarely been investigated in outpatient settings, particularly for migrant population. With growing numbers of migrants in Belgium, identifying potential differences in drug use is essential. Also, previous research regarding social determinants of health highlight important disparities for migrant population. Difficulties in communication between health caregivers and patients from different cultural and ethnic backgrounds has been underlined.
Methods
Using a sequential mixed method design, the MADESIO protocol explores the adherence to oral anticancer medications in patients with haematological malignancies and among first and second generation migrants of varied origin. Conducted in the ambulatory setting, a first quantitative strand will measure adherence rates and associated risk factors in two sub-groups of patients with haematological malignancies (group A: first and second generation migrants and group B: non-migrants). The second qualitative strand of this study uses semi-structured interviews to address address the patients’ subjective meanings and understand the statistical associations observed in the quantitative study (strand one). MADESIO aims to provide a first assessment of whether and why migrants constitute a population at risk concerning adherence to oral anticancer medications.
Discussion
Our protocol is designed to provide a comprehensive understanding of adherence in a specific population. The methodological choices applied allow to explore adherence among patients from diverse linguistic and cultural backgrounds. A particular emphasis has been paid to minimize the biases and increase the reliability of the data collected. Easily reproductible, the MADESIO design may help healthcare services to screen adherence to Oral anticancer medications and to guide providers in choosing the best strategies to address medication adherence of migrants or minority diverse population.
1. Background
For the last two decades, the use of oral anticancer medications (OAMs) has been increasing, and oncological teams must deal with the challenge of ensuring optimal adherence to prescribed treatments. Poor adherence impacts the quality of response and the risk of relapse [1, 2]. However, data are scarce regarding how cancer patients adhere to their medication plan, particularly those with malignancies other than breast cancer [3].
Moreover, oncohaematology appears to be the poor cousin to cancer adherence studies. Of the 51 studies involved in the latest 2016 systematic review [4] focusing to adherence to OAMs, 30 focused on breast cancer compared to 9 on haematological cancer patients, all pertaining to patients with either chronic myelogeneous leukemia (CML) or acute lymphoïd leukemia (ALL).
Due to the wide variety of methods used, the lack of standardisation in defining optimal adherence and the considerable differences in length of study follow-up or methodological quality, adherence values range widely across studies. Although no reliable estimate of adherence to OAMs can be gleaned from the literature, the data suggest that a substantial proportion of patients struggle to adhere to these medications as prescribed, with adherence declining over time [4].
In oncohaematology, reported adherence ranges from 20% to 53% for CML patients and from 6% to 35% for ALL patients [5]. Today, apart from CML and ALL, several other haematological malignancies are increasingly treated by self-administered OAMs [6], many of which involve long and complex treatment regimens. Frequent dose adjustment due to toxicities or comorbidities, in Belgium, are associated with the non-recording of prescribed dosages and considerably limit large-scale retrospective measures of adherence in this context. This paucity in research results in an overview of factors that impact only CML and ALL patients.
In Belgium, to our knowledge, the only available data were reported by the ‘ADAGIO’ study [1]. Limited to CML patients, this study allowed us to recognise that, despite the gravity of their disease, as well as the fear of relapse or death, non-adherence to the oral chemotherapy medication imatinib was more frequent than physicians and their family members believed. Patient-physician interactions were innovatively investigated and identified as a contributing variable to adherence. Unfortunately, the study suffered from serious underrepresentation of patients from diverse minority populations. This is even more restricting, in that studies suggest considerable disparities and difficulties in communication between doctors and patients from different cultural and ethnic backgrounds [7–10]. Considering that medication adherence is a complex interplay of predisposing factors, patients’ knowledge and beliefs and patient-physician interactions [2, 9, 10], the underrepresentation of migrants and ethnic minorities (MEM) in adherence studies is critical. It may challenge the generalisability of the results of these cancer clinical trials to ethnic minority patients [11].
The scientific literature on health disparities mainly focuses on the use or access to healthcare services. The topic of MEM adherence to OAM is far less studied. The ethnic background has been frequently reported to be a predictor of poor adherence in other chronic diseases [12–15], but even in this field, those studies presented important limitations that prevented definite conclusions [16].
Yet, disquieting facts may raise concerns about a higher risk of poor adherence among MEM. They have been identified as one of the most vulnerable groups in health provision and have worse outcomes in cancer [17–20]. If these health disparities are largely explained by the fact that MEM are often part of the most vulnerable category of patients due to their lower educational, social and socioeconomic status, even after adjustment for socioeconomic variables, the risk of morbidity for MEM remains higher [7]. This suggests these patients are exposed to other risk factors [8, 9]. In Belgium, MEM are also confronted with barriers when using health services [21].
Disparities in health and health care not only affect the groups facing disparities, but also limit overall gains in quality of care and health for the broader population and result in unnecessary costs. Addressing health disparities is increasingly important as the population becomes more diverse [22]. The Brussels capital region has the highest concentration of population of foreign nationality in Belgium, with 34.7% foreigners on 1 January 2018 and 56.7% without Belgian nationality at birth [23]. In the context of the emergence of novel oral therapeutics in oncohaematology, it becomes urgent to identify any potential differences in the use of OAMs between our patients with or without a migrant background.
1.A Adherence, a concept to be considered
A number of terms, e.g. ‘compliance’, ‘adherence’, ‘observance’, ‘persistence’ or ‘concordance’ are currently used to define different aspects of the act of seeking medical attention, acquiring prescriptions and taking medicines appropriately. Sometimes, these are used interchangeably, but they however impose different views about the relationship between the patient and the health care provider [24].
Since the initial definition of compliance, referring to the extent to which patient behaviour coincides with clinical prescription [25], the definition of adherence has progressively evolved with an ever-increasing emphasis on patient agreement. In 2003, the World Health Organisation (WHO) defined adherence to long-term therapy as ‘the extent to which a person’s behaviour–taking medication, following a diet, and/or executing lifestyle changes–corresponds with agreed recommendations from a health care provider’ [26]. Some continue to criticise this reductive definition as the patient seems to only have the option to agree with the subscriber’s recommendations. In our opinion, this is going down the wrong path.
Adherence can be described as two complementary dimensions: adherence to medication and therapeutic adherence. The first can be defined as the degree to which the patient follows the prescribed medication. It refers to the behaviour, i.e. the measurable, visible and quantifiable part of adherence and comprises three components: 1) the initiation of treatment, referring to when the patient takes the first dose of a prescribed medication; 2) the implementation of the dosing regimen, defined as the extent to which a patient’s actual dosing corresponds to the prescribed dosing regimen, from initiation until the last dose taken; and 3) discontinuation, corresponding to the end of therapy, when the next dose to be taken is omitted and no more doses are taken thereafter [24]. Therapeutic adherence rather refers to the patient’s level of acceptance of the therapeutic project. This dimension recognises that the patient, as an empowered actor, must ‘adhere’ to therapy and not only ‘submit’ to its prescription. Considering this, allows us to understand adherence not as a stable behaviour over time but rather submitted to changing environmental and psychological factors. To grasp all the stakes of care practice, we propose a mixed method able to measure both quantifiable behaviour and explore the patient’s subjectivity regarding disease and treatment.
2. Methods/Design
2.A. Objectives
The objectives are: 1) to measure, in two subgroups of migrant and non-migrant patients with haematological malignancies, medication adherence and persistence regarding OAMs; 2) to identify the associated risk factors and; 3) to understand any observed differences between the two subgroups.
To meet these objectives, we developed a mixed method approach able of tackling the challenges of studying adherence in haematology and in first generation (FG) and second generation (SG) migrants of different origins.
2.B. Design
Conducted in the outpatient clinics of two hospitals, the MADESIO mixed-method explanatory design will combine sequentially a quantitative questionnaire-based study with an in depth qualitative approach [Fig 1]. The first four-visit questionnaire-based survey will measure adherence to OAMs and identify associated risk factors in two sub-groups of at least 60 migrant and 53 non-migrant haematological cancer patients. The second qualitative phase, using semi-structured interviews, will more deeply address the patients’ subjective meanings, trying to understand any association observed in the quantitative results.
Fig 1. MADESIO explanatory design.
The quantitative and qualitative phases are connected on two levels. Firstly, the quantitative results will instruct the appropriate participants to be selected for the qualitative phase. Secondly, the quantitative phase will focus the results that need to be examined in more detail in the qualitative study. A six-month intermediate analysis will be performed on the quantitative results.
2.C. Clinical setting
The Institut Jules Bordet (IJB) and the Centre Hospitalier Universitaire Saint-Pierre (CHUStP) are two hospitals located in Brussels, the capital of Belgium, and separated by a single street.
The CHUStP is a local general university hospital and the IJB is a comprehensive cancer centre involved in research and teaching. Both are part of the Iris network of Brussels public and academic hospitals whose mission is to attend every patient. To prevent any discrimination in their large population of foreign origin, they have implemented a linguistic assistance and intercultural mediation service.
Located in the centre of Brussels, their oncohaematological outpatient settings offer an ideal setting to explore adherence in the context of interethnic and intercultural medical consultations. The patients managed in oncohaematology include a high proportion of FG and SG migrants [27]. Half of them were born abroad and 40% do not have French or Dutch as their mother language. In contrast, but similar to the rest of Belgium [28], the number of foreigner doctors is limited and to a large extent from neighbouring countries and with no language barrier (the Netherlands, France, Germany) or to two traditional immigration countries for Belgium, namely Italy and Morocco (the latter also with limited language barriers since French is a widespread language).
2.D. Population and methods
This prospective trial is two-fold.
2.D.1. Quantitative questionnaire-based study
To measure adherence and identify associated risk factors, relevant data will be collected and questionnaires distributed on four successive visits, as detailed in Fig 2. For patient comfort, visits are dispersed according to the usual follow-up frequency of the patient, which depends on the haematological malignancy and disease stage. Visit 2 might be in Month 1, 2 or 3, visit 3 in Month 3, 4 or 6, and visit 4 in Month 6 or 9.
Fig 2. MADESIO questionnaire-based survey participant timeline.
Study population. About 113 patients with haematological malignancies will be enrolled over 12 months, divided into two subgroups of 60 migrants and 53 non-migrants.
As the definition and operationalisation of ‘migrant’ will determine the categories of health inequalities and thus influence both the measurement of these inequalities and the actions taken to tackle them, defining who counts as a migrant is of crucial importance [29].
There is still no consensus on a single definition of ‘migrant’. Migrants might be defined by foreign birth, by foreign citizenship or by their movement into a new country to stay temporarily (sometimes for as little as a year) or to settle for the long term [30]. If we do not consider the duration of movement, a migrant is generally accepted as any person who is moving or has moved across an international border or within a state away from his/her habitual place of residence, regardless of (1) the person’s legal status; (2) whether the movement is voluntary or involuntary; (3) what the causes of movement are; or (4) what the length of stay is [31]. But, if the concept of usual residence exists and is certainly valuable for large migration statistical frameworks [32], the challenges posed by its definition makes it inoperative at the prospective clinical scale.
According to our local context and study objectives, it seems relevant to define as ‘first generation (FG) migrants’ the group of foreign-born persons and as ‘second generation (SG) migrants’ people native-born but holding either a foreign nationality or having one or both parents foreign-born. This definition, by integrating SG migrants, avoids restricting the issue to the newly arrived immigrants and allows us to address groups that have been part of the country’s history for over half a century [33]. SG native-born migrants may potentially be part of a group that share minority status in Belgium due to ethnicity, place of birth, language, religion, citizenship and other cultural differences. As they may practice a different mother tongue and or different cultural norms and values from the majority culture, they may also be affected by ethnic inequalities in health.
Sample size estimation. The sample sizes were calculated to answer our first objective of measuring adherence to OAMs in migrants and non-migrants with haematological malignancies. In the absence of pre-existing data in migrant populations with haematological malignancies in Belgium, adherence prevalence was estimated based on mainly foreign scientific literature. The number of 60 migrant and 53 non-migrant patients were respectively calculated based on an estimated prevalence of adherence of 60% [5, 34] and 70% [3, 34, 35], considering a confidence interval of 95% with a precision of 10% and a non-response rate of 20%.
Eligibility of participants. Eligible patients are adult patients with a haematological malignancy, having taken at least one OAM since minimum 30 days and having a minimum of six months life expectancy. Patients should be able to read and fully understand French, Dutch, English, Arabic, Polish or Romanian. Language selection was based on Belgian and Brussels migration statistics, hospital statistics and the experience of the haematologists [36, 37].
We decided to exclude illiterate patients as they are particularly vulnerable to communication issues. Expressing oneself and reflecting on one’s health condition requires some ability for abstraction which is not equally present in illiterate people. Certainly, the enrolment of these patients would have required an alternative oral qualitative approach requiring more time and human resources than what was available. By restricting the study to literate patients, we considerably reduced the need for assistance from intercultural mediators (IM). This is particularly valuable as the participation in research activities is not foreseen in the tasks of IM and their availability is extremely variable.
Recruitment strategies. The screening and enrolment process was conducted by a researcher who had access to consultation planning and patient medical files. All eligible patients are listed and selected in running order.
Briefly, the purpose of the study is presented during an initial phone call. If the patient agrees, a half-hour information visit is scheduled during the next regular follow-up visit. In case of a language barrier, the contact person mentioned in the patient’s medical file is invited and the information visit is organised in the presence of an IM. After this information session, informed consent is obtained from the patient.
Self-reported measures of adherence and theoretical framework. Numerous methods for measuring adherence have been described, but no single method performs well on all criteria [38, 39]. All objective and subjective measures have advantages and disadvantages [40–47]. Whether objective or subjective, more than presenting different tools, each method appears to capture different information on medication taking [48] and should be assumed to be complementary. A combination is therefore recommended to increase the validity and reliability of the collected adherence data. Among the key elements that need to be considered when selecting self-reported measures of adherence is the fact that adherence tools must be developed based on a theoretical framework and a qualitative exploratory phase [49].
The nature, extent, and determinants of non-adherent behaviours are complex. Despite extensive research, a limited understanding of adherence phenomena and the absence of a standard theoretical framework suitable for all populations for empirically testing adherence outcomes against the determinants are common challenges for adherence research [50]. There are no theories of adherence per se, but various models and theories [51–53] exist to predict the variability that characterises behavioural adherence.
From a theoretical medical anthropology perspective, Kleinman [54] argued that health care outcomes such as adherence are directly related to the degree of cognitive disparity between the explanatory models of practitioner and patient. People’s health beliefs from other cultures are often not concordant with those of western healthcare professionals, increasing beyond the language barrier the risk of misunderstanding or disagreement. Others confirm that misunderstandings are more frequent in ethnic minority patients and often result in non-adherence [8].
However, we cannot ignore the fact that there are many other theoretical models assessing other factors to explain adherence behaviours patients with migrant background. Lower socioeconomic conditions, disparities in beliefs and expectations about disease or treatment and/or miscommunication between patients and physicians are a few examples of them [12]. Patients may exhibit different types of non-adherent behaviours. Unintentional non-adherence may be due to forgetfulness, or the inability to follow treatment instructions due to poor understanding or physical problems such as poor eyesight or dexterity, whereas intentional non-adherence arises when the patient rejects either the doctor’s diagnosis or the doctor’s recommended treatment [55, 56]. It would be biased to restrict our analysis to lower socioeconomic conditions, language discordance and cultural differences to explain any disparities regarding adherence behaviours in the migrant population.
Considering what each existing scale really measures and how they have been validated [58], we selected complementary tools assessing the factors identified as relevant domains of interest in the study of adherence behaviours, both generally and within ethnic minorities.
Outcome measures of adherence. Among the scales able to measure medication-taking behaviour, the Tool for Adherence Behaviour Screening (TABS) [51] and the Morisky Medication Adherence Scale (MMAS-8) [57] allow for screening both intentional and unintentional non-adherence. If the reasons behind intentional an unintentional non-adherence are distinct, the strategies for addressing them are as well. Therefore, separate measures for these two types of non-adherence are essential, especially when regression is used to determine the predictors of non-adherence.
The MMAS-8 was designed to both measure medication-taking behaviour and to identify barriers to adherence. It has demonstrated a significant correlation with objective measures of adherence [58, 59] and it is already validated for cancer conditions in several languages. Items of the TABS are worded both positively and negatively within the same section to avoid acquiescence, affirmation or agreement bias. Furthermore, the thoughtful wording of the items allows the screening of both underutilisation and overutilisation.
These two 8-item scales have a short pass time and can easily be repeated at every visit. As medication adherence may vary and decrease over time due to a range of factors [3], this repetition is essential to evaluate treatment continuity.
Beliefs about medicines that may influence adherence must be screened, particularly when exploring adherence among culturally diverse population. On visit 2, the patient additionally completes the Beliefs and Behaviour Questionnaire (BBQ) [51] and the Beliefs about Medicine Questionnaire (BMQ) [60].
The BBQ appears particularly relevant for a first exploration. Not limited to any specific model for predicting non-adherence, clinically useful, simple, socially and culturally relevant, this 21 close-ended questionnaire covers the various themes of adherence in adequate depth [61]. The 18 items of the BMQ quantify and compare a patient’s personal beliefs about the necessity of their prescribed medication and their concerns about taking it. Patients who believe their medication to be necessary and have more concerns regarding how to take it have consistently been shown to be more adherent in a range of diseases [62–65].
Additional outcome measures. On visit 3, the patient completes the Human Connection Scale (HCS) to measure his/her appraisal of the therapeutic alliance, i.e. the collaborative bond between the patient and the haematologist [66]. Considering that patients with a migrant background are less likely to form a therapeutic alliance with their physician [67], the patient’s appraisal of interactions seems a critical domain of interest in the study of adherence in the context of interethnic or intercultural consultation.
Additionally, the patient completes the Hospital and Anxiety Depression Scale (HADS) [68], a simple, reliable tool to screen both anxiety and depression in people with physical health problems. Depression, anxiety, fears or anger about the illness can bring about an adverse attitude towards therapy, which can affect medication adherence [69]. Our study will invariably enrol patients with various haemopathies and prognosis, more or less further along in treatment, who experience different levels of stress or anxiety. Furthermore, migrants have specific risks and exposures to violence and stressful life experiences related to the migration process [70, 71].
Other variables. During the first visit, the researcher collects the full socio-demographic, economic, educational, linguistic and migratory characteristics necessary for the analysis. For patient declining to participate, we seek their consent to collect main sociodemographic data in order to assess if the respondent sample is representative of the eligible population.
Taking advantage of the presence of the IM, the researcher orally collects a 5-item medication questionnaire exploring patient’s general behaviours regarding medicines to determine whether they receive assistance in preparing or taking medicines, they use alternative or complementary medicines or have implemented changes in their lifestyle to improve their health.
At the end of the visit, the patient receives and completes an auto-administered social desirability scale. The social desirability is usually defined as ‘the tendency of individuals to present themselves favourably with respect to current social norms and standard’ [72] and is considered a potential and typical bias in the measurement of self-reported adherence [73]. Considering that social desirability concerns may differ across cultures, this tendency must be captured when studying differences in self-reported health behaviours, beliefs or experience between different cultural groups. By adding the Social Desirability Scale-17 (SDS-17) [74], we improve our ability to assess whether observed differences may be the reflection of differences in willingness to report such behaviour or beliefs.
In parallel with every visit, clinical data are collected from patient medical records: diagnosis, disease stage, prescribed drug regimen, side effects, concomitant medications, comorbidities and performance status. Also, contextual information such as the presence of family members, language(s) used for communication, assistance of an (non)-professional interpreter, estimation of patient’s level of French proficiency or the duration of the consultation, are provided by the physician.
Strategies to enhance the quality of self-reported measures of adherence. The quality of self-reported measures of adherence may be enhanced through efforts to use validated scales, assess the proper construct or reduce social desirability bias [75, 76]. Those were considered and implemented throughout the study design.
Firstly, we only selected questionnaires that demonstrated adequate reliability and validity in the same or similar target groups. Furthermore, compared to interview-based self-reports, we preferred auto-administrated questionnaires as they are less susceptible to information bias and interviewer effects [77].
Secondly, several efforts were made to strengthen the patient’s feeling of anonymity and confidentiality. Patients are contacted and informed by the same researcher, initially trained in anthropology and familiar with interview techniques. Dressed in civilian clothes, the researcher provides the information and collects informed consent in a private consultation box, away from other patients and professionals. In parallel, questionnaires are pre-coded with the patient study number and visit date and distributed in the same Identity-coded envelope. The patient can therefore complete the questionnaires when convenient during the hospital visit and slide it into one of the numerous mailboxes available before leaving the hospital. Only the researcher can link the identity study number to the patient’s name and has access to respondent answers.
Thirdly, both written and orally, the study objectives are presented without ever using the word ‘adherence’. The words used further underline the willingness to understand their disease and treatment experience preventing them from feeling the need to be a ‘good patient’, which may lead to overinflating of adherence rates. It is key to emphasise that there is no judgment about taking or not taking their treatment as prescribed and to indicate that our interest is about their feelings and experience. With the same objective, each adherence scale starts with a statement normalising non-adherence, recognising the challenges of taking regular medications.
Finally, to avoid any unwilling changes in haematologist attitudes or any increased motivation related to the Hawthorne effect, patient enrolment and data processing are wholly independent of them. For the same reason, participating haematologists ignore the content of the questionnaires.
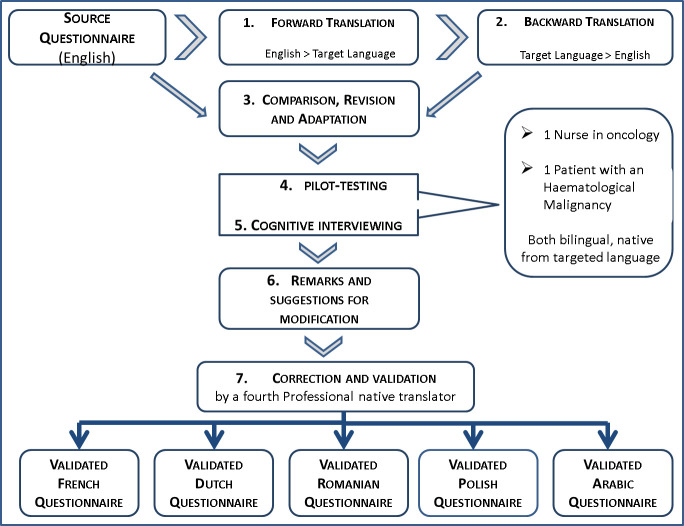
Translation and validation of selected instruments. Although using an existing validated questionnaire will save time and resources, it may not be readily available in the language required for the targeted respondents [78]. Among the selected questionnaires, only the HADS was already validated in six translated versions. For others, we established a rigorous, appropriate and user-friendly valid translation method. According to several recommendations, we combined different translation techniques and applied the steps inspired by WHO guidelines and described in Fig 3 [78–82].
Fig 3. Translation and validation process of selected instruments.
1)The original English source questionnaire was translated by a linguistic and culturally competent professional translator. 2) Another independent translator converted back the translated version to English and 3) the researcher ranked each item in terms of comparability of language and interpretability. All discrepancies, misunderstandings or unclear wordings were discussed with a third independent translator and retranslated if needed. 4) The pre-final translated version was pilot-tested with one bilingual patient with a haematological malignancy and one bilingual nurse in oncology. 5) A cognitive debriefing allowed to check if the translated items retained the same meaning as the original, and to assess the clarity and appropriateness of wording. 6) All suggestions for linguistic and cultural adaptation were discussed with the researcher, the two bilingual respondents and the third professional translator until a consensus was reached (7).
In some cases, the return of the instrument to the authors was necessary to clarify the original meaning of a statement; in other cases, translated versions were adapted to treatment or language specificities.
The MMAS-8 was originally built to assess medication-taking behaviours in chronic diseases and includes the following question: ‘Did you take your medication yesterday?’. Haematological cancer patients might face various intricate medication regimens. Some take an OAM only 21 days on 30, so that if the study visit is scheduled during the 7 interruption days, the question is not relevant to measuring adherence. As patients always came in for consultation on the first day of their new 21-day cycle, the question was changed to ‘Did you take your medication today?’
The terms ‘cancer’ and ‘oral anticancer medication’ were respectively changed to ‘haematological disease’ and the patient’s specific drug name. This allowed us to bypass the dilemma existing in some languages or cultures regarding the use of the word ‘cancer’ while respecting the patient’s perception, who may not define their malignancy as a cancer but as a disease.
The interpretability of the BBQ item ‘I put up with my medical problems before taking any action’ was unobvious. The confusing interpretation of this item was not revealed by the forward and back-translation process. But, during the cognitive interviews, the meanings given by the respondents varied greatly. Some understood it as making an effort to not take a medicine for unpleasant symptoms, and by others as making an effort to not seek medical care when they have a medical problem. Others wanted to ensure that all their cancer-related problems were under control before starting any sport activity. After clarifying the meaning with the developers of the questionnaire, we changed the original item to: ‘When I experience medical problems, I delay seeking medical treatment or help from doctors’. The authors of BBQ think that more adherent patients are more likely to seek medical care (as opposed to self-treatment) in the presence of symptoms, and that worry about health in general is positively correlated with action.
Finally, the BBQ item ‘It is physically difficult to handle some of my medications’ also required clarification from the developers. After backward translation, ‘to handle’ became ‘to take’ in Polish and ‘to use’ in Dutch, with the related risk of some interpreting this as difficulty with manipulating the medication or others as difficulty with swallowing. The BBQ authors confirmed the original meaning of the item referring to the ‘difficulty with handling my medication due to dexterity issues’.
Now that the translated versions are no longer confusing, and the meaning has been preserved, the final version will be implemented for all patients enrolled in the study.
Statistical analysis. Data will be recorded using the Survey Processing System 7.4© and will be analysed using STATA version 15. Descriptive statistics will be performed. For each subgroup, the mean and standard deviation (SD) or the median and interquartile range, depending on the distribution, will be calculated for each score. For categorical variables, Pearson’s chi-squared test will be used; for continuous variables, Student’s t-test will be used. P-values <0.05 will be considered significant.
2.D.2. Qualitative semi-structured interviews
Quantitative research methods have significant limitations in capturing the complexity of human behaviour and experience, tending to ignore the social and discursive contexts in which individual and collective understandings of illness experience emerge [83]. Understanding the meaning that patients give to their illness and treatment experience may be crucial to explain the statistical associations between variables observed in the quantitative study.
At this stage, we can only hypothesise that adherence rates or associated risk factors may be different between the subgroups of migrants and non-migrants. In any case, the qualitative study will help us to understand why these results differ or not.
McGill Illness Narrative Interview. The McGill Illness Narrative interview (MINI) was designed to elicit the individual meaning of illness, the modes of reasoning, the historical sequences and the sociocultural contexts of illness experience [84]. Addressing the discursive contexts in which individual and collective understandings of illness experience emerge may contribute to understanding any potential association between personal or cultural beliefs and adherence behaviours.
The semi-structured framework of the MINI allows for a wide range of interpretative strategies, from critical and interpretative anthropology [85] to literary theory [84, 86, 87] via grounded theory [88]. At this stage and given the lack of previous existing data in similar population or context, the fact that theory can inductively emerge from collected data is an asset. If Kleinman [54] argues that medication adherence is directly related the degree of cognitive disparity between the explanatory models of practitioner and patient, Weiner [89] reminds us that patients facing a serious illness such as haematological malignancy do not always offer causal attributions for their illness. Lay accounts of illness experience not always form logical and coherent schemas organised around causal attribution. Therefore, Groleau in ‘Déterminants culturels et l’approche écologique’ underlines that restrict explanatory models to patients’ causal attributions of their disease may only reveal a small portion of the many representations that come into play with regards to illness and health-related behaviour. Thanks to the MINI, we will be able to elicit three distinct types of reasoning about symptoms or illness experience: 1) a basic narrative account structured by contiguity, 2) a prototype narrative, focusing on previous experiences with similar conditions, and 3) an explanatory model narrative organised in terms of explicit knowledge of causes, mechanisms or other cultural models of process.
Unstructured elements of the MINI will provide useful narratives to study individual subjective meanings, including the patient’s level of acceptance of the therapeutic project. Contrarily, the structured dimension allows us to compare the sociocultural contexts of illness experience between groups of patients from different migrant backgrounds.
Sample size and selection. According to a sequential mixed-method explanatory design, an intermediate analysis of quantitative results is needed to purposively select appropriate participants for sample stratification. At this stage, we can consider selecting outliers from the regression analysis or decide to learn more about the differences between people who score either high or low on adherence variables.
This purposive sampling will be followed by concurrent data generation and data analysis. Through various stages of coding, undertaken in conjunction with constant comparative analysis, we will employ theoretical sampling until theoretical saturation is reached.
Eligible patients will be identified, informed, and enrolled with the same process as for the quantitative study. The researcher will conduct the interviews for 45–90 minutes in a private room, with the patient alone or in the presence of the IM when needed.
Analysis. The interviews will be audio taped and transcribed verbatim. The narratives will be analysed according to their form or structure or their content at a collective level, exploring any recurrent themes or structures among narrators.
At this stage and in the absence of previous existing data, we cannot assume any common patterns of meaning in specific groups of FG or SG migrants. But, an individual’s understanding of illness may be viewed as a co-construction of the meaning and reflects at the same time 1) the previously acquired and organized interpretations of illness, 2) new reflections of illness experience and 3) the unfolding relationship between the interviewer and the respondent [6]. Therefore, if the MINI does not distinguish between personal and cultural meanings linked to disease and treatment, the distinction between this level of knowledge can be determined by adding explicit questions or by comparing interviews across groups of individuals from specific cultural backgrounds or social positions.
2.D.3. Ethics approval and consent to participate
The MADESIO protocol has been submitted and approved by the Institut Jules Bordet Academic Trials Promoting Team (Comité des Projets Cliniques–CPC) on the 24.04.2019 and both by the Ethics Committee (EC) of the Institut Jules Bordet (accreditation number OM011) as central EC on the 11.07.2019 and the EC of the Centre Hospitalier Universitaire Saint-Pierre (accreditation number OM011) as local EC on the 03.09.2019, with the reference number 2030. A Privacy Impact Assessment (PIA) has been issued in accordance with GDPR regulations and validated with a minor impact by the two Data Protection Officers of both hospitals. Written informed consent will be sought from patients before starting to participate. All data will be securely stored and password protected, accessible only to the researcher. Only pre-coded data will be transferred to the research team and only disaggregated data will be used in publications resulting from this study. Protocol details are recorded and amendments will be reported to ClinicalTrials.gov. The research team will follow the ethical standards of the Declaration of Helsinki.
3. Discussion
The MADESIO Protocol was designed to tackle the challenges of studying adherence in patients with various haematological malignancies and with diverse language and migrant backgrounds.
Based on broad theoretical approach, our study will suit to migrant populations by exteding the explored adherence predictive factors to contextual variables, miscommunication issues and factors shaped by culture.
Methodologically, we implemented diverse strategies to enhance the quality of data collected.
We combined validated scales to strengthen the reliability and validity of self-reported measures. Based on a rigorous translation and validation process we ensured robust data comparability for our sample. Throughout the selection, the distribution and the collection, we worked to minimize the social desirability bias and strengthen the patients’ feeling of anonymity and confidentiality. In parallel, the questionnaire distribution process was designed to not increase the patient’s burden, and therefore enhance their participation and reduce the non-response rate. Strategically, we also favored auto-administrated questionnaires with closed-ended questions in order to reduce the necessary human ressources for data collection of translation assistance. Finally, with our qualitative approach we will overcome the limits of descriptive analysis and provide a comprehensive understanding of adherence behaviours.
Definitely, a number of limitations can be identified. First of all, our study is exploratory with a small sample size. The comparison of observed adherence rates between the migrant and non-migrant subgroups will not be sufficient for statistical significance. A larger sample would increase the representativeness of the results and limit any centre effect. Furthermore, the validation of the translation process, even rigorous, is limited by the restricted available resources. The bilingual individuals involved in the process represent a separate population that cannot be automatically generalised to the monolingual target population [85]. The translation would benefit from being validated with a pilot phase on larger monolingual patient samples.
The upcoming MADESIO mixed-method study will provide a first overview of how non-migrants and migrants with haematological malignancies, in our two Brussels Hospitals, adhere to their OAMs. This comprehensive approach will offer a solid starting point to see beyond the identification of language and ethnicity as risk factors and better understand whether and why FG and SG migrants constitute a risky population regarding adherence to OAMs. The knowledge generated may be extremely valuable for healthcare services in order to simplify the development of patient-tailored complex interventions and to guide providers in choosing the strategies to address medication adherence of migrants or diverse minority populations.
Singularly, our protocol may highlight the feasibility for healthcare services to fight against cancer inequities by routinely detecting poor adherence in diverse minority populations. By disseminating this protocol in the peer-reviewed literature and in national and European scientific forums, we could convince haematologists and young investigators to be part of the next generation of haemato-oncologists who put ethnicity and culture in the scope of cancer research. A collaborative action from the community of clinicians and researchers will be highly beneficial for the sharing of cross-culturally adapted instruments and protocols.
Acknowledgments
We warmly thank all patients who participated in the study. More particularly, we thank the bilingual patients and nurses who participated in the preliminary pilot testing. We thank all the haematologists who supported the research and agreed to answer to the clinical and contextual questions throughout the study visits: Prof N. Meuleman, Prof P. Lewalle, Dr A. Salaroli, Dr C. Spilleboudt, Dr M. Maerevoet, Dr M. Vercruyssen, Dr S. Wittnebel, Dr F. Massaro, Dr F. Andreozzi.
We thank also Mr. Ibrahima Diallo for the developpement and programmation of the Survey Processing System allowing encoding and registration of the quantitative data collected. We thank MMAS Research LLC. for providing the 3rd July 2018, upon payment of licence fees, the right to use the MMAS-8 form. We thank Prof J. George from Monash University who gave us the authorisation to use the TABS and BBQ free of charge (authorisation obtained on 15 May 2018). We thank Prof R. Horne from University College London, who gave us the permission to use the BMQ without charge (authorisation obtained on 28 August 2019). We thank Prof J. W. Mack from the Harvard University and Prof H.G. Prigerson from Weill Cornell Medicine Centre for Research on End-of-Life Care who gave their agreement to use the HCS without charge (authorisation obtained on 3 July 2018). We also thank the GL Assessment team for their support in obtaining the licence to use the Hospital Anxiety and Depression Scale against payment of license fees (permission obtained on 26 November 2019, ref. PRF0002647). We thank Prof Stoeber who allowed us to use the SD-17 free of charge as of 12 February 2019. Finally, we would like to thank Prof D. Groleau and Dr L.J. Kirmayer from McGill University who authorised us to use the McGill semi-structured Interview on 10 July 2018.
Funding Statement
The first author, SM, obtained two research grants from the Fondation Kisane and les Amis de l’Institut to cover the part-time dedicated to the research. In 2018 the study project was awarded by the Belgian Society of Haematology as part of a broader project aiming to support migrant patients and won the PAtient CEntricity Award (PACE Award). The 10.000 € PACE Award allowed to cover the purchasing licensing fees for the use of the questionnaires and the translation fees. The funders had and will not have a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Noens L, Van Lierde MA, De Bock R, Verhoef G, Zachée P, Berneman Z, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009. May 28;113(22):5401–11. doi: 10.1182/blood-2008-12-196543 [DOI] [PubMed] [Google Scholar]
- 2.Bhatia S, Landier W, Shangguan M, Hageman L, Schaible AN, Carter AR, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a Children’s Oncology Group study. J Clin Oncol. 2012. Jun 10;30(17):2094–101. doi: 10.1200/JCO.2011.38.9924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009. Jan-Feb;59(1), 56–66. doi: 10.3322/caac.20004 [DOI] [PubMed] [Google Scholar]
- 4.Greer JA, Amoyal N, Nisotel L, Fishbein JN, MacDonald J, Stagl J, et al. A systematic review of adherence to oral antineoplastic therapies. Oncologist. 2016. Mar;21:354–376. doi: 10.1634/theoncologist.2015-0405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall AE, Paul C, Bryant J, Lynagh MC, Rowlings P, Enjeti A, et al. To adhere or not to adhere: Rates and reasons of medication adherence in hematological cancer patients. Crit Rev Oncol Hematol. 2016. Jan;97:247–262. doi: 10.1016/j.critrevonc.2015.08.025 [DOI] [PubMed] [Google Scholar]
- 6.Betcher J, Dow E, Khera N. Oral chemotherapy in patients with hematological malignancies—care process, pharmacoeconomic and policy implications. Curr Hematol Malig Rep. 2016. Aug;11(4):288–294. doi: 10.1007/s11899-016-0325-2 [DOI] [PubMed] [Google Scholar]
- 7.Lepièce B. Discrimination dans la consultation médicale interethnique de médecine générale? Public Health thesis. Presses universitaires de Louvain; 2016. Available from: https://dial.uclouvain.be/pr/boreal/object/boreal:174622. French [Google Scholar]
- 8.Van Wieringen JCM, Harmsen JAM, Bruijnzeels MA. Intercultural communication in general practice. Eur J Public Health. 2002. Mar;12:63–68. doi: 10.1093/eurpub/12.1.63 [DOI] [PubMed] [Google Scholar]
- 9.Sleath B, Rubin RH, Huston SA. Hispanic ethnicity physician-patient communication, and antidepressant adherence. Compr Psychiatry. 2003. May-Jun;44:198–204. doi: 10.1016/S0010-440X(03)00007-5 [DOI] [PubMed] [Google Scholar]
- 10.Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003. Dec 2;139:907–915. doi: 10.7326/0003-4819-139-11-200312020-00009 [DOI] [PubMed] [Google Scholar]
- 11.Costa LJ, Hari PN, Kumar SK. Differences between unselected patients and participants in multiple myeloma clinical trials in US: a threat to external validity. Leuk Lymphoma. 2016. Apr 22;57(12): 2827–2832. doi: 10.3109/10428194.2016.1170828 [DOI] [PubMed] [Google Scholar]
- 12.Mourao SSM, Bernarders SG. Ethnic minorities’ and immigrants’ therapeutic (non)adherence: What is the role of social and cultural contexts? Análise Psicológica. 2014. Sept;32(3):341–351. [Google Scholar]
- 13.Stout E, Sexton P, Meghani SH. Racial differences in adherence to prescribed analgesia in cancer patients: an integrated review of quantitative research. J Clin Outcomes Manag. 2017. Jan;24(1):39–48. [Google Scholar]
- 14.Yang Y, Thumula V, Pace PF, Banahan BF, Wilkin NE, Lobb WB. Predictors of medication nonadherence among patients with diabetes in Medicare Part D programs: a retrospective cohort study. Clinl Ther. 2009. Oct;31(10):2178–88; discussion 2150–1. doi: 10.1016/j.clinthera.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 15.Peeters B, Van Tongelen I, Boussery K, Mehuys E, Remon JP, Willems S. Factors associated with medication adherence to oral hypoglycaemic agents in different ethnic groups suffering from type 2 diabetes: a systematic literature review and suggestions for further research. Diab Med. 2011. Mar;28(3):262–75. doi: 10.1111/j.1464-5491.2010.03133.x [DOI] [PubMed] [Google Scholar]
- 16.Crombez P, Michiels S, Bron D. Are we ready for intercultural cancer care? Curr Opin Oncol. 2018. Jul; 30:205–211. doi: 10.1097/CCO.0000000000000449 [DOI] [PubMed] [Google Scholar]
- 17.Arber A, Odelius A, Williams P, Lemanska A, Faithfull S. Do patients on oral chemotherapy have sufficient knowledge for optimal adherence? A mixed methods study. Eur J Cancer Care (Eng). 2017. Mar;26(2). doi: 10.1111/ecc.12413 [DOI] [PubMed] [Google Scholar]
- 18.Mathes T, Jaschinski T, Pieper D. Adherence influencing factors—a systematic review of systematic reviews. Arch Public Health. 2014. Oct 27;72:37. doi: 10.1186/2049-3258-72-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harmsen H, Bernsen R, Meeuwesen L, Thomas S, Dorrenboom G, Pinto D, et al. The effect of educational intervention on intercultural communication: results of a randomised controlled trial. Br J Gen Pract. 2005. May 1;55(514):343–350. [PMC free article] [PubMed] [Google Scholar]
- 20.Surbone A. Cultural Competence, Survivorship, and Supportive Care. In: MASCC Society News. The Multinational Association of Supportive Care in Cancer. 2016. Nov Available from: http://www.mascc.org/assets/documents/Newsletters/masccnews_november2016.pdf [Google Scholar]
- 21.Lorant V, Derluyn I, Dauvrin M, Coune I, Verrept H. Vers des soins de santé interculturels: Recommandations du groupe ETHEALTH en faveur de la réduction des inégalités de santé parmi les migrants et minorités ethniques. In: DIAL.pr Research Publications. 2011. Dec Available from: http://hdl.handle.net/2078.1/100686. French. [Google Scholar]
- 22.Artiga S, Orgera K, Pham O. Disparities in Health and Health Care: Five Key Questions and Answers. In: Kaiser Family Foundation. 2020. March. Available from: https://www.kff.org/racial-equity-and-health-policy/issue-brief/disparities-in-health-and-health-care-five-key-questions-and-answers/ [Google Scholar]
- 23.STATBEL [Internet]. La Belgique en chiffres. [cited 2020 Feb 20]. Available from: https://statbel.fgov.be/fr/themes/population/migrations#figures. French
- 24.Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012. May;73(5):691–705. doi: 10.1111/j.1365-2125.2012.04167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakcett DL, Haynes R. Compliance with therapeutic regimes. Johns Hopkins University Press, Baltimore; 1976. [Google Scholar]
- 26.World Health Organization. Adherence to long-term therapies—Evidence for action. 2003. 211p. [Google Scholar]
- 27.Michiels S, Bron D, Crombez P, Vercruyssen M, Tricas-Sauras S, Kirakoya F. A prospective approach of migrants and ethnic minorities in order to overcome poor adherence and to guarantee an equal access to innovative transplant procedures. Proceedings of The 45th Annual Meeting of the European Society for Blood and Marrow Transplantation: Nurses Group–Oral Session; 2019 Mar 24–26; Frankfurt, Germany. Bone Marrow Transplant;2019. [Google Scholar]
- 28.Dussault G, Fronteira I, Cabral J. Migration of health personnel in the WHO European Region. [Internet]. World Health Organization; 2009. [cited 2019 Jul 18]. Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwj3pd2Tw7TvAhXPuaQKHZCXDQkQFjAAegQIAhAD&url=http%3A%2F%2Fwww.euro.who.int%2F__data%2Fassets%2Fpdf_file%2F0010%2F95689%2FE93039.pdf&usg=AOvVaw1dEld7jcsK_xfeSTKO6Fnc. [Google Scholar]
- 29.Dauvrin M. Cultural competence in health care: challenging inequalities, involving institutions. Public Health Thesis. Presses universitaires de Louvain. 2013. Available from: https://dial.uclouvain.be/pr/boreal/object/boreal:135385 [Google Scholar]
- 30.Levecque K, Garcia Benavides F, Ronda E, Van Rossem R. Using existing health information systems for migrant health research in Europe: challenges and opportunities. In: Devide I, Allan K, Lorant V, Razum O. Health inequalities and Risk Factors among migrants and ethnic minorities. Presses universitaires de Louvain; 2014.p.53–68 [Google Scholar]
- 31.International Organization for Migration (IOM). Who is a migrant? Glossary on Migration. 2019;34. 248p. [Google Scholar]
- 32.Nations United. Handbook on Measuring International Migration through Population Censuses. Department of Economic and Social Affairs, Statistics Division. New York. 2020; 157p. [Google Scholar]
- 33.Scheppers E, van Dongen E, Dekker J, Geertzen J, Dekker J. Potential barriers to the use of health services among ethnic minorities: a review. Family Practice. 2006. June;23(3):325–348. doi: 10.1093/fampra/cmi113 [DOI] [PubMed] [Google Scholar]
- 34.Bickell NA, Wang JJ, Oluwole S, Schrag D, Godfrey H, Hiotis K, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006. Mar 20;24(9):1357–1362. doi: 10.1200/JCO.2005.04.5799 [DOI] [PubMed] [Google Scholar]
- 35.Bouwman L, Eeltink C, Visser O, Janssen JJW, Maaskant JM. Prevalence and associated factors of medication non-adherence in hematological-oncological patients in their home situation. BMC Cancer. 2017. Nov 9;17(739):1–8. doi: 10.1186/s12885-017-3735-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Institut bruxellois de statistique et d’analyse (Ibsa perspective.brussels) [Internet]. Nationalités. Population.[cited 2020 Oct 22]. Available from: https://ibsa.brussels/themes/population/nationalites. French
- 37.Milica P. Belgium: a country of permanent immigration. Migration Policy Institute [Internet]. 2012. Nov 15 [cited 2020 Jan 15]. Available from: https://www.migrationpolicy.org/article/belgium-country-permanent-immigration [Google Scholar]
- 38.Nguyen T-M-U., La Caze A., & Cottrell N. What are validated self-report adherence scales really measuring? A systematic review. Br J Clin Pharmacol. 2014. Mar; 77(3):427–445. doi: 10.1111/bcp.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clinical Therapeutics. 1999;21(6):1074–1090. doi: 10.1016/S0149-2918(99)80026-5 [DOI] [PubMed] [Google Scholar]
- 40.Remington G, Kwon J, Collins A, Laporte D, Mann S, Christensen B. The use of electronic monitoring (MEMS) to evaluate antipsychotic compliance in outpatients with schizophrenia. Schizophr Res. 2007. Feb;90:229–37. doi: 10.1016/j.schres.2006.11.015 [DOI] [PubMed] [Google Scholar]
- 41.Santschi V, Wuerzner G, Schneider MP, Bugnon O, Burnier M. Clinical evaluation of IDAS II, a new electronic device enabling drug adherence monitoring. Eur J Clin Pharmacol. 2007. Dec; 63:1179–84. doi: 10.1007/s00228-007-0364-7 [DOI] [PubMed] [Google Scholar]
- 42.van Onzenoort HA, Neef C, Verberk WW, van Iperen HP, de Leeuw PW, van der Kuy PH. Determining the feasibility of objective adherence measurement with blister packaging smart technology. Am J Health Syst Pharm. 2012. May 15;69:872–879. doi: 10.2146/ajhp100592 [DOI] [PubMed] [Google Scholar]
- 43.van den Boogaard J, Lyimo RA, Boeree MJ, Kibiki GS, & Aarnoutse RE. Electronic monitoring of treatment adherence and validation of alternative adherence measures in tuberculosis patients: a pilot study. Bull World Health Organ. 2011. Sep 1;89: 632–39. doi: 10.2471/BLT.11.086462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu JR, Moser DK, Chung ML, Lennie TA. Objectively measured, but not self-reported, medication adherence independently predicts event-free survival in patients with heart failure. J Card Fail. 2008. Apr;14:203–210. doi: 10.1016/j.cardfail.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Velligan DI, Wang M, Diamond P, Glahn DC, Castillo D, Bendle S, et al. Relationships among subjective and objective measures of adherence to oral antipsychotic medications. Psychiatr Serv. 2007. Sep;58:1187–1192. doi: 10.1176/ps.2007.58.9.1187 [DOI] [PubMed] [Google Scholar]
- 46.Pai ALH, Drotar D, Kodish E. Correspondence between objective and subjective reports of adherence among adolescents with acute lymphoblastic leukemia. Child Health Care. 2008. Jul 24;37:225–235. doi: 10.1080/02739610802151597 [DOI] [Google Scholar]
- 47.Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner L, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000. Mar 10;14:357–366. doi: 10.1097/00002030-200003100-00008 [DOI] [PubMed] [Google Scholar]
- 48.Lehman A, Aslani P, Ahmed R, Celio J, Gauchet A, Bedouch P, et al. Assessing medication adherence: options to consider. Int J Clin Pharm. 2014. Feb;36(1):55–69. doi: 10.1007/s11096-013-9865-x [DOI] [PubMed] [Google Scholar]
- 49.George J, Mackinnon A, Kong D, Stewart K. Development and validation of the Beliefs and Behaviour Questionnaire (BBQ). Patient Educ Couns. 2006. Dec;64(1–3):50–60. doi: 10.1016/j.pec.2005.11.010 [DOI] [PubMed] [Google Scholar]
- 50.Leventhal H, Meyer D, Narenz D. The common sense representation of illness danger. In: Rachman S. Contributions to medical psychology. Oxford: Pergamon Press; 1980. P.17–30. [Google Scholar]
- 51.Mootz M. De patiënt en zijn naasten: de invloed van houdingen in het persoonlijk netwerk van de patiënt op zijn medische consumptie (The patient and his nearest). [thesis] The Hague. J-H. Pasmans; 1981. Dutch. [Google Scholar]
- 52.Rosenstock I. The health belief model and preventative health behaviour. Health Education Monograph. 1974. Dec 1:354–86. doi: 10.1177/109019817400200405 [DOI] [Google Scholar]
- 53.Rotter J. Generalized expectancies for internal versus external control of reinforcements. Psychology Monitor. 1966; 80(1)whole No. 609:1–28. [PubMed] [Google Scholar]
- 54.Kleinman MA. The patients and healers in the context of culture. Berkeley: University of California Press; 1980. p. 448. [Google Scholar]
- 55.Horne R. Representations of medications and treatment: Advances in theory and measurement. In: Petrie KJ, Weinman JA. Perceptions of health and illness. London: Harwood Academic; 1997. pp. 155–88. [Google Scholar]
- 56.Royal Pharmaceutical Society of Great Britain, Marinker M. From compliance to concordance: Achieving shared goals in medicine taking. London: Royal Pharmaceutical Society, in partnership with Merck Sharp & Dohme; 1997. [Google Scholar]
- 57.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive Validity of A Medication Adherence Measure in an Outpatient Setting. J Clin Hypertens (Greenwich, Conn.). 2008. May 2;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care. 2009. Jan;15:59–66. [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Kong MC, Ko Y. Psychometric properties of the 8-item Morisky Medication Adherence Scale in patients taking warfarin. Thromb Haemost. 2012. Oct;108:1–7. doi: 10.1160/TH12-05-0312 [DOI] [PubMed] [Google Scholar]
- 60.Horne R, Weinman J, Hankins M. Beliefs about medicines questionnaire (BMQ): The development and evaluation of a new method for assessing the cognitive representation of medication. Psychology and Health. 1999;14:1–24. [Google Scholar]
- 61.Horne R, Weinman J. Self-regulation and self-management in asthma: exploring the role of illness perceptions and treatment beliefs in explaining non-adherence to preventer medication. Psychology and Health. 2002;17:17–32. [Google Scholar]
- 62.Ross S, Walker A, MacLeod MJ. Patient compliance in hypertension: role of illness perceptions and treatment beliefs. Journal of Human Hypertension. 2004;18:607–613. doi: 10.1038/sj.jhh.1001721 [DOI] [PubMed] [Google Scholar]
- 63.Berglund E, Lytsy P, Westerling R. Adherence to and beliefs in lipid-lowering medical treatments: a structural equation modeling approach including the necessity-concern framework. Patient Educ Couns. 2013. Apr;91:105–112. doi: 10.1016/j.pec.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 64.Alhalaiqa F, Deane KH, Nawafleh AH, Clark A, Gray R. Adherence therapy for medication non-compliant patients with hypertension: a randomised controlled trial. J Hum Hypertens. 2012. Feb;26:117–126. doi: 10.1038/jhh.2010.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petrie KJ, Perry K, Broadbent E, Weinman J. A text message programme designed to modify patients’ illness and treatment beliefs improves self-reported adherence to asthma preventer medication. Br J Health Psychol. 2012. Feb;17:74–84. doi: 10.1111/j.2044-8287.2011.02033.x [DOI] [PubMed] [Google Scholar]
- 66.Mack JW, Block SD, Nilsson M, Wright A, Trice E, Friedlander R. Measuring therapeutic alliance between oncologists and patients with advanced cancer: the Human Connection Scale. Cancer. 2009. Jul 15;115(14):3302–3311. doi: 10.1002/cncr.24360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roberts LW, Johnson ME, Brems C, Warner TD. When providers and patients come from different backgrounds: perceived value of additional training on ethical care practices. Transcult Psychiatry. 2008. Dec;45(4):553–565. doi: 10.1177/1363461508100782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Snaith RP. The Hospital Anxiety And Depression Scale. Health Qual Life Outcomes. 2003. Aug 1;1:29. doi: 10.1186/1477-7525-1-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jing J, Sklar GE, Min Sen Oh V, Chuen Li S. Factors affecting therapeutic compliance: A review from the patient’s perspective. Ther Clin Risk Manag. 2008. Feb;4(1):269–286. doi: 10.2147/tcrm.s1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cleary SD, Snead R, Dietz-Chavez D, Rivera I, Edberg MC. Immigrant Trauma and Mental Health Outcomes Among Latino Youth. J Immigr Minor Health. 2018. Oct;20(5):1053–1059. doi: 10.1007/s10903-017-0673-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Porter M, Haslam N. Predisplacement and postdisplacement factors associated with mental health of refugees and internally displaced persons: a meta-analysis. JAMA. 2005. Aug 3;294(5):602–612. doi: 10.1001/jama.294.5.602 [DOI] [PubMed] [Google Scholar]
- 72.Zerbe WJ, Paulhus DL. Socially desirable responding in organizational behaviour: A reconception. Acad of Manage Rev. 1987. Apr 1;12(2):250–264. doi: 10.5465/amr.1987.4307820 [DOI] [Google Scholar]
- 73.Nieuwkerk PT, de Boer-van der Kolk IM, Prins JM, Locadia M, Sprangers MA. Self-reported adherence is more predictive of virological treatment response among patients with a lower tendency towards socially desirable responding. Antivir Ther. 2010;15(6):913–6. doi: 10.3851/IMP1644 [DOI] [PubMed] [Google Scholar]
- 74.Stöbber J. The Social Desirability Scale-17 (SDS-17). Eur J Psychol Assess, 2001;17:222–232. doi: 10.1027//1015-5759-7.3.222 [DOI] [Google Scholar]
- 75.Garfield S, Clifford S, Eliasson L, Barber N, Willson A. Suitability of measures of self-reported medication adherence for routine clinical use: a systematic review. BMC Med Res Methodol. 2011. Nov 3;11:149–157. doi: 10.1186/1471-2288-11-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stirratt MJ, Dunbar-Jacob J, Crane HM, Simoni JM, Czajkowski S, Hilliard ME, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015. Dec;5(4):470–82. doi: 10.1007/s13142-015-0315-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Armstrong BK, White E, Saracci R. Principles of exposure measurement in epidemiology. Monographs in Epidemiology and Biostatistics. Vol. 21. New York, NY: Oxford University Press; 1995. [Google Scholar]
- 78.Tsang S, Royse CF, Terkaw AS. Guidelines for developing, translating, and validating a questionnaire in perioperative and pain medicine. Saudi J Anaesth. 2017. May;11(Suppl 1):S80–S89. doi: 10.4103/sja.SJA_203_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sperber AD. Translation and validation of study instruments for cross-cultural research. Gastroenterology. 2014. Jan;126(1 Suppl 1):S124–S128. doi: 10.1053/j.gastro.2003.10.016 [DOI] [PubMed] [Google Scholar]
- 80.World Health Organization. Process of translation and adaptation of instruments. Management of substance abuse. [Cited 2019 Dec 6]. Available from https://www.who.int/substance_abuse/research_tools/translation/en/.
- 81.Cha E-S, Kim HK, Erlen JA. Translation of scales in cross-cultural research: issues and techniques. J Adv Nurs. 2007. May;58(4):386–395. doi: 10.1111/j.1365-2648.2007.04242.x Epub 2007 Apr 17. [DOI] [PubMed] [Google Scholar]
- 82.Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol. 1993. Dec;46(12): 1417–1432. doi: 10.1016/0895-4356(93)90142-n [DOI] [PubMed] [Google Scholar]
- 83.Groleau D, Young A, Kirmayer LJ. The McGill Illness Narrative Interview (MINI): an interview schedule to elicit meanings and modes of reasoning related to illness experience. Transcult Psychiatry. 2006. Dec;43(4):671–691. doi: 10.1177/1363461506070796 [DOI] [PubMed] [Google Scholar]
- 84.Smith RC. Analytic strategies for oral history interviews. In: Gubrium JF, Holstein J. Handbook of interview research: Context and method. Thousand Oaks; 2002. pp.711–731 [Google Scholar]
- 85.Good B. Medicine, rationality, and experience: An anthropological perspective. New York: Cambridge University Press; 1994. [Google Scholar]
- 86.Czarniawska B. Narrative, interviews, and organizations. In: Gubrium JF, Holstein J. Handbook of interview research: Context and method. Thousand Oaks; 2002. pp.733–749. [Google Scholar]
- 87.Riessman CK. Analysis of personal narratives. In: Gubrium JF, Holstein J. Handbook of interview research: Context and method. Thousand Oaks; 2002. pp. 695–710. [Google Scholar]
- 88.Glaser BG, Strauss AL. The discovery of grounded theory: Strategies for qualitative research. Chicago: Aldine; 1967. [Google Scholar]
- 89.Weiner B. Sponteneous’ causal thinking. Psychol Bull. 1985;97:74–84. [PubMed] [Google Scholar]