Abstract
Background:
Intra-amniotic infection is present in 10% of patients with an episode of preterm labor, and is a risk factor for impending preterm delivery and neonatal morbidity/mortality. Intra-amniotic inflammation is often associated with intra-amniotic infection, but sometimes is present in the absence of detectable microorganisms. Antibiotic treatment of intra-amniotic infection has traditionally been considered to be ineffective. Intra-amniotic inflammation without microorganisms has a similar prognosis to intra-amniotic infection.
Objective:
To determine whether antibiotics can eradicate intra-amniotic infection or intra-amniotic inflammation in patients with preterm labor and intact membranes.
Study design:
The study population consisted of women who met the following criteria: 1) singleton gestation between 20–34 weeks; 2) preterm labor and intact membranes; 3) transabdominal amniocentesis performed for the evaluation of the microbiologic/inflammatory status of the amniotic cavity; 4) intra-amniotic infection and/or inflammation; and 5) received antibiotic treatment which consisted of ceftriaxone, clarithromycin, and metronidazole. Follow-up amniocentesis was performed in a subset of patients. Amniotic fluid was cultured for aerobic and anaerobic bacteria and genital mycoplasmas, and polymerase chain reaction (PCR) was performed for Ureaplasma spp. Intra-amniotic infection was defined as a positive amniotic fluid culture or positive PCR, and intra-amniotic inflammation was suspected when there was an elevated amniotic fluid white blood cell count or a positive rapid test for matrix metalloproteinase-8. For this study, the final diagnosis of intra-amniotic inflammation was made by measuring the interleukin-6 concentration in stored amniotic fluid (>2.6 ng/mL). These results were not available to managing clinicians. Treatment success was defined as eradication of intra-amniotic infection and/or inflammation or delivery ≥37 weeks.
Results:
1) Of 62 patients with intra-amniotic infection and/or inflammation, 50 received the antibiotic regimen. Of those, 29 were undelivered for ≥ 7 days and 19 underwent follow-up amniocenteses; 2) microorganisms were identified by culture or PCR of amniotic fluid obtained at admission in 21% (4/19) of patients who had follow-up amniocentesis, and were eradicated in 3 out of 4; 3) resolution of intra-amniotic inflammation was confirmed in 79% (15/19) of patients, and one other patient delivered at term, although the resolution of intra-amniotic inflammation could not be confirmed because further follow-up amniocentesis was not performed; thus, resolution of intra-amniotic inflammation/infection or term delivery (treatment success) occurred in 84% (16/19) of patients who had a follow-up amniocentesis; 4) treatment success occurred in 32% (16/50) of patients with intra-amniotic infection/inflammation who received antibiotics; and 5) the median amniocentesis-to-delivery interval was significantly longer among women who received the combination of antibiotics than among those who did not (11.4 days versus 3.1 days: p=0.04).
Conclusion:
Eradication of intra-amniotic infection/inflammation occurred in 79% of patients with preterm labor, intact membranes, and intra-amniotic infection/inflammation. Treatment success occurred in 84% of patients who underwent follow-up amniocentesis and in 32% of women who received the antibiotic regimen.
Keywords: intra-amniotic inflammation, interleukin-6, ceftriaxone, clarithromycin, metronidazole, amniotic fluid, prematurity, chorioamnionitis, pregnancy, MMP-8, white blood cell, pregnancy, antibiotics, amniotic fluid
Introduction
Preterm labor is a syndrome caused by multiple pathologic processes.1 The following mechanisms of disease have been implicated: intra-amniotic infection,2–25 “sterile” intra-amniotic inflammation,26–39 uterine overdistention,40,41 maternal anti-fetal rejection,42–46 decidual senescence,47–50 and possibly other mechanisms that are yet to be identified.
One of every ten patients with preterm labor and intact membranes will have intra-amniotic infection2–7,12,16,17,22–25,33,35,51 which is largely subclinical,2,6,22,26,33,38,39,52,53 and these patients are at increased risk for early preterm delivery,3,6–8,22,33,53 neonatal complications,6,8,21,26,33,54–66 and maternal morbidity (such as acute pulmonary edema, when treated with tocolytics and steroids67–69) or maternal sepsis.70 Similar risks occur in patients with preterm PROM and intra-amniotic infection.4,13,17,71,72
Given the frequency and importance of intra-amniotic infection in the pathogenesis of preterm labor with intact membranes, several randomized clinical trials have tested the efficacy and safety of antibiotic administration.73–76 Despite initial enthusiasm,10,73,75,77 subsequent trials have not shown beneficial effects,74,78–83 and currently, antibiotic administration is restricted to patients with an episode of premature labor who are carriers of group B streptococcus (GBS)84,85 or have unknown GBS status86 to prevent vertical transmission and neonatal sepsis.87,88
Intra-amniotic inflammation, defined as an elevated concentration of interleukin-6 or matrix metalloproteinase-8 (MMP-8) in amniotic fluid in the absence of demonstrable microorganisms detected with culture or molecular methods (“sterile” intra-amniotic inflammation), has also been associated with adverse pregnancy outcomes, including acute histologic chorioamnionitis and funisitis.25–28,33,71,89 Activation of the inflammasome has been implicated in the mechanisms responsible for preterm labor induced by “sterile” intra-amniotic inflammation.31,32,90–92
Important advances have been made in the identification of patients at risk of spontaneous preterm delivery by assessing cervical length in the midtrimester,93–103 as well as in the treatment of patients with a sonographic short cervix with vaginal progesterone.104–115 However, the optimal treatment of patients with an episode of preterm labor, intact membranes, and intra-amniotic infection or intra-amniotic inflammation has not been determined. Previous reports demonstrated the eradication of microorganisms in the amniotic cavity of patients with a short cervix116,117 and preterm PROM.27,118 A recent report suggests that a subset of patients with preterm labor and intra-amniotic infection may benefit from antibiotic administration.119
We have recently reported that the antibiotic treatment of patients with preterm PROM can reduce the rate of intra-amniotic infection and intra-amniotic inflammation, as well as funisitis and the fetal systemic inflammatory response, using a combination of antibiotics (ceftriaxone, clarithromycin, and metronidazole) which target microorganisms frequently isolated from the amniotic cavity in these cases.118,120
The purpose of this study was to determine whether antibiotics could eradicate intra-amniotic infection or intra-amniotic inflammation without demonstrable microorganisms in patients with preterm labor and intact membranes.
Materials and Methods
Study design
This is a retrospective case series study of pregnant women admitted to Seoul National University Hospital between January 2004 and March 2014, who met the following criteria; 1) singleton gestations between 20–34 weeks; 2) preterm labor and intact amniotic membranes determined by sterile speculum examination; 3) transabdominal amniocentesis performed for the evaluation of the microbiologic and inflammatory status of the amniotic cavity; 4) positive amniotic fluid culture or intra-amniotic inflammation; and 5) antibiotic treatment (regimen consisted of ceftriaxone, clarithromycin and metronidazole). Follow-up amniocentesis was performed in a subset of patients at the discretion of the managing physician.
At the Seoul National University Hospital, a transabdominal amniocentesis is routinely offered to all patients admitted with the diagnosis of preterm labor to assess the microbiologic status of the amniotic cavity and fetal lung maturity. Retrieval of amniotic fluid was performed after written informed consent was obtained. Preterm labor was diagnosed as the presence of regular uterine contractions (four or more contractions in 20 minutes or eight or more in 60 minutes). The Institutional Review Board of the Seoul National University Hospital approved the collection and use of these samples and clinical information for research purposes. The Seoul National University has a Federal Wide Assurance with the Office for Human Research Protection (OHRP) of the Department of Health and Human Services (DHHS) of the United States.
Amniotic fluid analysis
Amniotic fluid was cultured for aerobic and anaerobic bacteria, as well as genital mycoplasmas. Beginning in 2007, samples were also assayed for Ureaplasma spp. using polymerase chain reaction (PCR) with specific primers using methods previously described8. An aliquot of amniotic fluid was examined in a hemocytometer chamber to determine the white blood cell count.33,121 In a subset of patients, MMP-8 concentration in amniotic fluid was measured using a commercially-available enzyme-linked immunosorbent assay (ELISA) (Amersham Pharmcia Biotech, Inc., Bucks, UK) and the results were available to clinicians. Intra-amniotic inflammation was suspected when the concentration of MMP-8 in the amniotic fluid was higher than 23 ng/mL, as previously reported.71,89,122–125
Between March 2005 and December 2010, a rapid MMP-8 bedside test (MMP-8 PTD Check test, SK Pharma Co, Ltd, Kyunggi-do, Korea) was performed and used in patient management. Details of the MMP-8 rapid test have been previously described.53,60,126,127 Amniotic fluid not used for diagnostic tests was centrifuged at 800g and stored at −80C.
Intra-amniotic infection was defined as a positive amniotic fluid culture or positive PCR for Ureaplasma spp. For the purposes of this study, a definitive diagnosis of intra-amniotic inflammation was made when the interleukin-6 concentration of stored amniotic fluid was higher than 2.6 ng/mL.33 The amniotic fluid interleukin-6 concentration was measured with a commercially available enzyme-linked immunoassay (R&D Systems, Minneapolis, MN) in 2017 and 2018. The sensitivity of the assay was 0.7 pg/mL. The intra- and inter-assay coefficients of variation were <10%. These results were not available to managing clinicians.
Clinical management
Intra-amniotic inflammation was suspected when there was an elevated amniotic fluid white blood cell count (defined as ≥ 19 cells/mm),122 a positive MMP-8 rapid test result,53,126,127 or an elevated concentration of amniotic fluid MMP-8 (>23 ng/mL) measured by ELISA.71,89,122 Suspicion of intra-amniotic inflammation, isolation of microorganisms by amniotic fluid culture, or the detection of Ureaplasma nucleic acids was an indication for the administration of antibiotics. We used a combination of antimicrobial agents previously prescribed in the management of patients with preterm PROM,118,120 including ceftriaxone 1g (intravenous) every 24 hours, clarithromycin 500mg (oral) every 12 hours, and metronidazole 500mg (intravenous) every 8 hours. Metronidazole was administered for a maximum of 4 weeks. A follow-up amniocentesis was offered to monitor the microbiologic and inflammatory status of amniotic cavity and fetal lung maturity. The use, discontinuation, or change of antibiotic regimen or tocolytics, or interval to follow-up amniocentesis, were left to the discretion of the treating clinicians because there was no uniformity among attending physicians about these issues. Tocolytics used were ritodrine, magnesium sulphate, or atosiban. Nonsteroidal anti-inflammatory agents, such as indomethacin, were not used as tocolytic agents. Group B streptococcus (GBS) screening and intrapartum treatment are not used in our institution because neonatal GBS sepsis is extremely rare in our patient population.128,129
Definition of treatment success in this study
Treatment success was defined as: a) eradication of intra-amniotic infection or intra-amniotic inflammation; or b) delivery at or after 37 weeks of gestation.
Diagnosis of acute histologic chorioamnionitis and clinical chorioamnionitis
Acute histologic chorioamnionitis was diagnosed in the presence of acute inflammatory changes in tissue samples including amnion and chorion-decidua.130 Funisitis was diagnosed in the presence of neutrophil infiltration into the umbilical vessel walls or Wharton’s jelly.71,89,122,131-134
Clinical chorioamnionitis was diagnosed by the presence of maternal fever (temperature >37.8°C) accompanied by two or more of the following criteria: 1) maternal tachycardia (heart rate >100 beats/min); 2) uterine tenderness; 3) foul-smelling amniotic fluid; 4) fetal tachycardia (heart rate >160 beats/min); and 5) maternal leukocytosis (leukocyte count >15,000 cells/mm3).135 The limitations of these criteria in the identification of intra-amniotic infection have been recently described.118,125,136–138 The criteria for the diagnosis of neonatal morbidity can be found in Supplementary Material S1.
Statistical analysis
Continuous variables were compared between two groups with the Mann-Whitney U test. Proportions were compared with the Fisher’s exact test. The amniocentesis-to-delivery interval was compared by using the generalized Wilcoxon test for survival analysis. A p-value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS (Version 22; SPSS Inc., Chicago, IL, USA).
Results
Characteristics of study population
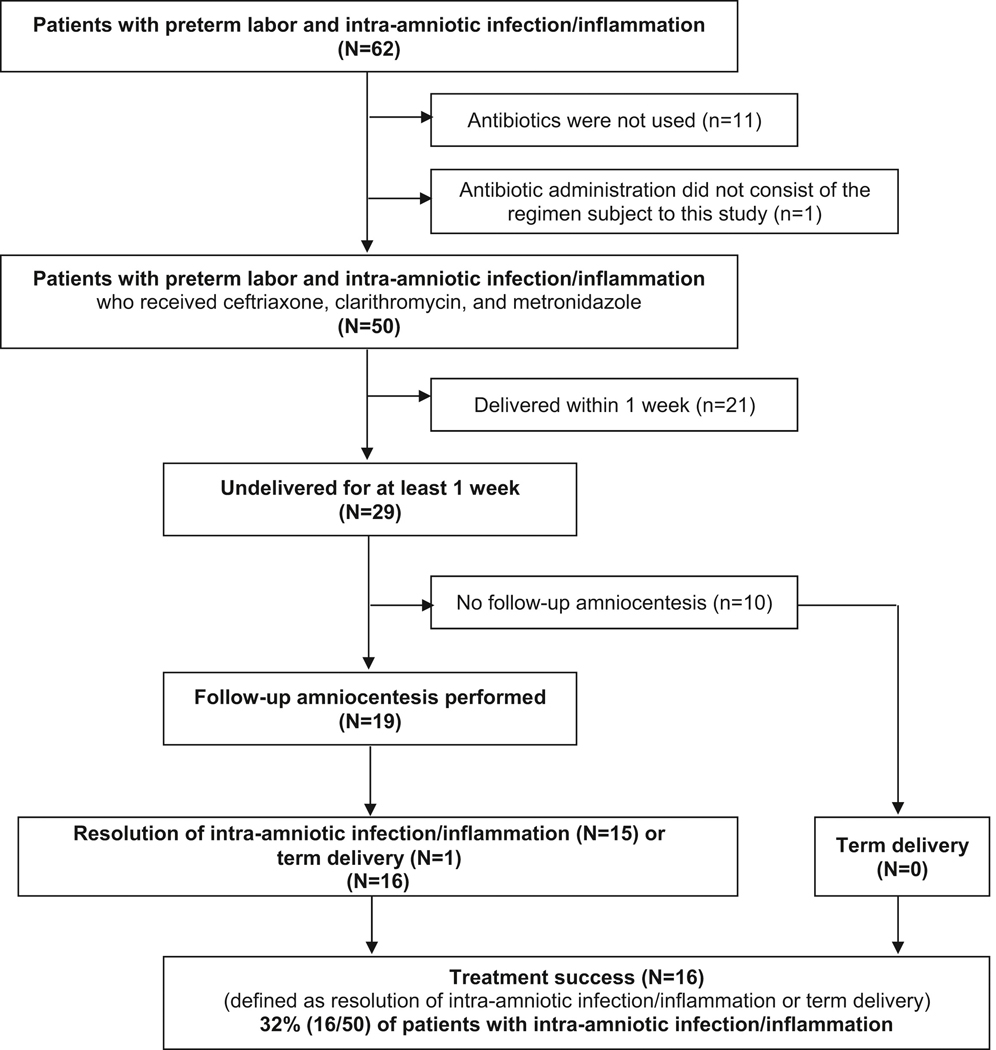
Figure 1 shows a flow diagram of patients included in this study. Sixty-two patients with intra-amniotic infection and/or inflammation were identified. A positive amniotic fluid culture was present in 11 patients; Ureaplasma spp. was detected by PCR method in 8 patients; and intra-amniotic inflammation was identified in 51 patients with a negative amniotic fluid culture for microorganisms. Bacteria identified by culture included Ureaplasma urealyticum (n=9), Mycoplasma hominis (n=2), and one isolate each of Streptococcus anginosus and Gardnerella vaginalis.
Figure 1.
Flow diagram of the study population
Of 62 patients with intra-amniotic infection and/or inflammation, 50 received the combination of ceftriaxone, clarithromycin, and metronidazole. The remaining 12 patients did not receive this antibiotic regimen (11 patients did not receive any antibiotics; one received an alternative regimen, consisting of ceftriaxone, azithromycin, and metronidazole). Of the 11 patients who did not receive any antibiotics, one had a positive amniotic fluid culture for Ureaplasma urealyticum, and antibiotics were not administered because of rapid progression of preterm labor to delivery.
The lack of antibiotic administration in the other 10 patients was because: 1) intra-amniotic infection/inflammation was not suspected because the patients had a low amniotic fluid white blood cell count when the MMP-8 rapid test was not available (n=4); however, intra-amniotic inflammation was diagnosed by an elevated interleukin-6 retrospectively; 2) the managing clinician preferred to rely on the results of the amniotic fluid white blood cell count rather than on those of the rapid MMP-8 test (n=2); 3) rapid progression of labor (n=2); 4) declined antibiotic treatment (n=1); and 5) transfer to another hospital because of unavailability of a neonatal intensive care bed (n=1).
Table 1 compares the characteristics and outcomes of patients who received the antibiotic regimen with those of patients who did not. There were no significant differences between the study groups in maternal age, cerclage use, gestational age at amniocentesis, interleukin-6 concentration, frequency of a positive amniotic fluid culture, use of tocolytics and antenatal corticosteroids, delivery within 7 and 14 days of amniocentesis, delivery <30, <34 and ≥37 weeks, clinical and acute histologic chorioamnionitis, and funisitis (p>0.1 for each). Patients who received the antibiotic regimen had a significantly higher median amniotic fluid white blood cell count (79 cells/mm3 vs. 3 cells/mm3), longer median amniocentesis-to-delivery interval (11.4 days vs. 3.1 days) and lower rate of delivery within 4 weeks of amniocentesis (58% vs. 91.7%) than those in whom the antibiotic regimen was not used (p<0.05 for each).
Table 1.
Clinical characteristics and outcomes of patients who did vs. did not use the regimen of antibiotics consisting of ceftriaxone, clarithromycin, and metronidazole
| Use of ceftriaxone, clarithromycin, and metronidazole (n=50) | No antibiotics or use of other antibiotics (n=12) | p-value | |
|---|---|---|---|
| Maternal age (years) | 31 (29–34) | 34 (31–36) | 0.12 |
| Nulliparity (%) | 62.0% (31/50) | 25.0% (3/12) | 0.027 |
| Cerclage before the onset of preterm labor | 12.0% (6/50) | 8.3% (1/12) | 0.99 |
| Cerclage after the onset of preterm labor and preterm labor stopped | 4.0% (2/50) | 8.3% (1/12) | 0.48 |
| Initial amniocentesis | |||
| Gestational age at amniocentesis (weeks) | 25.4 (22.1–27.5) | 25.7 (22.6–28.6) | 0.63 |
| Positive amniotic fluid culture (%) | 20.0% (10/50) | 9.1% (1/12) | 0.68 |
| Positive amniotic fluid PCR for Ureaplasma spp. | 21.2% (7/33) | 11.1% (1/9) | 0.66 |
| Amniotic fluid WBC count (cells/mm3) | 79 (2–860) | 3 (0–65) | 0.048 |
| Amniotic fluid WBC count (≥ 19 cells/mm3) | 58.3% (28/48) | 25.0% (3/12) | 0.054 |
| Amniotic fluid interleukin-6 (ng/mL) | 18.2 (4.1–43.0) | 7.8 (3.2–16.9) | 0.12 |
| Amniotic fluid interleukin-6 (>2.6 ng/mL) | 100% (49/49) | 100% (12/12) | >0.99 |
| Cervical dilatation > 3cm (%) | 10.0% (5/50) | 16.7% (2/12) | 0.61 |
| Use of tocolytics (%) | 98.0% (49/50) | 91.7% (11/12) | 0.35 |
| Antenatal corticosteroids administration (%) | 62.0% (31/50) | 58.3% (7/12) | >0.99 |
| Gestational age at delivery (weeks) | 28.9 (25.5–33.9) | 27.3 (23.4–31.7) | 0.29 |
| Interval between amniocentesis to delivery (days) | 11.4 (2.8–57.0) | 3.1 (0.3–17.8) | 0.04 b |
| Delivery within 7 days of amniocentesis | 42.0% (21/50) | 58.3% (7/12) | 0.35 |
| Delivery within 14 days of amniocentesis | 52.0% (26/50) | 67% (8/12) | 0.52 |
| Delivery within 4 weeks of amniocentesis | 58.0% (29/50) | 91.7% (11/12) | 0.042 |
| Delivery before 30 weeks a | 57.4% (27/47) | 81.8% (9/11) | 0.18 |
| Delivery before 34 weeks | 76.0% (38/50) | 91.7% (11/12) | 0.43 |
| Delivery at term (>=37 weeks) | 8.0% (4/50) | 8.3% (1/12) | >0.99 |
| Clinical chorioamnionitis | 12.0% (6/50) | 0% (0/12) | 0.59 |
| Acute histologic chorioamnionitis | 69.2% (27/39) | 88.9% (8/9) | 0.41 |
| Funisitis | 30.8% (12/39) | 22.2% (2/9) | >0.99 |
Data are median (interquartile range) or % (n/N).
PCR, polymerase chain reaction; WBC, white blood cell.
Cases who underwent amniocentesis at or beyond 30 weeks were excluded from the analysis.
The amniocentesis-to-delivery interval was compared by using the generalized Wilcoxon test for survival analysis.
Of 50 patients treated with the antibiotic regimen, 29 remained undelivered for at least one week (figure 1). Microorganisms identified in the amniotic fluid of 29 patients undelivered for at least one week included Ureaplasma urealyticum (n=4) and Mycoplasma hominis (n=1). One patient had a mixed infection of Ureaplasma urealyticum and Mycoplasma hominis. Microorganisms identified in the amniotic fluid of 21 patients delivered within 7 days of amniocentesis included Ureaplasma urealyticum (n=4), and one isolate each of Mycoplasma hominis, Streptococcus anginosus and Gardnerella vaginalis. One patient had a mixed infection of Ureaplasma urealyticum and Mycoplasma hominis.
Table 2 compares the characteristics and outcomes of patients delivered within seven days of amniocentesis and those who were undelivered for at least seven days. There were no significant differences in the median gestational age at amniocentesis and the frequency of a positive amniotic fluid culture for microorganisms between the two groups (p>0.1 for each). Patients who remained undelivered for at least one week had a significantly lower median concentration of amniotic fluid interleukin-6 and white blood cell count than those who delivered before one week (p<0.005 for both).
Table 2.
Characteristics and outcomes of patients delivered within 7 days of amniocentesis and those who were undelivered for at least 7 days
| Delivery before 1 week (n=21) | Undelivered for ≥ 1 week (n=29) | p-value | |
|---|---|---|---|
| Maternal age (years) | 33 (30–36) | 30 (28–33) | 0.07 |
| Nulliparity (%) | 47.6% (10/21) | 72.4% (21/29) | 0.09 |
| Initial amniocentesis | |||
| Gestational age at amniocentesis (weeks) | 26.4 (22.6–28.4) | 24.3 (21.9–26.9) | 0.13 |
| Positive amniotic fluid culture (%) | 28.6% (6/21) | 13.8% (4/29) | 0.29 |
| Positive amniotic fluid PCR for Ureaplasma spp. | 14.3% (2/14) | 26.3% (5/19) | 0.67 |
| Amniotic fluid WBC count (cells/mm3) | 725 (94->1000) | 5 (1–100) | 0.002 |
| Amniotic fluid WBC count (≥ 19 cells/mm3) | 81.0% (17/21) | 40.7% (11/27) | 0.008 |
| Amniotic fluid interleukin-6 (ng/mL) | 28.2 (14.0–46.5) | 10.3 (3.4–21.8) | 0.001 |
| Amniotic fluid interleukin-6 (>2.6 ng/mL) | 100% (21/21) | 100% (28/28) | >0.99 |
| Cervical dilatation > 3cm (%) | 14.3% (3/21) | 6.9% (2/29) | 0.64 |
| Use of tocolytics (%) | 95.2% (20/21) | 100% (29/29) | 0.42 |
| Antenatal corticosteroids administration (%) | 52.4% (11/21) | 69.0% (20/29) | 0.26 |
| Gestational age at delivery (weeks) | 26.6 (22.9–28.7) | 33.1 (27.3–34.9) | <0.001 |
| Delivery within 14 days of amniocentesis | 100% (21/21) | 17.2% (5/29) | <0.001 |
| Delivery within 4 weeks of amniocentesis | 100% (21/21) | 27.6% (8/29) | <0.001 |
| Delivery before 30 weeks a | 94.7% (18/19) | 32.1% (9/28) | <0.001 |
| Delivery before 34 weeks | 100% (21/21) | 58.6% (17/29) | 0.001 |
| Delivery at term (>=37 weeks) | 0% (0/21) | 13.8% (4/29) | 0.13 |
| Clinical chorioamnionitis | 23.8% (5/21) | 3.4% (1/29) | 0.07 |
| Acute histologic chorioamnionitis | 81.3% (13/16) | 60.9% (14/12) | 0.29 |
| Funisitis | 37.5% (6/16) | 26.1% (6/23) | 0.50 |
Data are median (interquartile range) or % (n/N).
PCR, polymerase chain reaction; WBC, white blood cell.
Cases who underwent amniocentesis at or beyond 30 weeks were excluded from the analysis.
Of 29 patients undelivered for ≥ 7 days, 10 did not have a follow-up amniocentesis (five declined the procedure, 2 had severe oligohydramnios due to rupture of membranes, 2 were transferred to another hospital, and in one the treating physician did not recommend the procedure). The remaining 19 patients had a follow-up amniocentesis to determine if intra-amniotic infection had been eradicated, if intra-amniotic inflammation was being treated, and to determine whether antibiotic treatment should be continued or stopped. Generally, antibiotics were discontinued if patients had a negative MMP-8 test result or if the amniotic fluid white blood cell count became normal. However, the final decision was made by the attending obstetrician.
There were no significant differences in the median gestational age at amniocentesis, amniotic fluid interleukin-6 concentration and white blood cell count and the frequency of a positive amniotic fluid culture between patients who were undelivered for at least one week and had follow-up amniocentesis and those who had not (p>0.1 for each). Patients who did not have a follow-up amniocentesis delivered significantly earlier than those who had follow-up amniocenteses (27.3 weeks [interquartile range, 25.0–33.9 weeks] vs 34.1 weeks [interquartile range, 31.7–35.6 weeks]; p<0.05).
Treatment success with antibiotics in this study
Of the 23 patients who had follow-up amniocentesis, intra-amniotic inflammation was determined to be successfully eradicated in 15, and intra-amniotic infection was eradicated in three (one with a positive culture and a positive PCR for Ureaplasma spp., and two with a negative culture but a positive PCR for Ureaplasma spp.). All patients with intra-amniotic infection also had intra-amniotic inflammation.
Microbiologic or biochemical evidence of successful treatment was demonstrated in 79% (15/19). One patient who did not have confirmation of eradication of intra-amniotic infection/inflammation at follow-up amniocentesis delivered at term. None of the 10 patients who did not have a follow-up amniocentesis delivered at term. Thus, treatment success of antibiotics (defined as eradication of intra-amniotic infection/inflammation or delivery ≥ 37 weeks of gestation) occurred in 84% (16/19) of patients who had follow-up amniocentesis and was possible in at least 32% (16/50) of patients with intra-amniotic infection/inflammation who received the antimicrobial agents.
Clinical outcome of patients treated with antimicrobial agents and who had a follow-up amniocentesis
Table 3 shows the details and Table 4 summarizes the characteristics and outcomes of 19 patients who were treated with the antimicrobial agents and had a follow-up amniocentesis. A detailed description of each patient can be found in the Supplementary Material S2.
Table 3.
Details of presentations of initial amniocentesis, and outcomes of patients who were treated with antibiotics (ceftriaxone, clarithromycin and metronidazole) and had follow-up amniocentesis
| Gestational age, weeks |
Initial amniocentesis |
Interval between initial amniocentesis and resolution (days)a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case No. | Amniocentesis | Delivery | Culture | PCR for Ureaplasm a spp. | interleukin-6 (ng/mL) | WBC count (cells/mm3) | MMP-8 rapid test | Birth weight (gm) | Steroid for fetal lung maturity (weeks) | Acute histologic chorioamnionitis/funisitis | Neonatal outcomes | |
| Group A : | Resolution confirmed and delivered after 34 weeks of gestation | |||||||||||
| 1 | 25.1 | 35.6 | Neg. | Pos. | 5.0 | 11 | Pos. | 28 | unknown | 25.3 | N/A | Survival without morbidity |
| 2 | 22.7 | 38.3 | Neg. | N/A | 33.4 | 100 | Pos. | 14 | 2620 | 22.9 | −/− | Survival without morbidity |
| 3 | 26.0 | 40.1 | Neg. | N/A | 2.7 | 5 | Pos. | 7 | 3860 | 26.0 | −/− | Survival without morbidity |
| 4 | 29.6 | 34.1 | Neg. | N/A | 2.7 | 0 | Pos. | 7 | 2200 | 33.3 | −/− | Survival without morbidity |
| 5 | 22.9 | 38.0 | Neg. | Neg. | 2.8 | 5 | Pos. | 38 | 2850 | −/− | Survival without morbidity | |
| 6 | 26.7 | 34.7 | Neg. | N/A | 4.1 | 0 | Pos. | 7 | 2610 | 26.3 | +/− | Survival without morbidity |
| 7 | 27.6 | 35.4 | Neg. | Neg. | 4.0 | 1 | Pos. | 10 | unknown | N/A | Survival without morbidity | |
| 8 | 20.9 | 34.7 | Neg. | Neg. | 3.3 | 1 | Pos. | 67 | 2040 | +/− | Survival without morbidity | |
| 9 | 25.6 | 35.0 | Neg. | Neg. | 3.6 | 25 | N/A | 48 | 2290 | +/− | Survival without morbidity | |
| Group B : | Resolution confirmed but delivered before 34 weeks of gestation | |||||||||||
| 10 | 28.4 | 31.7 | Neg. | Pos. | 42.6 | 720 | N/A | 13 | 1840 | 28.4 | +/− | Survival without morbidity |
| 11 | 24.0 | 32.6 | Neg. | Neg. | 2.9 | 2 | N/A | 30 | 1990 | 29.7 | +/− | Survival without morbidity |
| 12 | 25.4 | 30.4 | Neg. | N/A | 2.6 | 0 | Pos. | 15 | 1400 | 24.0 | +/+ | Survival without morbidity |
| 13 | 21.0 | 33.3 | Neg. | Neg. | 51.5 | 105 | Pos. | 22 | 1800 | 27.9 | −/− | Survived and diagnosed as BPD |
| 14 | 21.0 | 29.6 | Pos. | Pos. | 4.8 | 0 | N/A | 30 | 1640 | 29.4 | +/− | Survival without morbidity |
| 15 | 20.1 | 25.4 | Neg. | Neg. | 11.1 | 16 | Pos. | 15 | 700 | 25.4 | +/+ | Shortly died (5 hours from birth) |
| Group C : | Resolution not confirmed but delivered after 37 weeks of gestation | |||||||||||
| 16 | 21.6 | 38.0 | Neg. | Neg. | 19.4 | 2 | N/A | - | 2760 | −/− | Survival without morbidity | |
| Group D : | Resolution not confirmed and delivered before 34 weeks of gestation | |||||||||||
| 17 | 31.4 | 32.9 | Neg. | Neg. | 22.0 | 100 | Pos. | - | 1710 | 30.9 | +/+ | Survival without morbidity |
| 18 | 26.4 | 32.6 | Pos. | N/A | 18.2 | 50 | Pos. | - | 2060 | +/− | Survival without morbidity | |
| 19 | 22.1 | 23.6 | Neg. | N/A | 23.8 | 54 | Pos. | - | 620 | +/− | Survived and diagnosed as RDS, BPD, IVH, PVL | |
PCR, polymerase chain reaction; MMP-8, matrix metalloproteinase-8; Pos., positive result; Neg., negative result; N/A, not assessed; BPD, bronchopulmonary dysplasia; RDS, respiratory distress syndrome; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia
the first amniocentesis without evidence of intra-amniotic infection/inflammation
Table 4.
Characteristics and outcomes of 19 patients who were treated with antibiotics (ceftriaxone, clarithromycin and metronidazole) and had follow-up amniocentesis
| Resolution of intra-amniotic inflammation | ||||
|---|---|---|---|---|
| Confirmed (n=15) | Not confirmed (n=4) | |||
| Group A: Delivery at or after 34 weeks (n=9) | Group B: Delivery before 34 weeks (n=6) | Group C: Delivery at or after 34 weeks (n=1) | Group D: Delivery before 34 weeks (n=3) | |
| Nulliparity | 88.9% (8/9) | 66.7% (4/6) | 0% (0/1) | 66.6% (2/3) |
| History of preterm delivery | 0% (0/9) | 16.7% (1/6) | 0% (0/1) | 0% (0/3) |
| Progesterone treatment | 0 | 0 | 0 | 0 |
| Cerclage before the onset of preterm Labor | 11.1% (1/9) | 16.7% (1/6) | 0% (0/1) | 33.3% (1/3) |
| Cerclage after the onset of preterm labor and labor stopped | 0% (0/9) | 16.7% (1/6) | 0% (0/1) | 0% (0/1) |
| Initial amniocentesis | ||||
| Gestational age at amniocentesis | 25.6 (22.9–26.7) | 22.5 (21.0–25.4) | 21.6 | 26.4 (22.1–31.4) |
| Positive amniotic fluid culture (%) | 0% (0/9) | 16.7% (1/6) | 0% (0/1) | 33.3% (1/3) |
| Positive amniotic fluid PCR for Ureaplasma spp. | 20% (1/5) | 40% (2/5) | 0% (0/1) | 0% (0/1) |
| Amniotic fluid WBC count (cells/mm3) | 5 (1–11) | 16 (2–105) | 2 | 54 (50–100) a |
| Positive MMP-8 Rapid test | 87.5% (7/8) | 100% (3/3) | 0% (0/0) | 100% (3/3) |
| Amniotic fluid interleukin-6 (ng/mL) | 3.56 (2.79–4.09) | 7.02 (2.6–42.6) | 19.40 | 21.97 (18.22–23.84) |
| Days from initial amniocentesis to resolution | 14 (7–38) | 18 (13–22) | N/A | N/A |
| Duration of new antibiotic regimen use (days)b | 21 (14–25) | 25.5 (21–31) | 14 | 10 (10–33) |
| Number of amniocentesis | 4 (4–4) | 3.5 (3–4) | 2 | 2 (2–3) |
| Gestational age at delivery (weeks) | 35.4 (34.7–38.0) | 31.1 (29.6–32.6) a | 38.0 | 32.6 (23.6–32.9) a |
| Days from initial amniocentesis to delivery | 73 (56–103) | 48 (32–66) | 115 | 10 (10–43) a |
| Delivery within 14 days of amniocentesis | 0% (0/9) | 0% (0/6) | 0% (0/1) | 66.7% (2/3) a |
| Delivery within 4 weeks of amniocentesis | 0% (0/9) | 16.7% (1/6) | 0% (0/1) | 66.7% (2/3) a |
| Delivery before 30 weeks | 0% (0/9) | 33.3% (2/6) | 0% (0/1) | 50.0% (1/2) c |
| Delivery before 34 weeks | 0% (0/9) | 100% (6/6) a | 0% (0/1) | 100% (3/3) a |
| Delivery at term (>=37 weeks) | 66.7% (6/9) | 0% (0/6) | 100% (1/1) | 0% (0/3) |
| Birth weight (grams) | 2610 (2200–2850) | 1720 (1225–1878) a | 2760 | 1710 (620–2060) a |
| Clinical chorioamnionitis | 0% (0/9) | 0% (0/6) | 0% (0/1) | 0% (0/3) |
| Acute histologic chorioamnionitis | 28.6% (2/7) | 83.3% (5/6) | 0% (0/1) | 100% (3/3) |
| Funisitis | 0% (0/7) | 50% (3/6) | 0% (0/1) | 33.3% (1/3) |
| Significant neonatal morbidity | 0% (0/9) | 33.3% (2/6) | 0% (0/1) | 33.3% (1/3) |
Data are median (interquartile ranges for group A and B, range for group D) or % (n/N).
PCR, polymerase chain reaction; WBC, white blood cell; MMP-8, matrix metalloproteinase-8.
p < 0.05 compared to Group A.
Some patients restarted antibiotic administration because they developed preterm rupture of membranes or preterm labor and intra-amniotic infection/inflammation after the discontinuation of antibiotics as intra-amniotic infection/inflammation resolved and preterm labor stopped. This duration was not included in this analysis
One case who underwent amniocentesis at or beyond 30 weeks was excluded from the analysis.
Comment
Principal findings of the study:
1) antibiotics were effective in treating intra-amniotic infection/inflammation in women with preterm labor and intact membranes as demonstrated by analysis of amniotic fluid obtained before and after antibiotics were administered; 2) resolution of intra-amniotic infection/inflammation was objectively demonstrated in 79% (15/19) of patients who received the antimicrobial agents and had follow-up amniocentesis; 3) the overall treatment success (defined as resolution of intra-amniotic inflammation or infection, or delivery ≥37 weeks) rate among patients who underwent follow-up amniocentesis was 84% (16/19). The overall success rate among all women with intra-amniotic infection/inflammation who received the antimicrobial agents was 32% (16/50).
The prevalence and clinical import of intra-amniotic infection/inflammation in patients with preterm labor and intact membranes
The frequency of a positive amniotic fluid culture for microorganisms in patients presenting with an episode of preterm labor and intact membranes is approximately 10%,3,6,12,14,33,139 and these patients are more likely to develop maternal complications such as clinical chorioamnionitis3 and pulmonary edema while receiving tocolytics,68,69 and deliver a preterm neonate shortly after admission.3,14,16,33,140,141 In addition, patients with intra-amniotic infection are more likely to show evidence of histologic chorioamnionitis (a maternal host response) or funisitis/chorionic vasculitis (pathologic hallmarks of the fetal inflammatory response syndrome, or FIRS).59,124,131–133 One of every four preterm neonates is born to a mother with microorganisms in the amniotic cavity.3,11,12,22,142–146
When microorganisms invade the human fetus, a systemic inflammatory response can be elicited, and this condition is referred to as FIRS (diagnosed by an elevated umbilical cord blood plasma IL-6 concentration). This condition is associated with a higher rate of neonatal complications147,148 because, before birth, these fetuses have multi-systemic involvement or dysfunction.149 Examples include leukocyte activation,150 leukocytosis,151 adrenal gland hyperactivity (elevated concentrations of cortisol in peripheral blood),152 cardiac dysfunction,153,154 and increased concentrations of matrix-degrading enzymes in amniotic fluid and fetal blood.155–160 FIRS is a risk factor for neonatal morbidity, as well as long-term complications such as cerebral palsy 54,161 and chronic lung disease.162–165
In summary, a strong body of evidence indicates that fetal exposure to microorganisms or intra-amniotic inflammation is associated with adverse outcome.1,5,6,8,9,39,55,64,71,131,161,166–170 Despite this overwhelming evidence, obstetricians in practice do not routinely ascertain whether patients with preterm labor have intra-amniotic infection/inflammation. The reason is two-fold: first, the best method to determine the presence of intra-amniotic infection/inflammation is analysis of amniotic fluid, which requires an invasive procedure (amniocentesis); second, the evidence that treatment with antimicrobial agents can eradicate intra-amniotic infection has been based on case reports. Therefore, in practice, clinicians rely on signs and symptoms of clinical chorioamnionitis (e.g. fever, maternal tachycardia, etc.) to exclude intra-amniotic infection. However, it is now well-established that these clinical signs are both insensitive and non-specific for the identification of intra-amniotic infection in both preterm6,33,121,125,171 and term gestations136,137,172–176. This is also the case for maternal circulating white blood cell count and other biomarkers of the acute phase response (such as serum C-reactive protein).18,121,167,177,178 One argument against the analysis of amniotic fluid used to be that results were not immediately available to affect patient management, as culture for microorganisms may take several days. However, rapid tests are now available for the diagnosis of intra-amniotic inflammation (such as amniotic fluid white blood cell count, glucose, amniotic fluid MMP-8, or interleukin-6, among others),4,19,38,53,60,126,127,167,176,177,179–184, and for the diagnosis of infection using PCR. 8,23,123,140,185,188
Antibiotic administration to patients in preterm labor with intact membranes
The evidence that intra-amniotic infection is causally linked to spontaneous preterm labor and delivery coalesced in the 1980s:2,3,13,14,144,189,190 this led to the conduct of several randomized clinical trials in which patients with an episode of preterm labor were allocated to antimicrobial agents vs. placebo or no treatment.73,74,77–80,82,191–194 Although the initial trials reported pregnancy prolongation,75,77,191 and, in some cases, a lower frequency in the rate of preterm delivery, 73,77 these findings were not supported by subsequent clinical trials78,81 or systematic reviews and meta-analyses.195–197 This led professional organizations, including the American College of Obstetricians and Gynecologists198 and Society of Maternal-Fetal Medicine,86 to recommend that antibiotics not be administered to patients with preterm labor and intact membranes, with the objective of prolonging pregnancy or reducing the rate of preterm birth. Antibiotics have been recommended in the context of preterm labor with intact membranes when delivery is impending and the patient is a carrier of Group B streptococci or Streptococcus agalactiae.85,86,199,200
Why are antibiotics considered ineffective in prolonging pregnancy and preventing preterm delivery in patients with preterm labor and intact membranes?
Preterm labor is a syndrome defined by the presence of increased uterine contractility, cervical dilatation, and decidual membrane activation, each caused by multiple pathologic processes.15,201,202 Intra-amniotic infection is only one of the potential mechanisms of disease responsible for this syndrome. If the frequency of intra-amniotic infection is only 10%22; then antimicrobial agents can only be effective in that small fraction of patients.26
The ORACLE II trial randomized 6,295 women with an episode of preterm labor with intact membranes to placebo or antibiotics: these patients did not have clinical evidence of infection, and amniocenteses were not performed to diagnose intra-amniotic infection.78 Therefore, 90% of patients enrolled in the ORACLE II trial could not have benefitted from antibiotic administration, and the negative results are not surprising. The same applies to all other randomized clinical trials of antibiotics in patients with preterm labor and intact membranes.74,79–82 However, these results should not be interpreted as indicating that antibiotics are ineffective when administered to the “right” patients: those who have proven intra-amniotic infection.
Experimental evidence that anti-microbial agents can eradicate intrauterine infection and prolong pregnancy
McDuffie et al. reported that, in pregnant rabbits, antibiotic administration (ampicillin and sulbactam) at or before inoculation with E. coli led to fewer preterm deliveries and more live pups than those whose treatment was delayed for more than four hours.203 Subsequently, Fidel et al., using the same experimental model, showed that antibiotic administration within 12 hours of inoculation – but not after 18 hours – increased the duration of pregnancy and reduced perinatal mortality.204 Collectively, these results suggest that antibiotics can be beneficial in cases of intrauterine infection.
These observations were subsequently confirmed in non-human primates. Investigators at the Oregon Primate Center administered Group B Streptococci to pregnant Rhesus monkeys (Macaca mulatta) on day 130 of gestation (term: 167 days), and observed an increase in uterine contractility at a median of 28 hours (range: 14–40 hours) after inoculation.5 This model of intra-amniotic infection has many features in common with intra-amniotic infection in women. Importantly, the onset of contractions was preceded by an increase in amniotic fluid concentrations of proinflammatory cytokines (IL-1β, TNFα, IL-6) and prostaglandins (E2 and F2a). None of the animals became febrile or had leukocytosis; yet, all delivered preterm. Subsequent studies demonstrated that dexamethasone,205 indomethacin,206 and IL-10 blocked IL1–induced uterine contractility (a model of intra-amniotic inflammation), suggesting a role for anti-inflammatory agents in the treatment of inflammation-induced preterm labor.207
Antibiotics used in this study to treat intra-amniotic infection/inflammation
An important principle in the treatment of infectious diseases is that antibiotic selection should be tailored to the microorganisms causing the infection. The rationale for the antibiotic regimen used in our study was described in previous studies of patients with preterm PROM.118,120 Briefly, two macrolides, erythromycin or azithromycin, have been used to treat intra-amniotic infection in women, and there is experimental evidence in non-human primates that azithromycin can eradicate Ureaplasma from the amniotic cavity and reduces fetal lung injury.208,209 Clarithromycin was chosen at our Institution because it has a much higher rate of transplacental passage than erythromycin or azithromycin, and this agent is effective against Ureaplasma species, the most common microorganism identified in the amniotic fluid of patients at risk for preterm delivery.210 Ceftriaxone was included because of its enhanced coverage of aerobic bacteria and high rate of transplacental passage.211,212 Metronidazole was selected because anaerobic organisms are frequently present in the amniotic cavity, and this drug provides optimal coverage for these microorganisms. We reported that, in patients with preterm PROM, this antibiotic combination eradicated intra-amniotic infection and/or inflammation in at least 33% of patients as demonstrated by repeat analysis of amniotic fluid.118 Whether other antimicrobial combinations can achieve the same result would need to be determined.
Evidence that intra-amniotic infection can be treated
Intra-amniotic infection has been successfully treated in patients with a sonographic short cervix without clinical manifestations of infection (fever, uterine tenderness, etc.).117 Eradication of intra-amniotic infection has also been reported in cases of preterm PROM118,213and preterm labor.214,215 Whether this approach is effective in patients with preterm labor with intact membranes had not been studied until recently.119 In patients with preterm labor and proven intra-amniotic infection, there was a shorter diagnosis-to-delivery interval.3,6–8,22,33,53 Indeed, it was generally believed that once patients present with preterm labor, an intra-amniotic “cytokine storm” would inevitably lead to preterm delivery.
The results reported herein represent the first objective confirmation that antibiotic treatment can eradicate intra-amniotic infection in preterm labor with intact membranes in a case series. This was demonstrated in three patients: the first had microbial invasion of the amniotic cavity with Ureaplasma spp. detected by culture; the other two had microbial nucleic acids detected by PCR.
In all three cases, a repeat amniocentesis yielded a negative amniotic fluid culture and negative PCR for microorganisms. Details of each specific case are illustrated in Table 3 (see cases 1, 10 and 14). It is interesting that in case 14, the first amniocentesis at 21 weeks was positive for Ureaplasma spp. and showed an elevated interleukin-6 (4.8 ng/mL). Antibiotic treatment eradicated both the microorganisms and evidence of the intra-amniotic inflammatory process (interleukin-6: 1.93 ng/mL). The treating physician elected to continue oral clarithromycin. Four weeks after successful treatment, the patient was suspected to have rupture of membranes and the amniotic fluid became positive for Morganella morganii, a Gram negative bacilli frequently implicated in nosocomial infections.216 Intra-amniotic inflammation (interleukin-6: 6.89 ng/mL) recurred, labor progressed, and the patient delivered at 29.4 weeks a 1640 g neonate that had no major complications. This case indicates that patients with an intra-amniotic infection may be susceptible to recurring infection with other microorganisms. Whether this indicates a deficit in host defense or an opportunistic infection during the course of antimicrobial therapy is unclear. Chorioamnionitis caused by Morganella morganii has been reported in an immunocompetent patient.217 Recent evidence derived from whole exome sequencing indicates that some patients may have deleterious mutations in genes encoding for proteins implicated in host defense against microbial invasion.218–221 There is evidence that acute chorioamnionitis may be recurrent in successive pregnancies222 and therefore, the predisposition to intra-amniotic infection may have a genetic basis.29,223–227
Recently, a group of investigators reported that antimicrobial agents in patients with intra-amniotic infection may result in prolongation of pregnancy and a decreased rate of admission to the neonatal intensive care unit without a change in perinatal morbidity.119 No follow-up amniocenteses were performed in that study; therefore, there was no objective evidence to determine whether antimicrobial therapy was effective in treating intra-amniotic infection/inflammation. Nonetheless, the reports of such studies represent indirect evidence consistent with our findings.
Successful treatment of intra-amniotic inflammation in preterm labor with intact membranes with antibiotics
Intra-amniotic inflammation in the absence of demonstrable microorganisms is more frequent than intra-amniotic infection in patients with preterm labor and intact membranes,24,26,33,36,39 a sonographic short cervix,28,117 and even preterm PROM.27,71 This type of intra-amniotic inflammation may be caused by either microorganisms which escaped detection27,28 or by danger signals or alarmins30–32,91,228,229 which are released by cells under stress or during the course of cell death such as necrosis.230–233 Examples of danger signals include high mobility group box (HMGB1), S100 calcium-binding protein B (S100B), and interleukin-1α, which can induce preterm labor by the activation of the inflammasome.31,90,92,234–238
The treatment of sterile inflammation is a major challenge in medicine. The conventional approach is to use anti-inflammatory agents, such as glucocorticoids239,240 or non-steroidal antiinflammatory agents.241,242 In some cases, treatment is possible with a specific agent that decreases the concentration of the danger signal such as allopurinol to decrease the concentration of uric acid in gout. However, in the case of intra-amniotic inflammation without demonstrable microorganisms, the optimal treatment is uncertain. Preliminary evidence from our laboratory suggests that inhibitors of the inflammasome may have therapeutic benefits in preventing preterm labor induced by specific danger signals such as S100B.236
How can antibiotics be effective in cases of intra-amniotic inflammation without demonstrable microorganisms? The antibiotic combination used at the Seoul National University Hospital included clarithromycin, which has been shown to have immunomodulatory properties and specifically inhibits AP-1 and NF-kappa B, two transcription factors which induce production of proinflammatory cytokines and effectively act as anti-inflammatory agents.243,244 We have previously shown that NFkB is upregulated by IL-1B.245–249
Our study shows that intra-amniotic inflammation/infection was successfully treated in 84% (16/19) of cases in which follow-up amniocentesis was performed. It is unlikely that this therapeutic success can be attributed to glucocorticoids because these agents were not used in 31% (5/16) of patients in whom intra-amniotic inflammation improved.
The results of the current study are consistent with our previous observations in the context of preterm PROM. The antimicrobial agents used in this study were able to treat and prevent intra-amniotic infection/inflammation, prolong the latency period, reduce acute histologic chorioamnionitis and funisitis, and improve neonatal outcomes in patients with preterm PROM.120
Strengths and limitations
Strengths of the study include: 1) this is the first case series in which women with intra-amniotic infection/inflammation were treated with antibiotics and monitored with serial amniocentesis to determine if there was therapeutic success in patients with preterm labor and intact membranes; 2) assessment of intra-amniotic infection/inflammation was performed by analysis of amniotic fluid using the amniotic fluid white blood cell count or a rapid test for MMP-8; 3) the retrospective diagnosis of intra-amniotic inflammation was performed using amniotic fluid concentrations of interleukin-6, which has been demonstrated to be a reliable marker, widely used in many reports to diagnose this condition; and 4) this study used serial evaluation of amniotic fluid. This is the only objective method to ascertain whether there is therapeutic efficacy.
Limitations of this study include its observational nature. This is not a randomized clinical trial in which there was a placebo arm. However, clinicians in our institution are unwilling to randomize patients with intra-amniotic infection/inflammation to placebo because such patients are at increased risk for clinical chorioamnionitis, maternal sepsis, and neonatal complications, such as early neonatal sepsis, among others. In a previous observational study, we reported that 91% of patients with intra-amniotic inflammation delivered within one week of amniocentesis.33 In contrast, in the current study, only 42% (21/50) delivered within one week: this is also indirect evidence of efficacy.
We have grouped together patients with intra-amniotic infection and intra-amniotic inflammation without demonstrable microorganisms. A limitation of our study is that there were only three patients with intra-amniotic infection who were successfully treated and that most of the patients had intra-amniotic inflammation without detectable microorganisms with the methods used in our institution. It is possible that more organisms could have been detected by using assays for the conserved region of the microbial genome or sequencing of microbial cell-free DNA.250–252 Further studies using molecular microbiologic methods are required to address this issue.
Another potential limitation to our interpretation of the results of this case series is that we used as a definition of “success” as delivery at ≥37 weeks of gestation, rather than ≥34 weeks. This may be a very stringent criterion to assess the prognosis of a patient with preterm labor and intra-amniotic infection/inflammation; however, using this definition strengthens the evidence of the effectiveness of antibiotics, as patients may benefit from antimicrobial agents without delivering at term (e.g. delivery at 36 weeks). Indeed, we performed a sensitivity analysis, and if treatment success was defined as eradication of intra-amniotic infection/inflammation or delivery at or after 32 weeks of gestation, the overall efficacy would be 44% (22/50).
Conclusion
The administration of antibiotics to patients with preterm labor and intact membranes with proven intra-amniotic infection/inflammation is associated with eradication of infection and inflammation in a subset of patients.
Supplementary Material
AJOG at a Glance:
-
What is the research question?
Can intra-amniotic infection or intra-amniotic inflammation be treated with antibiotics in patients with preterm labor and intact membranes?
-
What are the key findings?
Resolution of intra-amniotic inflammation or intra-amniotic infection was objectively demonstrated by analysis of amniotic fluid after treatment with antibiotics in 83% of patients.
-
What does this add to what is known?
Contrary to what is widely believed, antimicrobial treatment of intra-amniotic infection or intra-amniotic inflammation can be successful in a subset of patients with preterm labor and intact membranes. These observations open new therapeutic alternatives and call for personalized assessment of patients with preterm labor and intact membranes to identify those who can benefit from this intervention.
Acknowledgement
The authors acknowledge the contributions of the patients who were seen at the Seoul University Hospital in South Korea, the obstetricians, nurses, and research personnel who made the collection of data for this retrospective study possible.
Source of funding: This research was supported in part by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning, Republic of Korea (2017R1A2B2007958); in part by the Perinatology Research Branch, Program for Perinatal Research and Obstetrics, Division of Intramural
Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part by Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
Footnotes
Condensation: Antibiotics can eradicate intra-amniotic inflammation in preterm labor
Disclosure: The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014;345:760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gravett MG, Hummel D, Eschenbach DA, Holmes KK. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol 1986;67:229–37. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intra-amniotic infection in women with preterm labor and intact membranes. American journal of obstetrics and gynecology 1989;161:817–24. [DOI] [PubMed] [Google Scholar]
- 4.Gauthier DW, Meyer WJ, Bieniarz A. Correlation of amniotic fluid glucose concentration and intra-amniotic infection in patients with preterm labor or premature rupture of membranes. American journal of obstetrics and gynecology 1991;165:1105–10. [DOI] [PubMed] [Google Scholar]
- 5.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intra-amniotic infection and preterm labor in rhesus monkeys. American journal of obstetrics and gynecology 1994;171:1660–7. [DOI] [PubMed] [Google Scholar]
- 6.Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol 1998;92:77–82. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo G, Capponi A, Vlachopoulou A, Angelini E, Grassi C, Romanini C. Ultrasonographic assessment of the uterine cervix and interleukin-8 concentrations in cervical secretions predict intrauterine infection in patients with preterm labor and intact membranes. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 1998;12:86–92. [DOI] [PubMed] [Google Scholar]
- 8.Yoon BH, Romero R, Lim JH, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. American journal of obstetrics and gynecology 2003;189:919–24. [DOI] [PubMed] [Google Scholar]
- 9.Novy MJ, Duffy L, Axthelm MK, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci 2009;16:56–70. [DOI] [PubMed] [Google Scholar]
- 10.Morales WJ, Angel JL, O’Brien WF, Knuppel RA, Finazzo M. A randomized study of antibiotic therapy in idiopathic preterm labor. Obstet Gynecol 1988;72:829–33. [DOI] [PubMed] [Google Scholar]
- 11.Romero R, Emamian M, Quintero R, et al. The value and limitations of the Gram stain examination in the diagnosis of intra-amniotic infection. American journal of obstetrics and gynecology 1988;159:114–9. [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Mazor M, Wu YK, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol 1988;12:262–79. [PubMed] [Google Scholar]
- 13.Romero R, Quintero R, Oyarzun E, et al. Intra-amniotic infection and the onset of labor in preterm premature rupture of the membranes. American journal of obstetrics and gynecology 1988;159:661–6. [DOI] [PubMed] [Google Scholar]
- 14.Skoll MA, Moretti ML, Sibai BM. The incidence of positive amniotic fluid cultures in patients preterm labor with intact membranes. American journal of obstetrics and gynecology 1989;161:813–6. [DOI] [PubMed] [Google Scholar]
- 15.Romero R, Avila C, Brekus CA, Morotti R. The role of systemic and intrauterine infection in preterm parturition. In: Garfield R, ed Uterine contractility Norwell, Massachusetts: Serono Symposia, 1990:319–53. [Google Scholar]
- 16.Armer TL, Duff P. Intra-amniotic infection in patients with intact membranes and preterm labor. Obstetrical & gynecological survey 1991;46:589–93. [DOI] [PubMed] [Google Scholar]
- 17.Coultrip LL, Grossman JH. Evaluation of rapid diagnostic tests in the detection of microbial invasion of the amniotic cavity. American journal of obstetrics and gynecology 1992;167:1231–42. [DOI] [PubMed] [Google Scholar]
- 18.Mazor M, Kassis A, Horowitz S, et al. Relationship between C-reactive protein levels and intra-amniotic infection in women with preterm labor. J Reprod Med 1993;38:799–803. [PubMed] [Google Scholar]
- 19.Hussey MJ, Levy ES, Pombar X, Meyer P, Strassner HT. Evaluating rapid diagnostic tests of intra-amniotic infection: Gram stain, amniotic fluid glucose level, and amniotic fluid to serum glucose level ratio. American journal of obstetrics and gynecology 1998;179:650–6. [DOI] [PubMed] [Google Scholar]
- 20.Blackwell SC, Berry SM. Role of amniocentesis for the diagnosis of subclinical intra-amniotic infection in preterm premature rupture of the membranes. Curr Opin Obstet Gynecol 1999;11:541–7. [DOI] [PubMed] [Google Scholar]
- 21.Hitti J, Tarczy-Hornoch P, Murphy J, Hillier SL, Aura J, Eschenbach DA. Amniotic fluid infection, cytokines, and adverse outcome among infants at 34 weeks’ gestation or less. Obstet Gynecol 2001;98:1080–8. [DOI] [PubMed] [Google Scholar]
- 22.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Mental retardation and developmental disabilities research reviews 2002;8:3–13. [DOI] [PubMed] [Google Scholar]
- 23.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One 2008;3:e3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cobo T, Kacerovsky M, Jacobsson B. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. American journal of obstetrics and gynecology 2014;211:708. [DOI] [PubMed] [Google Scholar]
- 25.Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. American journal of obstetrics and gynecology 2014;210:125 e1- e15. [DOI] [PubMed] [Google Scholar]
- 26.Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014;72:458–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2015;28:1394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med 2014:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. American journal of obstetrics and gynecology 2015;213:S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero R, Grivel JC, Tarca AL, et al. Evidence of perturbations of the cytokine network in preterm labor. American journal of obstetrics and gynecology 2015;213:836 e1- e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez-Lopez N, Romero R, Plazyo O, et al. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am J Reprod Immunol 2016;75:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plazyo O, Romero R, Unkel R, et al. HMGB1 Induces an Inflammatory Response in the Chorioamniotic Membranes That Is Partially Mediated by the Inflammasome. Biol Reprod 2016;95:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. American journal of obstetrics and gynecology 2001;185:1130–6. [DOI] [PubMed] [Google Scholar]
- 34.Maymon E, Romero R, Chaiworapongsa T, et al. Amniotic fluid matrix metalloproteinase-8 in preterm labor with intact membranes. American journal of obstetrics and gynecology 2001;185:1149–55. [DOI] [PubMed] [Google Scholar]
- 35.Romero R, Espinoza J, Rogers WT, et al. Proteomic analysis of amniotic fluid to identify women with preterm labor and intra-amniotic inflammation/infection: the use of a novel computational method to analyze mass spectrometric profiling. J Matern Fetal Neonatal Med 2008;21:367–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SM, Romero R, Lee J, et al. The frequency and clinical significance of intra-amniotic inflammation in women with preterm uterine contractility but without cervical change: do the diagnostic criteria for preterm labor need to be changed? J Matern Fetal Neonatal Med 2012;25:1212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park CW, Yoon BH, Kim SM, Park JS, Jun JK. The frequency and clinical significance of intra-amniotic inflammation defined as an elevated amniotic fluid matrix metalloproteinase-8 in patients with preterm labor and low amniotic fluid white blood cell counts. Obstet Gynecol Sci 2013;56:167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A rapid interleukin-6 bedside test for the identification of intra-amniotic inflammation in preterm labor with intact membranes. J Matern Fetal Neonatal Med 2016;29:349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh KJ, Hong JS, Romero R, Yoon BH. The frequency and clinical significance of intra-amniotic inflammation in twin pregnancies with preterm labor and intact membranes. J Matern Fetal Neonatal Med 2017:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams Waldorf KM, Singh N, Mohan AR, et al. Uterine overdistention induces preterm labor mediated by inflammation: observations in pregnant women and nonhuman primates. American journal of obstetrics and gynecology 2015;213:830 e1- e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rouse DJ, Skopec GS, Zlatnik FJ. Fundal height as a predictor of preterm twin delivery. Obstet Gynecol 1993;81:211–4. [PubMed] [Google Scholar]
- 42.Kim CJ, Romero R, Kusanovic JP, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol 2010;23:1000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee J, Romero R, Xu Y, et al. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS One 2011;6:e16806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee J, Romero R, Xu Y, et al. Detection of anti-HLA antibodies in maternal blood in the second trimester to identify patients at risk of antibody-mediated maternal anti-fetal rejection and spontaneous preterm delivery. Am J Reprod Immunol 2013;70:162–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee J, Romero R, Chaiworapongsa T, et al. Characterization of the fetal blood transcriptome and proteome in maternal anti-fetal rejection: evidence of a distinct and novel type of human fetal systemic inflammatory response. Am J Reprod Immunol 2013;70:265–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wegorzewska M, Nijagal A, Wong CM, et al. Fetal intervention increases maternal T cell awareness of the foreign conceptus and can lead to immune-mediated fetal demise. J Immunol 2014;192:1938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirota Y, Daikoku T, Tranguch S, Xie H, Bradshaw HB, Dey SK. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest 2010;120:803–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 2012;196:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cha J, Bartos A, Egashira M, et al. Combinatory approaches prevent preterm birth profoundly exacerbated by gene-environment interactions. J Clin Invest 2013;123:4063–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomez-Lopez N, Romero R, Plazyo O, et al. Preterm labor in the absence of acute histologic chorioamnionitis is characterized by cellular senescence of the chorioamniotic membranes. American journal of obstetrics and gynecology 2017;217:592 e1- e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holst RM, Mattsby-Baltzer I, Wennerholm UB, Hagberg H, Jacobsson B. Interleukin-6 and interleukin-8 in cervical fluid in a population of Swedish women in preterm labor: relationship to microbial invasion of the amniotic fluid, intra-amniotic inflammation, and preterm delivery. Acta Obstet Gynecol Scand 2005;84:551–7. [DOI] [PubMed] [Google Scholar]
- 52.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. American journal of obstetrics and gynecology 1992;166:1515–28. [DOI] [PubMed] [Google Scholar]
- 53.Nien JK, Yoon BH, Espinoza J, et al. A rapid MMP-8 bedside test for the detection of intra-amniotic inflammation identifies patients at risk for imminent preterm delivery. American journal of obstetrics and gynecology 2006;195:1025–30. [DOI] [PubMed] [Google Scholar]
- 54.Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. American journal of obstetrics and gynecology 1997;177:19–26. [DOI] [PubMed] [Google Scholar]
- 55.Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG 2003;110 Suppl 20:124–7. [DOI] [PubMed] [Google Scholar]
- 56.Murthy V, Kennea NL. Antenatal infection/inflammation and fetal tissue injury. Best Pract Res Clin Obstet Gynaecol 2007;21:479–89. [DOI] [PubMed] [Google Scholar]
- 57.Kirchner L, Helmer H, Heinze G, et al. Amnionitis with Ureaplasma urealyticum or other microbes leads to increased morbidity and prolonged hospitalization in very low birth weight infants. Eur J Obstet Gynecol Reprod Biol 2007;134:44–50. [DOI] [PubMed] [Google Scholar]
- 58.Korzeniewski SJ, Romero R, Cortez J, et al. A “multi-hit” model of neonatal white matter injury: cumulative contributions of chronic placental inflammation, acute fetal inflammation and postnatal inflammatory events. J Perinat Med 2014;42:731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pugni L, Pietrasanta C, Acaia B, et al. Chorioamnionitis and neonatal outcome in preterm infants: a clinical overview. J Matern Fetal Neonatal Med 2016;29:1525–9. [DOI] [PubMed] [Google Scholar]
- 60.Kim SM, Romero R, Lee J, et al. About one-half of early spontaneous preterm deliveries can be identified by a rapid matrix metalloproteinase-8 (MMP-8) bedside test at the time of midtrimester genetic amniocentesis. J Matern Fetal Neonatal Med 2016;29:2414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paules C, Pueyo V, Marti E, et al. Threatened preterm labor is a risk factor for impaired cognitive development in early childhood. American journal of obstetrics and gynecology 2017;216:157 e1- e7. [DOI] [PubMed] [Google Scholar]
- 62.Catov JM, Scifres CM, Caritis SN, Bertolet M, Larkin J, Parks WT. Neonatal outcomes following preterm birth classified according to placental features. American journal of obstetrics and gynecology 2017;216:411 e1- e14. [DOI] [PubMed] [Google Scholar]
- 63.Chevallier M, Debillon T, Pierrat V, et al. Leading causes of preterm delivery as risk factors for intraventricular hemorrhage in very preterm infants: results of the EPIPAGE 2 cohort study. American journal of obstetrics and gynecology 2017;216:518 e1- e12. [DOI] [PubMed] [Google Scholar]
- 64.Oh KJ, Park JY, Lee J, Hong JS, Romero R, Yoon BH. The combined exposure to intra-amniotic inflammation and neonatal respiratory distress syndrome increases the risk of intraventricular hemorrhage in preterm neonates. J Perinat Med 2018;46:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vaz A, Malheiro MF, Severo M, Rodrigues T, Guimaraes H, Montenegro N. Effect of antenatal corticosteroids on morbidity and mortality of preterm singletons and twins. J Matern Fetal Neonatal Med 2018;31:754–60. [DOI] [PubMed] [Google Scholar]
- 66.Ting JY, Kingdom JC, Shah PS. Antenatal glucocorticoids, magnesium sulfate, and mode of birth in preterm fetal small for gestational age. American journal of obstetrics and gynecology 2018;218:S818–S28. [DOI] [PubMed] [Google Scholar]
- 67.Takeuchi K, Mochizuki M, Moriyama T, Funakoshi T, Nakago S, Maruo T. Pulmonary edema as an acute complication of ritodrine therapy in the presence of maternal intrauterine infection. Clin Exp Obstet Gynecol 1998;25:99–100. [PubMed] [Google Scholar]
- 68.Bax A, Middeldorp AM, Harinck B, Holleboom C, van Roosmalen J. Unilateral pulmonary edema as a life-threatening complication of ritodrine. Acta Obstet Gynecol Scand 1999;78:915–6. [PubMed] [Google Scholar]
- 69.Kayacan N, Dosemeci L, Arici G, Karsli B, Erman M. Pulmonary edema due to ritodrine. Int J Clin Pharmacol Ther 2004;42:350–1. [DOI] [PubMed] [Google Scholar]
- 70.Kilpatrick SJ, Abreo A, Gould J, Greene N, Main EK. Confirmed severe maternal morbidity is associated with high rate of preterm delivery. American journal of obstetrics and gynecology 2016;215:233 e1–7. [DOI] [PubMed] [Google Scholar]
- 71.Shim SS, Romero R, Hong JS, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. American journal of obstetrics and gynecology 2004;191:1339–45. [DOI] [PubMed] [Google Scholar]
- 72.Yoon BH, Romero R, Park JS, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. American journal of obstetrics and gynecology 1998;179:1254–60. [DOI] [PubMed] [Google Scholar]
- 73.McGregor JA, French JI, Reller LB, Todd JK, Makowski EL. Adjunctive erythromycin treatment for idiopathic preterm labor: results of a randomized, double-blinded, placebo-controlled trial. American journal of obstetrics and gynecology 1986;154:98–103. [DOI] [PubMed] [Google Scholar]
- 74.Romero R, Sibai B, Caritis S, et al. Antibiotic treatment of preterm labor with intact membranes: a multicenter, randomized, double-blinded, placebo-controlled trial. American journal of obstetrics and gynecology 1993;169:764–74. [DOI] [PubMed] [Google Scholar]
- 75.Norman K, Pattinson RC, de Souza J, de Jong P, Moller G, Kirsten G. Ampicillin and metronidazole treatment in preterm labour: a multicentre, randomised controlled trial. Br J Obstet Gynaecol 1994;101:404–8. [DOI] [PubMed] [Google Scholar]
- 76.Rajaei M, Sultani M, Zare S. A randomized controlled trial of adjunctive erythromycin in women with idiopathic preterm labor. J Matern Fetal Neonatal Med 2006;19:17–20. [DOI] [PubMed] [Google Scholar]
- 77.Svare J, Langhoff-Roos J, Andersen LF, et al. Ampicillin-metronidazole treatment in idiopathic preterm labour: a randomised controlled multicentre trial. Br J Obstet Gynaecol 1997;104:892–7. [DOI] [PubMed] [Google Scholar]
- 78.Kenyon SL, Taylor DJ, Tarnow-Mordi W, Group OC. Broad-spectrum antibiotics for spontaneous preterm labour: the ORACLE II randomised trial. ORACLE Collaborative Group. Lancet 2001;357:989–94. [DOI] [PubMed] [Google Scholar]
- 79.Cox SM, Bohman VR, Sherman ML, Leveno KJ. Randomized investigation of antimicrobials for the prevention of preterm birth. American journal of obstetrics and gynecology 1996;174:206–10. [DOI] [PubMed] [Google Scholar]
- 80.Gordon M, Samuels P, Shubert P, Johnson F, Gebauer C, Iams J. A randomized, prospective study of adjunctive ceftizoxime in preterm labor. American journal of obstetrics and gynecology 1995;172:1546–52. [DOI] [PubMed] [Google Scholar]
- 81.Keuchkerian SE, Sosa CG, Fernandez A, Alonso JG, Laborde A, Cuadro JC. Effect of amoxicillin sulbactam in threatened preterm labour with intact membranes: a randomised controlled trial. Eur J Obstet Gynecol Reprod Biol 2005;119:21–6. [DOI] [PubMed] [Google Scholar]
- 82.Newton ER, Dinsmoor MJ, Gibbs RS. A randomized, blinded, placebo-controlled trial of antibiotics in idiopathic preterm labor. Obstet Gynecol 1989;74:562–6. [PubMed] [Google Scholar]
- 83.Newton ER, Shields L, Ridgway LE 3rd, Berkus MD, Elliott BD. Combination antibiotics and indomethacin in idiopathic preterm labor: a randomized double-blind clinical trial. American journal of obstetrics and gynecology 1991;165:1753–9. [DOI] [PubMed] [Google Scholar]
- 84.Ohlsson A, Shah VS. Intrapartum antibiotics for known maternal Group B streptococcal colonization. Cochrane Database Syst Rev 2014:CD007467. [DOI] [PubMed] [Google Scholar]
- 85.Mayor S. Antibiotics are recommended in preterm labour to stop group B streptococcal transmission. BMJ 2017;358:j4271. [DOI] [PubMed] [Google Scholar]
- 86.ACOG Committee Opinion No. 445: antibiotics for preterm labor. Obstet Gynecol 2009;114:1159–60. [DOI] [PubMed] [Google Scholar]
- 87.Hutzal CE, Boyle EM, Kenyon SL, et al. Use of antibiotics for the treatment of preterm parturition and prevention of neonatal morbidity: a metaanalysis. American journal of obstetrics and gynecology 2008;199:620 e1–8. [DOI] [PubMed] [Google Scholar]
- 88.Patel K, Williams S, Guirguis G, Gittens-Williams L, Apuzzio J. Genital tract GBS and rate of histologic chorioamnionitis in patients with preterm premature rupture of membrane. J Matern Fetal Neonatal Med 2018;31:2624–7. [DOI] [PubMed] [Google Scholar]
- 89.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intra-amniotic inflammation in patients with cervical insufficiency. American journal of obstetrics and gynecology 2008;198:633 e1–8. [DOI] [PubMed] [Google Scholar]
- 90.Gotsch F, Romero R, Chaiworapongsa T, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med 2008;21:605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gomez-Lopez N, Romero R, Xu Y, et al. Are amniotic fluid neutrophils in women with intra-amniotic infection and/or inflammation of fetal or maternal origin? American journal of obstetrics and gynecology 2017;217:693 e1- e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gomez-Lopez N, Romero R, Xu Y, et al. A Role for the Inflammasome in Spontaneous Preterm Labor With Acute Histologic Chorioamnionitis. Reprod Sci 2017;24:1382–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med 1996;334:567–72. [DOI] [PubMed] [Google Scholar]
- 94.Iams JD, Goldenberg RL, Mercer BM, et al. The Preterm Prediction Study: recurrence risk of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. American journal of obstetrics and gynecology 1998;178:1035–40. [DOI] [PubMed] [Google Scholar]
- 95.Goldenberg RL, Iams JD, Das A, et al. The Preterm Prediction Study: sequential cervical length and fetal fibronectin testing for the prediction of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. American journal of obstetrics and gynecology 2000;182:636–43. [DOI] [PubMed] [Google Scholar]
- 96.To MS, Skentou C, Liao AW, Cacho A, Nicolaides KH. Cervical length and funneling at 23 weeks of gestation in the prediction of spontaneous early preterm delivery. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2001;18:200–3. [DOI] [PubMed] [Google Scholar]
- 97.Uquillas KR, Fox NS, Rebarber A, Saltzman DH, Klauser CK, Roman AS. A comparison of cervical length measurement techniques for the prediction of spontaneous preterm birth. J Matern Fetal Neonatal Med 2017;30:50–3. [DOI] [PubMed] [Google Scholar]
- 98.Sharvit M, Weiss R, Ganor Paz Y, Tzadikevitch Geffen K, Danielli Miller N, BironShental T. Vaginal examination vs. cervical length - which is superior in predicting preterm birth? J Perinat Med 2017;45:977–83. [DOI] [PubMed] [Google Scholar]
- 99.Hernandez-Andrade E, Maymon E, Luewan S, et al. A soft cervix, categorized by shearwave elastography, in women with short or with normal cervical length at 18–24 weeks is associated with a higher prevalence of spontaneous preterm delivery. J Perinat Med 2018;46:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Melamed N, Pittini A, Hiersch L, et al. Serial cervical length determination in twin pregnancies reveals 4 distinct patterns with prognostic significance for preterm birth. American journal of obstetrics and gynecology 2016;215:476 e1- e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Melamed N, Pittini A, Hiersch L, et al. Do serial measurements of cervical length improve the prediction of preterm birth in asymptomatic women with twin gestations? American journal of obstetrics and gynecology 2016;215:616 e1- e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kiefer DG, Peltier MR, Keeler SM, et al. Efficacy of midtrimester short cervix interventions is conditional on intra-amniotic inflammation. American journal of obstetrics and gynecology 2016;214:276 e1–6. [DOI] [PubMed] [Google Scholar]
- 103.Nikolova T, Uotila J, Nikolova N, Bolotskikh VM, Borisova VY, Di Renzo GC. Prediction of spontaneous preterm delivery in women presenting with premature labor: a comparison of placenta alpha microglobulin-1, phosphorylated insulin-like growth factor binding protein-1, and cervical length. American journal of obstetrics and gynecology 2018;219:610 e1- e9. [DOI] [PubMed] [Google Scholar]
- 104.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH, Fetal Medicine Foundation Second Trimester Screening G. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med 2007;357:462–9. [DOI] [PubMed] [Google Scholar]
- 105.DeFranco EA, O’Brien JM, Adair CD, et al. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2007;30:697–705. [DOI] [PubMed] [Google Scholar]
- 106.Berghella V. Novel developments on cervical length screening and progesterone for preventing preterm birth. BJOG 2009;116:182–7. [DOI] [PubMed] [Google Scholar]
- 107.Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2011;38:18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Romero R, Yeo L, Chaemsaithong P, Chaiworapongsa T, Hassan SS. Progesterone to prevent spontaneous preterm birth. Semin Fetal Neonatal Med 2014;19:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decreases preterm birth </= 34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2016;48:308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vintzileos AM, Visser GH. Interventions for women with midtrimester short cervix: which ones work? Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2017;49:295–300. [DOI] [PubMed] [Google Scholar]
- 111.Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a metaanalysis of individual patient data. American journal of obstetrics and gynecology 2018;218:161–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanchez-Ramos L. Vaginal progesterone is an alternative to cervical cerclage in women with a short cervix and a history of preterm birth. American journal of obstetrics and gynecology 2018;219:5–9. [DOI] [PubMed] [Google Scholar]
- 113.Romero R, Conde-Agudelo A, Nicolaides KH. There is insufficient evidence to claim that cerclage is the treatment of choice for patients with a cervical length <10 mm. American journal of obstetrics and gynecology 2018;219:213–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. American journal of obstetrics and gynecology 2018;219:10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Campbell S. Prevention of spontaneous preterm birth: universal cervical length assessment and vaginal progesterone in women with a short cervix: time for action! American journal of obstetrics and gynecology 2018;218:151–8. [DOI] [PubMed] [Google Scholar]
- 116.Morency AM, Rallu F, Laferriere C, Bujold E. Eradication of intra-amniotic Streptococcus mutans in a woman with a short cervix. J Obstet Gynaecol Can 2006;28:898–902. [DOI] [PubMed] [Google Scholar]
- 117.Hassan S, Romero R, Hendler I, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med 2006;34:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee J, Romero R, Kim SM, Chaemsaithong P, Yoon BH. A new antibiotic regimen treats and prevents intra-amniotic inflammation/infection in patients with preterm PROM. J Matern Fetal Neonatal Med 2016;29:2727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yoneda S, Shiozaki A, Yoneda N, et al. Antibiotic Therapy Increases the Risk of Preterm Birth in Preterm Labor without Intra-Amniotic Microbes, but may Prolong the Gestation Period in Preterm Labor with Microbes, Evaluated by Rapid and High-Sensitive PCR System. Am J Reprod Immunol 2016;75:440–50. [DOI] [PubMed] [Google Scholar]
- 120.Lee J, Romero R, Kim SM, et al. A new anti-microbial combination prolongs the latency period, reduces acute histologic chorioamnionitis as well as funisitis, and improves neonatal outcomes in preterm PROM. J Matern Fetal Neonatal Med 2016;29:707–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yoon BH, Yang SH, Jun JK, Park KH, Kim CJ, Romero R. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstet Gynecol 1996;87:231–7. [DOI] [PubMed] [Google Scholar]
- 122.Park JS, Romero R, Yoon BH, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. American journal of obstetrics and gynecology 2001;185:1156–61. [DOI] [PubMed] [Google Scholar]
- 123.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med 2010;38:261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim SM, Romero R, Park JW, Oh KJ, Jun JK, Yoon BH. The relationship between the intensity of intra-amniotic inflammation and the presence and severity of acute histologic chorioamnionitis in preterm gestation. J Matern Fetal Neonatal Med 2015;28:1500–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oh KJ, Kim SM, Hong JS, et al. Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intra-amniotic infection or intra-amniotic inflammation. American journal of obstetrics and gynecology 2017;216:604 e1- e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim KW, Romero R, Park HS, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intra-amniotic inflammation in women with preterm premature rupture of membranes. American journal of obstetrics and gynecology 2007;197:292 e1–5. [DOI] [PubMed] [Google Scholar]
- 127.Chaemsaithong P, Romero R, Docheva N, et al. Comparison of rapid MMP-8 and interleukin-6 point-of-care tests to identify intra-amniotic inflammation/infection and impending preterm delivery in patients with preterm labor and intact membranes(). J Matern Fetal Neonatal Med 2018;31:228–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Heo JS, Shin SH, Jung YH, et al. Neonatal sepsis in a rapidly growing, tertiary neonatal intensive care unit: Trends over 18 years. Pediatr Int 2015;57:909–16. [DOI] [PubMed] [Google Scholar]
- 129.Lee SM, Chang M, Kim KS. Blood Culture Proven Early Onset Sepsis and Late Onset Sepsis in Very-Low-Birth-Weight Infants in Korea. J Korean Med Sci 2015;30 Suppl 1:S67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yoon BH, Romero R, Kim CJ, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. American journal of obstetrics and gynecology 1995;172:960–70. [DOI] [PubMed] [Google Scholar]
- 131.Maymon E, Romero R, Pacora P, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. American journal of obstetrics and gynecology 2000;183:94–9. [DOI] [PubMed] [Google Scholar]
- 132.Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11:18–25. [DOI] [PubMed] [Google Scholar]
- 133.Yoon BH, Romero R, Shim JY, Shim SS, Kim CJ, Jun JK. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med 2003;14:85–90. [DOI] [PubMed] [Google Scholar]
- 134.Lee SE, Romero R, Kim CJ, Shim SS, Yoon BH. Funisitis in term pregnancy is associated with microbial invasion of the amniotic cavity and intra-amniotic inflammation. J Matern Fetal Neonatal Med 2006;19:693–7. [DOI] [PubMed] [Google Scholar]
- 135.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intra-amniotic infection at term. The Journal of infectious diseases 1982;145:1–8. [DOI] [PubMed] [Google Scholar]
- 136.Romero R, Chaemsaithong P, Korzeniewski SJ, et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med 2016;44:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term VI: acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. J Perinat Med 2016;44:33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sung JH, Choi SJ, Oh SY, Roh CR, Kim JH. Revisiting the diagnostic criteria of clinical chorioamnionitis in preterm birth. BJOG 2017;124:775–83. [DOI] [PubMed] [Google Scholar]
- 139.Gonzalez-Bosquet E, Cerqueira MJ, Dominguez C, Gasser I, Bermejo B, Cabero L. Amniotic fluid glucose and cytokines values in the early diagnosis of amniotic infection in patients with preterm labor and intact membranes. J Matern Fetal Med 1999;8:155–8. [DOI] [PubMed] [Google Scholar]
- 140.Romero R, Miranda J, Chaiworapongsa T, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014;71:330–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Park JY, Romero R, Lee J, Chaemsaithong P, Chaiyasit N, Yoon BH. An elevated amniotic fluid prostaglandin F2alpha concentration is associated with intra-amniotic inflammation/infection, and clinical and histologic chorioamnionitis, as well as impending preterm delivery in patients with preterm labor and intact membranes. J Matern Fetal Neonatal Med 2016;29:2563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bobitt JR, Ledger WJ. Amniotic fluid analysis. Its role in maternal neonatal infection. Obstet Gynecol 1978;51:56–62. [PubMed] [Google Scholar]
- 143.Miller JM Jr., Pupkin MJ, Hill GB. Bacterial colonization of amniotic fluid from intact fetal membranes. American journal of obstetrics and gynecology 1980;136:796–804. [DOI] [PubMed] [Google Scholar]
- 144.Wahbeh CJ, Hill GB, Eden RD, Gall SA. Intra-amniotic bacterial colonization in premature labor. American journal of obstetrics and gynecology 1984;148:739–43. [DOI] [PubMed] [Google Scholar]
- 145.Berg TG, Philpot KL, Welsh MS, Sanger WG, Smith CV. Ureaplasma/Mycoplasmainfected amniotic fluid: pregnancy outcome in treated and nontreated patients. Journal of perinatology : official journal of the California Perinatal Association 1999;19:275–7. [DOI] [PubMed] [Google Scholar]
- 146.Jacobsson B, Mattsby-Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand 2003;82:120–8. [DOI] [PubMed] [Google Scholar]
- 147.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. American journal of obstetrics and gynecology 1998;179:194–202. [DOI] [PubMed] [Google Scholar]
- 148.Romero R, Gomez R, Ghezzi F, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. American journal of obstetrics and gynecology 1998;179:186–93. [DOI] [PubMed] [Google Scholar]
- 149.Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol 2007;50:652–83. [DOI] [PubMed] [Google Scholar]
- 150.Berry SM, Romero R, Gomez R, et al. Premature parturition is characterized by in utero activation of the fetal immune system. American journal of obstetrics and gynecology 1995;173:1315–20. [DOI] [PubMed] [Google Scholar]
- 151.Chaiworapongsa T, Romero R, Berry SM, et al. The role of granulocyte colonystimulating factor in the neutrophilia observed in the fetal inflammatory response syndrome. J Perinat Med 2011;39:653–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yoon BH, Romero R, Jun JK, et al. An increase in fetal plasma cortisol but not dehydroepiandrosterone sulfate is followed by the onset of preterm labor in patients with preterm premature rupture of the membranes. American journal of obstetrics and gynecology 1998;179:1107–14. [DOI] [PubMed] [Google Scholar]
- 153.Romero R, Espinoza J, Goncalves LF, et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2004;16:146–57. [DOI] [PubMed] [Google Scholar]
- 154.Mitchell T, MacDonald JW, Srinouanpranchanh S, et al. Evidence of cardiac involvement in the fetal inflammatory response syndrome: disruption of gene networks programming cardiac development in nonhuman primates. American journal of obstetrics and gynecology 2018;218:438 e1- e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Athayde N, Edwin SS, Romero R, et al. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. American journal of obstetrics and gynecology 1998;179:1248–53. [DOI] [PubMed] [Google Scholar]
- 156.Locksmith GJ, Clark P, Duff P, Schultz GS. Amniotic fluid matrix metalloproteinase-9 levels in women with preterm labor and suspected intra-amniotic infection. Obstet Gynecol 1999;94:1–6. [DOI] [PubMed] [Google Scholar]
- 157.Maymon E, Romero R, Pacora P, et al. Matrilysin (matrix metalloproteinase 7) in parturition, premature rupture of membranes, and intrauterine infection. American journal of obstetrics and gynecology 2000;182:1545–53. [DOI] [PubMed] [Google Scholar]
- 158.Locksmith GJ, Clark P, Duff P, Saade GR, Schultz GS. Amniotic fluid concentrations of matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 during pregnancy and labor. American journal of obstetrics and gynecology 2001;184:159–64. [DOI] [PubMed] [Google Scholar]
- 159.Maymon E, Romero R, Pacora P, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intra-amniotic infection. J Perinat Med 2001;29:308–16. [DOI] [PubMed] [Google Scholar]
- 160.Vadillo-Ortega F, Sadowsky DW, Haluska GJ, et al. Identification of matrix metalloproteinase-9 in amniotic fluid and amniochorion in spontaneous labor and after experimental intrauterine infection or interleukin-1 beta infusion in pregnant rhesus monkeys. American journal of obstetrics and gynecology 2002;186:128–38. [DOI] [PubMed] [Google Scholar]
- 161.Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. American journal of obstetrics and gynecology 2000;182:675–81. [DOI] [PubMed] [Google Scholar]
- 162.Yoon BH, Romero R, Jun JK, et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. American journal of obstetrics and gynecology 1997;177:825–30. [DOI] [PubMed] [Google Scholar]
- 163.Lee J, Oh KJ, Yang HJ, Park JS, Romero R, Yoon BH. The importance of intra-amniotic inflammation in the subsequent development of atypical chronic lung disease. J Matern Fetal Neonatal Med 2009;22:917–23. [DOI] [PubMed] [Google Scholar]
- 164.Lee J, Oh KJ, Park CW, Park JS, Jun JK, Yoon BH. The presence of funisitis is associated with a decreased risk for the development of neonatal respiratory distress syndrome. Placenta 2011;32:235–40. [DOI] [PubMed] [Google Scholar]
- 165.Yoon BH, Romero R, Kim KS, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. American journal of obstetrics and gynecology 1999;181:773–9. [DOI] [PubMed] [Google Scholar]
- 166.Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol 1993;30:167–83. [DOI] [PubMed] [Google Scholar]
- 167.Romero R, Yoon BH, Mazor M, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. American journal of obstetrics and gynecology 1993;169:805–16. [DOI] [PubMed] [Google Scholar]
- 168.Yoon BH, Kim CJ, Romero R, et al. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. American journal of obstetrics and gynecology 1997;177:797–802. [DOI] [PubMed] [Google Scholar]
- 169.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med 2007;25:21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Strunk T, Inder T, Wang X, Burgner D, Mallard C, Levy O. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis 2014;14:751–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Revello R, Alcaide MJ, Abehsera D, et al. Prediction of chorioamnionitis in cases of intra-amniotic infection by ureaplasma urealyticum in women with very preterm premature rupture of membranes or preterm labour. J Matern Fetal Neonatal Med 2018;31:1839–44. [DOI] [PubMed] [Google Scholar]
- 172.Romero R, Miranda J, Kusanovic JP, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med 2015;43:19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term IV: the maternal plasma cytokine profile. J Perinat Med 2016;44:77–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. J Perinat Med 2016;44:53–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Martinez-Varea A, Romero R, Xu Y, et al. Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. J Perinat Med 2017;45:523–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chaiyasit N, Romero R, Chaemsaithong P, et al. Clinical chorioamnionitis at term VIII: a rapid MMP-8 test for the identification of intra-amniotic inflammation. J Perinat Med 2017;45:539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yoon BH, Jun JK, Park KH, Syn HC, Gomez R, Romero R. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet Gynecol 1996;88:1034–40. [DOI] [PubMed] [Google Scholar]
- 178.Lee SY, Park KH, Jeong EH, Oh KJ, Ryu A, Park KU. Relationship between maternal serum C-reactive protein, funisitis and early-onset neonatal sepsis. J Korean Med Sci 2012;27:674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Romero R, Quintero R, Nores J, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. American journal of obstetrics and gynecology 1991;165:821–30. [DOI] [PubMed] [Google Scholar]
- 180.Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. American journal of obstetrics and gynecology 1993;169:839–51. [DOI] [PubMed] [Google Scholar]
- 181.Dildy GA, Pearlman MD, Smith LG, Tortolero-Luna G, Faro S, Cotton DB. Amniotic fluid glucose concentration: a marker for infection in preterm labor and preterm premature rupture of membranes. Infect Dis Obstet Gynecol 1994;1:166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Greig PC, Ernest JM, Teot L. Low amniotic fluid glucose levels are a specific but not a sensitive marker for subclinical intrauterine infections in patients in preterm labor with intact membranes. American journal of obstetrics and gynecology 1994;171:365–70; discussion 70–1. [DOI] [PubMed] [Google Scholar]
- 183.Park CW, Lee SM, Park JS, Jun JK, Romero R, Yoon BH. The antenatal identification of funisitis with a rapid MMP-8 bedside test. J Perinat Med 2008;36:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra-amniotic inflammation/infection. J Matern Fetal Neonatal Med 2016;29:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yoon BH, Romero R, Kim M, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. American journal of obstetrics and gynecology 2000;183:1130–7. [DOI] [PubMed] [Google Scholar]
- 186.DiGiulio DB, Romero R, Kusanovic JP, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm prelabor rupture of membranes. Am J Reprod Immunol 2010;64:38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rowlands S, Danielewski JA, Tabrizi SN, Walker SP, Garland SM. Microbial invasion of the amniotic cavity in midtrimester pregnancies using molecular microbiology. American journal of obstetrics and gynecology 2017;217:71 e1- e5. [DOI] [PubMed] [Google Scholar]
- 188.Rehbinder EM, Lodrup Carlsen KC, Staff AC, et al. Is amniotic fluid of women with uncomplicated term pregnancies free of bacteria? American journal of obstetrics and gynecology 2018;219:289 e1- e12. [DOI] [PubMed] [Google Scholar]
- 189.Broekhuizen FF, Gilman M, Hamilton PR. Amniocentesis for gram stain and culture in preterm premature rupture of the membranes. Obstet Gynecol 1985;66:316–21. [PubMed] [Google Scholar]
- 190.Romero R, Roslansky P, Oyarzun E, et al. Labor and infection. II. Bacterial endotoxin in amniotic fluid and its relationship to the onset of preterm labor. American journal of obstetrics and gynecology 1988;158:1044–9. [DOI] [PubMed] [Google Scholar]
- 191.Winkler M, Baumann L, Ruckhaberle KE, Schiller EM. Erythromycin therapy for subclinical intrauterine infections in threatened preterm delivery--a preliminary report. J Perinat Med 1988;16:253–6. [PubMed] [Google Scholar]
- 192.Tarnow-Mordi W, Phillips G, Taylor D. Randomized controlled trials of antibiotics in preterm labor. American journal of obstetrics and gynecology 1994;171:865–6. [DOI] [PubMed] [Google Scholar]
- 193.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. Randomized trial of antibiotics in addition to tocolytic therapy to treat preterm labor. Infect Dis Obstet Gynecol 1994;1:220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Oyarzun E, Gomez R, Rioseco A, et al. Antibiotic treatment in preterm labor and intact membranes: a randomized, double-blinded, placebo-controlled trial. J Matern Fetal Med 1998;7:105–10. [DOI] [PubMed] [Google Scholar]
- 195.King J, Flenady V. Prophylactic antibiotics for inhibiting preterm labour with intact membranes. Cochrane Database Syst Rev 2002:CD000246. [DOI] [PubMed] [Google Scholar]
- 196.Lamont RF. Can antibiotics prevent preterm birth--the pro and con debate. BJOG 2005;112 Suppl 1:67–73. [DOI] [PubMed] [Google Scholar]
- 197.Flenady V, Hawley G, Stock OM, Kenyon S, Badawi N. Prophylactic antibiotics for inhibiting preterm labour with intact membranes. Cochrane Database Syst Rev 2013:CD000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.American College of O, Gynecologists’ Committee on Practice B-O. Practice Bulletin No. 171: Management of Preterm Labor. Obstet Gynecol 2016;128:e155–64. [DOI] [PubMed] [Google Scholar]
- 199.ACOG committee opinion. Prevention of early-onset group B streptococcal disease in newborns. Number 173--June 1996. Committee on Obstetric Practice. American College of Obstetrics and Gynecologists. Int J Gynaecol Obstet 1996;54:197–205. [PubMed] [Google Scholar]
- 200.Koenig JM, Keenan WJ. Group B streptococcus and early-onset sepsis in the era of maternal prophylaxis. Pediatr Clin North Am 2009;56:689–708, Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Romero R, Sepulveda W, Baumann P, et al. The preterm labor syndrome: biochemical, cytologic, immunologic, pathologic, microbiologic, and clinical evidence that preterm labor is a heterogeneous disease (Abstract). American journal of obstetrics and gynecology;168:288. [Google Scholar]
- 202.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG 2006;113 Suppl 3:17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.McDuffie RS Jr., Blanton SJ, Shikes RH, Gibbs RS. A rabbit model for bacterially induced preterm pregnancy loss: intervention studies with ampicillin-sulbactam. American journal of obstetrics and gynecology 1991;165:1568–74. [DOI] [PubMed] [Google Scholar]
- 204.Fidel P, Ghezzi F, Romero R, et al. The effect of antibiotic therapy on intrauterine infection-induced preterm parturition in rabbits. J Matern Fetal Neonatal Med 2003;14:57–64. [DOI] [PubMed] [Google Scholar]
- 205.Sadowsky DW, Novy MJ, Witkin SS, Gravett MG. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. American journal of obstetrics and gynecology 2003;188:252–63. [DOI] [PubMed] [Google Scholar]
- 206.Sadowsky DW, Haluska GJ, Gravett MG, Witkin SS, Novy MJ. Indomethacin blocks interleukin 1beta-induced myometrial contractions in pregnant rhesus monkeys. American journal of obstetrics and gynecology 2000;183:173–80. [DOI] [PubMed] [Google Scholar]
- 207.Gravett MG, Adams KM, Sadowsky DW, et al. Immunomodulators plus antibiotics delay preterm delivery after experimental intra-amniotic infection in a nonhuman primate model. American journal of obstetrics and gynecology 2007;197:518 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Grigsby PL, Novy MJ, Sadowsky DW, et al. Maternal azithromycin therapy for Ureaplasma intra-amniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. American journal of obstetrics and gynecology 2012;207:475 e1- e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Acosta EP, Grigsby PL, Larson KB, et al. Transplacental transfer of Azithromycin and its use for eradicating intra-amniotic ureaplasma infection in a primate model. The Journal of infectious diseases 2014;209:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Witt A, Sommer EM, Cichna M, et al. Placental passage of clarithromycin surpasses other macrolide antibiotics. American journal of obstetrics and gynecology 2003;188:816–9. [DOI] [PubMed] [Google Scholar]
- 211.Kafetzis DA, Brater DC, Fanourgakis JE, Voyatzis J, Georgakopoulos P. Ceftriaxone distribution between maternal blood and fetal blood and tissues at parturition and between blood and milk postpartum. Antimicrob Agents Chemother 1983;23:870–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lamb HM, Ormrod D, Scott LJ, Figgitt DP. Ceftriaxone: an update of its use in the management of community-acquired and nosocomial infections. Drugs 2002;62:1041–89. [DOI] [PubMed] [Google Scholar]
- 213.Romero R, Hagay Z, Nores J, Sepulveda W, Mazor M. Eradication of Ureaplasma urealyticum from the amniotic fluid with transplacental antibiotic treatment. American journal of obstetrics and gynecology 1992;166:618–20. [DOI] [PubMed] [Google Scholar]
- 214.Mazor M, Chaim W, Horowitz S, Leiberman JR, Glezerman M. Successful treatment of preterm labour by eradication of Ureaplasma urealyticum with erythromycin. Arch Gynecol Obstet 1993;253:215–8. [DOI] [PubMed] [Google Scholar]
- 215.Mazor M, Chaim W, Meirovitz M, Yohay D, Leiberman JR, Glezerman M. Eradication of viridans streptococci from the amniotic cavity by parenteral antibiotic administration. A case report. J Reprod Med 1995;40:820–2. [PubMed] [Google Scholar]
- 216.Falagas ME, Kavvadia PK, Mantadakis E, et al. Morganella morganii infections in a general tertiary hospital. Infection 2006;34:315–21. [DOI] [PubMed] [Google Scholar]
- 217.Johnson JR, Feingold M. Case of chorioamnionitis in an immunocompetent woman caused by Morganella morganii. J Matern Fetal Med 1998;7:13–4. [DOI] [PubMed] [Google Scholar]
- 218.Ferrario C, Borgo F, de Las Rivas B, Munoz R, Ricci G, Fortina MG. Sequencing, characterization, and gene expression analysis of the histidine decarboxylase gene cluster of Morganella morganii. Current microbiology 2014;68:404–11. [DOI] [PubMed] [Google Scholar]
- 219.Olaitan AO, Diene SM, Gupta SK, Adler A, Assous MV, Rolain JM. Genome analysis of NDM-1 producing Morganella morganii clinical isolate. Expert review of anti-infective therapy 2014;12:1297–305. [DOI] [PubMed] [Google Scholar]
- 220.Al-Muhanna AS, Al-Muhanna S, Alzuhairi MA. Molecular investigation of extendedspectrum beta-lactamase genes and potential drug resistance in clinical isolates of Morganella morganii. Annals of Saudi medicine 2016;36:223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lata P, Govindarajan SS, Qi F, Li JL, Maurya SK, Sahoo MK. Draft Genome Sequences of Extended-Spectrum-Beta-Lactamase-Producing Morganella morganii Strains AA1 and AV1, Isolated from a Freshwater Lake and Eicchorniacrassipes Roots. Genome announcements 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Himes KP, Simhan HN. Risk of recurrent preterm birth and placental pathology. Obstet Gynecol 2008;112:121–6. [DOI] [PubMed] [Google Scholar]
- 223.Strauss JF 3rd, Romero R, Gomez-Lopez N, et al. Spontaneous preterm birth: advances toward the discovery of genetic predisposition. American journal of obstetrics and gynecology 2018;218:294–314 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Vasconcelos Carvalho da Silva L, Javorski N, Andre Cavalcanti Brandao L, de Carvalho Lima M, Crovella S, Helena Eickmann S. Influence of MBL2 and NOS3 polymorphisms on spontaneous preterm birth in North East Brazil: genetics and preterm birth. J Matern Fetal Neonatal Med 2018:1–9. [DOI] [PubMed] [Google Scholar]
- 225.Toure DM, ElRayes W, Barnes-Josiah D, Hartman T, Klinkebiel D, Baccaglini L. Epigenetic modifications of human placenta associated with preterm birth: a systematic review. J Matern Fetal Neonatal Med 2018;31:530–41. [DOI] [PubMed] [Google Scholar]
- 226.Paquette AG, Shynlova O, Kibschull M, et al. Comparative analysis of gene expression in maternal peripheral blood and monocytes during spontaneous preterm labor. American journal of obstetrics and gynecology 2018;218:345 e1- e30. [DOI] [PubMed] [Google Scholar]
- 227.Frey HA, Stout MJ, Pearson LN, et al. Genetic variation associated with preterm birth in African-American women. American journal of obstetrics and gynecology 2016;215:235 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kim YM, Romero R, Oh SY, et al. Toll-like receptor 4: a potential link between “danger signals,” the innate immune system, and preeclampsia? American journal of obstetrics and gynecology 2005;193:921–7. [DOI] [PubMed] [Google Scholar]
- 229.Romero R, Chaiworapongsa T, Alpay Savasan Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med 2011;24:1444–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Martin SJ. Cell death and inflammation: the case for IL-1 family cytokines as the canonical DAMPs of the immune system. FEBS J 2016;283:2599–615. [DOI] [PubMed] [Google Scholar]
- 231.Sachet M, Liang YY, Oehler R. The immune response to secondary necrotic cells. Apoptosis 2017;22:1189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Frascoli M, Coniglio L, Witt R, et al. Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-gamma and TNF-alpha. Sci Transl Med 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yan H, Li H, Zhu L, Gao J, Li P, Zhang Z. Increased TLR4 and TREM-1 expression on monocytes and neutrophils in preterm birth: further evidence of a proinflammatory state. J Matern Fetal Neonatal Med 2018:1–9. [DOI] [PubMed] [Google Scholar]
- 234.Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. American journal of obstetrics and gynecology 1992;167:1041–5. [DOI] [PubMed] [Google Scholar]
- 235.Romero R, Mazor M, Brandt F, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992;27:117–23. [DOI] [PubMed] [Google Scholar]
- 236.Friel LA, Romero R, Edwin S, et al. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med 2007;35:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lu HY, Ma JL, Shan JY, Zhang J, Wang QX, Zhang Q. High-mobility group box-1 and receptor for advanced glycation end products in preterm infants with brain injury. World J Pediatr 2017;13:228–35. [DOI] [PubMed] [Google Scholar]
- 238.Gomez-Lopez N, Romero R, Xu Y, et al. A Role for the Inflammasome in Spontaneous Labor at Term with Acute Histologic Chorioamnionitis. Reprod Sci 2017;24:934–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Dougherty TF, Schneebeli GL. The use of steroids as anti-inflammatory agents. Annals of the New York Academy of Sciences 1955;61:328–48. [DOI] [PubMed] [Google Scholar]
- 240.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Molecular and cellular endocrinology 2011;335:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Cortet B, Duquesnoy B. [Action of non-steroidal anti-inflammatory agents on the immune system]. Revue du rhumatisme et des maladies osteo-articulaires 1991;58:379–86. [PubMed] [Google Scholar]
- 242.Fullerton JN. Use of non-steroidal anti-inflammatory drugs (NSAIDs) as immunomodulatory agents. BMJ 2013;347:f4984. [DOI] [PubMed] [Google Scholar]
- 243.Siddiqui J. Immunomodulatory effects of macrolides: implications for practicing clinicians. The American journal of medicine 2004;117 Suppl 9A:26S–9S. [DOI] [PubMed] [Google Scholar]
- 244.Yamamoto S, Ogasawara N, Yamamoto K, et al. Mitochondrial proteins NIP-SNAP-1 and −2 are a target for the immunomodulatory activity of clarithromycin, which involves NFkappaB-mediated cytokine production. Biochemical and biophysical research communications 2017;483:911–6. [DOI] [PubMed] [Google Scholar]
- 245.Hertelendy F, Molnar M, Romero R. Interferon gamma antagonizes interleukin-1betainduced cyclooxygenase-2 expression and prostaglandin E(2) production in human myometrial cells. J Soc Gynecol Investig 2002;9:215–9. [PubMed] [Google Scholar]
- 246.Hertelendy F, Rastogi P, Molnar M, Romero R. Interleukin-1beta-induced prostaglandin E2 production in human myometrial cells: role of a pertussis toxin-sensitive component. Am J Reprod Immunol 2001;45:142–7. [DOI] [PubMed] [Google Scholar]
- 247.Belt AR, Baldassare JJ, Molnar M, Romero R, Hertelendy F. The nuclear transcription factor NF-kappaB mediates interleukin-1beta-induced expression of cyclooxygenase-2 in human myometrial cells. American journal of obstetrics and gynecology 1999;181:359–66. [DOI] [PubMed] [Google Scholar]
- 248.Li R, Ackerman WEt, Summerfield TL, et al. Inflammatory gene regulatory networks in amnion cells following cytokine stimulation: translational systems approach to modeling human parturition. PLoS One 2011;6:e20560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Vora S, Abbas A, Kim CJ, et al. Nuclear factor-kappa B localization and function within intrauterine tissues from term and preterm labor and cultured fetal membranes. Reprod Biol Endocrinol 2010;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kowarsky M, Camunas-Soler J, Kertesz M, et al. Numerous uncharacterized and highly divergent microbes which colonize humans are revealed by circulating cell-free DNA. Proc Natl Acad Sci U S A 2017;114:9623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Dinakaran V, Rathinavel A, Pushpanathan M, Sivakumar R, Gunasekaran P, Rajendhran J. Elevated levels of circulating DNA in cardiovascular disease patients: metagenomic profiling of microbiome in the circulation. PLoS One 2014;9:e105221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Renko J, Koskela KA, Lepp PW, et al. Bacterial DNA signatures in carotid atherosclerosis represent both commensals and pathogens of skin origin. Eur J Dermatol 2013;23:53–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.