Abstract
Porcine epidemic diarrhea virus (PEDV), a leading cause of piglet diarrhea outbreaks, poses a significant danger to the swine industry. The aim of this study was to investigate the epidemic characteristics of PEDV that was circulating in Guangdong province, one of China’s major pig producing provinces. Clinical samples were collected from eight pig farms in Guangdong province between 2018 and 2019 and tested for the major porcine enteric pathogens, including PEDV, transmissible gastroenteritis virus (TGEV), Swine enteric coronavirus (SeCoV), Swine acute diarrhea syndrome coronavirus (SADS-CoV), porcine deltacoronavirus (PDCoV), and porcine rotavirus (RV). As a result, only PEDV and RV were detected at a rate of 47.0% (16/34) and 18.6% (8/34), respectively. Coinfectoin with PEDV and RV occurred at a rate of PEDV 12.5% (2/16). Subsequently, the full-length S gene sequences of 13 PEDV strains were obtained, and phylogenetic analysis suggested the presence of GII-c group PEDV strains in this region (non-S-INDEL). Two novel common amino acid insertions (55T/IG56 and 551L) and one novel glycosylation site (1199G+) were detected when the CV777 and ZJ08 vaccine strains were compared. Furthermore, intragroup recombination events in the S gene regions 51–548 and 2478–4208 were observed in the PEDV strains studied. In summary, the observations provide current information on the incidence of viral agents causing swine diarrhea in southern China and detailed the genetic characteristics and evolutionary history of the dominant PEDV field strains. Our findings will aid in the development of an updated vaccine for the prevention and control of PEDV variant strains.
Introduction
Porcine epidemic diarrhea, which is characterized by vomiting, acute watery diarrhea, dehydration, and weight loss in pigs, is a highly contagious and acute infectious disease caused by the porcine epidemic diarrhea virus (PEDV). PEDV can infect pigs of any age, but particularly suckling piglets, and has a high mortality rate of up to 100% [1, 2]. Although the first PEDV outbreak was reported in 1971 in the United Kingdom, the PEDV cv777 strain was not isolated until 1977 in Belgium [1, 3, 4]. In the 1980s and 1990s, PEDV spread throughout many pig-producing countries in Europe and Asia, causing significant economic losses [5]. Since its discovery in China in the 1980s, PEDV has caused sporadic diarrhea outbreaks in Asian pig herds, causing even greater economic losses than in Europe [1, 6–10]. Except for an epidemic outbreak in northern Italy in 2005, only sporadic outbreaks of PEDV were reported in Europe [7]. Until 2010, a remarkable increase in PEDV outbreaks was reported in China’s pig-producing provinces [11]. A mutant strain was identified in which infected piglets defecate yellow watery stools, lose weight, and eventually die of dehydration, with a high mortality rate of up to 80%–100% in suckling piglets [12].
PEDV was first detected in the United States of America in 2013 and quickly spread throughout the country and into Europe [12–14]. Since then, a larger-scale epidemic outbreak of PEDV has impacted pig farms in China, Japan, and Korea, as well as countries in central and Eastern Europe, resulting in significant economic losses for the pig industry.
PEDV is a member of the Coronaviridae family’s Alphacoronavirus genus and the Orthocoronavirinae subfamily, which are enveloped viruses with an unsegmented positive sense RNA genome. The single-strand RNA genome of PEDV has a cap structure at 5’ and the poly (A) tail at the 3 ’end, with a size of approximately 30 kb. Four of the six open reading frames of the PEDV genome encode structural protein Spike (S), envelope (E), membrane (M), and nucleocapsid (N). The remaining 2/3 region of the 5 ’-terminal of ORF1a/1b and ORF3 encodes two viral RNA polymerase complex proteins. The S, E, M, and N structural proteins are located at the downstream of ORF1a and ORF1b [15]. ORF3 is an accessory gene that encodes a helper protein that aids in viral genome replication and translation [16]. The PEDV E glycoprotein is a component of the viral envelope that is involved in the formation and budding of the viral envelope [17]. The M protein contains 226 amino acids and has a molecular weight of 27–32 kDa. It is made up of two extracapsular parts, a trimer, and a carboxyl terminal within the virus to form a three-dimensional conformation [18]. The N protein is a phosphorylated basic protein with three functional domains that are all conserved: the N-terminal functional domain, the intermediate RNA binding domain, and the C-terminal binding domain. N protein is involved in the formation of viral sacs and is essential for coronavirus replication and transcription [19]. PEDV S protein, a type I fibrinoprotein, is involved in receptor binding, inducing neutralization antibodies, and membrane fusion. PEDV S protein consists of a signal peptide, an extracellular domain, a transmembrane region, and a cytoplasmic domain [20]. Cell proteases can splice S protein into S1 and S2, and the N-terminal region of S1 can bind to host cell receptors and mediate virus entry. In comparison to S1, the S2 region is more conserved, and it can mediate virus-cell membrane fusion and then internalization into host cells [21]. Remarkably, the S gene of PEDV contains the most variable regions in the entire PEDV genome and has the highest degree of diversity among all genes. Thus, the S gene has been used as a phylogenetic marker.
The diversity of the S gene is important in PEDV surveillance research because it provides a solid foundation for vaccine development, as well as disease prevention and control. A number of variant PEDV strains with insertions and deletions (INDEL) in the S gene were reported in the United States in 2014, altering viral antigenicity and pathogenicity [22, 23]. Although several studies on the genetic characterization and prevalence of PEDV have been conducted in central [24] and western China [25] prior to 2018, there has been little research on the prevalence of PEDV in southern China in recent years. The purpose of this study was to determine the viral agent prevalence in piglets suffering from acute diarrhea in Guangdong, China, and to provide updated information for genetic characterization of PEDV field strains.
Materials and methods
Ethics statement
This study was approved by the Research Ethics Committee of the College of Life Science and Engineering, Foshan University. Written informed consent was obtained from all owners whose animals were used in the study.
Sample collection and cDNA synthesis
During 2018–2019, 19 feces and 15 fecal swabs were collected from diarrheal pigs in eight Guangdong province swine farms (Table 1). The pigs did not receive vaccines against PEDV, TGEV or RV. The samples were suspended in phosphate-buffered saline (PBS, pH = 7.4) and then clarified for 10 min at 4,000 rpm using centrifugation. The viral RNA was extracted using the Body Fluid Viral DNA/RNA Miniprep kit (Axygen, China) as directed by the manufacturer. Maxima H Minus First Strand cDNA Synthesis Kit was used to reverse transcribe viral RNA (Thermo Fisher Scientific, USA).
Table 1. Details of sample information in this study.
| Swine farms N0. | Surveillance city | Collection date | Number of samples | PEDV positive | RV positive |
|---|---|---|---|---|---|
| 1 | Shixing | November, 2018 | 3 | 3/3 | 0/3 |
| 2 | Lechang | January, 2019 | 5 | 3/5 | 0/5 |
| 3 | Wujiang | January, 2018 | 5 | 0/5 | 0/5 |
| 4 | Zhenjiang | January, 2018 | 4 | 4/4 | 1/4 |
| 5 | Renhua | November,2018 | 6 | 0/6 | 3/6 |
| 6 | Ruyuan | November, 2018 | 3 | 0/3 | 1/3 |
| 7 | Qujiang | November, 2018 | 3 | 1/3 | 2/3 |
| 8 | Gaofang | January, 2018 | 5 | 5/5 | 1/5 |
Virus detection
The presence of swine alphacoronaviruses, such as PEDV, Transmissible gastroenteritis virus (TGEV), Swine enteric coronavirus (SeCoV), and Swine acute diarrhea syndrome coronavirus (SADS-CoV), was tested using a universal primer (Swine Cov F: 5’-AAACTGGAAYTTCASMTGG-3’; Swine Cov R: 5’-ACATARWAAGCCCAWC) designed by this study. Furthermore, the porcine deltacoronavirus (PDCoV) was detected using a primer set described previously by Sun et al. [26]. RV VP6 F: 5’-GAAACGGAATAGCTCCACAAT-3’ and RV VP6 R: 5’-GAATAATCAAATCCAGCCACC-3’ are primers. The presence of porcine rotavirus was detected by targeting the VP6 gene with an expected size of 271 bp. Thermo Fisher Scientific’s Phusion Hot Start II High-Fidelity PCR Master Mix (Thermo Fisher Scientific, USA) was used to amplify the M gene of swine alphacoronavirus and the S gene of PDCoV, which have expected sizes of 547 bp and 1763 bp, respectively. Table 1 lists the primers used in this study. The PCR products were purified using the Gene JET Extraction Kit from Thermo Fisher Scientific (Thermo Fisher Scientific, USA) and then subcloned into the pMD-18T vector (Takara, Japan). Sanger sequencing (Sangon Biotech, China) was used to obtain the M gene sequences, which were then BLAST searched against the GenBank database.
PEDV S gene sequencing
The M gene positive samples were subjected to obtain the full-length sequence of the S gene. Two primers sets (PEDV-S1F, 5’-ATGACGCCATTTGTGGTTTTTC-3’ PEDV-S1R, 5’-GCCAGACTGAGATGGGACG-3’; PEDV-S2F:5’-TGGCAGTATTGGCTACGTCC-3’ PEDV-S2 R:5’-TGACGACTGTGTCAATCGTGT-3’) (Table 2) based on the conserved region of PEDV genome were designed to amplify the full-length sequence of S gene of PEDV. The S gene of PEDV was amplified using the Phusion Hot Start II High-Fidelity PCR Master Mix (Thermo Fisher Scientific, USAPEDV). Pre-denaturation at 98 °C for 30 s, followed by 35 cycles of denaturation at 98 °C for 10 s, annealing at 53 °C for 30s, and extension at 72 °C for 2 min, followed by extension fully at 72 °C for 10 min. Table 2 lists the primers available. Gene JET Gel Extraction Kit (Thermo Fisher Scientific, USA) was used to purify the amplified products, which were then subcloned into the pMD-18T vector (Takara, Japan) and sequenced using the Sanger method (Sangon Biotech, China). The sequences were assembled using MEGA. 7(Version 7.0.26).
Table 2. List of primers used in this study.
| Gene name | Primer sequence | Length(bp) | Target gene |
|---|---|---|---|
| Swine-CoV-M | F: AAACTGGAAYTTCASMTGG | 654 | M |
| R: ACATARWAAGCCCAWCCAGT | |||
| PEDV-S1 | F: ATGACGCCATTTGTGGTTTTTC | 2356 | S1 |
| R: GCCAGACTGAGATGGGACG | |||
| PEDV-S2 | F: TGGCAGTATTGGCTACGTCC | 1925 | S2 |
| R: TGACGACTGTGTCAATCGTGT | |||
| PDCOV-S1 | F: ATGCAGAGAGCTCTATTGATTATGAC | 1763 | S1 |
| R: AACTTGCAAGTACTCCGTCTGAACG | |||
| PDCOV-S2 | F: ATTTTCTCTTTCCGTTCAGACGGAG | 1750 | S2 |
| R: CTACCATTCCTTAAACTTAAAGGACG | |||
| RVA-VP6 | F: GAAACGGAATAGCTCCACAAT | 271 | VP6 |
| R: GAATAATCAAATCCAGCCACC |
Phylogenetic analysis
The S gene sequences of 98 PEDV representative strains (Table 3) were extracted from the GenBank database for phylogenetic analysis to understand the evolution of the most common PEDV strains in Guangdong, China. The majority of the reference sequences were found in Asia, Europe, and North America. The MAFFT (Multiple Alignment using Fast Fourier Transform) embedded in the UGENE software was used to align multiple sequences (Version 36.0). The nucleotide (nt) and amino acid (aa) homology of S genes among the 13 strains were calculated using the Geneious software (Version 11.0.9) after alignment. The phylogenetic trees of S genes were constructed using the maximum likelihood (ML) method with 1,000 bootstrap replicates in IQ-TREE (Version 1.6.12) based on representative PEDV strains deposited in the GenBank. The phylogenetic tree was further annotated by FigTree (Version 1.4.3).
Table 3. PDEV reference strains described in this study.
| Virus strain | Countries | Time | GenBank accession no. | Virus strain | Countries | Time | GenBank accession no. |
|---|---|---|---|---|---|---|---|
| CV777 | Beigium | 1988 | AF353511 | USA Colorado | USA | 2013 | KF272920 |
| LZC | China | 2006 | EF185992 | USA Indiana 17,846 | USA | 2013 | KF452323 |
| CH/S | China | 1986 | JN547228 | USA/IA/2013/19321 | USA | 2013 | KM975738 |
| BJ-2011-1 | China | 2011 | JN825712 | USA/low107/2013 | USA | 2013 | KJ645696 |
| CHGD-01 | China | 2011 | JN980698 | USA/Minnesota52/2013 | USA | 2013 | KJ645704 |
| ZJCZ4 | China | 2011 | JX524137 | OH851 | USA | 2014 | KJ399978 |
| GD-1 | China | 2011 | JX647847 | OH1414 | USA | 2014 | KJ408801 |
| AJ1102 | China | 2012 | JX188454 | PC21A | USA | 2014 | KR078299 |
| CH/GDGZ | China | 2012 | KF384500 | FJzz1 | China | 2011 | MK288006 |
| GD-A | China | 2012 | JX112709 | MH748550 | China | 2019 | MH748550 |
| AH2012 | China | 2012 | KC210145 | LZC | China | 2007 | EF185992 |
| CH/YNKM-8 | China | 2013 | KF761675 | GD-B | China | 2012 | JX088695 |
| KPEDV-9 | Korea | 1997 | KF898124 | IA2 | USA | 2013 | KF468754 |
| DR13 | Korea | 1999 | DQ862099 | SM98 | Korea | 2011 | GU937797 |
| attenuated-DR13 | Korea | 2002 | JQ023162 | YN144 | China | 2015 | KT021232 |
| KNU-0802 | Korea | 2008 | GU180143 | JS-HZ2012 | China | 2013 | KC210147 |
| KNU-0902 | Korea | 2009 | GU180145 | MEX/124/2014 | USA | 2015 | KJ645700 |
| CNU-091222-01 | Korea | 2009 | JN184634 | MMN | USA | 2013 | KF468752 |
| KNU-1303 | Korea | 2013 | KJ451038 | PC21A | USA | 2015 | KR078299 |
| K13JA12 | Korea | 2013 | KJ539151 | USA/Colorado/2013 | USA | 2013 | KF272920 |
| KNU-1401 | Korea | 2014 | KJ451047 | CH/JX-2/2013 | China | 2015 | KJ526096 |
| KNU-1402 | Korea | 2014 | KJ451048 | CH/JX-1/2013 | China | 2015 | KF760557 |
| K14JB01 | Korea | 2014 | KJ539154 | CH/HNAY/2015 | China | 2015 | KR809885 |
| IA1 USA | USA | 2013 | KF468753 | LC | China | 2012 | JX489155 |
| FL2013 | China | 2015 | KP765609 | CH/JXJA/2017 | China | 2018 | MF375374 |
| JS2008 | China | 2013 | KC109141 | CH/SCZY44/2017 | China | 2018 | MH061338 |
| PEDV-1556-Valencia-Requena | Spain | 2020 | MN692763 | Hawaii/39249/2014 | USA | 2015 | KP688354 |
| PEDV_1611_Murcia_Lorca | Spain | 2020 | MN692768 | EAS1 | Thailand | 2014 | KR610991 |
| SLOreBAS-2/2015 | Slovenia | 2016 | KY019624 | AVCT12 | Thailand | 2010 | LC053455 |
| SLO/JH-11/2015 | Slovenia | 2016 | KU297956 | ZJU/G1/2013 | China | 2013 | KU664503 |
| SNJ-P | China | 2019 | MK702008 | 85–7_China | China | 2013 | KX839246 |
| LW/L | China | 2019 | MK392335 | SC1402 | China | 2014 | KP162057 |
| OKN-1/JPN/2013 | Japan | 2015 | LC063836 | PPC-14 | Korea | 2014 | MG781192 |
| MYG-1/JPN/2014 | Japan | 2015 | LC063838 | SQ2014 | China | 2014 | KP728470 |
| EAS2 | Thailand | 2015 | KR610992 | SD-M | China | 2012 | JX560761 |
| PEDV_1613_Murcia_Fuentealamo | Spain | 2020 | MN692769 | YN15 | China | 2013 | KT021228 |
| PEDV_GER_L01014-K01_15–04_2015 | Germany | 2018 | LT898420 | YN1 | China | 2013 | KT021227 |
| PEDV_GER_L00906-K16_14–01_2014 | Germany | 2018 | LT898430 | VN/VAP1113 | Vietnam | 2013 | KJ960179 |
| PEDV_GER_L01020-K01_15–10_2015 | Germany | 2018 | LT898413 | YN30 | China | 2013 | KT021229 |
| HUA-14PED96 | Viet Nam | 2016 | KT941120 | CBR1 | Thailand | 2014 | KR610993 |
| SCDY523 | China | 2018 | MH593144 | IWT1 | Japan | 2014 | LC063834 |
| CHM | China | 2013 | KM887144 | KB2013-4 | China | 2013 | KX580953 |
| CV777 | China | 2016 | KT323979 | SHQP/YM/2013 | China | 2013 | KJ196348 |
| JSLS-1/2015 | China | 2016 | KX534205 | CH/HNLH/2015 | China | 2015 | KT199103 |
| GDS01 | China | 2015 | KM089829 | NW8 | China | 2015 | MF782687 |
| CH/SCCD/2014 | China | 2017 | KU975389 | YC | China | 2014 | KU252649 |
| CH/SCZG/2017 | China | 2018 | MH061337 | CH/GX/2015/750A | China | 2015 | KY793536 |
| 15V010/BEL/2015 | Belgium | 2015 | KR003452 | GER/L00719/2014 | Germany | 2014 | LM645058 |
| L00721/GER/2014 | Germany | 2014 | LM645057 | FR/001/2014 | France | 2014 | KR011756 |
Recombination analysis and N-linked glycosylation prediction
The evolution of coronaviruses, including PEDV, was aided by genome recombination. The recombination detection program (RDP v5) was used to determine the recombination event in the PEDV S gene, which included ninedetection algorithms (RDP, GENECONV, Bootscan, Maxchi, Chimaera, SiSscan, PhylPro, LARD, and 3Seq) [27]. The detection of potential recombinants was done using a P<0.01 threshold. As previously stated, N-linked glycosylation was predicted [28].
Results
Sample screening and sequencing
Sixteen of the thirty-four field samples (47.0%) were found to be positive for PEDV. In the fecal samples or fecal swabs, the TGEV and PDCoV were not found. From fecal samples and fecal swabs, seven and nine PEDV positive samples were identified, respectively. Based on the RT-PCR results, eight samples (23.5%) were tested to be porcine rotavirus (RV) positive. The co-infection rate of PEDV and RV was 12.5% (2/16) (Table 1). The complete S gene and a portion of the M gene were amplified and sequenced using Sanger sequencing. GDsg01-GDsg13 are the names of the 13 S genes that were discovered. The S genes from the five swine farms ranged in length from 4158 to 4164 nucleotides (nt), with nt and amino acid (aa) homology of 97.09%-99.95% and 96.77% -99.79%, respectively (S1 Fig). The sequences were determined using Sanger sequencing and deposited in GenBank with accession No. MW478760-MW478772 respectively.
Phylogenetic analysis of S gene
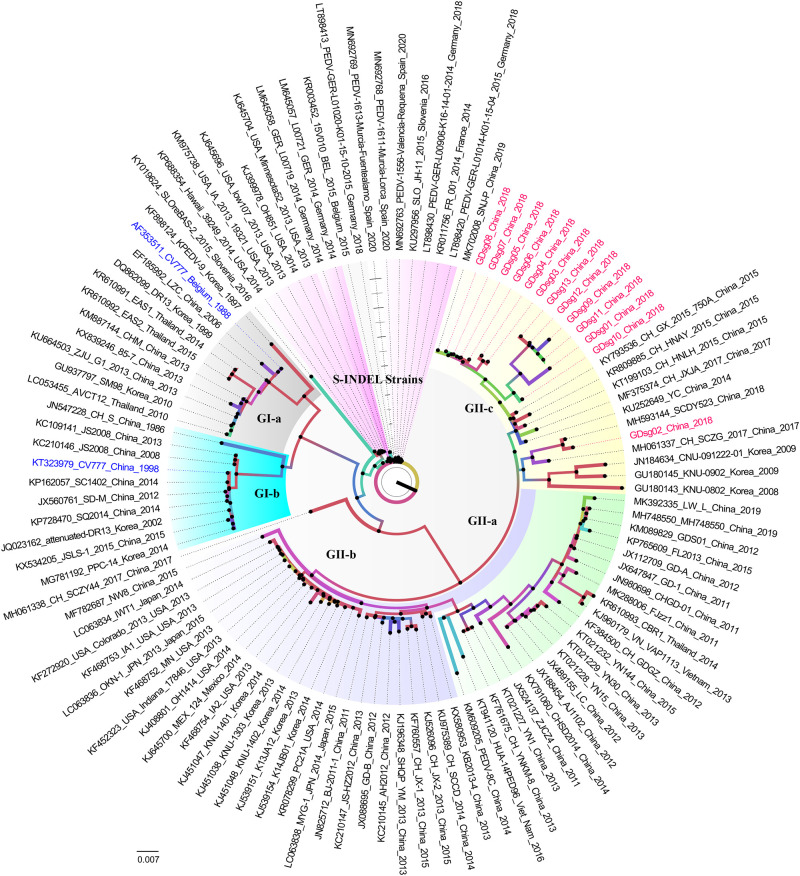
A phylogenetic tree was constructed using full-length S genes from the 98 reference PEDV strains available in GenBank, and the 13 S-gene sequences obtained in this study to understand the phylogenetic relationship of these PEDV strains. PEDV strains were divided into two categories: traditional G1 and variant G2. The CV777 and SM98 strains were in the G1-a group. The attenuated vaccine strains were found in the G1-b group (CV777 and DR13). The 13 PEDV strains reported in this study had a strong association with the GII-c subgroup, according to our phylogenetic analysis (Fig 1).
Fig 1. Phylogenetic analysis of full-length S gene of 13 PEDV strains collected in this study.
MAFFT (Multiple Alignment using Fast Fourier Transform) in the UGENE software was used to align 98 PEDV reference strains with 13 PEDV strains (Version 36.0). With IQ-TREE, the phylogenetic tree was built using the maximum likelihood (ML) method with 1,000 bootstrap replicates (Version 1.6.12). PEDV’s S gene PEDV was divided into six categories: GI-a (light grey), GI-b (blue), GII-a (light green), GII-b (light cyan), and GII-c (light cyan) (light yellow). The CV777 reference strains are highlighted in blue, while the 13 strains reported in this study are highlighted in red. Nucleotide substitutions per site are indicated by a 0.007 bar.
Sequence comparative analysis of S gene
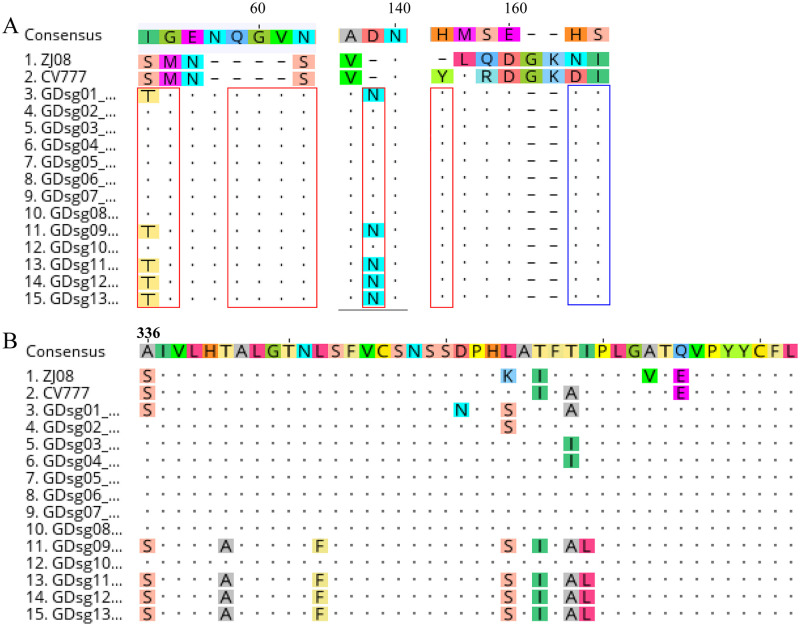
The S gene sequences were compared with classic strains and vaccine strains to further investigate the genetic characteristics of the 13 detected strains. As a result, the 13 PEDV strains shared homology with CV777 (KT323979), ZJ08, and AJ1102 (JX188454) of 93.4–93.8%, 93.3–93.6%, and 96.9–97.8%, respectively (Table 4). Compared with ZJ08, a total of 104 aa mutations were observed in the 13 PEDV strains of this study. S1 proteins exhibited 72.1% (75/104) of aa mutations and were majorly distributed in the S1-NTD and S1-CTD domains. The 13 strains shared a common aa deletion (163DI164) and five common aa insertions (55T/IG56, 59QGVN62, 136N, 153H, and 551L) (Fig 2A) compared to the reference strains CV777 and ZJ08. In addition, a high polymorphism (>30% in a specific aa position) of mutations (N = 19) such as 62Y→66H/Y, 135N→135N/D was observed in the S1 protein, especially the S1-CTD region (Fig 2B). In addition, two novel common mutations, including A520S and G612V, were identified in this study. The detailed list of aa mutations in the S protein of the 13 PEDV strains in comparison with vaccine strain ZJ08 is shown in Table 5.
Table 4. Sequence comparison of S gene of 13 PEDV strains and 3 vaccine strains.
| PEDV strains | Percentage of nucleotide identity (%) | ||
|---|---|---|---|
| CV777 (KT323979) | ZJ08 | AJ1102 (JX188454) | |
| GDsg01 China 2018 | 93.4 | 93.3 | 97.2 |
| GDsg02 China 2018 | 93.6 | 93.4 | 97.8 |
| GDsg03 China 2018 | 93.7 | 93.5 | 97.3 |
| GDsg04 China 2018 | 93.7 | 93.4 | 97.3 |
| GDsg05 China 2018 | 93.8 | 93.6 | 97.6 |
| GDsg06 China 2018 | 93.8 | 93.6 | 97.5 |
| GDsg07 China 2018 | 93.8 | 93.6 | 97.6 |
| GDsg08 China 2018 | 93.8 | 93.6 | 97.6 |
| GDsg09 China 2018 | 93.7 | 93.5 | 97.0 |
| GDsg10 China 2018 | 93.7 | 93.4 | 97.4 |
| GDsg11 China 2018 | 93.7 | 93.5 | 96.9 |
| GDsg12 China 2018 | 93.6 | 93.4 | 96.9 |
| GDsg13 China 2018 | 93.7 | 93.4 | 96.9 |
Fig 2. Analysis of animo acid mutations in the S protein of 13 PEDV strains.
MUSCLE was used to align the sequences, and Geneious software (Version 11.0.9) was used to visualize them. (A) The common insertions (red box) and deletions (blue box) of amino acid (aa) mutations compared with the reference strains ZJ08 and CV777. (B). The regions in the S1 protein with a relative high polymorphism of mutations. (C) The predicted N-linked glycosylation sites of reference strain (CV777 and ZJ08), and 13 PEDV strains collected in this study. MUSCLE and the Geneious software were used to align the sequences (Version 11.0.9). The purple arrow represents the predicted N-linked glycosylation site based on the consensus N-X-S/T (X can be any amino acid except proline) glycosylation motif.
Table 5. Statistics of mutations in the S protein of the 13 PEDV strains in comparison with ZJ08.
| Domain | Mutations | Domain | Mutations | Domain | Mutations |
|---|---|---|---|---|---|
| SP | 2TP3→2KS3 | 231S→231I/L | 763L→763S | ||
| 5I→5T/N | 241DS242→241EP/L242 | 765D→765S | |||
| 15L→15S | S1-CTD | 265L/265V | 773M→773T | ||
| S1-NTD | 27QSTI30→27S/AANT30 | 281W→281L | 778I→783M/I | ||
| ΔΔ→55T/IG56 | 283I→283M | S2 | 801S→801S/T | ||
| 56MNS58→56GEN58 | 298MM299→298TI299 | 805V→805A | |||
| ΔΔΔΔ→59QGVN62 | 308A→308V | 853E→853E/D | |||
| 60S→60T | 323F→323S | 890G→890R | |||
| 62Y→66H/Y | 330S→330S/A | 958A→958V | |||
| 64GTGIE68→64AGQHP68 | 335T→335T/A | 962L→962F | |||
| 80Y→80H | 341L→341F/L | 964T→964S | |||
| 82DS83→82RG83 | 353K→353S/L | 972H→972Y | |||
| 85Q→85H | 355I→355I/T | 994L→994M/L | |||
| 114S→114N | 362TI363→362AL/I363 | 1027N→1027K | |||
| 116I→116T | 362V→362A | 1043S→1043A | |||
| 126DN127→126NI127 | 364E→364Q | 1050I→1050V | |||
| 134V→134A | 373V→373L/V | 1095A→1095A/S | |||
| 135N→135N/D | 377K→377N | 1112Q→1112Q/L | |||
| Δ136N | 392K→392R | 1139E→1134E/D | |||
| 429D→434G/D | 1141I→1141I/V | ||||
| Δ153H | 437V→442I | 1161N→1161N/D | |||
| 154LQDGK158→154MSEHS158 | 473S→473A | 1166D→1166A | |||
| 159NI160→159ΔΔ160 | 495I→495I/T | 1172GD1173→1172DE1173 | |||
| 173A→173S | 516A→521S/A | 1192TY1193→1192NH1193 | |||
| 181I→181F | Δ551L | 1231S→1231R | |||
| 191R→191K | 600G→600S | 1259I→1259T | |||
| 195KRS197→195SGG197 | 608G→613V/G | 1261P→1261S/P | |||
| 205T→210E | 632Q→632E | TM | 1275L→1275L/T | ||
| 222Y→222S | S1 other domain | 718N→718N/S | Cyto | 1297R→1297Q | |
| 224E→224Q | 723N→723S | 1358G→1358C/G | |||
| 1375A→1375V |
S gene recombination analysis
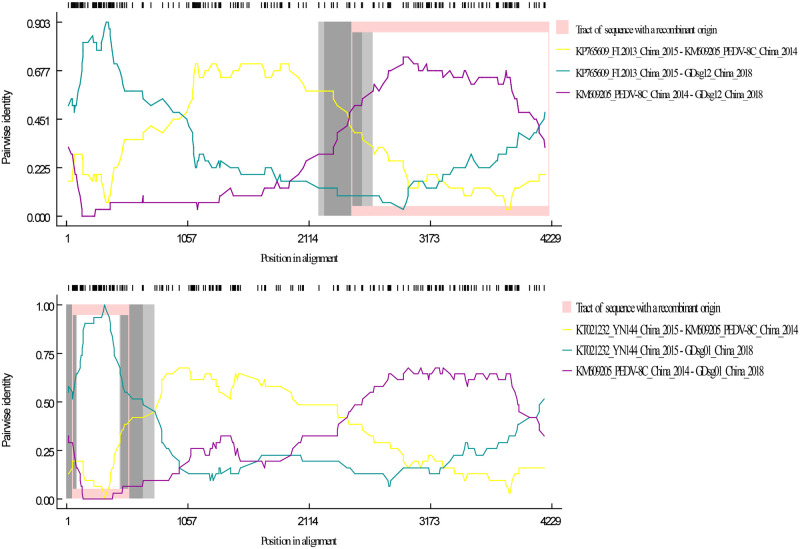
We performed a recombination analysis based on the 13 PEDV strains collected in this study as well as the 98 reference strains described above to understand the recombination events that occurred during the evolution of the PEDV strains circulating in Guangdong, China. The position 2478–4208 of the S gene of GDsg12 strain was predicted as a recombinant between KP765609 (major parent, GII-a) and KM609205 (minor parent, GII-a) (Fig 3A), which was supported by 6 detection methods (RDP, P-values ≤ 1.17× 10−6; Bootscan, P-values ≤ 1.08 ×10−7; Maxchi P-values ≤ 5.43×10−9; Chimaera, P-values ≤ 7.38×10−9; SiSscan, P-values ≤ 2.82×10−8; 3Seq, P-values ≤ 3.67×10−10). Similarly, 6 detection methods indicated that position 51–548 of the S gene of the GDsg01 strain was likely produced by intragroup recombination between KT021232 (major parent, GII-a) and KM609205 (minor parent, GII-a) (Fig 3B) (RDP, P-values ≤ 2.17×10−11; GENECONV, P-values ≤ 2.24×10−9; Bootscan, P-values ≤ 1.76 10−11; Maxchi, Chimaera, P-values ≤ 2.17×10−8; 3Seq, P-values ≤ 2.86×10−6) with a high degree of reliability.
Fig 3. Detection of possible recombination events in the PEDV strains.
The recombination detection software (RDP v5) was used to recognize recombination events in the GDsg12 (A) and GDsg01 (B) genomes using nine detection algorithms (RDP, GENECONV, Bootscan, Maxchi, Chimaera, SiSscan, PhylPro, LARD, 3Seq). The Y-axis represents the pairwise identity between the recombinant and its putative parents. The X-axis represents the position in alignment with a 30-nt sliding window. The comparison of recombinant-major parent, recombinant-minor parent, major-minor parent was indicated as cyan, purple, and yellow lines, respectively.
N-linked glycosylation prediction
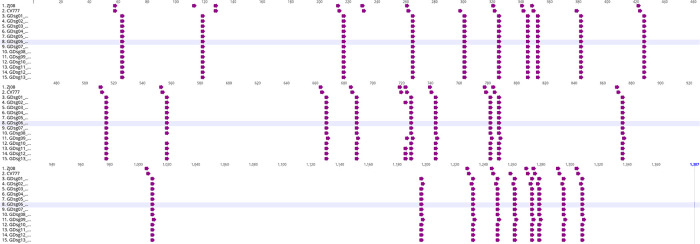
The N-linked glycosylation sites were predicted based on the consensus N-X-S/T glycosylation motif. As a result, the 13 PEDV strains had 28–29 predicted N-glycosylation sites (G+) and showed a more similar pattern to CV777 than ZJ08 (Fig 4). Two (131G-, 235G-) and one common loss of glycosylation sites (235G-) were observed when compared with the reference strains ZJ08 and CV777, respectively. In contrast, the PEDV strains reported in this study gained three (302G+, 1199 G+, and 1264 G+) and one (1199G+) potential glycosylation sites compared with the ZJ08 and CV777, respectively. In addition, 61.5% (8/13) of PEDV strains reported in this study lost the glycosylation site at residue 725.
Fig 4. Predicted N-linked glycosylation sites of reference strains (CV777 and ZJ08) and 13 PEDV strains collected in this study.
The sequences were aligned using MUSCLE with the Geneious software (Version 11.0.9). The purple arrow represents the predicted N-linked glycosylation site based on the consensus N-X-S/T (X can be any amino acid except proline) glycosylation motif.
Discussion
Several coronaviruses, including PEDV, TGEV, SADS-CoV, SeCoV, and PDCoV, have been identified in piglets suffering from acute diarrhea and vomiting [29–32]. CoV entry is mediated by the S glycoprotein, which is a critical factor in determining the virus’s tissue tropism and antigenicity. Additionally, the S gene of the coronavirus is prone to change through recombination or accumulation of point mutations, which can result in the virus losing its antigenicity and even the vaccine failing to work [33–35]. The molecular characterization of PEDVs is a major focus of swine diarrhea virus research due to the virus’s high prevalence.
During 2018–2019, we investigated the presence of swine diarrhea virus in eight pig farms in Guangdong, China, and then focused on the genetic characterization of PEDV. Our findings supported previous research [36], which indicated that PEDV is a leading cause of swine acute diarrhea in south China. We reported a detection rate of 47.0% for PDEV strains in fecal samples and fecal swabs collected from diarrheal pigs in eightswine farms. The reduction in TGEV reported in that study [36] is in agreement with our results. The gradual disappearance of TGEV has been attributed to the spread of porcine respiratory coronavirus, which conserved the majority of antigenic sites and caused a cross-protection against TGEV [37]. PDCoV, a newly identified member of the viral agents that cause swine diarrhea, was previously considered to be the second most prevalent swine diarrhea pathogen, following PEDV [38]. Sun et al. previously reported the genetic characterization of PDCoV in Shandong province during the same time period as this study and found a 50% co-infection rate of PDEV and PDCoV [26]. However, PDCoV was not detected in our study. Those observations indicated that the molecular epidemiology of the swine diarrhea virus varies greatly across China. Rotaviruses (RV), which belong to the Reoviridae family, have been identified as a major cause of viral gastroenteritis in young animals, such as piglets [39, 40]. We found an 18.6% detection rate for RV and a 12.5% co-infection rate for PEDV and RV. Surprisingly, a recent survey of pig farms in Brazil found porcine rotavirus B to be the primary viral agent (71.1%) in newborn piglets with acute viral gastroenteritis. In contrast, another study found that RT-PCR had a low detection rate of porcine RV (3%). The detection rate of porcine RV; however, varies depending on the detection method [41]. In this study, all PEDV stains were found to be clustered into GII-c (non-S-INDEL) subgroups. In a recent study, Tian et al. found that PEDV strains with the S gene of the GII-c subgroup were the most prevalent in Sichuan [25]. Furthermore, the nt identity of the PEDV strains in this study was similar to vaccine strains CV777 and ZJ08, but slightly higher than AJ1102 to. A genomic variation hotspot was also discovered in the S1-CTD region. It’s been suggested that the surface of PEDV’s S protein PEDV contains four major epitopes [42, 43]. Our findings revealed a number of novel common mutations, including A520S and G612V. These aa changes may have an effect on the virus’s antigenicity, leading to immune escape and vaccine failure. It’s worth noting that all 13 PEDV strains have the same newly discovered B-cell epitope (722SSTFNSTREL731) [42] of S protein PEDV. In addition to the previously reported common aa deletion (163DI164) and aa insertions (59QGVN62, 136N, and 153H), we report two new common aa insertions for PEDV strains circulating in south China: 55T/IG56 and 551L. Nevertheless, more research is needed into the impact of those common mutations on virus characterization, such as antigenicity and pathogenicity.
In addition to the accumulation of point mutations, homologous recombination among members of the same genus is a common way for the genetic evolution of coronaviruses. The recent SARS-CoV-2 outbreak was thought to be the result of cross-species recombination between bat and pangolin coronaviruses [44, 45]. Similarly, PEDV is intensively recombined between other members of the Alphacoronavirus, such as TGEV. In 2016, Italy [37], Germany [46] and Slovakia [47] reported the discovery of a novel swine enteric coronavirus with a backbone derived from TGEV and the S gene derived from PEDV [47]. Notably, a recent retrospective study indicated that the recombinant SeCoV circulating in Spain may have been misidentified as PEDV using S-protein or S-gene assays [48]. Our studies indicated that recombination occurred in both the S1 and S2 regions of GII-c PEDV strains, as supported by at least six highly reliable methods. The recombination events could be a result of pigs being transported and traded frequently in China.
In conclusion, our findings revealed that PEDV and porcine RV were the two main viral agents responsible for the outbreak of diarrhea on swine farms in China’s Guangdong province. A number of novel mutations were discovered, including common insertions like 55T/IG56 and 551L. In addition, when compared to the vaccine strain, one common loss of glycosylation site (235G-) was observed. Intragroup recombination events were discovered in the S gene of the PEDV strains studied. Our findings highlight the critical need for the development of novel vaccines to combat recent new PEDV variants.
Supporting information
The sequence homology was calculated by the Geneious software (Version 11.0.9) after multiple sequence alignment.
(TIF)
Data Availability
All sequence files are available from the GenBank database (accession No. MW478760-MW478772).
Funding Statement
This study was supported by National Key R&D Program of China (2018YFD0501200); National Natural Science Foundation of China (Grant No. 32002320); Guangdong Basic and Applied Basic Research Foundation (Grant No. 2020A1515010116 and 2019A1515110785). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pensaert MB, de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch Virol. 1978;58(3):243–7. Epub 1978/01/01. doi: 10.1007/BF01317606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shibata I, Tsuda T, Mori M, Ono M, Sueyoshi M, Uruno K. Isolation of porcine epidemic diarrhea virus in porcine cell cultures and experimental infection of pigs of different ages. Vet Microbiol. 2000;72(3–4):173–82. Epub 2000/03/23. doi: 10.1016/s0378-1135(99)00199-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood EN. An apparently new syndrome of porcine epidemic diarrhoea. Vet Rec. 1977;100(12):243–4. Epub 1977/03/19. [DOI] [PubMed] [Google Scholar]
- 4.Chasey D, Cartwright SF. Virus-like particles associated with porcine epidemic diarrhoea. Res Vet Sci. 1978;25(2):255–6. Epub 1978/09/01. doi: 10.1016/S0034-5288(18)32994-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song D, Park B. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes. 2012;44(2):167–75. Epub 2012/01/25. doi: 10.1007/s11262-012-0713-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kusanagi K, Kuwahara H, Katoh T, Nunoya T, Ishikawa Y, Samejima T, et al. Isolation and serial propagation of porcine epidemic diarrhea virus in cell cultures and partial characterization of the isolate. J Vet Med Sci. 1992;54(2):313–8. Epub 1992/04/01. doi: 10.1292/jvms.54.313 . [DOI] [PubMed] [Google Scholar]
- 7.Martelli P, Lavazza A, Nigrelli AD, Merialdi G, Alborali LG, Pensaert MB. Epidemic of diarrhoea caused by porcine epidemic diarrhoea virus in Italy. Vet Rec. 2008;162(10):307–10. Epub 2008/03/11. doi: 10.1136/vr.162.10.307 . [DOI] [PubMed] [Google Scholar]
- 8.Pijpers A, van Nieuwstadt AP, Terpstra C, Verheijden JH. Porcine epidemic diarrhoea virus as a cause of persistent diarrhoea in a herd of breeding and finishing pigs. Vet Rec. 1993;132(6):129–31. Epub 1993/02/06. [DOI] [PubMed] [Google Scholar]
- 9.Pospischil A, Hess RG, Bachmann PA. Light microscopy and ultrahistology of intestinal changes in pigs infected with epizootic diarrhoea virus (EVD): comparison with transmissible gastroenteritis (TGE) virus and porcine rotavirus infections. Zentralbl Veterinarmed B. 1981;28(7):564–77. Epub 1981/01/01. doi: 10.1111/j.1439-0450.1981.tb01774.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben Salem AN, Chupin Sergei A, Bjadovskaya Olga P, Andreeva Olga G, Mahjoub A, Prokhvatilova Larissa B. Multiplex nested RT-PCR for the detection of porcine enteric viruses. J Virol Methods. 2010;165(2):283–93. Epub 2010/02/23. doi: 10.1016/j.jviromet.2010.02.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Fang L, Xiao S. Porcine epidemic diarrhea in China. Virus Res. 2016;226:7–13. Epub 2016/06/05. doi: 10.1016/j.virusres.2016.05.026 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Li H, Liu Y, Pan Y, Deng F, Song Y, et al. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg Infect Dis. 2012;18(8):1350–3. Epub 2012/07/31. doi: 10.3201/eid1808.120002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stadler J, Zoels S, Fux R, Hanke D, Pohlmann A, Blome S, et al. Emergence of porcine epidemic diarrhea virus in southern Germany. BMC Vet Res. 2015;11:142. Epub 2015/07/03. doi: 10.1186/s12917-015-0454-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevenson GW, Hoang H, Schwartz KJ, Burrough ER, Sun D, Madson D, et al. Emergence of Porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J Vet Diagn Invest. 2013;25(5):649–54. Epub 2013/08/22. doi: 10.1177/1040638713501675 . [DOI] [PubMed] [Google Scholar]
- 15.Brian DA, Baric RS. Coronavirus genome structure and replication. Curr Top Microbiol Immunol. 2005;287:1–30. Epub 2004/12/22. doi: 10.1007/3-540-26765-4_1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian Y, Yu Z, Cheng K, Liu Y, Huang J, Xin Y, et al. Molecular characterization and phylogenetic analysis of new variants of the porcine epidemic diarrhea virus in Gansu, China in 2012. Viruses. 2013;5(8):1991–2004. Epub 2013/08/21. doi: 10.3390/v5081991 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corse E, Machamer CE. Infectious bronchitis virus E protein is targeted to the Golgi complex and directs release of virus-like particles. J Virol. 2000;74(9):4319–26. Epub 2001/02/07. doi: 10.1128/jvi.74.9.4319-4326.2000 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen JF, Sun DB, Wang CB, Shi HY, Cui XC, Liu SW, et al. Molecular characterization and phylogenetic analysis of membrane protein genes of porcine epidemic diarrhea virus isolates in China. Virus Genes. 2008;36(2):355–64. Epub 2008/01/25. doi: 10.1007/s11262-007-0196-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi D, Shi H, Sun D, Chen J, Zhang X, Wang X, et al. Nucleocapsid Interacts with NPM1 and Protects it from Proteolytic Cleavage, Enhancing Cell Survival, and is Involved in PEDV Growth. Sci Rep. 2017;7:39700. Epub 2017/01/04. doi: 10.1038/srep39700 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao L, Ge X, Gao Y, Zarlenga DS, Wang K, Li X, et al. Putative phage-display epitopes of the porcine epidemic diarrhea virus S1 protein and their anti-viral activity. Virus Genes. 2015;51(2):217–24. Epub 2015/08/22. doi: 10.1007/s11262-015-1234-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang SH, Bae JL, Kang TJ, Kim J, Chung GH, Lim CW, et al. Identification of the epitope region capable of inducing neutralizing antibodies against the porcine epidemic diarrhea virus. Mol Cells. 2002;14(2):295–9. Epub 2002/11/22. . [PubMed] [Google Scholar]
- 22.Vlasova AN, Marthaler D, Wang Q, Culhane MR, Rossow KD, Rovira A, et al. Distinct characteristics and complex evolution of PEDV strains, North America, May 2013-February 2014. Emerg Infect Dis. 2014;20(10):1620–8. Epub 2014/10/04. doi: 10.3201/eid2010.140491 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Byrum B, Zhang Y. New variant of porcine epidemic diarrhea virus, United States, 2014. Emerg Infect Dis. 2014;20(5):917–9. Epub 2014/04/23. doi: 10.3201/eid2005.140195 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui JT, Qiao H, Hou CY, Zheng HH, Li XS, Zheng LL, et al. Characteristics of the spike and ORF3 genes of porcine epidemic diarrhea virus in Henan and Shanxi provinces of China. Arch Virol. 2020;165(10):2323–33. Epub 2020/07/28. doi: 10.1007/s00705-020-04744-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian Y, Yang X, Li H, Ma B, Guan R, Yang J, et al. Molecular characterization of porcine epidemic diarrhea virus associated with outbreaks in southwest China during 2014–2018. Transbound Emerg Dis. 2020. Epub 2020/12/12. doi: 10.1111/tbed.13953 . [DOI] [PubMed] [Google Scholar]
- 26.Sun W, Wang L, Huang H, Wang W, Cao L, Zhang J, et al. Genetic characterization and phylogenetic analysis of porcine deltacoronavirus (PDCoV) in Shandong Province, China. Virus Res. 2020;278:197869. Epub 2020/01/22. doi: 10.1016/j.virusres.2020.197869 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin DP, Murrell B, Golden M, Khoosal A, Muhire B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015;1(1):vev003. Epub 2015/05/26. doi: 10.1093/ve/vev003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen F, Li W, Guo J, Yang J, Zhang X, Mei K, et al. Genetic characterization of a novel genotype H9N2 avian influenza virus from chicken in South China. J Infect. 2020;81(5):816–46. Epub 2020/09/22. doi: 10.1016/j.jinf.2020.09.011 . [DOI] [PubMed] [Google Scholar]
- 29.Fu X, Fang B, Liu Y, Cai M, Jun J, Ma J, et al. Newly emerged porcine enteric alphacoronavirus in southern China: Identification, origin and evolutionary history analysis. Infect Genet Evol. 2018;62:179–87. Epub 2018/04/29. doi: 10.1016/j.meegid.2018.04.031 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Z, Zhong H, Zhou Q, Du Y, Chen L, Zhang Y, et al. A Highly Pathogenic Strain of Porcine Deltacoronavirus Caused Watery Diarrhea in Newborn Piglets. Virol Sin. 2018;33(2):131–41. Epub 2018/03/24. doi: 10.1007/s12250-018-0003-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou L, Sun Y, Lan T, Wu R, Chen J, Wu Z, et al. Retrospective detection and phylogenetic analysis of swine acute diarrhoea syndrome coronavirus in pigs in southern China. Transbound Emerg Dis. 2019;66(2):687–95. Epub 2018/09/02. doi: 10.1111/tbed.13008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou P, Fan H, Lan T, Yang XL, Shi WF, Zhang W, et al. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature. 2018;556(7700):255–8. Epub 2018/04/06. doi: 10.1038/s41586-018-0010-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen N, Li S, Zhou R, Zhu M, He S, Ye M, et al. Two novel porcine epidemic diarrhea virus (PEDV) recombinants from a natural recombinant and distinct subtypes of PEDV variants. Virus Res. 2017;242:90–5. Epub 2017/09/28. doi: 10.1016/j.virusres.2017.09.013 . [DOI] [PubMed] [Google Scholar]
- 34.Lu Y, Su X, Du C, Mo L, Ke P, Wang R, et al. Genetic Diversity of Porcine Epidemic Diarrhea Virus With a Naturally Occurring Truncated ORF3 Gene Found in Guangxi, China. Front Vet Sci. 2020;7:435. Epub 2020/08/15. doi: 10.3389/fvets.2020.00435 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun J, Li Q, Shao C, Ma Y, He H, Jiang S, et al. Isolation and characterization of Chinese porcine epidemic diarrhea virus with novel mutations and deletions in the S gene. Vet Microbiol. 2018;221:81–9. Epub 2018/07/10. doi: 10.1016/j.vetmic.2018.05.021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J, Shi BJ, Huang XG, Peng MY, Zhang XM, He DN, et al. A multiplex RT-PCR assay for rapid and differential diagnosis of four porcine diarrhea associated viruses in field samples from pig farms in East China from 2010 to 2012. J Virol Methods. 2013;194(1–2):107–12. Epub 2013/08/31. doi: 10.1016/j.jviromet.2013.08.008 . [DOI] [PubMed] [Google Scholar]
- 37.Boniotti MB, Papetti A, Lavazza A, Alborali G, Sozzi E, Chiapponi C, et al. Porcine Epidemic Diarrhea Virus and Discovery of a Recombinant Swine Enteric Coronavirus, Italy. Emerg Infect Dis. 2016;22(1):83–7. Epub 2015/12/23. doi: 10.3201/eid2201.150544 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song D, Zhou X, Peng Q, Chen Y, Zhang F, Huang T, et al. Newly Emerged Porcine Deltacoronavirus Associated With Diarrhoea in Swine in China: Identification, Prevalence and Full-Length Genome Sequence Analysis. Transbound Emerg Dis. 2015;62(6):575–80. Epub 2015/08/08. doi: 10.1111/tbed.12399 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martella V, Banyai K, Matthijnssens J, Buonavoglia C, Ciarlet M. Zoonotic aspects of rotaviruses. Vet Microbiol. 2010;140(3–4):246–55. Epub 2009/09/29. doi: 10.1016/j.vetmic.2009.08.028 . [DOI] [PubMed] [Google Scholar]
- 40.Molinari BL, Possatti F, Lorenzetti E, Alfieri AF, Alfieri AA. Unusual outbreak of post-weaning porcine diarrhea caused by single and mixed infections of rotavirus groups A, B, C, and H. Vet Microbiol. 2016;193:125–32. Epub 2016/09/08. doi: 10.1016/j.vetmic.2016.08.014 . [DOI] [PubMed] [Google Scholar]
- 41.Zhang F, Luo S, Gu J, Li Z, Li K, Yuan W, et al. Prevalence and phylogenetic analysis of porcine diarrhea associated viruses in southern China from 2012 to 2018. BMC Vet Res. 2019;15(1):470. Epub 2019/12/29. doi: 10.1186/s12917-019-2212-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong N, Meng Q, Jiao Y, Wu Y, Zuo Y, Wang H, et al. Identification of a novel B-cell epitope in the spike protein of porcine epidemic diarrhea virus. Virol J. 2020;17(1):46. Epub 2020/04/05. doi: 10.1186/s12985-020-01305-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li RF, Qiao SL, Yang YY, Su YF, Zhao P, Zhou EM, et al. Phylogenetic analysis of porcine epidemic diarrhea virus (PEDV) field strains in central China based on the ORF3 gene and the main neutralization epitopes. Archives of Virology. 2014;159(5):1057–65. doi: 10.1007/s00705-013-1929-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Giorgi EE, Marichannegowda MH, Foley B, Xiao C, Kong XP, et al. Emergence of SARS-CoV-2 through recombination and strong purifying selection. Sci Adv. 2020;6(27). Epub 2020/09/17. doi: 10.1126/sciadv.abb9153 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wen F, Yu H, Guo J, Li Y, Luo K, Huang S. Identification of the hyper-variable genomic hotspot for the novel coronavirus SARS-CoV-2. J Infect. 2020;80(6):671–93. Epub 2020/03/08. doi: 10.1016/j.jinf.2020.02.027 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akimkin V, Beer M, Blome S, Hanke D, Hoper D, Jenckel M, et al. New Chimeric Porcine Coronavirus in Swine Feces, Germany, 2012. Emerg Infect Dis. 2016;22(7):1314–5. Epub 2016/04/14. doi: 10.3201/eid2207.160179 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandelik R, Sarvas M, Jackova A, Salamunova S, Novotny J, Vilcek S. First outbreak with chimeric swine enteric coronavirus (SeCoV) on pig farms in Slovakia—lessons to learn. Acta Vet Hung. 2018;66(3):488–92. Epub 2018/09/29. doi: 10.1556/004.2018.043 . [DOI] [PubMed] [Google Scholar]
- 48.de Nova PJG, Cortey M, Diaz I, Puente H, Rubio P, Martin M, et al. A retrospective study of porcine epidemic diarrhoea virus (PEDV) reveals the presence of swine enteric coronavirus (SeCoV) since 1993 and the recent introduction of a recombinant PEDV-SeCoV in Spain. Transbound Emerg Dis. 2020. Epub 2020/06/09. doi: 10.1111/tbed.13666 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The sequence homology was calculated by the Geneious software (Version 11.0.9) after multiple sequence alignment.
(TIF)
Data Availability Statement
All sequence files are available from the GenBank database (accession No. MW478760-MW478772).