Abstract
Functionalized trans-cyclooctenes are useful bioorthogonal reagents that are typically prepared using a flow photoisomerization method where the product is captured by AgNO3 on silica gel. While this method is effective, the leaching of silver can be problematic when scaling up syntheses. It is shown here that Ag(I) immobilized on tosic silica gel can be used to capture trans-cyclooctene products at higher loadings without leaching. It is demonstrated that the sulfonated silica gel can be regenerated and reused with similar yields over multiple runs. Nine different trans-cyclooctenes were synthesized, including those commonly utilized in bioorthogonal chemistry as well as new amine and carboxylic acid derivatives.
Keywords: flow, photochemistry, trans-cyclooctene, large scale, bioorthogonal
Graphical Abstract
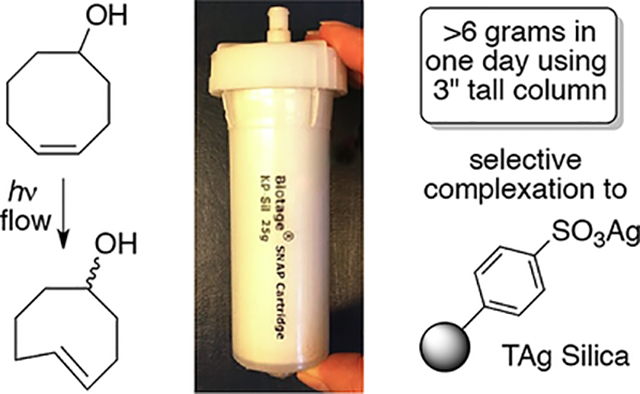
Advances in the field of bioorthogonal chemistry have been accompanied by a renaissance in the chemistry of strained molecules including cyclooctynes, cyclopropenes and trans-cyclooctenes.1–11 Derivatives of trans-cyclooctene have emerged as highly reactive coupling partners for bioorthogonal inverse electron demand Diels-Alder reactions12–17 with rates as fast as 3.3 × 106 M−1s−1·18 A number of multistep routes for the preparation of trans-cyclooctenes from cis-cyclooctenes had been described,19–22 but relatively few methods that have been applied to the synthesis of functionalized trans-cyclooctene derivatives.23–26 Several approaches have been described to produce trans-cycloalkenes that contain heteroatoms in the backbone.27–29 Woerpel has demonstrated that oxasila-trans-cycloalkenes can be prepared via silylene additions to vinylepoxides,30–34 and Tomooka has synthesized and studied the properties of a range of planar chiral heterocycles.35–38 Bicyclic trans-cycloalkenes have been prepared via a sequence of enantioselective cyclopropanation and skeletal rearrangment.39 Hsung,40,41 and Takasu42 have shown that torquoselective opening of bicyclic cyclobutenes can be used to transiently produce trans-cycloalkenes.
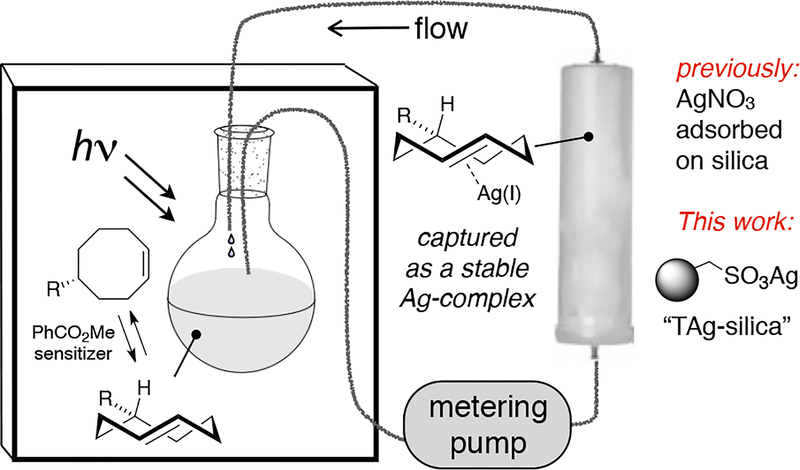
The singlet sensitized photoisomerization of cis-cyclooctene, pioneered by Inoue,43 is a direct method for the synthesis of trans-cyclooctene. To improve the practicality of photoisomerization, we designed a closed-loop flow reactor that perturbs the equilibrium through selective metal complexation of the trans-isomer.44,45 A schematic of the apparatus for preparing trans-cyclooctenes is shown in Scheme 1. At completion (usually 6 h), the silica is removed and stirred with ligand (typically NH4OH) to liberate the trans-cyclooctene, which is then recovered by extraction.
Scheme 1.
Schematic of flow reactor
Our first generation system (AgNO3 adsorbed on SiO2- referred to as AgNO3/SiO2) has been used successfully for a range of trans-cyclooctene preparations.44 A modification of this flow system utilizes a quartz tube in conjunction with a UV light,46 and a microflow system for cyclooctene photoisomerization has been described.47 Recently, we described a flow photoisomerization system with in-line cooling that can be used to synthesize trans-cycloheptenes.48 Several groups have also described procedures where the flow photoisomerization protocol is mimicked by periodically stopping irradiation, capturing the trans-cyclooctene by filtering through AgNO3 on silica, and resubjecting the filtrate to photoisomerization.49,50 Tomooka has prepared (E)-4-[7]orthocyclophene without flow chemistry by directly adding AgNO3/SiO2 to a photoisomerization reaction in pentane.37
While flow photoisomerization using AgNO3/SiO2 is a reliable method for producing gram quantities of trans-cyclooctenes, leaching of silver (plausibly as the trans-alkene•AgNO3 complex) from the column can be problematic when scaling up syntheses of trans-cyclooctenes, especially for trans-cyclooctenes of high polarity. Herein, we describe a modification for flow photoisomerization that utilizes immobilized Ag(I) on commerical tosic acid silica gel. Throughput is enabled by higher Ag-loadings that can be achieved because the metal is covalently bound to the solid support. The silica gel can be regenerated and reused multiple times without significant change in yield.
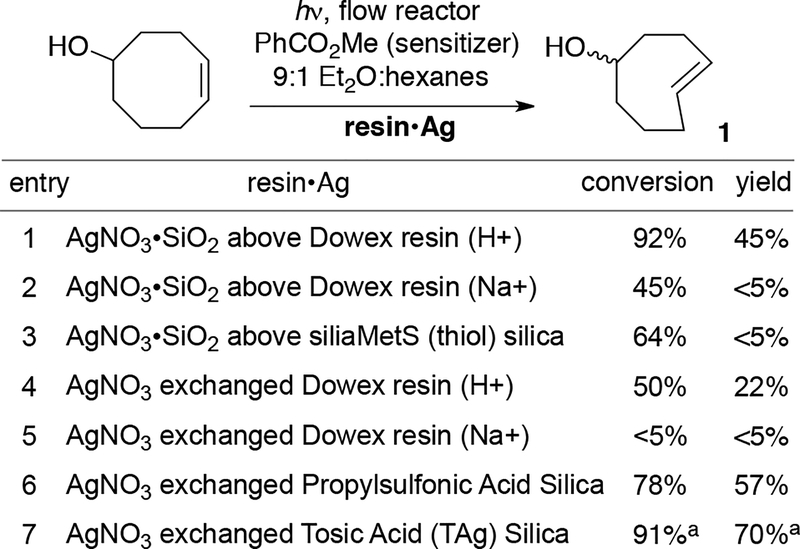
Commercially available Dowex ion exchange resins in acid (H+) and sodium salt (Na+) form were evaluated for their ability to prevent silver leaching from AgNO3/SiO2 (Scheme 2). Here, Dowex was used as a ‘catch’ resin that was layered beneath the AgNO3/SiO2. The acidic form of Dowex gave a moderate result (92% conversion, 45% yield, entry 1) whereas the sodium salt was ineffective (entry 2). Similarly, the use of thiolfunctionalized silica as a catch resin gave only traces of product (entry 3). Attempts to directly exchange Ag ions onto Dowex were also ineffective, leading to only 22% of TCO product with acidic Dowex, and trace product with the sodium salt form (entries 4 and 5). More successful was the use of silver-ion exchanged propylsulfonic acid silica (entry 6) or tosic acid silica gel (entry 7). Both types resulted in good conversions and yields of the trans-cyclooctene. The silver exchanged toluenesulfonic acid cartridge provided the highest yield of TCO and was used for further studies.
Scheme 2.
Screening of Resins for Flow-photoisomerization
All resins were packed in a Biotage Snap Cartridge. For entries 1–3, two resins were packed in the same cartridge. Conversions were calculated by adding dodecane to the reaction mixture followed by GC analysis. a average of 4 runs. See Scheme 3.
Silver nitrate adsorbed onto silica gel has long served as a tool for improving chromatography,51 and sulfonated silica gel that has been subjected to ion exchange with silver (I) has been utilized as a stationary phase for separations of lipids and other fatty acids.52–56 We subjected commercially available tosic acid silica gel (Silicycle, catalog R60530B) to Ag-ion exchange by eluting with 0.5 M AgNO3 in 9:1 acetonitrile:water until it was no longer acidic. The Ag-ion exchanged tosic acid silica gel (TAg silica) was washed sequentially with methanol, acetone, and hexanes, after which it was dried under a stream of compressed air. As shown in Scheme 2, entry 7, TAg silica functioned efficiently as a solid support for the flow-photoisomerization, providing a 70% isolated yield of isomers of 5-hydroxy-trans-cyclooctene 1. As shown in Scheme 2, entry 6, similarly prepared Ag-ion exchanged propylsulfonic acid silica gel was less effective than tosic acid silica.
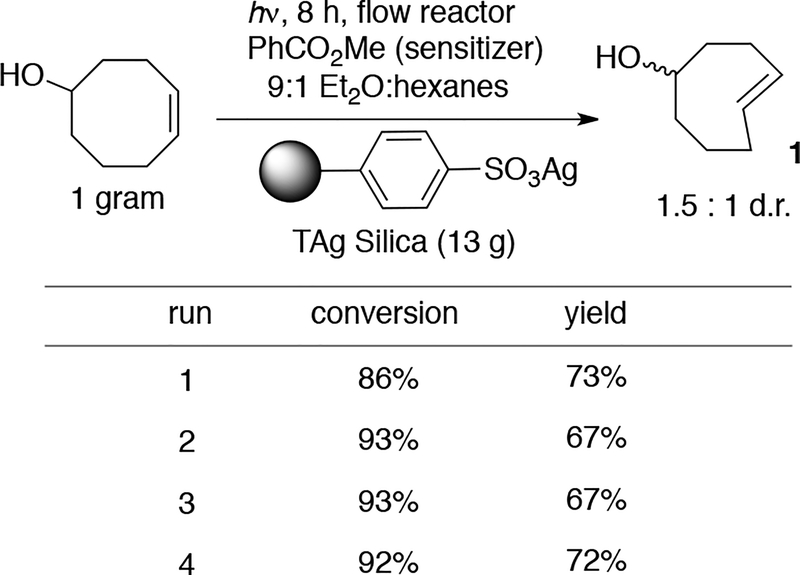
For routine photoisomerizations, 13 grams of TAg silica was loaded into a small (‘10 gram’) Biotage Snap Cartridge that was subsequently wrapped with aluminum foil. Using this cartridge with the experimental setup described in Scheme 1, a 13 mM solution of 5-hydroxy-cis-cyclooctene (1 gram) in 9:1 Et2O:hexanes was continually cycled through this column while irradiated by a Rayonet light source (254 nm). Liberation of the trans-cyclooctene by treatment with NH3-saturated methanol and extractive workup gave 5-hydroxy-trans-cyclooctene in 73% isolated yield and 1.5:1 d.r. (Scheme 3, run 1). No leaching was observed with TAg silica. By contrast, much more stationary phase is required with AgNO3 on silica gel, and leaching is occasionally problematic despite inclusion of a large bed of unmodified silica at the bottom of the cartridge.44
Scheme 3.
Flow photoisomerization requires minimal TAg silica. Reactions run on 1 gram scale were carried out using a ‘10 gram’ cartridge that could be recycled multiple times with similar results.
We demonstrated the ability to reuse the tosic silica gel, which is moderately expensive.57 As shown in Scheme 3, a SNAP cartridge containing 13 grams of tosic silica gel could be recycled without a significant change in yield (68 ± 5%) in four consecutive reactions that each began with 1 gram of 5-hydroxy-cis-cyclooctene. Between each photoreaction, the sulfonated silica gel was eluted with NH3-saturated methanol to release the 5-hydroxy-trans-cyclooctene. The sulfonated silica gel, which remained in the cartridge, was then neutralized, and Ag-ion exchange was carried out as previously described. Lower yields were observed with TAg silica gel that already been used 4 times, with a 63% isolated yield on run 5 and a 53% yield on run 6.
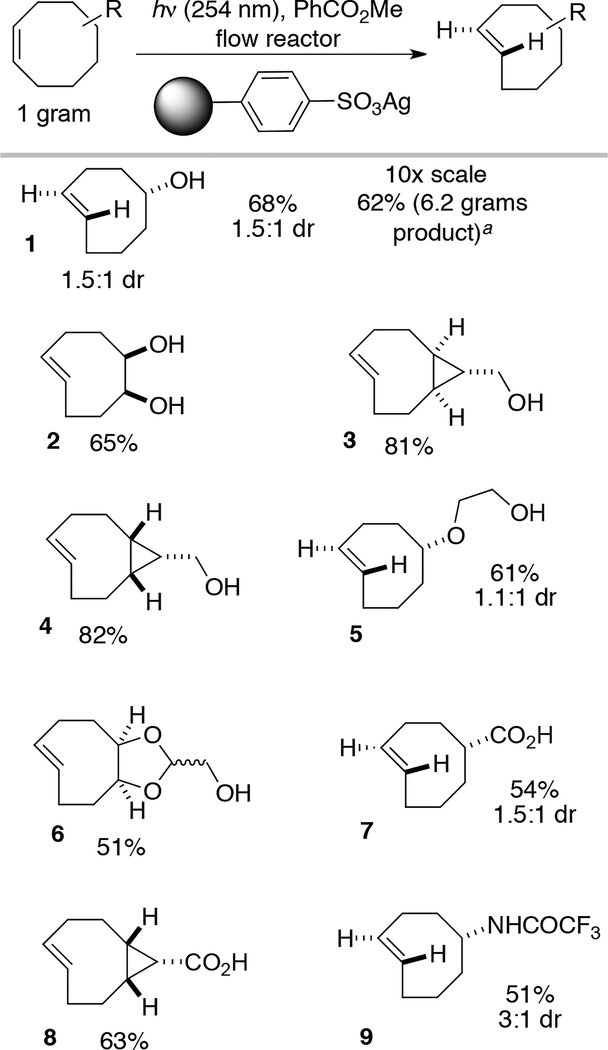
Commerical tosic silica has an average functionality loading of 0.7 mmol/g,58 which enables high silver loadings and thereby allows a reduction in the solvent volume and cartridge size required for photoisomerization. In our initial report that used AgNO3/SiO2, we demonstrated that 5-hydroxy-cis-cycloctene could be photoisomerized to produce 3 grams of 5-hydroxy-trans-cycloctene in a single run.44 However, to minimize leaching we used a large, custom column with a bed of unmodified silica gel at the base, which served as a catch resin for leaching silver. With TAg silica, the photoisomerization could be carried out on the same scale with ~90% less stationary phase than is required with AgNO3 on silica. With 40 grams of tosic silica in a 3″ tall Biotage Snap Cartridge, it was possible to produce 6.2 grams of 5-hydroxy trans-cyclooctene [Scheme 4.]. In this large scale experiment, 1 was eluted and the column recharged with silver at the 6 h midpoint.
Scheme 4.
Substrate scope for silver ion exchanged sulfonated silica gel used in flow photoisomerization. All reactions were conducted starting with 1 gram of cis-cyclooctene starting material using 13 grams of tosic silica gel in a small Biotage SNAP cartridge. Compound 1 was also photoisomerized on larger scale using 40 grams of tosic silica gel in a 3″ tall Biotage SNAP cartridge.
The scope of trans-cyclooctenes produced using TAg silica in the flow-photochemical apparatus is shown in Scheme 4. This modification to the closed-loop flow system was used to synthesize trans-cyclooctenes with a variety of substitution including: hydroxyl, carboxylic acid, hydroxyethylether, diol, as well as protected amines. Compounds 1–6 are known, and are compounds that have been used broadly for bioorthogonal chemistry.44,59,60 Additionally, the new amine and carboxylic acid derivatives 7–10 have been synthesized.
In conclusion, a method for photoisomerization of cyclooctenes is reported in which Ag(I) tosic silica gel is used to capture trans-cyclooctene products at high loadings without leaching, facilitating large scale synthesis using a minimal amount of solid support. The sulfonated silica gel can be regenerated and reused with similar yields over four runs. A variety of trans-cyclooctenes were synthesized, including those commonly utilized in bioorthogonal chemistry as well as new amine and carboxylic acid derivatives.
General Considerations:
All reactions were conducted under N2 in flame-dried glassware. Tetrahydrofuran was distilled under nitrogen from a mixture of sodium and benzophenone. Unstabilized OmniSolv diethyl ether was purchased from MilliporeSigma and dried by passing through a column of activated molecular sieves. Solutions of NH3-saturated methanol were prepared by sparging methanol with gaseous ammonia at 0 °C. Reagents were purchased from Aldrich or Alfa Aesar and used as received. Compounds were chromatographed via silica gel (ICN SiliTech 32–62D, 60Å). High resolution mass spectra were obtained using a Thermo Q-Exactive Orbitrap high resolution mass spectrometer using a heated electrospray (HESI) source, or a Waters GCT Premier using a liquid injection field desorption ionization (LIFDI) source. An APT pulse sequence was used for 13C NMR spectra, where methylene and quaternary carbons appear ‘up’ (u), and methine and methyl carbons appear ‘down’ (dn).
Photoisomerizations were carried out using a Southern New England Ultraviolet Company Rayonet® reactor model RPR-100 or RPR-200, equipped with 8 low-pressure mercury lamps (2537 Å). Photoisomerizations were carried out in a quartz flask (Southern New England Ultraviolet Company). Biotage® SNAP cartridges (Biotage part No. FSK0–1107) were used to house the AgNO3-exchanged sulfonated silica gel. The bottom of the column was interfaced to PTFE tubing (1/8″ OD × 0.063″ ID, flanged with a thermoelectric flanging tool), equipped with flangeless nylon fittings (1/4–28 thread, IDEX part no. P-582), using a female luer (1/4–28 thread, IDEX part no. P-628). The top of the column was interfaced using a male luer (1/4–28 thread, IDEX part no. P-675). The pump used for recirculating solvents through the photolysis apparatus was purchased from Fluid Metering, Inc. (FMI pump model RP-D equipped with pump head FMI R405). Adapters for interfacing the FMI pump to the PTFE tubing were purchased from IDEX (part no. U-510).
Procedures
Ag-ion exchanged tosic acid silica gel (TAg silica)
Sulfonic acid modified silica gel was purchased from Silicycle (0.57 – 0.89 mmol/g, SKU R60530B, particle size: 40–63 μm/ 230–400 mesh, 60 Å, endcapped). Silica gel (100 g) was transferred to a 100 g Biotage SNAP cartridge (Biotage #FSK0–1107) wrapped in aluminum foil. A solution of AgNO3 (0.5M in 9:1 acetonitrile:water) was passed through the column by gravity until pH neutral. The column was washed with 2 × 100 mL volumes each of methanol, acetone, and hexanes, respectively. The silica gel was dried under a stream of air, transferred to an amber bottle wrapped in aluminum foil, and stored for further use.
(Z)-N-(Cyclooct-4-en-1-yl)-2,2,2-trifluoroacetamide
To a solution of (Z)-cyclooct-4-en-1-amine61 (1.20 g, 9.58 mmol) in CH2Cl2 (20 mL) at 0 °C was added triethylamine (4.0 mL, 29 mmol) and trifluoroacetic anhydride (1.47 mL, 10.5 mmol) dropwise. The solution was allowed to stir overnight at rt, after which TLC analysis showed complete conversion of starting material. The solution was poured into water (20 mL) and extracted twice with ethyl acetate. The combined organics were dried over MgSO4, filtered, and concentrated to give an amber solid. Silica gel chromatography (25% ether/hexanes) provided the compound as a white solid (1.49 g, 6.74 mmol, 70% yield). m.p. 73–76 °C. 1H NMR (400 MHz, C6D6) δ: 5.87 (s, 1H), 5.56 – 5.37 (m, 2H), 3.85 – 3.71 (m, 1H), 1.97 – 1.66 (m, 4H), 1.45 – 1.14 (m, 5H), 0.93 – 0.84 (m, 1H). 13C NMR (101 MHz, C6D6) δ: 155.7 (q, JCF = 36.2 Hz, u), 130.7 (dn), 129.4 (dn), 116.7 (q, JCF = 288.4 Hz, u), 50.3 (dn), 34.1 (u), 34.1 (u), 26.1 (u), 25.9 (u), 23.1 (u). 19F NMR (565 MHz, C6D6) δ: 75.8. vmax (CH2CL2/cm−1: 3300, 3093, 3024, 2931, 2854, 1696, 1554, 1207, 1181, 878, 720. HRMS (LIFDI-TOF) [M+] m/z: calcd for C10H14F3NO: 221.1027; found: 221.1042.
(1R,8S,9S,Z)-Bicyclo[6.1.0]non-4-ene-9-carboxylic acid
Sodium hydroxide (7.21 g, 0.18 mol) was dissolved in water (90 mL) and added to a stirring solution of ethyl (1R,8S,9S,Z)-bicyclo[6.1.0]non-4-ene-9-carboxylate62 (3.50 g, 0.018 mol) in ethanol (78 mL). The mixture was stirred for 48 h at rt. The mixture was then diluted with CHCl3 and washed with 1M HCl. The organic layer was separated dried over Na2SO4, filtered, and concentrated into a white solid that was used without further purification (2.48 g, 0.015 mol, 83% yield). Spectral properties matched those from the literature.63 1H NMR (400 MHz, CDCl3) δ: 10.54 (s, 1H), 5.73 – 5.57 (m, 2H), 2.60 – 2.47 (m, 2H), 2.33 – 2.19 (m, 2H), 2.17 – 2.03 (m, 2H), 1.96 – 1.84 (m, 2H), 1.78 (t, J = 8.7 Hz, 1H), 1.59 – 1.46 (m, 2H). 13C NMR (100 MHz, CDCl3) δ: 178.7 (u), 129.5 (dn), 27.0 (u), 25.4 (dn), 22.6 (u), 21.1 (dn).
General Photoisomerization Procedure
A ‘10 g’ Biotage SNAP cartridge wrapped in aluminum foil was used to house the modified silica gel (13.2 g). The (Z)-cyclooctene derivative (0.012–0.024 M in solvent) and methyl benzoate (2.0 equiv.) were dissolved in the indicated solvent in a quartz flask. After sparging the contents with N2 for 15 minutes, the flask was placed in a Rayonet® reactor and connected via PTFE tubing to a column (Biotage® SNAP) and an FMI pump. The column was then packed with silver nitrate exchanged sulfonated silica gel. Any remaining space between the silica and the cartridge cap was filled with cotton. The column was flushed with the reaction solvent, followed by equilibration via circulation with the FMI pump (~100 mL per minute). The solution in the quartz flask was then irradiated at 254 nm under continuous flow for the indicated time. Th SNAP cartridge was washed with additional solvent, and then dried by a stream of compressed air. A solution of NH3-saturated methanol was allowed to pass through the column and the effluent was collected until no more trans-cyclooctene product was detected by TLC. The collected solution was then concentrated by rotary evaporation and the residue was purified by flash silica gel chromatography to obtain pure trans-cyclooctenes.
rel-(1R, 4E, pR)-Cyclooct-4-en-1-ol (eq-1) and rel-(1R, 4E, pS)-Cyclooct-4-en-1-ol (ax-1)
With recycling of TAg Silica.
The general photoisomerization procedure was followed using (Z)-cyclooct-4-en-1-ol (840 mg, 6.65 mmol) in 9:1 ether:hexanes (500 mL) and methyl benzoate (2.06 g, 15.1 mmol), in a 500 mL quartz tube. Dodecane (1.28 g, 7.51 mmol) was added as an internal reference for GC analysis. A 25 g Biotage® SNAP cartridge was filled with TAg silica gel (12.8 g, 11.4 mmol). Irradiation was carried out until no change in conversion could be observed by GC analysis. The tubing was disconnected and the column flushed with ether, then dried by a stream of compressed air. NH3-saturated methanol (100 mL) was passed through the column until no more trans-cyclooctene product could be detected by TLC. Methanol (100 mL) was passed through and the column was dried under a stream of air pressure. The crude residue was purified through a short pad of flash silica gel (100% ether) to provide 616 mg (73%) of a 1.6:1 mixture of eq-1:ax-1 as a pale yellow oil. The diastereomers were separated by silica gel column chromatography (5–20% Et2O/hexanes). Additional column chromatography completely separated the diastereomers. 1H and 13C NMR spectra agreed with previously reported data.44 ax-1: 1H NMR (400 MHz, C6D6) δ 5.37 (m, 1H),5.19 (m, 1H), 3.14 (m, 1H), 2.18–2.04 (m, 3H), 1.80–1.62 (m, 5H), 1.35–1.25 (m, 2H), 0.85 (s, 1H); 13C NMR (100 MHz, C6D6) δ 135.3 (dn), 132.7 (dn), 77.3 (dn), 44.9 (u), 41.3 (u), 34.7 (u), 33.0 (u), 31.6 (u); eq-1: 1H NMR (400 MHz, C6D6) δ 5.62 (m, 1H), 5.40 (m, 1H), 3.71 (m, 1H), 2.40 (m, 1H), 2.18 (m, 1H), 2.02–1.67 (m, 5H), 1.67–1.50 (m, 1H), 1.31 (m, 1H), 0,98 (m, 1H), 0.71 (s, 1H); 13C NMR (100 MHz, C6D6) δ 134.9 (dn), 132.4 (dn), 66.9 (dn), 43.3 (u), 34.7 (u), 34.1 (u), 29.7 (u), 27.8 (u).
The sulfonated silica in the column was reused by washing the column sequentially with methanol, water, and then eluting with 1M HNO3 until the effluent was pH 2. Water was passed through the column to remove excess acid, and then the column was eluted with a 0.5 M solution of silver nitrate (9:1 CH3CN:H2O) until the effluent was no longer acidic. The silica in the column was washed sequentially with methanol, acetone, and hexanes, and then equilibrated to the reaction solvent conditions, and reused for flow-isomerization.
Large Scale Preparation
The general photoisomerization procedure was followed using (Z)-cyclooct-4-en-1-ol (9.96 g, 0.078 mol) in ether (500 mL) and methyl benzoate (21.2 g, 0.155 mol), in a 500 mL quartz tube. Dodecane (13.23 g, 0.078 mol) was added as an internal reference for GC analysis. A 25 g Biotage® SNAP cartridge was filled with TAg silica gel (40.9 g, 0.023 mol). Irradiation was carried out until no change in conversion could be observed by GC analysis (6 h, 30% conversion). The trans-cyclooctene product was isolated and the sulfonated silica was reloaded with silver ions following the procedures described above. The column was reconnected to the flow apparatus and irradiation carried out with the same batch for a second time, until no change in conversion could be observed by GC analysis (12 h, 98% conversion). More trans-cyclooctene product was isolated and combined with the first batch. Column chromatography provided the products as a 1.4:1 mixture of diastereomers (6.20 g, 0.048 mol, 62% yield). 1H and 13C NMR spectra agreed with previously reported data.44
rel-(1R,2S,E)-cyclooct-5-ene-1,2-diol (2)
The general photoisomerization procedure was followed using rel-(1R,2S,Z)-cyclooct-5-ene-1,2-diol (566 mg, 3.98 mmol) in 5% IPA:Et2O (350 mL) and methyl benzoate (1.08 g, 7.96 mmol) in a 500 mL quartz tube. A 10 g Biotage® SNAP cartridge was filled with Ag(I) tosic silica gel (9.15 g, 5.22 mmol, based on 0.57 mmol/g silica). Irradiation was carried out for 16.5 hours. The tubing was disconnected and the column flushed with 10% IPA: Et2O, then dried by a stream of compressed air. A solution of methanolic ammonia (100 mL) was passed through the column by gravity and the solution was concentrated via rotary evaporation. The crude product was purified by silica gel column chromatography (EtOAc) to afford 370 mg (65% yield) of the title compound as a white solid. 1H and 13C NMR spectra agreed with previously reported data.44 1H NMR (400 MHz, CDCl3) δ: 5.77 – 5.64 (m, 1H), 5.50 – 5.37 (m, 1H), 3.97 (s, 1H), 3.69 (m, 1H), 2.44 – 1.96 (m, 6H), 1.82 – 1.61 (m, 3H), 1.56 (s, 1H). 13C NMR (100 MHz, CD3OD) δ: 135.2, 129.1, 78.6, 76.8, 37.8, 36.0, 31.9, 27.6.
rel-((1R, 8S, 9R, 4E)-bicyclo[6.1.0]non-4-en-9-yl)m ethanol (3)
The general photoisomerization procedure was followed using ((1R,8S,9r,Z)-bicyclo[6.1.0]non-4-en-9-yl)methanol64–66 (1.00 g, 6.57 mmol) in 1:1 ether/hexanes (500 mL) and methyl benzoate (1.79 g, 13.1 mmol), in a 500 mL quartz tube. Dodecane (1.12 g, 6.57 mmol) was added as an internal standard. A ‘10 g’ Biotage® SNAP cartridge was filled with silver nitrate exchanged sulfonated silica gel (14.49 g, 9.85 mmol) and irradiation carried out for 4 h, after which GC analysis complete conversion. The tubing was disconnected and the column was flushed with additional solvent (1:1 ether/hexanes), dried by a stream of compressed air, followed by treatment with NH3-saturated methanol (150 mL). After concentration of the solution, the residue was purified by flash column chromatography using silica gel (10–40% ether/hexanes as eluent) to afford the product as an oil (0.805g, 5.29 mmol, 81% yield). 1H and 13C NMR spectra agreed with previously reported data.67 1H NMR (600 MHz, CD3OD) δ: 5.93 – 5.85 (m, 1H), 5.15 (ddd, J = 15.7, 10.5, 3.9 Hz, 1H), 3.50 – 3.41 (m, 2H), 2.44 – 2.36 (m, 1H), 2.34 – 2.17 (m, 3H), 1.98 – 1.90 (m, 2H), 0.98 – 0.87 (m, 1H), 0.70 – 0.59 (m, 1H), 0.55 – 0.46 (m, 1H), 0.42 – 0.32 (m, 2H). vmax (film)/cm−1: 3330, 2024, 2854, 1638, 1445, 1059.
rel-((1R, 8S, 9S, 4E)-bicyclo[6.1.0]non-4-en-9-yl)methanol (4)
The general photoisomerization procedure was followed using ((1R,8S,9s,Z)-bicyclo[6.1.0]non-4-en-9-yl)methanol (1.00 g, 6.57 mmol) in 1:1 ether/hexanes (500 mL) and methyl benzoate (1.79 g, 13.1 mmol), in a 500 mL quartz tube. Dodecane (1.12 g, 6.57 mmol) was added as an internal standard. A ‘10 g’ Biotage® SNAP cartridge was filled with silver nitrate exchanged sulfonated silica gel (14.49 g, 9.85 mmol) and irradiation carried out for 8 h, after which GC analysis showed complete conversion. The tubing was disconnected and the column was flushed with additional solvent (1:1 ether/hexanes), dried by a stream of compressed air, followed by treatment with ammonium hydroxide (30% aqueous). The aqueous solution was extracted with twice with CH2Cl2. The combined organics were washed with water, dried over Na2SO4, filtered, and concentrated to afford the crude product as a pale yellow oil (0.820 g, 5.38 mmol, 82% yield). 1H and 13C NMR spectra agreed with previously reported data.60 1H NMR (400 MHz, MeOD) δ 5.94 – 5.81 (m, 1H), 5.23 – 5.10 (m, 1H), 3.50 (d, J = 7.7 Hz, 2H), 2.36 – 2.05 (m, 4H), 2.00 – 1.82 (m, 2H), 1.27 – 1.00 (m, 2H), 0.90 – 0.67 (m, 2H), 0.66 – 0.53 (m, 1H). 13C NMR (100 MHz, C6D6) δ 138.5 (dn), 131.4 (dn), 59.4 (u), 34.5 (u), 34.2 (u), 27.78 (u), 27.76 (u), 21.5 (dn), 19.4 (dn), 18.3 (dn).
rel-(1R, 4E, pR) −2-(Cyclooct-4-en-1-yloxy)ethan-1-ol (eq-5) and rel-(1R, 4E, pS)-2-(Cyclooct-4-en-1-yloxy)ethan-1-ol (ax-5)
The general photoisomerization procedure was followed using (Z)-2-(cyclooct-4-en-1-yloxy)ethan-1-ol59 (1.08 g, 6.33 mmol) in 7:3 ether:hexanes (500 mL) and methyl benzoate (1.73 g, 12.7 mmol) in a 500 mL quartz tube. Dodecane (1.08 g, 6.36 mmol) was added as an internal standard. A ‘10 g’ Biotage® SNAP cartridge was filled with silver nitrate exchanged sulfonated silica gel (16.7 g, 9.51 mmol). Irradiation was carried out for 7 h, after which GC analysis showed 85% conversion. The tubing was disconnected and the column flushed with ether, then dried by a stream of compressed air. The silica gel was poured into a flask and stirred with 30% ammonium hydroxide (100 mL) for approximately 15 minutes. CH2G2 (100 mL) was added and the solution was stirred an additional 10 minutes. The solution was filtered and the filtrate was extracted with 3 × CH2Cl2. The combined organic layers were dried over MgSO4, filtered, and concentrated via rotary evaporation. The crude oil was purified by flash silica gel chromatography (15% ether in hexanes) to provide a 1.4:1 mixture of eq-5:ax-5 as a pale yellow oil (0.662 g, 3.89 mmol, 61% yield). Additional column chromatography completely separated the diastereomers. 1H and 13C NMR spectra agreed with previously reported data.59
Ax-5:
1H NMR (400 MHz, C6D6) δ: 5.44 – 5.32 (m, 1H), 5.24 – 5.12 (m, 1H), 3.62 – 3.48 (m, 2H), 3.31 – 3.16 (m, 1H), 3.16 – 3.06 (m, 1H), 2.80 (dd, J = 10.6, 5.2 Hz, 1H), 2.38 – 2.25 (m, 1H), 2.25 – 2.12 (m, 2H), 2.12 – 1.97 (m, 2H), 1.88 – 1.61 (m, 4H), 1.41 – 1.15 (m, 2H).). 13C NMR (100 MHz, C6D6) δ: 135.5 (dn), 132.3 (dn), 86.1 (dn), 69.7 (u), 62.2 (u), 41.2 (u), 38.0 (u), 34.8 (u), 33.3 (u), 31.9 (u).
Eq-5:
1H NMR (400 MHz, C6D6) δ: 5.78 – 5.64 (m, 1H), 5.47 – 5.34 (m, 1H), 3.58 – 3.46 (m, 2H), 3.25 (m, 1H), 3.21 – 3.11 (m, 1H), 3.11 – 3.01 (m, 1H), 2.35 (m, 1H), 2.26 – 2.18 (m, 1H), 2.11 – 2.01 (m, 1H), 1.97 – 1.85 (m, 2H), 1.85 – 1.71 (m, 3H), 1.63 – 1.53 (m, 1H), 1.23 – 1.12 (m, 1H), 0.97 – 0.87 (m, 1H). 13C NMR (100 MHz, C6D6) δ: 136.0 (dn), 131.4 (dn), 75.0 (dn), 70.2 (u), 62.1 (u), 40.3 (u), 34.9 (u), 33.2 (u), 30.2 (u), 27.9 (u).
((2s,3aR,9aS,E)-3a,4,5,8,9,9a-Hexahydrocycloocta[d][1,3]dioxol-2-yl)m ethanol (6)
The general photoisomerization procedure was followed using ((2s,3aR,9aS,Z)-3a,4,5,8,9,9a-hexahydrocycloocta[d][1,3]dioxol-2-yl)methanol18 (syn/anti = 1.1:1) (1.11 g, 6.02 mmol) in 1:1 ether/hexanes (450 mL) and methyl benzoate (1.64 g, 12.1 mmol), in a 500 mL quartz tube. A ‘10 g’ Biotage® SNAP cartridge was filled with silver nitrate exchanged sulfonated silica gel (13.29 g, 9.04 mmol) and irradiation carried out for 12 h. The tubing was disconnected and the column was flushed with ether, dried by a stream of compressed air, followed by treatment with NH3-saturated methanol (100 mL). After concentration of the solution, the residue was purified by passing through a short pad of flash silica gel (50% ether/hexanes as eluent). The fractions were collected and concentrated to afford the product as a white solid (0.571 g, 3.10 mmol, 51% yield). 1H and 13C NMR spectra agreed with previously reported data.18 1H NMR (400 MHz, CDCl3) δ: 5.70 – 5.45 (m, 4H), 5.12 (t, J = 3.6 Hz, 1H), 4.86 (t, J =3.0 Hz, 1H), 4.15 – 3.89 (m, 4H), 3.72 – 3.62 (m, 2H), 3.61 – 3.52 (m, 2H), 2.48 – 2.07 (m, 8H), 1.96 – 1.48 (m, 10H). 13C NMR (C6D6, 100 MHz) δ: 136.3 (dn), 136.1 (dn), 131.3 (dn), 131.1 (dn), 101.5 (dn), 83.6 (dn), 82.8 (dn), 80.7 (dn), 79.2 (dn), 64.2 (u), 63.7(u), 39.6 (u), 39.0 (u), 34.0 (u), 32.3 (u), 31.9 (u), 31.5 (u), 25.8 (u), 25.0 (u).
rel-(1R, 4E, pR) -cyclooct-4-ene-1-carboxylic acid (eq-7) and rel-(1R, 4E, pS) -cyclooct-4-ene-1-carboxylic acid (ax-7)
The general photoisomerization procedure was followed using (Z)-cyclooct-4-ene-1-carboxylic acid68 (999.0 mg, 6.403 mmol) in ether (500 mL) and methyl benzoate (1.750 g, 12.85 mmol), in a 500 mL quartz tube. A 25 g Biotage® SNAP cartridge was filled with silver nitrate exchanged sulfonated silica gel (17.17 g, 9.787 mmol). Irradiation was carried out for 4.5 h, after which the tubing was disconnected and the column flushed with ether, then dried by a stream of compressed air. The silica was placed in a flask and stirred with brine (100 mL) and CH2G2 (100 mL) for approximately 15 minutes. The solution was filtered and extracted with CH2Cl2. The organic layer was dried over MgSO4, filtered, and concentrated. The crude product was purified by flash silica gel chromatography (0–20% ether in hexanes) to provide a 1.5:1 mixture of eq-7:ax-7 (536 mg, 3.43 mmol, 54% yield). Analytically pure samples were obtained by additional chromatography.
Axial diastereomer:
m.p. 68–70 °C. 1H NMR (400 MHz, C6D6) δ: 12.53 (bs, 0.1H), 5.71 – 5.50 (m, 1H), 5.50 – 5.32 (m, 1H), 2.90 (qd, J = 11.8, 4.7 Hz, 1H), 2.60 – 2.41 (m, 1H), 2.23 – 1.91 (m, 5H), 1.82 – 1.63 (m, 2H), 1.33 – 1.11 (m, 1H), 0.95 – 0.74 (m, 1H). 13C NMR (151 MHz, C6D6) δ: 182.3 (u), 134.48 (dn), 134.43 (dn), 41.2 (dn), 38.4 (u), 34.5 (u), 31.6 (u), 31.1 (u), 30.8 (u). vmax (CH2CL2/cm−1: 2926, 2860, 1699, 1441, 1237, 1219, 988. HRMS (LIFDI-TOF) [M+] m/z: calcd for C9H14O2: 154.0994; found: 154.0967.
Equatorial diastereomer :
m.p. 81–84 °C. 1H NMR(400 MHz, C6D6) δ: 12.00 (bs, 0.44H), 5.31 – 5.11 (m, 2H) 2.21 – 2.04 (m, 4H), 1.95 – 1.51 (m, 5H), 1.33 – 1.17 (m, 1H), 1.01 – 0.87 (m, 1H). 13C NMR(101 MHz, C6D6) δ: 185.7 (u), 133.97 (dn), 133.95 (dn), 41.2 (dn), 38.4 (u), 34.5 (u), 31.6 (u), 31.1 (u), 30.8 (u). . vmax (CH2Cl2)/cm−1: 2928, 2855, 1701, 1225, 1215, 1191, 991. HRMS (LIFDI-TOF) [M+] m/z: calcd for C9H14O2: 154.0994; found: 154.0968.
rel-(1R, 8S, 9S, 4E)-bicyclo[6.1.0]non-4-ene-9-carboxylic acid (8)
The general photoisomerization procedure was followed using (1R,8S,9s,Z)-bicyclo[6.1.0]non-4-ene-9-carboxylic acid (1.01 g, 6.09 mmol) in 50% ether:hexanes (500 mL) and methyl benzoate (1.66 g, 12.2 mmol), in a 500 mL quartz tube. A 25 g Biotage® SNAP cartridge was filled with silver nitrate exchanged sulfonated silica gel (13.78 g,12.26 mmol). Irradiation was carried out for 15 h, after which GC analysis showed complete conversion. The tubing was disconnected and the column was flushed with ether and dried by a stream of compressed air. The silica was placed in a flask and stirred with brine (100 mL) and CH2Cl2 (100 mL) for approximately 15 minutes. The solution was filtered and extracted with CH2Cl2. The organic layer was dried over MgSO4, filtered, and concentrated. The crude product was purified by flash silica gel chromatography (5% ether in hexanes) to provide the product as a white solid (0.634 g, 3.81 mmol, 63% yield). m.p. (°C): 124–127. 1H NMR (400 MHz, C6D6) δ: 12.57 (bs, 1H), 5.55 (ddd, J = 15.8, 9.2, 6.0 Hz, 1H), 5.16 – 5.05 (m, 1H), 2.23 – 2.12 (m, 2H), 1.80 – 1.70 (m, 4H), 1.70 – 1.59 (m, 2H), 1.57 – 1.44 (m, 1H), 0.66 – 0.55 (m, 1H), 0.55 – 0.44 (m, 1H). 13C NMR (101 MHz, C6D6) δ: 179.8 (u), 137.5 (dn), 132.3 (dn), 33.7 (u), 33.0 (u), 27.1 (u), 25.87 (dn), 25.85 (u), 24.8 (dn), 21.0 (dn). vmax (CH2CL2/cm−1: 3344, 3002, 2922, 2856, 1688, 1443, 1234, 1200, 1181. HRMS (LIFDI-TOF) [M+] m/z: calcd for C10H14O2: 166.0994; found: 166.0983.
rel-(1R, 4E, pR)-N-(Cyclooct-4-en-1-yl)-2,2,2-trifluoroacetamide (eq-9) and rel-(1R, 4E, pS)-N-(Cyclooct-4-en-1-yl)-2,2,2-trifluoroacetam ide (ax-9)
The general photoisomerization procedure was followed using (Z)-N-(cyclooct-4-en-1-yl)-2,2,2-trifluoroacetamide (1.01 g, 4.57 mmol) in 4:1 ether/hexanes (400 mL) and methyl benzoate (1.24 g, 9.13 mmol), in a 500 mL quartz tube. A 25 g Biotage® SNAP cartridge was filled with silver nitrate exchanged sulfonated silica gel (24.13 g, 13.75 mmol) and irradiation carried out for 8.5 h, after which GC analysis showed 82% conversion. The tubing was disconnected and the column was flushed with additional ether, dried by a stream of compressed air, followed by treatment with NH3-saturated methanol (100 mL). After concentration of the solution, the residue was purified by flash column chromatography using C2-silica gel69 (15% ether/hexanes) to afford a white solid as a 3:1 mixture of eq-9:ax-9 (0.514 g, 2.32 mmol, 51%). Analytically pure samples were obtained by additional chromatography.
Axial diastereomer:
m.p. 65–66 °C 1H NMR (400 MHz, C6D6) δ: 5.92 (br s, 1H), 5.17 – 5.10 (m, 1H), 5.03 – 4.95 (m, 1H), 4.04 (m, 1H), 1.89 – 1.82 (m, 2H), 1.76 – 1.66 (m, 2H), 1.66 – 1.38 (m, 3H), 1.19 – 0.99 (m, 2H), 0.68 – 0.61 (m, 1H). 13C NMR. (101 MHz, C6D6) δ: 155.9 (q, JCF = 36.1 Hz), 133.9 (dn), 133.4 (dn), 116.8 (JCF = 288.8 Hz), 45.8 (dn), 40.2 (u), 34.0 (u), 31.3 (u), 29.8 (u), 29.1 (u). 19F NMR (565 MHz, C6D6) δ: 75.7. vmax (CH2Cl2)/cm−1 3344, 2947, 2919, 1711, 1689, 1200, 1181, 1157. HRMS (LIFDI-TOF) [M+] m/z calcd for C10H14F3NO: 221.1027; found: 221.1028.
Equatorial diastereomer:
m.p. 80–81 °C. 1H NMR (400 MHz, C6D6) δ: 5.51 (br s, 1H), 5.29 – 5.14 (m, 2H), 3.21 (s, 1H), 2.17 – 2.09 (m, 1H), 2.05 – 1.86 (m, 2H), 1.75 – 1.42 (m, 3H), 1.35 – 1.14 (m, 2H), 1.10 – 0.91 (m, 2H). 13C NMR. (101 MHz, C6D6) δ: 155.4 (q, JCF = 36.0 Hz), 134.0 (dn), 133.7 (dn), 116.7 (q, JCF = 288.3 Hz), 54.5 (dn), 41.2 (u), 39.0 (u), 34.7 (u), 33.4 (u), 32.2 (u). 19F NMR (565 MHz, C6D6) δ: 75.7. vmax (CH2Cl2)/cm−1 3284, 2937, 1718, 1695, 1209, 1184, 991 HRMS (LIFDI-TOF) [M+] m/z calcd for C10H14F3NO: 221.1027; found 221.1045.
Supplementary Material
Acknowledgments
We thank Sri Chintala and William Lambert for experimental and spectroscopic assistance.
Funding Information This work was supported by NIH R01EB014354, R01DC014461, and NSF DMR-1506613. Spectra were obtained with instrumentation supported by NIH grants P20GM104316, P30GM110758, S10RR026962, S10OD016267 and NSF grants CHE-0840401, CHE-1229234, and CHE-1048367.
Footnotes
Supporting Information Copies of 1H NMR and 13C NMR spectra are provided.
References
- (1).Sletten EM; Bertozzi CR Angew. Chem. Int. Ed 2009, 48, 6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Patterson DM; Nazarova LA; Prescher JA ACS Chem. Biol 2014, 9, 592. [DOI] [PubMed] [Google Scholar]
- (3).Lang K; Chin JW ACS Chem. Biol 2014, 9, 16. [DOI] [PubMed] [Google Scholar]
- (4).McKay CS; Finn MG Chem. Biol 2014, 21, 1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ramil CP; Lin Q Chem. Commun 2013, 49, 11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).MacKenzie DA; Sherratt AR; Chigrinova M; Cheung LLW; Pezacki JP Curr. Opin. Chem. Biol 2014, 21, 81. [DOI] [PubMed] [Google Scholar]
- (7).Rossin R; Robillard MS Curr. Opin. Chem. Biol 2014, 21, 161. [DOI] [PubMed] [Google Scholar]
- (8).Meyer J-P; Adumeau P; Lewis JS; Zeglis BM Bioconjug. Chem 2016, acs. bioconjchem.6b00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Cycloadditions in Bioorthogonal Chemistry; Vrabel M; Carell T, Eds.; 2016. [DOI] [PubMed] [Google Scholar]
- (10).Nikić I; Lemke EA Curr. Opin. Chem. Biol 2015, 28, 164. [DOI] [PubMed] [Google Scholar]
- (11).Lang K; Chin JW Chem. Rev 2014, 114, 4764. [DOI] [PubMed] [Google Scholar]
- (12).Blackman ML; Royzen M; Fox JM J. Am. Chem. Soc 2008, 130, 13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Devaraj NK; Weissleder R; Hilderbrand SA Bioconjug. Chem 2008, 19, 2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Devaraj NK; Weissleder R Acc. Chem. Res 2011, 44, 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Wu H; Devaraj NK Top. Curr. Chem 2015, 374, 3. [DOI] [PubMed] [Google Scholar]
- (16).Selvaraj R; Fox JM Curr. Opin. Chem. Biol 2013, 17, 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Knall A-C; Slugovc C Chem. Soc. Rev 2013, 42, 5131. [DOI] [PubMed] [Google Scholar]
- (18).Darko A; Wallace S; Dmitrenko O; Machovina M; Mehl R; Chin JW; Fox J Chem. Sci 2014, 0, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Cope AC J. Am. Chem. Soc 1962, 84, 3191. [Google Scholar]
- (20).Hines JN; Peagram MJ; Thomas EJ; Whitham GH J. Chem. Soc. Perkin Trans 1 1973, 2332. [Google Scholar]
- (21).Vedejs E; Snoble KAJ; Fuchs PL J. Org. Chem 1973,38, 1178. [Google Scholar]
- (22).Corey EJ; Shulman JI Tetrahedron Lett 1968, 33, 3655. [Google Scholar]
- (23).Reese A, C. B. S J. Am. Chem. Soc 1970, 92, 2566. [Google Scholar]
- (24).Braddock DC; Cansell G; Hermitage SA; White AJP Tetrahedron-Asymmetry 2004, 15, 3123. [Google Scholar]
- (25).Whitham GH; Wright MJ Chem. Soc 1971, 886. [Google Scholar]
- (26).Shea KJ; Kim JS J. Am. Chem. Soc 1992, 114, 4846. [Google Scholar]
- (27).Jendralla H Chem. Ber 1982, 115, 201. [Google Scholar]
- (28).Jendralla H Tetrahedron 1983, 39, 1359. [Google Scholar]
- (29).Kozma E; Nikić I; Varga BR; Aramburu IV; Kang JH; Fackler OT; Lemke EA; Kele P ChemBioChem 2016, 17, 1518. [DOI] [PubMed] [Google Scholar]
- (30).Prévost M; Woerpel KA J. Am. Chem. Soc 2009, 131, 14182. [DOI] [PubMed] [Google Scholar]
- (31).Prévost M; Ziller JW; Woerpel KA Dalt. Trans 2010, 39, 9275. [DOI] [PubMed] [Google Scholar]
- (32).Hurlocker B; Hu C; Woerpel KA Angew. Chem. Int. Ed 2015, 54, 4295. [DOI] [PubMed] [Google Scholar]
- (33).Santucci J; Sanzone JR; Woerpel KA Eur. J. Org. Chem 2016, 2016, 2933. [Google Scholar]
- (34).Sanzone JR; Woerpel KA Angew. Chem. Int. Ed 2016, 55, 790. [DOI] [PubMed] [Google Scholar]
- (35).Tomooka K; Uehara K; Nishikawa R; Suzuki M; Igawa KJ Am. Chem. Soc 2010, 132, 9232. [DOI] [PubMed] [Google Scholar]
- (36).Igawa K; Ichikawa N; Ano Y; Katanoda K; Ito M; Akiyama T; Tomooka KJ Am. Chem. Soc 2015, 137, 7294. [DOI] [PubMed] [Google Scholar]
- (37).Igawa K; Machida K; Noguchi K; Uehara K; Tomooka KJ Org. Chem 2016, 81, 11587. [DOI] [PubMed] [Google Scholar]
- (38).Tomooka K; Komine N; Fujiki D; Nakai T; Yanagitsuru SJ Am. Chem. Soc 2005, 127, 12182. [DOI] [PubMed] [Google Scholar]
- (39).Miura T; Nakamuro T; Liang C-J; Murakami MJ Am. Chem. Soc 2014, 136, 15905. [DOI] [PubMed] [Google Scholar]
- (40).Wang X-N; Krenske EH; Johnston RC; Houk KN; Hsung RP J. Am. Chem. Soc 2015, 137, 5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Wang X-N; Krenske EH; Johnston RC; Houk KN; Hsung RP J. Am. Chem. Soc 2014, 136, 9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Arichi N; Yamada K-I; Yamaoka Y; Takasu KJ Am. Chem. Soc 2015, 137, 9579. [DOI] [PubMed] [Google Scholar]
- (43).Inoue Y; Mori T In CRC Handbook of Organic Photochemistry and Photobiology; Lenci F; Horspool W, Eds.; Boca Raton, Fl, 2003; pp. 1–16. [Google Scholar]
- (44).Royzen M; Yap GPA; Fox JM J. Am. Chem. Soc 2008, 130, 3760. [DOI] [PubMed] [Google Scholar]
- (45).Royzen M; Taylor MT; Deangelis A; Fox JM Chem. Sci 2011, 2, 2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Svatunek D; Denk C; Rosecker V; Sohr B; Hametner C; Allmaier G; Fröhlich J; Mikula H Monatsh. Chem 2016, 147, 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Billaud EMF; Shahbazali E; Ahamed M; Cleeren F; Noël T; Koole M; Verbruggen A; Hessel V; Bormans G Chem. Sci 2017, 8, 1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Fox J; Fang Y; Zhang H; Huang Z; Scinto S; Yang J; Ende C. W. am; Dmitrenko O; Johnson DS Chem. Sci 2018, 7, 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Devaraj NK; Upadhyay R; Haun JB; Hilderbrand SA; Weissleder R Angew. Chem. Int. Ed 2009, 48, 7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Schoch J; Staudt M; Samanta A; Wiessler M; Jäschke A Bioconjug. Chem 2012, 23, 1382. [DOI] [PubMed] [Google Scholar]
- (51).Mander LN; Williams CM Tetrahedron 2016, 72, 1133. [Google Scholar]
- (52).Christie WW J. Chromatogr. A 1988, 454, 273. [DOI] [PubMed] [Google Scholar]
- (53).Christie WW J. Lipid Res 1989, 30, 1471. [PubMed] [Google Scholar]
- (54).Christie WW J. Sci. Food Agric 1990, 52, 573. [Google Scholar]
- (55).Christie WW J. High Resolut. Chromatogr. Chromatogr. Commun 1987, 10, 148. [Google Scholar]
- (56).Momchilova S; Nikolova-Damyanova BJ Sep. Sci 2003, 26, 261. [Google Scholar]
- (57).The current cost of SiliaBond® Tosic Acid (SCX) silica gel, R60530B, is $3–5/gram, dependent upon quantity purchased.
- (58).For tosic silica gel, the loading of the tosic functional group is lot dependent ranging from 0.57 – 0.89 mmol/g.
- (59).Li Z; Cai H; Hassink M; Blackman ML; Brown RCD; Conti PS; Fox JM Chem. Commun 2010, 46, 8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Wang M; Svatunek D; Rohlfing K; Liu Y; Wang H; Giglio B; Yuan H; Wu Z; Li Z; Fox JM Theranostics 2016, 6, 887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Oh C; Kim K; Ham W Tetrahedron Lett 1998, 39, 2133. [Google Scholar]
- (62).O’Brien J; Chintala S; Fox JM J. Org. Chem 2018, DOI: 10.1021/acs.joc.7b02329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Dehmlow EV; Plückebaum OJ Prakt. Chem./Chem.-Zeit 1996, 338, 303. [Google Scholar]
- (64).Anciaux AJ; Hubert AJ; Noels AF; Petiniot N; Teyssie PJ Org. Chem 1980, 45, 695. [Google Scholar]
- (65).Ast W; Rheinwald G; Kerber R Die Makromol. Chemie 1976, 177, 1349. [Google Scholar]
- (66).Dommerholt J; Schmidt S; Temming R; Hendriks LJA; Rutjes FPJT; van Hest JCM; Lefeber DJ; Friedl P; van Delft FL Angew. Chem. Int. Ed. Engl 2010, 49, 9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Taylor MT; Blackman ML; Dmitrenko O; Fox JM J. Am. Chem. Soc 2011, 133, 9646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Bloodworth AJ; Melvin T; Mitchell JC J. Org. Chem 1988, 53, 1078. [Google Scholar]
- (69).Panne P; Fox JM J. Am. Chem. Soc 2007, 129, 22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.