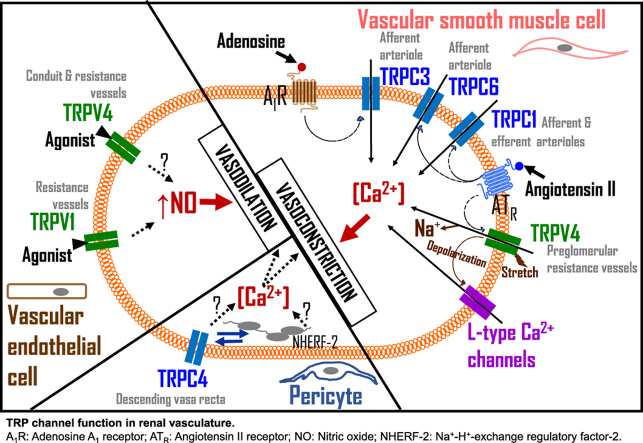
Abstract
Members of the transient receptor potential (TRP) channels that are expressed in the kidney have gained prominence in recent years following discoveries of their role in maintaining the integrity of the filtration barrier, regulating tubular reabsorption of Ca2+ and Mg2+, and sensing osmotic stimuli. Furthermore, evidence has linked mutations in TRP channels to kidney disease pathophysiological mechanisms, including focal segmental glomerulosclerosis, disturbances in Mg2+ homeostasis, and polycystic kidney disease. Several subtypes of TRP channels are expressed in the renal vasculature, from preglomerular arteries and arterioles to the descending vasa recta. Although investigations on the physiological and pathological significance of renal vascular TRP channels are sparse, studies on isolated vessels and cells have suggested their involvement in renal vasoregulation. Renal blood flow (RBF) is an essential determinant of kidney function, including glomerular filtration, water and solute reabsorption, and waste product excretion. Functional alterations in ion channels that are expressed in the endothelium and smooth muscle of renal vessels can modulate renal vascular resistance, arterial pressure, and RBF. Hence, renal vascular TRP channels are potential therapeutic targets for the treatment of kidney disease. This review summarizes the current knowledge of TRP channel expression in renal vasculature and their role in controlling kidney function in health and disease.
Keywords: TRP channels, Kidney, Renal blood flow, Renal vasculature
Graphical abstract
Highlights
-
•
TRP channels are widely distributed in mammalian kidneys in glomerular, tubular, and vascular cells.
-
•
TRPC and TRPV channels are functionally expressed in afferent arterioles.
-
•
TRPC4 may regulate Ca2+ signaling in the descending vasa recta.
-
•
Smooth muscle, endothelial, and pericyte TRP channels may participate in signal transduction mechanisms.
-
•
TRP channels underlie renal autoregulation and regional kidney perfusion in health and disease.
1. Introduction
The mammalian transient receptor potential (TRP) channels constitute a diverse superfamily of six-transmembrane, cation-selective ion channels with six major families, namely, TRPA (Ankyrin), TRPC (Canonical), TRPM (Melastatin), TRPV (Vanilloid), TRPP (Polycystin), and TRPML (Mucolipin). TRP channels are widely expressed in mammalian tissues and are activated by a myriad of physiological stimuli, including temperature, mechanical stress, intracellular and extracellular ligands, and redox signaling molecules (Clapham, 2003; Montell, 2005; Pedersen et al., 2005).
Like their nearly ubiquitous presence throughout the body, the TRP channels are widely distributed in mammalian kidneys in glomerular, tubular, and vascular cells (Carlstrom et al., 2015; Chubanov et al., 2017; Tomilin et al., 2016). Glomerular mesangial cells (GMCs) express TRPC channels and modulate cell surface area, proliferation, and survival (Adebiyi, 2014; Graham et al., 2007; Kong et al., 2015; Meng et al., 2014; Soni and Adebiyi, 2016, 2017; Sours et al., 2006; Wang et al., 2004). TRPP1 co-assemble with TRPC1 and TRPC4 and the resultant channel complexes mediate angiotensin II (AngII)-induced Ca2+ influx in human GMCs (Du et al., 2008). TRPC5- and TRPC6-mediated Ca2+ signal transduction mechanisms regulate podocytes' structure and function (Dryer and Reiser, 2010; Greka and Mundel, 2011, 2012; Staruschenko et al., 2019; Wieder and Greka, 2016). Gain-of-function mutations in TRPC6 result in hereditary glomerulopathy, including focal segmental glomerulosclerosis (Reiser et al., 2005; Winn et al., 2005). Recent studies have also proposed that TRPC5 channels contribute to podocyte derangement in animal models of progressive kidney disease (Schaldecker et al., 2013; Zhou et al., 2017).
TRP channels have been identified in different segments of the nephron. TRPC3 is abundantly expressed in the collecting ducts, colocalizes with aquaporin-2, and trafficked to the apical membrane in response to antidiuretic hormone (Goel et al., 2007, 2010). TRPC3 is a putative candidate for Ca2+ reabsorption and osmoregulation in the collecting ducts (Khayyat et al., 2020). Immunofluorescence indicated that TRPM2 is expressed in mouse proximal tubular epithelial cells (Gao et al., 2014). Pharmacological inhibition and genetic ablation of TRPM2 in mice ameliorated NADPH oxidase-dependent acute kidney injury (AKI) (Gao et al., 2014). TRPM3 is expressed in tubular epithelial cells and may contribute to Ca2+ homeostasis (Lee et al., 2003). TRPM6/7 control magnesium absorption in the distal convoluted tubule (Schlingmann and Gudermann, 2005). TRPP1 (polycystin-2), co-assembles with polycystin-1 (PC1) to form functional cation channels (Hanaoka et al., 2000; Koulen et al., 2002; Mochizuki et al., 1996). PC1 and TRPP1 are required for kidney development (Chauvet et al., 2002; van Adelsberg, 1999). Mutations of PKD1 and PKD2, the genes that encode PC1 and TRPP1, respectively, result in the progressive formation of numerous cysts in the kidney, common in autosomal dominant polycystic kidney disease (ADPKD) (Wilson, 2004). TRPP1 was detected in rat distal convoluted tubules, cortical collecting ducts, proximal tubules, and thick ascending limbs (Zhao et al., 2002). TRPP1 expression levels were increased in all segments of post-ischemic rat nephrons, suggesting that it may contribute to the mechanisms that underpin ischemic AKI (Zhao et al., 2002). Although TRPV1 has been implicated mainly in renal sensory responses, the channels are also expressed in the distal tubule apical membrane and collecting ducts (Feng et al., 2008). TRPV1 colocalizes with α-epithelial sodium channels (αENaC) in mouse cortical collecting ducts (Li et al., 2014). Capsaicin-induced activation of TRPV1 diminished αENaC-mediated sodium reabsorption (Li et al., 2014), but whether this effect alters blood volume and pressure is unclear. TRPV4 channels are abundant in the thin and thick ascending limb of the Loop of Henle and the distal convoluted and connecting tubules of adult mice and rats (Cohen, 2007; Tian et al., 2004). TRPV4-mediated osmoregulation in the kidney has been proposed (Cohen, 2007; Tian et al., 2004). Both TRPV5 and TRPV6 channels are expressed in the epithelial cells of the distal convoluted and connecting tubules (Hoenderop et al., 2001; Nijenhuis et al., 2003), where they control Ca2+ reabsorption (Bianco et al., 2007; Hoenderop et al., 2003; Nie et al., 2016; Peng et al., 2018).
Focused reviews on vascular TRP channel function are available (Di and Malik, 2010; Dietrich and Gudermann, 2011; Dietrich et al., 2010; Earley and Brayden, 2015; Jardín et al., 2013; Zholos and Curtis, 2013). However, despite extensive work in the cerebral, pulmonary, and mesenteric blood vessels, only a handful of studies have investigated TRP channels in the renal vascular bed. To the best of our knowledge, no review has yet been published on the role of TRP channels, specifically in the renal vasculature. Since renal vascular resistance controls critical physiological functions, including blood pressure, renal autoregulation, filtration, and electrolyte homeostasis, it is important to understand renal vascular TRP channels' distinctive roles. Therefore, this review aims to discuss the current knowledge of the expression and function of renal vascular TRP channels in health and disease.
2. Renal vascular TRPC channels
Expression of TRPC channels in renal vasculature. The TRPC family of the TRP channels is formed by seven mammalian members (TRPC1-7). RT-PCR indicated that TRPC1, TRPC3, TRPC4, TRPC5, and TRPC6 are expressed in rat preglomerular vessels (Facemire et al., 2004). However, only TRPC3, TRPC4, TRPC6, and TRPC7 mRNAs were found in SMCs isolated from canine renal arteries (Walker et al., 2001). TRPC3 mRNA expression level is ~ 3-fold more than TRPC1, TRPC5, and TRPC6 in rat renal microvessels (Facemire et al., 2004). Unlike TRPC1, TRPC5, and TRPC6, TRPC3 is ~ 7-fold more abundant in renal microvessels compared with the aorta (Facemire et al., 2004). SMC TRPC1 has been immunostained in rat afferent and efferent arterioles, although the study did not distinguish the level of TRPC1 expression between the arterioles (Takenaka et al., 2002). TRPC3 channels are expressed in the plasma membrane of neonatal pig afferent arteriolar SMCs (Soni et al., 2017a). TRPC3 protein expression levels in whole kidneys and afferent arterioles were higher in 20-days-old than newborn pigs, suggesting postnatal changes in the expression of the channels (Soni et al., 2017a). Whereas TRPC4 protein was detected in the rat descending vasa recta (DVR), TRPC5 was notably absent (Lee-Kwon et al., 2005). The expression of TRPC5 protein in renal medulla suggests its presence in medullary structures other than the DVR (Lee-Kwon et al., 2005).
The function of TRPC channels in renal vasculature. Endothelin-1 activated TRPC channels in primary SMCs that were cultured from rat renal microvessels (Palygin et al., 2016). L-type Ca2+ channel blocker nifedipine prevented AngII-induced Ca2+ entry in rat afferent arterioles, while a non-selective TPRC channel blocker SKF 96365 reduced AngII-induced Ca2+ influx in efferent arterioles (Loutzenhiser and Loutzenhiser, 2000). AngII-mediated constriction of rat afferent arterioles was also prevented by nifedipine (Takenaka et al., 2002). By contrast, AngII-induced constriction of the efferent arterioles was unaffected by nifedipine but inhibited by SKF 96365 (Takenaka et al., 2002). In another study, SKF 96365 and gadolinium (a non-selective TRPC channel blocker) reduced noradrenaline-induced increase in intracellular Ca2+ concentration in rat afferent arteriolar SMCs (Salomonsson et al., 2010). Taken together, these findings suggest that differential functions for TRPC channels in afferent and efferent arterioles may exist. However, since SKF 96365 and gadolinium are non-selective TRPC channel blockers, additional studies are necessary to characterize the role of TRPC channels in AngII-induced renal arteriolar reactivity.
Selective adenosine A1-receptor (A1R) activator 2-chloro-N6 cyclopentyladenosine (CCPA) stimulated receptor-operated calcium entry (ROCE) in neonatal pig afferent arterioles via TRPC3 channels (Soni et al., 2017a). Evidence indicated that induction of ROCE by CCPA is dependent on kidney maturation, as shown by a more significant increase in intracellular Ca2+ in 20 days-old piglets than in newborns, paralleling the increased protein expression of TRPC3 in the kidneys and afferent arterioles of the older pigs (Soni et al., 2017a). Postnatal kidney maturation did not alter A1R expression (Soni et al., 2017a). Thus, the observed changes in ROCE are likely due to increased TRPC3 expression (Soni et al., 2017a). These findings are significant because A1Rs control renal function, including the tubuloglomerular feedback (TGF) mechanism. Hence, adenosine-induced increase in renal vascular resistance during the TGF process may involve maturation-dependent Ca2+ influx via SMC TRPC3 channels.
The descending vasa recta (DVR) originating from the efferent arterioles of juxtamedullary glomeruli controls renal medullary perfusion (Pallone and Silldorff, 2001; Pallone et al., 1998; Zhang et al., 2002). DVR is primarily made up of pericytes and endothelial cells. Ca2+ influx into pericytes, the SMC-like contractile cells, regulates DVR reactivity, and hence, renal medullary blood flow (Pallone and Silldorff, 2001; Pallone et al., 1998; Zhang et al., 2002). The expression of TRPC4 in DVR pericytes and endothelial cells suggests the channel may be involved in Ca2+-dependent signal transduction mechanisms in DVR that control medullary microcirculation (Lee-Kwon et al., 2005).
Despite the abundance of TRPC6 in renal microvasculature (Facemire et al., 2004; Salomonsson et al., 2010; Walker et al., 2001), its physiological role in the microvessels remains elusive. A study reported that flufenamic acid, a TRPC6 activator triggered a sustained increase in intracellular Ca2+ concentration in afferent arterioles, which was insensitive to L-type Ca2+ channel blockers, diltiazem, and nifedipine (Fellner and Arendshorst, 2008). Since flufenamic acid can modulate various ion channels (Guinamard et al., 2013), the contribution of TRPC6 to renal vasoregulation remains to be determined.
3. Renal vascular TRPM and TRPP channels
To date, there are no known published studies on renal vascular TRPM channels. However, unpublished data from Dr. Earley's group suggest that TRPM4 is expressed in renal interlobar artery SMCs and that TRPM4 channel inhibitor 9-phenanthrol attenuates myogenic constriction in isolated interlobar arteries (Earley, 2013). Whether TRPM4 inhibition alters myogenic tone in resistance size vessels or renal autoregulation is unknown.
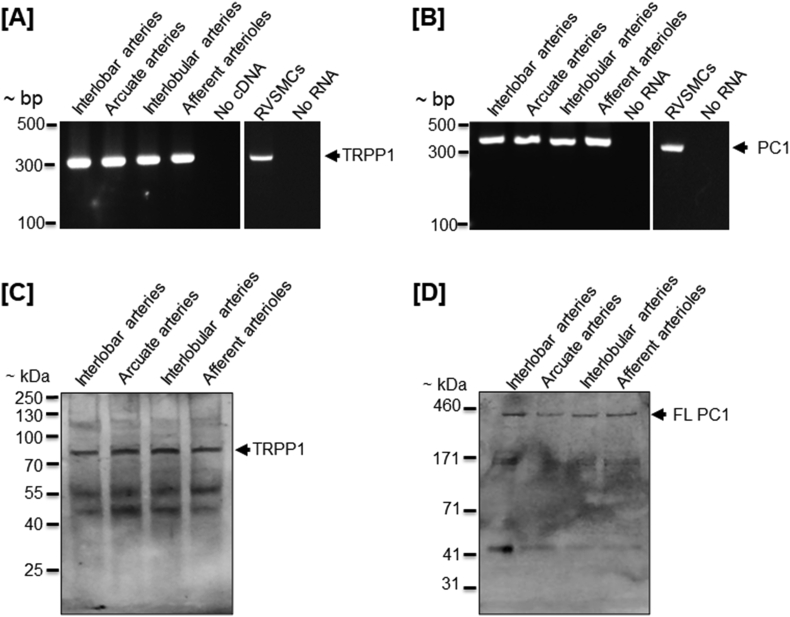
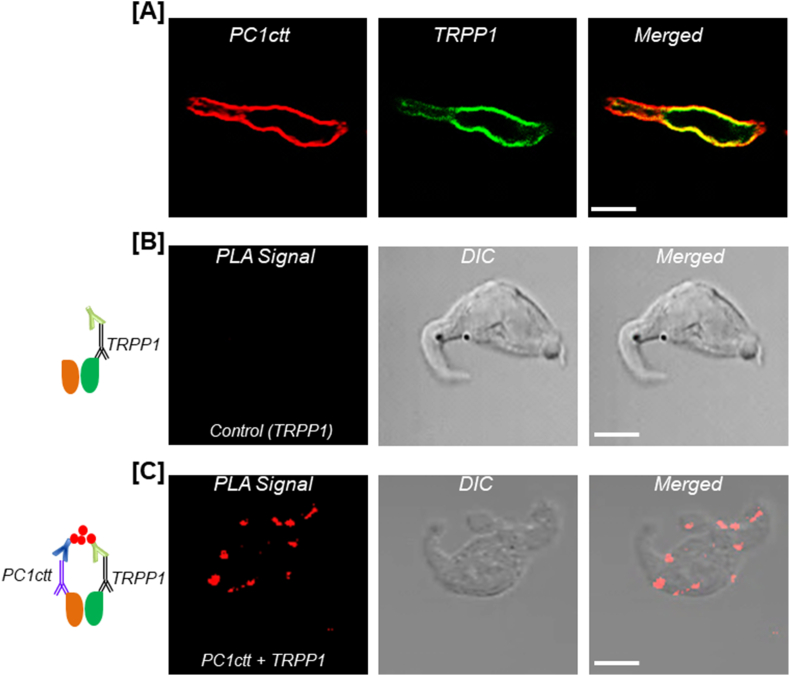
TRPP1 was detected in human fetal and adult renal vessels (Chauvet et al., 2002). Unpublished data from our laboratory indicate that PC1 and TRPP1 mRNAs and proteins are expressed in neonatal pig renal vasculature, ranging from large vessels interlobar and arcuate arteries to small resistance vessels interlobular arteries and afferent arterioles (Fig. 1). Both PC1 and TRPP1 are also expressed in SMCs isolated from preglomerular renal microvessels of the pigs (Fig. 1A and B). Antibodies directed against TRPP1 and the cytoplasmic COOH terminus of PC1 (PC1ctt) immunostained the proteins in the plasma membrane of neonatal pig renal vascular SMCs. (Fig. 2A). We also found that PC1ctt colocalizes and interacts with TRPP1 at the plasma membrane of the cells as determined by immunofluorescence and proximity ligation assay (Fig. 2B and C). These data indicate that endogenous TRPP1 interacts with PC1ctt in the plasma membrane of porcine renal vascular SMCs, the physiological implication of which is unclear. However, previous studies in other vascular beds indicated that PC1 and TRPP1 might regulate vascular myogenic response (Narayanan et al., 2013; Sharif-Naeini et al., 2009). Deleting PC1 in mouse SMCs caused a reduction in stretch-activated ion channel (SAC) current magnitude and mesenteric myogenic constriction (Sharif-Naeini et al., 2009). siRNA-mediated knockdown of TRPP1 (polycystin-2) did not alter myogenic tone in mesenteric arteries but restored SAC activity and tone in mesenteric arteries that lacked PC1 (Sharif-Naeini et al., 2009).
Fig. 1.
Expression of TRPP1 and PC1 in intact renal vessels and vascular smooth muscle cells (RVSMCs) of neonatal pigs. A and B: agarose gels demonstrating amplification of TRPP1 (bp: 314) and PC1 (bp: 352) in renal vessels and RVSMCs. Western immunoblotting with: C: a goat anti-TRPP1 antibody (EB09540; Everest Biotech, Ramona CA) and D: a mouse monoclonal anti-PC1 antibody (C1749; full length: FL; Sigma-Aldrich, St. Louis, MO) showing expression of full-length TRPP1 and PC1 proteins. Whereas TRPP1 bands ran slightly lower (~90 kDa) than its expected molecular weight (106 kDa), PC1 bands correspond to the approximate size (460 kDa). RVSMCs were individually selected using a patch pipette, as previously described (Soni et al., 2017b). NuPAGE Tris-Acetate (3–8%) gels and HiMark protein standard (Invitrogen, Carlsbad CA) were used to separate the large molecular weight full-length PC1 protein.
Fig. 2.
Endogenous TRPP1 and the cytoplasmic COOH terminus of PC1 (PC1ctt) colocalize and interact in the plasma membrane of renal vascular smooth muscle cells (RVSMCs) of neonatal pigs. A: Immunofluorescence staining of a RVSMC, demonstrating that both TRPP1 and PC1ctt are expressed in the plasma membrane and colocalize. B and C: In situ proximity ligation assay (PLA) detected an interaction between PC1ctt and TRPP1 in RVMSCs. Whereas PLA signals (red fluorescence) were absent in negative control cells labeled only with anti-TRPP1, the signals were detected in cells labeled with anti-PC1ctt and anti-TRPP1 antibodies. PLA was performed using the Duolink in situ PLA kit (Olink Bioscience, Uppsala, Sweden), as we have previously described (Adebiyi, 2014). Images were acquired with a Zeiss LSM Pascal laser-scanning confocal microscope. The rabbit anti-PC1ctt antibody was a kind gift from Dr. Oxana Beskrovnaya (Chauvet et al., 2004) (Genzyme Corporation). Bar = 10 μm.
Findings from other studies suggested that TRPP1 is required for myogenic constriction of rat cerebral arteries and mouse hindlimb arteries (Bulley et al., 2018; Narayanan et al., 2013). If future studies reveal a similar role for TRPP channels in the renal vasculature, this could potentially impact our understanding of the autoregulation of RBF and GFR. Furthermore, pathological alterations in renal vascular TRPP1 or PC1, or both could contribute to complications and homeostatic imbalance seen in ADPKD.
4. Renal vascular TRPV channels
Expression of TRPV channels in renal vasculature. TRPV1 mRNA, along with TRPV4, has been detected in the main renal artery (Chen et al., 2015). Moreover, the expression of TRPV1 was 10 times lower, and TRPV4 was 10 times higher in renal parenchymal tissue than renal arteries (Chen et al., 2015). In a 2008 study, Willette et al. (2008) reported positive immunostaining for TRPV4 in rat renal arcuate artery endothelium, but not smooth muscle. Moreover, Western immunoblotting and PCR indicated that TRPV4 is expressed in intact renal vessels isolated from neonatal pigs (Soni et al., 2017b). TRPV4 protein expression levels in whole kidneys and renal microvasculature are higher in adult pigs, suggesting age-dependent expression of the channels (Soni et al., 2017b). Neonatal pig TRPV1, 2, and 3 may be restricted to renal vascular endothelial cells as PCR demonstrated that intact renal interlobular arteries express TRPV1, 2, 3, and 4 channels, but only TRPV4 was found in SMCs isolated from them (Soni et al., 2017b). TRPV4 channels are functionally expressed in both SMCs and endothelial cells across several vascular beds (Filosa et al., 2013). However, we have demonstrated that TRPV4 is predominantly expressed in neonatal pig renal vascular SMCs compared with endothelial cells (Soni et al., 2017b). The prevalent location of TRPV4 in blood vessels may likely determine its physiological function.
Function of TRPV channels in renal vasculature. Renal vascular TRPV1 appears to be bestowed with widely varying functions depending on the location of the channels. Nanomolar concentrations of TRPV1 agonist capsaicin caused endothelium-dependent dilation of phenylephrine-precontracted mesenteric arteries of adult mice, an effect reversed by TRPV1 antagonist capsazepine (Chen et al., 2015). However, micromolar concentrations of capsaicin are required to produce relaxation of the main renal arteries isolated from the mice (Chen et al., 2015). Capsaicin-induced relaxation of the main renal arteries was unaltered by endothelial denudation or TRPV1 deletion, suggesting that endothelium- and TRPV1-independent mechanisms underlie capsaicin-induced relaxation of large renal arteries (Chen et al., 2015).
In contrast to the large arteries, TRPV1 activation by capsaicin produced vasodilation of resistance vessels of perfused mouse kidneys precontracted with phenylephrine (Chen et al., 2015). In line with this observation, capsaicin-induced renal vasorelaxation was reversed by capsazepine and absent in TRPV1 knockout mice (Chen et al., 2015). Furthermore, inhibition of endothelial nitric oxide synthesis with L-NAME attenuated TRPV1-dependent renal vasodilation in perfused kidneys suggesting a possible role for nitric oxide (Chen et al., 2015). In contrast to renal arteries, capsaicin did not alter the diameter of adult rat DVRs pre-constricted with noradrenaline (Chen et al., 2015). Hence, TRPV1 channels appear to possess vasoregulatory effects only in pre-constricted preglomerular vessels of the kidney. This aspect of TRPV1 could be related to the increase in GFR seen with TRPV1 activation (Li and Wang, 2008), and might be of interest for future studies on hypertension and ischemia-reperfusion injury (Chen et al., 2015).
The study mentioned above also investigated the role of TRPV4 activation on renal conduit and resistance vessels (Chen et al., 2015). Unlike TRPV1, TRPV4 activation by GSK1016790A produced endothelium-dependent vasodilation of both phenylephrine-precontracted mesenteric and main renal arteries at nanomolar concentrations, which was reduced by AB159908, a TRPV4 channel blocker (Chen et al., 2015). TRPV4 activation also caused renal vasodilation in experiments using isolated perfused kidneys, and the effect was inhibited by AB159908 and absent in TRPV4 knockout mice (Chen et al., 2015). Contrary to the observations with TRPV1, activation of TRPV4 caused vasodilation of norepinephrine-precontracted rat DVR (Chen et al., 2015). These observations showed that the role of TRPV4 channels in renal vasculature might be broader than that of TRPV1, encompassing both preglomerular and post-glomerular vessels. However, the effects of activation or blockade of TRPV1 or TRPV4 channels on renal microvessels that had developed spontaneous physiological tone was not investigated in this study.
Data from our recent work support a mechanosensitive role for vascular SMC TRPV4 channels in neonatal renal myogenic autoregulation (Soni et al., 2017b). We showed that pharmacological inhibition of TRPV4 channels did not alter nimodipine-sensitive, 75 mM K+-induced increase in intracellular Ca2+ concentration, and vasoconstriction in neonatal pig renal preglomerular microvessels. However, TRPV4 inhibition significantly diminished 1) pressure-induced membrane depolarization and spontaneous tone, 2) phospholipase A2-independent increase in intracellular Ca2+ concentration and renal vasoconstriction induced by hypoosmotic stretch, and 3) pressure-induced increase in intracellular Ca2+ concentration and myogenic vasoconstriction. In anesthetized and mechanically ventilated neonatal pigs, intrarenal artery infusion of nicardipine, an L-type Ca2+-channel blocker, reduced the mean arterial pressure (MAP) and abolished renal autoregulation triggered by a step increase in arterial pressure (Soni et al., 2017b). However, selective TRPV4 channel blockers HC 067047 and RN 1734 inhibited renal autoregulation without altering the MAP (Soni et al., 2017b). Thus, TRPV4-dependent renal myogenic mechanism in isolated microvessels is reproducible in vivo.
TRPV4-mediated cerebral and mesenteric vasodilation, aortic and pulmonary vasoconstriction, and endothelial store- and receptor-operated Ca2+ entry have been reported (Adapala et al., 2011; Earley et al., 2005; Goldenberg et al., 2015; Lorenzo et al., 2008; Ma et al., 2011; Saifeddine et al., 2015; Sonkusare et al., 2012, 2014; Xia et al., 2013). In a recent study, we showed that both GSK1016790A and 4α-PDD (selective TRPV4 channel agonists) constricted renal microvessels of neonatal pigs (Soni et al., 2019). Intrarenal artery infusion of a TRPV4 inhibitor into the kidney of neonatal pigs did not alter AngII-induced increase in MAP. However, it caused: 1) a reduction in AngII-induced receptor-operated Ca2+ entry in afferent arterioles and AngII-induced renal vasoconstriction, 2) a decrease in AngII-induced kidney hypoperfusion, and 3) a reduction in AngII-induced increase in renal vascular resistance (Soni et al., 2019). Hence, it is plausible to suggest that TRPV4 mediates multimodal Ca2+ signaling and physiological functions in the vasculature, depending on cell type, vascular bed, or animal species.
Ischemia-reperfusion (IR) injury to the kidneys of neonatal pigs resulted in an increase in TRPV4 protein expression in preglomerular resistance vessels and agonist-induced TRPV4 cation currents in renal vascular SMCs (Soni et al., 2019). Pharmacological inhibition of TRPV4 attenuated IR-induced increase in renal vascular resistance, IR-induced decrease in GFR, and IR-induced increase in the predictive biomarkers of AKI (Soni et al., 2019). Urinary AngII was increased in neonatal pigs subjected to renal IR (Soni et al., 2019). Since AngII-induced neonatal renal vascular reactivity is partly dependent on TRPV4 channels, AngII receptor-operated Ca2+ entry via SMC TRPV4 channels may contribute to kidney insufficiency in neonatal pig AKI (Soni et al., 2019). In addition to AngII, increased biosynthesis of other endogenous vasoactive mediators that activate TRPV4 channels may contribute to ischemic AKI.
5. Discussion
Various cardiovascular and kidney diseases are inextricably linked to alterations in renal microcirculation. The kidneys receive about 20% of the total cardiac output and play a vital role in removing toxic nitrogenous waste products and maintaining the balance of extracellular fluid volume and electrolyte compositions. Blood vessels of the kidney contribute to these functions by controlling regional kidney perfusion (Carlstrom et al., 2015; Thomson and Blantz, 2008). Evidence from the studies discussed here (summarized in Table 1) demonstrates the presence of TRP channels in vascular segments that play crucial functions in regulating renal microcirculation. The afferent arterioles, which form the effector end of the myogenic and TGF autoregulation mechanisms, express TRPC and TRPV channels. Although TRPC3 contributes to adenosine receptor-mediated vasoconstriction of afferent arterioles in neonatal pigs (Soni et al., 2017a), additional animal studies are required to provide information on the unresolved role of renal vascular ion channels in TGF mechanisms. The cellular signal transduction pathway that underpins myogenic autoregulation includes stretch-induced activation of mechanosensitive ion channels in SMC plasmalemma and succeeding depolarization of the membrane, the opening of L-type Ca2+ channels, and extracellular Ca2+ influx (Davis and Hill, 1999; Schubert and Mulvany, 1999). TRPV4 appears to be a candidate for renal vascular SMC mechanosensitive ion channels, but this has only been proven in infant pigs.
Table 1.
Expression and function of renal vascular TRP channels.
| Publication | Animal species | Vasculature | Channel subtype | Methods used to study the expression | Salient finding(s) |
|---|---|---|---|---|---|
| Walker, R. L. et al. (2001) | Dog | Main renal arteries | TRPC3, 4, 6, & 7 | RT-PCR and qRT-PCR | Canine renal arteries express TRPC3, 4, 6, and one splice variant of TRPC7. |
| Loutzenhiser and Loutzenhiser (2000) | Rat | Afferent & efferent arterioles | TRPC | Pharmacological interventions | AngII induces increase in [Ca2+]i in both the afferent and efferent arterioles, where Ca2+ entry in the afferent arterioles is mediated by L-type Ca2+ channels, and in efferent arterioles is mediated by store-operated Ca2+ entry sensitive to TRPC blockade. |
| Takenaka, T. et al. (2002) | Rat | Afferent & efferent arterioles | TRP-1 (TRPC1) | Immunohistochemistry | Significant differences in functional characteristics exist between afferent and efferent arterioles. AngII mediated vasoconstriction of the afferent arterioles is VDCC-dependent, whereas that of efferent arterioles is TPRC-mediated. |
| Facemire, C. S. et al. (2004) | Rat | Preglomerular resistance vessels | TRPC1, 3, 4, 5, & 6. | RT-PCR, Western blotting | mRNAs for all TRPC subtypes except TRPC2 & 7 are expressed. TRPC3 mRNA is highly expressed in the resistance vessels in comparison to the aorta. Protein expression levels show an abundance of TRPC6. |
| Lee-Kwon, W. et al. (2005) | Rat | Vasa recta, peritubular capillaries, | TRPC4 | RT-PCR, Western blotting, confocal microscopy | A potential association exists between TRPC4 channels and a PDZ domain adaptor protein, NHERF-2, expressed in the DVR. TRPC4 but not TRPC5 is co-expressed with NHERF-2 in the pericytes and endothelial cells of DVR. |
| Fellner, S. K. & W. J. Arendshorst (2008) | Rat | Afferent arterioles | TRPC6 | Pharmacological interventions | AngII-mediated [Ca2+]i increase in afferent arterioles is attenuated by TRPC blockade and exaggerated by TRPC6 activation. |
| Salomonsson, M. et al. (2010) | Rat | Afferent arterioles | TRPC1/3/6 | Pharmacological interventions | Non-specific TRPC channel blockers SKF 96365 and Gd3+ attenuate norepinephrine-induced increase in [Ca2+]i in preglomerular resistance vessels. |
| Soni, H. et al. (2017) | Pig | Afferent arterioles | TRPC3 | RT-PCR, Western blot, and confocal microscopy | TRPC3 expression in the afferent arteriole increases with the maturation of the kidney in the postnatal period. A1R-dependent increase in [Ca2+]i is brought about by receptor-operated mechanisms and is dependent on the level of TRPC3 channel expression. |
| Unpublished data cited in Earley, S. (2013) | Rat | Interlobar arteries | TRPM4 | RT-PCR Immunocytochemistry |
TRPM4 is expressed in renal interlobar artery SMCs. TRPM4 channel inhibitor 9-phenanthrol inhibits myogenic constriction of isolated interlobar arteries. |
| Unpublished data presented in this review | Pig | Interlobar, arcuate, and interlobular arteries and afferent arterioles | TRPP1 | RT-PCR, Western blotting, immunofluorescence | TRPP1 mRNA and protein are expressed by large and small resistance vessels of the porcine kidney. TRPP1 colocalizes and interacts with the cytoplasmic carboxy terminal of PC1. |
| Chen, L. et al. (2015) | Mice | Renal artery Preglomerular and afferent arterioles, vasa recta | TRPV1 and TRPV4 | RT-PCR | TRPV1 activation causes NO-mediated vasorelaxation of phenylephrine-precontracted renal preglomerular resistance vessels but does not affect conduit arteries or vasa recta. Contrary to TRPV1, TRPV4 activation causes NO-mediated vasorelaxation in both conduit and resistance vessels. |
| Willette, R. N. et al. (2008) | Rat | Arcuate artery | TRPV4 | Immunohistochemistry | Renal arcuate artery endothelium is positive for TRPV4 immunostaining, whereas arterial smooth muscle and glomeruli are negative. |
| Soni, H. et al. (2017) | Pig | Interlobular arteries | TRPV4 | RT-PCR, qRT-PCR, Western blotting | TRPV4 channels mediate myogenic response in renal resistance arteries by activating downstream VDCCs via a phospholipase A2-independent mechanism. |
| Soni, H. et al. (2019) | Pig | Interlobular arteries, afferent arterioles | TRPV4 | Western blotting | TRPV4 channel expression is upregulated in renal IR injury, and TRPV4 is involved in renal IR injury by contributing to AngII evoked ROCE in SMCs. |
Apart from their role in preglomerular resistance vessels, TRP channels might also serve essential functions in the renal medulla. TRPC4 may regulate Ca2+ entry into pericytes and endothelial cells that make up the DVR (Lee-Kwon et al., 2005), potentially controlling renal medullary vascular resistance. Blood flow through the vasa recta determines the medullary interstitial milieu, where juxtamedullary nephrons establish the medullary osmotic gradient via the countercurrent multiplier and the vasa recta maintain the gradient through the countercurrent exchange mechanism. Therefore, TRP channel regulation of vasa recta blood flow could, in turn, modulate urine concentration mechanisms.
6. Outlook
Among the six families of mammalian TRP channels, only renal vascular TRPC and TRPV have been modestly investigated. Evidence of their physiological function using whole animal models is minimal. Vasoregulation by these channels suggests that they may contribute not only to renal vascular bed perfusion but the processes of reabsorption, secretion, urine concentration, and maintenance of blood volume. The emerging evidence of TRP channel signaling in renal vasculature suggests that these proteins might become potential new therapeutic targets for kidney disease associated with changes in renal vascular reactivity. Because of renal vasculature's unique anatomical characteristics and highly specialized physiological roles, no other vascular bed can serve as its model in the truest sense. Therefore, future studies must continue to investigate vascular TRP channel functions in their native locations rather than attempting to extrapolate their roles in other vascular beds with starkly different structural and physiological characteristics. Given the non-selectivity of many pharmacological modulators of TRP channels, more studies using genetic animal models are necessary to fill the knowledge gaps in the current understanding of the physiological and pathophysiological significance of renal vascular TRP channels.
CRediT authorship contribution statement
Praghalathan Kanthakumar: Writing, Revision and final approval. Adebowale Adebiyi: Conceptualization, writing, Revision and final approval.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Dr. Adebiyi was supported by grants from the National Institutes of Health (R01DK101668, R56DK120595, R01HL151735, R01DK120595, and R01DK127625) and the American Heart Association Grant-in-Aid (16GRNT30990069). We thank Dr. Oxana Beskrovnaya (Genzyme Corporation) for the kind gift of the PC1ctt antibody. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Adapala R.K., Talasila P.K., Bratz I.N., Zhang D.X., Suzuki M., Meszaros J.G. PKCalpha mediates acetylcholine-induced activation of TRPV4-dependent calcium influx in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H757–H765. doi: 10.1152/ajpheart.00142.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebiyi A. RGS2 regulates urotensin II-induced intracellular Ca2+ elevation and contraction in glomerular mesangial cells. J. Cell. Physiol. 2014;229:502–511. doi: 10.1002/jcp.24470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco S.D.C., Peng J.-B., Takanaga H., Suzuki Y., Crescenzi A., Kos C.H. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J. Bone Miner. Res. 2007;22:274–285. doi: 10.1359/jbmr.061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulley S., Fernández-Peña C., Hasan R., Leo M.D., Muralidharan P., Mackay C.E. Arterial smooth muscle cell PKD2 (TRPP1) channels regulate systemic blood pressure. Elife. 2018;7 doi: 10.7554/eLife.42628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstrom M., Wilcox C.S., Arendshorst W.J. Renal autoregulation in health and disease. Physiol. Rev. 2015;95:405–511. doi: 10.1152/physrev.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet V., Qian F., Boute N., Cai Y., Phakdeekitacharoen B., Onuchic L.F. Expression of PKD1 and PKD2 transcripts and proteins in human embryo and during normal kidney development. Am. J. Pathol. 2002;160:973–983. doi: 10.1016/S0002-9440(10)64919-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet V., Tian X., Husson H., Grimm D.H., Wang T., Hiesberger T. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J. Clin. Invest. 2004;114:1433–1443. doi: 10.1172/JCI21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Kaßmann M., Sendeski M., Tsvetkov D., Marko L., Michalick L. Functional transient receptor potential vanilloid 1 and transient receptor potential vanilloid 4 channels along different segments of the renal vasculature. Acta Physiol. 2015;213:481–491. doi: 10.1111/apha.12355. [DOI] [PubMed] [Google Scholar]
- Chubanov V., Kubanek S., Fiedler D., Mittermeier L., Gudermann T., Dietrich A. In: Neurobiology of TRP Channels. TLR E., editor. CRC Press/Taylor & Francis; Boca Raton (FL): 2017. Functions of TRP channels in health and disease. [PubMed] [Google Scholar]
- Clapham D.E. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Cohen D.M. 2007. The Role of TRPV4 in the Kidney. [PubMed] [Google Scholar]
- Davis M.J., Hill M.A. Signaling mechanisms underlying the vascular myogenic response. Physiol. Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- Di A., Malik A.B. TRP channels and the control of vascular function. Curr. Opin. Pharmacol. 2010;10:127–132. doi: 10.1016/j.coph.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Dietrich A., Gudermann T. TRP channels in the cardiopulmonary vasculature. Adv. Exp. Med. Biol. 2011;704:781–810. doi: 10.1007/978-94-007-0265-3_41. [DOI] [PubMed] [Google Scholar]
- Dietrich A., Kalwa H., Gudermann T. TRPC channels in vascular cell function. Thromb. Haemostasis. 2010;103:262–270. doi: 10.1160/TH09-08-0517. [DOI] [PubMed] [Google Scholar]
- Dryer S.E., Reiser J. TRPC6 channels and their binding partners in podocytes: role in glomerular filtration and pathophysiology. Am. J. Physiol. Ren. Physiol. 2010;299:F689–F701. doi: 10.1152/ajprenal.00298.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Ding M., Sours-Brothers S., Graham S., Ma R. Mediation of angiotensin II-induced Ca2+ signaling by polycystin 2 in glomerular mesangial cells. Am. J. Physiol. Ren. Physiol. 2008;294:F909–F918. doi: 10.1152/ajprenal.00606.2007. [DOI] [PubMed] [Google Scholar]
- Earley S. TRPM4 channels in smooth muscle function. Pflügers Archiv. 2013;465:1223–1231. doi: 10.1007/s00424-013-1250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S., Brayden J.E. Transient receptor potential channels in the vasculature. Physiol. Rev. 2015;95:645–690. doi: 10.1152/physrev.00026.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S., Heppner T.J., Nelson M.T., Brayden J.E. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ. Res. 2005;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- Facemire C.S., Mohler P.J., Arendshorst W.J. Expression and relative abundance of short transient receptor potential channels in the rat renal microcirculation. Am. J. Physiol. Ren. Physiol. 2004;286:F546–F551. doi: 10.1152/ajprenal.00338.2003. [DOI] [PubMed] [Google Scholar]
- Fellner S.K., Arendshorst W.J. Angiotensin II-stimulated Ca2+ entry mechanisms in afferent arterioles: role of transient receptor potential canonical channels and reverse Na+/Ca2+ exchange. Am. J. Physiol. Ren. Physiol. 2008;294:F212–F219. doi: 10.1152/ajprenal.00244.2007. [DOI] [PubMed] [Google Scholar]
- Feng N.H., Lee H.H., Shiang J.C., Ma M.C. Transient receptor potential vanilloid type 1 channels act as mechanoreceptors and cause substance P release and sensory activation in rat kidneys. Am. J. Physiol. Ren. Physiol. 2008;294:F316–F325. doi: 10.1152/ajprenal.00308.2007. [DOI] [PubMed] [Google Scholar]
- Filosa J.A., Yao X., Rath G. TRPV4 and the regulation of vascular tone. J. Cardiovasc. Pharmacol. 2013;61:113–119. doi: 10.1097/FJC.0b013e318279ba42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G., Wang W., Tadagavadi R.K., Briley N.E., Love M.I., Miller B.A. TRPM2 mediates ischemic kidney injury and oxidant stress through RAC1. J. Clin. Invest. 2014;124:4989–5001. doi: 10.1172/JCI76042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel M., Sinkins W.G., Zuo C.D., Hopfer U., Schilling W.P. Vasopressin-induced membrane trafficking of TRPC3 and AQP2 channels in cells of the rat renal collecting duct. Am. J. Physiol. Ren. Physiol. 2007;293:F1476–F1488. doi: 10.1152/ajprenal.00186.2007. [DOI] [PubMed] [Google Scholar]
- Goel M., Zuo C.D., Schilling W.P. Role of cAMP/PKA signaling cascade in vasopressin-induced trafficking of TRPC3 channels in principal cells of the collecting duct. Am. J. Physiol. Ren. Physiol. 2010;298:F988–F996. doi: 10.1152/ajprenal.00586.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg N.M., Wang L., Ranke H., Liedtke W., Tabuchi A., Kuebler W.M. TRPV4 is required for hypoxic pulmonary vasoconstriction. Anesthesiology. 2015;122:1338–1348. doi: 10.1097/ALN.0000000000000647. [DOI] [PubMed] [Google Scholar]
- Graham S., Ding M., Sours-Brothers S., Yorio T., Ma J.X., Ma R. Downregulation of TRPC6 protein expression by high glucose, a possible mechanism for the impaired Ca2+ signaling in glomerular mesangial cells in diabetes. Am. J. Physiol. Ren. Physiol. 2007;293:F1381–F1390. doi: 10.1152/ajprenal.00185.2007. [DOI] [PubMed] [Google Scholar]
- Greka A., Mundel P. Balancing calcium signals through TRPC5 and TRPC6 in podocytes. J. Am. Soc. Nephrol. : JASN. 2011;22:1969–1980. doi: 10.1681/ASN.2011040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greka A., Mundel P. Calcium regulates podocyte actin dynamics. Semin. Nephrol. 2012;32:319–326. doi: 10.1016/j.semnephrol.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinamard R., Simard C., Del Negro C. Flufenamic acid as an ion channel modulator. Pharmacol. Ther. 2013;138:272–284. doi: 10.1016/j.pharmthera.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka K., Qian F., Boletta A., Bhunia A.K., Piontek K., Tsiokas L. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- Hoenderop J.G., Müller D., Van Der Kemp A.W., Hartog A., Suzuki M., Ishibashi K. Calcitriol controls the epithelial calcium channel in kidney. J. Am. Soc. Nephrol. : JASN. 2001;12:1342–1349. doi: 10.1681/ASN.V1271342. [DOI] [PubMed] [Google Scholar]
- Hoenderop J.G., van Leeuwen J.P., van der Eerden B.C., Kersten F.F., van der Kemp A.W., Merillat A.M. Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J. Clin. Invest. 2003;112:1906–1914. doi: 10.1172/JCI19826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardín I., Dionisio N., Lopez J.J., Salido G.M., Rosado J.A. Pharmacology of TRP channels in the vasculature. Curr. Vasc. Pharmacol. 2013;11:480–489. doi: 10.2174/1570161111311040011. [DOI] [PubMed] [Google Scholar]
- Khayyat N.H., Tomilin V.N., Zaika O., Pochynyuk O. Polymodal roles of TRPC3 channel in the kidney. Channels (Austin) 2020;14:257–267. doi: 10.1080/19336950.2020.1804153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F., Ma L., Zou L., Meng K., Ji T., Zhang L. Alpha1-Adrenergic receptor activation stimulates calcium entry and proliferation via TRPC6 channels in cultured human mesangial cells. Cell. Physiol. Biochem. 2015;36:1928–1938. doi: 10.1159/000430161. [DOI] [PubMed] [Google Scholar]
- Koulen P., Cai Y., Geng L., Maeda Y., Nishimura S., Witzgall R. Polycystin-2 is an intracellular calcium release channel. Nat. Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- Lee-Kwon W., Wade J.B., Zhang Z., Pallone T.L., Weinman E.J. Expression of TRPC4 channel protein that interacts with NHERF-2 in rat descending vasa recta. Am. J. Physiol. Cell Physiol. 2005;288:C942–C949. doi: 10.1152/ajpcell.00417.2004. [DOI] [PubMed] [Google Scholar]
- Lee N., Chen J., Sun L., Wu S., Gray K.R., Rich A. Expression and characterization of human transient receptor potential melastatin 3 (hTRPM3) J. Biol. Chem. 2003;278:20890–20897. doi: 10.1074/jbc.M211232200. [DOI] [PubMed] [Google Scholar]
- Li J., Wang D.H. Increased GFR and renal excretory function by activation of TRPV1 in the isolated perfused kidney. Pharmacol. Res. 2008;57:239–246. doi: 10.1016/j.phrs.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Wang F., Wei X., Liang Y., Cui Y., Gao F. Transient receptor potential vanilloid 1 activation by dietary capsaicin promotes urinary sodium excretion by inhibiting epithelial sodium channel α subunit-mediated sodium reabsorption. Hypertension. 2014;64:397–404. doi: 10.1161/HYPERTENSIONAHA.114.03105. [DOI] [PubMed] [Google Scholar]
- Lorenzo I.M., Liedtke W., Sanderson M.J., Valverde M.A. TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 2008;105:12611–12616. doi: 10.1073/pnas.0803970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutzenhiser K., Loutzenhiser R. Angiotensin II-induced Ca2+ influx in renal afferent and efferent arterioles: differing roles of voltage-gated and store-operated Ca2+ entry. Circ. Res. 2000;87:551–557. doi: 10.1161/01.res.87.7.551. [DOI] [PubMed] [Google Scholar]
- Ma X., Cheng K.T., Wong C.O., O'Neil R.G., Birnbaumer L., Ambudkar I.S. Heteromeric TRPV4-C1 channels contribute to store-operated Ca(2+) entry in vascular endothelial cells. Cell Calcium. 2011;50:502–509. doi: 10.1016/j.ceca.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng K., Xu J., Zhang C., Zhang R., Yang H., Liao C. Calcium sensing receptor modulates extracellular calcium entry and proliferation via TRPC3/6 channels in cultured human mesangial cells. PloS One. 2014;9 doi: 10.1371/journal.pone.0098777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T., Wu G., Hayashi T., Xenophontos S.L., Veldhuisen B., Saris J.J. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci. STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Narayanan D., Bulley S., Leo M.D., Burris S.K., Gabrick K.S., Boop F.A. Smooth muscle cell transient receptor potential polycystin-2 (TRPP2) channels contribute to the myogenic response in cerebral arteries. J. Physiol. 2013;591:5031–5046. doi: 10.1113/jphysiol.2013.258319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie M., Bal M.S., Yang Z., Liu J., Rivera C., Wenzel A. Mucin-1 increases renal TRPV5 activity in vitro, and urinary level associates with calcium nephrolithiasis in patients. J. Am. Soc. Nephrol. : JASN (J. Am. Soc. Nephrol.) 2016;27:3447–3458. doi: 10.1681/ASN.2015101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijenhuis T., Hoenderop J.G., van der Kemp A.W., Bindels R.J. Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J. Am. Soc. Nephrol. : JASN (J. Am. Soc. Nephrol.) 2003;14:2731–2740. doi: 10.1097/01.asn.0000094081.78893.e8. [DOI] [PubMed] [Google Scholar]
- Pallone T.L., Silldorff E.P. Pericyte regulation of renal medullary blood flow. Exp. Nephrol. 2001;9:165–170. doi: 10.1159/000052608. [DOI] [PubMed] [Google Scholar]
- Pallone T.L., Silldorff E.P., Turner M.R. Intrarenal blood flow: microvascular anatomy and the regulation of medullary perfusion. Clin. Exp. Pharmacol. Physiol. 1998;25:383–392. doi: 10.1111/j.1440-1681.1998.tb02220.x. [DOI] [PubMed] [Google Scholar]
- Palygin O., Miller B., Ilatovskaya D.V., Sorokin A., Staruschenko A. Two-photon imaging of endothelin-1-mediated intracellular Ca(2+) handling in smooth muscle cells of rat renal resistance arteries. Life Sci. 2016;159:140–143. doi: 10.1016/j.lfs.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S.F., Owsianik G., Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Peng J.B., Suzuki Y., Gyimesi G., Hediger M.A. In: Calcium Entry Channels in Non-excitable Cells. Kozak J.A., Putney J.W. Jr., editors. CRC Press/Taylor & Francis (c) 2017; Boca Raton (FL): 2018. TRPV5 and TRPV6 calcium-selective channels; pp. 241–274. Taylor & Francis Group, LLC. [Google Scholar]
- Reiser J., Polu K.R., Moller C.C., Kenlan P., Altintas M.M., Wei C. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifeddine M., El-Daly M., Mihara K., Bunnett N.W., McIntyre P., Altier C. GPCR-mediated EGF receptor transactivation regulates TRPV4 action in the vasculature. Br. J. Pharmacol. 2015;172:2493–2506. doi: 10.1111/bph.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomonsson M., Braunstein T.H., Holstein-Rathlou N.H., Jensen L.J. Na+-independent, nifedipine-resistant rat afferent arteriolar Ca2+ responses to noradrenaline: possible role of TRPC channels. Acta Physiol. 2010;200:265–278. doi: 10.1111/j.1748-1716.2010.02141.x. [DOI] [PubMed] [Google Scholar]
- Schaldecker T., Kim S., Tarabanis C., Tian D., Hakroush S., Castonguay P. Inhibition of the TRPC5 ion channel protects the kidney filter. J. Clin. Invest. 2013;123:5298–5309. doi: 10.1172/JCI71165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingmann K.P., Gudermann T. A critical role of TRPM channel-kinase for human magnesium transport. J. Physiol. 2005;566:301–308. doi: 10.1113/jphysiol.2004.080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert R., Mulvany M.J. The myogenic response: established facts and attractive hypotheses. Clin. Sci. 1999;96:313–326. [PubMed] [Google Scholar]
- Sharif-Naeini R., Folgering J.H., Bichet D., Duprat F., Lauritzen I., Arhatte M. Polycystin-1 and -2 dosage regulates pressure sensing. Cell. 2009;139:587–596. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- Soni H., Adebiyi A. TRPC6 channel activation promotes neonatal glomerular mesangial cell apoptosis via calcineurin/NFAT and FasL/Fas signaling pathways. Sci. Rep. 2016;6:29041. doi: 10.1038/srep29041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni H., Adebiyi A. Urotensin II-induced store-operated Ca(2+) entry contributes to glomerular mesangial cell proliferation and extracellular matrix protein production under high glucose conditions. Sci. Rep. 2017;7:18049. doi: 10.1038/s41598-017-18143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni H., Peixoto-Neves D., Buddington R.K., Adebiyi A. Adenosine A1 receptor-operated calcium entry in renal afferent arterioles is dependent on postnatal maturation of TRPC3 channels. Am. J. Physiol. Ren. Physiol. 2017;313:F1216. doi: 10.1152/ajprenal.00335.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni H., Peixoto-Neves D., Matthews A.T., Adebiyi A. TRPV4 channels contribute to renal myogenic autoregulation in neonatal pigs. Am. J. Physiol. Ren. Physiol. 2017;313:F1136. doi: 10.1152/ajprenal.00300.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni H., Peixoto-Neves D., Olushoga M.A., Adebiyi A. Clinical science; London, England : 1979: 2019. Pharmacological Inhibition of TRPV4 Channels Protects against Ischemia-Reperfusion-Induced Renal Insufficiency in Neonatal Pigs; p. 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkusare S.K., Bonev A.D., Ledoux J., Liedtke W., Kotlikoff M.I., Heppner T.J. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkusare S.K., Dalsgaard T., Bonev A.D., Hill-Eubanks D.C., Kotlikoff M.I., Scott J.D. AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci. Signal. 2014;7:ra66. doi: 10.1126/scisignal.2005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sours S., Du J., Chu S., Ding M., Zhou X.J., Ma R. Expression of canonical transient receptor potential (TRPC) proteins in human glomerular mesangial cells. Am. J. Physiol. Ren. Physiol. 2006;290:F1507–F1515. doi: 10.1152/ajprenal.00268.2005. [DOI] [PubMed] [Google Scholar]
- Staruschenko A., Spires D., Palygin O. Role of TRPC6 in progression of diabetic kidney disease. Curr. Hypertens. Rep. 2019;21:48. doi: 10.1007/s11906-019-0960-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka T., Suzuki H., Okada H., Inoue T., Kanno Y., Ozawa Y. Transient receptor potential channels in rat renal microcirculation: actions of angiotensin II. Kidney Int. 2002;62:558–565. doi: 10.1046/j.1523-1755.2002.00484.x. [DOI] [PubMed] [Google Scholar]
- Thomson S.C., Blantz R.C. Glomerulotubular balance, tubuloglomerular feedback, and salt homeostasis. J. Am. Soc. Nephrol. 2008;19:2272–2275. doi: 10.1681/ASN.2007121326. [DOI] [PubMed] [Google Scholar]
- Tian W., Salanova M., Xu H., Lindsley J.N., Oyama T.T., Anderson S. Renal expression of osmotically responsive cation channel TRPV4 is restricted to water-impermeant nephron segments. Am. J. Physiol. Ren. Physiol. 2004;287:F17–F24. doi: 10.1152/ajprenal.00397.2003. [DOI] [PubMed] [Google Scholar]
- Tomilin V., Mamenko M., Zaika O., Pochynyuk O. Role of renal TRP channels in physiology and pathology. Semin. Immunopathol. 2016;38:371–383. doi: 10.1007/s00281-015-0527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Adelsberg J.S. The role of the polycystins in kidney development. Pediatr. Nephrol. 1999;13:454–459. doi: 10.1007/s004670050639. [DOI] [PubMed] [Google Scholar]
- Walker R.L., Hume J.R., Horowitz B. Differential expression and alternative splicing of TRP channel genes in smooth muscles. Am. J. Physiol. Cell Physiol. 2001;280:C1184–C1192. doi: 10.1152/ajpcell.2001.280.5.C1184. [DOI] [PubMed] [Google Scholar]
- Wang X., Pluznick J.L., Wei P., Padanilam B.J., Sansom S.C. TRPC4 forms store-operated Ca2+ channels in mouse mesangial cells. Am. J. Physiol. Cell Physiol. 2004;287:C357–C364. doi: 10.1152/ajpcell.00068.2004. [DOI] [PubMed] [Google Scholar]
- Wieder N., Greka A. Calcium, TRPC channels, and regulation of the actin cytoskeleton in podocytes: towards a future of targeted therapies. Pediatr. Nephrol. 2016;31:1047–1054. doi: 10.1007/s00467-015-3224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette R.N., Bao W., Nerurkar S., Yue T.L., Doe C.P., Stankus G. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J. Pharmacol. Exp. Therapeut. 2008;326:443–452. doi: 10.1124/jpet.107.134551. [DOI] [PubMed] [Google Scholar]
- Wilson P.D. Polycystic kidney disease. N. Engl. J. Med. 2004;350:151–164. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- Winn M.P., Conlon P.J., Lynn K.L., Farrington M.K., Creazzo T., Hawkins A.F. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- Xia Y., Fu Z., Hu J., Huang C., Paudel O., Cai S. TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension. Am. J. Physiol. Cell Physiol. 2013;305:C704–C715. doi: 10.1152/ajpcell.00099.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Rhinehart K., Pallone T.L. Membrane potential controls calcium entry into descending vasa recta pericytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R949–R957. doi: 10.1152/ajpregu.00251.2002. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Haylor J.L., Ong A.C. vol. 17. official publication of the European Dialysis and Transplant Association - European Renal Association; 2002. Polycystin-2 expression is increased following experimental ischaemic renal injury; pp. 2138–2144. (Nephrology, Dialysis, Transplantation). [DOI] [PubMed] [Google Scholar]
- Zholos A.V., Curtis T.M. TRP channels in vascular disorders. Curr. Top. Med. Chem. 2013;13:295–309. doi: 10.2174/1568026611313030007. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Castonguay P., Sidhom E.H., Clark A.R., Dvela-Levitt M., Kim S. A small-molecule inhibitor of TRPC5 ion channels suppresses progressive kidney disease in animal models. Science. 2017;358:1332–1336. doi: 10.1126/science.aal4178. [DOI] [PMC free article] [PubMed] [Google Scholar]