Abstract
Background and Aims
Root sprouting (RS), i.e. the ability to form adventitious buds on roots, is an important form of clonal growth in a number of species, and serves as both a survival strategy and a means of spatial expansion, particularly in plants growing in severely and recurrently disturbed habitats. Occurrence and/or success of plants in severely and recurrently disturbed habitats are determined by two components, namely the ability to produce adventitious buds on roots and the vigour of their production. As mechanisms behind different magnitudes of RS remain unclear, our study investigates: (1) whether the presence or absence of specific tissues in roots can promote or limit RS; and (2) whether there is some relationship between RS ability, RS vigour and species niche.
Methods
We studied RS ability together with RS vigour in 182 Central European herbaceous species under controlled experimental conditions. We used phylogenetic logistic regressions to model the presence of RS, RS vigour, the relationship between RS and anatomical traits and the relationship between RS and parameters of species niches.
Key Results
A quarter of herbs examined were able to produce adventitious buds on roots. They were characterized by their preference for open dry habitats, the presence of secondary root thickening and the occurrence of sclerified cortical cells in roots. Root sprouting vigour was not associated with any specific anatomical pattern, but was correlated with the environmental niches of different species, indicating that preferred disturbed and dry habitats might represent a selection pressure for more vigorous root sprouters than undisturbed and wet habitats.
Conclusions
Our study shows that sprouting from roots is quite common in temperate dicotyledonous herbs. Two components of RS – ability and vigour – should be considered separately in future studies. We would also like to focus more attention on RS in herbs from other regions as well as on external forces and internal mechanisms regulating evolution and the functions of RS in both disturbed and undisturbed habitats.
Keywords: Adventitious bud, anatomical features, eudicot herbs, disturbance regime, Ellenberg indicator values, niche preference, root sprouting vigour
INTRODUCTION
Ecological disturbances, along with soil properties and climate, affect plant strategies and play a role in the evolutionary ecology of vegetation (Gleason, 1926; Grubb, 1977; Grime, 1979; Bellingham and Sparrow, 2000; Pausas and Bond, 2018). When disturbance is severe, leading to loss of all above-ground biomass, two plant recovery strategies are common – resprouting after damage and regeneration from seeds (seeding) (Vesk and Westoby 2004). While seeding is typical for ecosystems with rare or, conversely, very frequent disturbances, resprouting prevails at an intermediate disturbance frequency (Bellingham and Sparrow, 2000; Clarke et al., 2015; Herben et al., 2018). Resprouting relies on regenerative organs, usually located below-ground, where their bud bank and storage carbohydrates are protected from disturbance (Pausas et al., 2018). While some types of regenerative organs are well adapted for recurrent disturbances, e.g. lignotubers in fire-prone areas (Paula et al., 2016), we know less about the role of their anatomical and morphological features in regenerative processes.
Regenerative organs may be of stem or root origin (Fig. 1) (Groff and Kaplan, 1988) and they very often overlap with clonal growth organs. While stem-derived organs of clonal growth are more frequent (Herben and Klimešová, 2020), root-derived clonal growth is less common (Bartušková et al., 2017; Ott et al., 2019). In contrast to the stem-derived organs, which have modular structures with internodes, nodes, leaves and axillary buds, roots do not have any such structure. Root-borne buds, later transformed into shoots, must arise independently of the apical or axillary meristem. This fundamental difference in their origins suggests that root-derived shoots are subject to different regulatory mechanisms compared with stem-derived shoots (Klimešová et al., 2017; Herben and Klimešová, 2020).
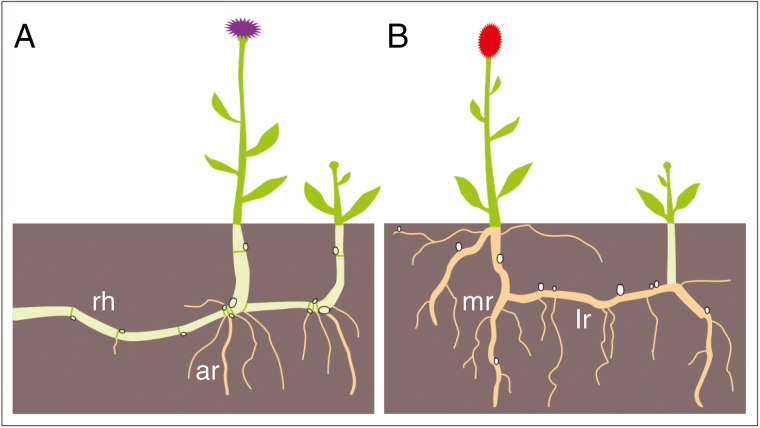
Fig. 1.
Below-ground regenerative organs of stem (A) and root origin (B) in herbaceous plants. A rhizomatous plant (A) with rhizome (rh; light green) bearing an axillary bud (green ovals) in each node. A root sprouting plant (B) with roots (light brown) bearing adventitious buds (pink ovals) formed out of the modular structure. Different types of roots are depicted: adventitious root (ar), main root (mr) and lateral root (lr).
Sprouting from roots may be an adaptation to severe and recurrent damage because bud formation on roots is often initiated by damage or fragmentation of the root system (Rauh, 1937; Klimešová et al., 2017). However, root sprouting (RS) is not always conditioned by injury and even occurs spontaneously, as part of normal development (Wittrock, 1884; Rauh, 1937). Previous studies on the distribution of RS species report them most commonly from disturbed habitats (Guerrero-Campo et al., 2006; Klimešová et al., 2008), but they are by no means restricted to them. Many RS species belong to expansive weeds (e.g Cirsium arvense, Sonchus arvensis or Convolvulus arvensis; Raju et al., 1966; Liew et al., 2013), clonal plants of open habitats, (e.g. Linaria vulgaris and Rumex acetosella; Rauh, 1937), in forest understorey (e.g. Rubus) or forming large forest stands (e.g. Populus tremuloides; Del Tredici, 2001). Some myco-heterotrophic plants from Orchidaceae and Ericaceae families represent cases of RS connected with specialized ontogeny (Klimešová, 2007). Root sprouting allows for a wide range of strategies, from the plant’s extreme ability to regenerate the whole body from a single piece of root to a way to achieve asexual reproduction via clonal growth (Klimešová and Martínková, 2004; Martínková and Klimešová, 2016; Herben and Klimešová, 2020). Simply showing the presence or absence of RS cannot capture the magnitude of this phenomenon. To understand the ecological function of RS, we should also quantify the vigorosity of bud production in RS species and focus on possible constraining factors.
Most root sprouters are eudicots (Bartušková et al., 2017) that are able to produce lateral meristems (i.e. cambium and phellogen) that cause secondary thickening and, at the same time, might be responsible for initiation of adventitious buds (Rauh, 1937; Dore, 1955; Peterson, 1975). Although this is probably the most common scenario, other sites of root bud formation are known. In herbs, adventitious buds on roots can be formed by epidermal cells, cortical parenchyma, pericyle or callus (Rauh, 1937; Charlton, 1965; Hamdoun, 1970; Polowick and Raju, 1982; Fambrini et al., 2003). In the case of woody root sprouters, the place and time of origin can significantly affect the success of the RS process. Pre-formed ‘additional buds’ that appeared endogenously (i.e. from the pericycle or secondary phloem) and thus have a functional vascular connection with the centre of the root stele, were able to sprout successfully. ‘Reparative buds’, which are formed exogenously (i.e. from proliferated pericycle, phellogen or callus) at the time of injury, lack the vascular connection to the stele and may fail to build properly and sprout into new shoots (Bosela and Ewers, 1997; Hayashi and Appezzato-da-Glória, 2009; Kataoka et al., 2019). No such limitations are known for herbaceous plants. Nevertheless, root anatomy is a potential candidate trait that accounts for variations in RS ability among herbaceous species and thus may determine a plant’s distribution along disturbance and environmental gradients.
Root sprouting in herbs has been known for centuries, and has often been studied from an agricultural point of view for targeting weed control. This provided important insights into internal and external regulatory mechanisms of RS in herbs, for example pathways regulating the dormancy of adventitious buds on roots (Horvath et al., 2003) and how the timing of disturbances affect RS (Klimešová et al., 2007; Liew et al., 2013; Taab et al., 2018). However, RS has not been studied systematically over a large set of species. We also lack standardized approaches for assessing RS that would go beyond casual observations of individual species under difficult to compare field conditions.
The aim of this study was to determine the frequency of RS in herbaceous species of central European flora, and to link this to the occurrence of these species along environmental gradients of humidity, light and disturbance. We then evaluated the hypothesis that anatomical traits can serve as predictors of the occurrence and vigour of RS. To address these objectives, we collected RS data on 182 herbaceous dicotyledonous species, selected to cover a range of clades and environmental conditions in central European flora, using a standard experimental protocol. In a sub-set of these species, we collected detailed data on their root anatomies. This enabled us to determine the presence/absence of RS and its vigour, to link these data with environmental information available for these species and to correlate anatomical traits with RS ability and vigour.
MATERIALS AND METHODS
Species selection
We sampled 182 Central European herbaceous plant species from 31 families. The selection of species spanned major angiosperm clades, but monocot species were generally excluded as these are known not to be RS (with an exception of one orchid clade; Rauh, 1937; Herben and Klimešová, 2020). One-third of selected species represented already reported RS species, although for many of them we had no experimental evidence regarding RS (Bartušková et al., 2017). The remainder of the species selected represented species for which there was no literature data regarding RS.
Species were collected in the field in 2016 and 2017 during their flowering periods in order to minimize differences in RS caused by phenological stage and to ensure proper species identification. In May 2016, a total of 80 species were sampled from dry and wet meadow habitats. In 2017, we focused on other types of habitats and concurrently covered early- and late-flowering species by starting sampling in April and continuing at different localities until October, which another 102 species. These species were collected from a range of natural and semi-natural habitats in the Czech Republic to cover the most common community types, comprising forest habitats, dry and wet meadows, and anthropogenic habitats. Some rare wetland species were obtained from the collection of aquatic and wetland plants of the Institute of Botany of the Czech Academy of Science, Třeboň (Supplementary data Table S1). The communities sampled allowed us to cover major environmental gradients in Czech vegetation: moisture, light availability and disturbance. We avoided rare habitats and habitats with extreme moisture or light conditions, as well as rare protected species. At each locality, we searched for plants referred to in the literature as RS (according to Bartušková, et al., 2017) to ensure that we had sufficient species for the analysis, as RS is referred to in only about 10 % of species in Central European flora (Bartušková et al., 2017). After collecting these RS species, we collected other common dicotyledonous herbs at the locality, focusing on the largest individuals available to ensure sufficient material for root fragments.
For most species, we excavated four fully developed individuals from one population. To ensure that sampled plants were different genetic individuals (genets), plants sampled were at least 10 m apart. The number of individuals was lower in 13 species (mean number of individuals 2.54) because of their rarity. In another 13 species, we sampled two populations (four individuals for each population). Excavated plants were placed in wet plastic bags and transported to the laboratory for further processing.
Experiment: bud formation on root fragments
Sampled roots were washed, and three root fragments (approx. 5 cm long) were selected from the root system of each plant, resulting, with a few exceptions, in 12 root fragments per plant species. Fragments were cut from the main root and thicker lateral roots; in the case of rhizomatous plants, fragments from thicker adventitious roots were taken (Fig. 1). We avoided roots of <1 mm in diameter because, in a pilot experiment, these were shown to be prone to rotting. In species with diverse root widths, we cut fragments of different diameters while avoiding damaged or senescing parts. Root fragments were weighed and lengths were measured. Fragments were then placed on wet sand in Petri dishes, watered regularly and kept in a climate chamber for 1 month (20 °C, 8 h light/16 h dark); this regime was successfully tested in our pilot experiments. Replicates (root fragments from one sampled individual in one Petri dish) of each species were organized in a randomized block design. After 1 month, samples with buds were harvested at different developmental stages and stored in 70 % ethanol. Fragments with no visible buds were discarded. We counted the number of adventitious buds, including those that had already grown into small shoots (Supplementary data Fig. S1). For further comparative analyses, we standardized the values of continuous variables per length of root fragment so that we obtained characteristics of sprouting vigour (the number of buds per unit length) and specific root biomass (root biomass per unit length) (Supplementary data Table S1).
Anatomical data collection
We analysed the anatomy of 68 species, of which 42 were RS that successfully developed buds during the 1 month experiment. Four species with well-developed buds were not analysed because of their tiny fragile roots. Because fragments of plants that were not successful root sprouters in our trial were often partly rotten at the end of experiment, we analysed the anatomies of non-RS plants as freshly collected plant roots. For this purpose, we selected 26 species out of 136 non-RS species used in our trial that were closely related to the successful RS species, i.e. species either from the same genus, if available, or from the same family (Supplementary data Table S2).
To evaluate RS anatomy, we used three root fragments, each of them originating from distinct plant individuals. To evaluate the anatomy for non-RS species, we always selected the thickest root found on an individual.
For RS samples, we carefully separated root portions (1 cm long) containing buds from each fragment. Buds were identified using an Olympus SZX16 stereomicroscope and Canon EOS 600D camera. For non-RS species, we selected the middle part of the root fragment for the analysis.
Cross- and longitudinal sections were obtained using a WSL sliding lab-microtome, where sections (20–60 µm thick) were clarified, stained with Safranin and Astra blue (Bukatsch, 1972), dehydrated in a crescent ethanol series and mounted on glass slides in Canada Balsam®. Digital photomicrographs were obtained using an Olympus BX53 microscope and an Olympus DP73 camera. CellSens Entry 1.9 software was used for image analysis.
The anatomical description was based on 14 characters, evaluated using a binary code (0 = absent; 1 = present), in root fragments at different stages of secondary development (Supplementary data Table S2). Traits included tissue dominating, covering tissue and presence of exodermis, cortex maintenance, sclerified parenchymal cells on the cortex, visibility of endodermis and proliferated pericycle, fibres on phloem and xylem, and the presence of a medulla, secretory structures, crystals and growth rings.
Additional data sources
We used Ellenberg indicator values (EIVs) (Ellenberg et al., 1991) to assess environmental preferences of the species studied, where EIVs describe the optima of most Central European species along gradients of nutrients, moisture and light (Diekmann, 2003). The EIVs were available for a sub-set of 154 (nutrients) to 169 (light) species. In order to place individual species on gradients of disturbance frequency and severity, we used species-level indicator values for disturbance regimes, expressed as the disturbance frequency indicator value and the disturbance severity indicator value (Herben et al., 2016). The indicator values express optimum values of disturbance frequency and severity (i.e. levels where the given species is most common) for individual species. These were available for a sub-set of 175 species.
Phylogenetic data were taken primarily from Daphne (Durka and Michalski, 2012), with data for an additional two species (Euphorbia virgata and Veronica vindobonensis) taken from Lososová et al. (2015). As the Daphne tree contains polytomies, these were replaced by randomly generated dichotomies with very short divergence times (0.001 million years ago) using the function multi2di from the R package (ape ver. 3.5). Differences among replicate runs using different randomly generated dichotomies were negligible.
Data analysis
We estimated phylogenetic signals in RS by fitting continuous-time reversible Markov models of binary trait evolution on the phylogenetic tree using the function fitDiscrete from the package geiger ver. 2.0.6 (Harmon et al., 2008), and used the estimated lambda as a measure of phylogenetic signal. We complemented this with stochastic character mapping to estimate the number of transitions between each trait state as a measure of the stability of the trait over the phylogeny. We first fitted a continuous-time reversible Markov model of binary trait evolution using the function make.simmap from the package phytools ver. 0.5-38 (Revell, 2012) to determine the transition matrix. We assumed an asymmetric process (forward and backward transitions being different). We used this transition matrix and the tip states on the tree to simulate 100 stochastic character histories. Prior distribution on the root node of the tree was estimated using the stationary character distribution of the transition matrix. We used these histories to estimate the expected number of evolutionary transitions over the tree and to reconstruct probabilities of the trait occurring on individual tree nodes.
We modelled the relationship between RS and anatomical traits using phylogenetic regressions. As anatomical traits are likely to be highly correlated, we first used principal components analysis (PCA) to identify sets of correlated variables (Supplementary data Table S3). Subsequently, we selected six traits that constituted a major share of variation among all anatomical traits, were not strongly correlated and were ecologically meaningful. These traits (covering tissue, sclereids on cortex, growth rings, proliferated pericycle, medulla and cortex maintenance) were chosen as predictors of RS in all subsequent analyses (Fig. 2). To this set of purely anatomical traits we added one morphological trait, i.e. specific root biomass (fragment weight/length ratio). We modelled the presence of RS using phylogenetic logistical regression employing the function MCMCglmm from the package MCMCglmm ver. 2.26 (Hadfield, 2010). We assumed a binomial (Bernoulli) distribution of RS, with a covariance structure determined by the inverse of the patristic (relatedness) matrix. We used 13 000 iterations, taking every tenth value to determine the likelihood function, and discarded the first 3000 iterations as burn-in. We visually assessed the output for convergence. The role of individual predictors was examined by assessing the posterior distribution of their corresponding coefficients. The RS vigour (number of buds per species) was modelled with a similar approach, but using Gaussian distribution to model the logarithm of the number of buds; we fitted the model only to species that showed RS. In addition to anatomical predictors, total fragment length was used to screen out differences among species due to different numbers of roots in the experiment.
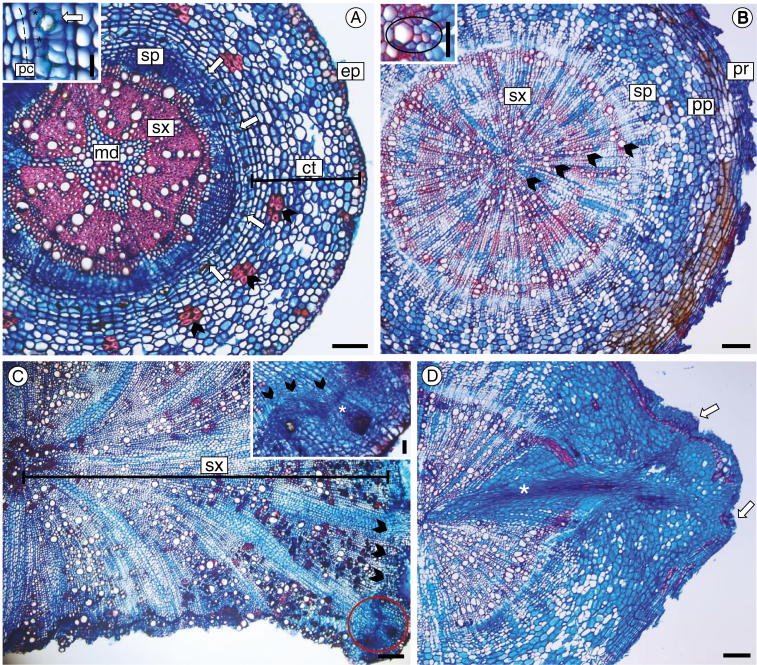
Fig. 2.
Root cross-sections at the secondary stage of development in Centaurea jacea (A), Euphorbia esula (B, D) and Knautia arvensis (C). The anatomical predictors used in the statistical analysis are shown. (A) Species with epidermis (ep) and cortex (ct) preserved; sclerified cortical cells (arrowheads) are scattered on the cortical parenchyma, and the endoderm (* inset) associated with secretory structures (arrows) constitutes the inner layer of the cortex; the vascular cylinder includes uniseriate pericycle (pc inset), secondary phloem (sp), secondary xylem (sx) and medulla (md), with lignified cells in the centre. (B) The species no longer has epidermis and cortex cells; they were lost during root thickening and replaced by periderm (pr), which was formed just adjacent to the endoderm. Proliferated pericycle (pp) and secondary phloem (sp) have indistinct limits, and four growth rings (arrowheads) were formed on the secondary xylem (sx). Note the presence of the exarch protoxylem (circled inset) feature that characterizes the root structure. (C) Overview of root with secondary xylem (sx) well developed, vascular cambium (arrowheads) and adventitious bud in an early stage of development (circled area). Note in the detail the cambial origin (arrowheads) of the root bud (*). (D) Advanced stage of bud development, with leaf primordium (arrows) and bud trace (*) on secondary xylem up to the centre of the root. Scale bars = 25 µm (A inset, B inset), 50 µm (C inset), 100 µm (A), 200 µm (B–D).
We used an identical approach to model the relationship between RS and parameters of species niche (EIVs and disturbance indicator values). Phylogenetic logistic regression was used in the same fashion as for anatomical traits, assuming a binomial distribution for the presence of RS, and Gaussian distribution for the logarithm of the number of buds (for sprouting species only). Total fragment length was always used to screen out differences among species due to different numbers of roots in the experiment.
For both sets of models (anatomy and parameters of the species niche), we also ran non-phylogenetic models, using the same distributions and the same set of predictors, but setting the covariance matrix to identity matrix. To visualize relationships of ability to sprout and number of buds to predictor variables, we used generalized additive models with penalized thin-plate regression smoothing from the package mgcv version 1.8-31 (Wood, 2003).
RESULTS
In the set of herbaceous plants studied, 46 out of 182 species were able to produce adventitious buds on root fragments (about one-third of reported RS perennial herbs of the Czech flora). The anatomical evaluation showed that in all RS samples, the secondary structure was already established, i.e. at least one of the lateral meristems (phellogen and cambium) was already activated (Fig. 2A–D). Periderm was observed in 33 species (Fig. 2B), and secondary phloem and xylem were present in all samples. Root growth rings varied from two to five rings and occurred in 11 species (Fig. 2B). Among non-RS plants, secondary structure was identified in the majority of species, except in two Plantaginaceae, Littorella uniflora and Plantago major, for which lateral meristems were not active.
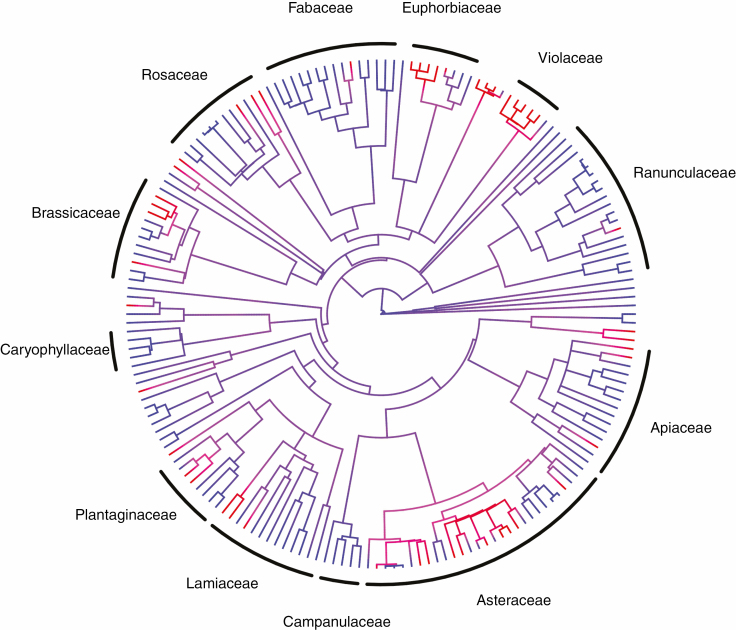
The ability of species to be RS was not strongly phylogenetically conserved (λ = 0.66), but still showed some phylogenetic pattern of differing incidence in individual lineages, although we found at least some RS species in all main angiosperm lineages examined (Fig. 3; Supplementary data Fig. S2). Root sprouting was particularly common in Asteraceae and in a number of families from the order Malpighiales (Violaceae, Euphorbiaceae and Hypericaceae). In contrast, it was rare in all species examined from the ranunculid clade (Ranunculaceae and Papaveraceae) and in Fabaceae.
Fig. 3.
Mapping of RS on the phylogenetic tree. Ancestral reconstruction is based on averaging of 100 stochastic character histories generated by a continuous Markov process with all rates different. The red colour indicates the presence of RS, and blue the absence of RS. Families with fewer than five species are not indicated in the figure; see Supplementary data Table S1 for the full listing of species and families.
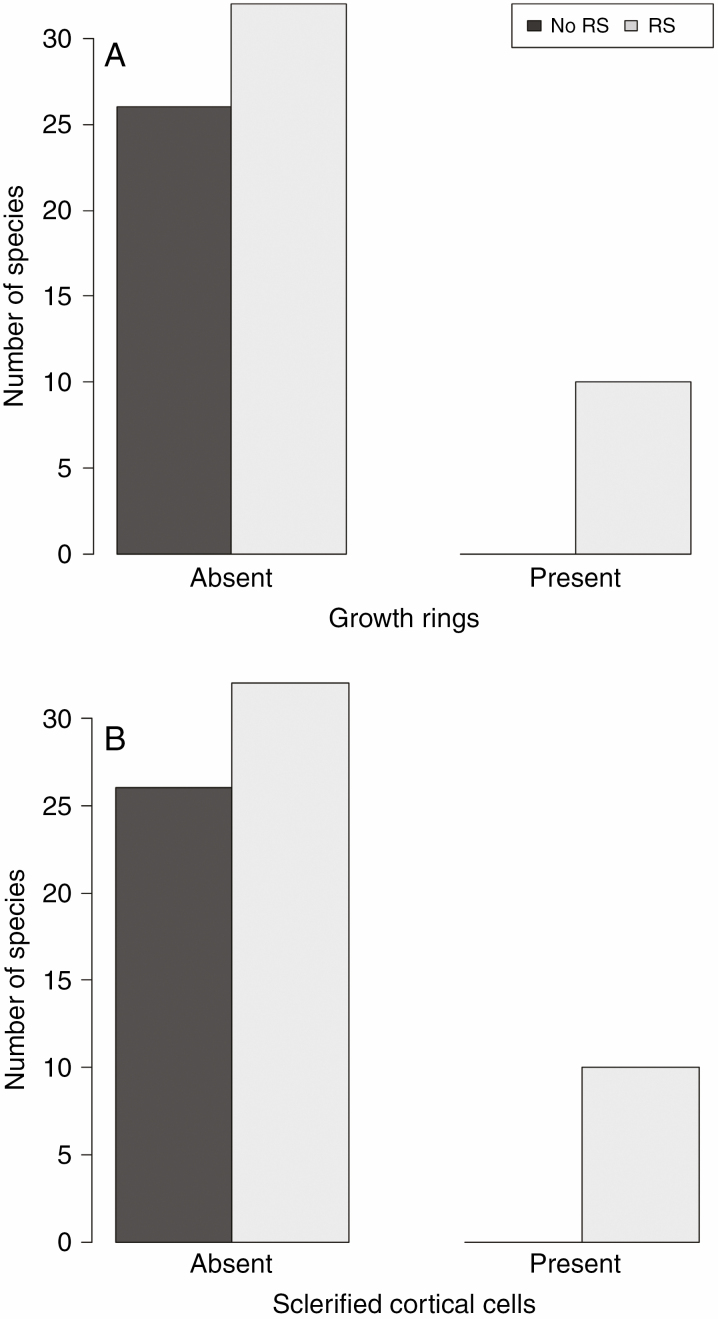
Among the anatomical traits statistically evaluated, the presence of growth rings and the presence of sclerified cortical cells in roots were considered to be the most significant predictors for RS ability (Table 1; Fig. 4). The combination of both traits was observed only in Geranium sanguineum and Hypericum perforatum, whereas none of the characteristics was observed in non-RS species (Fig. 4; Supplementary data Table S2). Marginally significant effects were also observed for covering tissue; RS species tended to have a periderm (i.e. secondary tissue replacing an epidermis), while non-RS species more often had only an epidermis (Table 1; Supplementary data Table S2). The results of phylogenetic and non-phylogenetic analyses were similar (Table 1). We were able to identify the tissue of origin of root buds only in Knautia arvensis, where reparative buds in early stages of development arose from the vascular cambium without vascular trace (Fig. 2C). In the other RS species, we observed only additional buds in different stages of development but always with distinct vascular connections with the centre of the root (Fig. 2D). The complete anatomical analysis of the 68 selected species is shown in Supplementary data Table S2.
Table 1.
Effects of anatomical traits on RS and RS vigour, analysed using non-phylogenetic and phylogenetic linear and logistic regressions with MCMC sampling (see the text for details)
| Explanatory variables | RS | RS vigour | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-phylogenetic | Phylogenetic | Non-phylogenetic | Phylogenetic | |||||||||
| Anatomical traits | Coefficient | CI | P | Coefficient | CI | P | Coefficient | CI | P | Coefficient | CI | P |
| Specific root biomass | 24.60 | –25.8 to 84.7 | 0.342 | 25.07 | –28.3 to 76.4 | 0.312 | 0.31 | 0.02–0.67 | 0.05. | 0.30 | –0.02 to 0.65 | 0.08· |
| Covering tissue | 166.76 | –26.4 to 400.0 | 0.09· | 159.68 | –22.7 to 388.0 | 0.092· | 0.25 | –0.91 to 1.50 | 0.68 | 0.23 | –0.87 to 1.44 | 0.7 |
| Sclerified cortical cells | 256.96 | 50.4–479.7 | 0.028* | 251.16 | 45.6–450.0 | 0.008** | 0.00 | –0.76 to 0.81 | 0.97 | –0.03 | –0.75 to 0.84 | 0.92 |
| Growth rings | 264.15 | 44.1–466.2 | 0.012* | 254.74 | 70.0–483.7 | 0.018* | 0.34 | –0.37 to 1.14 | 0.356 | 0.35 | –0.37 to 1.10 | 0.362 |
| Pericycle | –66.66 | –219.4 to 69.1 | 0.374 | –64.53 | –219.9 to 68.5 | 0.374 | -0.22 | –1.06 to 0.50 | 0.528 | –0.22 | –0.96 to 0.61 | 0.558 |
| Medulla | –58.38 | –249.6 to 113.3 | 0.496 | –58.53 | –216.9 to 116.5 | 0.454 | –0.13 | –0.91 to 0.76 | 0.76 | –0.12 | –0.93 to 0.74 | 0.8 |
| Dominating cortex | 149.86 | –51.6 to 372.0 | 0.13 | 147.04 | –55.0 to 385.1 | 0.132 | -0.07 | –1.11 to 0.88 | 0.878 | –0.07 | –0.99 to 1.03 | 0.894 |
| Fragment length | –0.31 | –3.63 to 2.40 | 0.854 | –0.46 | –3.51 to 2.18 | 0.74 | 0.01 | –0.01 to 0.02 | 0.25 | 0.01 | –0.01 to 0.02 | 0.234 |
RS is expressed as a binary variable, RS vigour is expressed as the number of buds. Coefficient, its confidence interval and probability (P) are shown. Significant effects are in bold. **P < 0.01; *P < 0.05; P < 0.1.
Fig. 4.
Root sprouting in species with the presence of growth rings (A) and in species with the presence of sclerified cortical cells (B).
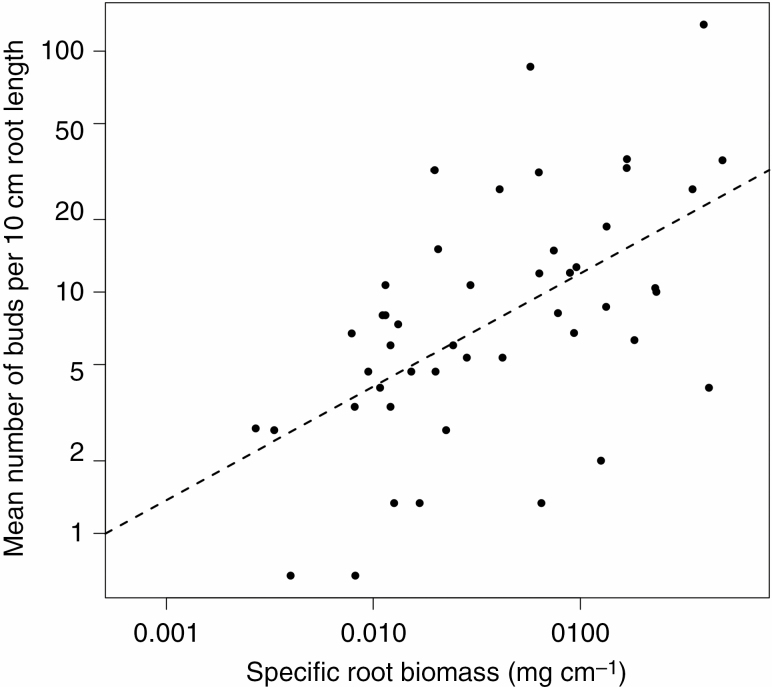
Root sprouting vigour, expressed as the number of adventitious buds per unit of root length, had a highly skewed distribution, ranging from values of 0.02 buds cm–1 of root length up to almost 4.3 buds cm–1 length (Supplementary data Table S1). It was positively affected by specific root biomass (i.e. root biomass per unit length, Table 1; Fig. 5), which can be considered a proxy for root thickness, since we used similar fragment lengths for individual species. No effect of anatomical traits on the sprouting vigour in RS species was observed (Table 1). The results of phylogenetic and non-phylogenetic analyses were similar (Table 1).
Fig. 5.
Relationship between specific root biomass and number of buds per 10 cm root length. The line shows ordinary least squares regression; adjusted R2 = 0.302. Each point represents one species. Only root sprouting species are shown. Note logarithmic scaling of the axes.
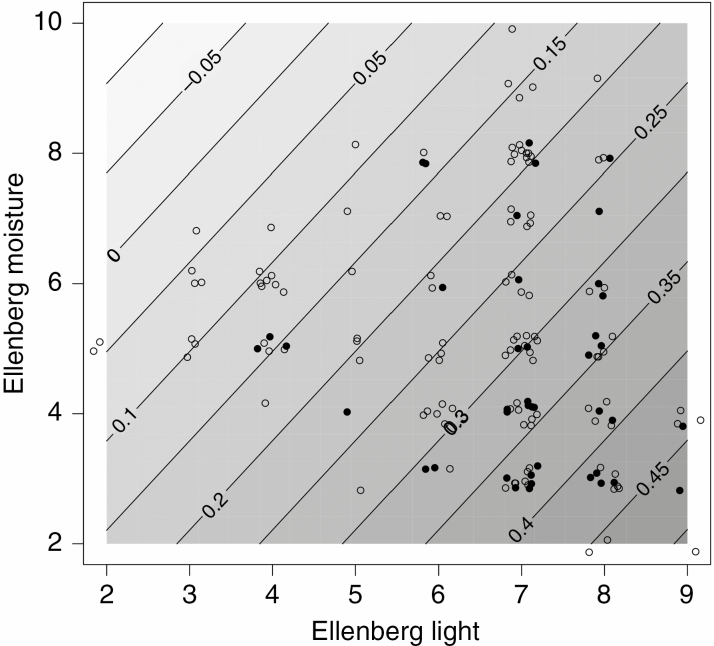
Root sprouting ability was correlated with species preferences on environmental gradients of light in a non-phylogenetic analysis (Table 2), and with both moisture and light species preferences in a phylogenetic analysis (Table 2). In very dry and open habitats, the likelihood of being an RS species was about 0.45, whereas it was essentially zero in shaded and moist habitats (Fig. 6). No significant effects of disturbance frequency and disturbance severity on RS ability were found in non-phylogenetic or phylogenetic analyses (Table 2).
Table 2.
Effects of environmental characteristics on RS and RS vigour, analysed using non-phylogenetic and phylogenetic linear and logistic regressions with MCMC sampling (see the text for details)
| Explanatory variables | RS | RS vigour | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Environmental characteristics |
Non-phylogenetic | Phylogenetic | Non-phylogenetic | Phylogenetic | ||||||||
| Coefficient | CI | P | Coefficient | CI | P | Coefficient | CI | P | Coefficient | CI | P | |
| Nitrogen | 4.55 | –59.8 to 70.2 | 0.898 | 2.17 | –35.0 to 42.1 | 0.916 | 0.13 | –0.14 to 0.40 | 0.304 | 0.13 | –0.13 to 0.39 | 0.316 |
| Moisture | –52.04 | –126.2 to 12.9 | 0.106 | –28.33 | –69.56 to 9.30 | 0.09· | –0.33 | –0.56 to –0.03 | 0.014* | –0.33 | –0.59 to –0.09 | 0.01** |
| Light | 76.27 | –17.4 to 170.3 | 0.096· | 19.84 | –32.9 to 71.6 | 0.436 | -0.34 | –0.76 to 0.07 | 0.122 | –0.33 | –0.73 to 0.08 | 0.12 |
| Disturbance frequency | 35.39 | –232.8 to 254.6 | 0.762 | 73.19 | –66.7 to 268.4 | 0.32 | 1.34 | 0.47–2.36 | 0.008** | 1.30 | 0.27–2.16 | 0.012* |
| Disturbance severity | 283.10 | –1132.8 to 645.8 | 0.514 | 306.92 | –843.7 to 154.4 | 0.164 | 2.94 | –1.40 to 6.94 | 0.152 | 2.69 | –1.18 to 6.77 | 0.19 |
| Fragment length | –0.66 | –6.42 to 5.42 | 0.828 | –2.57 | –7.82 to 1.02 | 0.182 | 0.02 | 0.003–0.04 | 0.028* | 0.02 | 0.007–0.04 | 0.018* |
RS is expressed as a binary variable, RS vigour is expressed as the number of buds. Coefficient, its confidence interval and probability (P) are shown. Significant effects are in bold. **P < 0.01; *P < 0.05; P < 0.1.
Fig. 6.
Likelihood of RS as a function of light and moisture niche of the species. Filled circles are RS species and open circles are non-RS species; isoclines show probability of RS as a function of the position in the light moisture plane predicted by a generalized additive model with cubic smooth spline and binomial distribution of the response variable.
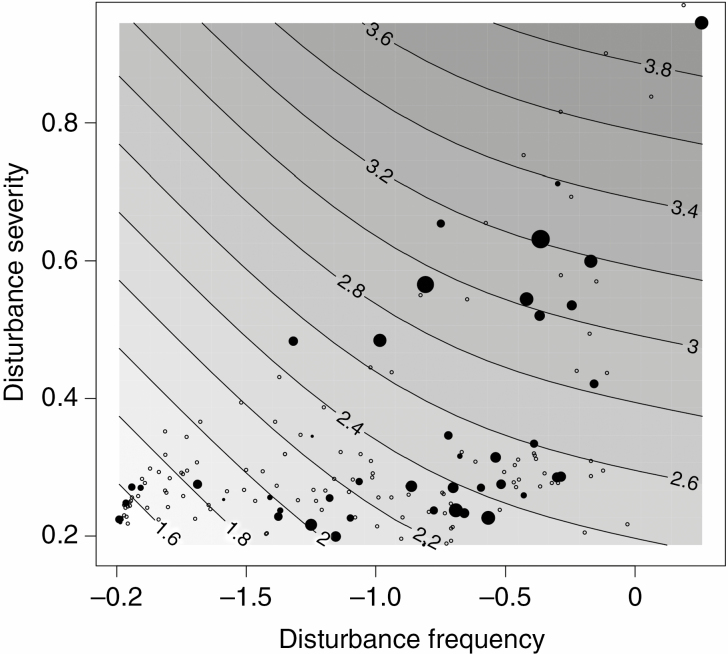
The RS vigour was significantly affected by species occurrence along environmental gradients of humidity and disturbance (Table 2). In non-phylogenetic analyses, the disturbance frequency had the largest impact on the number of buds, followed by the moisture gradient (Table 2). Root sprouting species in more frequently and severely disturbed habitats tended to have higher RS vigour (i.e. to produce more buds per unit of root length) than RS species in habitats with minor disturbances (Fig. 7). Similar results were obtained from a phylogenetic analysis (Table 2).
Fig. 7.
Root sprouting vigour as a function of disturbance severity and frequency indicator values. Open circles are non-RS species and filled circles are RS species; the size of the circle is proportional to the log number of buds. Numbers at isoclines show the number of buds per 5 cm fragment length predicted by a generalized additive model with cubic smooth spline. Disturbance frequency is expressed as common log of disturbance return time in years (e.g. a value of –2 indicates disturbance once in a century), and disturbance severity as a proportion of bare soil opened in the disturbance event.
DISCUSSION
Using a resprouting experiment with a diverse collection of 182 Central European herbs, we identified RS ability in a quarter of species studied. Our analysis supported previous observations that RS ability was often associated with the presence of lateral meristems responsible for secondary thickening of roots. Moreover, an association of RS with the presence of sclerified cells in the root cortex was newly identified. Separation of RS ability from RS vigour revealed a more frequent occurrence of RS species on dry and open habitats, whereas most vigorous root sprouters tended to occur on dry and frequently disturbed habitats. We observed the highest vigour of bud production among the largest roots, highlighting the importance of reserves and the presence of secondary growth for successful regeneration.
Phylogenetic distribution
Phylogenetic patterns of RS showed that it was a fairly flexible character, supporting findings of Herben and Klimešová (2020) who showed that it had a similar (or even slightly lower) phylogenetic conservation compared with stem-based clonality. Still, there was a discernible phylogenetic pattern in Eudicots, with RS being particularly common in Asteraceae (namely Cichorioidae, but also elsewhere), in Rosaceae and in a number of families from the order Malpighiales (Herben and Klimešová, 2020). While phylogenetic reconstructions of a larger, although less exactly measured, dataset by Herben and Klimešová (2020) implied that RS may be an ancestral character in Rosaceae, this does not seem to be the case in Asteraceae, where the analysis showed dotted patterns indicating a number of parallel evolution events. The presence of RS in almost all species examined (and all three lineages examined) from Malphighiales is particularly remarkable and may need further study, as a number of other lineages in this order are also well known for root sprouting (Rhizophora, Kandelia, Rhizophoraceae; Ogita et al., 2004; Populus and Salix, Salicaceae; Linum, Linaceae; both Klimešová et al., 2017; Indotristicha, Podostemmaceae, Mohan-Ram and Seghal, 1997). Our data, although fairly limited, extend the list of RS-capable genera and may imply that RS may be an ancestral trait of this order, although formal analysis is still impossible due to insufficient species sampled. Root sprouting may provide an additional vegetative morphological character common to this morphologically extremely diverse and species-rich order (Endress et al., 2013).
Anatomical correlates of root sprouting
Root sprouting and non-RS herbaceous species differ in root anatomy: RS species have a lateral meristem and exhibit secondary growth, and their roots contain sclerified cortical cells. This character may be a primary prerequisite for the production of adventitious meristems in eudicot herbs, and the second might be a response to injury.
Our findings imply that herbaceous RS prioritizes the development of pre-formed buds on roots as the majority of buds observed in our experiment are ‘additional’ and have a typical vascular connection with the centre of the root stele (Fig. 2D). We observed ‘reparative buds’ in early stages of development only in K. arvensis (Fig. 2C). The reparative buds arose from the vascular cambium and did not show a vascular connection with the centre of the root. The cambial origin of root buds has already been reported for the aquatic herb Sium latifolium L. (Rauh, 1937) and, in woody plants, root buds developed near the vascular cambium (Hayashi and Appezzato-da-Glória, 2009; Imatomi et al., 2014). The experimental approach used in this study did not allow us to compare a situation with bud formation before and after plant injury, as our observations were carried out only after the bud formation experiment. Therefore, we have only indirect evidence for pre-formation of the majority of adventitious buds, and their origins (additional vs. reparative buds) in herbs warrant further study.
Our analysis also showed a correlation between RS and the presence of sclerified cortical cells in roots, probably because both features were a consequence of plant injury. The process of cell sclerification activated by a changed hormone profile is considered a protective mechanism in plants following tissue injury (Lev-Yadun, 1994; Didi et al., 2015). Sclerified cells are indigestible by herbivores; they inhibit the growth of pathogens and are frequently seen as a response to infection. Their formation, therefore, is expected to be beneficial to injured RS individuals.
In contrast to the ability to resprout from roots, RS vigour, expressed as the number of buds produced per species, was not associated with any specific anatomical pattern. The only linked parameter was root thickness (Table 1; Fig. 5), supporting the hypothesis that differences in resprouting vigour are primarily constrained by the physiological status of plants and the total amount of resources available for regrowth. We speculate that RS species equip their roots with storage capacity in order to allow resprouting when fragmented.
Ecology of root sprouting
We found that RS frequency was greater in species from dry and open habitats (Fig. 6). The combined effects of moisture and light on the distribution of RS species were more pronounced than the effect of disturbance. Wet habitats seem to be unsuitable for RS species (Grace 1993). In the study by Sosnová et al. (2010), RS species were either missing or under-represented in wetland communities of the Netherlands. This could be attributed to the short persistence of roots due to anoxia (Klimešová et al., 2018) that prohibits their secondary thickening, and therefore adventitious bud formation, and to the high incidence of stem-derived organs of clonal growth with increasing habitat wetness (Ye et al., 2014; Klimešová and Herben, 2015). In contrast, disturbed and dry habitats are normally occupied by plants with long persisting roots, i.e. with secondary thickening and with a low number of stem-derived clonal growth organs (Klimešová and Herben, 2015) that are conducive to RS. In this respect, it is remarkable that RS is almost entirely absent from monocots (Herben and Klimešová, 2020), which are likely to have evolved in wet or aquatic habitats (Givnish et al., 2018).
The weak effect of the disturbance regime on the ability to resprout from roots does not support our initial hypothesis that RS is associated with disturbance (see also Klimešová et al., 2017). While we cannot exclude some effects of disturbance because the openness of many habitats is often driven by the combined effects of disturbance severity and frequency, we believe that a weak relationship between RS ability and disturbance is a true pattern, contrasting with the relationship between RS vigour and disturbance. An earlier analysis (Klimešová et al., 2017) used RS data based on field observations, not from controlled experiments as in the current study. Such field observations are likely to have confounded RS ability with the quantity of buds produced (i.e. RS vigour), and may have been responsible for earlier reports claiming a relationship between RS ability and disturbance.
Our analyses did confirm an association between high RS vigour and occurrence on frequently disturbed habitats (e.g. habitats disturbed more than once per year) (Fig. 7) but not in severely disturbed habitats (e.g. habitats where not is only above-ground biomass damaged but the disturbance also reaches the soil profile and below-ground plant organs). This might be explained by a lower number of root sprouters in our analysis in comparison with previous publications (Klimešová et al., 2017) and correlations between disturbance severity and disturbance frequency indices (Herben et al., 2016).
Disturbance regime is the main factor favouring RS vigour (e.g. weeds of arable land). Root sprouting vigour, in our set of species, was also connected with the moisture gradient. The overall preference of RS species for dry open habitats possibly indicates their tolerance for low water availability and a tendency to avoid competition for light. Also thicker roots (higher specific root biomass in our study) could protect from desiccation in a dry environment and support species with vigorous resprouting.
CONCLUSIONS
This study, based on a trial with 182 species of dicotyledonous herbs from Central Europe, constitutes the first experimental examination of RS ability together with RS vigour. Importantly, the experimental approach enabled us to separate the two components of RS – ability and vigour – which were likely to be confounded in earlier studies. It also enabled us to show that these two components were correlated with different anatomical features and different components of each ecological niche. While adventitious sprouting from roots in herbs is not limited to the same degree by anatomy as is sprouting in woody plants, it is clearly associated with secondary thickening of roots as an important prerequisite. In contrast, for resprouting vigour, we should seek an explanation in the physiology of the plants (such as reserves available in the root) and not in their anatomy. Further studies should be devoted to ecophysiological aspects of RS in plants from different habitats differing in sprouting vigour. Such research may reveal new information on the evolution of troublesome weeds.
Our results are based on analysis from a single temperate flora that has been under high selection pressure from human activities (e.g. agriculture) since the beginning of the Holocene. Other biomes and floras with different evolutionary histories, disturbance regimes and environmental factors should be studied to understand the topic properly and to be able to draw conclusions about the generality of our findings. While root sprouting is an easy-to-lose and easy-to-acquire trait, its highly uneven distribution among individual lineages may be associated with different abilities to thrive in dry or disturbed habitats. Further searches for the occurrence of this remarkable trait in several candidate lineages (e.g. Malpighiales, Rosales and Asteraceae) may show more consistent patterns which are now obscured by insufficient or low-quality data.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: list of species, their RS vigour, specific root biomass, families and localities. Table S2: list of species evaluated for anatomy and the whole set of anatomical traits studied. Table S3: correlation matrix of the whole set of anatomical traits. Figure S1: regenerated root fragments of different species. Figure S2: mapping of RS on the phylogenetic tree with full species names.
ACKNOWLEDGEMENTS
We thank Ondřej Mudrák for help with species collection. We also thank Jana Navrátilová, curator of The Collection of aquatic and wetland plants – Hortus Botanicus Třeboň, for providing the species for this study; and the Anatomical Laboratory (Department of Functional Ecology, Institute of Botany of the CAS) for providing their equipment. We are grateful to John D. Brooker for language revision, and two anonymous reviewers for their useful comments.
FUNDING
This research was supported by the Grant Agency of the Czech Republic (GA19-13103S) and long-term research development project of the Czech Academy of Sciences [no. RVO 67985939].
LITERATURE CITED
- Bartušková A, Malíková L, Klimešová J. 2017. Checklist of root-sprouters in the Czech flora: mapping the gaps in our knowledge. Folia Geobotanica 52: 337–343. [Google Scholar]
- Bellingham PJ, Sparrow AD. 2000. Resprouting as a life history strategy in woody plant communities. Oikos 89: 409–416. [Google Scholar]
- Bosela MJ, Ewers FW. 1997. The mode of origin of root buds and root sprouts in the clonal tree Sassafras albidum (Lauraceae). American Journal of Botany 84: 1466–1481. [PubMed] [Google Scholar]
- Bukatsch F. 1972. Bemerkungen zur Doppelfarbung: Astrablau-Safranin. Mikrokosmos 61: 255. [Google Scholar]
- Charlton WA. 1965. Bud initiation in excised roots of Linaria vulgaris. Nature 207: 781–782. [Google Scholar]
- Clarke PJ, Bell DM, Lawes MJ. 2015. Testing the shifting persistence niche concept: plant resprouting along gradients of disturbance. The American Naturalist 185: 747–755. [DOI] [PubMed] [Google Scholar]
- Del Tredici P. 2001. Sprouting in temperate trees: a morphological and ecological review. Botanical Review 67: 121–140. [Google Scholar]
- Didi V, Jackson P, Hejátko J. 2015. Hormonal regulation of secondary cell wall formation. Journal of Experimental Botany 66: 5015–5027. [DOI] [PubMed] [Google Scholar]
- Diekmann M. 2003. Species indicator values as an important tool in applied plant ecology – a review. Basic and Applied Ecology 4: 493–506. [Google Scholar]
- Dore J. 1955. Studies in regeneration of horseradish: I. A re-examination of the morphology and anatomy of regeneration. Annals of Botany 19: 127–137. [Google Scholar]
- Durka W, Michalski SG. 2012. Daphne: a dated phylogeny of a large European flora for phylogenetically informed ecological analyses. Ecology 93: 2297–2297. [Google Scholar]
- Ellenberg H, Weber HE, Düll R, Wirth V, Werner W, Paulissen D. 1991. Zeigerwerte von Pflanzen in Mitteleuropa. Scripta Geobotanica 18: 1– 248. [Google Scholar]
- Endress PK, Davis CC, Matthews ML. 2013. Advances in the floral structural characterization of the major subclades of Malpighiales, one of the largest orders of flowering plants. Annals of Botany 111: 969–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrini M, Cionini G, Conti A, Michelotti V, Pugliesi C. 2003. Origin and development in vitro of shoot buds and somatic embryos from intact roots of Helianthus annuus × H. tuberosus. Annals of Botany 92: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givnish TJ, Zuluaga A, Spalink D, et al. 2018. Monocot plastid phylogenomics, timeline, net rates of species diversification, the power of multi-gene analyses, and a functional model for the origin of monocots. American Journal of Botany 105: 1888–1910. [DOI] [PubMed] [Google Scholar]
- Gleason HA. 1926. The individualistic concept of the plant association. Bulletin of the Torrey Botanical Club 53: 7–26. [Google Scholar]
- Grace JB. 1993. The adaptive significance of clonal reproduction in angiosperms: an aquatic perspective. Aquatic Botany 44: 159–180. [Google Scholar]
- Grime JP. 1979. Plant strategies and vegetation processes. Chichester: Wiley. [Google Scholar]
- Groff PA, Kaplan DR. 1988. The relation of root systems to shoot systems in vascular plants. Botanical Review 54: 387–422. [Google Scholar]
- Grubb PJ. 1977. The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biological Reviews 52: 107–145. [Google Scholar]
- Guerrero-Campo J, Palacio S, Pérez-Rontomé C, Montserrat-Martí G. 2006. Effect of root system morphology on root-sprouting and shoot-rooting abilities in 123 plant species from eroded lands in North-east Spain. Annals of Botany 98: 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R Package. Journal of Statistical Software 33: 1–22. [PMC free article] [PubMed] [Google Scholar]
- Hamdoun AM. 1970. The anatomy of subterranean structures of Cirsium arvense (L.) Scop. Weed Research 12: 128–136. [Google Scholar]
- Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. 2008. GEIGER: investigating evolutionary radiations. Bioinformatics (Oxford, England) 24: 129–131. [DOI] [PubMed] [Google Scholar]
- Hayashi AH, Appezzato-da-Glória B. 2009. Resprouting from roots in four Brazilian tree species. Revista De Biologia Tropical 57: 789–800. [DOI] [PubMed] [Google Scholar]
- Herben T, Klimešová J. 2020. Evolution of clonal growth forms in angiosperms. New Phytologist 225: 999–1010. [DOI] [PubMed] [Google Scholar]
- Herben T, Chytrý M, Klimešová J. 2016. A quest for species-level indicator values for disturbance. Journal of Vegetation Science 27: 628–636. [Google Scholar]
- Herben T, Klimešová J, Chytrý M. 2018. Effects of disturbance frequency and severity on plant traits: an assessment across a temperate flora. Functional Ecology 32: 799–808. [Google Scholar]
- Horvath DP, Anderson JV, Chao WS, Foley ME. 2003. Knowing when to grow: signals regulating bud dormancy. Trends in Plant Science 8: 534–540. [DOI] [PubMed] [Google Scholar]
- Imatomi M, Souza JP, Gualtieri SCJ, Ferreira AG. 2014. The role of root buds in the regeneration of Casearia sylvestris Swartz (Salicaceae) in the Cerrado, São Carlos, São Paulo State, Brazil. Hoehnea 41: 345–352. [Google Scholar]
- Kataoka EY, Alves DM, Koch I, Souto LS. 2019. Are there buds in the roots of Aspidosperma spp. (Apocynaceae)? A comparative morphoanatomical study of underground organs. Flora 256: 92–99. [Google Scholar]
- Klimešová J. 2007. Root-sprouting in myco-heterotrophic plants: prepackaged symbioses or overcoming meristem limitation? New Phytologist 173: 8–10. [DOI] [PubMed] [Google Scholar]
- Klimešová J, Herben T. 2015. Clonal and bud bank traits: patterns across temperate plant communities. Journal of Vegetation Science 26: 243–253. [Google Scholar]
- Klimešová J, Martínková J. 2004. Intermediate growth forms as a model for the study of plant clonality functioning: an example with root sprouters. Evolutionary Ecology 18: 669–681. [Google Scholar]
- Klimešová J, Sosnová M, Martínková J. 2007. Life-history variation in the short-lived herb Rorippa palustris: effect of germination date and injury timing. Plant Ecology 189: 237–246. [Google Scholar]
- Klimešová J, Kociánová A, Martínková J. 2008. Weeds that can do both tricks: vegetative versus generative regeneration of the short-lived root-sprouting herbs Rorippa palustris and Barbarea vulgaris. Weed Research 48: 131–135. [Google Scholar]
- Klimešová J, Herben T, Martínková J. 2017. Disturbance is an important factor in the evolution and distribution of root-sprouting species. Evolutionary Ecology 31: 387–399. [Google Scholar]
- Klimešová J, Martínková J, Ottaviani G. 2018. Belowground plant functional ecology: towards an integrated perspective. Functional Ecology 32: 2115–2126. [Google Scholar]
- Lev-Yadun S. 1994. Induction of sclereid differentiation in the pith of Arabidopsis thaliana (L.) Heynh. Journal of Experimental Botany 45: 1845–1849. [Google Scholar]
- Liew J, Andersson L, Bostrom U, Forkman J, Hakman I, Magnuski E. 2013. Regeneration capacity from buds on roots and rhizomes in five herbaceous perennials as affected by time of fragmentation. Plant Ecology 214: 1199–1209. [Google Scholar]
- Lososová Z, Šmarda P, Chytrý M, et al. 2015. Phylogenetic structure of plant species pools reflects habitat age on the geological time scale. Journal of Vegetation Science 26: 1080–1089. [Google Scholar]
- Martínková J, Klimešová J. 2016. Enforced clonality confers a fitness advantage. Frontiers in Plant Science 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan Ram HY, Sehgal A. 1997. In vitro studies on developmental morphology of Indian Podostemaceae. Aquatic Botany 57: 97–132. [Google Scholar]
- Ogita S, Yeung EC, Sasamoto H. 2004. Histological analysis in shoot organogenesis from hypocotyl explants of Kandelia candel (Rhizophoraceae). Journal of Plant Research 117: 457–464. [DOI] [PubMed] [Google Scholar]
- Ott JP, Klimešová J, Hartnett DC. 2019. The ecology and significance of below-ground bud banks in plants. Annals of Botany 123: 1099–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula S, Naulin PI, Arce C, Galaz C, Pausas JG. 2016. Lignotubers in Mediterranean basin plants. Plant Ecology 217: 661–676. [Google Scholar]
- Pausas JG, Bond WJ. 2018. Humboldt and the reinvention of nature. Journal of Ecology 00: 1–7. [Google Scholar]
- Pausas JG, Lamont BB, Paula S, Appezzato-da-Glória B, Fidelis A. 2018. Unearthing belowground bud banks in fire-prone ecosystems. New Phytologist 217: 1435–1448. [DOI] [PubMed] [Google Scholar]
- Peterson RL. 1975. The initiation and development of root buds. In: Torrey JG, Clarcson DT, eds. The development and function of roots. London: Academic Press, 125–161. [Google Scholar]
- Polowick PL, Raju MVS. 1982. The origin and development of root buds in Asclepias syriaca. Canadian Journal of Botany 60: 2119–2125. [Google Scholar]
- Raju MVS, Coupland RT, Steeves TA. 1966. On the occurrence of root buds on perennial plants in Saskatchewan. Canadian Journal of Botany 44: 33–36. [Google Scholar]
- Rauh W. 1937. Die Bildung von Hypocotyl- und Wurzelsprossen und ihre Bedeutung für die Wuchsformen der Pflanzen. Nova Acta Leopoldina 4: 395–555. [Google Scholar]
- Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Sosnová M, van Diggelen R, Klimešová J. 2010. Distribution of clonal growth forms in wetlands. Aquatic Botany 92: 33–39. [Google Scholar]
- Taab A, Andersson L, Boström U. 2018. Modelling the sprouting capacity from underground buds of the perennial weed Sonchus arvensis. Weed Research 58: 348–356. [Google Scholar]
- Vesk PA, Westoby M. 2004. Sprouting ability across diverse disturbances and vegetation types worldwide. Journal of Ecology 92: 310–320. [Google Scholar]
- Wittrock VB. 1884. Ueber Wurzelsprossen bei krautartigen Gewächsen, mit besonderer Rücksicht auf ihre verschiedene biologische Bedeutung. Botanisches Centralblatt 17: 227–232, 257–264. [Google Scholar]
- Wood SN. 2003. Thin-plate regression splines. Journal of the Royal Statistical Society (B) 65: 95–114. [Google Scholar]
- Ye D, Hu Y, Song M, et al. 2014. Clonality–climate relationships along latitudinal gradient across China: adaptation of clonality to environments. PLoS One 9: e94009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.