Abstract
Background and Aims
The centre–periphery hypothesis posits that higher species performance is expected in geographic and ecological centres rather than in peripheral populations. However, this is not the commonly found pattern; therefore, alternative approaches, including the historical dimension of species geographical ranges, should be explored. Morphological functional traits are fundamental determinants of species performance, commonly related to environmental stability and productivity. We tested whether or not historical processes may have shaped variations in tree and leaf traits of the Chaco tree Bulnesia sarmientoi.
Methods
Morphological variation patterns were analysed from three centre–periphery approaches: geographical, ecological and historical. Tree (stem and canopy) and leaf (leaf size and specific leaf area) traits were measured in 24 populations across the species range. A principal component analysis was performed on morphological traits to obtain synthetic variables. Linear mixed-effects models were used to test which of the implemented centre–periphery approaches significantly explained trait spatial patterns.
Key Results
The patterns retrieved from the three centre–periphery approaches were not concordant. The historical approach revealed that trees were shorter in centre populations than in the periphery. Significant differences in leaf traits were observed between the geographical centre and the periphery, mainly due to low specific leaf area values towards the geographical centre. We did not find any pattern associated with the ecological centre–periphery approach.
Conclusions
The decoupled response between leaf and tree traits suggests that these sets of traits respond differently to processes occurring at different times. The geographical and historical approaches showed centres with extreme environments in relation to their respective peripheries, but the historical centre has also been a climatically stable area since the Last Glacial Maximum. The historical approach allowed for the recovery of historical processes underlying variation in tree traits, highlighting that centre–periphery delimitations should be based on a multi-approach framework.
Keywords: Centre–periphery hypothesis, Gran Chaco Americano, ecological niche modelling, geographical centre, intraspecific traits, Bulnesia sarmientoi, marginal plant populations, niche centroid, refugium
INTRODUCTION
The centre–periphery hypothesis (CPH) is one of the main frameworks used to study population performance across a species range (see a comprehensive review in Pironon et al., 2017). This hypothesis assumes that the geographic central area is the site where conditions are more favourable and stable (Brown, 1984), and where species should be more abundant or have better performance, decreasing towards the geographic periphery (Brown, 1984; Sexton et al., 2009; Abeli et al., 2020). A central assumption of CPH is that geographical and environmental spaces are concordant (Brown, 1984); however, in recent years, predictions derived from the CPH are not the commonly found patterns (Sagarin and Gaines, 2002; Abeli et al., 2014; Santini et al., 2019). The main explanation for the absence of centre–periphery patterns under CPH predictions is the widely accepted statement that the geographical and environmental spaces are not concordant (Martínez-Meyer et al., 2013; Van Couwenberghe et al., 2013; Santini et al., 2019), i.e. not all ecologically marginal populations are located on the geographical periphery, and vice versa (Soulé, 1973). More recent studies have found that population dynamics is associated with distance to the ecological centre, also called the niche centroid (e.g. Martínez-Meyer et al., 2013; Weber et al., 2017; Osorio-Olvera et al., 2019).
Species performance has been extensively studied along a geographical and ecological centre–periphery gradient, focusing on the current distribution of the focal species (e.g. Carey et al., 1995; Nantel and Gagnon, 1999; Jump and Woodward, 2003; Costa et al., 2016), and on the current ecological environment, more recently based on ecological niche modelling (ENM) (e.g. Martínez-Meyer et al., 2013; Weber et al., 2017; Osorio-Olvera et al., 2019). However, these studies do not take into account historical dimensions of species range, such as past environmental conditions [e.g. from the Last Glacial Maximum (LGM)]. These conditions, which change through time, create stable/unstable areas that could affect species traits that respond to long-term environmental conditions, consequently influencing species performance. Therefore, there is a need to incorporate a historical approach to species performance studies (Keppel et al., 2012; Tzedakis et al., 2013; Pironon et al., 2015). Further, ENM is a tool to identify climatically stable areas for long periods of time, under a historical niche perspective. Indeed, reconstruction of palaeo- and current distributions through ENM, together with the study of genetic patterns, is a multidisciplinary framework to identify stable areas (e.g. Waltari et al., 2007; Collevatti et al., 2012; Baranzelli et al., 2017).
The historical approach has been recently used in genetic (e.g. Eckert et al., 2008; Duncan et al., 2015; Pironon et al., 2015) and demographic (e.g. abundance, germination and growth rate; Pironon et al., 2015; Douda et al., 2019) studies, evidencing its importance in significantly explaining species performance patterns (e.g. Pironon et al., 2015; Douda et al., 2019). Nonetheless, other important traits, such as morphological traits, remain unexplored from this perspective. Historically stable areas preserve the evolutionary history of lineages (Keppel et al., 2012; Tzedakis et al., 2013), and could harbour populations (and communities) where morphological traits are expressed differently from unstable areas (e.g. Myking and Yakovlev, 2006; Hatziskakis et al., 2011; Varsamis et al., 2020), including some traits related to the species performance. Variation or changes of some morphological traits may be linked to past species distribution rather than current ecological conditions (Douda et al., 2019), especially traits that have a long-term response to environmental conditions, or are strongly influenced by abiotic factors modelled over long periods of time (such as edaphic factors that influence tree height).
The study of morphological traits that determine species’ performance has fundamental implications, both in the field of evolutionary ecology and in conservation biology (Sagarin et al., 2006). Traits that influence performance are important resources to find high genetic diversity or geographic locations of genetic uniqueness (Lesica and Allendorf, 1995; Lira-Noriega and Manthey, 2014; Macdonald et al., 2017), to describe the relationships between species and the community (Fulton, 1999; Clark et al., 2011; Lebrija-Trejos et al., 2016), to understand environmental preferences (Díaz et al., 2004; Wright et al., 2004; Moles et al., 2009; Marks et al., 2016; Baranzelli et al., 2020) and also as descriptors of the environment of a population or species (Díaz and Cabido, 2001), among other aspects. Specifically, functional traits in plants, such as leaf size, longevity, and canopy height and structure, are subject to environmental selection pressures (Díaz and Cabido, 2001); therefore, they are a fundamental determinant of species performance, in general related to environmental stability and productivity (Violle et al., 2007), that should be explored under the historical centre–periphery approach. Unlike other traits that influence species performance, such as demographic, genetic or physiological traits, morphological functional traits are useful to understand environmental selective pressures and climatic changes more directly (Díaz and Cabido, 2001; Violle et al., 2007), and to compare attributes (i.e. measurements) of each trait from distant ecosystems (Reich et al., 1997; Díaz et al., 2004); additionally, obtaining those attributes arises as an easy and inexpensive method (Pérez-Harguindeguy et al., 2013).
In this study, we propose the inclusion of morphological traits in centre–periphery studies adding a historical approach to the geographical and ecological approaches. Specifically, we present the case of the forest tree Bulnesia sarmientoi Lorentz ex Griseb. (Zygophyllaceae), an endangered species, distributed in central–northern Argentina, south-east Bolivia, western Paraguay and marginally in Brazil (Zuloaga et al., 2008; Waller et al., 2012). We selected B. sarmientoi as a model species because a previous phylogeographical and niche modelling study (Camps et al., 2018) revealed that the genetic diversity, past demography and distribution range of the species were significantly affected by Pleistocene climatic fluctuations. Moreover, a Pleistocene refugium for B. sarmientoi was proposed in a climatically stable area from the LGM up to the present, which harbours the highest values of genetic diversity (Camps et al., 2018). Additionally, a previous ecological study revealed a significant variation in diameter, height and population density of B. sarmientoi, suggesting differences in growth dynamics across its range (Loto et al., 2018).
Based on the previous phylogeographical study of B. sarmientoi (Camps et al., 2018) showing a significant imprint of past climatic changes on the evolutionary history of the focal species, our general hypothesis is that the historical processes would have shaped variations in B. sarmientoi tree and leaf traits; therefore, the historical approach is expected to be a better predictor of population performance than the approaches that consider the geographical or ecological space, finding differences in morphological traits between the historical centre, representing a climatically stable area, and the historical periphery. To test our hypothesis, we used and compared three approaches (geographical, ecological and historical) to define central and peripheral populations, and examined which of them best explains morphological variation in B. sarmientoi. The ecological (i.e. geographical distance to the current niche centroid) and the historical (i.e. geographical distance to the LGM niche centre) approaches were based on Camps et al. (2018).
MATERIALS AND METHODS
Study area and species data
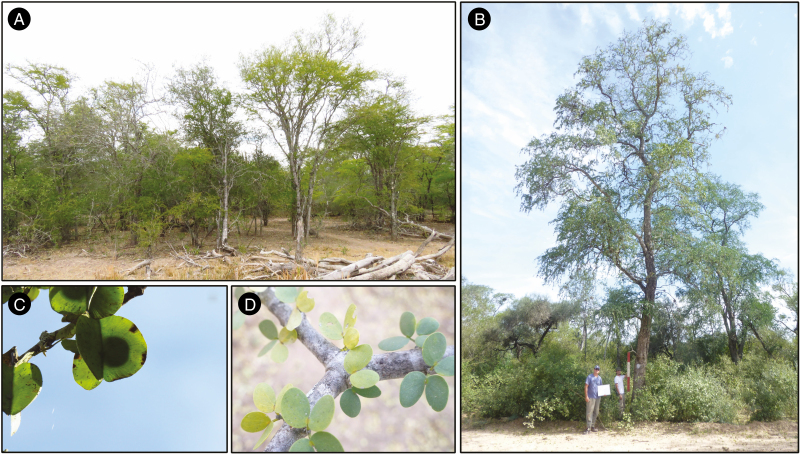
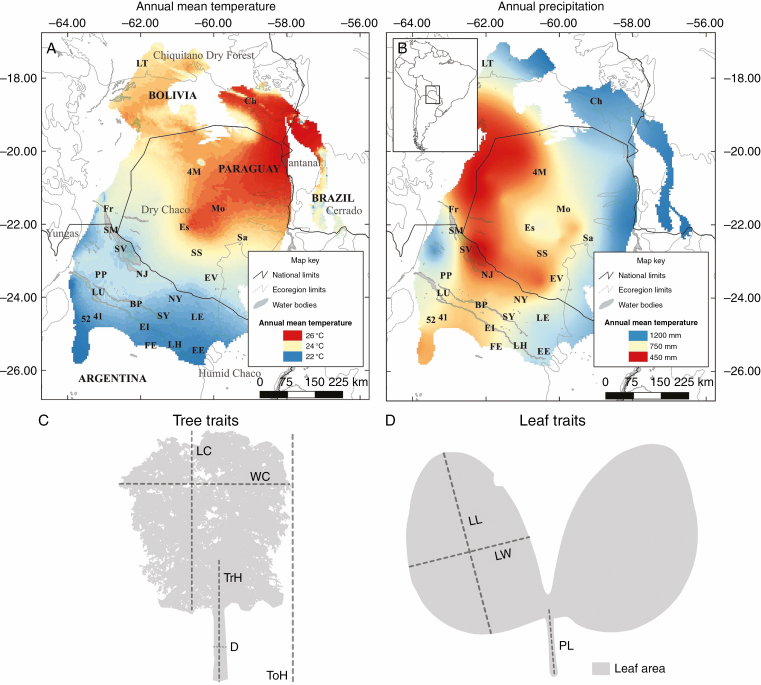
Bulnesia sarmientoi is a large tree species (up to 20 m) (Fig. 1); it is dominant in heavy clay-rich soils, with highly impeded drainage and temporary anaerobiosis (Adamoli et al., 1972; Prado, 1993). The species is distributed in central–northern Argentina, south-east Bolivia, western Paraguay and marginally in Brazil (Zuloaga et al., 2008; Waller et al., 2012) (Fig. 2). The distribution area mainly includes the Dry Chaco ecoregion and marginally the Humid Chaco, Chiquitano Dry Forest and Pantanal, according to the classification of Olson et al. (2001). Temperature in the B. sarmientoi distribution range can reach an annual mean close to 26 °C, whereas the minimum annual mean temperature in the area is 22 °C [MERRAclim database (Vega et al., 2017)]; the entire region is a warm, seasonal environment. In contrast, the precipitation gradient is more marked, with higher rainfall in the north (1200 mm) and south-east (850 mm), and lower in the centre–west (450 mm) [Worldclim database (Hijmans et al., 2005)] (Fig. 2). The precipitation gradient ranges from semi-arid to humid, determining different ecoregions and ecotone zones (Cabrera, 1976; Olson et al., 2001).
Fig. 1.
Bulnesia sarmientoi in its natural habitat. (A) ‘Palosantal’, i.e. a particular vegetation type usually monospecific and conformed by small trees. (B) Single or very few tall B. sarmientoi trees in a degraded mixed forest. (C) Immature fruit. (D) Leaves.
Fig. 2.
Distribution of Bulnesia sarmientoi, bioclimatic variables and morphological traits measured across the species distribution range. (A) Annual mean temperature. (B) Annual precipitation. (C) Traits measured on trees. D, trunk diameter; LC, canopy length; ToH, total height; TrH, trunk height; WC, canopy width. (D) Traits measured on leaf. LL, leaf length; LW, leaf width; PL, petiole length. Bioclimatic variables obtained from MERRAclim (Vega et al., 2017) and the Worldclim database (Hijmans et al., 2005). Population codes are given in Supplementary data Table S1.
Sampling and trait measurements
The morphological traits of stem, canopy (both hereafter called ‘tree traits’) and leaves (hereafter called ‘leaf traits’) of mature individuals of B. sarmientoi were studied in 24 populations. Sampled populations covered the entire distribution range of B. sarmientoi, except for the Pantanal ecoregion populations. At this point, it is important to mention that the few samples from the northern portion of the range reflect the natural distribution and abundance of the species, so it is not a sampling bias. The collecting procedure and minimum number of samples was performed following Pérez-Harguindeguy et al. (2013). To obtain tree traits, digital photographs of the five tallest mature individuals per population (118 trees total; in two populations only four trees were measured) were taken in a straight line to the tree, with the lens pointing towards the trunk, at breast height. Next to the trunk, a 150 cm wooden plank (demarcated every 50 cm) was placed (see Fig. 1B), which served as a reference to measure the tree traits in the software HOJA 3.6 (Verga, 2015). The tree traits measured were: canopy length (LC), canopy width (WC), total height (ToH), trunk height (TrH), trunk diameter (D), total height/canopy width ratio (RHC) and total height/trunk diameter ratio (RHD) (Fig. 2). These traits were measured because B. sarmientoi showed variation in size (e.g. diameter and height) according to the type of forest where they grew (Loto et al, 2018). In addition, height is recognized as a trait indicating resource availability (Fulton, 1999; Marks et al., 2016), while canopy structure showed intraspecific variation associated with environmental conditions and interspecific competition (e.g. Benavides et al., 2019; Lang et al., 2019; Ding et al., 2020).
Five leaves per tree, from five trees per population, were collected and measured (600 leaves in total). The leaves were selected randomly from the middle portion of different branches that were in intermediate parts of the treetop, whenever possible. Digital images obtained from scanned leaves were used to take leaf measurements using HOJA 3.6. The leaf traits measured were: dry mass (DM), leaf area (LA), leaf length, leaf width (LW), petiole length (PL), specific leaf area (SLA), total leaf area (TLA) and length/width ratio (RLW) (Fig. 2). Measured leaf traits were selected based on previous studies, suggesting that SLA variation is associated with resource availability (Díaz et al., 2004; Wright et al., 2004), and leaf size variation with moisture availability or aridity (e.g. Kleinschmit, 1993; Souza et al., 2018; Li et al., 2020). To obtain DM, the leaves were oven-dried at 70 °C for 80 h. SLA was calculated as a one-sided area of a fresh leaf, divided by its oven-dry mass.
Geographical approach
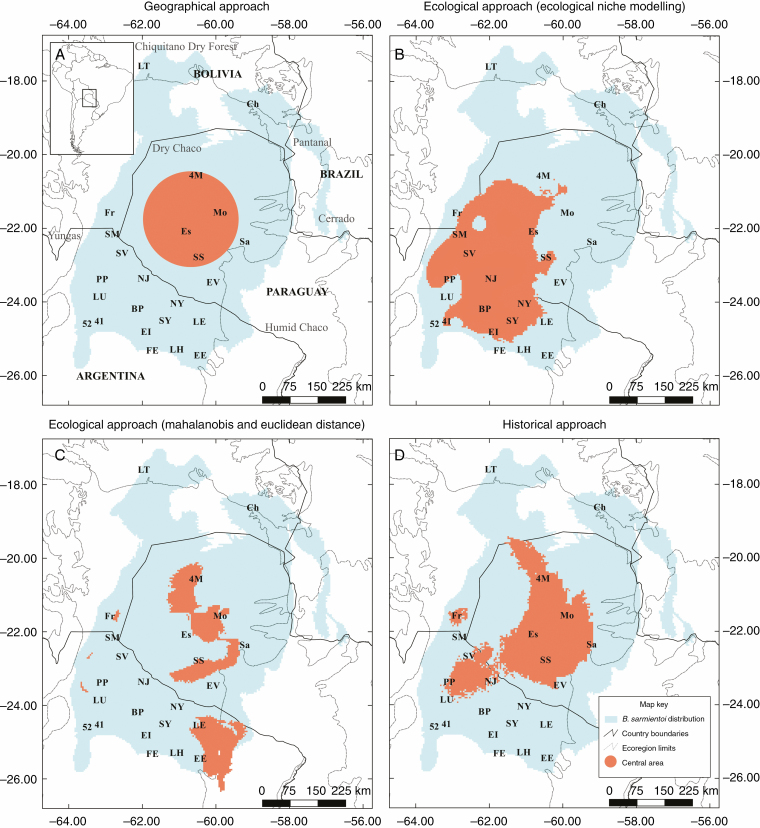
The geographical approach consisted of evaluating how tree and leaf traits vary across the distribution range with respect to the geographical centre (GC) and the geographical periphery (GP). The GC was calculated with the polygon centroid tool, implemented with SAGA 2.3.2 in Quantum GIS 2.18 (QGIS Development Team, http://www.qgis.org/). The centre has an area of 61 930 km2, which is located equidistant to the north/south and east/west largest dimensions of the total distribution range of B. sarmientoi (412 864 km2). Each population was classified as GC or GP (Fig. 3).
Fig. 3.
Maps of centre–periphery approaches explored in Bulnesia sarmientoi. (A) Geographical approach. (B) Ecological approach based on ecological niche modelling analysis. (C) Ecological approach based on Mahalanobis and Euclidean distances to the climatic centroid. (D) Historical approach. Bulnesia sarmientoi distribution was delimited by Camps et al. (2018). Population codes are given in Supplementary data Table S1.
Ecological approach
The ecological approach consisted of evaluating how tree and leaf traits vary in two components related to the niche centroid, calculated from current climatic conditions. Two methods were used to calculate the niche centroid: the first one was calculated from ENM analysis, and the other from Mahalanobis and Euclidean distances. We consider the calculation of two different niche centroids because ENM is widely used to test the CPH, but the distances seem to be better indicators of environmental suitability, mainly the Mahalanobis distance (Osorio-Olvera et al., 2019).
The niche centroid calculation was derived from an ENM analysis of B. sarmientoi previously reported (Camps et al., 2018). In that study, seven algorithms were tested and the best three were selected according to the validation metrics (Camps et al., 2018). We used those three best algorithms (Table 1) to obtain a niche centre of the species considering a probabilistic range. The niche centre was calculated as a range that represented the highest 15 % probability of each algorithm (Table 1), taking the threshold as the minimum value of that range, and the highest probabilistic value as the maximum value. The ranges (i.e. niche centre) inferred with the three algorithms were overlapped on a map to obtain a consensus area representing the climatically most favourable area for the species (Fig. 3). Then, each studied population was classified as niche centroid (NC_ENM) when it was included in the consensus area, or as niche periphery (NP_ENM) when it was outside the favourability area.
Table 1.
Minimum value (threshold), greater probabilistic value and 15 % upper range of the three algorithms used for the Bulnesia sarmientoi ecological niche modelling
| Algorithm | Threshold | Greater probabilistic value | 15 % upper range |
|---|---|---|---|
| Bioclim (Busby, 1991) | 0.013 | 0.759 | 0.647–0.759 |
| Support vector machine (Vapnik, 1998) | 0.027 | 0.040 | 0.038–0.040 |
| Maxent (Phillips et al., 2006) | 0.115 | 0.717 | 0.627–0.717 |
The second niche centroid was calculated using the Mahalanobis and Euclidean distances, also taken from Camps et al. (2018), and was based on the distance to the fundamental niche of the species, defined by the three variables that contributed most to the ENM mentioned above (mean temperature of the most humid quarter, specific humidity mean of the coldest quarter and annual mean specific humidity). The average of Mahalanobis and Euclidean distances to the climatic centroid, called suitability (varying between 0 and 1), was used to classify the studied populations as central or peripheral (Fig. 3). Populations with suitability values >0.85 were considered to be in the niche centroid (NC_ME), and populations with suitability values <0.85 were considered to be in the niche periphery (NP_ME).
A buffer area of 6.28 km (maximum diagonal distance of one pixel) was considered for these two niche centroid calculations. Thus, any sampled population that was found outside the centroid, but <6.28 km away from that area, was considered part of the niche centroid. The buffer distance was calculated in Quantum GIS 2.18.
Historical approach
The historical centre–periphery approach consisted of defining a historical central area based on previous phylogeographic and paleodistribution analyses (Camps et al., 2018). In that study, a putative climatic refugium for B. sarmientoi was proposed; this area has been climatically stable from the LGM up to the present, and is currently a hotspot of genetic diversity (Camps et al., 2018). In the same way as the ecological approach, a buffer area of 6.28 km was considered. Thus, we considered populations to be in the historical centre (HC) if they were found in the area of the climatic refugium, or to be in the historical periphery (HP) if they were found outside the refugium (Fig. 3).
Statistical analyses
Measured tree and leaf traits were standardized, and only those traits not strongly correlated (Pearson correlation value <0.8) were included for subsequent analyses (see Supplementary data Figs S1 and S2). To assess the presence of distance-based patterns of variation in morphological traits, a spatial autocorrelation analysis was performed for each leaf and tree trait. Significance levels of Moran’s I coefficients of spatial autocorrelation were obtained using Monte Carlo methods with 999 simulations. Three different distances were tested, according to the minimum, average and maximum distance that separates the studied populations (40, 70 and 100 km, respectively). These analyses were performed in InfoStat v.2018 (Di Rienzo et al., 2018).
For each centre–periphery approach, a principal component analysis (PCA) and a linear mixed-effects model (LMM) were performed, each with one set of morphological traits (i.e. tree and leaf). The PCA was used to obtain new, fully uncorrelated variables, which synthesize the information from the original dataset (McGarigal et al., 2000). For the PCA, the ‘princomp’ methodology and the ‘ggbiplot’ library (Vu, 2016) were used in R 3.4.4 (R Core Team, 2018 through RStudio 1.1.4 (RStudio Team, 2015). The number of components to be retained was based on parallel analysis (Horn, 1965) with 5000 iterations (adjusted eigenvalue >1) using the ‘paran’ R package (Dinno, 2018). The retained components of the PCA were saved and used in the LMM.
The LMM was performed to test which of the implemented centre–periphery approaches significantly explained morphological patterns, using the new variables obtained with the PCA as response variables, and the population location (centre vs. periphery) as a fixed factor. A mixed-effects model was chosen to incorporate and control the absence of independence of the data from the same population, using ‘population’ as a random factor. The LMM was performed using the ‘nlme’ library (Pinheiro et al., 2020) of R 3.4.4 through RStudio 1.1.4. Each retained component of leaf and tree variation presented a normal distribution (Shapiro–Wilks test P > 0.05; the residues were adjusted approximately to the theoretical normal line).
RESULTS
Analyses of morphological traits
The tree traits measured in Bulnesia sarmientoi that were not strongly correlated (Pearson correlation value <0.8) were: tree height/canopy width ratio (RHC), height/trunk diameter ratio (RHD), total height (ToH) and trunk height (TrH). On the other hand, the leaf traits that did not show a strong correlation (Pearson correlation value <0.8) were: dry mass (DM), leaf length (LL), petiole length (PL), length/width ratio (RLW) and specific leaf area (SLA). Correlation matrices and a summary of the morphological measures obtained for each sampled population are shown in Supplementary data Figs S1 and S2, and Table S1, respectively). The spatial autocorrelation analysis indicated that none of the measured morphological traits (i.e. tree and leaf traits) shows a significant distance-based pattern (Moran’s I P-value >0.05) in any of the three tested spatial distances.
For the PCA performed to obtain the new synthetic variables from tree traits, two components were retained, representing 80.9 % of the total tree trait variation. ToH and TrH traits made the greatest contribution to principal component (PC) 1 (–0.656 and –0.605, respectively), and the RHC trait to PC2 (0.807). For the PCA of leaf traits, two PCs were retained, summarizing 68 % of the total variance. DM and LL were the most variable traits along PC1 (–0.594 and –0.565, respectively) and SLA was the most variable along PC2 (0.825).
Geographic centre–periphery
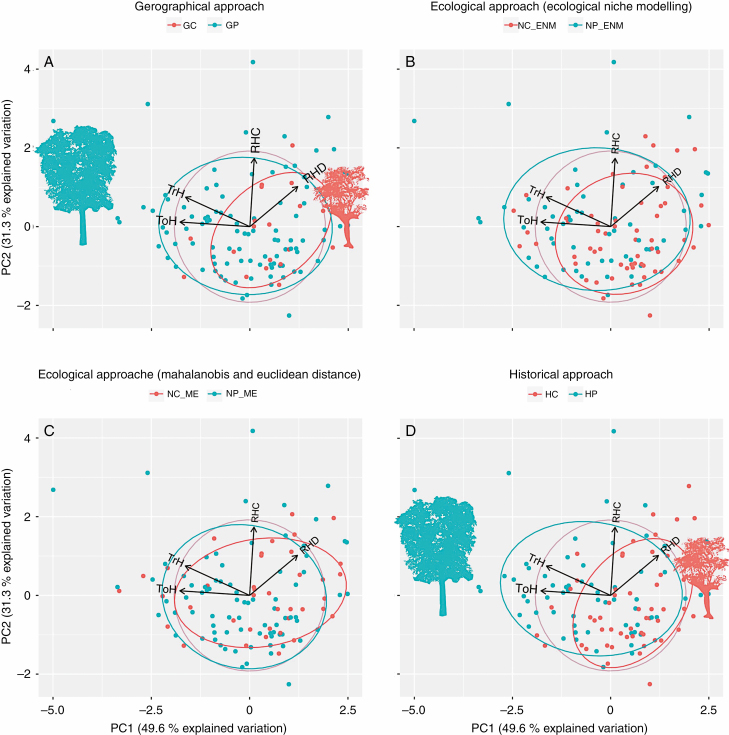
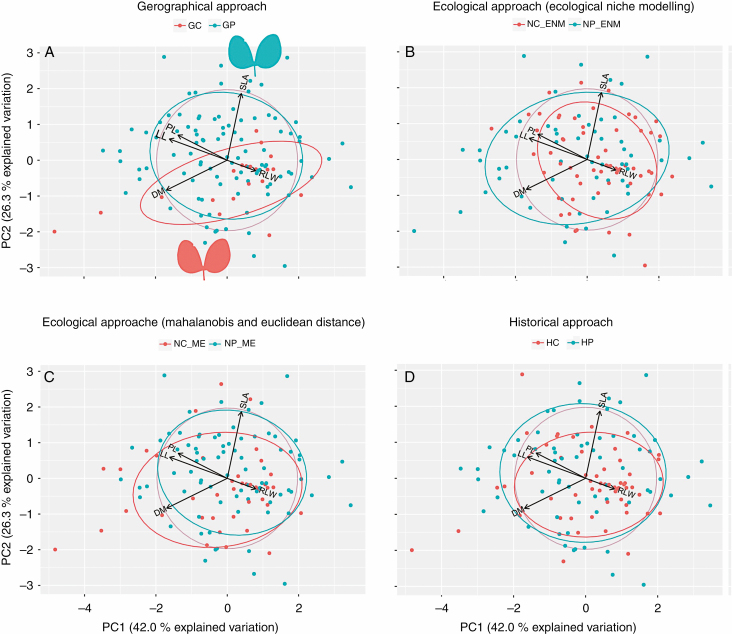
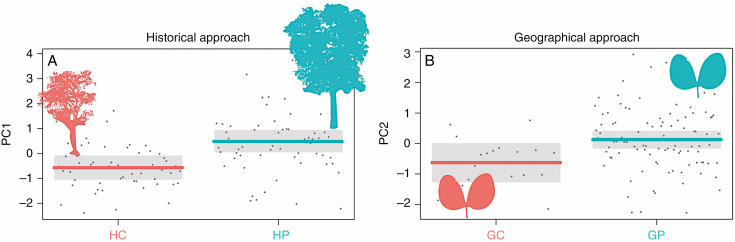
Climatically, the geographical centre was a dry warm environment, while the periphery was a warm rainy environment (see Fig. 2). Geographical central and peripheral populations showed a differentiation in the multivariate space of tree traits (Fig. 4A). The biplot of the PCA shows trees from central localities to the right of the plots, presenting lower values of TrH and ToH than those located on the periphery. However, the LMM showed no significant differences between the centre and periphery in tree traits, on either PC axis (Table 2). Regarding leaf variation, the PCA presented a differentiation between the geographical centre and the periphery in the multivariate space (Fig. 5A). The biplot of the PCA shows those individuals that belong to the geographical centre mainly differentiated from peripheral populations in relation to PC2, indicating small SLA values in the geographical centre. LMM confirmed the significant differences between the centre and periphery in the leaf traits (PC2) (Table 2; Fig. 6B).
Fig. 4.
Principal component analysis graphics of tree traits. (A) Geographical approach. (B) Ecological approach based on ecological niche modelling analysis. (C) Ecological approach based on Mahalanobis and Euclidean distances to the climatic centroid. (D) Historical approach. The contribution of each trait is proportional to the length of its corresponding vector. RHC, height/canopy width ratio; RHD, height/trunk diameter ratio; ToH, total height; TrH, trunk height; GC, geographical centre; GP, geographic periphery; NC_ENM, niche centroid; NP_ENM, niche periphery; NC_ME, niche centroid; NP_ME, niche periphery; HC, historic centre; HP. historical periphery.
Table 2.
Results of the linear mixed-effects models to test the effect of centre–periphery in leaf and tree traits summarized on principal component axes
| Approach and variables | Tree traits | Leaf traits | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | s.e. | d.f. | t-value | P-value | Estimate | s.e. | d.f. | t-value | P-value | |
| 1. Geographical | ||||||||||
| PC1 | 0.66 | 0.52 | 22 | 1.3 | 0.22 | 0.13 | 0.52 | 22 | 0.25 | 0.80 |
| PC2 | 0.115 | 0.33 | 22 | 0.35 | 0.73 | 0.76 | 0.36 | 22 | 2.1 | 0.048 |
| 2a. Ecological (ecological niche modelling) | ||||||||||
| PC1 | 0.7 | 0.38 | 22 | 1.9 | 0.077 | 0.56 | 0.37 | 22 | 1.5 | 0.15 |
| PC2 | 0.8 | 0.24 | 22 | 1.6 | 0.12 | 0.058 | 0.29 | 22 | 0.2 | 0.84 |
| 2b. Ecological (Mahalanobis and Euclidean distances) | ||||||||||
| PC1 | 0.36 | 0.42 | 22 | 0.84 | 0.41 | -0.41 | 0.40 | 22 | –1.01 | 0.32 |
| PC2 | –0.089 | 0.26 | 22 | –034 | 0.74 | 0.48 | 0.29 | 22 | 1.6 | 0.12 |
| 3. Historical | ||||||||||
| PC1 | 1.06 | 0.34 | 22 | 3.1 | 0.005 | 0.161 | 0.39 | 22 | 0.41 | 0.68 |
| PC2 | 0.37 | 0.24 | 22 | 1.6 | 0.13 | 0.32 | 0.29 | 22 | 1.12 | 0.27 |
Significant effects (P ≤ 0.05) are shown in bold. PC1, principal component 1; PC2, principal component 2.
Fig. 5.
Principal component analysis graphics of tree traits. (A) Geographical approach. (B) Ecological approach based on ecological niche modelling analysis. (C) Ecological approach based on Mahalanobis and Euclidean distances to the climatic centroid. (D) Historical approach. The contribution of each trait is proportional to the length of its corresponding vector. DM, dry mass; LL, leaf length; PL, petiole length; RLW, length/width ratio; SLA, specific leaf area; GC, geographical centre; GP, geographical periphery; NC_ENM, niche centroid; NP_ENM, niche periphery; NC_ME, niche centroid; NP_ME, niche periphery; HC, historical centre; HP, historical periphery.
Fig. 6.
Graphics for inear mixed-effects models (LMMs). Only the LMMs that indicated significant differences (P < 0.05) are shown. (A) LMM of tree trait PC1 in the historical climatic centre–periphery approach and (B) LMM of leaf trait PC2 in the geographical centre–periphery approach. GC, geographical centre; GP, geographical periphery; HC, historical centre; HP, historical periphery.
Ecological centre–periphery
The centre derived from the ENM was mainly dry, and contains the lowest temperatures in the species range (see Fig. 2). The periphery derived from the ENM was warmer and wetter towards the north and east. Conversely, the centre derived from the Mahalanobis and Euclidean distances showed a north–south gradient of temperature and precipitation, with the north being warmer and drier than the south (see Fig. 2). Meanwhile, the periphery presented temperature and precipitation values representative of the range of values found across the species’ distribution area, without specific climatic conditions. Regarding the PCA performed with tree traits, ecological central and peripheral populations were not differentiated in the PCA biplot (Fig. 4B, C). LMMs confirmed that there were no differences between populations located in the ecological centre compared with those located in the ecological periphery for either of the two components of the variation of tree traits (Table 2). In the same way, the PCA performed with leaf traits did not show a clear differentiation in traits between central and peripheral populations (Fig. 5B, C). LMMs confirmed that there were no differences between populations located in the ecological centre compared with those located in the ecological periphery for either of the two components of the leaf trait variation (Table 2).
Historical centre–periphery
Besides being a climatically stable area, this historical centre presented, both during the LGM and current times, lower precipitation levels than the historical periphery (see Supplementary Figs S3 and S4, and Table S2). For the PCA performed with tree traits, historical central and peripheral populations showed a differentiation in the multivariate space (Fig. 4D). The biplot of the PCA showed trees from central localities to the right of the plots presenting lower values of TrH and ToH than those located on the periphery. LMMs confirmed the significant differences between the centre and periphery in the PC1 of the tree traits (Table 2; Fig. 6A). Conversely, the PCA performed with leaf traits did not show a clear differentiation in traits between central and peripheral populations (Fig. 5D). LMM confirmed no significant differences between the centre and periphery in leaf traits, on either PC axis (Table 2).
DISCUSSION
In this study, we investigated which of three centre–periphery approaches better explains the morphological differentiation of the tree species Bulnesia sarmientoi. The spatial localizations of the three centres considered were not coincident, as was also reported in previous studies (e.g. Pironon et al., 2015). In particular, the non-correspondence between the geographic location of ecological and historical centres suggests that the location of the niche optimum has changed over time. The historical centroid approach evidenced a centre–periphery pattern only for tree traits, showing smaller trees in the historical centre than in the historical periphery. Otherwise, we found significant differences in leaf traits between the geographical centre and the periphery, mainly due to smaller SLA values toward the geographical centre with respect to the periphery. Previous studies had already reported that a historical approach significantly explained genetic and demographic traits (e.g. Pironon et al., 2015; Douda et al., 2019), but no previous studies have used these approaches to analyse morphological traits determinant of species performance.
Historical and geographic approaches explain trait variation: possible processes underlying the retrieved centre–periphery patterns
Spatially, the geographic, ecological and historical centre–periphery approaches were not concordant. Given these spatial and climatic differences, not all approaches explained trait variation. Climatically, there were similarities across approaches: the geographic, ecological and historical centres were drier than peripheral areas; however, they did not coincide in the temperature ranges, with the geographic centre being the warmest. The tree trait pattern obtained using the historical approach showed lower values of total height and trunk height towards the historical centre than towards the historical periphery. The historical centre encloses a putative climatic refugium for B. sarmientoi, which is currently a hotspot of genetic diversity and has been a climate-stable area from the LGM up to the present (Camps et al., 2018). Regarding environmental conditions, the LGM refugium (i.e. historical centre) persists in sites where the soil was formed by Andean sediments from the Pliocene (Ramos and Ghiglione, 2008; Iriondo, 2010), and where the driest climate conditions in the entire range have occurred over time (see Supplementary data Fig. S3 and S4, and Table S2). Interestingly, according to field observations, most sites within the proposed LGM refugium (i.e. the historical centre) showed a special type of forest commonly named ‘palosantales’; this association with palosantales was not identified with the other approaches. In palosantales, which are usually monospecific and with a higher population density than in mixed forests (see Fig. 1A, B), B. sarmientoi individuals showed much smaller trunk diameter and did not develop the size observed in mixed forests (Loto et al., 2018). Thus, the historical component explaining tree trait variation would be related to the persistence of this kind of forest in the historical centre. This could be either due to two not mutually exclusive factors that could have been modulated by historical processes, namely the lack of competition due to the absence of other forest species in this stressful area, and the direct effect of stressful conditions affecting tree traits. In this line, previous studies showed tree height is lower in low precipitation sites (Fulton, 1999; Moles et al., 2009). In addition, low height may indicate low soil fertility and richness (Fulton, 1999; Marks et al., 2016). Ongoing studies of species that coexist with B. sarmientoi in mixed forests, such as Aspidosperma quebracho-blanco and Prosopis spp., will enhance the understanding of whether this refugium was a favourable site only for B. sarmientoi.
According to the three centre–periphery approaches studied here, the geographical centre approach is the one that best represents the precipitation gradient, from a dry warm environment in the geographical centre to a warm rainy environment towards the periphery. The centre derived from the ecological approach was dry, but its temperature was highly variable, having areas that presented the highest temperature values and others that presented the lowest temperature values across the species range; therefore, the ecological centre as a whole did not represent an ‘extreme’ environment. The ecological periphery presented a similar situation. Given the climatic gradient captured by the geographical approach (and not by the ecological approach), the obtained pattern showing the highest specific leaf area values in the geographical periphery makes sense, since it is indicative of high resource availability and productive environments towards peripheral populations (Reich et al., 1998; Albert et al., 2010). Low specific leaf area values indicate dense leaves and low growth rates, and are associated with resource conservation and dominance in areas of stressful conditions (Díaz et al., 2004; Wright et al., 2004; Albert et al., 2010). This environmental response of specific leaf area is current and not historical (i.e. it was not revealed using the historical approach), which is consistent with the expected phenotypic plasticity of functional traits (see Díaz and Cabido, 2001; Wright et al., 2004; Violle et al., 2007).
The environmental characteristics of the geographic and historical centre–periphery approaches (i.e. low precipitation, high temperatures and resource-poor soils), and the observed trait patterns, show that B. sarmientoi is a stress-tolerant species. Furthermore, the mean value of specific leaf area for the studied populations was low to intermediate (see Supplementary data Table S1) compared with specific leaf area measurements taken at the global scale (Reich et al., 1998; Pérez-Harguindeguy et al., 2013), suggesting that it is a conservative species (sensuDíaz et al., 2004), i.e. it would have a conservative strategy in the use of resources, with low performance and productivity rates. On the other hand, in resource-rich environments, such as those occupied by the species in the Humid Chaco, Chiquitano Dry Forest or Pantanal (all peripheral environments under the geographic and historical approaches), tree height or the specific leaf area present values associated with a better performance (high tree height and high specific leaf area). However, our field observations registered low population abundance in these peripheral sites, suggesting that interspecific competition could be conditioning species performance. It seems that in the geographic and historical peripheral sites, individual traits are maximized, while in central sites (stressful environments), population performance is maximized.
The differential pattern between morphological traits and species abundance highlights that the former are better indicators of environmental pressures than species abundance, thus there is a need to include them more frequently in centre–periphery studies (Dallas et al., 2017; Santini et al., 2019). Although we analysed the tree and leaf traits as two datasets, and not as individual traits, we detected that the statistical differences were strongly influenced mainly by the tree height and the specific leaf area, as mentioned above. These results are consistent with previous studies, suggesting that both traits have a great response to environmental conditions (Reich et al., 1998; Fulton, 1999; Díaz et al., 2004; Moles et al., 2009; Albert et al., 2010; Cosacov et al., 2014). Instead, the remaining measured traits which did not show a strong centre–periphery pattern probably are less variable at the intraspecific level (Siefert et al., 2015), or they could be variable at a different spatial scale from the one considered in this study, associated with other environmental factors (e.g. soil properties, or environmental variables not considered here).
On the absence of some centre–periphery patterns
In disagreement with other studies (e.g. on population abundance, Martínez-Meyer et al., 2013; Van Couwenberghe et al., 2013; Weber et al., 2017), our results show that the ecological approach is a poor predictor of centre–periphery patterns, despite having tested two niche centroids derived from ENM output and from Mahalanobis and Euclidean distances. However, we know that an ecological approach does not necessarily explain trait variation, as reviewed for population abundance (Santini et al., 2019). Probably, as suggested by Dallas et al. (2017), there are important factors other than the environment involved in the regulation of population dynamics, such as dispersal boundaries and unmeasured ecological interactions. It should be taken into account that the ENM, used to define the niche centroid, is based on climatic similarities rather than on fitness (Osorio-Olvera et al., 2019). Another important consideration is that ENM is a current ‘snapshot’ of a particular moment in the history of the focal species, and the environment it occupies today may not be the one that maximizes its fitness. These theoretical limitations of the ecological centre–periphery approach are also applicable to the historical approach, since the latter is methodologically derived from the former. However, a key difference should be considered: areas that are climatically stable over time are informative about the persistence of the species in a specific geographical area, and therefore the approach is useful in demographic terms (see evidence of climatically stable areas inferred by ENM and genetic demographic analysis in Carstens and Richards, 2007; Waltari et al., 2007; Collevatti et al., 2012; Baranzelli et al., 2017, among others). Climatic stability (i.e. the historic centre) resulted in an area where the environmental conditions persisted over time and left their imprint, especially in tree traits that have a long-term environmental response. Hence, we suggest that the historical approach performs better than the ecological one in reflecting the suitability of a geographical area for a species. Future research should explore other methodologies to define centres and peripheries under an ecological approach, considering key factors shaping the form and function of morphological traits.
Another outstanding result was the lack of significant differences in tree traits in the geographical centre with respect to the geographical periphery. In the geographical central area, the most extreme climatic conditions or low resources occur, these being the conditions reported as causes of low values for total height (Marks et al., 2016). One possible explanation is the time scale, because tree height variation was associated with long-term processes, in contrast to more plastic traits, such as leaf size or specific leaf area (traits that evidenced a geographic centre–periphery pattern). On the other hand, the absence of a geographic gradient in tree traits is possibly the consequence of the fact that this gradient does not detect the optimal microsites for the species. These microsites present environmental characteristics, different from the surroundings, which positively affect the performance of a species (Dunwiddie and Martin, 2016; Mayoral et al., 2019). We consider that, unlike the other approaches, the historical approach returns these microsites because the historical centre is inferred based on long-term climatic suitability (with genetic patterns corroborating the long-lasting persistence of the species in that area), and climate is the main structuring factor (Keppel et al., 2012) also affecting other environmental factors such as edaphic conditions. However, in addition, the absence of a geographical gradient in tree traits may be due to the advanced process of deforestation and selective logging in the Gran Chaco (Mereles and Pérez de Molas, 2008; Hansen et al., 2013; Vallejos et al., 2015; Pometti et al., 2021). These threats, which are particularly intense in the mixed forests, could reduce the chances of sampling high trees.
Conclusions
Our results show that each trait dataset (i.e. tree and leaf trait) studied in Bulnesia sarmientoi showed a different pattern, explained by different approaches. The geographic approach explained leaf trait variation, probably associated with environmental suitability; the geographic centre presents the most severe climatic conditions of the distribution area, since it is an area characterized by dry conditions and high temperatures; and the historical approach allows the recovery of historical processes underlying tree trait variation. In the historical centre, a greater number of palosantales forests are present; historically this centre has been the driest area across the species geographic range. These patterns observed for the two morphological datasets suggest that both sets respond to environmental processes following a centre–periphery dynamic; while tree trait variation was associated with long-term processes, leaf trait patterns were associated with short-term conditions. The ecological approach was a poor predictor of centre–periphery patterns, probably because the measured traits could not capture the effect of meaningful ecological processes. Due to the dynamic nature of the geographical ranges, delimitation of the centre and the periphery should be based not only on current geographical and ecological perspectives, but also on historical ones.
Based on our research, we highlight the use of a historical perspective in the explanation of centre–periphery patterns, and the inclusion of morphological traits to understand species performance across their range, in addition to demographic, physiological or genetic traits. Our results showed that the historical approach could elucidate historical processes underlying morphological trait variation, reinforcing the importance of this approach and the use of morphological functional traits to understand environmental selective pressures. Future studies could explore centre–periphery morphological patterns under the three approaches used in our study in co-distributed species, to elucidate if there is a geographical, ecological or historical signal at the community level.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Supplementary Data 1: correlation matrices and mean values of tree and leaf morphological traits of Bulnesia sarmientoi. Supplementary Data 2: present and past climate in the historical centre and periphery of Bulnesia sarmientoi. Figure S1: correlation matrices of tree traits. Figure S2: correlation matrices of leaf traits. Figure S3: random points created in the Bulnesia sarmientoi distribution area and in the LGM refuge area. Figure S4: dispersion plot of the present and past climate. Table S1: mean values of tree and leaf morphological traits in the 24 populations studied. Table S2: differences of annual mean temperature and annual precipitation, in the historical centre and periphery, given by the Tukey test applied to ANOVA
ACKNOWLEDGEMENTS
A.C. and A.N.S. acknowledge the National Research Council of Argentina (CONICET) and the Universidad Nacional de Córdoba (UNC) as researchers, and G.A.C. as a doctoral fellowship holder. The authors thank Viviana Rojas Bonzi for helpful comments on the manuscript. We thank Jorgelina Brasca for reviewing the English text. We are grateful to IMBIV (UNC-CONICET) and IFRGV (CIAP, INTA). Finally, we thank the Handling Editor, Dr Alex Fajardo, and the anonymous reviewers for their helpful comments. We declare that the research work was not carried out in the presence of any personal, professional or financial relationships that could potentially be construed as a conflict of interest.
FUNDING
This work was supported by Instituto Nacional de Tecnología Agropecuaria (grant no. PNFOR-1104064 to S.N.M.P.), Fondo para la Investigación Científica y Tecnológica (grant nos PICT-2015-3089 and PICTO-2014-0013 to A.N.S.) and Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina (grant no. PIP-11220150100690CO to A.N.S.).
LITERATURE CITED
- Abeli T, Gentili R, Mondoni A, Orsenigo S, Rossi G. 2014. Effects of marginality on plant population performance. Journal of Biogeography 41: 239–249. [Google Scholar]
- Abeli T, Ghitti M, Sacchi R. 2020. Does ecological marginality reflect physiological marginality in plants?. Plant Biosystems 154: 149–157. [Google Scholar]
- Adamoli J, Neumann R, De Colina AD, Morello J. 1972. El Chaco aluvional salteño. Revista de Investigaciones Agropecuarias 9: 165–237. [Google Scholar]
- Albert CH, Thuiller W, Yoccoz NG, Douzet R, Aubert S, Lavorel, S. 2010. A multi-trait approach reveals the structure and the relative importance of intra- vs. interspecific variability in plant traits. Functional Ecology 24: 1192–1201. [Google Scholar]
- Baranzelli MC, Cosacov A, Ferreiro G, Johnson LA, Sérsic AN. 2017. Travelling to the south: phylogeographic spatial diffusion model in Monttea aphylla (Plantaginaceae), an endemic plant of the Monte Desert. PLoS One 12: e0178827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranzelli MC, Cosacov A, Rocamundi N, et al. 2020. Volcanism rather than climatic oscillations explains the shared phylogeographic patterns among ecologically distinct plant species in the southernmost areas of the South American Arid Diagonal. Perspectives in Plant Ecology, Evolution and Systematics 45: 125542. [Google Scholar]
- Benavides R, Valladares F, Wirth C, Müller S, Scherer-Lorenzen M. 2019. Intraspecific trait variability of trees is related to canopy species richness in European forests. Perspectives in Plant Ecology, Evolution and Systematics 36: 24–32. [Google Scholar]
- Brown JH. 1984. On the relationship between abundance and distribution of species. The American Naturalist 124: 255–279. [Google Scholar]
- Busby JR. 1991. BIOCLIM: a bioclimate analysis and prediction system. In: Margules CR, Austin MP, eds. Nature conservation: cost effective biological surveys and data analysis. Canberra: CSIRO, 64–68. [Google Scholar]
- Cabrera A. 1976. Regiones fitogeográficas argentinas. Enciclopedia Argentina de Agricultura y Jardinería, 2nd edn. Buenos Aires: ACME. [Google Scholar]
- Camps GA, Martínez-Meyer E, Verga AR, Sérsic AN, Cosacov A. 2018. Genetic and climatic approaches reveal effects of Pleistocene refugia and climatic stability in an old giant of the Neotropical Dry Forest. Biological Journal of the Linnean Society 125: 401–420. [Google Scholar]
- Carey PD, Watkinson AR, Gerard FFO. 1995. The determinants of the distribution and abundance of the winter annual grass Vulpia ciliata ssp. ambigua. Journal of Ecology 83: 177–187. [Google Scholar]
- Carstens BC, Richards CL. 2007. Integrating coalescent and ecological niche modeling in comparative phylogeography. Evolution 61: 1439–1454. [DOI] [PubMed] [Google Scholar]
- Clark JS, Bell DM, Hersh MH, et al. 2011. Individual-scale variation, species-scale differences: inference needed to understand diversity. Ecology Letters 14: 1273–1287. [DOI] [PubMed] [Google Scholar]
- Collevatti RG, Terribile LC, Lima-Ribeiro MS, et al. 2012. A coupled phylogeographical and species distribution modelling approach recovers the demographical history of a Neotropical seasonally dry forest tree species. Molecular Ecology 21: 5845–5863. [DOI] [PubMed] [Google Scholar]
- Cosacov A, Cocucci AA, Sérsic AN. 2014. Geographical differentiation in floral traits across the distribution range of the Patagonian oil-secreting Calceolaria polyrhiza: do pollinators matter? Annals of Botany 113: 251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J, Castro S, Loureiro J, Barrett SC. 2016. Variation in style morph frequencies in tristylous Lythrum salicaria in the Iberian Peninsula: the role of geographical and demographic factors. Annals of Botany 117: 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas T, Decker RR, Hastings A. 2017. Species are not most abundant in the centre of their geographic range or climatic niche. Ecology Letters 20: 1526–1533. [DOI] [PubMed] [Google Scholar]
- Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW. 2018. InfoStat versión 2018.Argentina: Grupo InfoStat, FCA, Universidad Nacional de Córdoba. http://www.infostat.com.ar. [Google Scholar]
- Díaz S, Cabido M. 2001. Vive la difference: plant functional diversity matters to ecosystem processes. Trends in Ecology and Evolution 16: 646–655. [Google Scholar]
- Díaz S, Hodgson JG, Thompson K, et al. 2004. The plant traits that drive ecosystems: evidence from three continents. Journal of Vegetation Science 15: 295–304. [Google Scholar]
- Ding J, Travers SK, Eldridge DJ. 2020. Grow wider canopies or thicker stems: variable response of woody plants to increasing dryness. Global Ecology Biogeography 30: 183–195. [Google Scholar]
- Dinno A. 2018. paran: Horn’s test of principal components/factors. R package version 1.5.2. https://CRAN.R-project.org/package=paran.
- Douda J, Doudová J, Hodková E, Vít P, Krak K, Mandák B. 2019. Population history explains the performance of an annual herb – within and beyond its European species range. Journal of Ecology 108: 958–968. [Google Scholar]
- Duncan SI, Crespi EJ, Mattheus NM, Rissler LJ. 2015. History matters more when explaining genetic diversity within the context of the core–periphery hypothesis. Molecular Ecology 24: 4323–4336. [DOI] [PubMed] [Google Scholar]
- Dunwiddie PW, Martin RA. 2016. Microsites matter: improving the success of rare species reintroductions. PLoS One 11: e0150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert CG, Samis KE, Lougheed SC. 2008. Genetic variation across species’ geographical ranges: the central–marginal hypothesis and beyond. Molecular Ecology 17: 1170–1188. [DOI] [PubMed] [Google Scholar]
- Fulton MR. 1999. Patterns in height–diameter relationships for selected tree species and sites in eastern Texas. Canadian Journal of Forest Research 29: 1445–1448. [Google Scholar]
- Hansen MC, Potapov PV, Moore R, et al. 2013. High-resolution global maps of 21st-century forest cover change. Science 342: 850–853. [DOI] [PubMed] [Google Scholar]
- Hatziskakis S, Tsiripidis I, Papageorgiou AC. 2011. Leaf morphological variation in beech (Fagus sylvatica L.) populations in Greece and its relation to their post-glacial origin. Botanical Journal of the Linnean Society 165: 422–436. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- Horn JL. 1965. A rationale and test for the number of factors in factor analysis. Psychometrika 30: 179–185. [DOI] [PubMed] [Google Scholar]
- Iriondo MH. 2010. Geología del Cuaternario en la Argentina. Santa Fe: Museo Provincial de Ciencias Naturales Florentino Ameghino. [Google Scholar]
- Jump AS, Woodward FI. 2003. Seed production and population density decline approaching the range-edge of Cirsium species. New Phytologist 160: 349–358. [DOI] [PubMed] [Google Scholar]
- Keppel G, Van Niel KP, Wardell-Johnson GW, et al. 2012. Refugia: identifying and understanding safe havens for biodiversity under climate change. Global Ecology and Biogeography 21: 393–404. [Google Scholar]
- Kleinschmit J. 1993. Intraspecific variation of growth and adaptive traits in European oak species. Annales des sciences forestières. France: EDP Sciences. [Google Scholar]
- Lang B, Geiger A, Oyunbileg M, et al. 2019. Intraspecific trait variation patterns along a precipitation gradient in Mongolian rangelands. Flora 254: 135–146. [Google Scholar]
- Lebrija-Trejos E, Reich PB, Hernández A, Wright SJ. 2016. Species with greater seed mass are more tolerant of conspecific neighbours: a key driver of early survival and future abundances in a tropical forest. Ecology Letters 19: 1071–1080. [DOI] [PubMed] [Google Scholar]
- Lesica P, Allendorf FW. 1995. When are peripheral populations valuable for conservation?. Conservation Biology 9: 753–760. [Google Scholar]
- Li Y, Zou D, Shrestha N, et al. 2020. Spatiotemporal variation in leaf size and shape in response to climate. Journal of Plant Ecology 13, 87–96. [Google Scholar]
- Lira-Noriega A, Manthey JD. 2014. Relationship of genetic diversity and niche centrality: a survey and analysis. Evolution 68: 1082–1093. [DOI] [PubMed] [Google Scholar]
- Loto DE, Gasparri I, Azcona M, García S, Spagarino C. 2018. Estructura y dinámica de bosques de palo santo en el Chaco Seco. Ecología Austral 28: 064-073. [Google Scholar]
- Macdonald SL, Llewelyn J, Moritz C, Phillips BL. 2017. Peripheral isolates as sources of adaptive diversity under climate change. Frontiers in Ecology and Evolution 5: 88. [Google Scholar]
- Marks CO, Muller-Landau HC, Tilman D. 2016. Tree diversity, tree height and environmental harshness in eastern and western North America. Ecology Letters 19: 743–751. [DOI] [PubMed] [Google Scholar]
- Martínez-Meyer E, Díaz-Porras D, Peterson AT, Yáñez-Arenas C. 2013. Ecological niche structure and rangewide abundance patterns of species. Biology Letters 9: 20120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoral C, van Breugel M, Turner BL, Asner GP, Vaughn NR, Hall JS. 2019. Effect of microsite quality and species composition on tree growth: a semi-empirical modeling approach. Forest Ecology and Management 432: 534–545. [Google Scholar]
- McGarigal K, Stafford S, Cushman S. 2000. Ordination: principal components analysis. In: Multivariate statistics for wildlife and ecology research. New York, NY: Springer, 19–80. [Google Scholar]
- Mereles F, Pérez de Molas L. 2008. Bulnesia sarmientoi Lorentz ex Griseb. (Zygophyllaceae): estudio de base para su inclusión en el Apéndice II de la Convención CITES. Lambaré: WWF Paraguay. [Google Scholar]
- Moles AT, Warton DI, Warman L, et al. 2009. Global patterns in plant height. Journal of Ecology 97: 923–932. [Google Scholar]
- Myking T, Yakovlev I. 2006. Variation in leaf morphology and chloroplast DNA in Ulmus glabra in the northern suture zone: effects of distinct glacial refugia. Scandinavian Journal of Forest Research 21: 99–107. [Google Scholar]
- Nantel P, Gagnon D. 1999. Variability in the dynamics of northern peripheral versus southern populations of two clonal plant species, Helianthus divaricatus and Rhus aromatica. Journal of Ecology 87: 748–760. [Google Scholar]
- Olson DM, Dinerstein E, Wikramanayake ED, et al. 2001. Terrestrial ecoregions of the world: a new map of life on Earth. Bioscience 51: 933–938. [Google Scholar]
- Osorio-Olvera L, Soberón J, Falconi M. 2019. On population abundance and niche structure. Ecography 42: 1415–1425. [Google Scholar]
- Pérez-Harguindeguy N, Díaz S, Garnier E, et al. 2013. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61: 167–234. [Google Scholar]
- Phillips SJ, Anderson RP, Schapire RE. 2006. Maximum entropy modeling of species geographic distributions. Ecological Modelling 190: 231–259. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team . 2020. nlme: linear and nonlinear mixed effects models. R package version 3.1-147, https://CRAN.R-project.org/package=nlme.
- Pironon S, Villellas J, Morris WF, Doak DF, García MB. 2015. Do geographic, climatic or historical ranges differentiate the performance of central versus peripheral populations?. Global Ecology and Biogeography 24: 611–620. [Google Scholar]
- Pironon S, Papuga G, Villellas J, Angert AL, García MB, Thompson JD. 2017. Geographic variation in genetic and demographic performance: new insights from an old biogeographical paradigm. Biological Reviews of the Cambridge Philosophical Society 92: 1877–1909. [DOI] [PubMed] [Google Scholar]
- Pometti C, Camps GA, Soldati MC, et al. 2021. Species without current breeding relevance but high economic value: Acacia caven, Acacia aroma, Acacia visco, Prosopis affinis, Prosopis caldenia and Gonopterodendron sarmientoi. In: Pastorino MJ, Marchelli P, eds. Low intensity breeding of native forest trees in Argentina. Cham: Springer, 295–318. [Google Scholar]
- Prado DE. 1993. What is the Gran Chaco vegetation in South America? II. A redefinition. Contribution to the study of flora and vegetation of the Chaco. VII. Candollea 48: 615–629. [Google Scholar]
- Ramos VA, Ghiglione MC. 2008. Tectonic evolution of the Patagonian Andes. Developments in Quaternary Science 11: 57–71. [Google Scholar]
- R Core Team . 2018. R: a language and environment for statistical computing.Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. 1997. From tropics to tundra: global convergence in plant functioning. Proceedings of the National Academy of Sciences, USA 94: 13730–13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Ellsworth DS, Walters MB. 1998. Leaf structure (specific leaf area) modulates photosynthesis–nitrogen relations: evidence from within and across species and functional groups. Functional Ecology 12: 948–958. [Google Scholar]
- RStudio Team . 2015. RStudio: integrated development for R. Boston, MA: RStudio, Inc. [Google Scholar]
- Sagarin RD, Gaines SD. 2002. The ‘abundant centre’ distribution: to what extent is it a biogeographical rule? Ecology letters 5: 137–147. [Google Scholar]
- Sagarin RD, Gaines SD, Gaylord B. 2006. Moving beyond assumptions to understand abundance distributions across the ranges of species. Trends in Ecology & Evolution 21: 524–530. [DOI] [PubMed] [Google Scholar]
- Santini L, Pironon S, Maiorano L, Thuiller W. 2019. Addressing common pitfalls does not provide more support to geographical and ecological abundant-centre hypotheses. Ecography 42: 696–705. [Google Scholar]
- Sexton JP, McIntyre PJ, Angert AL, Rice KJ. 2009. Evolution and ecology of species range limits. Annual Reviews of Ecology, Evolution, and Systematics 40: 415–436. [Google Scholar]
- Siefert A, Violle C, Chalmandrier L, et al. 2015. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecology Letters 18: 1406–1419. [DOI] [PubMed] [Google Scholar]
- Soulé M. 1973. The epistasis cycle: a theory of marginal populations. Annual Review of Ecology and Systematics 4: 165–187. [Google Scholar]
- Souza ML, Duarte AA, Lovato MB, Fagundes M, Valladares F, Lemos-Filho JP. 2018. Climatic factors shaping intraspecific leaf trait variation of a neotropical tree along a rainfall gradient. PLoS One 13: e0208512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzedakis PC, Emerson BC, Hewitt GM. 2013. Cryptic or mystic? Glacial tree refugia in northern Europe. Trends in Ecology & Evolution 28: 696–704. [DOI] [PubMed] [Google Scholar]
- Vallejos M, Volante JN, Mosciaro MJ, Vale LM, Bustamante ML, Paruelo JM. 2015. Transformation dynamics of the natural cover in the Dry Chaco ecoregion: a plot level geo-database from 1976 to 2012. Journal of Arid Environments 123: 3–11. [Google Scholar]
- Van Couwenberghe R, Collet C, Pierrat JC, Verheyen K, Gégout JC. 2013. Can species distribution models be used to describe plant abundance patterns? Ecography 36: 665–674. [Google Scholar]
- Vapnik V. 1998. Statistical learning theory. New York: Wiley. [Google Scholar]
- Varsamis G, Karapatzak E, Tseniklidou K, Merou Th, Tsiftsis S. 2020. Plant morphological variability at the distribution edges: the case of Dryas octopetala (Rosaceae) in northern Greece. Willdenowia 50: 267–277. [Google Scholar]
- Vega GC, Pertierra LR, Olalla-Tárraga MÁ. 2017. MERRAclim, a high-resolution global dataset of remotely sensed bioclimatic variables for ecological modelling. Scientific Data 4: 170078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verga A. 2015. Hoja 3.6. Instituto de Fisiología y Recursos Genéticos Vegetales, CIAP, INTA. Free distribution software (contact: anibal.r.verga@gmail.com).
- Violle C, Navas ML, Vile D, et al. 2007. Let the concept of trait be functional! Oikos 116: 882–892. [Google Scholar]
- Vu VQ. 2016. Ggbiplot: a ggplot2 based biplot. R package version 0.55. 2011. http://github.com/vqv/ggbiplot.
- Waller T, Barros M, Draque J, Micucci P. 2012. Conservation of the Palo Santo tree, Bulnesia sarmientoi Lorentz ex Griseb, in the South America Chaco Region. Medicinal Plant Conservation 15: 4–9. [Google Scholar]
- Waltari E, Hijmans RJ, Peterson AT, Nyári AS, Perkins SL, Guralnick RP. 2007. Locating pleistocene refugia: comparing phylogeographic and ecological niche model predictions. PloS One 2: e563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MM, Stevens RD, Diniz-Filho JAF, Grelle CEV. 2017. Is there a correlation between abundance and environmental suitability derived from ecological niche modelling? A meta-analysis. Ecography 40: 817–828. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Zuloaga OF, Morrone O, Belgrano MJ. (eds).2008. Catálogo de Las Plantas Vasculares Del Cono Sur: Argentina, Sur de Brasil, Chile, Paraguay y Uruguay. St. Louis: Missouri Botanical Garden Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.